Abstract
Panic disorder (PD) is a severe, chronic disorder characterized by one or more unexpected panic attacks followed by worry about additional attacks and/or the implications of the attacks. If attacks are sufficiently severe or frequent, they can promote marked, sometimes debilitating behavioral changes. Many panic disorder sufferers appear to be incompletely responsive to treatment and are subject to relapse after remission. In this article, we highlight the current understanding of the pathophysiology of PD using a “fear circuit” model. Using this model as a reference point, we review the evidence base supporting existing and emerging treatments and suggest strategies for optimizing initial treatment response. Finally, a differential diagnostic approach for clinical evaluation of unsatisfactory response to treatment in PD is presented.
Keywords: panic disorder, treatment resistance, drug treatment, cognitive behavioral, comorbidity
Introduction
Panic disorder (PD) is a severe, persistent, and potentially disabling anxiety disorder that affects an estimated 3.5 to 5 percent of the general population at some point in their lives.1 Twice as many female subjects as male subjects are affected. PD is characterized by one of more unexpected panic attacks followed by at least one month of concern of additional attacks, the physical implications of these attacks, or changes in behavior (e.g., help-seeking, fearful avoidance) related to these attacks. Agoraphobia is a term that describes avoidance situations in which an attack may occur or apprehensive endurance of these situations, which can confer significant limitations in social, work, and family functioning. Two-thirds of PD-affected individuals will develop major depression at some time2 and are at increased risk for developing one or more additional psychiatric disorders within a six-month period.1
A unifying hypothesis explaining why panic attacks occur in some individuals and not in others is still lacking. However, basic and clinical research supports the concept that neurobiological alterations (including distorted cognition) in key brain areas are important in mediating the appearance and persistence of PD. The scientific rationale for pharmacological treatment of PD is based on substantial empirical evidence supporting the effectiveness of pharmacotherapy for this condition. Despite our increased knowledge of the biological underpinnings of PD and our expanding treatment armamentarium, nearly 20 percent of those individuals diagnosed as having PD will remain seriously ill five years later.3
Neurobiology of PD
Different brain structures within the fear circuit are thought to be involved in mediating panic attacks, panic-related phobic avoidance, and fearful anticipation of additional attacks. Based on the available preclinical and clinical research findings available, Gorman and colleagues proposed a hypothetical neuroanatomic model that takes into account the observed efficacy of both pharmacotherapy and cognitive-behavior therapy (CBT) in individuals with PD (Figure 1).4,5 Activation of the central nucleus of the amygdala activates the “alarm” (the typically observed symptoms that occur during conditioned fear) via projections to structures in the brainstem and hypothalamus and communicates with other key neuroanatomical fear circuit nuclei. Projections to the amygdala from the hippocampus and medial prefrontal cortex have inhibitory inputs to the amygdala and are also important in the mediating distorted cognitions, disrupted coping strategy, and fearful avoidance observed following unexpected, uncontrolled panic attacks.
Figure 1.
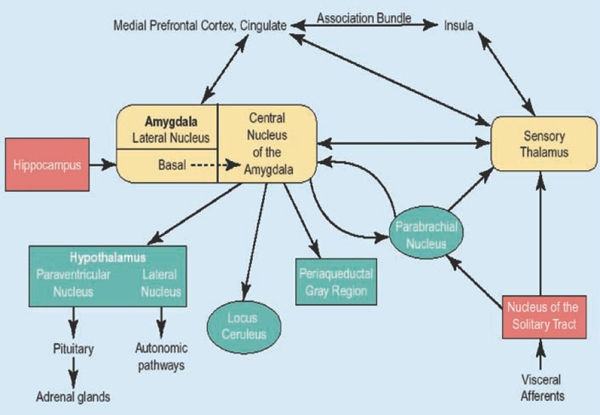
Fear circuit model
Source: Gorman, et al., AJP 2000;157:493-505 (used with permission)
Table 1.
Panic symptoms and panic disorder
| A panic attack is a discrete period of intense fear or discomfort, in which four (or more) of the following symptoms develop abruptly and reach a peak within 10 minutes: |
|
| Panic disorder is characterized by recurrent unexpected panic attacks as described above, at least one of which has been followed by one month (or more) of one (or more) of the following: |
|
Source: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Washington, DC: American Psychiatric Press, Inc., 2000
The potent anxiogenic brain neuropeptide, corticotropin releasing factor (CRF), is thought to play an important role in initiating panic attacks.4 Most effective pharmacological treatments for PD appear to exert significant direct or indirect inhibitory effects on CRF function.6 Many other neurobiological hypotheses regarding the etiology of PD are compatible with this fear circuit model, including loss of homeostasis of brain monamines,7 abnormal C02 sensitivity,8 hypothalamic-pituitary-adrenal dysfunction,9,10 alterations in brain cholecystokin activity,11 disrupted inter-regulation between inhibitory gamma-aminobutyric acid (GABA),12 and excitatory (glutamate) neuronal activity.12 Whether there are distinct subtypes of PD or overlapping pathophysiologic factors is unclear. What is clear is that not all individuals with PD respond equally well to one treatment.
There is convicing evidence that both inherited vulnerability and subsequent life events, especially early adverse events, play important roles in the onset and course of PD. Kendler and colleagues have used data from large-scale family and twin studies to examine the role of genetic and environmental factors in anxiety disorders and have consistently found PD to be among the most highly inheritable.13,14 Early life events, such as sexual or physical abuse, also appear to substantially increase the risk for subsequent development of mood and anxiety symptoms.15,16 For example, in one recent study, nearly one-third of a sample of PD patients reported a history of physical and/or sexual abuse.17 Interaction between nature and nurture is suggested by the observed association between early abuse and the subsequent severity of symptoms of anxiety and depression.18 Both clinical and epidemiologic studies have shown that PD commonly co-exists with other psychiatric disorders, such as depression, other anxiety disorders, and alcohol abuse.1 As such, optimal treatment planning for PD should include careful assessment of comorbid disorders and an understanding of the variety of factors that can influence the course, severity, and presentation of the illness. Other factors, including gender, socioculture, and religious orientation, are important sources of variability. Since there are potentially many interacting etiologies for PD, it is easier to understand why we often clinically observe that no one treatment is universally effective. The various research techniques employed to enhance our understanding of the pathophysiology of PD (e.g., measuring neurotransmitter or metabolite concentrations in plasma and cerebrospinal fluid, challenge studies, neuroimaging) are useful for hypothesis testing, but are of no practical use for the diagnosis and treatment of PD.9,19,20,21 The neuroanatomic fear circuit model does not rely on the presence or absence of any particular biological abnormality, but has provided a fruitful model for developing and testing various hypotheses.7 It also provides a clinically useful tool that can assist the clinician in formulating a differential diagnostic plan for increasing the intensity of the pharmacological, cognitive, or both treatments as the next step when the initial treatment does not go well. As such, it provides a useful framework for the purposes of day-to-day clinical assessment and improved management of individuals with unsatisfactory treatment outcome for PD.
