Abstract
Semantically reversible sentences are prone to misinterpretation and take longer for typically developing children and adults to comprehend; they are also particularly problematic for those with language difficulties such as aphasia or Specific Language Impairment. In our study we used fMRI to compare the processing of semantically reversible and nonreversible sentences in 41 healthy participants to identify how semantic reversibility influences neuronal activation. By including several linguistic and nonlinguistic conditions within our paradigm, we were also able to test whether the processing of semantically reversible sentences places additional load on sentence-specific processing, such as syntactic processing and syntactic-semantic integration, or on phonological working memory. Our results identified increased activation for reversible sentences in a region on the left temporal–parietal boundary, which was also activated when the same group of participants carried out an articulation task which involved saying “one, three” repeatedly. We conclude that the processing of semantically reversible sentences places additional demands on the subarticulation component of phonological working memory.
INTRODUCTION
Some sentences are harder to process than others. Although the overall complexity of a sentence may be modulated in terms of its grammatical structure, there are additional properties that can increase sentence complexity. A prominent class of such sentence types is semantically reversible sentences (e.g., “The leopard races the young lion”; see Figure 1). These sentences have an interesting property in that when the subject (e.g., leopard) and the object (e.g., lion) are swapped or reversed (e.g., “The lion races the young leopard”), these sentences remain meaningful, although the exact meaning of the sentence is changed (for instance, the animal doing the racing changes). By contrast, in a nonreversible sentence (e.g., “The dog chews the bone”; see Figure 1), swapping the subject (e.g., dog) and the object (e.g., bone) results in a sentence with no real meaning (“The bone chews the dog”).
Figure 1.
Examples of semantically reversible and nonreversible sentences. The subject and the object of a reversible sentence may be reversed and still produce a meaningful sentence, whereas nonreversible sentences become semantically anomalous when they are reversed. In thematic role assignment, the agent is the entity acting on the object or person in the sentence, whereas the entity or the person being acted upon is referred to as the patient.
Both typically developing children and adults alike take longer to comprehend semantically reversible sentences, which are also more prone to misinterpretation than nonreversible sentences (Kemper & Catlin, 1979; Herriot, 1969; Turner & Rommetveit, 1967; Slobin, 1966). This added difficulty may be attributed to a reduction in the constraints on (theta) role assignment of the subject and the object for reversible sentences. Reversible sentences can become even more difficult to interpret when their grammatical structure deviates from the subject–verb–object word order typically found in English. For instance, reversible passives (e.g., “the dog was bitten by the fox”) are consistently misinterpreted by typical adults across a range of sentence types (Ferreira, 2003). An explanation for this extra complexity is that individuals cannot rely on a simple word order heuristic for role assignment. In some instances, it may prove useful to assess the semantic likelihood of events occurring in the sentence referenced by the verb (e.g., it is more likely that a cat would be chasing a mouse than vice versa) but this may also lead to misinterpretation. Thus, consistently correct interpretation of reversible sentences is dependent on a full evaluation of syntactic structure, a property that makes these sentences particularly important in the assessment of syntactic processing capabilities. For instance, semantically reversible sentences are used to determine the preservation of syntactic processing skills in acquired and developmental disorders of language, such as “agrammatic” aphasia and Specific Language Impairment (SLI), as well as degenerative disorders such as Alzheimer's disease (Bickel, Pantel, Eysenbach, & Schröder, 2000; Waters & Rochon, 1998). However, there is some debate as to whether difficulty in processing semantically reversible sentences is purely indicative of a syntactic deficit or whether difficulties in processing these sentences arise from other sources.
The account that difficulty in processing reversible sentences is indicative of a syntactic deficit was put forward by Caramazza and Zurif (1976), who found that “agrammatic” Broca's aphasics struggle to comprehend reversible sentences. They argued that agrammatic aphasics are unable to evaluate syntactic structures and must therefore rely upon simple heuristic strategies for sentence comprehension, which are prone to failure. Grodzinsky (1990) explained the sentence processing difficulties of Broca's aphasics in terms of damage to a specific sentence processing mechanism that connects an antecedent with its trace. Processing semantically reversible sentences is also particularly problematic for young children with SLI, which is a developmental disorder of language occurring in the absence of cognitive impairment or brain damage (Leonard, 1998). Grammar-specific accounts of this disorder are also a prevalent feature of the literature (van der Lely, 2005; Rice, 2000; van der Lely & Christian, 2000; van der Lely & Stollwerck, 1996). However, there is no consensus view. Indeed, there is considerable debate as to whether the cause is specific to grammar in both the SLI (Ullman & Pierpont, 2005; Gathercole & Baddeley, 1990) and the aphasia literature (Grodzinsky, Pinango, Zurif, & Drai, 1999; Berndt, Mitchum, & Haedings, 1996).
A second explanation for the sentence processing difficulties of Broca's aphasics emphasizes the role of semantic processing in sentence comprehension, suggesting that their difficulty in understanding sentences arises from an inability to integrate the syntactic structure of a sentence with semantic information (Berndt, Mitchum, Burton, & Haendiges, 2004; Saffran, Schwartz, & Linebarger, 1998). A third alternative proposes that the deficit lies in phonology. For instance, Gathercole and Baddeley (1990) argue that phonological problems are the principal cause of SLI, pointing to data which indicate that children with SLI have a reduced phonological working memory capacity in comparison with both their age-matched peers and their language-matched control participants (Montgomery, 1995a, 1995b, 2004; Ellis Weismer et al., 2000; Dollaghan & Campbell, 1998; Gathercole & Baddeley, 1990). A fourth perspective is that reduced capacity across the whole sentence processing network will have a greater detriment on semantically reversible sentences (Caplan, Waters, DeDe, Michaud, & Reddy, 2007).
In summary, semantic reversibility increases the processing difficulty of a sentence across a range of grammatical constructions. Moreover, these sentences are particularly vulnerable in both developmental and acquired disorders of language. The present study aims to identify brain regions associated with the processing of semantically reversible sentences over a range of sentences with different syntactic structures, thus examining the overall property of semantic reversibility on sentence processing. We compared the processing of semantically reversible versus nonreversible sentences in auditory and visual processing modalities in normal individuals with no history of developmental or acquired language difficulties. The inclusion of both modalities allowed us to focus on amodal sentence processing rather than modality-specific effects. Our paradigm also included additional linguistic and nonlinguistic tasks that allowed us to functionally localize systems that were differentially responsive to the syntactic and semantic demands of sentence level or articulatory processing. This allowed us to determine whether the functions of the brain regions associated with semantically reversible sentences are most consistent with syntactic/syntactic-semantic processing, phonological processing, amodal semantics, or all of the above (for further details, see the Experimental Paradigm section). Moreover, by deliberately including a large sample of participants (47) with a wide age range (7–73 years) and verbal ability range, we were able to test whether the effect of reversible relative to nonreversible sentences was dependent on level of vocabulary knowledge, memory, age, and general cognitive ability.
METHODS
Participants
The participants were 47 right-handed volunteers (24 males) aged between 7 and 73 years who had English as their first language. All participants had normal or corrected-to-normal vision, with no reported hearing difficulties or disturbances in speech comprehension, speech production, or reading. Six participants were excluded due to an incomplete coverage of temporal brain regions in the functional scans (remaining total of 41 participants). This study was approved by the joint ethical committee of the Institute of Neurology and the National Hospital for Neurology and Neurosurgery, London, UK. Informed consent (written consent from a parent or guardian in the case of young children under 16) was obtained from all participants.
Behavioral Tests
All participants carried out two psychometric tests: (i) the British Picture Vocabulary Scale, Second Edition (BPVS-II; Dunn, Dunn, Whetton, & Burlcy, 1997) and (ii) the Matrices task from the British Ability Scale, Second Edition (BAS-II; Elliot, Smith, & McCulloch, 1997). All participants between 7 and 11 years also carried out the Reading test from the BAS-II to ensure that they had sufficient reading ability to cany out the functional imaging paradigm. The reading test consists of 90 words divided into nine blocks of 10 words. Children start the test at an age-appropriate starting point and read aloud a series of words presented on a card. The words increase in complexity as the test progresses. The test is continued until the child makes eight or more consecutive errors. An ability score that takes into account the difficulty of the test items completed is then obtained using a lookup table supplied with the test. Children with a minimum reading age of 7 years were considered to be at an appropriate level to cany out the reading task used in the fMRI paradigm given that the sentence stimuli were designed to be suitable for children of this age (for further details, see section on Sentence Stimuli). All children who took part in this study had a reading level in line with or in advance of their chronological age (reading age range of 7 years and 4 months to 15 years and 3 months). Therefore, although older participants were expected to be more proficient readers, the younger children included in this study were capable of comprehending the sentence stimuli.
