Abstract
Background
Since the discovery of deep-sea chemosynthesis-based communities, much work has been done to clarify their organismal and environmental aspects. However, major topics remain to be resolved, including when and how organisms invade and adapt to deep-sea environments; whether strategies for invasion and adaptation are shared by different taxa or unique to each taxon; how organisms extend their distribution and diversity; and how they become isolated to speciate in continuous waters. Deep-sea mussels are one of the dominant organisms in chemosynthesis-based communities, thus investigations of their origin and evolution contribute to resolving questions about life in those communities.
Methodology/Principal Finding
We investigated worldwide phylogenetic relationships of deep-sea Bathymodiolus mussels and their mytilid relatives by analyzing nucleotide sequences of the mitochondrial cytochrome c oxidase subunit I (COI) and NADH dehydrogenase subunit 4 (ND4) genes. Phylogenetic analysis of the concatenated sequence data showed that mussels of the subfamily Bathymodiolinae from vents and seeps were divided into four groups, and that mussels of the subfamily Modiolinae from sunken wood and whale carcasses assumed the outgroup position and shallow-water modioline mussels were positioned more distantly to the bathymodioline mussels. We provisionally hypothesized the evolutionary history of Bathymodilolus mussels by estimating evolutionary time under a relaxed molecular clock model. Diversification of bathymodioline mussels was initiated in the early Miocene, and subsequently diversification of the groups occurred in the early to middle Miocene.
Conclusions/Significance
The phylogenetic relationships support the “Evolutionary stepping stone hypothesis,” in which mytilid ancestors exploited sunken wood and whale carcasses in their progressive adaptation to deep-sea environments. This hypothesis is also supported by the evolutionary transition of symbiosis in that nutritional adaptation to the deep sea proceeded from extracellular to intracellular symbiotic states in whale carcasses. The estimated evolutionary time suggests that the mytilid ancestors were able to exploit whales during adaptation to the deep sea.
Introduction
Deep-sea mussels of the genus Bathymodiolus (Mytilidae, Bathymodiolinae) are one of the dominant macroorganisms in chemosynthesis-based communities in hydrothermal vents on spreading ridges and back-arc basins and in cold-water seeps along subduction zones. Since the original description of the genus [1], 22 Bathymodiolus species have been described [2]–[13], and their biogeographic distributions are as follows. There are: 1) 14 Pacific species, B. japonicus Hashimoto & Okutani 1994, B. platifrons Hashimoto & Okutani 1994, B. septemdierum Hashimoto & Okutani 1994, B. hirtus Okutani et al. 2004, B. securiformis Okutani et al. 2004, B. aduloides Hashimoto & Okutani 1994, B. taiwanensis Cosel 2008, B. brevior Cosel et al. 1994, B. elongates Cosel et al. 1994, B. tangaroa Cosel & Marshall 2003, B. manusensis Hashimoto & Furuta 2007, B. edisonensis Cosel and Janssen 2008, and B. anteumbonatus Cosel and Janssen 2008 from the West Pacific and B. thermophilus Kenk & Wilson, 1985 from the East Pacific; 2) seven Atlantic species, B. childressi Gustafson et al. 1998, B. heckerae Gustafson et al. 1998, and B. brooksi Gustafson et al. 1998 from the West Atlantic, B. azoricus Cosel & Comtet 1999 and B. puteoserpentis Cosel et al. 1994 from the Mid-Atlantic Ridge, and the trans-Atlantic B. mauritanicus Cosel 2002 and B. boomerang Cosel & Ole 1998; and 3) one Indian Ocean species, B. marisindicus Hashimoto 2001. Two species of the genus Gigantidas from the West Pacific, G. horikoshii Hashimoto &Yamane 2005 and G. gladius Cosel & Marshall 2003, and one species of the genus Tamu from the Atlantic, T. fisheri Gustafson et al. 1998, belong to the subfamily Bathymodiolinae [5], [9], [14]. Active exploration of new localities and careful surveys of known localities suggests the existence of many cryptic species. The species diversity is very high in the West Pacific compared with other areas, and thus the origin of the bathymodioline mussels seems to be located in the West Pacific. However, the mismatch distributions of the West Pacific B. septemdierum and B. brevior and the Indian Ocean B. marisindicus suggest that the Southern Central Indian Ridge of the Indian Ocean might be the more ancient residence rather than the Izu-Ogasawara Island-arc and the North Fuji Basin of the West Pacific, if periods from formation to expansion of their populations were not significantly different among them [15].
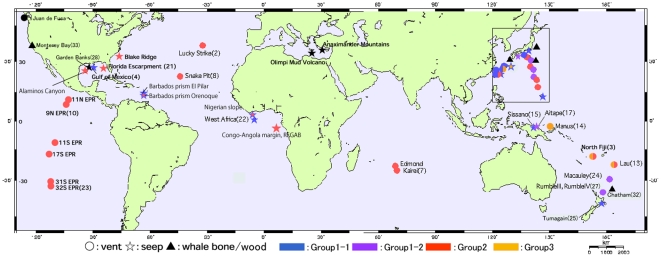
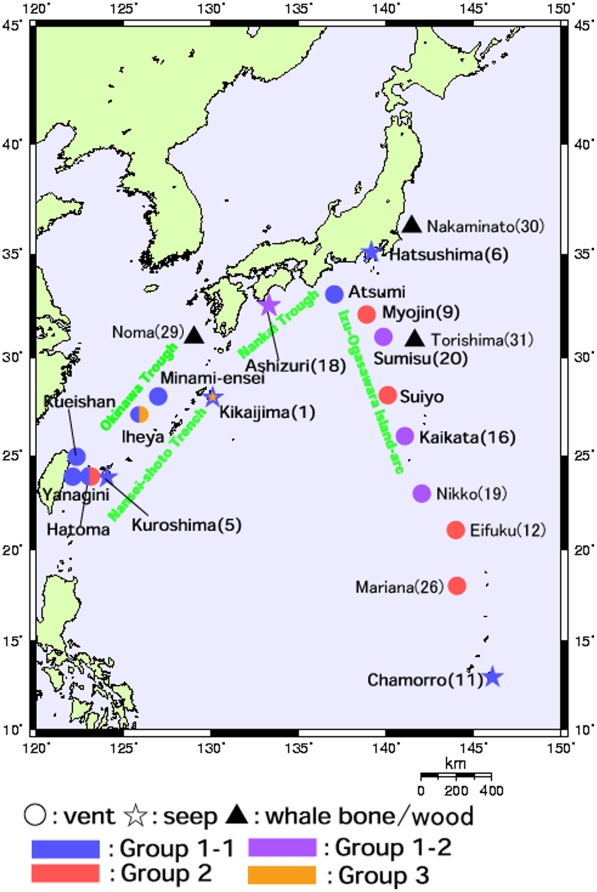
In Japanese waters (Figs. 1 and 2), six Bathymodiolus and one Gigantidas species have steady residences as evidenced by a stable, constant supply of their propagules [3, 10. 