The Therapeutic Trial
Assuring the adequacy of the initial treatment is perhaps the best way to avoid inadequate response and perhaps the accrual of comorbidity treatment.22 Unfortunately, there is no evidence addressing the adequacy of treatment in PD. It should be noted that the time-course for improvement is similar to that seen with antidepressant treatment; however, full response to medication will depend on comorbidity, severity of panic and avoidance, and intercurrent psychosocial stressors. Thus, it may take several months for substantial treatment gains. The order of onset of improvement is usually reduction in the severity and frequency of panic attacks, with gradual reduction in fearful avoidance and anticipatory anxiety.23
There is empirical support for the efficacy of both psychopharmacological and CBT for PD. The clinical decision to prescribe medication versus CBT to treat PD involves an ongoing collaborative interplay between the wishes and beliefs of the patient, the orientation of the clinician, and the severity of the symptoms.24
Pharmacological treatment should be accompanied by appropriate patient education, which serves to support the rationale for treatment and to enhance adherence by providing the appropriate cognitive “set.” Establishing a therapeutic alliance is a critical component of the therapeutic trial. Beginning at the first encounter, preparation of the patient should include discussing the potential need for more than one medication trial, as well as expected adverse effects of medication. Discussing the likelihood of inherited vulnerability to PD can ease the self blame and feelings of failure due to the inability of a patient to manage frightening panic symptoms on his or her own. It is also helpful to discuss the expected duration of treatment and to acknowledge that it is often unclear how long treatment should continue and that periodic decreases in medication after optimizing treatment gains are part of the long-term plan. It should be made clear to the patient that improvement is gradual rather than immediate. Family members or spouses, with the patient's consent, may be included in these initial discussions to increase their understanding of the illness and to temper their frustrations at PD-related limitations imposed on the patient.
During treatment it is important to document clinical change. It has been shown that panic attack frequency is not a particularly good measure for assessing the effects of treatment.25 In order to assess the severity of panic symptoms and their impact upon daily functioning and quality of life, we suggest the clinician become familiar with some assessment tools that can be used to assess change during treatment. The Panic and Agoraphobia Scale26 and the Panic Disorder Severity Scale27 assess illness severity and are sensitive to change. The Sheehan Disability Scale28 measures illness-related impairment, and the Quality of Life Enjoyment and Satisfaction Questionnaire29 measures life satisfaction and quality of life.30 Such measures are particularly valuable in evaluating both PD-related impairment and quality of life over the course of treatment.
Optimizing Treatment
There are four classes of pharmacological treatment with comparable efficacy for PD: Selective serotonin reuptake inhibitors (SSRIs), selective norepinephrine-serotonin reuptake inhibitor (SNRIs), tricyclic antidepressants (TCAs), and benzodiazepines (BZ). Prior treatment history, cost, concurrent medical and psychiatric conditions, and side effects are important considerations. The properties of the individual agents (e.g., half-life, drug-drug interactions) are also important.
Antidepressant medications, such as SSRIs, recommended as first-line treatments for PD,31 and multiple, randomized, controlled trials (RCTs) have demonstrated their efficacy in PD.24 Studies of the SNRI venlafaxine have also shown efficacy in PD that is superior to placebo and comparable to SSRIs.32,33 TCAs and monoamine oxidase inhibitors (MAOIs) are well-studied and have been shown to be effective in PD.24 The older MAOIs are used infrequently due to their side effect burden and toxicity in overdose;34 the selective MAOIs are either not available or have not been studied in PD (e.g., selgiline). Concerns over the emergence of suicidal behavior have been raised by the US Food and Drug Administration (FDA) for all antidepressants in children and adolescents.35 There have not yet been similar concerns expressed for adults; however, considering the potential risk for suicidal behavior associated with panic and other anxiety disorders, caution and discussion with the patient prior to instituting antidepressant treatment is prudent.36
Patients with PD can be exquisitely sensitive to the activating effects of SSRIs and TCAs during initiation of treatment.24 Advising patients that activation (e.g., jitteriness, increased anxiety, insomnia) during initiation of treatment may occur, is not dangerous, and actually may be an indication that the diagnosis of panic is correct can assist in adherence early in treatment. Slow upward titration is often sufficient to limit activation, but for some patients, initial activation can limit dose titration. There is evidence suggesting that initiating treatment with the short-term adjunctive use of benzodiazepines accelerates initial improvement.37 Insomnia, gastrointestinal symptoms, and weight gain can also limit treatment acceptance, and these side effects should be addressed prior to and revisited throughout treatment. Nonadherence due to sexual side effects during antidepressant treatment should also be addressed at the outset, and patients should be provided with information about the use of adjunctive agents to blunt or counteract sexual dysfunction as well as the option of switching to a different class of anti-panic agent.
It can sometimes be difficult to distinguish between symptoms and side effects or to tell if panic attacks are resistant to treatment or simply recurrent in the face of poor adherence or external stress. In the absence of empirical data, our experience in clinical practice has indicated that a second trial with a different SSRI or venlafaxine may be beneficial if initial treatment is not sufficiently effective. We recommend a brief period of co-administration, generally 2 to 4 weeks, while the new agent is initiated and the old agent is discontinued. The patient should be closely monitored during the taper of the first agent and concomitant upward titration of the second agent.
BZs have proven efficacy as monotherapy and in long-term use for PD.38 As described above, they can be especially helpful when used in combination with SSRIs at the initiation of treatment. Patients with PD may require doses higher than the doses many clinicians are accustomed to prescribing.
Some clinicians hesitate to use BZs due to concerns about dependence, abuse potential, and cognitive dulling.39 While important to recognize,40,41 there is little empirical support for any significant abuse or harmful effects from BZs on neurocognitive function,42 or a significant risk for abuse among patients with anxiety disorders.43,44 For PD sufferers who have a prior history of substance abuse, however, the risk is increased and caution is indicated.45 Thorough patient education and close monitoring of side effects and usage patterns are critical components to therapeutic success. If BZs are used, it is important to remember that they do not treat depressive symptoms and may even worsen depression.46 Moreover, there is some limited evidence suggesting that BZ use may interfere with exposure therapy.47 All of the medications currently used to treat PD, however, have limitations and risks in addition to their benefits.