The BPVSTI is a measure of an individual's receptive vocabulary for standard English. In this test, participants are asked to select (from four options) the picture that most accurately matches a word (such as “ladder” or “collision”) read aloud by the tester. The test consists of 14 sets of words of increasing levels of difficulty, each containing 12 items. Each set has an approximate age-range indicator, which is used to select the appropriate starting set. Providing the performance of the participant meets the criterion of one or no errors on this initial set, the base set is established (should the participant make more than one error, preceding sets are administered until a base set is determined). The test is then conducted until the participant makes eight or more incorrect responses within a set (the ceiling set). The raw test score is calculated by taking the item number of the ceiling set and by subtracting from it the total errors made over all sets from the base set onward.
The Matrices task from the BAS-II was used as a measure of general cognitive ability. In this test, participants are shown an incomplete matrix of black and white abstract figures, with each matrix consisting of either four or nine cells. Participants are required to select the most appropriate pattern to complete the matrix from six potential tiles by pointing to or by reading the number of the tile that best completes the matrix. Participants first complete four practice items and then begin the test at an age-appropriate level, which is indicated on the test (previous items are administered should they fail on the first three test items). The test is discontinued if the participant makes five failures out of six consecutive items. An ability score, which takes into account the number and level of difficulty of the test items completed, is then obtained from a lookup table supplied with the test.
Experimental Paradigm
The experimental paradigm consisted of four activation tasks: (1) auditory sentence processing, (2) visual sentence processing, (3) hand action retrieval in response to pictures of familiar objects, and (4) articulation. Details of these activation conditions and their corresponding baselines are provided below. In brief, auditory and visual sentences were either reversible or nonreversible. Direct comparison of these sentence types identified regions associated with reversible sentence processing. To assign a functional role to the areas associated with reversible sentences, we considered the previous literature and also the pattern of activation across a range of tasks in our own subjects. Syntactic and syntactic-semantic areas were expected to be included in the set of areas activated for both auditory and visual sentence processing over and above all other conditions. Likewise, articulatory areas were expected to be included in the set of areas activated for the articulation task over and above all other conditions. We also identified amodal semantic areas as those that were activated for auditory sentences, visual sentences, and hand action retrieval in response to pictures of objects. An important point to note here, before describing the conditions in detail, is that our experimental design and the interpretation of our data were not based solely on subtractive logic. Thus, we acknowledge that the comparison of auditory and visual sentences to all other conditions will include processes other than syntactic-semantic processing (e.g., working memory). The interpretation of our results therefore rests on the integration of our findings with those in the previous literature. The inclusion of multiple conditions in the present design has two strong advantages over the previous literature: (1) it avoids the well-known pitfalls of reverse inference (problems with deductive validity; see Poldrack, 2006), and (2) it tests whether a novel effect (reversible sentences vs. nonreversible sentences) overlaps with activation for other conditions within the same subjects. In other words, by including multiple conditions, we provide our own subject-specific localizers.
Auditory and Visual Sentence Processing
Participants listened passively to auditory sentence stimuli and silently read visual sentences. These activation tasks consisted of three types of sentence stimuli: (i) reversible sentences, (ii) nonreversible sentences, and (iii) scrambled sentences (strings of words that did not constitute a meaningful sentence). The baseline task in the auditory modality consisted of listening to the same speech recordings after they had been rendered meaningless by digital reversal. In the visual modality, the baseline task consisted of viewing the same words presented in an unrecognizable (false) font.
We chose passive listening/reading tasks for three reasons. First, they have the advantage of avoiding task-induced strategies over and above the speech comprehension processes that we were interested in. Second, they allow us to test the effect of reversible versus nonreversible sentences under the same conditions as behavioral studies that have demonstrated misinterpretation of reversible sentences in adults and children (Ferreira, 2003; Kemper & Catlin, 1979; Herriot, 1969; Turner & Rommetveit, 1967; Slobin, 1966). Third, they do not confound sentence level processing with activation related to the production of a motor response. Although passive paradigms make it difficult to assess what the subject is doing in the scanner because there is no in-scanner behavioral measure, a significant effect of reversible versus nonreversible sentences would indicate active online sentence processing. Moreover, we also used an on-line video system and eye tracking to ensure that all participants were attending to the stimuli. Post-scanning memory tests (that the participants were not expecting) also ensured that the sentences had been processed because it was unlikely that participants would perform above chance on the memory test unless the sentences had been processed at the semantic and syntactic level (see below for more details).
Sentence Stimuli
Sentence stimuli consisted of 40 somantically reversible and 40 nonreversible sentences with six to eight words per sentence. Familiar words were selected to be suitable for children as young as 7 years. Sentences were constructed using high-frequency (>20 per million) monosyllabic and bisyllabic nouns, verbs, and adjectives and had a Flesch–Kincaid grade level readability score of 1.3. Reversible and nonreversible sentences were matched tor the number of words, letters, syllables, and phonemes in a sentence as well as the mean imageability of content words, mean age of acquisition, and Kucera–Francis frequency of content words based on information from the MRC Psycholinguistics database (http://www.psy.uwa.edu.au/mrcdatabase/uwa_mrc.htm). Both reversible and nonreversible sentence sets consisted of active, passive, subject cleft, object cleft, locative, and dative sentence types (these stimuli are in line with those used to identify language deficits in acquired and developmental disorders). Sentences were tested across this range of grammatical constructions to ensure that activations elicited during the processing of reversible sentence types could be attributed to the general property of sentence reversibility rather than a specific syntactic construction per se. These same sentence types were presented across both visual and auditory modalities to ensure consistency across tasks. Examples of sentence stimuli with further details regarding the composition of the stimuli can be seen in Table 1. Reversible and nonreversible sentence sets were each split into two groups (A and B) of equivalent composition for the purpose of presenting one set in an auditory and the other in a visual format. No sentence was repeated across modality. The presentation of subsets A and B in either an auditory or a visual format was counterbalanced across participants. Scrambled sentences were constructed from the same set of words as reversible and nonreversible sentences, consisting of initially grammatical sentences (e.g., “The cow chased the fat horse”), which were then assigned a pseudorandom word order that did not form a meaningful sentence (e.g., “Chased the the horse cow fat”). This condition is therefore fully matched to the sentences at the lexical level.
Table 1.
Details of Sentence Stimuli Composition with Examples of Sentence Types (M = mean; KF = Kucera–Francis)
| No. Sentences |
Total No. Words |
No. per Sentence (M) |
KF Frequency (M) |
Imageability of Content Words (M) |
Age of Acquisition (M) |
Concreteness (M) |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sentence Type | Example | Syllables | Letters | Phonemes | ||||||
| Reversible Sentences | ||||||||||
| Active | 8 | “The old dog bites the fox” | 6 | 7 | 25 | 17 | 122 | 558 | 208 | 539 |
| Passive | 8 | “The rat is sniffed by the gray squirrel” | 8 | 10 | 32 | 25 | 57 | 565 | 242 | 553 |
| Subject cleft | 8 | “It is the dancer that hugs the clown” | 8 | 9 | 30 | 22 | 108 | 572 | 293 | 562 |
| Object cleft | 8 | “It is the cook that the woman loves” | 8 | 9 | 29 | 22 | 179 | 565 | 228 | 544 |
| Locative | 4 | “The circle is in the gold star” | 7 | 8 | 25 | 19 | 88 | 573 | 245 | 580 |
| Dative | 4 | “Give the happy boy to the girl” | 7 | 8 | 24 | 19 | 139 | 534 | 204 | 509 |
| Total | 40 | 7 | 8 | 27 | 21 | 115 | 561 | 237 | 548 | |
| Nonreversible Sentences | ||||||||||
| Active | 8 | “The rich queen spends the money” | 6 | 8 | 26 | 20 | 115 | 525 | 306 | 515 |
| Passive | 8 | “The giant safe is locked by the guard” | 8 | 10 | 31 | 24 | 74 | 505 | 357 | 494 |
| Subject cleft | 8 | “It is the drunk that starts the fight” | 8 | 9 | 30 | 24 | 182 | 506 | 306 | 470 |
| Object cleft | 8 | “It is the dress the model hates” | 8 | 10 | 31 | 25 | 87 | 503 | 315 | 473 |
| Locative | 4 | “The marble temple is in the field” | 7 | 9 | 26 | 21 | 210 | 554 | 324 | 548 |
| Dative | 4 | “Put the salt on the plain meal” | 7 | 9 | 28 | 23 | 172 | 483 | 310 | 471 |
| Total | 40 | 7 | 9 | 28 | 23 | 140 | 513 | 320 | 495 | |
Nonreversible sentences were mainly strongly nonreversible, where the constraints set for the sentence stimuli permitted.
Articulation Task
Participants read aloud the visually presented digits “1” and “3” alternately. These digits were chosen because saying “one” involves pursing the lips and saying “three” involves the tongue protruding. Therefore, alternating between 1 and 3 maximized the use of the major articulators, and the repetitive pattern may activate the articulatory loop component of phonological working memory. To reduce susceptibility artifacts induced by airflow during speech production and to minimize auditory processing of the spoken response, we instructed participants to make the appropriate mouth movements with minimal voicing. Responses were recorded using a specialized microphone that canceled out the scanner noise. The baseline task consisted of making alternate mouth movements (of either pursed lips or separated lips with the tongue slightly protruding) when prompted by a gray scale image of the desired mouth shape displayed on-screen. We were able to distinguish which movement the participants were making using our on-line microphone.
