Abstract
Objective:
Prior studies have shown improved neurocognition with initiation of antiretroviral treatment (ART) in HIV. We hypothesized that stopping ART would be associated with poorer neurocognitive function.
Methods:
Neurocognitive function was assessed as part of ACTG 5170, a multicenter, prospective observational study of HIV-infected subjects who elected to discontinue ART. Eligible subjects had CD4 count >350 cells/mm3, had HIV RNA viral load <55,000 cp/mL, and were on ART (≥2 drugs) for ≥6 months. Subjects stopped ART at study entry and were followed for 96 weeks with a neurocognitive examination.
Results:
A total of 167 subjects enrolled with a median nadir CD4 of 436 cells/mm3 and 4.5 median years on ART. Significant improvements in mean neuropsychological scores of 0.22, 0.39, 0.53, and 0.74 were found at weeks 24, 48, 72, and 96 (all p < 0.001). In the 46 subjects who restarted ART prior to week 96, no significant changes in neurocognitive function were observed.
Conclusion:
Subjects with preserved immune function found that neurocognition improved significantly following antiretroviral treatment (ART) discontinuation. The balance between the neurocognitive cost of untreated HIV viremia and the possible toxicities of ART require consideration.
Classification of evidence:
This study provides Class III evidence that discontinuing ART is associated with an improvement in 2 neuropsychological tests (Trail-Making Test A & B and the Wechsler Adult Intelligence Scale–Revised Digit Symbol subtest) for up to 96 weeks. Resuming ART was not associated with a decline in these scores for up to 45 weeks.
GLOSSARY
- ACTG
= AIDS Clinical Trials Group;
- ART
= antiretroviral treatment;
- EFV
= efavirenz;
- HAART
= highly active antiretroviral therapy;
- HAD
= HIV-associated dementia;
- NP
= neuropsychological summary score;
- TI
= treatment interruption;
- VL
= viral load.
HIV enters the CNS within days of infection, and can result in nervous system disease including HIV-associated dementia (HAD) and the less severe dysfunction mild neurocognitive disorder. Antiretroviral therapy (ART) has led to prolonged survival in patients with HIV,1–3 and a dramatic decrease in the incidence of HAD.4
In the past, many HIV-positive patients with preserved immune function were prescribed ART, consistent with treatment guidelines at the time.5–7 After the recognition that ART led to significant toxicities and did not eradicate latent HIV infection, updated guidelines recommended delayed treatment initiation.8,9 There are conflicting results as to whether these patients can safely discontinue ART.10–12
Studies have shown that cognitive deficits are associated with ongoing viral replication in the CNS and that neurocognitive functioning improves in patients who initiate ART.13–18 This recovery of function suggests that the underlying mechanism includes an alteration of neuronal function prior to actual cell death. Since CNS viral replication is known to occur from the initial days of infection, it is likely that neuronal function is being impaired in the long asymptomatic period of HIV infection, resulting in subclinical cognitive impairment.
Since neurocognitive functioning improves with ART, we hypothesized that neurocognition would get worse in patients who discontinue treatment. We sought to evaluate neurocognitive functioning after treatment interruption (TI) in a population who had early treatment initiation. We also sought to evaluate potential neurocognitive improvement on ART in those patients who resumed ART, following a TI.
METHODS
AIDS Clinical Trials Group (ACTG) 5170 was a multicenter, observational, prospective, 2-step study in asymptomatic HIV-infected subjects who wished to discontinue ART in the United States.11 In step 1, eligible subjects elected to stop antiretroviral therapy; in step 2, patients from step 1 reinitiated antiretroviral therapy. This study design provided a Class III level of evidence.
Standard protocol approvals, registrations, and patient consents.
This study, ACTG A5170, is registered with ClinicalTrials.gov under the identifier NCT00050284. The study was approved by the institutional review boards at each site. All subjects gave written consent prior to enrollment.
Subjects.
Subjects were included for step 1 if they had confirmed HIV-1 infection, age >12 years, CD4 count >350 cells/mm3 immediately prior to first ART, CD4 count >350 cells/mm3 and plasma HIV-1 RNA viral load (VL) <55,000 copies/mL at screening, currently receiving ART with ≥2 drugs for ≥6 months, and a Karnofsky score ≥70. Subjects were excluded if they had a prior Centers for Disease Control and Prevention category B or C event, active drug or alcohol use, or serious medical condition that would negatively impact participation in the protocol. Complete inclusion and exclusion criteria have been reported in the primary study report.11
Study design.
Subjects who underwent TI upon entry to step 1 were followed for up to 96 weeks. Neurocognitive assessments were conducted every 24 weeks. Subjects were eligible for step 2 if subject or provider desired to reinitiate ART, and were followed for at least 24 weeks, or 96 weeks from step 1 entry (whichever was longer). The protocol strongly recommended that subjects resume ART if the CD4 cell count fell to ≤250 cells/mm3.
