Abstract
Using behavioral, structural, and functional imaging techniques, we demonstrate contrasting effects of vocabulary knowledge on temporal and parietal brain structure in 47 healthy volunteers who ranged in age from 7 to 73 years. In the left posterior supramarginal gyrus, vocabulary knowledge was positively correlated with gray matter density in teenagers but not adults. This region was not activated during auditory or visual sentence processing, and activation was unrelated to vocabulary skills. Its gray matter density may reflect the use of an explicit learning strategy that links new words to lexical or conceptual equivalents, as used in formal education and second language acquisition. By contrast, in left posterior temporal regions, gray matter as well as auditory and visual sentence activation correlated with vocabulary knowledge throughout lifespan. We propose that these effects reflect the acquisition of vocabulary through context, when new words are learnt within the context of semantically and syntactically related words.
INTRODUCTION
Vocabulary knowledge gradually increases across lifespan and is essential for both auditory and written sentence comprehension. Previous studies have suggested that gray matter density in the posterior supramarginal gyri (pSMG) provides a marker for the number of words learnt (Lee et al., 2007). However, the mechanisms underlying this effect remain poorly understood. The aim of the present study was to investigate the effect of vocabulary knowledge on gray matter density in multiple brain regions and to establish whether the observed effects were dependent on age and therefore potentially indicative of different vocabulary learning strategies. In the following, we describe the background and the rationale for our study.
The association of the pSMG with language learning was first reported by Mechelli et al. (2004), who identified greater gray matter density in this region for bilinguals than for monolinguals. Moreover, increases in gray matter density were found to be positively correlated with second language proficiency. The significance of this effect was investigated further by Lee et al. (2007), who found that vocabulary knowledge in English-speaking monolingual teenagers was positively correlated with gray matter density in the same pSMG region that was implicated in adult second language learning. In addition, using diffusion tensor imaging, Lee et al. identified direct anatomical connections from the pSMG to both the anterior supramarginal gyrus and the angular gyrus. These regions have been associated with phonological and semantic decisions, respectively (Gathercole, 2006; Golestani & Pallier, 2007; Devlin, Matthews, & Rushworth, 2003; Gathercole, Hitch, Service, & Martin, 1997; Price, More, Humphreys, & Wise, 1997; Demonet et al., 1992). Thus, Lee et al. proposed that the pSMG is an important site for linking phonological and semantic information, which is central to vocabulary acquisition in both first and second languages.
Word knowledge is mainly acquired through exposure to everyday language. In this instance, vocabulary is increased by reading or hearing new words set within the context of known words that have different but related meanings, and from which the meaning of the new word can be deduced. Here, vocabulary learning occurs via semantic and syntactic associations and prior knowledge of morphological structure (see Kilian, Nagy, Pearson, Anderson, & García, 1995; Nagy, Herman, & Anderson, 1985). However, new words can also be learnt by forming an explicit link between a new word and a lexical equivalent (e.g., “purchase” is another word for “buy”) or a definition (e.g., An “ATOM” is the smallest particle that comprises a chemical element; “GARGANTUAN” has a similar meaning to “gigantic”; “EGREGIOUS” means very bad). This kind of word learning may take place through instruction by a parent or caregiver, in a formal educational setting, or be self-motivated in a revision-based scenario, in which an individual is specifically focused on learning a new terminology. This type of expansion of vocabulary also increases knowledge of morphological relationships between words (e.g., “bake” and “baker”) that can be generalized when new words are introduced.