Object Actions Task
Participants viewed pictures of objects that had strongly associated hand actions, for example, scissors, spoon, and calculator. They were instructed to make the corresponding action with their right hand. In the baseline task, participants viewed pictures of objects or nonobjects that did not have a strongly associated hand action and were instructed to make a rocking motion with their right hand in response to viewing the picture. To remind participants what to do in the baseline task, we presented a red rainbow-shaped bidirectional arrow above each baseline stimulus. Examples of the stimuli are shown in Figure 2. All responses in this condition were recorded using a video camera, directed on the right hand of the participant in the scanner.
Figure 2.
Examples of stimuli presented in the object conditions. In the activation task (objects with hand actions), participants were instructed to use their right hand to make an action as if using the object. In each of the three baseline tasks (objects, animals, and nonobjects), participants made a rocking motion (also with their right hand) when viewing the stimulus.
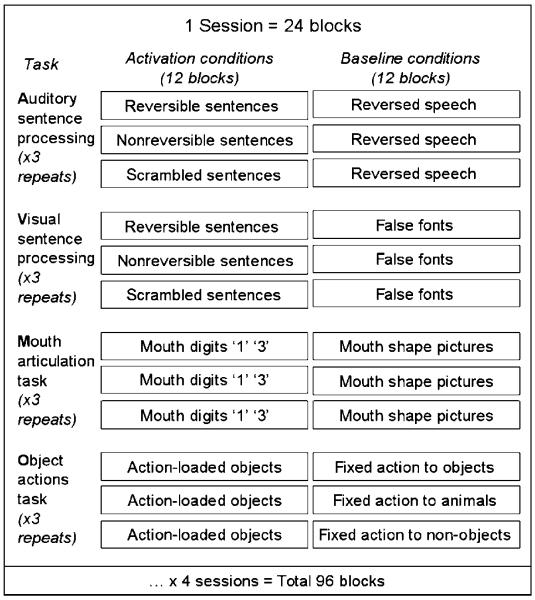
Condition order was blocked. There were 96 blocks in total, 12 for each of the activation and baseline conditions. All 96 blocks were presented across four different scanning sessions (runs), with 24 blocks in each session. The 12 blocks of auditory sentences were subdivided into 4 blocks of reversible sentences, 4 blocks of nonreversible sentences, and 4 blocks of scrambled sentences. The 12 blocks of visual sentences were subdivided in the same way. Within a session, there were 3 blocks of each activation condition and 3 blocks of baseline condition. For the auditory and visual blocks, there was one block of each sentence type (reversible, nonreversible, and scrambled). This design is depicted in Figure 3. Within an 18-sec block, there were 5 sentences comprising 37 words or 37 of the corresponding baseline stimuli; 18 digits in articulation blocks or 18 images in the corresponding baseline condition; and 15 pictures in hand action retrieval blocks and the corresponding baselines. Although the total number of stimuli varied in the sentence and hand action conditions (to optimize processing time), this stimulus difference was removed by including the baseline stimuli (e.g., sentences – baseline vs. hand action retrieval – baseline).
Figure 3.
Experimental design. All condition blocks for a single session are depicted here. One run through each type of task (A, V, M, and O) totalled 8 blocks. The order of each type of task was counterbalanced within session for each run (3 runs × 8 blocks each = 24 blocks). Session order was counterbalanced across participants (×4 sessions), as were sentence stimuli (×2 sets), giving a total of 8 (2 × 4) condition orders.
Each sentence, digit, or picture was modeled as a separate event within condition. Therefore, over sessions, there were a total of 18 × 12 = 216 digit events, 15 × 12 = 180 picture events, and 37 × 4 = 148 word events per sentence type. A block of an activation condition was always followed or preceded by a block of its corresponding baseline condition. Short blocks and event-related analyses were used to maximize experimental efficiency (Mechelli, Price, Henson, & Friston, 2003; Mechelli, Henson, Price, & Friston, 2003). The order of the activation conditions was counterbalanced within and between session and subject.
Procedure
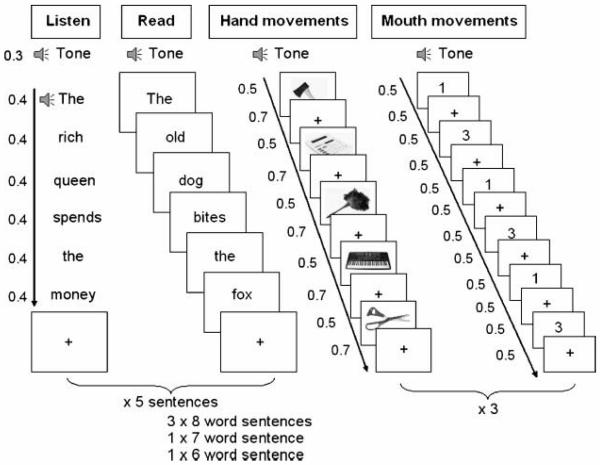
A summary of the procedure is detailed in Figure 4, showing the presentation and timing of the stimuli across all tasks.
Figure 4.
Procedure. Timing and presentation of tasks, from left to right: (1) auditory sentence processing, (2) visual sentence processing, (3) object action retrieval, and (4) articulation.
Each session commenced with a visual cue to “Get Ready…” followed by a count down, during which dummy scans were acquired. Each type of task (activation and baseline) was preceded by an appropriate visually displayed instruction (Helvetica, size 80): “Listen” (auditory comprehension task), “Read” (visual comprehension task), “Mouth movements,” or “Hand movements.” This instruction was displayed for 2.2 sec and was followed by an auditory pure tone, which sounded for 0.3 sec. Each activation and baseline task had a total duration of 18 sec. The presentation of activation and baseline tasks was separated by a brief auditory pure tone that sounded for 0.3 sec, followed by a 0.2-sec fixation cross. At the end of each activation and baseline task, there was a 1.5-sec pause before the onset of the next task. This resulted in a total duration of 40.5 sec for an activation and a baseline pair.
In the visual sentence processing task, a total of five sentences were presented per activation task. Each set of sentences consisted of one of each of the following sentence types: one active (six words), one passive (eight words), one subject cleft (eight words), one object cleft (eight words), one locative/dative (seven words). Further details of these sentence types are shown in Table 1. A total of 37 words were presented in each sentence condition. Each word within each sentence was presented on-screen at a rate of one word every 0.4 sec (resulting in a maximum duration of 3.2 sec for an eight word sentence). Each word was presented in a Helvetica font size 20. Each sentence was separated by 0.5 sec. The audi tory and the visual word presentation rates were equated by recording the auditory stimuli from a woman reading aloud the visual stimuli presented using the same script that was to be used in the scanner. Words were read with a flat intonation contour, minimizing effects of sentence prosody in the auditory condition. Sentence change was indicated by an auditory beep, whereas in the visual condition the first word of each sentence started with a capital letter.
In the articulation task, there were 18 presentations of stimuli per activation and baseline condition, which were displayed for 0.5 sec and separated by an ISI of 0.5 sec. In the object action retrieval task, there were 15 presentations of stimuli per activation and baseline condition, each with an event duration of 0.5 sec and an ISI of 0.7 sec. The presentation of stimuli was set at this rate to limit object naming and to allow participants to complete their hand action before the onset of the next stimulus.
Memory Tests
All participants carried out two pen and paper memory tests following scanning: (i) memory for sentences and (ii) memory for pictures. Participants were not informed of these tests before scanning. These tasks were used to ensure that participants had been attending to the stimuli while in the scanner and to determine whether memory for sentences had any effect on the processing of semantically reversible sentences. The memory-for-sentences test consisted of 24 sentences, 12 familiar sentences (6 presented in each modality—auditory and visual), and 12 previously unseen during scanning (6 using previously presented words and 6 using novel words). The picture memory test followed the same format, consisting of 24 names of animals and objects, 12 familiar and 12 previously unseen. All participants scored above chance on both of these tests (sentence memory test score, M = 70%, SD = 11%; picture memory test score, M = 68%, SD = 10%). The scores for the memory-for-sentences test were adjusted to account for incorrect as well as correct responses. This was done by subtracting the total of false-positive responses made from the total correct responses for familiar sentences. Analyses of variance were then used to assess whether there were any differences in (i) memory for sentences according to processing modality (auditory vs. visual) and (ii) sentence type (reversible vs. nonreversible). Group (children and teenagers vs. adults) was entered as a between subject factor to test for any potential age-related behavioral differences in performance. We did not detect any main effect of sentence processing modality, F(1,39) = 2.06, p = .16, or sentence type, F(1,39) = 0.22, p = .64, or any interaction of group with sentence processing modality, F(1,39) = 0.08, p = .78, or sentence type, F(1,39) = 1.18, p = .28. These results indicate (i) that there were no observable effects of processing modality or sentence type on the memory for sentences and (ii) that there were no significant differences in these scores between children and adults. However, to account for individual differences in sentence memory, we entered adjusted scores for auditory and visual memory for sentences into subsequent analyses in SPM.