14]. Some species possibly have transient residences through incident, leaky supply of propagules as mentioned below. Bathymodiolus japonicus and B. platifrons are distributed in seeps in Sagami Bay and vents of the Okinawa Trough, which are separated by approximately 1,500 km. Bathymodiolus aduloides is distributed in seeps in Sagami Bay, the Nankai Trough, and the subduction zone of the Nansei-shoto Trench and vents of the Okinawa Trough. However, our genetic analyses have not confirmed its existence in the Nankai Trough. The Nankai Trough is situated between Sagami Bay and the Okinawa Trough. There appear to be some barriers to gene flow between the Nankai Trough and Sagami Bay and between the Nankai Trough and Okinawa Trough. Only one specimen, identified genetically as B. aduloides, has been obtained so far from vents in the Izu-Ogasawara Island-arc. The three species of Bathymodiolus mussels can exploit both seeps and vents as habitats. No significant genetic differentiation was discernible between seep and vent populations of B. platifrons [15]. Our studies also suggested a genetic similarity between seep and vent populations of B. japonicus [16], indicating the high adaptability of these species to deep-sea environments, albeit the seemingly large environmental differences between seeps and vents. Bathymodiolus septemdierum is distributed in vents in the Izu-Ogasawara Island-arc, but not in Sagami Bay, which is approximately 500 km from the Myojin Knoll and Suiyo Seamount in the Izu-Ogasawara Island-arc. Only one specimen, identified genetically as B. septemdierum, has been obtained so far from the Okinawa Trough. There are relatively large obstacles to gene flow between Sagami Bay and the Izu-Ogasawara Island-arc. Our genetic studies suggested that this species was conspecific to B. brevior and might possibly be conspecific to B. marisindicus [15]. If this is the case, this species has the widest habitat range among Bathymodiolus species from Japanese waters southeastward to the North Fuji Basin in the West Pacific Ocean and southwestward to the Kairei Field in the Indian Ocean. The two remaining species, B. hirtus and B. securiformis, are distributed in seeps of the subduction zone of the Nansei-shoto Trench. The latter is also distributed in seeps in the Nankai Trough. Gigantidas horikoshii is distributed in vents in the Izu-Ogasawara Island-arc. One specimen from Sagami Bay has been identified as Sissano B. sp. 1, which resides mainly in Sissano in the West Pacific Ocean.
Figure 1. The sampling sites for deep-sea Bathymodiolus mussels and their relatives used in this study.
Refer to Table 2 for details of the sampling sites. ○, hydrothermal vent; •, cold-water seep; ▪, wood/whale bone; ▴, shallow.
Figure 2. The sampling sites for deep-sea Bathymodiolus mussels and their relatives in Japanese waters.
The boxed region in Fig. 1 is enlarged. Refer to Table 2 for details of the sampling sites. ○, hydrothermal vent; •, cold-water seep; ▪, wood/whale bone; ▴, shallow.
Organisms initially invading the deep sea encounter serious difficulties and must alter their feeding strategies to overcome poor nutrition and acquire tolerance to high pressure and cold seawater. Furthermore, organisms in vents and seeps must establish symbiosis with chemosynthetic bacteria as an effective feeding strategy and tolerance to toxic H2S. The “Evolutionary stepping stone hypothesis” has been proposed, in which the ancestors of bathymodioline mussels exploited sunken wood and whale carcasses in their progressive adaptation to deep-sea environments [17], [18]. Further studies are required to elucidate the origin and adaptive process of bathymodioline mussels as a representative of organisms in chemosynthesis-based communities. Our previous research suggested an evolutionary transition from shallow water to vent/seep sites via sunken wood/whale carcass sites and supported the hypothesis [15], [19], although deeper branching was poorly supported. However, the process did not occur in a single, unidirectional manner. Our research also suggested independent invasion into vents and seeps and reversion into whale carcass sites from vent or seep sites in the mytilid lineages.
Only some Bathymodiolus species from limited areas were the subjects of earlier molecular phylogenetic studies [18], [20]–[22]. Subsequently, using updated databases, molecular phylogenetics searched for the phylogeny of about 10 species [16], [23], [24], and mytilid relatives from whale carcasses and wood were included to trace the origins of Bathymodiolus mussels [25], [26]. In our previous studies [15], [19], we sequenced the mitochondrial genes of more than 15 nominal and cryptic species, and showed that mussels in the subfamily Bathymodiolinae comprised four groups. The first group (Group1) includes seven West Pacific and Atlantic Bathymodiolus species (Group 1-1) and two West Pacific Gigantidas species (Group 1-2). The group includes members of the B. childressi clade (B. childressi, B. mauritanicus, B. platifrons, B. hirtus, B. anteumbonatus, B. japonicus, B. tangaroa. B. securiformis, B. edisonensis, and two Gigantidas species) based on morphological traits [13]. The second group (Group 2) includes six or seven Bathymodiolus species, which are subdivided into three subclusters consisting of the Indo-West Pacific, Atlantic, and East Pacific species, respectively, with the exception of B. brooksi that diverges basally to the subclusters. Group 2 includes members of Cosel's B. thermophilus clade (B. thermophilus and East Pacific B. sp., B. brevior, B. azoricus, B. elongates, B. puteoserpentis, B. septemdierum, B. boomerang, B. heckerae, and B. brooksi). The third group (Group 3) includes two West Pacific Bathymodiolus species, and the fourth group includes only one species, T. fisheri. The group includes members of Cosel's B. aduloides clade (B. aduloides and B. manusensis). Our studies also showed that the subfamily Bathymodiolinae and the genus Bathymodiolus were not monophyletic, suggesting the need to reevaluate the classification.
In the present study, we investigated worldwide phylogenetic relationships of Bathymodiolus mussels and their mytilid relatives by analyzing concatenated sequences of the mitochondrial cytochrome c oxidase subunit I (COI) and NADH dehydrogenase subunit 4 (ND4) genes. We also investigated the evolutionary processes of Bathymodiolus mussels by estimating evolutionary divergence times with variable rates over time.
