Abstract
Kinetochores have been proposed to play multiple roles in mitotic chromosome alignment, including initial microtubule (MT) capture, monitoring MT attachments, prometaphase and anaphase chromosome movement and tension generation at metaphase. In addition, kinetochores are essential components of the spindle assembly checkpoint (SAC), and couple chromosome alignment with SAC silencing at metaphase. Although the molecular details of these activities remain under investigation, cytoplasmic dynein has been implicated in several aspects of MT and SAC regulation. Recent work clarifies the contribution of dynein to MT interactions and to events that drive anaphase onset. This review summarizes these studies and provides new models for dynein function.
Keywords: dynein, mitosis, kinetochores, microtubules, spindle, checkpoint, phosphorylation
1. Introduction
Kinetochores are transient, chromosome-associated protein assemblies that form during mitosis to mediate force-producing interactions with MTs, MT binding proteins and additional components required for mitotic regulation [1–2]. Perhaps the most distinctive feature of kinetochores in higher eukaryotes is the tri-laminar plate structure revealed by EM analysis [3]. Functional roles in providing an interface between chromatin and MTs are suggested by the structures adjacent to the inner and outer plates. Roles in sensing tension and stretch are suggested by the increasing distance between inner and outer plates at metaphase. Furthermore, kinetochores are implicated in depositing signaling molecules at the spindle midzone after anaphase onset, dictating the position of the cytokinetic cleavage furrow [4]. How kinetochores orchestrate these distinct activities has only recently been deciphered.
Among the most challenging aspects of kinetochore analysis is the growing number of proteins that accumulate there and the hierarchy of these proteins in the kinetochore structure [5]. Approximately 80 proteins have been identified as core kinetochore components and the organization of many of these proteins is conserved among eukaryotes. Some features of the outer kinetochore are more complicated in higher eukaryotes including components of the cytoplasmic dynein pathway and the RZZ complex (rod, zw10, zwilch) [2]. The reasons for this additional complexity in higher eukaryotes are not known, but could include: 1) increased chromosome number, 2) the rapid dispersal of chromosomes into the cytoplasmic volume after nuclear envelope breakdown and 3) the need to initiate MT-chromosome interactions de novo in a larger space.
An intriguing component of the more highly-elaborated outer kinetochore is the MT motor cytoplasmic dynein. Dynein is a multi-subunit, minus end-directed motor responsible for many aspects of intracellular motility, including organelle transport [6–7], retrograde axonal transport [7], and cell migration [8]. Dynein is also implicated in multiple mitotic functions. During the G2/M transition, dynein releases from interphase cargos and localizes to three novel mitotic loci: 1) the cell cortex, 2) spindle poles, and 3) kinetochores. There is consensus that polar and cortical localizations of dynein reflect roles in spindle formation/integrity and spindle rotation/positioning [9–13]. In contrast, the specific contributions of dynein at kinetochores remain controversial [14]. Kinetochore dynein has been implicated in MT-attachment [15–17], chromosome movement [18–20] and regulation of the SAC [21–23]. In addition, several proteins have been proposed to interact, directly or indirectly, with kinetochore dynein as a means to regulate dynein targeting and/or function. The mechanisms that coordinate these interactions/functions are not well understood.
2. When is Dynein at the Kinetochore?
Immunofluorescence microscopy (IFM) studies with dynein antibodies demonstrate that dynein localizes initially to kinetochores during prometaphase [15–17, 24]. Dynein accumulates at kinetochores prior to MT attachment (Fig. 1), and displays exaggerated recruitment after MT depolymerization [17, 24]. The latter suggests that dynein is a component of the fibrous corona implicated in sensing MT attachment and providing feedback to the kinetochore. The timing of kinetochore localization together with dynein inhibition studies suggest that dynein is involved in initial interactions with MTs and in early aspects of chromosome movement during prometaphase [19, 24–27]. Interestingly, as chromosomes achieve bipolar attachment and approach the metaphase plate (Fig. 1), kinetochore dynein becomes less prominent by IFM analysis [28]. This reduction in kinetochore dynein coincides with enhanced dynein labeling along spindle fibers and spindle poles [22–23]. This transition from kinetochores to spindle fibers is consistent with recent models that link dynein to checkpoint silencing at metaphase [2, 21–23, 29–30]. After alignment, dynein is undetectable at kinetochores [15–16, 23], and is not detected on kinetochores throughout anaphase and cytokinesis (Fig. 1). Although the loss of dynein labeling at metaphase kinetochores could reflect antibody inaccessibility issues, dynein is detected on spindle fibers and at spindle poles after alignment [23]. Another possibility is that the enhanced motility of dynein after alignment reduces dynein accumulation to very low levels. Consistent with this possibility, live imaging of dynein in Drosophila reveals kinetochore labeling that persists into anaphase [18]. Additional live imaging studies will be needed to determine the degree of dynein binding at kinetochores after anaphase onset.