fMRI Data Acquisition
A Siemens 1.5-T Sonata scanner was used to acquire a total of 768 T2*-weighted echo-planar images with BOLD contrast (192 scans per four sessions). Each echo-planar image comprised 30 axial slices of 2-mm thickness with a 1-mm interslice interval and a 3 × 3-mm in-plane resolution. Volumes were acquired with an effective repetition time of 2.7 sec per volume, and the first six (dummy) volumes of each run were discarded to allow for Tl equilibration effects. In addition, a Tl-weighted anatomical volume image was acquired from all participants to ensure that there were no anatomical abnormalities.
fMRI Data Analysis
Preprocessing was conducted using statistical parametric mapping (SPM2, Wellcome Trust Centre for Neuroimaging, London, UK; http://www.fil.ion.ucl.ac.uk/spm) running under Matlab 6.5.1 (Mathworks Inc., Sherbon, MA). All volumes (excluding dummy scans) from each participant were realigned using the first as a reference image and unwarped (Jesper et al., 2001), adjusting for residual motion-related signal changes. The functional images were then spatially normalized (Friston, Ashburner, et al., 1995) to a standard MNI-305 template using nonlinear basis functions. Functional data were spatially smoothed with a 6-mm FWHM isotropic Gaussian kernel to compensate for residual variability after spatial normalization and to permit application of Gaussian random-field theory for corrected statistical inference (Friston, Holmes, et al., 1995).
First Level Statistical Analysis
For each participant, data were analyzed in SPM2 with high-pass filtering using a set of discrete cosine basis functions with a cutoff period of 128 sec. Each stimulus (sentence, digit, picture, instruction, etc.) was modeled as a separate event within each condition and convolved with a canonical hemodynamic response function. This resulted in 13 different conditions at the first level, which were as follows:
(A) Auditory sentences: reversible
(B) Auditory sentences: nonreversible
(C) Auditory sentences: scrambled
(D) Auditory sentences: baseline (reversed speech)
(E) Visual sentences: reversible
(F) Visual sentences: nonreversible
(G) Visual sentences: scrambled
(H) Visual sentences: baseline (false font)
(I) Hand action retrieval
(J) Hand action baseline
(K) Articulation
(L) Mouth movements
(M) Instructions
For each participant, the following 13 contrasts were generated at the first level:
(1) Reversibility effect—auditory: [reversible] − [nonreversible] (= A − B)
(2) Reversibility effect—visual: [reversible] − [nonreversible] (= E − F)
(3) Auditory reversible sentences: [reversible] − [baseline] (= A − D)
(4) Auditory-nonreversible sentences: [nonreversible] − [baseline] (= B − D)
(5) Visual reversible sentences: [reversible] − [baseline] (= E − H)
(6) Visual-nonreversible sentences: [nonreversible] − [baseline] (= F − H)
(7) Auditory sentences: [sentences] − [baseline] (= A + B − 2D)
(8) Visual sentences: [sentences] − [baseline] (= E + F − 2H)
(9) Auditory words: [scrambled sentences] − [baseline] (= C − D)
(10) Visual words: [scrambled sentences] − [baseline] (= G − H)
(11) Hand action retrieval − hand action baseline (= I − J)
(12) Articulation − hand action baseline (= K − J)
(13) Mouth movements − hand action baseline (= L − J)
Second Level Statistical Analyses
There were three different statistical models at the second (group) level.
Analysis 1: The effect of reversible > nonreversible sentences across all participants
To identify areas that were more activated by reversible than nonreversible sentences over and above all other variables, we used a two-sample t test with six covariates (in SPM5). The two samples included the contrast images from each of the 41 participants for (1) auditory-reversible sentences relative to auditory-nonreversible sentences and (2) visual-reversible sentences relative to visual-nonreversible sentences. The six covariates were test scores from the following cognitive measures: vocabulary knowledge (raw scores from the BPVS-II), nonverbal problem solving ability (ability scores from the BAS-II: matrices), scores for auditory memory and visual memory for sentences (derived from scores on the sentence memory test carried out after scanning), and age in months (linear and nonlinear).
The purpose of including the covariates was twofold. First, it allowed us to identify the main effect of reversible versus nonreversible sentences after potential variance from all the covariates had been factored out. Second, it enabled us to determine whether the effect of reversible versus nonreversible sentences was dependent on age or any of the cognitive measures. The combined analysis of child and adult data is valid upon the basis of previous methodological study (Kang, Burgund, Lugar, Petersen, & Schlaggar, 2003). However, to ensure that we had not missed any effects of reversible versus nonreversible sentences that were specific to age group, we repeated the analysis (two-sample t test with four covariates: vocabulary, matrices, and auditory and visual memory for sentences) with children and teenagers only (21 participants, 10 males; mean age = 14 years, range = 7–17 years) and adults only (20 participants, 9 men; mean age = 43.6 years, range = 24–73 years).
The statistical threshold was set at p < .05 after correcting for multiple comparisons across the whole brain in either height (family wise correction) or extent. Within these regions, we also looked for the effect of covariates at p < .05 uncorrected.
Analysis 2: Reversible and nonreversible sentences in children and adults
To examine the pattern of activation for reversible and nonreversible sentences in more detail, we carried out an ANOVA to plot the activation for reversible and nonreversible sentences separately according to processing modality (auditory vs. visual) and age group (children and teenagers vs. adults). The following four contrast images were entered into this analysis for each age group: (1) auditory-reversible sentences—baseline, (2) auditory-nonreversible sentences—baseline, (3) visual-reversible sentences—baseline, and (4) visual-nonreversible sentences—baseline. This analysis also included two covariates, which were auditory and visual memory for sentences. The inclusion of these scores allowed us to control for any individual differences in sentence memory test scores.
Group level Analysis 3: Functional localizers at the group level
The aim of this analysis was to establish whether any activation elicited for semantically reversible sentences over nonreversible sentences could be attributed to the syntactic and semantic demands of sentence processing or to articulatory processes used to index phonological working memory. To dissociate these different processing networks and to additionally identify regions associated with amodal semantic processing, we entered contrasts (7 to 13) from the first level analysis into a second level ANOVA in SPM5.
Sentence-specific processing areas were identified as those activated (a) by auditory and visual sentences only and (b) by auditory and visual sentences relative to all other conditions.
Articulatory areas were identified as those activated (a) by the articulation task and (b) by the articulation task relative to all other conditions. This combination of conditions identified regions most strongly engaged in the articulatory process (as all other conditions did not require an articulatory response) while also including areas that may be engaged in both articulation and sentence processing.
Amodal semantic processing areas were identified as those activated (a) by [auditory sentences relative to baseline] + [visual sentences relative to baseline] + [hand action retrieval relative to baseline] and (b) by each of the same contrasts individually. The combination of these conditions included regions that represent a common semantic system across tasks (Vandenberghe, Price, Wise, Josephs, & Frackowiak, 1996).
The statistical threshold for each main effect (a) was set at p < .05 after correcting for multiple comparisons across the whole brain in either height (family-wise correction) or extent. In these regions with significant main effects, we report the effect of (b) at p < .001 uncorrected.
RESULTS
Analysis 1: The Effect of Reversible > Nonreversible Sentences across All Participants
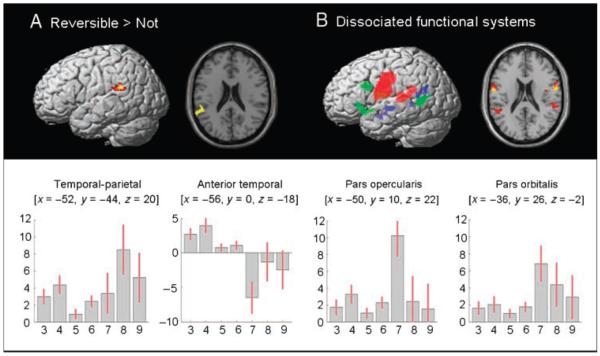
When age and cognitive ability were factored out, reversible compared with nonreversible visual sentences activated a region in the left temporal–parietal boundary, as shown in Figure 5A. This activation bridged a lateral region of the left posterior superior temporal gyrus and the neighboring inferior parietal region (for coordinates, see Table 2). We will henceforth refer to it as the left T-P region. As shown in Table 2, there was a corresponding trend for auditory-reversible versus nonreversible sentences (p = .003 uncorrected) but there was also an interaction of stimulus modality with [reversible vs. nonreversible] (Z = 3.1, p < .001), indicating that the effect was stronger for visual than auditory sentences.
Figure 5.