Critically, the findings of Lee et al. (2007) were based on monolingual adolescents aged between 12 and 15 years. These individuals were in full-time education and were therefore expected to be actively engaged in expanding their vocabulary, learning new words as labels for new concepts, and lexicalizing existing concepts. Of the two learning strategies for acquiring vocabulary knowledge, explicit learning mainly through instruction is more likely to be exploited by children than by adults, who are no longer in formal education and who most probably continue to learn vocabulary mainly through context with a minimal contribution from explicit instruction. Therefore, as the influence of different learning strategies alters across lifespan, we would expect the relationship between vocabulary and brain structure to be age dependent. To investigate this hypothesis, we combined structural and functional imaging using a cross-section of participants of different ages. This study used a different sample of participants and different vocabulary measures to those reported by Lee et al. Replication of the vocabulary effects using a different vocabulary test allows us to assess the generalizability of the effect across different vocabulary measures. Our expectation was that learning vocabulary through context would be reflected by a positive correlation between vocabulary knowledge and gray matter density that is (a) continuous across lifespan and (b) in brain regions that are activated by semantic and syntactic associations during reading and listening tasks. By contrast, we expected that learning through an explicit strategy would be reflected by a positive correlation between vocabulary knowledge and gray matter density that was (a) limited to those undergoing formal education and (b) in regions that were not necessarily involved in reading or listening to speech.
To address these hypotheses, we studied a group of 47 healthy participants between the ages of 7 to 73 years. We assessed gray matter density using structural magnetic resonance imaging (MRI), whereas activation for reading and listening to language stimuli was assessed using functional MRI in the same sample of participants. We also tested whether the local differences in gray matter we identified were susceptible to influence from age-related differences in gray matter. For instance, numerous studies have reported that the well-known decline in gray matter post-adolescence (Paus, 2005; Gogtay et al., 2004; Giedd et al., 1999) is characterized by different trajectories across frontal, temporal, and parietal brain regions (Wilke, Krägeloh-Mann, & Holland, 2007; Lenroot & Giedd, 2006; Allen, Bruss, Kice Brown, & Damasio, 2005, Gogtay et al., 2004; Sowell et al., 2003; Giedd et al., 1999). Consequently, a decline in gray matter with age may make it more difficult to detect small increases in gray matter with vocabulary, and this confound may be stronger in some regions than others and change with age. We therefore investigated whether differences in the trajectory of decline in gray matter with age could account for any age-dependent differences in the effect of vocabulary knowledge.
To our knowledge, the combination of behavioral, structural, and functional differences has not been tested before in the context of normal development, although previous studies of abnormal development have suggested that dyslexic individuals can have atypical activation and gray matter in the same regions (Chételat et al., 2008; Hoeft et al., 2007; Hyde, Lerch, Griffiths, Evans, & Peretz, 2007; Brambati et al., 2004, 2006; Hyde, Zatorre, Griffiths, Lerch, & Peretz, 2006; Silani et al., 2005). Evidence that vocabulary acquisition affects the same regions in structural and functional images would also suggest that increased gray matter might be a consequence of increased neuronal activation or vice versa.
METHODS
Participants
The participants were 48 right-handed volunteers (24 males), aged between 7 and 73 years, who had English as their first language. Children and teenagers were recruited through existing contacts in local schools in the London area, and adults were recruited mainly through advertisement in local further education institutions. All participants had normal or corrected-to-normal vision, with no reported hearing difficulties or disturbances in speech comprehension, speech production, or reading. None of the participants had a preference or a history of preference for using their left hand or left foot, none had right-lateralized language activation (see below), and none were bilingual, multilingual, or had any special linguistic training.
One male child was excluded from the functional analyses due to an incomplete coverage of temporal brain regions. One teenager was excluded from the structural analyses due to a poor quality image caused by motion artifacts, leaving a total of 47 participants for each analysis. Participant demographics can be seen in Table 1. This study was approved by the joint ethical committee of the Institute of Neurology and the National Hospital for Neurology and Neurosurgery, London, UK. Informed consent (written consent from a parent or guardian in the case of children under 16 years old) was obtained from all participants.
Table 1.
Participant Demographics
| Age Range | n | M | SD | |
|---|---|---|---|---|
| Children | 7.4–11.7 | 9 (4 males) | 9.5 | 1.2 |
| Adolescents | 12.4–17.5 | 17 (8 males) | 14.7 | 1.8 |
| Adults | 21.3–72.6 | 22 (11 males) | 45.3 | 7.0 |
Age range displayed in years and months.