Results
Phylogenetic relationships of Bathymodiolus mussels and their relatives
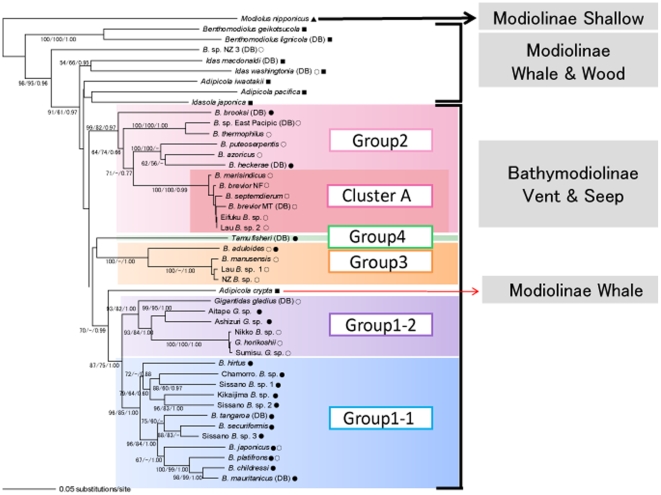
Mussels of the subfamily Bathymodiolinae were divided into four groups (Fig. 3). The first group (Group 1) was subdivided into two subgroups. One subgroup (Group 1-1) consisted of seven nominal species, B. hirtus, B. japonicus, B. platifrons, and B. securiformis from Japanese waters, B. tangaroa from the West Pacific, B. mauritanicus and B. childressi from the Atlantic, and five unidentified (not morphologically examined) Bathymodiolus-related mussels from Sissano (Sissano B. sp. 1, B. sp. 2, and B. sp. 3), the Chamorro Seamount (Chamorro B. sp.), and off Kikaijima Island (Kikaijima B. sp.) in the West Pacific. All the members so far examined in Group 1-1 (6 nominal species and Sissano B. sp.) have methanotrophic endosymbionts [27]–[28]. Group 1-2 included two nominal species, G. horikoshii and G. gladius, and four unidentified Gigantidas-related mussels from the Nikko Seamount (Nikko G. sp.), Sumisu Caldera (Sumisu G. sp.), Aitape (Aitape G. sp.), and off Ashizuri Cape (Ashizuri G. sp.) in the West Pacific. The former two unidentified mussels are likely to be conspecific with G. horikoshii because of their genetic similarity. The species has thioautotrophic endosymbionts (the data will be published elsewhere).
Figure 3. Phylogenetic relationships of deep-sea Bathymodiolus mussels and their relatives based on the 401-bp COI and 423-bp ND4 sequences.
The NJ tree was constructed based on the genetic distances calculated according to Kimura's two-parameter method using Modiolus nipponicus as an outgroup species. The MP and Bayesian trees presented essentially the same topology as the NJ tree. Only the NJ (left) and MP (middle) bootstrap values >50% and Bayesian posterior probabilities (right) >0.50 are specified. The scale bar indicates 0.01 substitutions per site. See Table 1 for abbreviations of Bathymodiolus mussels and their relatives. ○, hydrothermal vent; •, cold-water seep; ▪, wood/whale bone; ▴, shallow.
The second group (Group 2) consisted of eight nominal and one undescribed (morphologically examined but not yet described) Bathymodiolus species. This group was subdivided into three subclusters including the Indo-West Pacific B. septemdierum, B. brevior, and B. marisindicus, Atlantic B. azoricus, B. puteoserpentis, and B. heckerae, and East Pacific B. thermophilus and undescribed species (East Pacific B. sp.), with the exception of the Atlantic B. brooksi, which diverged basally to the three clusters. Bathymodiolus septemdierum, B. brevior, and B. marisindicus comprised the closely related species group (Cluster A [16]). We showed in our previous study that a high gene flow occurred between B. septemdierum and B. brevior and that the gene flow between B. marisindicus and B. septemdierum or B. brevior was low but not negligible, although their habitats are approximately 5,000–10,000 km apart [15]. Mussels from the Lau Basin (Lau B. sp.1) and Eifuku Seamount (Eifuku B. sp.) included in the cluster are likely to be conspecific to B. septemdierum because of their genetic similarity. Four species in Group 2, B. septemdierum, B. brevior, B. marisindicus, and B. thermophilus, contain solely thioautotrophs, and B. puteoserpentis, B. azoricus, B. heckerae, and B. brooksi harbor both thioautotrophs and methanotrophs [27], [29]–[35].
The third group (Group 3) consisted of two nominal species, B. aduloides and B. manusensis, restricted to the West Pacific. Mussels from the Lau Basin (Lau B. sp. 2) and off New Zealand (NZ B. sp.) are likely to be conspecific with B. manusensis because of their genetic similarity. Species in this group contain thioautotrophic endosymbionts [36]. The above three groups, two subgroups, and three subclusters were well supported. The fourth group (Group 4) consisted only of T. fisheri. Groups 3 and 4 were allied and in turn they were allied with Group 1, but the relationships were poorly supported.
Mussels of the subfamily Modiolinae from sunken wood and whale carcasses assumed the outgroup position to the bathymodioline mussels from vents and seeps, with the exception of Adipicola crypta (Dall et al. 1938) from whale carcasses. The species was allied with Group 1 with marginal support. The cluster including vent/seep bathymodioline mussels and wood/whale modioline mussels was well supported. Mytilid mussels flourishing in shallow water were positioned more distantly to the vent/seep bathymodioline mussels. Although only the modioline Modiolus nipponicus (Oyama 1950) was included in the present tree, modioline Modiolus modiolus (Linnaeous 1758) and three species of the subfamily Mytilinae were also positioned, as was M. nipponicus in our previous studies [15]. The unity of Bathymodiolus mussels was not supported, because two species of Gigantidas in the Bathymodiolinae and A. crypta in the Modiolinae perturbed the unity.
Estimation of divergence time
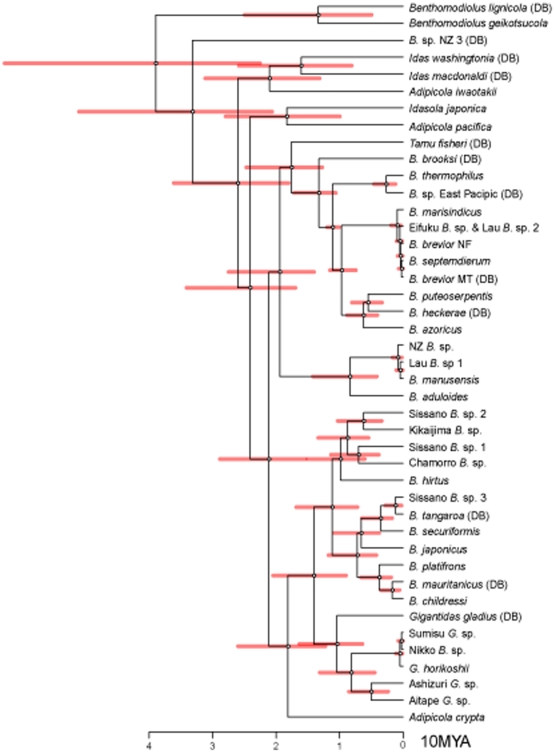
Estimating divergence time is useful to reconstruct evolutionary history. The mean evolutionary rate of mitochondrial DNA has been estimated to be 1∼2% per million years [37]. However, the application of a molecular clock is problematic in some cases, because the rate constancy of molecular evolution is a prerequisite [38], [39]. Our preliminary study showed that the rate of molecular evolution varied among lineages of Bathymodiolus mussels, and thus we adopted Thorne and Kishino's approach (see [40] for details of the application of this approach). We show estimates of evolutionary time on the ML tree (Fig. 4). For calibration, we used reference time associated with the split between the Atlantic and East Pacific subclusters (12 to 10 MYA) in Group 2. Our results (Table 1) showed that diversification of bathymodioline mussels initiated in the early Miocene (about 20 MYA). Subsequently, Groups 1 to 3 started differentiating in the early to middle Miocene (about 19 to 14 MYA).