Some patients may elect to forego initial pharmacotherapy in favor of a psychological intervention, such as CBT or exposure therapy. CBT is comparable to medication treatment of mild to moderate panic symptoms48 and may provide a more durable post-treatment response.47,49,50 Many clinicians use elements of CBT, psychoeducation, and other psychosocial interventions in the course of treating PD. If psychotherapy is to be effective as monotherapy, the clinician must be adequately trained and experienced in the delivery of the particular modality, and the patient must be willing to commit to an adequate frequency and number of visits.51 With respect to intensity of treatment, at least 12 sessions of CBT by a qualified professional are considered the minimum effective dose for PD. It should also be noted that CBT may be particularly useful during a BZ taper.52,53
CBT and other psychotherapeutic treatments are thought to exert their antipanic effects via cortical and hippocampal projections to and from the amygdala.49 Functional imaging studies comparing subjects treated with either CBT or medication have both shown considerable overlap as well as treatment-specific patterns of regional cortical activation.54,55 While combined, these observations suggest the possibility that psychotherapeutic and pharmacological treatments could exert synergistic anxiolytic effects. CBT and other psychological therapies in combination with pharmacotherapy have been reported to be beneficial in PD patients who do not respond to initial pharmacotherapy56,57,58,59 and to help manage symptoms during a medication taper.52,53 Controlled clinical studies of combined treatments (psychotherapy and pharmacotherapy) versus psychotherapy or pharmcotherapy alone in uncomplicated PD patients have not shown advantages of combined treatment over single treatment methods, but combined treatments do show advantages over single treatments in patients with persistent avoidance and cognitive distortion.47,49,60,61,62
Evaluating Treatment Resistance
Among patients who tolerate and adhere fully with initial therapy, a significant number will still not respond fully to treatment. As there are no standardized criteria for a therapeutic trial in panic disorder, treatment resistance in this review is defined as an inadequate response to what is generally considered adequate treatment. Assuming that an adequate dosage is employed, the usual time course for improvement in PD patients receiving BZs is a reduction in the severity and frequency of panic attacks within the first week.39 For those receiving antidepressants, some reduction in the intensity and frequency of panic attacks should be seen within the first 3 to 4 weeks.24 Following the improvement in panic attack symptoms, reduction in anticipatory anxiety and, if present, phobic anxiety usually improves within 6 to 8 weeks. However, for full remission, the required time may be several months. For PD patients who remain symptomatic after initial treatment, re-consideration of the diagnosis, intensity of treatment (dose and duration of treatment), adherence, and initially overlooked psychiatric and medical comorbidities may be necessary. Unrecognized substance/alcohol abuse or personality disorder should also be considered. The clinician should employ a differential diagnostic approach to the component(s) of PD, such as concurrent psychosocial stressors, and related complications that have not responded, including panic attacks, phobic avoidance, anticipatory anxiety, and cognitive factors within that individual patient (Table 2). It is important to keep in mind that empirical studies show significant percentage of PD patients fail to respond to a given treatment and that many patients require a different agent, combined treatment, augmentation with another agent, or other novel approaches.
Table 2.
Differential diagnosis for treatment-resistant panic disorder
| PROBLEM REVIEW | DIFFERENTIAL DIAGNOSIS | COMMENT |
|---|---|---|
| Persistent panic attacks |
|
|
| Persistent anticipatory/generalized anxiety |
|
|
| Residual phobia |
|
|
| Other disorders |
|
|
| Environmental event/stressor(s) |
|
|
| Other |
|
|
- BZ
benzodiazepines
- CBT
binge eating disorder
- MAOI
monoamine oxidase inhibitor
- OCD
obsessive compulsive disorder
- PTSD
posttraumatic stress disorder
- SSRI
selective serotonin reuptake inhibitor
- TCA
tricyclic antidepressants
Comorbid major depression is a common complication in PD. Recent findings of a 12-year follow up study conducted by the Harvard/Brown Anxiety Research Program (HARP) highlight both the difficulty in treating PD and the impact of depressive comorbidity on the course of PD.63 In this large study, patients with co-occurring PD and major depression were half as likely to recover. Even when unaccompanied by depression, PD patients had the highest rates of recurrence of all anxiety disorders. Moreover, while 80 percent of subjects in the study with simple PD attained recovery, fewer that half of those with panic and agoraphobia did so. This study underscores the intrinsic relapsing and remitting nature of PD, and suggests that certain PD features, such as the presence of agoraphobia and social phobia, may be intrinsically more difficult to remit. Consideration of unrecognized personality disorders and substance or alcohol abuse can also complicate both the diagnosis and treatment of PD.45,63,64,65,66 Accumulating evidence suggests a substantial overlap between anxiety, panic, and bipolar spectrum disorders,67,68 and consideration of previously unrecognized bipolar spectrum disorders in frequently relapsing or nonresponsive PD is indicated. In cases of treatment failure where no additional psychiatric condition is present, a high index of suspicion must be maintained for a variety of medical disorders that often accompany, exacerbate, or even cause panic symptoms. These include temporal lobe epilepsy, migraine, hypertension, mitral valve prolapse, coronary artery disease (CAD), chronic obstructive pulmonary disease (COPD), hyperthyroidism, and irritable bowel syndrome (IBS).69
In cases of inadequate response to pharmacotherapy, pharmacodynamic factors70 and pharmacokinetic factors (drug interactions and interindividual variation in drug metabolism) should be considered.71 Several recent studies indicate the presence of a variety of structural and functional neurotransmitter receptor alterations that may impact the activity of various medications in patients with PD. In particular, differences in the GABA-A receptor distribution, density and binding properties have been noted in PD subjects when compared to healthy controls.37,72 It has been shown that drug-naïve PD patients require significantly higher doses of BZs than controls to induce sedation and oculomotor slowing,73 which may represent differences in the conformation of the BZ receptors in these individuals. The use of plasma levels to assess concentrations of antipanic agents in patients who appear to require above average oral doses but exhibit low or moderate plasma drug levels may be useful for identifying rapid metabolizer patients who require higher doses. In a six-week, double-blind, placebo-controlled, dose-response study, Lesser and colleagues74 evaluated 96 patients with PD who received six weeks treatment with 2mg or 6mg per day of alprazolam or placebo. The authors found that 67 percent of patients with alprazolam plasma levels greater than 40ng/mL, 57 percent of patients with alprazolam levels between 20 and 40ng/mL, and 42 percent of patients with levels lower than 20ng/mL achieved complete suppression of their panic attacks. Fifty-three percent of patients achieved moderate response on global improvement scales at plasma concentrations of 20ng/mL and 80 percent at plasma concentrations of 60ng/mL. Still higher concentrations of alprazolam were necessary to achieve significant reduction in phobias (over 70ng/mL). Mavissakalian and Perel23 reported a dose-response relationship between plasma levels of imipramine plus desipramine combined and clinical response in patients receiving imipramine for PD with agoraphobia. Doses of over 150mg daily were usually necessary for optimal balance of antipanic effects and side effects; antiphobic effects were apparent at higher doses (2.5mg/kg) with the offset of reduced tolerability.
In addition to potential abnormalities in the GABA-ergic system, it has been reported that subjects with PD have reduced 5-HT1a receptor density and activity in key areas of the brain.75 While the clinical implications of these findings are unclear, they are consistent the clinical observation that PD patients often require higher than average doses of antidepressants to achieve response. The clinical requirement for high dose levels for efficacy and the need for initiating treatment at low doses and slow titration can be a source of frustration for both clinician and patient. This underscores the importance of establishing a clear and realistic framework for the therapeutic trial as discussed previously. Table 3 provides dosing guidelines for the agents most commonly used to treat PD.
Table 3.