Activation for (A) reversible versus nonreversible sentences (B) syntactic and semantic sentence processing in blue, amodal semantics in green, and articulation in red. The statistical threshold for both (A) and (B) was p < .001 uncorrected for height but p < .05 corrected for extent. Plots show the parameter estimates for each condition in each of the labeled regions. The red bars are the 90% confidence intervals. On the x-axis, the conditions correspond to contrasts 7–13 that were entered into group level analysis 3 (for details, see Methods section): auditory sentences (AS), visual sentences (VS), auditory words (AW), visual words (VW), object action retrieval (O), articulation (A), and mouth movements (M). The y-axis shows effect sizes as the mean of the beta value from the first level analysis (i.e., the percentage increase in activation relative to the global mean). These plots show that inferior frontal regions responded to both nonlinguistic and linguistic stimuli. Activation of the posterior superior temporal gyrus is greatest in sentence contrasts (AS and VS). Activation on the left temporal–parietal boundary is greatest in the articulation contrast (A). The peak for semantically reversible sentence falls within this region.
Table 2.
Regions That Showed Increased Activation for Reversible Relative to Nonreversible Sentences
| Reversible > Nonreversible: Left Temporal–Parietal Boundary | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Main Effect |
Visual Sentences |
Auditory Sentences |
|||||||||||||
| Analysis | x | y | z | Z Score | n Voxels | x | y | z | Z Score | n Voxels | x | y | z | Z Score | n Voxels |
| All participants | −58 | −42 | 22 | 3.5 | 22 | −58 | −44 | 20 | 4.0 | 89 | −58 | −40 | 24 | 2.3 | 62 |
| −62 | −48 | 22 | 3.3 | −52 | −44 | 26 | 3.9 | −64 | −40 | 28 | 2.8 | ||||
| Children only | −58 | −44 | 20 | 2.7 | −52 | −34 | 20 | 4.2 | −64 | −38 | 28 | 2.4 | |||
| Adults only | −64 | −46 | 24 | 2.4 | −62 | −48 | 20 | 2.3 | −62 | −42 | 26 | 2.4 | |||
Coordinates [x, y, z] are reported in Montreal Neurological Institute space. Activation for visual sentences across all participants was significant at p < .05 corrected for extent across the whole brain, n voxels = the number of voxels significant at p < .001 uncorrected for visual sentences and p < .05 uncorrected for auditory sentences. Peak voxels are shown in boldface. Those from all participants come from Analysis 1; those for Children and Adults alone come from Analysis 2.
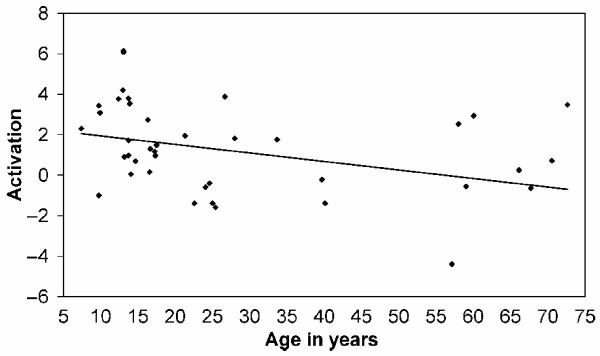
Of the six covariates, only linear age had an impact on activation for reversible compared with nonreversible sentences (at [x =−54, y =−38, z = 20], Z = 4.2, 19 voxels at p < .001), indicating that the effect of reversible versus nonreversible visual sentences was higher in younger participants, as shown in Figure 6.
Figure 6.
Scatter plot showing the relationship between age and activation at the peak voxel [x =−54, y =−38, z = 20] for the significant effect of age on visual-reversible over and above nonreversible sentences (Z = 4.2, 19 voxels at p < .001). The values on the y-axis represent effect size derived from β values for each participant.
There were no other significant effects of reversible relative to nonreversible sentences, even when the analysis was repeated in each age group separately. Therefore, the whole group analysis captured the most prominent source of variance related to reversible versus nonreversible sentences.
Analysis 2: Reversible and Nonreversible Sentences in Children and Adults
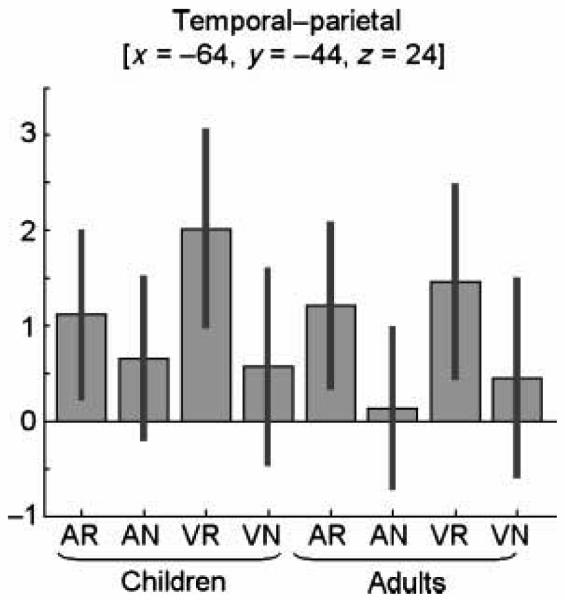
Consistent with Analysis 1, a main effect of reversible versus nonreversible sentences was identified in left T-P at [x =−64, y =−44, z = 24]. The effect was greater for visual than auditory sentences and observed in both age groups (see Figure 7).
Figure 7.
Shows a plot of the parameter estimates according to age group, processing modality, and sentence type at the peak coordinate of the main effect for reversible versus nonreversible sentences in this analysis [x =−64, y =−44, z = 24]. The red bars are the 90% confidence intervals. On the x-axis, the conditions correspond to contrasts 1–4 from each age group as entered into group level analysis 2 (for details, see Methods section): (1) auditory-reversible sentences, (2) auditory-nonreversible sentences, (3) visual-reversible sentences, and (4) visual-nonreversible sentences. The y-axis shows effect sizes as the mean of the beta value from the first level analysis (i.e., the percentage increase in activation relative to the global mean). This plot shows that both age groups show a similar activation profile across sentence types.
In summary, we identified one signifiaint effect of reversible versus nonreversible sentences in a left T-P region. The effect was observed in both younger than older participants (see Figure 7), but it was greater in the younger participants (see Figure 6).
Analysis 3: Functional Localizers at the Group Level
Activations for (i) syntactic and semantic sentence processing, (ii) articulation, and (iii) amodal semantics are shown in Figure 5B. As can be seen, the left T-P region associated above with reversible compared with nonreversible sentences was more activated during the articulation task than any other condition (shown in red). This result indicates that articulatory processes are implicitly engaged during silent sentence processing, most notably for semantically reversible sentences. In contrast, syntactic and semantic sentence activation (shown in blue) was observed in the left anterior and posterior middle temporal gyrus. These areas were activated by both reversible and nonreversible sentences, and there was no effect of sentence type in any of these identified regions. In articulation, other areas associated with articulation were observed in bilateral superior temporal and precentral gyri, and amodal semantic activation (shown in green) was observed in left lateralized regions in the inferior and middle temporal gyri, the pars opercularis and pars orbitalis, and the left putamen (for details, see Table 3C).
Table 3.
Activation for Functional Systems
| (A) Syntax/Syntactic-Semantic Processing | ||||||
|---|---|---|---|---|---|---|
| Sentences |
Sentence > All |
|||||
| Anatomical Location | x | y | z | n Voxels | Z Score | Z Score |
| Anterior temporal gyrus | −56 | 0 | −16 | 10 | Inf. | 6.7 |
| −58 | −18 | −6 | 49 | Inf. | 6.9 | |
| Posterior temporal gyrus | −48 | −42 | 0 | 10 | Inf. | Inf. |
| −64 | −50 | 10 | 14 | Inf. | Inf. | |
| −48 | −60 | 20 | 27 | 5.2 | 6.2 | |
| (B) Articulation | |||||||
|---|---|---|---|---|---|---|---|
| Articulation |
Articulation > All |
||||||
| Hemisphere | Anatomical Location | x | y | z | n Voxels | Z Score | Z Score |
| Left | Temporal–parietal boundary | −50 | −46 | 20 | 40 | 4.9 | 3.7 |
| −62 | −40 | 14 | 5.0 | 4.1 | |||
| Left | Pre/postcentral gyrus | −46 | −14 | 36 | 550 | Inf. | Inf. |
| −50 | −12 | 32 | Inf. | Inf. | |||
| −60 | −4 | 18 | Inf | Inf. | |||
| Right | Temporal–parietal boundary | 62 | −40 | 14 | 71 | 5.8 | 5.5 |
| 58 | −34 | 18 | 5.3 | 5.3 | |||
| Right | Pre/postcentral gyrus | 50 | −12 | 44 | 586 | Inf. | Inf. |
| 48 | −10 | 34 | Inf. | Inf. | |||
| 52 | −6 | 28 | Inf. | Inf. | |||
| 58 | −4 | 24 | Inf. | Inf. | |||
| (C) Amodal Semantics | |||||
|---|---|---|---|---|---|
| Left Hemisphere |
|||||
| Anatomical Location | x | y | z | Z Score | n Voxels |
| Middle temporal gyrus | −58 | −58 | 2 | Inf. | 125 |
| −54 | −64 | 10 | 5.0 | ||
| Inferior frontal opercularis | −50 | 10 | 22 | 6.5 | 90 |
| −48 | 16 | 28 | 4.8 | ||
| Orbitalis | −36 | 26 | −2 | 4.9 | 58 |
| −50 | 18 | −8 | 4.0 | ||
| Putamen | −24 | −4 | 12 | 6.4 | 43 |
| −22 | 0 | 12 | 6.3 | ||
| −22 | −6 | 16 | 6.0 | ||
n voxels = the number of voxels significant at p < .001 uncorrected. Coordinates shown in boldface are those closest to the peak activation for reversible sentences.