Figure 4. Posterior distribution of evolutionary divergence times.
Phylogenetic relationships of deep-sea Bathymodiolus mussels based the concatenated 401-bp COI and 423-bp ND4 sequences. The ML tree was constructed using Modiolus nipponicus as an outgroup species. The red lines represent 95% credibility intervals of sampled values. See Table 2 for abbreviations of Bathymodiolus mussels.
Table 1. Estimated evolutionary time (t).
| t (MYA) | |
| Onset of diversification of the subfmaily Bathumodiolinae | 21.1±3.6 |
| Split of Groups 2 and 3 | 19.4±3.4 |
| Split of Groups 1-1 and 1-2 | 14.1±3.0 |
| Split of B. brooksi and the other members in Group2 | 13.3±1.7 |
| Split of East Pacific subcluster from the common ancestor of Indo-West Pacfic and Atlantic subclusters in Group 2 | (12∼10)a |
| Onset of diversification in Bathymodiolus spp. of Group 1-1 | 11.2±2.5 |
| Onset of diversification in Gigantidas spp. of Group 1-2 | 10.4±2.5 |
| Split of B. hirtus from the common ancestor of Sissano B. spp. 1 and 2, Kikaijima B. sp., and Chamorro B. sp. in Group 1-1 | 9.8±2.3 |
| Split between Indo-West Pacfic and Atlantic subclusters in Group 2 | 9.7±1.0 |
| Split of B. aduloides and B. manusensis in Group 3 | 8.4±2.6 |
| Split of B. japonicus from the common ancestor of B. securiformis, B. tangaroa, and Sissano B. sp. 3 in Group 1-1 | 6.6±1.8 |
| Onset of diversification of Atlantic species in Group 2 | 6.2±1.2 |
| Split of B. platifrons from the common ancestor of B. mauritanicus and B. childressi in Group 1-1 | 3.8±1.2 |
| Split of B. securiformis from the common ancestor of B. tangaroa and Sissano B. sp. 3 in Group 1-1 | 3.5±1.5 |
| Split of B. thermophilus and East Pacific sp. in Group 2 | 2.6±0.9 |
| Onset of diversification in Cluster A of Group 2 | 0.9±0.4 |
reference time.
Discussion
Phylogenetic relationships of Bathymodiolus mussels and their relatives
Bathymodioline species from vents and seeps were divided into four well-supported groups (Fig. 3). Together they comprised the poorly-supported bathymodioline cluster. Concatenated sequence data, however, provided better resolution of the phylogeny of Bathymodiolus mussels and their relatives than those derived from single COI [19] or ND4 [15] sequence data, although some OTUs could not be used because of the lack of sequence data on either gene. Modioline species from sunken wood and whale carcasses assumed the outgroup position to the bathymodioline mussels, with the exception of A. crypta from whale carcasses. Mytilid species from shallow water such as M. nipponicus were positioned more distantly to the bathymodioline mussels. The results support the “Evolutionary stepping stone hypothesis,” which advocates adaptive progress of deep-sea organisms from shallow-water to vent/seep sites via wood/whale carcass sites [17], [18].
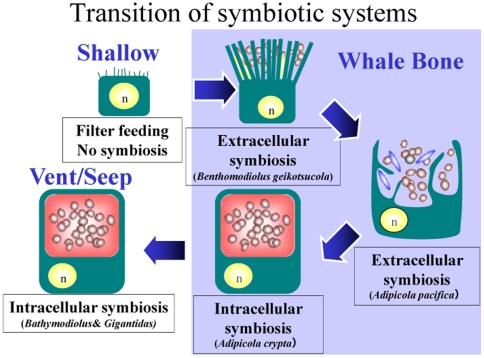
Three modioline species, Benthomodiolus geikotsucola Okutani & Miyazaki 2007, A. pacifica (Dall et al. 1938), and A. crypta, were obtained from whale carcasses in Japanese waters, and the epidermal cells of their gills harbored thioautotrophic bacterial symbionts [15], although no mytilid mussels haboring symbionts have previously been reported from shallow water. As shown schematically in Fig. 5, Benthomodiolus geikotsucola from naturally sunken Bryde's whale carcasses at the Torishima Seamount (approximately 4,000 m in depth) had extracellular symbionts trapped by microvilli of the host cells (the data will be published elsewhere). Adipicola pacifica and A. crypta inhabit artificially settled sperm whale carcasses off Noma Cape (approximately 250 m in depth). The former species had extracellular symbionts enclosed by the protrudent host cell membrane (the data will be published elsewhere). Enclosure by the cell membrane appears more effective to maintain extracellular symbionts than microvilli trapping. The symbionts of the latter species existed inside the host cells, as in Bathymodiolus mussels. These findings suggest that nutritional adaptation to the deep sea proceeded from the extracellular symbiotic state to the intracellular symbiotic state in whale carcasses. The evolutionary transition of symbiosis also supports the “Evolutionary stepping stone hypothesis”. Benthomodiolus geikotsucola is one of mytilid mussels that can live in the deepest sea and thus is more adaptive to abyssal waters than vent/seep bathymodioline mussels (their habitats are up to 4,000 m in depth), but maintains primitive state of symbiosis. However, some species live in sunken wood in shallower water and trap symbionts using microvilli [41].
Figure 5. Schematic representation of evolutionary symbiostic transition.
Mytilid mussels from shallow water with no symbionts; Benthomodiolus geikotsucola from whale carcasses haboring extracellular symbionts trapped by microvilli of the host cells; Adipicola pacifica from whale carcasses haboring extracellular symbionts enclosed by the protrudent host cell membrane; A. crypta from whale carcasses haboring intracellular symbionts; Bathymodiolus mussels with intracellular symbionts.
Modioline A. pacifica, A. iwaotakii (Habe 1958), and Idasola japonica Habe 1976 comprised the most closely related outgroup to the bathymodioline cluster, while A. crypta was included in it. Adipicola crypta does not differ from bathymodioline mussels in the phylogenetic position and symbiotic status. Since our results indicated that monophylies of the subfamily Bathymodiolinae and the genus Bathymodiolus were not supported, the classification should be reevaluated. Moreover, our results showed the existence of several cryptic species, and thus more extensive morphological investigation is indispensable.
Our studies suggested that B. brevior was conspecific to B. septemdierum and might possibly be conspecific to B. marisindicus. Similar situations are discernible in other species. Bathymodiolus childressi was clearly distinguished from B. mauritanicus by the mitochondrial COI gene, but the two species did not form a separate clade based on the nuclear rDNA spacer ITS2 [24]. Bathymodiolus boomerang was not genetically discriminated from B. heckerae by either the COI gene or nuclear rDNA spacer ITS2 [24]. Systematics including possible synonyms should be revised, while cryptic species must be formally described.