Behavioral Tests
All participants carried out two psychometric tests: (i) the British Picture Vocabulary Scale II (BPVS-II; Dunn, Dunn, Whetton, & Burley, 1997) and (ii) the matrices task from the British Ability Scale II (BAS-II; Elliot, Smith, & McCulloch, 1997). All participants between 7 and 11 years also carried out the Reading test from the BAS-II to ensure that they had sufficient reading ability to carry out the functional imaging paradigm. The Reading test consists of 90 words divided into 9 blocks of 10 words. Children start the test at an age-appropriate point and read aloud a series of words presented on a card. The words increase in complexity as the test progresses. The test is continued until the child makes eight or more consecutive errors. An ability score that takes into account the difficulty of the test items completed is then obtained using a lookup table supplied with the test. Children with a minimum reading age of 7 years were considered to be at an appropriate level to carry out the reading task used in the fMRI paradigm. All children who took part in this study had a reading level in line with or in advance of their chronological age (reading age range = 7.4 to 15.3).
The BPVS-II is a measure of an individual's receptive vocabulary for Standard English (Dunn et al., 1997). In this test, participants are asked to select (from four options) the picture that most accurately matches a word (such as “ladder” or “collision”) read aloud by the tester. The test consists of 14 sets of words of increasing levels of difficulty, each containing 12 items. Each set has an approximate age-range indicator, which is used to select the appropriate starting point of the test. The test is conducted until the participant makes eight or more incorrect responses within a set. The raw score from this test takes into account the level of difficulty of the test items administered and the number of incorrect responses over the duration of the test.
The matrices task from the BAS-II was used as a measure of general cognitive ability. In this test, participants are shown an incomplete matrix of black and white abstract figures, with each matrix consisting of either four or nine cells. Participants are required to select the most appropriate pattern to complete the matrix from six potential tiles by pointing to or reading the number of the tile that best completes the matrix. Participants first complete four practice items and then begin the test at an age-appropriate level, which is indicated on the test (previous items are administered should they fail on the first three test items). The test is discontinued if the participant makes five failures out of six consecutive items. An ability score, which takes into account the number and the level of difficulty of the test items completed, is then obtained from a look-up table supplied with the test.
Functional Imaging
Experimental Paradigm
The experimental paradigm included a sentence processing task that was carried out in (i) auditory and (ii) visual modalities, where participants listened passively to auditory sentence stimuli, and silently read visual sentences. They also listened to or read scrambled sentences (strings of words that did not constitute a meaningful sentence)—tapping comprehension at the individual word level. The baseline task in the auditory modality consisted of listening to the same speech recordings after they had been rendered meaningless by digital reversal. In the visual modality, the baseline task consisted of viewing the same words presented in an unrecognizable (false) font. A passive listening/reading paradigm has the advantage of avoiding task-induced strategies over and above the speech comprehension processes in which we were interested. Although there was no in-scanner behavior to assess on-line speech comprehension, we were able to correlate activation with vocabulary knowledge measured outside the scanner. In addition, it should be noted that, as the power in our analysis came from intersubject variation in auditory or written language processing, it was unnecessary to equate in-scanner performance across participants. Instead, we ensured that each participant was attending to the words and processing the sentences by conducting an unexpected memory task at the end of each scanning session. The resulting memory scores were then included as covariates in our second level statistical analyses of vocabulary ability (see below for more details).
Sentence Stimuli
Sentence stimuli consisted of 80 sentences with six to eight words per sentence. Familiar words were selected to be suitable for children as young as 7 years and had a Flesch–Kincaid grade level readability score of 1.3. Sentences were constructed using high frequency (>20 per million) monosyllabic and bisyllabic nouns, verbs, and adjectives. Sentence sets were split into two groups (A and B) of equivalent composition for the purpose of presenting one set in an auditory format and the other set in a visual format. Sentences were matched at the lexical level in terms of mean frequency, imageability, and age of acquisition and at the sentence level in terms of mean word length and syllable length. These same sentence types were presented across both visual and auditory modalities to ensure consistency across tasks. No sentence was repeated across modality. The presentation of subsets A and B in either an auditory or a visual format was counterbalanced across participants. Scrambled sentences were constructed from the same set of words as meaningful sentences, consisting of initially grammatical sentences (e.g., “The cow chased the fat horse”), which were then assigned a pseudo-random word order that did not form a meaningful sentence (e.g., “Chased the the horse cow fat”). This condition is therefore fully matched to the sentences at the lexical level. Examples of sentence stimuli can be seen in the Appendix.