The neurocognitive examination consisted of Trail-Making Test A & B19 and the Wechsler Adult Intelligence Scale–Revised Digit Symbol subtest.20 These tests are sensitive in detecting both HIV-related neurocognitive changes21,22 and antiretroviral effects upon CNS functions.13 The raw test score of each measurement was standardized using demographic-adjusted normative means, which adjust for gender, age, education, and ethnicity (Caucasian and African American).23 A standardized z score was calculated by subtracting the appropriate normative mean from the raw score then dividing by the appropriate normative SD for each test. A neuropsychological summary score (NP) was created for the analyses, by averaging the z scores across the 3 tests. Neurocognitive impairment was defined as 1 SD below the mean on 2 of the 3 tests or 2 SDs below the mean on 1 of 3 tests. Sustained impairment required subjects to meet the impairment criteria for 2 consecutive visits.
The changes in NP score from the baseline to weeks 24, 48, 72, and 96 in step 1 and step 2 were analyzed using t test for paired data. The linear and quadratic trends of the NP scores in both steps were examined using generalized estimating equation embedded in SAS PROC GENMOD procedure (Cary, NC). To take into account the practice effect and possible informative dropout, study week and the dropout time were included in the regression model as covariates.24 In addition, 2 sensitivity analyses were performed using the t test for paired data. In one analysis, we compared the change in NP score after week 48 when the practice effect was considered to be leveled out. In the other analysis, we repeated the above analyses by only including subjects who completed the 96 weeks of follow-up without resumption of ART. Subgroup analyses assessing the effect of efavirenz (EFV) (Sustiva, Princeton, NJ, Bristol-Myers Squibb), ABC (Ziagen, GSK, RTP, NC), ZDV (Retrovir, GSK), D4T (Zerit, BMS, New York, NY), and baseline HIV RNA category (>50 cps/mL vs ≤50 cps/mL) in step 1 were performed. All tests were 2-sided. The p values were not adjusted for multiple tests and p < 0.05 was considered significant.
RESULTS
Study population.
Demographics characteristics of the 167 subjects enrolled are presented in tables 1 and 2. The median duration of ART prior to TI was 4.5 years, and the median duration of follow-up was 96 weeks after TI and 45 weeks after reinitiation of ART. A total of 144 subjects completed the study, including 102 who remained off ART for 96 weeks. A total of 137 (82%) subjects had VL ≤400 copies/mL at entry. The immunologic and virologic outcomes are detailed elsewhere.11
Table 1 Demographics at baseline
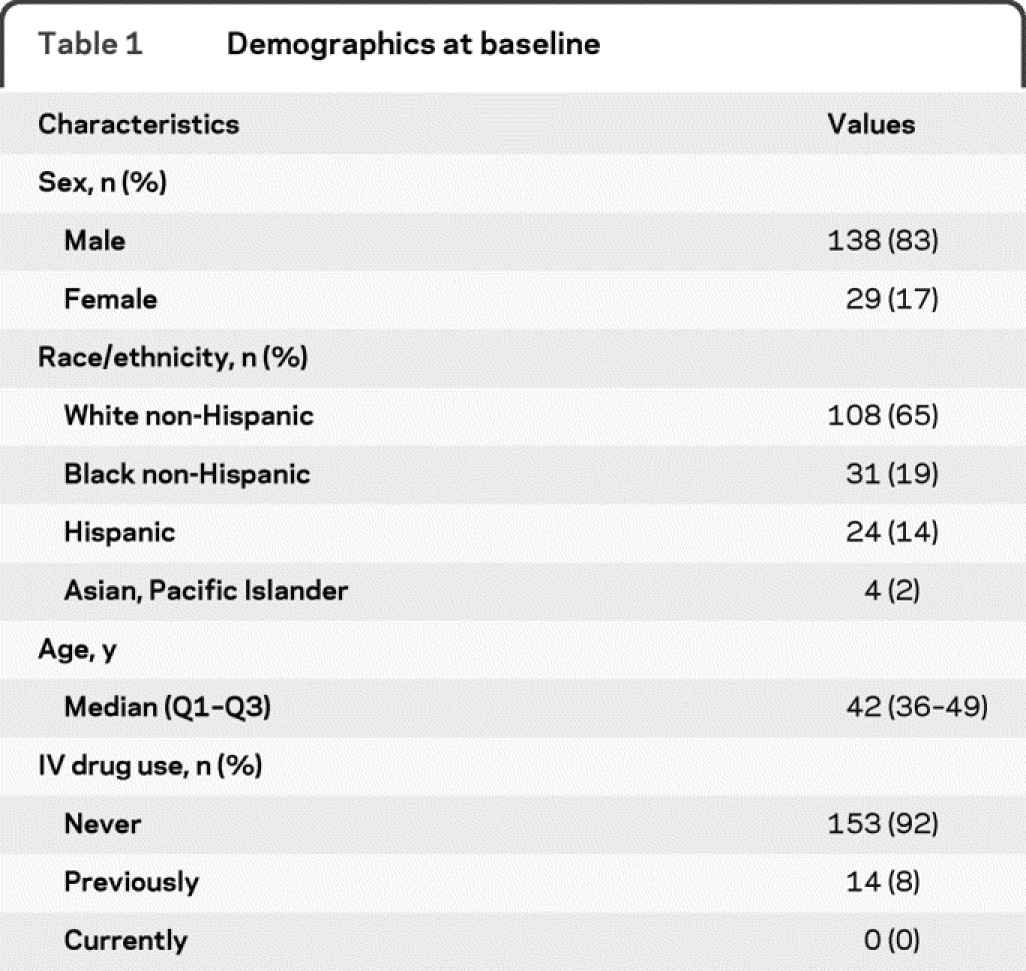
Table 2 Disease and treatment characteristics at baseline

Improved neurocognition when stopping antiretrovirals.