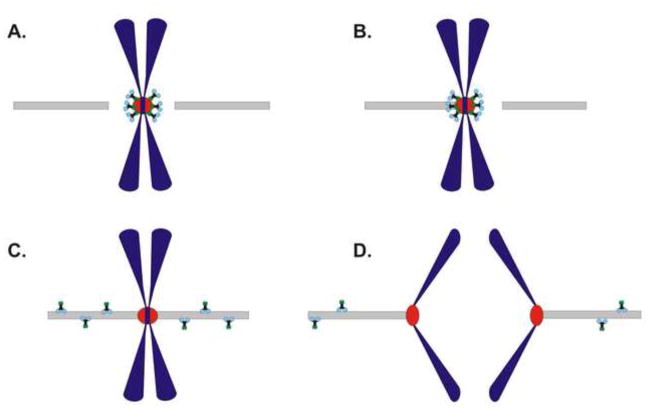
Figure 1.
Changes in Kinetochore Dynein During Mitosis. Dynein is initially recruited to kinetochores before MT attachment (A.) and remains at kinetochores during the process of mono-orientation and association with the spindle (B.) After bi-oriented MT attachments are achieved (C.), dynein undergoes poleward movement and labels K-fibers and spindle poles. By anaphase onset (D.), dynein is prominent on spindle fibers but undetectable at kinetochores.
3. Proposed Kinetochore Binding Partners
One approach that has been used to address the function of kinetochore dynein involves manipulating candidate binding partners. A growing number of proteins have been proposed to recruit or mediate binding of dynein to the kinetochore, including: dynactin, zw10, LIS-1, nudE/EL, nudC and Spindly. This section summarizes the evidence linking each to kinetochore dynein (Table 1).
Table 1.
Summary of Dynein Binding Candidates, the Impact of Depletion/Disruption on Dynein Levels and Mitotic Progression.
| Kinetochore Dynein Binding/Recruitment Partner | Molecular Interactions with Dynein | Impact on Kinetochore Dynein Levels | Mitotic Progression Phenotypes |
|---|---|---|---|
| Zw10 | Dynein IC | reduced | Premature Anaphase Onset |
| Dynactin | Dynein IC | reduced | Prometaphase/Metaphase Arrest |
| NudE/EL | Dynein HC, IC, LC8 | reduced | Metaphase Arrest |
| LIS-1 | Dynein HC, IC | normal | Metaphase Arrest |
| NudC/CL | Dynein IC | NudC-N/A NudCL-reduced | Prometaphase/Metaphase Arrest |
| Spindly | N/A | reduced | Prometaphase/Metaphase Arrest |
3.1: Dynactin
Dynactin is a multi-subunit complex first identified as a dynein cofactor capable of stimulating dynein-based transport events in vitro [31–34]. A direct interaction between the dynein intermediate chains (ICs) and the p150Glued subunit of dynactin implicates dynactin as a dynein cargo receptor [35–36]. Consistent with this possibility, dynactin is involved in most examples of dynein-driven transport [7, 37]. A role for dynactin in linking dynein to kinetochores was suggested by initial studies using p50(dynamitin) [24]. This overexpression approach disrupts the dynactin complex and interferes with dynactin populations at the kinetochore. Because dynactin depletion from kinetochores has a significant effect on kinetochore dynein, dynactin was proposed to act as a recruitment factor for dynein at the kinetochore [24]. Although the specific role in initial recruitment of kinetochore dynein remains under investigation [23], it is clear that a subset of kinetochore dynein requires the presence of dynactin.
3.2: Zw10
First identified as a kinetochore checkpoint protein in Drosophila [38], zw10 is an essential component of the Rod/Zw10/Zwilch (RZZ) complex that is required for kinetochore dynein activity (reviewed in [30]). Mutations in Drosophila zw10 abolish the localization of dynein to kinetochores, but do not impact dynein at other mitotic loci [39]. Furthermore, siRNA-based depletion of human zw10 reduced dynein levels at kinetochores substantially [40], suggesting this pathway is conserved among higher eukaryotes. Initially, dynein was thought to require zw10 indirectly because dynactin is dependent on zw10 for kinetochore binding [39]. However, recent work has revealed a direct interaction between mitotically-phosphorylated dynein and zw10 [23]. In addition, the kinetochore population of Spindly appears to require zw10 [41–42], potentially providing another method of dynein linkage. Although roles in linking dynein by both direct and indirect mechanisms complicates interpretation, it remains clear that zw10 and the RZZ complex are intimately involved in kinetochore dynein recruitment.
3.3: LIS-1
Lissencephaly is a brain development disease resulting from mutations in the human LIS1 gene [43]. In addition to playing a role in neuronal migration during development, LIS-1 is also required for proper cell division [12]. During mitosis, LIS-1 localizes to prometaphase kinetochores, but is diminished in response to chromosome alignment [12]. This coincides with the kinetochore to spindle fiber transition for dynein [23]. Biochemical and yeast two hybrid assays reveal a direct interaction between LIS-1, dynein, and dynactin [12, 44]. Overexpression of full-length LIS-1 or LIS-1 fragments did not displace dynactin or dynein from kinetochores, but did induce defects in dynein function [12, 44]. Interestingly, p50(dynamitin) overexpression effectively depletes LIS-1 from kinetochores, providing evidence of a protein hierarchy at the kinetochore [44].