Evolutionary process of Bathymodiolus mussels
After basal trichotomous divergence of the three groups, the East Pacific subcluster diverged in Group 2. Next the Atlantic and Indo-West Pacific subclusters split (Fig. 3). However, the divergence of the three subclusters also appears trichotomous, because alliance of the latter subclusters was marginally supported. Divergence of the Atlantic and East Pacific subclusters may have been caused by the closure of the Isthmus of Panama. The rise of the Isthmus of Panama began in the middle Miocene (15.5 to 12 MYA), an island chain emerged 13 to 12 MYA, and in the late Miocene (11.5∼8 MYA) terrestrial species were able to move between North and South America [42]. Although the final closure was only accomplished by 3 to 3.5 MYA, faunal changes between the East Pacific Ocean and the Caribbean Sea had begun long before. The formation of the Isthmus of Panama has exerted profound effects on shallow-water animals since the late Miocene. Diversification of reef corals in the Caribbean, followed by an increase in carbonate-associated benthic foraminiferans, began in the late Miocene [43]. Snapping shrimps, living at depths of less than 20 m in mangrove stands and shallow waters, diverged between the East Pacific and the Caribbean 9 to 3 MYA [44]. Transisthmian divergence of shallow-water gastropods occurred 8.5 to 5.3 MYA [45]. It seems reasonable to consider that the transisthmian isolation of deep-sea animals preceded that of shallow-sea animals, although this depends on larval behavior and physiology. It is not known whether larvae of Bathymodiolus mussels are transported by bottom currents, but the blocking of larval transport through the closing transisthmian seaway is more likely for deep-sea animals than for shallow-water animals. The isotope composition of hydrogenous ferromanganese crusts from the western North Atlantic and the central Pacific Oceans suggests that the compositional shift around 12 MYA might have been related to the initial shallowing of the Central American Isthmus, which prevented the access of deep water from the North Atlantic [46].
It is very difficult to set unequivocal reference times. Nevertheless, we tentatively set reference times of 12 to 10 MYA for the split between the Atlantic and East Pacific subclusters. We provisionally propose here a hypothesis on the evolutionary process of Bathymodiolus mussels to provide a basis for discussions of the evolution of deep-sea animals.
According to the evolutionary time estimated by Thorne and Kishino's approach (Table 2), the three groups are estimated to have diverged in the Miocene, when climates changed markedly. It is generally accepted that transgression and regression concomitant with global warming and cooling lead to worldwide dispersal and diversification of sea animals. If this is the case for deep-sea animals, the ancestor of Bathymodiolus established worldwide distribution in the sea enlarged by transgression in the early Miocene. Subsequently, in the early to middle Miocene, diversification was caused by vicariance due to regression and plate tectonic events. Our estimate of the onset of bathymodiolin diversification (ca. 21 MYA) is roughly consistent with the younger estimate based on 18S rRNA data that showed that the common ancestor of modern bathymodioline vent and seep species might have lived as early as 22 MYA [47], [48]. If the ancestor of the Bathymodiolinae originated in the Miocene, it is possible that it used whale carcasses as evolutionary stepping stones for progressive adaptation from shallow to deep waters, because whale carcasses have been available since the late Eocene (ca. 39 MYA [49]).
Table 2. Sample list.
| Species | Sample abbreviation | Sampling site (locality number in Fig. 1) | Depth (m) | Habitat type |
| Bathymodiolinae | ||||
| Bathymodiolus aduloides | AK1 | Off Kikaijima Island (1) | 1 451 | Seep |
| B. azoricus | AZL1 | Lucky Strike, Mid-Atlantic Ridge (2) | Unknown | Vent |
| B. brevior NF | BN | Mussele Valley, North Fiji Basin (3) | Unknown | Vent |
| B. childressi | ChiG1 | Gulf of Mexico(4) | 1 859 | Seep |
| B. hirtus | HK1 | Kuroshima Knoll, Off Yaeyama Islands (5) | 637 | Seep |
| B. japonicus | JH1 | Off Hatsushima, Sagami Bay (6) | 1 170–1 180 | Seep |
| B. marisindicus | MK1 | Kairei Field, Southern Central Indian Ridge (7) | 2 443–2 454 | Vent |
| B. platifrons | PH1 | Off Hatsushima, Sagami Bay (6) | 1 170–1 180 | Seep |
| B. puteoserpentis | PUS1 | Snake Pit, Mid-Atlantic Ridge (8) | 3 023–3 510 | Vent |
| B. securiformis | LK1 | Kuroshima Knoll, Off Yaeyama Islands (5) | 641 | Seep |
| B. septemdierum | SM1 | Myojin Knoll, Izu-Ogasawara Island-arc (9) | 1 288–1 290 | Vent |
| B. thermophilus | ThE1 | 9N East Pacific Rise (10) | 2 524 | Vent |
| Chamorro B. sp. | C1 | South Chamorro Seamount, Mariana (11) | 2 899 | Seep |
| Eifuku B. sp. | EF1 | Northwest Eifuku Seamount (12) | 1 625 | Vent |
| Kikaijima B. sp. | Kikaijima | Off Kikaijima Island (1) | 1 430 | Seep |
| Lau B. sp. 1 | Lau1 | Hine Hina, Lau Basin (13) | 1 818 | vent |
| Lau B. sp. 2 | BR1 | Hine Hina, Lau Basin (13) | 1 818 | Vent |
| B. manusensis | BE1 | PACKMANUS Field E, Manus Basin (14) | 1 627–1 629 | Vent |
| NZ B. sp. | Ne1 | Off New Zea land (unknown) | Unknown | Vent |
| Sissano B. sp. 1 | Si2-1 | Sissano, Papua New Guinea (15) | 1 646 | Seep |
| Sissano B. sp. 2 | Si1-1 | Sissano, Papua New Guinea (15) | 1 881 | Seep |
| Sissano B. sp. 3 | Si3-3 | Sissano, Papua New Guinea (15) | 1 881 | Seep |
| Gigantidas horikoshii | Kaikata | Kaikata Seamount (16) | 486 | Vent |
| Aitape G. sp. | Aitape1 | Aitape, Papua New Guinea (17) | 470 | Seep |
| Ashizuri G. sp. | Ashizuri | Off Ashizuri Cape (18) | 575 | Seep |
| Nikko G. sp. | NK1 | Nikko Seamount (19) | 485 | Vent |
| Sumisu G. sp. | Su1 | Sumisu Caldera (20) | 676–686 | Vent |
| Database | ||||
| B. brooksi | B. brooksiWFE(DB) | West Florida Escarpment (21) | 3 314 | Seep |