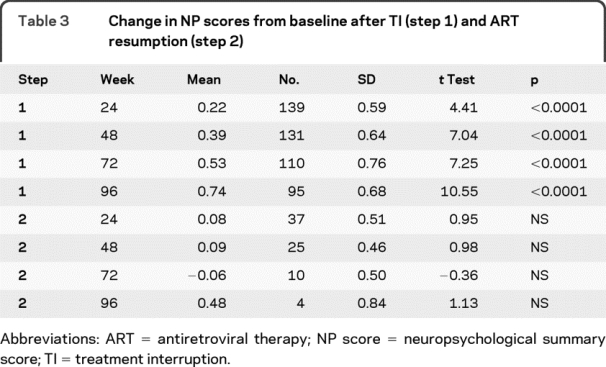
Mean NP scores increased when subjects stopped ART (figure 1). Significant mean NP score improvements of 0.22, 0.39, 0.53, and 0.74 were found at weeks 24, 48, 72, and 96 (all p < 0.001) (see table 3). The effect sizes (d) associated with the observed changes ranged from medium (0.37 at 24 weeks) to large (1.08 at 96 weeks). Sensitivity analysis including only the 95 subjects who stayed in step 1 for 96 weeks gave similar results.

Figure 1 Plot of mean neuropsychological summary score following treatment interruption
Table 3 Change in NP scores from baseline after TI (step 1) and ART resumption (step 2)
Subsequent analyses were undertaken to better understand the unexpected results. Practice or learning effects are known to occur with test-retest in neurocognitive studies, tend to diminish with repeated testing, and level out at the third repeated assessment.24 Therefore, analyses were undertaken that examined changes at the third assessment on (48 weeks and after). From week 48 to 72, the 107 subjects had NP score improvements of 0.11 (p < 0.05); from week 48 to 96, the 95 subjects had NP score improvements of 0.33 (p < 0.0001). Practice or learning effect does not seem to account for the improving performance off antiretroviral therapy.
No neurocognitive improvements with ART resumption.
Forty-two subjects restarted ART and enrolled in step 2. There were no significant improvements in NP scores (figure 2). From ART resumption to week 24 on ART, NP scores improved 0.08 in the 37 subjects with data available; at week 48 of ART resumption, the mean improvement in NP scores was 0.09 in the 25 subjects still on study; at week 72 of ART resumption, NP scores decreased by 0.06 in the 10 subjects still on study; and at week 96 of ART resumption, the mean improvement in NP scores was 0.48 in the four subjects still on study at that time.

Figure 2 Plot of mean neuropsychological summary score following treatment resumption
Neurocognitive impairment.
For subjects stopping antiretroviral therapy (step 1), 68 out of 166 with baseline results were impaired (41%). There were 41 of 167 (25%) subjects with sustained impairment at some point during step 1. There were 40 out of 166 with impairment at baseline but who later became unimpaired on step 1 (24%). For subjects who resumed ART on step 2, 9 out of 39 subjects were impaired at baseline (23%). There were 9 of 41 with sustained impairment at some point during step 2 (22%). There were 3 out of 39 with impairment at step 2 baseline but who later became unimpaired (8%).
Informative dropout.
We were concerned that potential dropout of impaired subjects during step 1 created the appearance of improved neurocognitive scores over the course of step 1. Therefore, we compared the NP scores in step 1 between subjects who stayed on step 1 for 96 weeks to those who later went to step 2. Between-group comparisons at week 24, 48, and 72 found no significant differences between these subjects (all p > 0.17, data not shown, comparison at week 96 was not done because of insufficient data). This suggests that the increases in performance were not due to informative dropout. Among the subjects reinitiating ART, we also compared the baseline NP scores at step 1 to the baseline NP scores at step 2. Of the 39 subjects who had NP scores at entry to both step 1 and step 2, there was a mean increase of 0.34 (p < 0.001) at step 2 baseline compared to step 1 baseline.
In the regression analysis that simultaneously modeled the change in NP score and time of dropout using generalized estimating equation, the time of dropout was not significant in step 1, and was marginally significant in step 2. However, the dropout effect was estimated to be −0.19 for every 24 weeks, suggesting that subjects who dropped out of step 2 early had a greater 0.19 increase in NP score from baseline compared with those who dropped out 24 weeks later.