In short, activation for processing semantically reversible sentences is located in an area that is more strongly associated with articulation than with syntactic or semantic processing.
DISCUSSION
Semantically reversible sentences are more difficult to process than nonreversible sentence types. As the property of semantic reversibility contributes to the overall difficulty of a sentence across a wide range of grammatical constructions, we set out to identify a main effect of semantic reversibility by comparing activation for semantically reversible sentences to that of nonreversible sentences. The results of this whole brain analysis identified a significant effect for reversible relative to nonreversible sentences in a left T-P region.
By including additional linguistic and nonlinguistic conditions within our paradigm, we were also able to test whether the activation in this left T-P region corresponded to that seen for syntactic/syntactic-semantic processing, subarticulatory processing, or amodal semantics. This analysis indicated that selective activation for reversible sentences identified in a left T-P region was part of the neuronal system that was more activated by articulation than by any other condition. The pattern of activation in the left T-P (as shown in the graph on the bottom panel of Figure 5) indicates that while this region was active during sentence processing, it was most active during the repetitive articulation conditions (saying “1” and “3”). This contrasts with the response of other components of the sentence processing network. For instance, in the left anterior temporal cortex, activation was higher for sentences in comparison to all other conditions (see bottom panel of Figure 5). Likewise, left inferior frontal regions (pars opercularis and pars orbitalis) were strongly activated for sentences but most strongly activated by non-linguistic conditions such as hand action retrieval (see Table 3C and Figure 5).
Previous studies have identified the left temporal–parietal boundary as being actively engaged in both speech perception and speech production tasks (Hickok, Buschsbaum, Humphries, & Muftuler, 2003; Buchsbaum, Hickok, & Humphries, 2001) and therefore an important site of overlap between the phonological systems for speech input and output (Buchsbaum et al., 2001). For example, Hickok et al. (2003) report coordinates in proximity to our region for speech and music perception and rehearsal tasks at [x =−51, y =−46, z = 16]. Consistent with these findings, similar coordinates are reported by Wise et al. (2001) for a silent word generation task [x =−57, y =−42, z = 22] and by Wildgruber, Kischka, Ackermann, Klose, and Grodd (1999) when participants covertly resequenced word strings [x =−56, y =−40, z = 20]. These studies therefore support the conclusion that this region is activated by tasks that engage verbal working memory. Indeed, the contribution of this region to verbal working memory has been consistently highlighted in the literature (Chein, Ravizza, & Fiez, 2003; Hickok et al., 2003; Martin, Wu, Freedman, Jackson, & Lesch, 2003). Hickok et al. suggest that this region supports verbal working memory through its involvement in the maintenance of phonological and acoustic information. Set in this context, our results suggest that the sub-articulatory component of phonological working memory is important in the processing of semantically reversible sentences.
Contrary to some previous studies of sentence complexity (Caplan et al., 2001; Cooke et al., 2001; Caplan, Waters, & Alpert, 1999), the comparison of semantically reversible and nonreversible sentences did not result in increased inferior frontal activation, even when we lowered the statistical threshold to p < .05 uncorrected. Instead, two different inferior frontal regions (pars orbitalis and pars triangularis) were consistently activated by reversible and nonreversible sentences (see Figure 5). The absence of inferior frontal activation in the comparison of reversible versus nonreversible sentences is likely to be explained by our experimental paradigm. Contrary to previous studies of syntactic complexity, we were able to compare reversible and nonreversible sentences while controlling across a range of sentences with different syntactic structures (e.g., active, passive, subject cleft, object cleft, etc.; see Table 1). In addition, we used passive listening and reading tasks that did not require “meta-linguistic” analysis (Birdsong, 1989) of either the semantic or the syntactic content of the sentences. This would have reduced the demands on executive processing while focusing on the type of processing that occurs during everyday speech perception and reading. The effect of reversible versus nonreversible sentences in passive processing tasks is also consistent with the behavioral literature showing that adults can misinterpret reversible sentences across a range of grammatical constructions (Ferreira, 2003; Kemper & Catlin, 1979; Herriot, 1969; Turner & Rommetveit, 1967; Slobin, 1966). Finally, we note that although we did not see increased inferior frontal activation for reversible compared with nonreversible sentences in our passive listening/reading paradigm, this does not exclude the possibility that there would be an effect of reversibility in paradigms that used on-line executive tasks (e.g., semantic or syntactic decisions) or for longer or more complex sentences as used in many of the studies reported by Caplan, Waters, and Alpert (2003) and Caplan et al. (1999, 2001).
With respect to the role of the inferior frontal activation during both reversible and nonreversible sentences, we found that the pattern of response during passive listening and reading was most consistent with amodal semantic processing in accord with many other studies (Noppeney & Price, 2003, 2004; Wagner, Paré, Clarke, & Poldrack, 2001; Thompson-Schill, Aguirre, D'Esposito, & Farah, 1999; Vandenberghe et al., 1996). In particular, we found that inferior frontal activation was not specific to sentence processing (see also Wartenburger et al., 2004) but was most active during a hand action retrieval task (see Figure 5).
Areas associated with passive syntactic and syntactic-semantic processing were located in the left anterior and posterior temporal cortex, consistent with many previous studies (Awad, Warren, Scott, Turkheimer, & Wise, 2007; Lindenberg & Scheef, 2007; Crinion, Warburton, Lambon Ralph, Howard, & Wise, 2006; Humphries, Binder, Medler, & liebenthal, 2006; Spitsyna, Warren, Scott, Turkheimer, & Wise, 2006; Humphries, Love, Swinney, & Hickock, 2005; Constable et al., 2004; Crinion, Lambon Ralph, Warburton, Howard, & Wise, 2003; Friederici & Kotz, 2003; Friederici, Rüschemeyer, Hahne, & Friebach, 2003; Vandenberghe, Nobre, & Price, 2002; Scott, Blank, Rosen, & Wise, 2000). This system is likely to include activation related to both syntactic processing and syntactic-semantic integration (hypotheses i and ii, respectively). For instance, Humphries et al. (2006) associate anterior temporal regions with the processing of syntactic structure, and Friederici and Kotz (2003) and Friederici et al. (2003) have specifically advocated the role of the posterior superior temporal gyrus as being involved in sentence evaluation and syntactic-semantic integration: Both these regions are included in our syntax/syntactic-semantic processing network (see Table 3A). Although we cannot conclusively dissociate the functions of these different regions, we can report that there was no evidence for increased activation for reversible relative to nonreversible sentences (at p > .05 uncorrected within a 6-mm diameter) in either the anterior temporal or the superior posterior temporal regions. In sum, increased activation for reversible sentences was only detected in a left T-P region that did not correspond to regions engaged in syntactic/syntactic-semantic or amodal semantic processing but was active for the same subjects during an articulation task.
Our results suggest that semantically reversible sentences increase the demands on a brain region associated with phonological working memory (Wildgruber et al., 1999). However, we still need to consider why the passive processing of semantically reversible sentences should increase the demands on phonological working memory. A potential explanation is that when the use of simple heuristic strategies for sentence processing (such as attending to the semantically relevant content words of a sentence) fails, the representation of a reversible sentence needs to be maintained for longer in phonological working memory to allow parts of the sentence to be reaccessed during sentence comprehension.
We also observed a stronger effect of reversible relative to nonreversible sentences in our T-P region for children in comparison to adults (see Figure 6). Consistent with this pattern of results, Grossman et al. (2002) found that younger subjects showed more posterior temporal activation [x =−40, y =−36, z = 6] during sentence processing than older subjects, and Wildgruber et al. (1999) found increased parietal activation [x =−40, y =−44, z = 40] with increasing demands on phonological memory. The effect of age is likely to be a consequence of the proficiency of language use that increases as a product of experience throughout life or the number of years in education. Indeed, Caplan et al. (2003) found increased left temporal–parietal activation [x =−54, y =−32, z = 32] in older subjects as opposed to young adults during a sentence plausibility task, but this difference was not apparent when older and younger participants were matched for the number of years in education. In summary, we are proposing that reversible sentences need to be maintained for longer in working memory but this effect is reduced with language proficiency. Thus, as previously suggested by Waters, Caplan, Alpert, and Stanczak (2003), activation during sentence processing is more likely to vary as a function of processing speed than working memory capacity.
