Introduction
Together, the world's eight acknowledged nuclear powers—the United States (US), Russia, United Kingdom (UK), France, China, India, Pakistan, and the Democratic People's Republic of Korea (North Korea)—have amassed an arsenal of almost 30,000 nuclear weapons since 1945. In addition, Israel is believed to be a nuclear power while Iran (and possibly Syria as well) is also suspected of developing nuclear weapons. Despite the technological sophistication that has enabled the 11 nuclear weapons states to produce and deliver nuclear bombs, most of these nations simultaneously also suffer from high internal rates of poverty and endemic neglected diseases. They include high prevalence rates of neglected tropical diseases in India, China, Pakistan, Iran, and Syria, and related neglected infections of poverty in the US and Europe. Indeed, the 11 nuclear weapons states together account for up to one-half of the global disease burden from all neglected diseases. However, for a tiny fraction (less than 1/10,000th) of the costs of producing and maintaining a nuclear arsenal the 11 nuclear powers could eliminate most of their neglected diseases and engage in joint neglected disease research and development efforts that help to reduce international tensions and promote world peace.
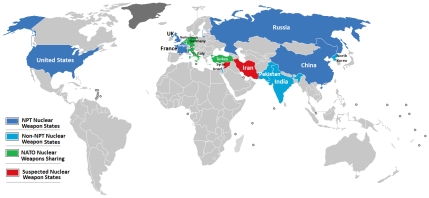
Shown in Table 1 and Figure 1 are the 11 established and suspected global nuclear powers. Following the development and deployment of the atomic bomb by the US in 1945 (at an estimated cost of US$20 billion), Russia became the second nuclear power in 1949, and in every decade since then at least one new country has joined the nuclear club [1], [2]. In addition three countries, South Africa, Argentina, and Brazil, began active nuclear weapons programs, but subsequently abandoned them by mutual treaty [1]. Today, only the first five nations to produce nuclear weapons,,the US, Russia, UK, France, and China, have signed the nuclear nonproliferation treaty [1]. The costs to maintain these nuclear arsenals are staggering. According to the Brookings Institution, which in 1998 published their US Nuclear Weapons Cost Study Project, the US alone spent $35 billion that year on nuclear weapons technology [2]. Further estimates indicate that the US may have spent more than $5.5 trillion in developing their nuclear arsenal, while France has invested approximately $1.5 trillion [3]. Although the data are unavailable, the costs for other nuclear weapon states are believed to be similar [3]. Therefore it is likely that the 11 nuclear weapons states together have invested at least $10 trillion on weapons production and maintenance.
Table 1. Estimated number of nuclear weapons by Country, 1945–2008.
| Country | Estimated number of nuclear weapons | Date first test conducted | Member of Nuclear Non-Proliferation Treaty? |
| United States | 10,000 | 1945 | Yes |
| USSR/Russia | 16,000 | 1949 | Yes |
| United Kingdom | 200 | 1952 | Yes |
| France | 350 | 1960 | Yes |
| China | 410 | 1964 | Yes |
| India | 75–110 | 1974 | No |
| Israel | 100–200 | 1979? | No |
| Pakistan | 60 | 1998 | No |
| DPR Korea (North Korea) | <10 | 2006 | No |
| Iran | Suspected nuclear weapons state | Not determined | No |
| Syria | Suspected nuclear weapons state | Not determined | No |
Figure 1. Map of countries with nuclear weapons.
NPT, nuclear nonproliferation treaty. Source: http://commons.wikimedia.org/wiki/File:Nuclear_weapons.png.
Despite this massive expenditure, each of the 11 nuclear weapons states, with the possible exception of the U.K., also suffers from high rates of neglected tropical diseases (and related neglected infections of poverty), defined as chronic and debilitating parasitic and other infectious diseases that occur in association with extreme poverty [4]. In addition to their health effects, the neglected tropical diseases also cause poverty through their ability to impair child physical and intellectual development, pregnancy outcomes, and worker productivity, while simultaneously promoting conflict and war through their agriculturally and socially destabilizing effects [4], [5]. Although it is common to think of neglected diseases as confined to low-income countries in sub-Saharan Africa, Southeast Asia, and Latin America, as shown in Table 2 these infections also exhibit a high prevalence in middle-income countries such as China, India, Pakistan, North Korea, Iran, and Syria, as well as in selected areas of poverty found in the US, Russia, and Eastern Europe [6]. Indeed, with the possible exceptions of the UK, high neglected disease burdens are present in all of the nuclear weapons states, particularly the helminth infections, leishmaniasis and Chagas disease, toxoplasmosis, and trachoma.