Neurocognition and virologic/immune system outcomes.
To assess potential differences related to HIV VL, we stratified the sample into those with baseline HIV RNA below (n = 89) and at or above 50 cps/mL (n = 50). No differences in neurocognitive functioning were found between the HIV RNA VL groups at 24, 48, 72, or 96 weeks. Sensitivity analyses restricting the sample to only those who completed 96 weeks off ART found similar results with no differences over time between the 2 groups. To assess the effect of immune system functioning, we stratified the group by using the median nadir CD4+ cell count (436) into those below (n = 70) and above (n = 69). Those below had mean lower NP scores (0.29) at week 72 than those above the median (0.69). In the sensitivity analyses restricting the sample to those who remained off ART for 96 weeks, we found a trend only (p = 0.08).
Neurocognition and EFV, ABC, ZDV, and D4T.
Previous studies have found that neurocognitive side effects were associated with the use of EFV.25 We stratified the sample by EFV use to see if there were differences in neurocognitive improvement between the groups. Significant increases were noted in both subgroup analyses of subjects discontinuing EFV-containing regimens (baseline n = 40; NP −0.77) and non-EFV-containing regimens (baseline n = 126; NP −0.62). For the non-EFV group subjects, the mean NP score improved 0.14, 0.34, 0.50, and 0.67 over weeks 24, 48, 72, and 96 (p < 0.05); and for the EFV group subjects, the mean NP score improved 0.38, 0.59, 0.77, and 0.96 over weeks 24, 48, 72, and 96 (p < 0.001). The difference in mean NP scores between the 2 groups was significant (p = 0.024, Wei-Johnson test). Although there was some evidence of greater neurocognitive improvement in subjects discontinuing EFV-based regimens, both EFV and non-EFV groups showed significant increases in mean neurocognitive scores at each week compared to baseline. Similar analyses for subgroups who reported stopping ZDV (n = 74) and those not on ZDV (n = 65); stopping D4T (n = 49) and those not (n = 90); and stopping ABC (n = 36) and those not (n = 103) did not find differences in neurocognitive functioning over weeks 24, 48, 72, and 96 between these subgroups.
DISCUSSION
HIV enters the CNS within days of initial infection, and is resident within the CNS throughout the prolonged asymptomatic period of HIV infection. CNS damage is thought to be associated with HIV viremia and inflammatory responses.26,27 It is known that highly active antiretroviral therapy (HAART) improves neurocognitive functioning.13,14,28–30 We expected that neurocognitive functioning would become worse when patients stopped taking antiretroviral therapy. Contrary to our hypothesis, neurocognitive functioning significantly improved after patients stopped treatment. This improvement continued over the course of the 96-week follow-up of the study among the patients remaining off ART. Based on the effect sizes, the magnitude of the observed improvement is likely to be clinically meaningful.
Why might this have occurred in this healthy population stopping antiretroviral therapy? There are several potential explanations. It is possible that these patients had subtle CNS drug-related toxicity that improved when treatment was stopped. It is known that repeated neuropsychological assessment can result in practice or learning effects, where improvement in functioning is due to practice or learning. However, studies have shown that practice or learning effects level off after 3 assessments.24 We assessed whether the observed improvement could be attributed to practice or learning effects. We observed continued improvement from 48 weeks out (third testing) on the study, indicating that the improvements are not likely to be solely attributed to practice or learning effects. It is possible that the follow-up period on study was not long enough to document changes. However, this seems unlikely since the median duration of follow-up was 96 weeks, a duration 4 times longer than those associated with improvement when starting antiretrovirals. Although unlikely, it is possible that prior antiretroviral regimens did not have benefit on the CNS. Antiretrovirals thought to enter the CNS were widely represented in the baseline ART regimens.28 It is also possible that there was a patient selection bias, so that subjects who elected to stop antiretrovirals were somehow more likely to have neurocognitive improvement. It may also be that the brief neuropsychological battery was not sensitive enough to detect declines. However, significant improvements were detected, indicating that the battery was sensitive enough in this study. Although brief, these tests have been found to be sensitive21,22 and specific31 in detecting HIV-related neurocognitive changes.