3.4: nudE/nudEL
Mammalian nudE and nudEL (nudE-like) are homologues of a dynein regulatory factor first identified in Aspergillus nidulans [45]. Along with nudF/LIS-1, these proteins were initially proposed to regulate dynein during neuronal migration [46]. However, more recent studies also implicate nudE/EL in regulation of dynein kinetochore function. NudE/EL localize to prophase kinetochores prior to the arrival of dynein and other proteins implicated in dynein targeting (dynactin, zw10, and LIS-1; [47]). In addition, nudE/EL remain associated with kinetochores after dynein redistribution at the metaphase-anaphase transition[47]. Despite differences in the timing of localization, biochemical assays demonstrate a direct interaction between nudE/EL and specific dynein subunits [47]. Several studies have investigated the role of nudE/EL in kinetochore dynein targeting. Microinjection of anti-nudE/EL antibodies displaced dynein, LIS-1, and dynactin from kinetochores [47]. RNAi-based depletion of nudE but not nudEL displaced dynein from kinetochores [48]. In a second study, RNAi-based depletion of nudEL, reduced levels of kinetochore dynein, LIS-1, and dynactin [49]. Although interactions of nudE/EL with dynein are clear, the specific contribution to kinetochore targeting requires further investigation.
3.5: nudC
NudC was first identified in Aspergillus nidulans as a regulator of nuclear migration [50–51], a process dependent on proper dynein function. The mammalian homologue of nudC is required for proper cell division and can associate with dynein [52], however it is unclear if this interaction occurs at the kinetochore. Recently, a mammalian nudC-like (nudCL) has been implicated in regulating mitotic dynein function [53]. Biochemical analysis revealed an association between nudCL and dynein, and RNAi-based depletion of nudCL induced mislocalization of dynein from kinetochores coupled with dynein IC degradation [53]. The degree to which nudCL mediates kinetochore-specific localization is not clear, especially because nudCL is largely soluble in mitotic cells and appears to affect dynein stability at multiple mitotic loci [53]. Further work will be needed to assess protein stability as a mechanism of mitotic dynein regulation and the function of nudC/CL.
3.6: Spindly
Identified as part of a RNAi screen of mitotic Drosophila S2 cells, Spindly is also implicated in targeting dynein to kinetochores [41]. Depletion of Spindly reduced kinetochore dynein levels in Drosophila [41], C. elegans [42] and human cells [41, 54]. However, the defective phenotypes resulting from Spindly depletion are somewhat variable. In Drosophila, Spindly is dispensable for recruitment of dynactin to kinetochores [41], whereas kinetochore dynactin is dependent on Spindly in worm and human systems [42, 54]. The consequences of Spindly depletion on kinetochore function are also divergent, including misregulation of the SAC [41] and defects in the retention of MT attachments [42], and spindle rotation [54]. Despite these discrepancies, a common theme is that Spindly is dependent on the RZZ complex for kinetochore localization and interacts with the RZZ complex biochemically [42, 54]. A significant gap in this model has been the lack of evidence for an interaction with dynein itself. Given the intimate connection between dynein, dynactin and RZZ at kinetochores [23–24, 39–40], Spindly is likely to play an important role in dynein function.
Proposed Kinetochore Functions
Dynein is implicated in several aspects of kinetochores function, most of which are essential for accurate chromosome segregation. Dynein activity has been explored largely through manipulation of candidate dynein binding partners. Given our incomplete understanding of these binding partners, it is likely that some experiments affect more than just dynein, thereby complicating interpretation. This section discusses and evaluates proposed functions for kinetochore dynein (Fig. 1).
4.1: MT-attachment
Kinetochores mediate the linkage of chromosomes to the mitotic spindle, providing the interface for direct interactions with MT plus ends. Initial MT-attachment to kinetochores is coupled to rapid poleward movement of the mono-oriented chromosomes [19, 25]. As chromosomes approach the poles, kinetochores recruit additional MTs which form parallel bundles known as K-fibers [55]. Several MT-binding kinetochore proteins have been proposed to initiate and stabilize kinetochore-MT-attachments (K-MTs), including dynein. Dynein was initially implicated in this process based on the observation that mono-oriented chromosomes move poleward at rates similar to the in vitro motility of dynein [25]. Additional studies have used manipulation of kinetochores to investigate a requirement for dynein. The first studies on zw10 demonstrated normal chromosome movement in zw10 mutant cells [38]. Because dynein recruitment is dependent on zw10[23, 40], this suggests that dynein is not required for MT-attachment. In contrast, cells expressing high levels of p50(dynamitin) displayed randomly oriented chromosomes which fail to align at the metaphase plate [24]. This phenotype could reflect a dynein/dynactin-mediated attachment defect, or the more globally disrupted state of spindles after p50(dynamitin) expression [24]. More recent studies have started to clarify potential roles for dynein in initial MT-attachment. Although multiple studies highlight the roles of the Ndc80/Hec1 complex in stabilizing and maintaining end- on MT attachments [56–57], dynein could be involved in reorienting tangential attachments to end-on attachments [26]. Furthermore, displacement of the motor domain of dynein from kinetochores destabilized K-fibers, suggesting a potential role for dynein in stabilizing K-MTs [27]. Complicating these interpretations, some experiments affect both dynein and dynactin, the latter containing an additional MT binding subunit. Recent experiments revealing the presence of dynactin-dependent and dynactin-independent populations of dynein have the potential to focus on dynein-specific phenotypes [23].