Table 2. The major neglected tropical diseases or neglected infection of poverty endemic to countries with nuclear weapons.
| Country | Neglected infection | Neglected infection | Neglected infection | Neglected infection | Neglected infection | Reference |
| (Est. Cases) | (Est. Cases) | (Est. Cases) | (Est. Cases) | (Est. Cases) | ||
| United States | Toxocariasis1–3 million | Trichomoniasis1 million | Chagas disease<1 million | Cysticercosis<0.2 million | Strongyloidiasis0.1 million | [10], [29] |
| USSR/Russia | ToxoplasmosisPrevalence ND | Opisthorchiasis12.5 million at risk | Congenital syphilisIncidence 170/100,000 in 1996 | [13], [16] [29], [32] | ||
| United Kingdom | ToxocariasisPrevalence ND | ToxoplasmosisPrevalence ND | [29] | |||
| France | Toxoplasmosis59%seroprevalence in pregnancy | LeishmaniasisIncidence 0.02–0.19/100,000 | Chagas diseasePrevalence ND | StrongyloidiasisPrevalence ND | [11], [21], [22], [28] [29] | |
| China | Ascariasis86 million | Hookworm39 million | Trichuriasis29 million | Trachoma27 million | Paragonimiasis Clonorchiasis13 million | [9], [13], [14] [30] |
| India | Ascariasis140 million | Trichuriasis73 million | Hookworm71 million | Lymphatic filariasis30 million | Trachoma1 millionToxoplasmosis12–45%seroprevalenceIn pregnancyLeishmaniasis0.3 million | [8], [12], [17], [29] [30] |
| Israel | LeishmaniasisIncidence 0.13–7/100,000 | [27] | ||||
| Pakistan | Ascariaisis21 million | Hookworm2 million | Trichuriasis1.5 million | Trachoma0.3 million | LeishmaniasisPrevalence ND | [8], [18], [26], [30] |
| DPR Korea(North Korea) | Ascariasis8 million | Hookworm1 million | Trichuriasis0.2 million | ClonorchiasisPrevalence ND | [8], [15] | |
| Iran | Ascariasis5 million | Trichuriasis1.6 million | Hookworm0.4 million | Toxoplasmosis29–64% seroprevalence in pregnancy or women | LeishmaniasisPrevalence NDTrachoma15,000 | [8], [19], [24], [25], [29] [30] |
| Syria | LeishmaniasisPrevalence ND | [20], [23] |
ND, not determined.
Helminthic Neglected Infections
The four major soil-transmitted helminth infections of humans include ascariasis (roundworm), trichuriasis (whipworm), hookworm infection, and strongyloidiasis (threadworm) [7]. These intestinal worm infections represent the most common neglected tropical diseases of humans living in poverty [7], [8]. Of the estimated 800 million ascariasis infections found predominantly in low- and middle-income countries [7], approximately one-third of the cases occur in nuclear weapons states including India (140 million), China (86 million), North Korea (8 million), Pakistan (7 million), and Iran (5 million) [8], [9]. These nations also account for 20% of the world's cases of hookworm infection, which is associated with anemia and extreme poverty resulting from impairments in child development and cognition, maternal morbidity during pregnancy, and diminished agricultural worker productivity [4], [7], [8], [9]. Trichuriasis and strongyloidiasis are endemic to these countries as well, and the US and France have pockets of endemic strongyloidiasis in, respectively, Appalachia and in Region Midi-Pyrenees in the Southwest [10], [11]. In the US and elsewhere, toxocariasis is a soil-transmitted helminth zoonosis associated with larval migrans syndromes, asthma, and developmental delays in up to 3 million African Americans living in poverty [10].