A further observation was that the effect of reversible versus nonreversible sentences was more prominent in the visual modality, which may simply reflect differing task demands in the auditory and the visual modalities. For example, subarticulation is greater for silent reading than listening (Michael, Keller, Carpenter, & Just, 2001). Consistent with this explanation, we found greater left T-P activation at [x =−54, y =−46, z = 24] for the main effect of visual relative to auditory words (Z = 4.2). The types of reversible sentences that we used may also have been more familiar in the visual than auditory domain because some sentence types—namely, cleft sentences are not typically experienced in the auditory modality. This may have impeded sentence comprehension in the auditory modality, particularly in children whose experience of language is less extensive than their adult counterparts. Further studies are therefore required for a better understanding of how stimulus modality, age, and comprehension ability influence the processing of different types of reversible sentences. Although we predict that the effect of reversible relative to nonreversible sentences is likely to be task dependent, the present study has enabled us to identify reversible sentence processing effects during a passive comprehension task that was not confounded by “meta-linguistic” or executive processes.
Finally, with respect to language disorders that show abnormally high difficulty with reversible sentences, there are multiple potential causes. Caplan et al. (2007) recently suggested that sentence processing difficulties in agrammatic aphasics may be the result of an intermittent reduction in general processing capacity (Caplan et al., 2007). A reduction in processing capacity when processing more complex sentences such as reversible sentences may result in a degraded representation of the linguistic input, which could make the comprehension of complex sentences more challenging when they cannot be solved with simple heuristic strategies. Although difficulties in processing semantically reversible sentences may also potentially arise from deficits to syntactic or syntactic-semantic processing, our data are consistent with the perspective that a deficit in phonological working memory may be one cause of apparent problems in syntax comprehension. This account is particularly pertinent in relation to SLI because phonological problems have been cited as a potential cause of the disorder in the literature (Gathercole & Baddeley 1990).
In conclusion, our interpretation is that when processing semantically reversible sentences, subarticulatory codes must be maintained for a longer period while thematic roles are assigned and the appropriate meaning of the sentence is established.
Acknowledgments
This research was funded by the Wellcome Trust. Fiona Richardson was supported by an MRC Career Establishment Grant G0300188, and a British Academy Grant SG-40400 awarded to Dr. Michael Thomas. The authors thank Janice Glensman, Amanda Brennan, David Bradbury, Helen Harth, Roberto Filippi, and Jenny Crinion for their assistance during scanning and neuropsychological testing.
REFERENCES
- Awad M, Warren JE, Scott SK, Turkheimer FE, Wise RJ. A common system for the comprehension and production of narrative speech. Journal of Neuroscience. 2007;27:11455–11464. doi: 10.1523/JNEUROSCI.5257-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt RS, Mitchum C, Burton MW, Haendiges AN. Comprehension of reversible sentences in aphasia: The effects of verb meaning. Cognitive Neuropsychology. 2004;21:229–244. doi: 10.1080/02643290342000456. [DOI] [PubMed] [Google Scholar]
- Berndt RS, Mitchum C, Haedings A. Comprehension of reversible sentences in “agrammatism”: A meta-analysis. Cognition. 1996;58:289–308. doi: 10.1016/0010-0277(95)00682-6. [DOI] [PubMed] [Google Scholar]
- Bickel C, Pantel J, Eysenbach K, Schröder J. Syntactic comprehension deficits in Alzheimer's disease. Brain and Language. 2000;71:432–448. doi: 10.1006/brln.1999.2277. [DOI] [PubMed] [Google Scholar]
- Birdsong D. Metalinguistic performance and interlinguistic competence. Springer-Verlag; New York: 1989. [Google Scholar]
- Buchsbaum B, Hickok G, Humphries C. Role of the left posterior superior temporal gyrus in phonological processing for speech perception and production. Cognitive Science. 2001;25:663–678. [Google Scholar]
- Caplan D, Vijayan S, Kuperberg G, West C, Waters G, Greve D, et al. Vascular responses to syntactic processing: Event-related fMRI study of relative clauses. Human Brain Mapping. 2001;15:26–38. doi: 10.1002/hbm.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan D, Waters G, Alpert N. Pet studies of sentence processing with auditory sentence presentation. Neuroimage. 1999;9:343–351. doi: 10.1006/nimg.1998.0412. [DOI] [PubMed] [Google Scholar]
- Caplan D, Waters G, Alpert N. Effects of age and speed of processing on rCBF correlates of syntactic processing in sentence comprehension. Human Brain Mapping. 2003;19:112–131. doi: 10.1002/hbm.10107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan D, Waters G, DeDe G, Michaud J, Reddy A. A study of syntactic processing in aphasia: I. Behavioural (psycholinguistic) aspects. Brain and Language. 2007;101:103–150. doi: 10.1016/j.bandl.2006.06.225. [DOI] [PubMed] [Google Scholar]
- Caramazza A, Zurif E. Dissociation of algorithmic and heuristic processes in language comprehension: Evidence from aphasia. Brain and Language. 1976;3:572–582. doi: 10.1016/0093-934x(76)90048-1. [DOI] [PubMed] [Google Scholar]
- Chein JM, Ravizza SM, Fiez JA. Using neuroimaging to evaluate models of working memory and their implications for language processing. Journal of Linguistics. 2003;16:315–339. [Google Scholar]
- Constable RT, Pugh KR, Berroya E, Mencl WE, Westerveld M, Ni W, et al. Sentence complexity and input modality effects in sentence comprehension: An fMRI study. Neuroimage. 2004;22:11–21. doi: 10.1016/j.neuroimage.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Cooke A, Zurif EB, DeVita C, Alsop D, Koenig P, Detre J, et al. Neural basis for sentence comprehension: Grammatical and short term memory components. Human Brain Mapping. 2001;15:80–94. doi: 10.1002/hbm.10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crinion JT, Lambon Ralph MA, Warburton EA, Howard S, Wise RJ. Temporal lobe regions engaged during normal speech comprehension. Brain. 2003;126:1193–1201. doi: 10.1093/brain/awg104. [DOI] [PubMed] [Google Scholar]
- Crinion JT, Warburton EA, Lambon Ralph MA, Howard S, Wise RJ. Listening to narrative speech after aphasic stroke: The role of the left anterior temporal lobe. Cerebral Cortex. 2006;16:1116–1125. doi: 10.1093/cercor/bhj053. [DOI] [PubMed] [Google Scholar]
- Dollaghan C, Campbell T. Nonword repetition and child language impairment. Journal of Speech, Language, and Hearing Research. 1998;41:1136–1146. doi: 10.1044/jslhr.4105.1136. [DOI] [PubMed] [Google Scholar]
- Dunn LM, Dunn LM, Whetton C, Burley J. The British Picture Vocabulary Scale. 2nd ed. NFER-Nelson; Windsor: 1997. [Google Scholar]
- Elliot CD, Smith P, McCulloch K. British Ability Scales Second Edition (BAS II) NFER-Nelson; London: 1997. [Google Scholar]
- Ellis Weismer S, Tomblin B, Zhang X, Buckwalter P, Chynoweth J, Jones M. Nonword repetition performance in school-age children with and without language impairment. Journal of Speech, Language, and Hearing Research. 2000;43:865–878. doi: 10.1044/jslhr.4304.865. [DOI] [PubMed] [Google Scholar]
- Ferreira F. The misinterpretation of noncanonical sentences. Cognitive Psychology. 2003;47:164–203. doi: 10.1016/s0010-0285(03)00005-7. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Kotz SA. The brain basis of syntactic processes: Functional imaging and lesion studies. Neuroimage. 2003;20:S8–S17. doi: 10.1016/j.neuroimage.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Ruschemeyer S-A, Hahne A, Friebach CJ. The role of the left inferior frontal and superior temporal cortex in sentence comprehension: Localizing syntactic and semantic processes. Cerebral Cortex. 2003;13:170–177. doi: 10.1093/cercor/13.2.170. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Frith CD, Poline JB, Heather JD, Frackowiak RSJ. Spatial registration and normalization of images. Human Brain Mapping. 1995;2:1–25. [Google Scholar]
- Friston KJ, Holmes A, Worsley KJ, Poline JB, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: A general linear approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Gathercole SE, Baddeley AD. Phonological memory deficits in language disordered children: Is there a causal connection? Journal of Memory and Language. 1990;2:103–127. [Google Scholar]
- Grodzinsky Y. Theoretical perspectives on language deficits. MIT Press; Cambridge, MA: 1990. [Google Scholar]
