Dorsal column spinal stimulation in dopamine-depleted rodents was recently reported to disrupt pathologic corticostriatal synchronization, alleviate akinesia, and restore locomotion.1 This claim has prompted consideration that spinal stimulation “might become an efficient and less invasive alternative for treatment of Parkinson disease (PD) in the future.”
In this study, we investigated whether dorsal column stimulation was of therapeutic benefit in 2 patients with PD.
Level of evidence.
This study provides Class II evidence that for patients with moderate to severe motor impairment from PD, high-frequency epidural cervical spinal cord stimulation does not significantly improve motor function as measured by the motor subsection of the Unified Parkinson's Disease Rating Scale (UPDRS).
Methods.
Two patients with PD had spinal stimulators (Medtronic models 3487a or 3898) implanted surgically into the high cervical epidural space without complication, as described previously.2 Patient 1, 75 years old, had moderate motor impairment (off/on medication motor UPDRS = 30/18). Patient 2, 77 years old, had more severe motor impairment (off/on medication UPDRS = 51/38) and was unable to walk without dopaminergic medication. Both patients met UK Brain Bank criteria for PD.3
Ten days postoperatively, both patients participated in a double-blind crossover study of the motor effects of spinal stimulation. In an initial exploratory phase, an antiparkinsonian effect of spinal stimulation was sought over a range of frequencies (30–300 Hz) and intensities (up to 4.0 V and 240 μs). Having failed to establish a clear benefit for any particular set of parameters, for the purposes of the study, patient 1 was assigned to receive 130 Hz stimulation (a common frequency employed for deep brain stimulation and beneficial in the rodent study) and patient 2 300 Hz (the most effective frequency in the rodent study). At those frequencies, stimulation intensity thresholds for the production of paresthesiae were established. Patients were then assessed in the off medication state (after overnight withdrawal of dopaminergic medication) during 3 conditions: off stimulation, subthreshold stimulation (without paresthesiae), and suprathreshold stimulation (with paresthesiae). Subthreshold and suprathreshold stimulation parameters were as follows: patient 1 130 Hz/2 V/240 μs and 130 Hz/3 V/240 μs; patient 2 300 Hz/3 V/200 μs and 300 Hz/4 V/200 μs. The ordering of conditions was counterbalanced so that each patient had 9 assessments. Outcomes were assessed ≥20 minutes after switching stimulation conditions (a period sufficient for most parkinsonian signs to return following subthalamic stimulation).4 The primary outcome measure was the motor subsection of the UPDRS (motor UPDRS, mean score of 2 blinded neurologists rating from videotape).5 Secondary outcomes included the timed Hand-Arm Movement test (number of alternating movements between 2 fixed points 30 cm apart in 30 s, mean of both sides), timed foot tapping score (number of foot taps in 30 s, mean of both sides), and time to walk 10 m test.6 Paresthesiae were rated by the subjects with a Visual Analogue Scale (10 cm VAS).7
Standard protocol approvals, registrations, and patient consents.
Ethical approval was granted by the local ethics committee and patients gave written informed consent.
Results.
Startle was not observed with changes to stimulation in either patient. VAS scores for the suprathreshold condition were greater than the off stimulation condition (mean 2.1 vs 0.68 cm, Z = −2.03, p = 0.04 Wilcoxon). VAS scores for the subthreshold and off stimulation conditions were not different (1.9 vs 0.68 cm, Z = −0.94, p = 0.35 Wilcoxon). There was strong interrater reliability between the motor UPDRS scoring of the 2 blinded neurologists (Spearman rho = 0.92).
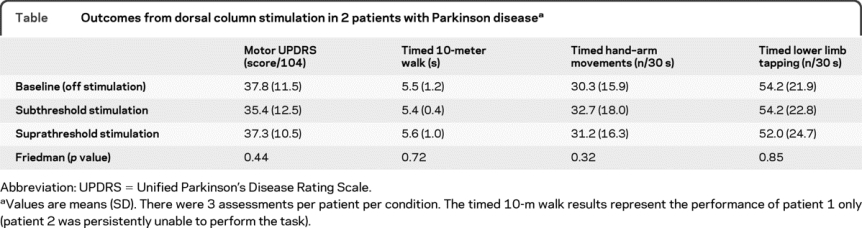
There was no difference in the primary outcome measure of motor UPDRS between stimulation conditions (χ2 = 1.65, p = 0.44 Friedman). There were no differences in any other outcome measure with stimulation (table). Dorsal column stimulation did not restore the locomotion of patient 2, who remained unable to walk in the off medication state.