We also noted a lack of substantial neurocognitive improvement with resumption of ART. Neurocognitive improvement with initiation of HAART has been documented in many studies of patients who were naïve or failing current therapy. However, these studies were of patients who initiated treatment later in the disease course. Patients later in the disease may have greater inflammation within the CNS, or greater VL, and therapeutic intervention may have a greater benefit. As the virus is present in the CNS early in the disease process, it is likely that a threshold of neuronal damage must be reached for subclinical and clinical findings to be demonstrable. Once the viral replication has exceeded this threshold, clinical findings are readily apparent. Early in disease, cognitive reserves may allow for continued near-normal functioning. Treatment initiation at this early stage of disease may not result in the acute cognition gains that have been found with treatment at later disease stages.32
There was fluctuation in the level of impairment observed during this study. At baseline, 41% of the subjects were impaired, the majority of whom (59%) later became unimpaired. Twenty-five percent of subjects had sustained impairment off treatment at some point, and a similar number (22%) of those who resumed ART had sustained impairment at some point. These findings are similar to a much larger study, the ACTG Linked Longitudinal Randomized Trial, which utilized the same brief neuropsychological battery and assessed 1,160 subjects.33 It is well-known that there are cognitive side effects of EFV,25 which could have played a role in this study. Due to these cognitive side effects, those subjects on EFV regimens might be expected to have greater neurocognitive improvement than those with non-EFV regimens. We investigated the potential cognitive side effect implications for this study by stratifying subjects into those who had been on an EFV-containing regimen vs those who had not. When comparing between the EFV and non-EFV groups, we found that the EFV group had greater improvement than the non-EFV group. We also found that both groups had significant cognitive improvement at all time points after discontinuing antiretroviral treatment. When other agents with possible impact on the CNS such as ZDV, ABC, and D4T were analyzed, there were no differences between groups who stopped regimens with these agents over time. We also investigated the relationship of VL and immune functioning to neurocognition in these patients as they stopped treatment. We found no relationship between neurocognitive functioning and baseline VL. There was a relationship with nadir CD4, but only at one time point.
There are several limitations to the present study that could be addressed in future studies investigating neurocognition and treatment interruption. We were unable to include an HIV+ untreated control group with similar disease severity in the current study, which may have allowed a more precise determination of the practice/learning effects. We did not assess for depression, anxiety, or substance abuse, although these were exclusionary criteria at diagnostic levels. We found improved neurocognitive functioning in this unique cohort of subjects who initiated antiretroviral treatment prior to immune impairment, and then later discontinued therapy. We were not able to attribute these results to practice and learning effects or the selective reinitiation of ART. It is possible that subtle antiretroviral toxicity may underlie the improvements measured after ART was stopped. Contrary to prior studies, significant neurocognitive improvement was not observed with resumption of ART in this unique cohort. This lack of observed improvement may be related to the earlier initiation of ART, where benefits occurred in other studies at a later disease stage, but may also be due to limited power. With the recent suggestion that earlier initiation of ART may improve clinical outcomes, the effect of ART as compared to that of unchecked HIV replication on neurocognitive function will require careful prospective study.34
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. Zhaohui Su and Dr. Scott Evans.
COINVESTIGATORS
A5170 team—Daniel Skiest, MD (University of Texas Southwestern Medical Center at Dallas, local site investigator [LSI], ACTU Grant 3 U01 AI046376-05); Tianna Petersen, MS (University of Texas Southwestern Medical Center at Dallas, study coordinator [SC], ACTU Grant 3 U01 AI046376-05); David Margolis, MD (University of North Carolina at Chapel Hill, LSI, ACTU Grant AI069423-03); Kevin Robertson, PhD (University of North Carolina at Chapel Hill, LSI, ACTU Grant AI069423-03); Deborah McMahon, MD (University of Pittsburgh, LSI, ACTU Grant AI069494-01); Nancy Mantz, MSN, CRNP (University of Pittsburgh, SC, ACTU Grant AI069494-01); Diane Havlir, MD (San Francisco General Hospital, LSI); Michele Downing, RN (San Francisco General Hospital, SC); Beverly Sha, MD (Rush University Medical Center, LSI, ACTU Grant AI069471); Jessica Shore, RN (Rush and Northwestern University, SC, ACTU Grant AI069471); Sylvia Stoudt, RN (Stanford University, SC, ACTU grant UOI-AI069556); Debbie Slamowitz, RN (Stanford University, SC, ACTU grant UOI-AI069556); Linda Meixner, RN (University of California, San Diego, SC, ACTU Grant AI069432); Susan Cahill, RN (University of California, San Diego, SC, ACTU Grant AI069432); Peter Frame, MD (University of Cincinnati, LSI, ACTG Grant