Dosing guidelines for agents used to treat panic disorder
| CLASS/AGENT | STARTING DOSE (MG/D) | TYPICAL EFFECTIVE DOSE (MG/D)* |
|---|---|---|
| SSRIs | ||
| Sertraline | 12.2–25 | 150–300 |
| Escitalopram | 5 | 10–30 |
| Fluoxetine | 2–5 | 40–80 |
| Fluvoxamine | 25 | 150–300 |
| Paroxetine | 5–10 | 40–60 |
| SNRI | ||
| Venlafaxine | 18.75–37.5 | 150–300 |
| Benzodiazepines | ||
| Alprazolam | 0.5–1.0 | 2–10 |
| Clonazepam | 0.25–0.5 | 2–6 |
| Tricyclics | ||
| Clomipramine | 10 | >200 |
| Desipramine | 10 | >300 |
| Imipramine | 10 | >300 |
| MAOIs | ||
| Phenelzine | 15 | >90 |
| Tranylcypromine | 10 | >70 |
| Antiepileptics | ||
| Valproate | 250–500 | 1,000–2,000 |
| Gabapentin | 100–200 | 600–3,400 |
| Levitracitam | 250–500 | 1500–3000** |
| Tiagabine | unknown | unknown |
| Vigabatrin | unknown | unknown |
4–6 weeks should be allowed to assess effectiveness
Information on dosing is anecdotal
Management of Treatment-Resistant Panic disorder
Even after adequate treatment, some patients still fail to experience satisfactory relief of panic symptoms. The clinician is then faced with the decision to increase the dose, use an augmenting agent, or switch to another agent. There are limited evidence-based data available to assist in developing a strategy for refractory PD. In general, we recommend that for patients with a partial response, increasing the dose of the agent being used is a simple first step toward assuring adequate intensity of treatment. This can be followed by augmentation with another antidepressant or other agent. When considering the use of non-standard, unapproved, or off-label medications, the existing literature, the relative risks and benefits, and the rationale for treatment should be carefully explained to the patient and their consent documented. If there is a total lack of response after six weeks, it seems prudent to switch to another agent. To our knowledge, there are no switching studies or published data on the use of any approved medication as monotherapy for treatment-resistant panic. Assuming that there is no known pharmacokinetic contraindication, a smooth transition from one agent to another can often be accomplished by “layering in” the new agent with a gradual dose reduction of the initial agent over 3 to 4 weeks.
The only published, double-blind, placebo-controlled study of augmentation in treatment-resistant PD demonstrated moderate benefit when pindolol was added to fluoxetine.76 An open label trial of the selective norepinephrine reuptake inhibitor (SNRI), reboxetine, in 26 patients who had failed initial SSRI treatment showed promising results,77 as did a small, open-label study of the combination of fluoxetine and imipramine in seven cases of resistant panic.78 Although this might suggest that the consecutive or concomitant use of two antidepressants from different classes may be one approach of overcoming refractory symptoms, additional studies are needed. Moreover, combining SSRIs with TCAs may cause additional side effects, including serotonin syndrome. Another open-label study found that olanzapine (average dose 12.3mg daily) was helpful in 10 patients with resistant PD.79 Buspirone is ineffective as monotherapy for PD,80 and there is no empirical evidence supporting its use in augmentation. One study reported benefical effects of buspirone for residual generalized anxiety symptoms in a small series of PD patients.81
In addition to the well-studied antidepressant agents discussed earlier, a number of other medications currently in use as antidepressants possess pharmacologic profiles that suggest possible efficacy in PD, although none have been examined in the context of treatment resistance. Mirtazapine, a noradrenergic agent with 5-HT3 antagonist properties, has shown promise in small, uncontrolled studies.82,83,84 Duloxetine, an SNRI approved for use in generalized anxiety disorder (GAD), was reportedly beneficial for PD in a single, small, uncontrolled setting.85 Bupropion, a dopaminergic and noradrenergic agent, was found ineffective in PD in a study performed in the early 1980s,86 but has since shown promise in one small, controlled trial and an open-label study.87 Given the current dearth of data, the consideration of any of these agents in patients with resistant panic should be undertaken only after other options have been explored and with specific justification documented.
The use of anticonvulsant medications in treatment-resistant PD appears to be common in clinical practice, but has not been studied systematically either as monotherapy or as augmentation. In small, placebo-controlled studies and numerous case reports anticonvulsants appear to have potential utility as anxiolytics.88 Putative mechanisms include enhancing GABA activity, modulating inhibitory/excitatory neurotransmission by decreasing glutamatergic activity, and modulating intracellular signaling pathways or voltage-dependent sodium and calcium ion channels. One small, open-label study of sodium valproate indicated effectiveness in panic patients with mood instability who had failed prior treatments,89 and in another report, a combination of clonazepam and sodium valproate showed efficacy in four patients who had failed prior treatments.90 A small study comparing carbamazepine with placebo showed no significant effect of the anticonvulsant.91 In the only placebo-controlled study to date, gabapentin was not superior to placebo on the main outcome measure, but was superior to placebo on all secondary variables,92 but this has not been replicated. Levetiracetam has shown promise in an open-label study93 in 18 PD patients and in a preliminary report to be an effective augmentation treatment in a small group of drug-resistant PD patients. Case reports suggest that tiagabine may be beneficial in panic alone or in augmentation.94,95 Similarly, vigabatrin has only a small case series and responses to experimentally induced panic supporting its use.95,96 Taken together, these data suggest that anticonvulsants may potentially play a larger role in the management of PD but require further study before their routine use or use in resistant PD patients.
Atypical neuroleptics, especially those that have serotonergic properties, may seem good candidates for treatment of anxiety disorders. No data is currently available detailing the extent to which these agents are prescribed off-label for treatment of PD, but anecdotal evidence suggests that it is a not-infrequent occurrence. Small open-label studies of the addition of either low-dose risperidone or olanzapine to standard therapy with SSRIs have indicated some benefit in reducing symptom burden and improving quality of life.97,98 Prospective studies on augmentation with other atypical agents have not yet been published, nor have these agents been studied as monotherapy or in treatment-resistant PD under controlled conditions.
Conclusion
In summary, PD is a severe, potentially disabling illness characterized by episodic remission and relapse, even in the presence of the best currently available treatments. Most patients can achieve at least modest benefit from a therapeutic trial of one of the empirically proven treatments. The keys to success in initial pharmacotherapy include adequate education, careful titration, and close follow-up visits early in treatment. The decision to use concomitant BZs or psychotherapy is made on an individual basis. Evidence for neurobiological differences between individuals with PD and normal individuals suggests higher than average doses of medication may be required to achieve remission of panic symptoms. Apparent treatment failures should be evaluated via a differential diagnostic approach for adherence, specific panic-related residual symptoms, and the presence of a comorbid anxiety or mood disorder, substance/alcohol abuse, and personality or medical disorders. Concurrent psychosocial stressors should also be evaluated. In cases where apparently adequate trials of at least two medications have been unsuccessful, switching or augmentation strategies may be employed based on clinical assessment. In the absence of empirical evidence, the art of medicine remains an important part of the process for optimizing the treatment of PD.