Procedure
Condition order was blocked, but each stimulus was analyzed as a separate event. Within each of the four different scanning sessions (runs), there were two blocks of auditory sentences, two blocks of visual sentences, one block of scrambled auditory sentences, one block of scrambled visual sentences, three blocks of auditory baseline, and three blocks of visual baseline. Within each block, there were 37 words/baseline stimuli, grouped into five sequences/sentences with 0.5 sec between sequences/sentences, during which a fixation cross was presented in the center of the screen. Within each sequence/sentence, stimuli were presented at a rate of one per 0.4 sec resulting in a maximum duration of 3.2 sec for an eight-stimulus sequence/sentence. The auditory and the visual word presentation rates were equated by recording the auditory stimuli from a female reading aloud the visual stimuli presented using the same script that was to be used in the scanner. Each word was presented in a Helvetica font size 20. Sentence change was indicated by an auditory beep, whereas in the visual condition, the first word of each sentence started with a capital letter.
Each task, consisting of an activation and a baseline pair, was preceded by an appropriate visually displayed instruction (Helvetica, size 80): “listen” (auditory sentence processing task) or “read” (visual sentence processing task). This instruction was displayed for 2.2 sec and was followed by an auditory pure tone, which sounded for 0.3 sec. Each activation and baseline condition had a total duration of 18 sec. The presentation of activation and baseline conditions was separated by a brief auditory pure tone, which sounded for 0.3 sec, followed by a 0.2-sec fixation cross. At the end of each activation and baseline task, there was a 1.5-sec pause before the onset of the next task. This resulted in a total duration of 40.5 sec for an activation (meaningful or scrambled sentences) and baseline pair. Each session commenced with a visual cue to “Get ready…” followed by a count down, during which dummy scans were acquired.
Memory Test
All participants carried out a pen-and-paper memory test following scanning to test their memory for sentences presented during the scanning session. Participants were not informed of this test before scanning. The main purpose of this test was to ensure that participants had been attending to the stimuli while in the scanner. These scores were also used to control for any potential effects that memory for sentences might have upon sentence processing in subsequent analyses conducted in SPM. This test consisted of 24 sentences, 12 familiar sentences seen during scanning, and 12 new sentences previously unseen during scanning. There were six familiar sentences for each of the two presentation modalities (auditory and visual). New sentences consisted of six sentences constructed from previously unseen words and six sentences constructed from novel words. All participants scored above chance on this test (test score, M = 70%, SD = 11%). These scores were adjusted to account for incorrect as well as correct responses before being entered into our analyses. The total of false positive responses was subtracted from the number of correct responses for familiar sentences from each presentation modality. This resulted in two scores—one for auditory memory for sentences and one for visual memory for sentences.
fMRI Data Acquisition
A Siemens 1.5-T Sonata scanner was used to acquire a total of 768 T2*-weighted echo-planar images with BOLD contrast (192 scans per 4 sessions). Each echo-planar image comprised 30 axial slices of 2-mm thickness with 1-mm interslice interval and 3 × 3-mm in-plane resolution. Volumes were acquired with an effective repetition time of 2.7 sec/volume, and the first six (dummy) volumes of each run were discarded to allow for T1 equilibration effects.