4.2: Prometaphase Chromosome Movement
Of the known motor proteins at kinetochores dynein is widely thought to play a role in chromosome movement because of its kinetochore localization and minus end-directed motility. When dynein is inhibited prior to MT-attachment, congression defects and prometaphase arrest are observed [24]. In contrast, when dynein is inhibited during prometaphase, either by injection of p50(dynamitin) or anti-dynein antibodies, chromosomes approach the metaphase plate normally [9, 21]. Together these findings suggest a role for kinetochore dynein in MT attachment, but not chromosome movement. Although counterintuitive, this is consistent with the fact that kinetochore dynein levels decrease after bioriented MT attachment when movement is most dynamic [23]. Additional approaches that deplete kinetochores of the dynein heavy chain [27] or disrupt the dynein-nudE/EL interaction [47] also reveal fairly normal metaphase alignment. Depletion of both zw10 [19, 40] and Spindly [41–42, 54] have been used to reduce dynein levels at kinetochores, however these experiments affect more than just dynein. Zw10 depletion is now known to reduce dynein, dynactin and Spindly from kinetochores [23, 39–42], complicating analysis of kinetochore-MT interactions. Spindly depletion also has the potential to affect multiple aspects of kinetochore function because Spindly is also a MT binding protein with plus end specificity [41]. Together these experiments challenge the specific role of dynein in prometaphase chromosome movement and encourage analysis of other candidates.
4.3: Anaphase Chromosome Movement
Similar to proposed roles for dynein in prometaphase chromosome movement, dynein is thought to be the dominant motor for anaphase chromosome movement because of the speed and direction of dynein-driven transport. However the evidence for kinetochore-associated dynein during anaphase is limited. In mammalian cells, dynein is not detectable at kinetochores after metaphase [15–16, 23]. In contrast, two studies in Drosophila suggest that depletion of kinetochore dynein either through disruption of RZZ components [20] or microinjection of either p50(dynamitin) or anti-DHC antibodies [18] reduced the rate of anaphase chromosome movement. Interestingly, both studies observed defective poleward chromosome movement after initial MT attachment. One possible resolution of these inconsistencies is that dynein affects anaphase chromosome movement indirectly. Either by determining MT orientation at kinetochores, stabilizing K-fibers, or regulating MT dynamics, dynein could contribute to anaphase chromosome movement without acting as a translocation motor.
4.4: Regulation of the SAC
The mitotic SAC ensures accurate chromosome segregation by resisting anaphase onset until all chromosomes have achieved alignment at the metaphase plate. In addition, the SAC provides recognition of successful alignment and timely progression into anaphase (Rev. in [2]). Central to the SAC are anaphase inhibitor proteins that undergo cyclical recruitment, “activation” and release from kinetochores to resist anaphase onset [2]. The RZZ complex functions as a platform for some of these anaphase inhibitors, partially explaining the phenotypes of RZZ disruption. At metaphase, the RZZ platform and other proteins are removed from kinetochores and transported towards spindle poles (Rev. in [30]). This event blocks anaphase inhibitor “activation” on aligned chromosomes in a process known as checkpoint silencing. Dynein has been implicated in this poleward transport of checkpoint proteins from kinetochores [30]. Consistent with this model, disruption of kinetochore dynein function by p50(dynamitin) or injection of anti-dynein IC antibodies results in metaphase arrest/delay [21, 23]. Mechanistically, the metaphase arrest/delay phenotype reflects the inability of dynein to transport the anaphase inhibitor platforms away from kinetochores, thereby allowing the continued production of anaphase inhibitors even after alignment. Several experimental approaches support this model for kinetochore dynein. Inhibition of dynein motility through ATP depletion blocks poleward streaming of checkpoint proteins and delays anaphase onset [21]. Studies in Drosophila demonstrated that dynein is required for efficient removal of the RZZ complex from kinetochores [22, 29]. Expression of moderate levels of p50(dynamitin) to affect kinetochore dynactin specifically results in retention of anaphase inhibitors on metaphase kinetochores and metaphase arrest [23]. Overall, these findings are consistent with the levels of kinetochore dynein across mitosis and the effects of dynein interference. However, crucial questions remain in this model. The specific changes in dynein responsible for initiating motility are not understood completely, nor are the role for dynein-cofactors such as dynactin. Dephosphorylation of the dynein ICs after alignment is required for productive streaming and checkpoint silencing [23]. However, the dynein LICs are also required for removal of the anaphase inhibitor mad2 [58–59]. Given that phosphorylation of the dynein ICs and LICs plays a role in metaphase dynein regulation, more studies will be needed to define the enzymology and regulation of this pathway.