AI-069513); Michelle Saemann, RN (University of Cincinnati, SC, ACTG Grant AI-069513); Alexandra Nesbitt, PA (University of North Carolina at Chapel Hill, LSI, ACTU Grant AI069423-01, GCRC Grant RR00046, Grant AI50410); Joseph Eron, Jr., MD (University of North Carolina at Chapel Hill, LSI, ACTU Grant AI069423-01, GCRC Grant RR00046, Grant AI50410); Timothy Wilkin, MD, MPH (Cornell Clinical Trials Unit, LSI, Columbia-Cornell ACTU Grant AI069470-01, Weill Medical College GCRC Grant M01 RR00047); Todd Stroberg, RN (Cornell Clinical Trials Unit, SC, Columbia-Cornell ACTU grant AI069470-01, Weill Medical College GCRC grant M01 RR00047); Charles Gonzalez, MD (New York University/NYC HHC at Bellevue Hospital Center, LSI, ACTU Grant 1U01AI069532-01, GCRC Grant M01-RR00096); Margarita Vasquez, RN (New York University/NYC HHC at Bellevue Hospital Center, SC, ACTU Grant 1U01AI069532-01, GCRC Grant M01-RR00096); Ann Collier, MD (University of Washington, Seattle, LSI, ACTU Grant AI069434); Shelia Dunaway, MD (University of Washington, Seattle, LSI, ACTU Grant AI069434); Karen T. Tashima, MD (The Miriam Hospital, LSI, Grant AI069472); Pamela Poethke, RN (The Miriam Hospital, SC, Grant AI069472); Ian Frank, MD (University of Pennsylvania, Philadelphia, LSI, ACTU Grant AI069467-01, CFAR Grant 5-P30-AI-045008-07); Joyce Okawa, RN (University of Pennsylvania, Philadelphia, SC, ACTU Grant AI069467-01, CFAR Grant 5-P30-AI-045008-07); Eric S. Daar, MD (Harbor-UCLA Medical Center, LSI, ACTU Grant AI069424); Sadia Shaik (Harbor-UCLA Medical Center, SC, ACTU Grant AI069424); Donna Mildvan, MD (Beth Israel Medical Center, LSI, Grant AI46370); Ronald D'Amico, DO (Beth Israel Medical Center, LSI, Grant AI46370); William A. O'Brien, MD (University of Texas Medical Branch at Galveston, LSI, ACTU Grant PHS 5U01AI032782); Gerianne Casey (University of Texas Medical Branch at Galveston, SC, ACTU Grant PHS 5U01AI032782); Connie Funk, RN, MPH (University of Southern California, SC, Grant 1UO1AI069428-01); Luis Mendez, BS (University of Southern California, SC, Grant 1UO1AI069428-01); Kimberly Gray, MSN, APRN, BC (Washington University in St. Louis, SC, ACTU Grant AI069495-01); Ge-Youl Kim, RN, BSN (Washington University in St. Louis, SC, ACTU Grant AI069495-01); Jane Reid, RNc, MS, ANP (University of Rochester, SC, ACTU Grant AI069511-01, GCRC Grant 5-MO1 RR00044); Carol Greisberger, RN (University of Rochester, SC, ACTU Grant AI069511-01, GCRC Grant 5-MO1 RR00044); Winston Cavert, MD (University of Minnesota, LSI, ACTU Grant AI27661); Christine Fietzer, RN (University of Minnesota, SC, ACTU Grant AI27661); Nathan Thielman, MD (Duke University Medical Center, LSI, ACTU Grant 1U01-AI069484); Lee McClurkin, RN (Duke University Medical Center, SC, ACTU Grant 1U01-AI069484); Deborah O'Connor, RN, MSN, CNP (Indiana University, SC, ACTU Grant AI25859); Mitchell Goldman, MD (Indiana University, LSI, ACTU Grant AI25859).
ACKNOWLEDGMENT
The A5170 study team thanks the patients who participated in 5170.
DISCLOSURE
Dr. Robertson has received funding for travel and speaker honoraria from GlaxoSmithKline, Boehringer Ingelheim, and Abbott, and research support from the NIH (NIAID 1U01AI068636 [NIMH PI], NIMH R01 MH067751 [Co-I], NINDS 5 U01 NS032228 [Co-I], and NIMH R01 MH081772 [Co-I]). Dr. Su reports no disclosures. Dr. Margolis serves on the editorial boards of the Journal of Virology and AIDS; has received speaker honoraria from Abbott, Gilead Sciences, Inc., Monogram Biosciences, Roche, GlaxoSmithKline, Boehringer Ingelheim, Merck Serono, Biotron Ltd., Trimeris, Inc., International AIDS Society USA, IAS-USA, and University of North Carolina Center for AIDS Research, UNC CFAR; serves on speakers' bureaus for Virco Pharmaceuticals L.L.C., Bristol-Myers Squibb, and Merck Serono; has received research support from Merck Serono, Bristol-Myers Squibb, the NIH (5 U01-AI067854-04 [Co-I], 5 U01 AI069423-03 [Co-I], NIAID 1 R01 MH085597-01A1 [PI], NIAID 1U19AI082608-01 [PI], NIAID 5 T32 AI007151-31 [PI], and NIAID 1 R34 AI084553-01 [PI]), and amfAR; and holds stock in Gilead Sciences, Inc. A. Krambrink reports no disclosures. Dr. Havlir serves on the editorial board of AIDS and EJIAS; and receives research support from the NIH (NIAID U01 AI069502 [PI], NICHD P01 HD059454 [PI], NIAID K24 AI51982 [PI], NIAID T32 AI060530 [PI], NIAID P30 AI27763 [Co-I], NIAID U01 AI062677 [PI], NIAID R01 AI051219 [Co-I]); CDC/Elizabeth Glaser Pediatric AIDS Foundation U62 CCU123541 [PI]; Adamas Pharmaceuticals ADS-TCAD-PO206 [PI]; and Abbott (drug donation for NIH funded study; Dr. Evans serves on scientific advisory boards for Genentech, Inc. and the HIV Neurobehavioral Research Center; serves as Guest Editor for the Drug Information Journal, Associate Editor for Case Studies in Business, Industry, and Government, and Academic Editor of PLoS Clinical Trials; has received honoraria from the NIH, the American Statistical Association, and the FDA for educational activities; serves as a consultant for CIS Biotech, Genentech, Inc., and Pfizer Inc.; serves on an advisory board for the FDA; and receives research support from the NIH (Statistical Center Support). Dr. Skiest has served on speaker's bureaus or received honoraria from for Gilead Sciences, Inc., Tibotec Therapeutics, GlaxoSmithKline, Bristol-Myers Squibb, and Merck; and receives research support from Gilead Sciences, Inc., Tibotec Therapeutics, Merck, Serono, Roche, GlaxoSmithKline, Pfizer, and the Schering-Plough Research Institute.