Functional Image Analysis
Preprocessing was conducted using statistical parametric mapping (SPM 2, Wellcome Trust Centre for Ncuroimaging, London, UK; http://www.fil.ion.ucl.ac.uk/spm) running under Matlab 6.5.1 (Mathworks Inc., Sherbon, MA). All volumes (excluding dummy scans) from each participant were realigned using the first as a reference image and unwarped (Jesper et al., 2001), adjusting for residual motion-related signal changes. The functional images were then spatially normalized (Friston, Ashburner, et al., 1995) to a standard MNI-305 template using nonlinear basis functions. Functional data were spatially smoothed with a 6-mm FWHM isotropic Gaussian kernel to compensate for residual variability after spatial normalization and to permit application of Gaussian random-field theory for corrected statistical inference (Friston, Holmes, et al., 1995).
Statistical Analysis of Functional Data
At the first level, data for each participant were analyzed with high-pass filtering using a set of discrete cosine basis functions with a cutoff period of 128 sec. Each sentence was modeled as a separate event within each condition and convolved with a canonical hemodynamic response function. This resulted in six different conditions at the first level, which were as follows:
(A) auditory sentences;
(B) auditory words (scrambled sentences);
(C) auditory baseline (reversed words);
(D) visual sentences;
(E) visual words (scrambled sentences); and
(F) visual baseline (false font).
The following four contrasts were generated for each participant:
(1) auditory sentences: [sentences] − [baseline] (= A − C);
(2) visual sentences: [sentences] − [baseline] (= D − F);
(3)auditory words: [scrambled sentences] − [baseline] (= B − C); and
(4) visual words: [scrambled sentences] − [baseline] (= E − F).
In addition, to verify that language processing was predominantly left lateralized, we generated two contrasts (one for each modality) combining activation for sentences and words at the first level for each participant. These contrasts were viewed for each participant and showed predominantly left-hemisphere activation for language processing in both modalities. None of the subjects had right-lateralized language.
Two statistical analyses were conducted at the second level using SPM 5. The aim of these analyses was to identify regions where functional activation is predicted by vocabulary score. Both analyses used the same model but differed in terms of the participant groups entered into the analysis:
Functional Analysis 1: the effect of vocabulary on functional activation across lifespan in all 47 participants; and
Functional Analysis 2: the effect of vocabulary on functional activation in teenagers only (17 participants, 8 males and 9 females, mean age = 14.7 years, range = 12.4–17.5 years).
The model used was a second-level ANOVA, which included contrasts 1 to 4 (listed above) and the following six covariates: (i) vocabulary score (BPVS-II raw score), (ii) nonverbal problem solving ability (BAS-II: matrices ability score), (iii) auditory memory for sentences, (iv) visual memory for sentences, (v) linear effects of age, and (vi) nonlinear effects of age. The inclusion of covariates allowed us to identify the effect of vocabulary upon the processing of auditory and visual sentences and words while factoring out any potential variance due to the other factors. Significant effects were corrected for multiple comparisons at p < .05, across the whole brain or in the left and right pSMG ROIs with a 10-mm radius centered on the peak coordinates in the left pSMG =[x = −44, y = −54, z = 46] and right pSMG [x = 52, y = −52, z = 44], as reported by Lee et al. (2007).
Structural Imaging
Structural Image Acquisition
Focal gray matter density was estimated based on T1-weighted anatomical whole-brain images acquired using a Siemens Sonata 1.5-T MRI scanner (Siemens Medical Systems, Erlangen, Germany). A T1-weighted modified driven equilibrium Fourier transform sequence (Deichmann, Schwarzbauer, & Turner, 2004) was used to acquire 176 sagittal partitions with an image matrix of 256 × 224, yielding a final resolution of 1 mm3 (repetition time/echo time/inversion time = 12.24/3.56/530 msec).
Structural Image Analysis
Scans were analyzed using SPM 5 (Wellcome Department of Imaging Neuroscience, http://www.fil.ion.ucl.ac.uk/spm). Preprocessing was performed using a generative model that combines bias correction, image registration, and tissue classification, using default gray and white matter tissue probability maps (as described in Ashburner & Friston, 2005). The resulting images were then smoothed using an isotropic kernel of 8 mm at FWHM. For consistency with previous VBM studies of language skill (Lee et al., 2007; Mechelli et al., 2004), we did not modulate the images with a correction for local gray matter but we did correct for global gray matter signal using proportional scaling in SPM. The information at each voxel was therefore an indication of the relative concentration of regional gray matter, typically referred to as gray matter density (for further details, see Mechelli, Price, Friston, & Ashburner, 2005).