| B. heckerae | B. heckeraeWFE(DB) | West Florida Escarpment (21) | 3 314 | Seep |
| B. mauritanicus | B. mauritanicus(DB) | West Africa (22) | 1 000–1 267 | Seep |
| B. sp. East Pacific | B. aff. thermophilus(DB) | 32S East Pacific Rise (23) | 2 331 | Vent |
| B. sp. NZ3 | B. sp. NZ3(DB) | Macauley Cone (24) | 200 | Vent |
| B. tangaroa | B. tangaroa(DB) | Off Turnagain Cape, New Zea land (25) | 920–1 205 | Seep |
| B. brevior MT | B. breviorMT(DB) | Mariana Trough (26) | 3 589 | Vent |
| Gigantidas gladius | Gigantidas gladius(DB) | Rumble III (27) | 300–460 | Vent |
| Tamu fisheri | Tamu fisheri(DB) | Garden Banks (28) | 546–650 | Seep |
| Modiolinae & Mytilinae | ||||
| Adipicola crypta | ACN1 | Off Noma Cape, Kagoshima (29) | 225–229 | Whale bone |
| Adipicola iwaotakii | AIH1 | Off Nakaminato, Ibaraki (30) | 490 | Wood |
| Adipicola pacifica | APN1 | Off Noma Cape, Kagoshima (29) | 225–229 | Whale bone |
| Idasola japonica | IJN1 | Off Noma Cape, Kagoshima (29) | 400∼425 | Wood |
| Benthomodiolus geikotsucola | Tori1-1 | Torishima Seamount (31) | 4 051 | Whale bone |
| Modiolus nipponicus | Modiolus nipponicus | Off Oura Harbor, Shizuika | - | Shallow |
| Database | ||||
| Benthomodiolus lignicola | Benthomodiolus lignicola(DB) | Chatham Rise (32) | 826–1 174 | Whale bone, Wood |
| Idas macdonaldi | Idas macdonaldi(DB) | Garden Banks (28) | 650 | Seep |
| Idas washingtonia | Idas washingtonia(DB) | Monterey Bay (33) | 960–1 910 | Whale bone, Wood, Vent |
Species in Group 3 could be relics of cosmopolitans, because there are only two species, the distribution of which is restricted to the West Pacific although they have a long history (since ca. 8.4 MYA). Species in Group 1-1 started differentiating ca. 11 MYA, and the four species in the Okinawa Trough, B. hirtus, B. japonicus, B. platifrons, and B. securiformis, speciated 3.5 to 9.8 MYA, long before the formation of the Okinawa Trough (ca. 2 MYA). Thus, members of Group 1-1 living in the Okinawa Trough speciated elsewhere and thereafter migrated to the Okinawa Trough [15]. It is unlikely that species in Group 1-1 are also relics of cosmopolitans. They are distributed in highly isolated localities, like Japanese waters and the East and West Atlantic, without any species in intervening areas. However, our results showed that B. platifrons from Japanese waters was very closely related to B. childressi from the West Atlantic and trans-Atlantic B. mauritanicus (Fig. 3). It is evident that further surveys of novel vent and seep sites and genetic examination of deep-sea mussels are needed to discover cryptic members of Groups 3 and 1-1 and to elucidate the evolutionary history of Bathymodiolus mussels.
Materials and Methods
Materials and sequencing of mitochondrial genes
Specimens used in this study are listed in Table 2 and collection sites are mapped in Figs. 1 and 2. Sequencing was performed as described previously [15], [18], [20]. Since the doubly uniparental inheritance of mitochondrial DNA is known in some mytilid mussels, we have included at least five specimens, if available, of each nominal and cryptic species in our previous analyses to detect divergent and highly heterogeneous DNA sequences [15], [16], [19]. However, we used one specimen of each species, because we have not seen any evidence of doubly uniparental inheritance so far. Nevertheless, it is not plausible to represent each species by a single sequence, especially for mytilid mussels from sunken whale carcasses and wood and shallow water, although many phylogenetic studies did so. Heteroplasmy of mitochondrial DNA was shown even in Bathymodiolus [50].
Phylogenetic analysis
DNA sequences were edited and aligned using DNASIS (Hitachi Software Engineering Co., Ltd., Tokyo, Japan) and MEGA 3.1 software [51], and the alignments were corrected by visual inspection for phylogenetic analysis. We used 401-bp COI and 423-bp ND4 sequences, excluding ambiguous sites. Dendrograms were constructed using the neighbor-joining (NJ) and maximum parsimony (MP) methods using PAUP*4.0 beta 10 software [52]. Genetic distances were computed using the Kimura's two-parameter method [53]. The reliability of trees was evaluated by producing 1,000 bootstrap replicates. The majority-rule consensus MP tree was constructed by conducting a heuristic search based on the 1,000 bootstrap replicates with an unweighted ts/tv ratio. The Bayesian tree was constructed using MrBayes version 3.1 software [54] based on the model evaluated by the Mrmodel test 2.2 [55]. The Monte Carlo Markov chain (MCMC) length was 5×106 generations, and we sampled the chain after every 100 generations. MCMC convergence was assessed by calculating the potential scale reduction factor, and the first 1×104 generations were discarded. We used Modiolus nipponicus (Mytilidae, Modiolinae) as an outgroup species.
Evolutionary divergence times were estimated using the relaxed molecular clock model implemented in the software Multidivtime [56], [57]. This model depends on the Maximum Likelihood topology, which was inferred with PAUP [52] using the Hasegawa-Kishino-Yano evolutionary model [58] assuming that rates across sites vary according to a discretized gamma distribution [59]. Under this relaxed molecular clock model the proportion of times from internal nodes to the ingroup root node – the root when we exclude the outgroup and then reroot the tree – are robust to time scaling [57]. Thus a preliminary run of Multidivtime without any calibration point was conducted to find an initial guess for the divergence time of the ingroup root node. The Multidivtime analysis was conducted assuming a gamma distribution with mean and standard deviation of 30MYA for the divergence time of the ingroup root node. Furthermore the splitting time between the Atlantic and East Pacific subclusters was calibrated to be between 10 and 12MYA. We then sampled 103 posterior estimates of divergence times and other parameters at every 104 iterations after discarding the first 105 samples.