Address correspondence and reprint requests to Dr. Kevin R. Robertson, Department of Neurology, University of North Carolina, 2128 Physician Office Building, 170 Manning Drive, Chapel Hill, NC 27599-7025 kevinr@neurology.unc.edu
Editorial, page 1248
e-Pub ahead of print on March 17, 2010, at www.neurology.org.
Study funding: Supported in part by the NIH/NIAID (AI-68636 to AIDS Clinical Trials Group and AI068634 to the Statistical and Data Management Center) and by the General Clinical Research Center Units funded by the National Center for Research Resources.
Disclosure: Author disclosures are provided at the end of the article.
Presented in part at the 14th Conference on Retroviruses and Opportunistic Infections, Oral Abstract 113, Los Angeles, CA, February 25–28, 2007.
Received June 26, 2009. Accepted in final form February 2, 2010.
REFERENCES
- 1.Hogg RS, Yip B, Chan KJ, et al. Rates of disease progression by baseline CD4 cell count and viral load after initiating triple-drug therapy. JAMA 2001;286:2568–2577. [DOI] [PubMed] [Google Scholar]
- 2.Palella FJ, Jr., Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection: HIV Outpatient Study Investigators. N Engl J Med 1998;338:853–860. [DOI] [PubMed] [Google Scholar]
- 3.Palella FJ, Jr., Deloria-Knoll M, Chmiel JS, et al. Survival benefit of initiating antiretroviral therapy in HIV-infected persons in different CD4+ cell strata. Ann Intern Med 2003;138:620–626. [DOI] [PubMed] [Google Scholar]
- 4.Dore GJ, Correll PK, Li Y, Kaldor JM, Cooper DA, Brew BJ. Changes to AIDS dementia complex in the era of highly active antiretroviral therapy. AIDS 1999;13:1249–1253. [DOI] [PubMed] [Google Scholar]
- 5.Department of Health, Human Services, Henry J. Kaiser Family Foundation. Guidelines for the use of antiretroviral agents in HIV-infected adults, adolescents. MMWR Recomm Rep 1998;47:43–82. [PubMed]
- 6.Carpenter CC, Fischl MA, Hammer SM, et al. Antiretroviral therapy for HIV infection in 1997: updated recommendations of the International AIDS Society-USA Panel. JAMA 1997;277:1962–1969. [PubMed] [Google Scholar]
- 7.Carpenter CC, Fischl MA, Hammer SM, et al. Antiretroviral therapy for HIV infection in 1998: updated recommendations of the International AIDS Society-USA Panel. JAMA 1998;280:78–86. [DOI] [PubMed] [Google Scholar]
- 8.DHHS Panel on Clinical Practices for Treatment of HIV Infection. Guidelines for use of antiretroviral agents in HIV-infected adults and adolescents. Available at: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed September 1, 2009.