Table Outcomes from dorsal column stimulation in 2 patients with Parkinson disease
Discussion.
We assessed whether the benefits of spinal stimulation, recently reported in parkinsonian rodents, could be translated to patients with PD. While it is not possible to prove that spinal stimulation in some form will never be of therapeutic value, we found that in 2 subjects, dorsal column stimulation delivered continuously at clinically acceptable stimulation intensities was not clinically beneficial.
The difference in outcomes between the 2 studies may reflect differences between species, models, or, alternatively, the differing methods of stimulation. In the rodent study, suprathreshold stimulation at intensities sufficient to produce startle was applied intermittently in 30–60 s bursts.1 Only during and shortly after stimulation did beneficial effects occur, which included reduced beta oscillations, alleviation of akinesia, and increased locomotion. The authors postulated that dorsal column stimulation may directly desynchronize pathologic oscillations, thereby providing a state permissive for movement.
However, an alternative mechanism is simply that bursts of startling stimulation increased arousal, thereby precipitating movement, as occurs with paradoxical kinesis. The authors of the rodent study controlled for startle-related phenomena by applying startling trigeminal stimulation, but at lower intensity thresholds than applied for spinal stimulation. Locomotion not only increased in parkinsonian rodents, but in healthy control rodents as well. Crucially, the effect of chronic stimulation was not reported so it is possible that habituation may have occurred.
Intermittent startling spinal stimulation that briefly precipitates locomotion by increasing arousal would not be an acceptable therapy in patients. However, we found that spinal stimulation administered continuously, at the same frequencies and at clinically acceptable intensities, failed to improve motor deficits in 2 carefully evaluated patients with PD.
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. W. Thevathasan and Dr. P. Brown.
*These authors contributed equally.
Study funding: Supported by the Medical Research Council (UK).
Disclosure: Dr. Thevathasan has received funding for travel from Medtronic, Inc. Dr. Mazzone has received funding for travel from Medtronic, Inc., and serves on the editorial board of Neuromodulation. Dr. Jha has received fellowship support from the Parkinson's Disease Society (UK). Dr. Djamshidian and Dr. Dileone report no disclosures. Dr. Di Lazzaro serves on scientific advisory boards for Medtronic, Inc.; serves on the editorial boards of Clinical Neurophysiology and Brain Stimulation and as editor of Case Report in Medicine; and receives research support from Institut des Recherches Internationales Servier. Dr. Brown has served as a consultant to Medtronic, Inc.
Received September 4, 2009. Accepted in final form January 28, 2010.
Address correspondence and reprint requests to Prof. Peter Brown, Sobell Department of Motor Neuroscience and Movement Disorders, UCL Institute of Neurology, 33 Queen Square, London, WCIN 3BG, UK; pbrown@ion.ucl.ac.uk
&NA;
- 1.Fuentes R, Petersson P, Siesser WB, Caron MG, Nicolelis MA. Spinal cord stimulation restores locomotion in animal models of Parkinson's disease. Science 2009;323:1578–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Insola A, Padua L, Mazzone P, Valeriani M. Unmasking of presynaptic and postsynaptic high-frequency oscillations in epidural cervical somatosensory evoked potentials during voluntary movement. Clin Neurophysiol 2008;119:237–245. [DOI] [PubMed] [Google Scholar]
- 3.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Temperli P, Ghika J, Villemure JG, Burkhard PR, Bogousslavsky J, Vingerhoets FJ. How do parkinsonian signs return after discontinuation of subthalamic DBS? Neurology 2003;60:78–81. [DOI] [PubMed] [Google Scholar]
- 5.Fahn S, Elton RL, UPDRS program members. Unified Parkinson's Disease Rating Scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M, ed. Recent Developments in Parkinson's Disease. Florham Park, NJ: Macmillan Healthcare Information; 1987:153–163, 293–304. [Google Scholar]
- 6.Defer GL, Widner H, Marie RM, Remy P, Levivier M. Core assessment program for surgical interventional therapies in Parkinson's disease (CAPSIT-PD). Mov Disord 1999;14:572–584. [DOI] [PubMed] [Google Scholar]
- 7.Huskisson E. Visual analogue scales. In: Pain Measurement and Assessment. New York: Raven Press; 1983. [Google Scholar]