Contributor Information
Richard L. Holt, Dr. Holt is from the Medical University of South Carolina Department of Psychiatry.
R. Bruce Lydiard, Lydiard is from Southeast Health Consultants, LLC, Charleston, South Carolina.
References
- 1.Kessler RC, Chiu WT, Jin R, et al. The epidemiology of panic attacks, panic disorder, and agoraphobia in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2006 Apr;63(4):415–24. doi: 10.1001/archpsyc.63.4.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robins LN, Locke BZ, Regier DA. An Overview of Psychiatric Disorders in America. New York: Free Press; 1991. [Google Scholar]
- 3.Katschnig H, Amering M, Stolk JM, Ballenger JC. Predictors of quality of life in a long-term followup study in panic disorder patients after a clinical drug trial. Psychopharmacol Bull. 1996;32(1):149–55. [PubMed] [Google Scholar]
- 4.Gorman JM, Kent JM, Sullivan GM, Coplan JD. Neuroanatomical hypothesis of panic disorder, revised. Am J Psychiatry. 2000;157(4):493–505. doi: 10.1176/appi.ajp.157.4.493. [DOI] [PubMed] [Google Scholar]
- 5.Sakai Y, Kumano H, Nishikawa M, et al. Cerebral glucose metabolism associated with a fear network in panic disorder. Neuroreport. 2005;16(9):927–31. doi: 10.1097/00001756-200506210-00010. [DOI] [PubMed] [Google Scholar]
- 6.Stout SC, Owens MJ, Nemeroff CB. Regulation of corticotropin-releasing factor neuronal systems and hypothalamic-pituitary-adrenal axis activity by stress and chronic antidepressant treatment. J Pharmacol Exp Ther. 2003;300:1085–92. doi: 10.1124/jpet.300.3.1085. [DOI] [PubMed] [Google Scholar]
- 7.Coplan JD, Lydiard RB. Brain circuits in panic disorder. Biologic Psychiatry. 1998;44(12):1264–76. doi: 10.1016/s0006-3223(98)00300-x. [DOI] [PubMed] [Google Scholar]
- 8.Sinha SS, Coplan JD, Pine DS, et al. Panic induced by carbon dioxide inhalation and lack of hypothalamic-pituitary-adrenal axis activation. Psychiatry Res. 1999;86(2):93–8. doi: 10.1016/s0165-1781(99)00029-3. [DOI] [PubMed] [Google Scholar]
- 9.Abelson JL, Curtis GC. Hypothalamic-pituitary-adrenal axis activity in panic disorder. 24-hour secretion of corticotropin and cortisol. Arch Gen Psychiatry. 1996;53(4):323–31. doi: 10.1001/archpsyc.1996.01830040059010. [DOI] [PubMed] [Google Scholar]
- 10.Abelson JL, Khan S, Liberzon I, Young EA. HPA axis activity in patients with panic disorder: Review and synthesis of four studies. Depress Anxiety. 2007;24(1):66–76. doi: 10.1002/da.20220. [DOI] [PubMed] [Google Scholar]
- 11.Pande AC, Greiner M, Adams JB, et al. Placebo-controlled trial of the CCK-B antagonist, CI-988, in panic disorder. Biologic Psychiatry. 1999;46(6):860–2. doi: 10.1016/s0006-3223(99)00090-6. [DOI] [PubMed] [Google Scholar]
- 12.Lydiard RB. The role of GABA in anxiety disorders. J Clin Psychiatry. 2003;64(3):21–7. [PubMed] [Google Scholar]
- 13.Hettema JM, Neale MC, Kendler KS. A review and meta-analysis of the genetic epidemiology of anxiety disorders. Am J Psychiatry. 2001;158(10):1568–78. doi: 10.1176/appi.ajp.158.10.1568. [DOI] [PubMed] [Google Scholar]
- 14.Hettema JM, Prescott CA, Myers JM, et al. The structure of genetic and environmental risk factors for anxiety disorders in men and women. Arch Gen Psychiatry. 2005;62(2):182–9. doi: 10.1001/archpsyc.62.2.182. [DOI] [PubMed] [Google Scholar]
- 15.Friedman S, Smith L, Fogel D, et al. The incidence and influence of early traumatic life events in patients with panic disorder: A comparison with other psychiatric outpatients. J Anxiety Dis. 2002;16(3):259–72. doi: 10.1016/s0887-6185(02)00097-x. [DOI] [PubMed] [Google Scholar]
- 16.Nemeroff CB, Bremner JD, Foa EB, et al. Posttraumatic stress disorder: A state-of-the-science review. J Psychiatr Res. 2006;40(1):1–21. doi: 10.1016/j.jpsychires.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Safren SA, Gershuny BS, Marzol P, et al. History of childhood abuse in panic disorder, social phobia, and generalized anxiety disorder. J Nerv Men Dis. 2002;190(7):453–6. doi: 10.1097/00005053-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Mancini C, Van Ameringen M, MacMillan H. Relationship of childhood sexual and physical abuse to anxiety disorders. J Nerv Mental Dis. 1995;183(5):309–14. doi: 10.1097/00005053-199505000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Bremner JD. Brain imaging in anxiety disorders. Expert Rev Neurother. 2004;4(2):275–84. doi: 10.1586/14737175.4.2.275. [DOI] [PubMed] [Google Scholar]
- 20.Butler J, O'Halloran A, Leonard BE. The Galway study of panic disorder. II: Changes in some peripheral markers of noradrenergic and serotonergic function in DSM III-R panic disorder. J Affect Dis. 1992;26(2):89–99. doi: 10.1016/0165-0327(92)90039-9. [DOI] [PubMed] [Google Scholar]
- 21.Keck ME, Strohle A. Challenge studies in anxiety disorders. Handb Exp Pharmacol. 2005(169):449–68. doi: 10.1007/3-540-28082-0_16. [DOI] [PubMed] [Google Scholar]
- 22.Goodwin R, Olfson M. Treatment of panic attack and risk of major depressive disorder in the community. Am J Psychiatry. 2001;158(7):1146–8. doi: 10.1176/appi.ajp.158.7.1146. [DOI] [PubMed] [Google Scholar]
- 23.Mavissakalian M, Perel J. Imipramine in the treatment of agoraphobia: Dose-response relationships. Am J Psychiatry. 1985;142(9):1032–6. doi: 10.1176/ajp.142.9.1032. [DOI] [PubMed] [Google Scholar]
- 24.Lydiard RB, Otto MW, Milrod B. Panic disorder. In: Gabbard GO, editor. Treatments of Psychiatric Disorders, Fourth Edition (TPD-IV) Washington, DC: American Psychiatric Press, Inc.; 2001. pp. 247–82. [Google Scholar]
- 25.Michelson D, Lydiard RB, Pollack MH, et al. Outcome assessment and clinical improvement in panic disorder: Wvidence from a randomized controlled trial of fluoxetine and placebo. The Fluoxetine Panic Disorder Study Group. Am J Psychiatry. 1998;155(11):1570–7. doi: 10.1176/ajp.155.11.1570. [DOI] [PubMed] [Google Scholar]