India accounts for roughly one-quarter of the world's 120 million cases of lymphatic filariasis, a disfiguring and stigmatizing vector-borne infection associated with elephantiasis [12], while China accounts for most of the world's food-borne trematode infections, including 13 million cases of clonorchiasis (liver fluke infection associated with liver fibrosis and bile duct cancer) and paragonimiasis (lung fluke infection associated with hemoptysis and other pulmonary disorders) [9], [13], [14]. Clonorchiasis is also endemic to North Korea [15], while a related liver fluke infection known as opisthorchiasis is highly endemic to Russia where an estimated 12.5 million people are at risk of infection, and in some parts of Siberia up to 95% of the population is infected [13], [16]. Up to 169,000 cases of cysticercosis occur among Hispanic Americans in the US [10].
Leishmaniasis and Other Protozoan Neglected Infections
Leishmaniasis is a serious sandfly-transmitted neglected tropical disease endemic to the Asian and Middle Eastern nuclear states where it is associated with extreme poverty [4], [17]. The visceral form (caused by Leishmania donovani in Asia and the Middle East) is the most severe and is associated with profound pancytopenia, hepatosplenomegaly, and failure to thrive when it occurs in childhood. Annually, there are approximately 500,000 new cases of visceral leishmaniasis worldwide with more than one-half of these cases (270,000) occurring in India alone [17]. Most of the Indian cases occur in the impoverished state of Bihar [17], while in Pakistan visceral leishmaniasis is endemic to Azad Jammu and Kashmir, on the Indian border [18]. Visceral leishmaniasis is also endemic to Iran and Syria [19], [20], and it occurs in southern France where it is a zoonosis caused by L. infantum with dogs as the major animal reservior, and recognized as an opportunistic infection with patients with HIV/AIDS [21], [22]. In most of the countries where visceral leishmaniasis is found [22]–[26], including Israel [27], the cutaneous form is also present and caused by either L. major or L. tropica. Cutaneous leishmaniasis is disfiguring and highly stigmatizing, especially for young women [4]. In the US, cutaneous leishmaniasis caused by L. mexicana and possibly other species has emerged along the border with Mexico [10]. Up to one million cases of Chagas disease are found in the US as well, mostly among Hispanic American immigrants, but there is also evidence of Chagas disease transmission within the borders of the US [10]. Chagas disease is also found in France [28]. Toxoplasmosis is an important congenital protozoan infection that results in blindness and profound mental disabilities found in all of the nuclear weapons states, but especially the US and France [29].
Bacterial Neglected Diseases
Almost one-half of the world's 60–80 million trachoma cases occur in nuclear weapons states, with China having the highest number of cases of any nation [30]. Similarly, India has the highest number of leprosy cases, with more than one-half of the new infections that occur annually in the world [31]. Following the collapse of the former Soviet Union in 1991, as a result of the disintegration of the state-run health care system and other political and socioeconomic changes, the rate of congenital syphilis rose at a rate 34 times higher than was seen in Western Europe [32].
Summary of Neglected Diseases in Nuclear Weapons States
Although the world's nuclear states have up to one-third of the world's cases of soil-transmitted helminth infections, more than one-half of the world's new cases of leishmaniasis and leprosy, and approximately one-half of the global disease burden of trachoma, they have chosen to devote their major resources to weapons production instead of neglected disease control [33]. For example, India allocates only $0.40 per individual per year for treatment of its population at risk for visceral leishmaniasis [17], while the projected US government annual budget in 2010 for global neglected tropical disease control is expected to be $65 million (part of a larger $350 million U.S. commitment), roughly 1,000 times less than its annual nuclear weapons budget [34]. The UK government has committed approximately £50 million to neglected tropical disease control [35]. A distinction can be made between states that can afford more nuclear weapons (i.e., US, UK, France) and those for which social spending must be curtailed to pursue nuclear ambitions (i.e., India, Pakistan, China). In terms of research and development (R&D) funding the George Institute estimates that the global budget for neglected tropical diseases is approximately $400 million including only $125 million for leishmaniasis and trypanosome infections, $80 million for dengue, $50 million for all helminth infections, and less than $10 million each for trachoma, leprosy and Buruli ulcer, trachoma, and typhoid/paratyphoid [36]. Together, all of the costs for both neglected disease control and R&D come close to $1 billion, or roughly less than 1/10,000th of the estimated $10 trillion committed for nuclear weapons.