5. Insights into Dynein Complexity at Kinetochores
The large number of interacting proteins and proposed functions for dynein at kinetochores has produced a complex literature that limits progress on understanding mitotic dynein regulation. One possibility is that multiple independent populations of dynein coexist at kinetochores, and that each population performs a different function (Fig. 2). This could explain how the same basic dynein complex can contribute to so many activities at so many different stages of mitosis. It would also suggest that each population might be recruited by a distinct receptor, thereby clarifying the complexity of binding partners. A simple prediction of this model is that experiments that affect one dynein population would not necessarily affect all populations. Consistent with this possibility, phosphorylated dynein can bind kinetochores in the absence of dynactin but not after depletion of zw10 [23]. A second hypothesis is that dynein is recruited through a single mechanism but undergoes changes in binding interactions that are coupled to MT attachment, chromosome alignment and checkpoint silencing (Fig. 3). This model predicts that disruption of early events would have a major impact on dynein levels, whereas defects in later events would be less dramatic. Consistent with this hypothesis, the interaction between phosphorylated dynein and zw10 has been proposed as a mechanism of initial recruitment to kinetochores, and disruption of RZZ reduces levels of total dynein at kinetochores across mitosis [23]. In contrast, kinetochore dynactin depletion reduces the levels of total dynein but does not affect levels of phosphorylated dynein. This is consistent with dynactin playing a role later in the sequence of interactions. Together these findings suggest that dynein is recruited through an interaction between phosphorylated dynein and zw10. Later in chromosome alignment, dynein leaves the kinetochores by streaming in a complex with dynactin (Fig. 3). Other binding partners could interact with dynein in between these extremes, perhaps to retain dynein under tension, facilitate transfer between binding partners or to stimulate dynein motility. This “sequential interaction” model makes a number of predictions that can be tested experimentally.
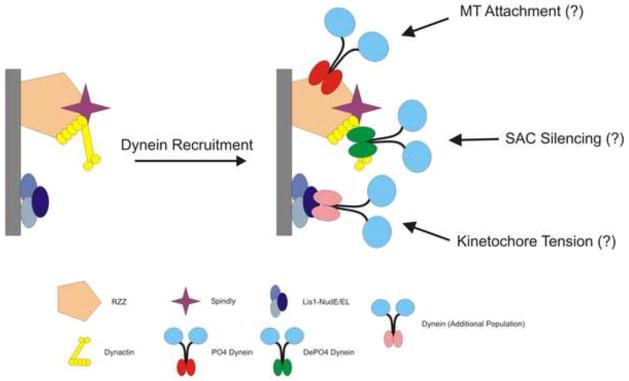
Figure 2.
Model 1: Multiple Populations of Dynein Coexist at Kinetochores. One possibility suggested by the literature is that multiple populations of dynein coexist, are recruited by distinct receptors and perform different functions. This would allow dynein to mediate diverse functions such as MT attachment, generation of tension and SAC silencing. Receptors for these dynein subsets include RZZ, LIS-1/nudE/EL/nudC/CL, dynactin and Spindly.
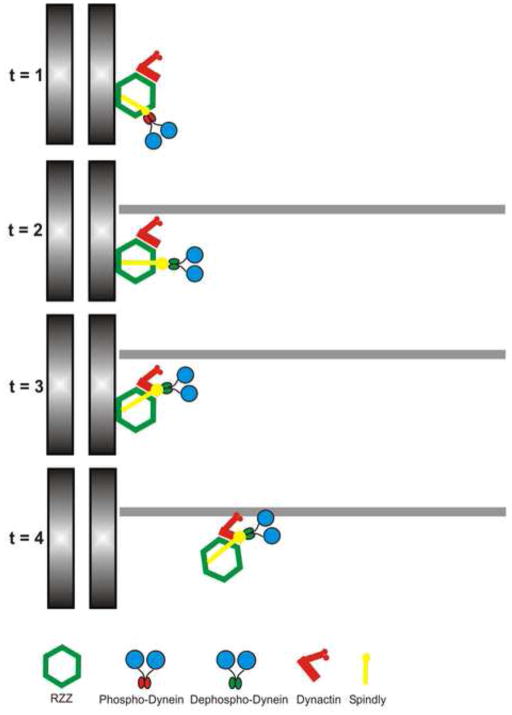
Figure 3.