- 26.Bandelow B, Brunner E, Broocks A, et al. The use of the Panic and Agoraphobia Scale in a clinical trial. Psychiatry Res. 1998;77(1):43–9. doi: 10.1016/s0165-1781(97)00118-2. [DOI] [PubMed] [Google Scholar]
- 27.Shear MK, Brown TA, Barlow DH, et al. Multicenter collaborative panic disorder severity scale. Am J Psychiatry. 1997;154(11):1571–5. doi: 10.1176/ajp.154.11.1571. [DOI] [PubMed] [Google Scholar]
- 28.Leon AC, Olfson M, Portera L, Farber L, Sheehan DV. Assessing psychiatric impairment in primary care with the Sheehan Disability Scale. Int J Psychiatry Med. 1997;27(2):93–105. doi: 10.2190/T8EM-C8YH-373N-1UWD. [DOI] [PubMed] [Google Scholar]
- 29.Endicott J, Nee J, Harrison W, Blumenthal R. Quality of Life Enjoyment and Satisfaction Questionnaire: A new measure. Psychopharmacol Bull. 1993;29(2):321–6. [PubMed] [Google Scholar]
- 30.Rapaport MH, Wolkow RM, Clary CM. Methodologies and outcomes from the sertraline multicenter flexible-dose trials. Psychopharmacol Bull. 1998;34(2):183–9. [PubMed] [Google Scholar]
- 31.American Psychiatric Association. APA Practice Guidelines for the Treatment of Psychiatric Disorders. Washington, DC: American Psychiatric Press, Inc.; 2006. [Google Scholar]
- 32.Pollack MH, Lepola U, Koponen H, et al. A double-blind study of the efficacy of venlafaxine extended-release, paroxetine, and placebo in the treatment of panic disorder. Depress Anxiety. 2006;24(1):1–14. doi: 10.1002/da.20218. [DOI] [PubMed] [Google Scholar]
- 33.Pollack MH, Worthington JJ, 3rd, Otto MW, et al. Venlafaxine for panic disorder: Results from a double-blind, placebo-controlled study. Psychopharmacol Bull. 1996;32(4):667–70. [PubMed] [Google Scholar]
- 34.Bakker A, van Balkom AJ, Spinhoven P. SSRIs vs. TCAs in the treatment of panic disorder: A meta-analysis. Acta psychiatrica Scandinavica. 2002;106(3):163–7. doi: 10.1034/j.1600-0447.2002.02255.x. [DOI] [PubMed] [Google Scholar]
- 35.Leslie LK, Newman TB, Chesney PJ, Perrin JM. The Food and Drug Administration's deliberations on antidepressant use in pediatric patients. Pediatrics. 2005;116(1):195–204. doi: 10.1542/peds.2005-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sareen J, Cox BJ, Afifi TO, et al. Anxiety disorders and risk for suicidal ideation and suicide attempts: A population-based longitudinal study of adults. Arch Gen Psychiatry. 2005;62(11):1249–57. doi: 10.1001/archpsyc.62.11.1249. [DOI] [PubMed] [Google Scholar]
- 37.Goddard AW, Brouette T, Almai A, et al. Early coadministration of clonazepam with sertraline for panic disorder. Arch Gen Psychiatry. 2001;58(7):681–6. doi: 10.1001/archpsyc.58.7.681. [DOI] [PubMed] [Google Scholar]
- 38.Pollack MH, Allgulander C, Bandelow B, et al. WCA recommendations for the long-term treatment of panic disorder. CNS Spect. 2003;8(8) 1:17–30. doi: 10.1017/s109285290000691x. [DOI] [PubMed] [Google Scholar]
- 39.Cross-National Collaborative Panic Study, Second Phase Investigators. Drug treatment of panic disorder. Comparative efficacy of alprazolam, imipramine, and placebo. Br J Psychiatry. 1992;160:191–202. doi: 10.1192/bjp.160.2.191. discussion 205. [DOI] [PubMed] [Google Scholar]
- 40.Mueller TI, Goldenberg IM, Gordon AL, et al. Benzodiazepine use in anxiety disordered patients with and without a history of alcoholism. J Clin Psychiatry. 1996;57(2):83–9. [PubMed] [Google Scholar]
- 41.Rickels K, Case WG, Schweizer E, Garcia-Espana F, Fridman R. Long-term benzodiazepine users 3 years after participation in a discontinuation program. Am J Psychiatry. 1991;148(6):757–61. doi: 10.1176/ajp.148.6.757. [DOI] [PubMed] [Google Scholar]
- 42.Gladsjo JA, Rapaport MH, McKinney R, et al. A neuropsychological study of panic disorder: negative findings. J Affect Dis. 1998;49(2):123–31. doi: 10.1016/s0165-0327(98)00006-8. [DOI] [PubMed] [Google Scholar]
- 43.Soumerai SB, Simoni-Wastila L, Singer C, et al. Lack of relationship between long-term use of benzodiazepines and escalation to high dosages. Psychiatr Serv. 2003;54(7):1006–11. doi: 10.1176/appi.ps.54.7.1006. [DOI] [PubMed] [Google Scholar]
- 44.Salzman C, Goldenberg I, Bruce SE, Keller MB. Pharmacologic treatment of anxiety disorders in 1989 versus 1996: Results from the Harvard/Brown anxiety disorders research program. J Clin Psychiatry. 2001;62(3):149–52. doi: 10.4088/jcp.v62n0302. [DOI] [PubMed] [Google Scholar]
- 45.Shelton RC, Harvey DS, Stewart PM, Loosen PT. Alprazolam in panic disorder: A retrospective analysis. Prog Neuropsychopharmacol Biol Psychiatry. 1993;17(3):423–34. doi: 10.1016/0278-5846(93)90076-5. [DOI] [PubMed] [Google Scholar]
- 46.Lydiard RB, Laraia MT, Ballenger JC, Howell EF. Emergence of depressive symptoms in patients receiving alprazolam for panic disorder. Am J Psychiatry. 1987;144(5):664–665. doi: 10.1176/ajp.144.5.664. [DOI] [PubMed] [Google Scholar]
- 47.Marks IM, Swinson RP, Basoglu M, et al. Alprazolam and exposure alone and combined in panic disorder with agoraphobia. A controlled study in London and Toronto. Br J Psychiatry. 1993;162:776–787. doi: 10.1192/bjp.162.6.776. [DOI] [PubMed] [Google Scholar]
- 48.Haby MM, Donnelly M, Corry J, Vos T. Cognitive behavioural therapy for depression, panic disorder and generalized anxiety disorder: A meta-regression of factors that may predict outcome. Aust N Z J Psychiatry. 2006;40(1):9–19. doi: 10.1080/j.1440-1614.2006.01736.x. [DOI] [PubMed] [Google Scholar]
- 49.Barlow DH, Gorman JM, Shear MK, Woods SW. Cognitive-behavioral therapy, imipramine, or their combination for panic disorder: A randomized controlled trial. JAMA. 2000;283(19):2529–36. doi: 10.1001/jama.283.19.2529. [DOI] [PubMed] [Google Scholar]