Statistical Analyses of Structural Data
A total of four statistical analyses were conducted on the structural data. The first two analyses were carried out in SPM 5 and differed only in terms of the participants entered into each analysis:
Structural Analysis 1: the effect of vocabulary on regional gray matter across lifespan in all 47 participants; and
Structural Analysis 2: the effect of vocabulary on regional gray matter in teenagers only (16 participants, 8 males and 8 females, mean age = 14.6 years, range = 12.4–17.5 years).
These multiple regression analyses included the same cognitive regressors as the functional imaging analysis, that is: (i) vocabulary knowledge (BPVS-II), (ii) nonverbal problem solving ability (BAS-II: matrices), (iii) scores for auditory memory for sentences, and (iv) scores for visual memory for sentences. To model age effects, we used proportional scaling that adjusts the signal at each voxel so that the overall global gray matter signal is the same for all images. This also has the effect of correcting for the known decrease in global gray matter signal that occurs with increasing age.
Statistical Threshold for Structural Analyses 1 and 2
In these analyses, we examined the effects of vocabulary score that were significant (p < .05) after family-wise correction for multiple comparisons either across the whole brain or in our ROIs: the left and the right pSMG were centered on the peak coordinates from Lee et al. (2007) at [x =−44, y=−54, z = 46] in the left and at x = 52, y =−52, z = 44] in the right with a 10-mm radius. In addition, any region showing an effect of vocabulary that survived correction for multiple comparisons across the whole brain in either the structural or the functional analyses was used as an ROI in the other analyses. Specifically, as described in the Results section (for Functional Analysis 1), the functional lifespan analysis revealed an effect of vocabulary that was significant after whole-brain correction. We created a mask of this functional effect that included only voxels where the positive correlation between vocabulary and activation was significant at p < .01 uncorrected. This functional mask was then used as an ROI in the structural analysis with a small volume correction for multiple comparisons at p < .05.
Two further analyses were conducted to examine the effect of age in regions where there was a significant positive correlation of gray matter density and vocabulary score [i.e., the pSMG, the left posterior STS (pSTS), and the left posterior temporo-parietal cortex (pT-P)]. As these analyses required the use of multivariate statistics to make comparisons across multiple brain regions, they were conducted in the statistical package SPSS:
Structural Analysis 3: region by age group interactions on the relationship between vocabulary and gray matter. ANCOVAs were used to investigate the effect of region on the interaction between vocabulary score, gray matter density, and age group. Region was modeled as a within-participants factor, group (teenagers vs. adults) as a between-participants factor, and vocabulary score as the covariate. Children were not included in this analysis due to small participant numbers.
Structural Analysis 4: The effect of region on the relationship between age and gray matter density. This analysis was carried out to determine whether the positive correlation between vocabulary and pSMG gray matter density was less sensitive in adults than in teenagers due to more age-related intersubject variability in gray matter signal during the teenage years. If so, then the effect of age-related gray matter decline should be greater in pSMG (where no adult correlation was found) than in pSTS or pT-P (where an adult correlation was observed). To test this hypothesis, regression analyses were carried out to examine the main effect of age using raw gray matter density values (without the voxel by voxel correction for global gray matter that is implemented when proportional scaling is used) extracted from the peak coordinates of our identified ROIs. ANCOVAs were then used to compare the trajectories of these different brain regions with region modeled as a within-participants factor and age as the covariate.