While some may argue that the trillions of dollars spent on nuclear weapons may have served as a successful deterrent for an all-out US-Soviet, a Sino-Soviet, or an Indo-Pakistan conflict and was therefore an actual investment in peace, imagine a world in which the nuclear weapons states increased their neglected disease control budgets more than 10-fold thereby, reducing the global nuclear weapon–to–neglected disease gap to 1,000 to 1. Ten billion dollars devoted to neglected diseases would be sufficient to effect large-scale control or elimination for most of the high-prevalence neglected tropical diseases in the 56 nations where five or more of these conditions are endemic [37]–[38]. Among the nuclear weapons states these 56 nations include India and China, although sufficient funds would remain to tackle neglected infections among pockets of poverty in the remaining wealthy nine nuclear countries. In addition to the enormous health impact of controlling or eliminating conditions with a combined disease burden that exceeds malaria or tuberculosis, because the neglected tropical diseases also contribute to poverty, reducing this disease burden would help reduce poverty and therefore help achieve the Millennium Development Goals [38]. Neglected tropical disease control and elimination would also help stabilize and build nations, reduce civil strife and international tensions, and contribute to world peace [5], [38]. Control of neglected infections of poverty would eliminate an important group of health disparities in the US and Europe [6], while simultaneously providing a vehicle by which nations such as India and China would attain new status as global leaders.
Scientific and technical cooperation between nuclear weapons states should also be enhanced in order to improve sanitation and water quality through collaborative ventures in engineering and urban planning, and to promote R&D for new drugs and vaccines to combat neglected tropical diseases [39], [40]. Similar “vaccine diplomacy” efforts between the US and Soviet Union during the Cold War led to the successful development, testing, and distribution of the oral polio vaccine and the smallpox vaccine [41], [42] Greater efforts are needed to engage leaders of the nuclear weapons states in a frank dialogue about reallocation of resources toward public health and scientific pursuits for neglected tropical disease R&D and control. Several possibilities exist for such engagement. Conferences are held every five years to review the operation of the nuclear nonprofileration treaty [43], and time should be set aside at these meetings for purposes of disease control and biomedical research. In 2009, the first BRIC (Brazil, Russia, India, China) summit took place in Russia [44], and in the future this vehicle could also be used to establish a meaningful dialogue on neglected diseases. In the coming decade, engaging the nuclear powers on neglected disease R&D and global implementation efforts would help to achieve Millennium Development Goals and would represent a significant diplomatic victory for the world.
Acknowledgments
The author acknowledges and thanks Professor Chris Beyrer from Johns Hopkins University and Dr. Juerg Utzinger from the Swiss Tropical Institute for their helpful suggestions on the manuscript.
Footnotes
The author has declared that no competing interests exist.
The author received no specific funding for this study.
References
- 1.The World Almanac And Book of Facts 2009 (C. 722. Alan Joyce, Editor-in-Chief), 2009; World Almanac Books;
- 2.U.S. Nuclear Weapons Cost Study Project. http://www.brookings.edu/projects/archive/nucweapons/50.aspx, accessed December 5, 2009.
- 3. http://www.ippnw-students.org/NWIP/factsheets/money.html, accessed December 5, 2009.