Model 2: Sequential Interaction Model. A second possibility consistent with the literature is that dynein undergoes changes in binding that are coupled to MT attachment, kinetochore stretch and chromosome alignment. Initially recruited through a direct interaction between phosphorylated dynein and zw10 (t=1), dynein is dephosphorylated (t=2), shifts binding from zw10 to dynactin (t=3), and translocates away from kinetochores (t=4). In this model, some binding partners are responsible for transferring dynein from zw10 to dynactin, whereas others are responsible for activating dynein as a motor.
6. Conclusions
The roles of dynein at kinetochores have expanded and evolved over the past several decades with the advent of new tools and approaches. This has led to a daunting literature. This review summarizes and re-examines some of the seminal findings in this field. With new insights and a better understanding of how phosphorylation regulates dynein targeting and activity, we propose a new model for dynein interactions at kinetochores. This model suggests that dynein undergoes a series of sequential interactions that allow dynein to perform different functions during chromosome alignment.
Acknowledgments
The authors thank members of the Vaughan laboratory for input and Dr. Ted Hinchcliffe for suggestions.
Abbreviations
- IFM
immunofluorescence microscopy
- MT
microtubule
- SAC
spindle assembly checkpoint
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Rieder CL. Mitosis: towards a molecular understanding of chromosome behavior. Curr Opin Cell Biol. 1991;3(1):59–66. doi: 10.1016/0955-0674(91)90166-v. [DOI] [PubMed] [Google Scholar]
- 2.Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol. 2007;8(5):379–93. doi: 10.1038/nrm2163. [DOI] [PubMed] [Google Scholar]
- 3.McEwen BF, et al. A new look at kinetochore structure in vertebrate somatic cells using high-pressure freezing and freeze substitution. Chromosoma. 1998;107(6–7):366–75. doi: 10.1007/s004120050320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carmena M, Ruchaud S, Earnshaw WC. Making the Auroras glow: regulation of Aurora A and B kinase function by interacting proteins. Curr Opin Cell Biol. 2009;21(6):796–805. doi: 10.1016/j.ceb.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheeseman IM, Desai A. Molecular architecture of the kinetochore-microtubule interface. Nat Rev Mol Cell Biol. 2008;9(1):33–46. doi: 10.1038/nrm2310. [DOI] [PubMed] [Google Scholar]
- 6.Paschal BM, Shpetner HS, Vallee RB. MAP 1C is a microtubule-activated ATPase which translocates microtubules in vitro and has dynein-like properties. J Cell Biol. 1987;105(3):1273–82. doi: 10.1083/jcb.105.3.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holzbaur ELF, Vallee RB. Dyneins: molecular structure and cellular function. Annu Rev Cell Biol. 1994;10:339–372. doi: 10.1146/annurev.cb.10.110194.002011. [DOI] [PubMed] [Google Scholar]
- 8.Dujardin DL, et al. A role for cytoplasmic dynein and LIS1 in directed cell movement. J Cell Biol. 2003;163(6):1205–11. doi: 10.1083/jcb.200310097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaisberg EA, Koonce MP, McIntosh JR. Cytoplasmic dynein plays a role in mammalian mitotic spindle formation. J Cell Biol. 1993;123(4):849–58. doi: 10.1083/jcb.123.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heald R, et al. Self-organization of microtubules into bipolar spindles around artificial chromosomes in Xenopus egg extracts [see comments] Nature. 1996;382(6590):420–5. doi: 10.1038/382420a0. [DOI] [PubMed] [Google Scholar]
- 11.Busson S, et al. Dynein and dynactin are localized to astral microtubules and at cortical sites in mitotic epithelial cells. Current Biology. 1998;8:541–544. doi: 10.1016/s0960-9822(98)70208-8. [DOI] [PubMed] [Google Scholar]
- 12.Faulkner NE, et al. A role for the lissencephaly gene LIS1 in mitosis and cytoplasmic dynein function. Nat Cell Biol. 2000;2(11):784–91. doi: 10.1038/35041020. [DOI] [PubMed] [Google Scholar]
- 13.O’Connell CB, Wang YL. Mammalian spindle orientation and position respond to changes in cell shape in a dynein-dependent fashion. Mol Biol Cell. 2000;11(5):1765–74. doi: 10.1091/mbc.11.5.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banks JD, Heald R. Chromosome movement: dynein-out at the kinetochore. Curr Biol. 2001;11(4):R128–31. doi: 10.1016/s0960-9822(01)00059-8. [DOI] [PubMed] [Google Scholar]
- 15.Pfarr CM, et al. Cytoplasmic dynein is localized to kinetochores during mitosis [see comments] Nature. 1990;345(6272):263–5. doi: 10.1038/345263a0. [DOI] [PubMed] [Google Scholar]
- 16.Steuer ER, et al. Localization of cytoplasmic dynein to mitotic spindles and kinetochores [see comments] Nature. 1990;345(6272):266–8. doi: 10.1038/345266a0. [DOI] [PubMed] [Google Scholar]