- 50.Biondi M, Picardi A. Increased probability of remaining in remission from panic disorder with agoraphobia after drug treatment in patients who received concurrent cognitive-behavioural therapy: A follow-up study. Psychother Psychosom. 2003;72(1):34–42. doi: 10.1159/000067186. [DOI] [PubMed] [Google Scholar]
- 51.Huppert JD, Bufka LF, Barlow DH, et al. Therapists, therapist variables, and cognitive-behavioral therapy outcome in a multicenter trial for panic disorder. J Consult Clin Psychol. 2001;69(5):747–55. doi: 10.1037//0022-006x.69.5.747. [DOI] [PubMed] [Google Scholar]
- 52.Otto MW, Pollack MH, Sachs GS, et al. Discontinuation of benzodiazepine treatment: Efficacy of cognitive-behavioral therapy for patients with panic disorder. Am J Psychiatry. 1993;150(10):1485–90. doi: 10.1176/ajp.150.10.1485. [DOI] [PubMed] [Google Scholar]
- 53.Gelder MG. Combined pharmacotherapy and cognitive behavior therapy in the treatment of panic disorder. J Clin Psychopharmacol. 1998;18(6) 2:2S–5S. doi: 10.1097/00004714-199812001-00002. [DOI] [PubMed] [Google Scholar]
- 54.Prasko J, Horacek J, Zalesky R, et al. The change of regional brain metabolism (18FDG PET) in panic disorder during the treatment with cognitive behavioral therapy or antidepressants. Neuro Endocrinol Lett. 2004;25(5):340–8. [PubMed] [Google Scholar]
- 55.Sakai Y, Kumano H, Nishikawa M, et al. Changes in cerebral glucose utilization in patients with panic disorder treated with cognitive-behavioral therapy. Neuroimage. 2006;33(1):218–26. doi: 10.1016/j.neuroimage.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 56.Pollack MH, Otto MW, Kaspi SP, et al. Cognitive behavior therapy for treatment-refractory panic disorder. J Clin Psychiatry. 1994;55(5):200–5. [PubMed] [Google Scholar]
- 57.Pollack MH, Otto MW, Roy-Byrne PP, et al. Novel treatment approaches for refractory anxiety disorders. Depress Anxiety. 2007 Apr 16; doi: 10.1002/da.20329. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 58.Heldt E, Gus Manfro G, Kipper L, et al. One-year follow-up of pharmacotherapy-resistant patients with panic disorder treated with cognitive-behavior therapy: Outcome and predictors of remission. Behav Res Ther. 2006;44(5):657–65. doi: 10.1016/j.brat.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 59.Otto MW. Treatment refractory anxiety disorders: CBT. Presented at Anxiety Disorders Association of America: Novel Approach to Refractory Anxiety Disorders. Jun 6, 2004. Fairfax, VA.
- 60.Azhar MZ. Comparison of fluvoxamine alone, fluvoxamine and cognitive psychotherapy, and psychotherapy alone in the treatment of panic disorder in Kelantan: Implications for management by family doctors. Med J Malaysia. 2000;55(4):402–8. [PubMed] [Google Scholar]
- 61.Bakker A, van Dyck R, Spinhoven P, van Balkom AJ. Paroxetine, clomipramine, and cognitive therapy in the treatment of panic disorder. J Clin Psychiatry. 1999;60(12):831–8. doi: 10.4088/jcp.v60n1205. [DOI] [PubMed] [Google Scholar]
- 62.Roy-Byrne PP, Craske MG, Stein MB, et al. A randomized effectiveness trial of cognitive-behavioral therapy and medication for primary care panic disorder. Arch Gen Psychiatry. 2005;62(3):290–8. doi: 10.1001/archpsyc.62.3.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bruce SE, Yonkers KA, Otto MW, et al. Influence of psychiatric comorbidity on recovery and recurrence in generalized anxiety disorder, social phobia, and panic disorder: A 12-year prospective study. Am J Psychiatry. 2005;162(6):1179–87. doi: 10.1176/appi.ajp.162.6.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sareen J, Chartier M, Paulus MP, Stein MB. Illicit drug use and anxiety disorders: Findings from two community surveys. Psychiatry Res. 2006;142(1):11–17. doi: 10.1016/j.psychres.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 65.Kushner MG, Abrams K, Thuras P, et al. Follow-up study of anxiety disorder and alcohol dependence in comorbid alcoholism treatment patients. Alcohol Clin Exp Res. 2005;29(8):1432–1443. doi: 10.1097/01.alc.0000175072.17623.f8. [DOI] [PubMed] [Google Scholar]
- 66.Sareen J, McWilliams L, Cox B, Stein MB. Does a U-shaped relationship exist between alcohol use and DSM-III-R mood and anxiety disorders? J Affect Dis. 2004;82(1):113–118. doi: 10.1016/j.jad.2002.12.001. [DOI] [PubMed] [Google Scholar]
- 67.Simon NM, Otto MW, Wisniewski SR, et al. Anxiety disorder comorbidity in bipolar disorder patients: data from the first 500 participants in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) Am J Psychiatry. 2004;161(12):2222–9. doi: 10.1176/appi.ajp.161.12.2222. [DOI] [PubMed] [Google Scholar]
- 68.Keck PE, Jr., Strawn JR, McElroy SL. Pharmacologic treatment considerations in co-occurring bipolar and anxiety disorders. J Clin Psychiatry. 2006;67(1):8–15. [PubMed] [Google Scholar]
- 69.Zaubler TS, Katon W. Panic disorder and medical comorbidity: A review of the medical and psychiatric literature. Bull Menninger Clin. 1996;60(2) A:A12–38. [PubMed] [Google Scholar]
- 70.Kaplan GB, Greenblatt DJ, Ehrenberg BL, et al. Differences in pharmacodynamics but not pharmacokinetics between subjects with panic disorder and healthy subjects after treatment with a single dose of alprazolam. J Clin Psychopharmacol. 2000;20(3):338–346. doi: 10.1097/00004714-200006000-00008. [DOI] [PubMed] [Google Scholar]
- 71.Bandelow B, Ruther E. Treatment-resistant panic disorder. CNS Spectr. 2004;9(10):725–739. doi: 10.1017/s1092852900022379. [DOI] [PubMed] [Google Scholar]
- 72.Goddard AW, Mason GF, Appel M, et al. Impaired GABA neuronal response to acute benzodiazepine administration in panic disorder. Am J Psychiatry. 2004;161(12):2186–2193. doi: 10.1176/appi.ajp.161.12.2186. [DOI] [PubMed] [Google Scholar]