- Grodzinsky Y, Piñango MM, Zurif E, Drai D. The critical role of group studies in neuropsychology: Comprehension regularities in Broca's aphasia. Brain and Language. 1999;67:134–147. doi: 10.1006/brln.1999.2050. [DOI] [PubMed] [Google Scholar]
- Grossman M, Cooke A, DeVita C, Alsop D, Detre J, Chen W, et al. Age-related changes in working memory during sentence comprehension: An fMRI study. Neuroimage. 2002;15:302–317. doi: 10.1006/nimg.2001.0971. [DOI] [PubMed] [Google Scholar]
- Herriot P. The comprehension of active and passive sentences as a function of pragmatic expectation. Journal of Verbal Learning and Verbal Behavior. 1969;8:166–169. [Google Scholar]
- Hickok G, Buschsbaum B, Humphries C, Muftuler T. Auditory-motor interaction revealed by fMRI: Speech, music, and working memory in area Spt. Journal of Cognitive Neuroscience. 2003;15:673–682. doi: 10.1162/089892903322307393. [DOI] [PubMed] [Google Scholar]
- Humphries C, Binder JR, Medler DA, Liebenthal E. Syntactic and semantic modulation of neural activity during auditory sentence comprehension. Journal of Cognitive Neuroscience. 2006;18:665–679. doi: 10.1162/jocn.2006.18.4.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries C, Love T, Swinney D, Hickock G. Response of anterior temporal cortex to syntactic and prosodic manipulations during sentence processing. Human Brain Mapping. 2005;26:128–138. doi: 10.1002/hbm.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesper LR, Amdersson JLR, Hutton C, Ashburner J, Turner R, Friston KJ. Modelling geometric defomiations in EPI time series. Neuroimage. 2001;13:903–919. doi: 10.1006/nimg.2001.0746. [DOI] [PubMed] [Google Scholar]
- Kang HC, Burgund ED, Lugar HM, Petersen SE, Schlaggar BL. Comparison of functional activation foci in children and adults using a common stereotactic space. Neuroimage. 2003;19:16–28. doi: 10.1016/s1053-8119(03)00038-7. [DOI] [PubMed] [Google Scholar]
- Kemper S, Catlin J. On the role of semantic constraints in sentence comprehension. Language and Speech. 1979;22:253–267. [Google Scholar]
- Leonard L. Children with specific language impairment. MIT Press; Cambridge, MA: 1998. [Google Scholar]
- Lindenberg R, Scheef L. Supramodal language comprehension: Role of the left temporal lobe for listening and reading. Neuropsychologica. 2007;45:2407–2415. doi: 10.1016/j.neuropsychologia.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Martin RC, Wu D, Freedman M, Jackson EF, Lesch M. An event-related fMRI investigation of phonological versus semantic short-term memory. Journal of Neurolinguistics. 2003;16:341–360. [Google Scholar]
- Mechelli A, Price CJ, Henson RNA, Friston KJ. Estimating efficiency a priori: A comparison of blocked and randomized designs. Neuroimage. 2003;18:798–805. doi: 10.1016/s1053-8119(02)00040-x. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Henson RNA, Price CJ, Friston KJ. Comparing event-related and epoch analysis in blocked design fMRI. Neuroimage. 2003;18:806–810. doi: 10.1016/s1053-8119(02)00027-7. [DOI] [PubMed] [Google Scholar]
- Michael EB, Keller TA, Carpenter PA, Just MA. fMRI investigation of sentence comprehension by eye and ear: Modality fingerprints on cognitive processes. Human Brain Mapping. 2001;13:239–353. doi: 10.1002/hbm.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery JW. Examination of phonological working memory in specifically language impaired children. Applied Psycholinguistics. 1995a;16:355–378. [Google Scholar]
- Montgomery JW. Sentence comprehension in children with specific language impairment: The role of phonological working memory. Journal of Speech and Hearing Research. 1995b;38:187–199. doi: 10.1044/jshr.3801.187. [DOI] [PubMed] [Google Scholar]
- Montgomery JW. Sentence comprehension in children with specific language impairment: Effects of input rate and phonological working memory. International Journal of Language and Communication Disorders. 2004;39:115–134. doi: 10.1080/13682820310001616985. [DOI] [PubMed] [Google Scholar]
- Noppeney U, Price CJ. Functional imaging of the semantic system: Retrieval of sensory-experienced and verbally leaned knowledge. Brain and Language. 2003;84:120–133. doi: 10.1016/s0093-934x(02)00525-4. [DOI] [PubMed] [Google Scholar]
- Noppeney U, Price CJ. Retrieval of abstract semantics. Neuroimage. 2004;22:164–170. doi: 10.1016/j.neuroimage.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Poldrack RA. Can cognitive processes be inferred from neuroimaging data? Trends in Cognitive Sciences. 2006;10:59–63. doi: 10.1016/j.tics.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Rice ML. Grammatical symptoms of specific language impairment. In: Bishop DVM, Leonard LB, editors. Speech and language impairments in children: Causes, characteristics, intervention and outcome. Psychology Press; Hove: 2000. pp. 17–34. [Google Scholar]
- Saffran EM, Schwartz MF, Linebarger MC. Semantic influences on thematic role assignment: Evidence from normals and aphasics. Brain and Language. 1998;62:255–297. doi: 10.1006/brln.1997.1918. [DOI] [PubMed] [Google Scholar]
- Scott SK, Blank SC, Rosen S, Wise RJS. Identification of a pathway for intelligible speech in the left temporal lobe. Brain. 2000;123:2400–2406. doi: 10.1093/brain/123.12.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slobin DI. Grammatical transformations and sentence comprehension in childhood and adulthood. Journal of Verbal Learning and Verbal Behavior. 1966;5:219–227. [Google Scholar]
- Spitsyna G, Warren JE, Scott SK, Turkheimer FE, Wise RJS. Converging language streams in the human temporal lobe. Journal of Neuroscience. 2006;26:7328–7336. doi: 10.1523/JNEUROSCI.0559-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL, Aguirre GK, D'Esposito M, Farah MJ. A neural basis for category and modality specificity of semantic knowledge. Neuropsychologia. 1999;37:671–676. doi: 10.1016/s0028-3932(98)00126-2. [DOI] [PubMed] [Google Scholar]
- Turner EA, Rommetveit R. The acquisition of sentence voice and reversibility. Child Development. 1967;38:649–660. [PubMed] [Google Scholar]
- Ullman MT, Pierpont EI. Specific Language Impairment is not specific to language: The procedural deficit hypothesis. Cortex. 2005;41:399–433. doi: 10.1016/s0010-9452(08)70276-4. [DOI] [PubMed] [Google Scholar]
- van der Lely HKJ. Domain-specific cognitive systems: Insight from grammatical-SLI. Trends in Cognitive Sciences. 2005;9:53–59. doi: 10.1016/j.tics.2004.12.002. [DOI] [PubMed] [Google Scholar]
- van der Lely HKJ, Christian V. Lexical word formation in grammatical SLI children: A grammar-specific or input processing deficit? Cognition. 2000;75:33–63. doi: 10.1016/s0010-0277(99)00079-7. [DOI] [PubMed] [Google Scholar]
- van der Lely HKJ, Stollwerck L. A grammatical specific language impairment in children: An autosomal dominant inheritance? Brain and Language. 1996;52:484–504. doi: 10.1006/brln.1996.0026. [DOI] [PubMed] [Google Scholar]
- Vandenberghe R, Nobre AC, Price CJ. The response of the left temporal cortex to sentences. Journal of Cognitive Neuroscience. 2002;14:550–560. doi: 10.1162/08989290260045800. [DOI] [PubMed] [Google Scholar]
- Vandenberghe R, Price CJ, Wise R, Josephs O, Frackowiak RSJ. Semantic system(s) for words or pictures: Functional anatomy. Nature. 1996;383:254–256. doi: 10.1038/383254a0. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Paré EJ, Clarke BJ, Poldrack RA. Recovering meaning: Left prefrontal cortex guides controlled semantic retrieval. Neuron. 2001;31:329–338. doi: 10.1016/s0896-6273(01)00359-2. [DOI] [PubMed] [Google Scholar]
- Wartenburger I, Heekeren HR, Buchert F, Heinemann S, De Bleser R, Villringer A. Neural correlates of syntactic transformations. Human Brain Mapping. 2004;22:72–81. doi: 10.1002/hbm.20021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters G, Caplan D, Alpert N, Stanczak L. Individual differences in rCBF correlates of syntactic processing in sentence comprehension: Effects of working memory and speed of processing. Neuroimage. 2003;19:101–112. doi: 10.1016/s1053-8119(03)00007-7. [DOI] [PubMed] [Google Scholar]
- Waters GS, Rochon E. Task demands and sentence comprehension in patients with dementia of the Alzheimer's type. Brain and Language. 1998;62:361–397. doi: 10.1006/brln.1997.1880. [DOI] [PubMed] [Google Scholar]
- Wildgruber D, Kischka U, Ackermann H, Klose U, Grodd W. Dynamic pattern of brain activation during sequencing of word strings evaluated by fMRI. Cognitive Brain Research. 1999;7:285–294. doi: 10.1016/s0926-6410(98)00031-7. [DOI] [PubMed] [Google Scholar]
- Wise RJS, Scott SK, Blank SC, Mummary CJ, Murphy K, Warburton EA. Separate neural subsystems within “Wernicke's area.”. Brain. 2001;124:83–95. doi: 10.1093/brain/124.1.83. [DOI] [PubMed] [Google Scholar]