- 9.Yeni PG, Hammer SM, Hirsch MS, et al. Treatment for adult HIV infection: 2004 recommendations of the International AIDS Society-USA Panel. JAMA 2004;292:251–265. [DOI] [PubMed] [Google Scholar]
- 10.Ananworanich J, Gayet-Ageron A, Le Braz M, et al. CD4-guided scheduled treatment interruptions compared with continuous therapy for patients infected with HIV-1: results of the Staccato randomised trial. Lancet 2006;368:459–465. [DOI] [PubMed] [Google Scholar]
- 11.Skiest DJ, Su Z, Havlir DV, et al. Interruption of antiretroviral treatment in HIV-infected patients with preserved immune function is associated with a low rate of clinical progression: a prospective study by AIDS Clinical Trials Group 5170. J Infect Dis 2007;195:1426–1436. [DOI] [PubMed] [Google Scholar]
- 12.El-Sadr WM, Lundgren JD, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med 2006;355:2283–2296. [DOI] [PubMed] [Google Scholar]
- 13.Robertson KR, Robertson WT, Ford S, et al. Highly active antiretroviral therapy improves neurocognitive functioning. J Acquir Immune Defic Syndr 2004;36:562–566. [DOI] [PubMed] [Google Scholar]
- 14.McCutchan JA, Wu JW, Robertson K, et al. HIV suppression by HAART preserves cognitive function in advanced, immune-reconstituted AIDS patients. AIDS 2007;21:1109–1117. [DOI] [PubMed] [Google Scholar]
- 15.Sacktor N, Nakasujja N, Skolasky R, et al. Antiretroviral therapy improves cognitive impairment in HIV+ individuals in sub-Saharan Africa. Neurology 2006;67:311–314. [DOI] [PubMed] [Google Scholar]
- 16.Tozzi V, Balestra P, Galgani S, et al. Positive and sustained effects of highly active antiretroviral therapy on HIV-1-associated neurocognitive impairment. AIDS 1999;13:1889–1897. [DOI] [PubMed] [Google Scholar]
- 17.Marra CM, Lockhart D, Zunt JR, Perrin M, Coombs RW, Collier AC. Changes in CSF and plasma HIV-1 RNA and cognition after starting potent antiretroviral therapy. Neurology 2003;60:1388–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sacktor N, McDermott MP, Marder K, et al. HIV-associated cognitive impairment before and after the advent of combination therapy. J Neurovirol 2002;8:136–142. [DOI] [PubMed] [Google Scholar]
- 19.Reitan RM, Wolfson, D. The Halstead-Reitan Neuropsychological Test Battery. Tucson, AZ: Neuropsychology Press; 1985. [Google Scholar]
- 20.Wechsler D. Wechsler Adult Intelligence Scale–Revised Manual. San Antonio: Psychological Corporation; 1981. [Google Scholar]
- 21.Tross S, Price RW, Navia B, et al. Neuropsychological characterization of the AIDS dementia complex: a preliminary report. AIDS 1988;2:81–88. [DOI] [PubMed] [Google Scholar]
- 22.Van Gorp WG, Miller EN, Satz P, Visscher B. Neuropsychological performance in HIV-1 immunocompromised patients: a preliminary report. J Clin Exp Neuropsychol 1989;11:763–773. [DOI] [PubMed] [Google Scholar]
- 23.Heaton RK, Grant I, Matthews CG. Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographic Corrections, Research Findings, and Clinical Applications. Lutz, FL: Psychological Assessment Resources; 1991. [Google Scholar]
- 24.Beglinger LJ, Gaydos B, Tangphao-Daniels O, et al. Practice effects and the use of alternate forms in serial neuropsychological testing. Arch Clin Neuropsychol 2005;20:517–529. [DOI] [PubMed] [Google Scholar]
- 25.Clifford DB, Evans S, Yang Y, et al. Impact of efavirenz on neuropsychological performance and symptoms in HIV-infected individuals. Ann Intern Med 2005;143:714–721. [DOI] [PubMed] [Google Scholar]
- 26.Meeker RB, Robertson K, Barry T, Hall C. Neurotoxicity of CSF from HIV-infected humans. J Neurovirol 1999;5:507–518. [DOI] [PubMed] [Google Scholar]
- 27.Sevigny JJ, Albert SM, McDermott MP, et al. An evaluation of neurocognitive status and markers of immune activation as predictors of time to death in advanced HIV infection. Arch Neurol 2007;64:97–102. [DOI] [PubMed] [Google Scholar]
- 28.Letendre SL, McCutchan JA, Childers ME, et al. Enhancing antiretroviral therapy for human immunodeficiency virus cognitive disorders. Ann Neurol 2004;56:416–423. [DOI] [PubMed] [Google Scholar]
- 29.Sacktor NC, Lyles RH, Skolasky RL, et al. Combination antiretroviral therapy improves psychomotor speed performance in HIV-seropositive homosexual men: Multicenter AIDS Cohort Study (MACS). Neurology 1999;52:1640–1647. [DOI] [PubMed] [Google Scholar]
- 30.Liner KJ 2nd, Hall CD, Robertson KR. Effects of antiretroviral therapy on cognitive impairment. Curr HIV/AIDS Rep 2008;5:64–71. [DOI] [PubMed] [Google Scholar]
- 31.Ellis RJ, Evans SR, Clifford DB, et al. Clinical validation of the NeuroScreen. J Neurovirol 2005;11:503–511. [DOI] [PubMed] [Google Scholar]
- 32.Giancola ML, Lorenzini P, Balestra P, et al. Neuroactive antiretroviral drugs do not influence neurocognitive performance in less advanced HIV-infected patients responding to highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2006;41:332–337. [DOI] [PubMed] [Google Scholar]
- 33.Robertson KR, Smurzynski M, Parsons TD, et al. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS 2007;21:1915–1921. [DOI] [PubMed] [Google Scholar]
- 34.Kitahata MM, Gange SJ, Abraham AG, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med 2009;360:1815–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]