- 17.Wordeman L, et al. Chemical subdomains within the kinetochore domain of isolated CHO mitotic chromosomes. J Cell Biol. 1991;114(2):285–94. doi: 10.1083/jcb.114.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharp DJ, Rogers GC, Scholey JM. Cytoplasmic dynein is required for poleward chromosome movement during mitosis in drosophila embryos [In Process Citation] Nat Cell Biol. 2000;2(12):922–30. doi: 10.1038/35046574. [DOI] [PubMed] [Google Scholar]
- 19.Yang Z, et al. Kinetochore dynein is required for chromosome motion and congression independent of the spindle checkpoint. Curr Biol. 2007;17(11):973–80. doi: 10.1016/j.cub.2007.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Savoian MS, Goldberg ML, Rieder CL. The rate of poleward chromosome motion is attenuated in Drosophila zw10 and rod mutants. Nat Cell Biol. 2000;2(12):948–52. doi: 10.1038/35046605. [DOI] [PubMed] [Google Scholar]
- 21.Howell BJ, et al. Cytoplasmic dynein/dynactin drives kinetochore protein transport to the spindle poles and has a role in mitotic spindle checkpoint inactivation. J Cell Biol. 2001;155(7):1159–72. doi: 10.1083/jcb.200105093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wojcik E, et al. Kinetochore dynein: its dynamics and role in the transport of the Rough deal checkpoint protein. Nat Cell Biol. 2001;3(11):1001–7. doi: 10.1038/ncb1101-1001. [DOI] [PubMed] [Google Scholar]
- 23.Whyte J, et al. Phosphorylation regulates targeting of cytoplasmic dynein to kinetochores during mitosis. J Cell Biol. 2008;183(5):819–34. doi: 10.1083/jcb.200804114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Echeverri CJ, et al. Molecular characterization of the 50-kD subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. J Cell Biol. 1996;132(4):617–33. doi: 10.1083/jcb.132.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rieder CL, Alexander SP. Kinetochores are transported poleward along a single astral microtubule during chromosome attachment to the spindle in newt lung cells. J Cell Biol. 1990;110(1):81–95. doi: 10.1083/jcb.110.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vorozhko VV, et al. Multiple mechanisms of chromosome movement in vertebrate cells mediated through the Ndc80 complex and dynein/dynactin. Chromosoma. 2008;117(2):169–79. doi: 10.1007/s00412-007-0135-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Varma D, et al. Direct role of dynein motor in stable kinetochore-microtubule attachment, orientation, and alignment. J Cell Biol. 2008;182(6):1045–54. doi: 10.1083/jcb.200710106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.King JM, Hays TS, Nicklas RB. Dynein is a transient kinetochore component whose binding is regulated by microtubule attachment, not tension. J Cell Biol. 2000;151(4):739–48. doi: 10.1083/jcb.151.4.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Basto R, et al. In vivo dynamics of the rough deal checkpoint protein during Drosophila mitosis. Curr Biol. 2004;14(1):56–61. doi: 10.1016/j.cub.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 30.Karess R. Rod-Zw10-Zwilch: a key player in the spindle checkpoint. Trends Cell Biol. 2005;15(7):386–92. doi: 10.1016/j.tcb.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Gill SR, et al. Dynactin, a conserved, ubiquitously expressed component of an activator of vesicle motility mediated by cytoplasmic dynein. J Cell Biol. 1991;115(6):1639–50. doi: 10.1083/jcb.115.6.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schroer TA, Sheetz MP. Two activators of microtubule-based vesicle transport. J Cell Biol. 1991;115(5):1309–18. doi: 10.1083/jcb.115.5.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holzbaur EL, et al. Homology of a 150K cytoplasmic dynein-associated polypeptide with the Drosophila gene Glued [published erratum appears in Nature 1992 Dec 17;360(6405):695] Nature. 1991;351(6327):579–83. doi: 10.1038/351579a0. [DOI] [PubMed] [Google Scholar]
- 34.King SJ, Schroer TA. Dynactin increases the processivity of the cytoplasmic dynein motor. Nat Cell Biol. 2000;2(1):20–4. doi: 10.1038/71338. [DOI] [PubMed] [Google Scholar]
- 35.Karki S, Holzbaur EL. Affinity chromatography demonstrates a direct binding between cytoplasmic dynein and the dynactin complex. J Biol Chem. 1995;270(48):28806–11. doi: 10.1074/jbc.270.48.28806. [DOI] [PubMed] [Google Scholar]
- 36.Vaughan KT, Vallee RB. Cytoplasmic dynein binds dynactin through a direct interaction between the intermediate chains and p150 Glued. J Cell Biol. 1995;131:1507–1516. doi: 10.1083/jcb.131.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vallee RB, et al. Dynein: An ancient motor protein involved in multiple modes of transport. J Neurobiol. 2004;58(2):189–200. doi: 10.1002/neu.10314. [DOI] [PubMed] [Google Scholar]