- 4.Hotez PJ, Fenwick A, Savioli L, Molyneux DH. Rescuing the bottom billion through control of neglected tropical diseases. Lancet. 2009;373:1570–1575. doi: 10.1016/S0140-6736(09)60233-6. [DOI] [PubMed] [Google Scholar]
- 5.Hotez PJ, Thompson TG. Waging peace through neglected tropical disease control: a U.S. foreign policy for the bottom billion. PLoS Negl Trop Dis. 2009;3:e346. doi: 10.1371/journal.pntd.0000346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hotez PJ. Neglected diseases amid wealth in the United States and Europe. Health Aff (Millwood) 2009;28:1720–5. doi: 10.1377/hlthaff.28.6.1720. [DOI] [PubMed] [Google Scholar]
- 7.Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, Diemert D, Hotez PJ. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2005;367:1521–32. doi: 10.1016/S0140-6736(06)68653-4. [DOI] [PubMed] [Google Scholar]
- 8.deSilva NR, Brooker S, Hotez PJ, Montresor A, Engels D, Savioili L. Soil-transmitted helminth infections: updating the global picture. Trends Parasitol. 2003;19:547–51. doi: 10.1016/j.pt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Ministry of Health of China. Report on the national survey of current situation of major human parasite diseases in china. National Institute of Parasitic Diseases. 2005:33. [Google Scholar]
- 10.Hotez PJ. Neglected infections of poverty in the United States of America. PLoS Negl Trop Dis. 2008;2 doi: 10.1371/journal.pntd.0000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michael E, Bundy DAP, Grenfell BT. Re-assessing the global prevalence and distribution of lymphatic filariasis. Parasitology. 1996;112:409–28. doi: 10.1017/s0031182000066646. [DOI] [PubMed] [Google Scholar]
- 13.Keiser J, Utzinger J. Emerging foodborne trematodiases. Emerg Infect Dis. 2005;11:1507–14. doi: 10.3201/eid1110.050614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keiser J, Utzinger J. Food-borne trematodiasis: current chemotherapy and advances with artemisinins and synthetic trioxolanes. Trends Parasitol. 2007;23:555–62. doi: 10.1016/j.pt.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 15.Kim HG, Han J, Kim MH, Cho KH, Shin IH, Kim GH, et al. Prevalence of clonorchiasis in patients with gastrointestinal disease: a Korean nationwide multicenter survey. World J Gastroenterol. 2009;15:86–94. doi: 10.3748/wjg.15.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yossepowitch O, Gotesman T, Assous M, Marva E, Zimlichman R, Dan M. Opisthorchiasis from imported raw fish. Emerg Infect Dis. 2004;10:2122–6. doi: 10.3201/eid1012.040410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joshi A, Narain JP, Prasittisuk C, Bhatia R, Hashim G, Jorge A, Banjara M, Kroeger A. Can visceral leishmaniasis be eliminated from Asia. J Vector Borne Dis. 2008;45:105–11. [PubMed] [Google Scholar]
- 18.Altaf C, Ahmed P, Ashraf T, Anwar M, Ahmed I. Childhood visceral leishmaniasis in Muzaffarabad, Azad Jammu and Kashmir: frequency and response to treatment in 61 cases. J Pak Med Assoc. 2005;55:475–8. [PubMed] [Google Scholar]
- 19.Ashkan MM, Rahim KM. Visceral leishmaniasis in paediatrics: a study of 367 cases in southwest Iran. Trop Doct. 2008;38:186–8. doi: 10.1258/td.2007.070259. [DOI] [PubMed] [Google Scholar]
- 20.Al-Nahhas S, Shabaan M, Hammoud L, Al-Taweel A, Al-Jorf S. Visceral leishmaniasis in the Syrian Arab Republic: early detection using rK39. East Mediterr Health J. 2003;9:856–62. [PubMed] [Google Scholar]
- 21.Marty P, Pomares-Estran C, Hasseine L, Delaunay P, Haas H, Rosenthal E. Leishmaniases in France: an update [article in French]. Arch Pediatr. 2009;Suppl 2:S96–100. doi: 10.1016/S0929-693X(09)75310-2. [DOI] [PubMed] [Google Scholar]
- 22.Dujardin J-C, Campino L, Canavate C, Dedet J-P, Gradoni L, Soteriadou K, Mazeris A, Ozbel Y, Boelaert M. Spread of vector-borne diseases and neglect of leishmaniasis, Europe. Emerg Infect Dis. 2008;14:1013–8. doi: 10.3201/eid1407.071589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Nahhas SA. Serodiagnosis of cutaneous leishmaniasis in the Syrian Arab Republic. Saudi Med J. 2009;30:382–8. [PubMed] [Google Scholar]