Acknowledgments
We wish to express our thanks to Drs. Takashi Okutani, Shigeaki Kojima, Hiromi Watanabe, Katsunori Fujikura, Shinji Tsuchida, Ken Takai, and Toshiyuki Yamaguchi for their useful advice and support throughout this work. We are also grateful to Drs. Nobuhiro Minaka and Hirohisa Kishino for helpful advice on phylogenetic analysis.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the Ministry of Education, Culture, Sports, Science and Technology of Japan (no. 21570093, http://www.jsps.go.jp/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kenk VC, Wilson BR. A new mussel (Bivalvia:Mytilidae) from hydrothermal vents in the Galapagos Rift Zone. Malacologia. 1985;26:253–271. [Google Scholar]
- 2.von Cosel R, Métivier B, Hashimoto J. Three new species of Bathymodiolus (Bivalvia: Mytilidae) from hydrothermal vents in the Lau Basin and the North Fiji Basin, western Pacific, and the Snake Pit area, Mid-Atlantic Ridge. Veliger. 1994;37:374–392. [Google Scholar]
- 3.Hashimoto J, Okutani T. Four new mytilid mussels associated with deepsea chemosynthetic communities around Japan. Venus. 1994;53:61–83. [Google Scholar]
- 4.von Cosel R, Olu K. Gigantism in Mytilidae. A new Bathymodiolus from cold seep areas on the Barbados accretionary prism. CR Acad Sci Paris Sci Vie. 1998;321:655–663. [Google Scholar]
- 5.Gustafson RG, Turner RD, Lutz RA, Vrijenhoek RC. A new genus and five new species of mussels (Bivalvia: Mytilidae) from deep-sea sulfide/hydrocarbon seeps in the Gulf of Mexico. Malacologia. 1998;40:63–112. [Google Scholar]
- 6.von Cosel R, Comtet T, Krylova EM. Bathymodiolus (Bivalvia: Mytilidae) from hydrothermal vents on the Azores Triple Junction and the Logatchev hydrothermal field, Mid-Atlantic Ridge. Veliger. 1999;42:218–248. [Google Scholar]
- 7.Hashimoto J. A new species of Bathymodiolus (Bivalvia: Mytilidae) from hydrothermal vent communities in the Indian Ocean. Venus. 2001;60:141–149. [Google Scholar]
- 8.von Cosel R. A new species of bathymodioline mussel (Mollusca, Bivalvia, Mytilidae) from Mauritania (West Africa), with comments on the genus Bathymodiolus Kenk & Wilson, 1985. Zoosystema. 2002;24:259–271. [Google Scholar]
- 9.von Cosel R, Marshall BA. Two new species of large mussels (Bivalvia: Mytilidae) from active submarine volcanoes and a cold seep off the eastern North Island of New Zealand, with description of a new genus. Nautilus. 2003;117:31–46. [Google Scholar]
- 10.Okutani T, Fujikura K, Sasaki T. Two new species of Bathymodiolus (Bivalvia: Mytilidae) from methane seeps on the Kuroshima Knoll off Yaeyama Islands, southwestern Japan. Venus. 2004;63:97–110. [Google Scholar]
- 11.Hashimoto J, Furuta M. A new species of Bathymodiolus (Bivalvia: Mytilidae) from hydrothermal vent communities in the Manus Basin, Papua New Guinea. Venus. 2007;66:57–68. [Google Scholar]
- 12.von Cosel R. A new bathymodioline mussel (Bivalvia: Mytiloidea: Mytilidae: Bathymodiolinae) from vent sites near Kueishan Island, north east Taiwan. Raffl Bull Zool. 2008;19:105–114. [Google Scholar]
- 13.von Cosel R, Janssen R. Bathymodiolus mussels of the Bathymodiolus (s. l.) childressi clade from methane seeps near Edison Seamount, New Ireland, Papua New Guinea (Bivalvia: Mytilidae). Arch. Molluskenkunde. 2008;137:195–224. [Google Scholar]
- 14.Hashimoto J, Yamane T. A new species of Gigantidas (Bivalvia: Mytilidae) from a vent site on the Kaikata Seamount southwest of the Ogasawara (Bonin) Islands, southern Japan. Venus. 2005;64:1–10. [Google Scholar]
- 15.Kyuno A, Shintaku M, Fujita Y, Matsumoto H, Utsumi M, et al. Dispersal and differentiation of deep-sea mussels of the genus Bathymodiolus (Mytilidae, Bathymodiolinae). J Mar Biol. 2009. 15. Article ID 625672.
- 16.Iwasaki H, Kyuno A, Shintaku M, Fujita Y, Fujiwara Y, et al. Evolutionary relationships of deep-sea mussels inferred by mitochondrial DNA sequences. Mar Biol. 2006;149:1111–1122. [Google Scholar]
- 17.Smith CR, Kukert H, Wheatcroft RA, Jumars PA, Deming JW. Vent fauna on whale remains. Nature. 1989;341:27–28. [Google Scholar]
- 18.Distel DL, Baco AR, Chuang E, Morrill W, Cavanaugh C, et al. Do mussels take wooden steps to deep-sea vents? Nature. 2000;403:725–726. doi: 10.1038/35001667. [DOI] [PubMed] [Google Scholar]
- 19.Fujita Y, Matsumoto H, Fujiwara Y, Hashimoto J, Galkin SV, et al. Phylogenetic relationships of deep-sea Bathymodiolus mussels with their mytilid relatives from sunken whale carcasses and wood. Venus. 2009;67:123–134. [Google Scholar]
- 20.Craddock C, Hoeh WR, Gustafson RG, Lutz RA, Hashimoto J, et al. Evolutionary relationships among deep-sea mytilids (Bivalvia: Mytilidae) from hydrothermal vents and cold-water methane/sulfide seeps. Mar Biol. 1995;121:477–485. [Google Scholar]
- 21.Miyazaki J-I, Shintaku M, Kyuno A, Fujiwara Y, Hashimoto J, et al. Phylogenetic relationships of deep-sea mussels of the genus Bathymodiolus (Bivalvia: Mytilidae). Mar Biol. 2004;144:527–535. [Google Scholar]
- 22.Smith PJ, McVeagh SM, Won Y, Vrijenhoek RC. Genetic heterogeneity among New Zealand species of hydrothermal vent mussels (Mytilidae: Bathymodiolus). Mar Biol. 2004;144:537–545. [Google Scholar]
- 23.Jones WJ, Won Y-J, Maas PAY, Smith PJ, Lutz RA, et al. Evolution of habitat use by deep-sea mussels. Mar Biol. 2006;148:841–851. [Google Scholar]
- 24.Roy KO-L, von Cosel R, Hourdeza S, Carney SL, Jollivet D. Amphi-Atlantic cold-seep Bathymodiolus species complexes across the equatorial belt. Deep-Sea Res I. 2007;54:1890–1911. [Google Scholar]
- 25.Samadi S, Ouéméré E, Lorion J, Tillier A, von Cosel R, et al. Molecular phylogeny in mytilids supports the wooden steps to deep-sea vents hypothesis. C R Biol. 2007;330:446–456. doi: 10.1016/j.crvi.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Lorion J, Deperron S, Gros O, Cruaud C, Samadi S. Several deep-sea mussels and their associated symbionts are able to live both on wood and on whale falls. Proc R Soc B. 2009;276:177–185. doi: 10.1098/rspb.2008.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujiwara Y, Takai K, Uematsu K, Tsuchida S, Hunt JC, et al. Phylogenetic characterization of endosymbionts in three hydrothermal vent mussels: influence on host distributions. Mar Ecol Prog Ser. 2000;208:147–155. [Google Scholar]