- 73.Roy-Byrne PP, Cowley DS, Greenblatt DJ, et al. Reduced benzodiazepine sensitivity in panic disorder. Arch Gen Psychiatry. 1990;47(6):534–538. doi: 10.1001/archpsyc.1990.01810180034006. [DOI] [PubMed] [Google Scholar]
- 74.Lesser IM, Lydiard RB, Antal E, et al. Alprazolam plasma concentrations and treatment response in panic disorder and agoraphobia. Am J Psychiatry. 1992;149(11):1556–62. doi: 10.1176/ajp.149.11.1556. [DOI] [PubMed] [Google Scholar]
- 75.Neumeister A, Bain E, Nugent AC, et al. Reduced serotonin type 1A receptor binding in panic disorder. J Neurosci. 2004;24(3):589–591. doi: 10.1523/JNEUROSCI.4921-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hirschmann S, Dannon PN, Iancu I, et al. Pindolol augmentation in patients with treatment-resistant panic disorder: A double-blind, placebo-controlled trial. J Clin Psychopharmacol. 2000;20(5):556–9. doi: 10.1097/00004714-200010000-00011. [DOI] [PubMed] [Google Scholar]
- 77.Dannon PN, Iancu I, Grunhaus L. The efficacy of reboxetine in the treatment-refractory patients with panic disorder: An open label study. Hum Psychopharmacol. 2002;17(7):329–33. doi: 10.1002/hup.421. [DOI] [PubMed] [Google Scholar]
- 78.Tiffon L, Coplan JD, Papp LA, Gorman JM. Augmentation strategies with tricyclic or fluoxetine treatment in seven partially responsive panic disorder patients. J Clin Psychiatry. 1994;55(2):66–9. [PubMed] [Google Scholar]
- 79.Hollifield M, Thompson PM, Ruiz JE, Uhlenhuth EH. Potential effectiveness and safety of olanzapine in refractory panic disorder. Depress Anxiety. 2005;21(1):33–40. doi: 10.1002/da.20050. [DOI] [PubMed] [Google Scholar]
- 80.Sheehan DV, Raj AB, Harnett-Sheehan K, et al. The relative efficacy of high-dose buspirone and alprazolam in the treatment of panic disorder: A double-blind placebo-controlled study. Acta Psychiatr Scand. 1993;88(1):1–11. doi: 10.1111/j.1600-0447.1993.tb03405.x. [DOI] [PubMed] [Google Scholar]
- 81.Gastriend D, Rosenbaum JF. Adjunctive buspirone in benzodiazepine-resistant treatment of four patients with panic disoder. Am J Psychiatry. 1989(146):914–16. doi: 10.1176/ajp.146.7.914. [DOI] [PubMed] [Google Scholar]
- 82.Boshuisen ML, Slaap BR, Vester-Blokland ED, den Boer JA. The effect of mirtazapine in panic disorder: An open-label pilot study with a single-blind placebo run-in period. Int Clin Psychopharmacol. 2001;16(6):363–8. doi: 10.1097/00004850-200111000-00008. [DOI] [PubMed] [Google Scholar]
- 83.Ribeiro L, Busnello JV, Kauer-Sant'Anna M, et al. Mirtazapine versus fluoxetine in the treatment of panic disorder. Braz J Med Biol Res. 2001;34(10):1303–7. doi: 10.1590/s0100-879x2001001000010. [DOI] [PubMed] [Google Scholar]
- 84.Sarchiapone M, Amore M, De Risio S, et al. Mirtazapine in the treatment of panic disorder: An open-label trial. Int Clin Psychopharmacol. 2003;18(1):35–8. doi: 10.1097/00004850-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 85.Crippa JA, Zuardi AW. Duloxetine in the treatment of panic disorder. Int J Neuropsychopharmacol. 2006;9(5):633–4. doi: 10.1017/S1461145705006206. [DOI] [PubMed] [Google Scholar]
- 86.Sheehan DV. The diagnosis and drug treatment of anxiety disorders. Prog Neuropsychopharmacol Biol Psychiatry. 1983;7(4-6):599–603. doi: 10.1016/0278-5846(83)90031-3. [DOI] [PubMed] [Google Scholar]
- 87.Simon NM, Emmanuel N, Ballenger J, et al. Bupropion sustained release for panic disorder. Psychopharmacol Bull. 2003;37(4):66–72. [PubMed] [Google Scholar]
- 88.Mula M, Pini S, Cassano GB. The role of anticonvulsant drugs in anxiety disorders: A critical review of the evidence. J Clin Psychopharmacol. 2007;27(3):263–72. doi: 10.1097/jcp.0b013e318059361a. [DOI] [PubMed] [Google Scholar]
- 89.Baetz M, Bowen RC. Efficacy of divalproex sodium in patients with panic disorder and mood instability who have not responded to conventional therapy. Can J Psychiatry. 1998;43(1):73–7. doi: 10.1177/070674379804300109. [DOI] [PubMed] [Google Scholar]
- 90.Ontiveros A, Fontaine R. Sodium valproate and clonazepam for treatment-resistant panic disorder. J Psychiatry Neurosci. 1992;17(2):78–80. [PMC free article] [PubMed] [Google Scholar]
- 91.Uhde TW, Stein MB, Post RM. Lack of efficacy of carbamazepine in the treatment of panic disorder. Am J Psychiatry. 1988;145(9):1104–9. doi: 10.1176/ajp.145.9.1104. [DOI] [PubMed] [Google Scholar]
- 92.Pande AC, Pollack MH, Crockatt J, et al. Placebo-controlled study of gabapentin treatment of panic disorder. J Clin Psychopharmacol. 2000;20(4):467–71. doi: 10.1097/00004714-200008000-00011. [DOI] [PubMed] [Google Scholar]
- 93.Papp LA. Safety and efficacy of levetiracetam for patients with panic disorder: Results of an open-label, fixed-flexible dose study. J Clin Psychiatry. 2006;67(10):1573–6. doi: 10.4088/jcp.v67n1012. [DOI] [PubMed] [Google Scholar]
- 94.Schwartz TL. The use of tiagabine augmentation for treatment-resistant anxiety disorders: A case series. Psychopharmacol Bull. 2002;36(2):53–7. [PubMed] [Google Scholar]
- 95.Zwanzger P, Baghai T, Boerner RJ, et al. Anxiolytic effects of vigabatrin in panic disorder. J Clin Psychopharmacol. 2001;21(5):539–40. doi: 10.1097/00004714-200110000-00017. [DOI] [PubMed] [Google Scholar]
- 96.Zwanzger P, Rupprecht R.Vigabatrin and tiagabine might have antipanic properties J Psychopharmacol (Oxford, England) 2004183440. [DOI] [PubMed] [Google Scholar]
- 97.Sepede G, De Berardis D, Gambi F, et al. Olanzapine augmentation in treatment-resistant panic disorder: A 12-week, fixed-dose, open-label trial. J Clin Psychopharmacol. 2006;26(1):45–9. doi: 10.1097/01.jcp.0000195108.01898.17. [DOI] [PubMed] [Google Scholar]
- 98.Simon NM, Hoge EA, Fischmann D, et al. An open-label trial of risperidone augmentation for refractory anxiety disorders. J Clin Psychiatry. 2006;67(3):381–5. doi: 10.4088/jcp.v67n0307. [DOI] [PubMed] [Google Scholar]