- 24.Emami MM, Yazdi M, Niforoushzadeh M. Emergence of cutaneous leishmaniasis due to Leishmania major in a new focus of central Iran. Trans R Soc Trop Med Hyg. 2009;103:1257–62. doi: 10.1016/j.trstmh.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 25.Talari SA, Talaei R, Shajari G, Vakili Z, Taghaviardakani A. Childhood cutaneous leishmaniasis: report of 117 cases from Iran. Korean J Parasitol. 2006;44:355–60. doi: 10.3347/kjp.2006.44.4.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khan SJ. , Muneeb S. Cutaneous leishmaniasis in Pakistan. Dermatol Online J. 2005;11:4. [PubMed] [Google Scholar]
- 27.Anis E, Leventhal A, Elkana Y, Wilamowski A, Pener H. Cutaneous leishmaniasis in Israel in the era of changing environment. Public Health Rev. 2001;29:37–47. [PubMed] [Google Scholar]
- 28.Lescure F-X, Canestri A, Melliez H, Jaureguiberry S, Develoux M, Dorent R, Guiard-Schmid J-P, Bonnard P, Ajana F, Rolla V, Carlier Y, Gay F, Elghouzzi M-H, Danis M, Pialoux G. Chagas disease, France. Emerg Infect Dis. 2008;14:644–6. doi: 10.3201/eid1404.070489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pappas G, Roussos N, Falagas ME. Toxoplasmosis snapshots: global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. Int J Parasitol. 2009;39:1385–94. doi: 10.1016/j.ijpara.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization. Global health atlas: global alliance for the elimination of blinding trachoma. 2009. available: http://www.who.int/globalatlas/ via the Internet. Data query for active trachoma by country.
- 31.World Health Organization. Global leprosy situation, beginning of 2008. Wkly Epidemiol Rec. 2008;83:293–300. [PubMed] [Google Scholar]
- 32.Simms I, Broutet N. Congenital syphilis re-emerging. J Dtsch Dermatol Ges. 2008;6:269–72. doi: 10.1111/j.1610-0387.2008.06490.x. [DOI] [PubMed] [Google Scholar]
- 33.Hotez PJ. Gandhi's hookworms. Foreign Policy, January 21, 2010, http://www.foreignpolicy.com/articles/2010/01/21/gandhis_hookworms, accessed January 30, 2010.
- 34. http://www.globalnetwork.org/press/2009/12/14/global-network-applauds-us-senate-approval-funds-help-eliminate-neglected-tropical-, accessed December 19, 2009.
- 35. http://www.dfid.gov.uk/Media-Room/Press-releases/2008/50-million-to-wipe-out-deadly-tropical-diseases/, accessed December 19, 2009.
- 36.Moran M, Guzman J, Ropars A-L, McDonald A, Sturm T, Jameson N, Wu L, Ryan S, Omune B The George Institute. G-Finder: Neglected Disease Research & Development: How Much Are We Really Spending? 2008. 70. Health Policy Division, The George Institute for International Health. [DOI] [PMC free article] [PubMed]
- 37.Hotez PJ, Molyneux DH, Fenwick A, Savioli L, Takeuchi T. A global fund to fight neglected tropical diseases: is the G8 Hokkaido Toyako 2008 summit ready? PLoS Negl Trop Dis. 2008;2:e220. doi: 10.1371/journal.pntd.0000220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hotez PJ. Forgotten People, Forgotten Diseases: The Neglected Tropical Diseases and Their Impact on Global Health and Development. Washington DC: ASM Press; 2008. 218 [Google Scholar]
- 39.Hotez PJ, Ferris M. The antipoverty vaccines. Vaccine. 2006;24:5787–99. doi: 10.1016/j.vaccine.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 40.Hotez PJ, Brown AS. Neglected tropical disease vaccines. Biologicals. 2009;37:160–4. doi: 10.1016/j.biologicals.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 41.Hotez PJ. Vaccine diplomacy. Foreign Policy. 2001 (May–June):68–9. [Google Scholar]
- 42.Hotez PJ. Peace through vaccine diplomacy. Science. 2010; Mar 12;;327(5971):1301. doi: 10.1126/science.1189028. [DOI] [PubMed] [Google Scholar]
- 43. The 2005 Review Conference of the Parties to the Treaty on the Non-Proliferation of Nuclear Weapons (NPT) 2–27 May 2005, http://www.un.org/events/npt2005/background.html, accessed January 30, 2010.
- 44. http://www.dw-world.de/dw/article/0,,4335954,00.html.