- 38.Williams BC, et al. The Drosophila l(1)zw10 gene product, required for accurate mitotic chromosome segregation, is redistributed at anaphase onset. J Cell Biol. 1992;118(4):759–73. doi: 10.1083/jcb.118.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Starr DA, et al. ZW10 helps recruit dynactin and dynein to the kinetochore. J Cell Biol. 1998;142(3):763–774. doi: 10.1083/jcb.142.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kops GJ, et al. ZW10 links mitotic checkpoint signaling to the structural kinetochore. J Cell Biol. 2005;169(1):49–60. doi: 10.1083/jcb.200411118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Griffis ER, Stuurman N, Vale RD. Spindly, a novel protein essential for silencing the spindle assembly checkpoint, recruits dynein to the kinetochore. J Cell Biol. 2007;177(6):1005–15. doi: 10.1083/jcb.200702062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gassmann R, et al. A new mechanism controlling kinetochore-microtubule interactions revealed by comparison of two dynein-targeting components: SPDL-1 and the Rod/Zwilch/Zw10 complex. Genes Dev. 2008;22(17):2385–99. doi: 10.1101/gad.1687508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dobyns WB, et al. Lissencephaly: A human brain malformation associated with deletion of the LIS1 gene located at chromosome 17p13. JAMA. 1993;270:2838–2842. doi: 10.1001/jama.270.23.2838. [DOI] [PubMed] [Google Scholar]
- 44.Tai CY, et al. Role of dynein, dynactin, and CLIP-170 interactions in LIS1 kinetochore function. J Cell Biol. 2002;156(6):959–68. doi: 10.1083/jcb.200109046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Efimov VP, Morris NR. The LIS1-related NUDF protein of Aspergillus nidulans interacts with the coiled-coil domain of the NUDE/RO11 protein. J Cell Biol. 2000;150(3):681–8. doi: 10.1083/jcb.150.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wynshaw-Boris A, Gambello MJ. LIS1 and dynein motor function in neuronal migration and development. Genes Dev. 2001;15(6):639–51. doi: 10.1101/gad.886801. [DOI] [PubMed] [Google Scholar]
- 47.Stehman SA, et al. NudE and NudEL are required for mitotic progression and are involved in dynein recruitment to kinetochores. J Cell Biol. 2007;178(4):583–94. doi: 10.1083/jcb.200610112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vergnolle MA, Taylor SS. Cenp-F links kinetochores to Ndel1/Nde1/Lis1/dynein microtubule motor complexes. Curr Biol. 2007;17(13):1173–9. doi: 10.1016/j.cub.2007.05.077. [DOI] [PubMed] [Google Scholar]
- 49.Liang Y, et al. Nudel modulates kinetochore association and function of cytoplasmic dynein in M phase. Mol Biol Cell. 2007;18(7):2656–66. doi: 10.1091/mbc.E06-04-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Osmani AH, Osmani SA, Morris NR. The molecular cloning and identification of a gene product specifically required for nuclear movement in Aspergillus nidulans. J Cell Biol. 1990;111:543–551. doi: 10.1083/jcb.111.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang D, et al. Cytoplasmic dynein participates in meiotic checkpoint inactivation in mouse oocytes by transporting cytoplasmic mitotic arrest-deficient (Mad) proteins from kinetochores to spindle poles. Reproduction. 2007;133(4):685–95. doi: 10.1530/rep.1.01167. [DOI] [PubMed] [Google Scholar]
- 52.Aumais JP, et al. Role for NudC, a dynein-associated nuclear movement protein, in mitosis and cytokinesis. J Cell Sci. 2003;116(Pt 10):1991–2003. doi: 10.1242/jcs.00412. [DOI] [PubMed] [Google Scholar]
- 53.Zhou T, et al. A mammalian NudC-like protein essential for dynein stability and cell viability. Proc Natl Acad Sci U S A. 2006;103(24):9039–44. doi: 10.1073/pnas.0602916103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chan YW, et al. Mitotic control of kinetochore-associated dynein and spindle orientation by human Spindly. J Cell Biol. 2009;185(5):859–74. doi: 10.1083/jcb.200812167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rieder CL, Salmon ED. Motile kinetochores and polar ejection forces dictate chromosome position on the vertebrate mitotic spindle. J Cell Biol. 1994;124:223–233. doi: 10.1083/jcb.124.3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheeseman IM, et al. The conserved KMN network constitutes the core microtubule-binding site of the kinetochore. Cell. 2006;127(5):983–97. doi: 10.1016/j.cell.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 57.DeLuca JG, et al. Kinetochore microtubule dynamics and attachment stability are regulated by Hec1. Cell. 2006;127(5):969–82. doi: 10.1016/j.cell.2006.09.047. [DOI] [PubMed] [Google Scholar]
- 58.Mische S, et al. Dynein light intermediate chain: an essential subunit that contributes to spindle checkpoint inactivation. Mol Biol Cell. 2008;19(11):4918–29. doi: 10.1091/mbc.E08-05-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sivaram MV, et al. Dynein light intermediate chain 1 is required for progress through the spindle assembly checkpoint. EMBO J. 2009;28(7):902–14. doi: 10.1038/emboj.2009.38. [DOI] [PMC free article] [PubMed] [Google Scholar]