- 28.Childress JJ, Fisher CR, Brooks JM, Kennicutt MC II, Bidigare R, et al. A methanotrophic marine molluscan (Bivalvia, Mytilidae) symbiosis: mussels fueled by gas. Science. 1986;233:1306–1308. doi: 10.1126/science.233.4770.1306. [DOI] [PubMed] [Google Scholar]
- 29.Cavanaugh CM, Levering PR, Maki JS, Mitchell R, Lidstrom ME. Symbiosis of methylotrophic bacteria and deep-sea mussels. Nature. 1987;325:346–348. [Google Scholar]
- 30.Distel DL, Lane DJ, Olsen GJ, Giovannoni SJ, Pace B, et al. Sulfur-oxidizing bacterial endosymbionts: analysis of phylogeny and specificity by 16S rRNA sequences. J Bacteriol. 1988;170:2506–2510. doi: 10.1128/jb.170.6.2506-2510.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Distel DL, Lee HK-W, Cavanaugh CM. Intracellular coexistence of methano- and thioautotrophic bacteria in a hydrothermal vent mussel. Proc Natl Acad Sci U S A. 1995;92:9598–9602. doi: 10.1073/pnas.92.21.9598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dubilier N, Windoffer R, Giere O. Ultrastructure and stable carbon isotope composition of the hydrothermal vent mussels Bathymodiolus brevior and B. sp. affinis brevior from the North Fiji Basin, western Pacific. Mar Ecol Prog Ser. 1998;165:187–193. [Google Scholar]
- 33.Fiala-Médioni A, McKiness ZP, Dando P, Boulegue J, Mariotti A, et al. Ultrastructural, biochemical, and immunological characterization of two populations of the mytilid mussel Bathymodiolus azoricus from the Mid-Atlantic Ridge: evidence for a dual symbiosis. Mar Biol. 2002;141:1035–1043. [Google Scholar]
- 34.Fisher CR, Brooks JM, Vodenichar JS, Zande JM, Childress JJ, et al. The co-occurrence of methanotrophic and chemoautotrophic sulfur-oxidizing bacterial symbionts in a deep-sea mussel. Mar Ecol. 1993;14:277–289. [Google Scholar]
- 35.Yamanaka T, Mizota C, Fujiwara Y, Chiba H, Hashimoto J, et al. Sulfur-isotopic composition of the deep-sea mussel Bathymodiolus marisindicus from currently active hydrothermal vents in the Indian Ocean. J Mar Biol Assoc UK. 2003;83:841–848. [Google Scholar]
- 36.Yamanaka T, Mizota C, Maki Y, Fujikura K, Chiba H. Sulfur isotope composition of soft tissues of deep-sea mussels, Bathymodiolus spp., in Japanese waters. Benthos Res. 2000;55:63–68. [Google Scholar]
- 37.Brown WM, Gorge M, Jr, Wilson AC. Rapid evolution of animal mitochondrial DNA. Proc Natl Acad Sci U S A. 1979;76:1967–1971. doi: 10.1073/pnas.76.4.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gillespie JH. Oxford: Oxford University Press; 1991. The Causes of Molecular Evolution.350 [Google Scholar]
- 39.Page RDM, Holmes EC. Oxford: Blackwell Sciences; 1998. Molecular Evolution: A Phylogenetic Approach.346 [Google Scholar]
- 40.Hasegawa M, Thorne JL, Kishino H. Time scale of eutherian evolution estimated without assuming a constant rate of molecular evolution. Genes Genet Syst. 2003;78:267–283. doi: 10.1266/ggs.78.267. [DOI] [PubMed] [Google Scholar]
- 41.Gros O, Gaill F. Extracellular bacterial association in gills of ≪wood mussels≫. Cah Biol Mar. 2007;48:103–109. [Google Scholar]
- 42.Duque-Caro H. Neogene stratigraphy, paleooceanography and paleobiogeography in northwest South America and the evolution of the Panama Seaway. Palaeogeol Palaeoclimatol Palaeoecol. 1990;77:203–234. [Google Scholar]
- 43.Collins LS, Budd AF, Coates AG. Earliest evolution associated with closure of the Tropical American Seaway. Proc Natl Acad Sci U S A. 1996;93:6069–6072. doi: 10.1073/pnas.93.12.6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Knowlton N, Weigt LA. New dates and new rates for divergence across the Isthmus of Panama. Proc Royal Soc London Ser B. 1998;265:2257–2263. [Google Scholar]
- 45.Collins TM. PhD dissertation, Yale University, New Haven, CT; 1989. Rates of mitochondrial DNA evolution in transisthmian geminate species. [Google Scholar]
- 46.Burton KW, Ling H-F, O'Nions RK. Closure of the Central American Isthmus and its effect on deep-water formation in the North Atlantic. Nature. 1997;386:382–385. [Google Scholar]
- 47.Van Dover CL, German CR, Speer KG, Parson LM, Vrijenhoek RC. Evolution and biogeography of deep-sea vent and seep invertebrates. Science. 2002;295:1253–1257. doi: 10.1126/science.1067361. [DOI] [PubMed] [Google Scholar]
- 48.Little CTS, Vrijenhoek RC. Are hydrothermal vent animals living fossils? TRENDS Ecol Evol. 2003;18:582–588. [Google Scholar]
- 49.Squires RL, Goedert JL, Barnes LG. Whale carcasses. Nature. 1991;349:574. [Google Scholar]
- 50.Craddock C, Hoeh WR, Lutz RA, Vrijenhoek RC. Extensive gene flow in the deep-sea hydrothermal vent mytilid Bathymodiolus thermophilus. Mar Biol. 1995;124:137–146. [Google Scholar]
- 51.Kumar S, Tamura K, Nei M. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform. 2004;5:150–156. doi: 10.1093/bib/5.2.150. [DOI] [PubMed] [Google Scholar]
- 52.Swofford DL. Sunderland, MA: Sinauer Associates; 2002. PAUP*: phylogenetic analysis using parsimony (and other methods), version 4.0 beta 10. [Google Scholar]
- 53.Kimura M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- 54.Huelsenbeck JP, Ronquist F, Nielsen R, Bollback JP. Bayesian inference of phylogeny and its impact on evolutionary biology. Science. 2003;294:2310–2314. doi: 10.1126/science.1065889. [DOI] [PubMed] [Google Scholar]
- 55.Posada D, Buckley TR. Model selection and model averaging in phylogenetics: advantages of the AIC and Bayesian approaches over likelihood ratio tests. Syst Biol. 2004;53:793–808. doi: 10.1080/10635150490522304. [DOI] [PubMed] [Google Scholar]
- 56.Kishino H, Thorne JL, Bruno WJ. Performance of a divergence time estimation method under a probabilistic model of rate variation. Mol Biol Evol. 2001;18:352–361. doi: 10.1093/oxfordjournals.molbev.a003811. [DOI] [PubMed] [Google Scholar]
- 57.Thorne JL, Kishino H, Painter IS. Estimating the rate of evolution of the rate of molecular evolution. Mol Biol Evol. 1998;15:1647–1657. doi: 10.1093/oxfordjournals.molbev.a025892. [DOI] [PubMed] [Google Scholar]
- 58.Hasegawa M, Kishino H, Yano T. Dating the human-ape splitting by a molecular clock of mitochondrial DNA. J Mol Evol. 1985;22:160–174. doi: 10.1007/BF02101694. [DOI] [PubMed] [Google Scholar]
- 59.Yang Z. Maximum likelihood phylogenetic estimation from DNA sequences with variable rates over sites: Approximate methods. J Mol Evol. 1994;39:306–314. doi: 10.1007/BF00160154. [DOI] [PubMed] [Google Scholar]