RESULTS
Functional Analyses
Functional Analysis 1: Lifespan Analysis in All 47 Participants
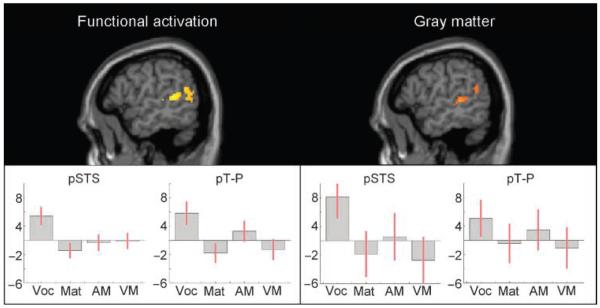
The whole-brain functional analysis across all participants identified a significant positive correlation between vocabulary score and sentence activation in the left pSTS that extended into the left pT-P. Peak coordinates and Z scores (shown in Table 2A) indicate that, in both regions, this effect was stronger for sentences (S) than for words (W) in scrambled sentences. These results are displayed in Figure 1. No significant effect of vocabulary score was identified in the left pSMG even when the threshold was lowered.
Table 2.
Functional Results for Left pSTS and Left pT-P Collapsed across Visual and Auditory Conditions
| Z Scores |
||||||||
|---|---|---|---|---|---|---|---|---|
| Anatomical Location | x | y | z | n Voxels | S and W | S | W | S–W |
| (A) Functional Lifespan Analysis | ||||||||
| Left pSTS | −44 | −36 | 2 | 231 | 4.3 | 4.1 | 3.9 | 2.8 |
| Left pT-P | −62 | −58 | 14 | 4.3 | 4.8 | 2.3 | 4.1 | |
| (B) Teenagers Only Functional Analysis | ||||||||
| Left pSTS | −46 | −36 | 4 | 105 | 2.5 | 3.6 | 3.3 | 3.3 |
| Left pT-P | −60 | −60 | 16 | 96 | 3.2 | 2.9 | 2.8 | 2.8 |
S = sentences, W = words.
Figure 1.
Analyses across lifespan. Lifespan analyses results showing the left pSTS and the left pT-P for functional and structural analyses, respectively. Graphs plot the effect of each of the following regressors upon brain activation (functional) and gray matter (structural): vocabulary (Voc), matrices (Mat), auditory memory for sentences (AM), and visual memory for sentences (VM) on each region. The y-axis shows effect sizes as the percentage increase relative to the global mean. The red bars are the 90% confidence intervals.
Activation for reading and listening was then examined to determine the functions of the identified regions. In both regions, we found main effects of sentences versus the words in scrambled sentences (Z = 5.2 in pSTS and Z = 5.7 in pT-P) and visual versus the auditory conditions (Z = 3.4 in pSTS and Z = 3.8 in pT-P). There was no interaction of sentences with visual versus auditory modality in either region. The results suggest that these areas, which show a positive correlation between functional activation and vocabulary score, are involved in sentence comprehension.
Functional Analysis 2: Teenagers Only
When the analysis was restricted to the teenage group, a similar pattern of results was observed, see Table 2B.
Structural Analyses
Structural Analysis 1: The Effect of Vocabulary on Regional Gray Matter Density across Lifespan
Using the results of the functional analysis as an ROI, we found a corresponding positive correlation between vocabulary score and gray matter density in both pSTS and pT-P (peaks at x =−48, y =−36, z = 6, Z = 4.0 and x =−50, y =−60, z = 16, Z = 3.2, respectively). Figure 1 illustrates the clear consistency between the effect of vocabulary on the functional data (significant after a whole-brain correction) and on the structural data (significant after a small volume correction limited to voxels that were significant in the functional data; for procedure, see Methods). However, contrary to expectation, we did not find an effect of vocabulary in the pSMG ROIs identified by Lee et al. (2007).
Structural Analysis 2: The Effect of Vocabulary Score on Regional Gray Matter in Teenagers Only
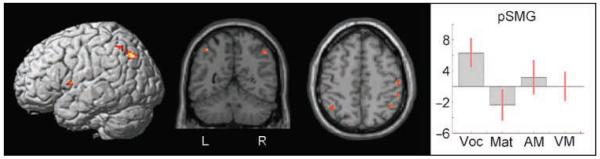
This analysis was restricted to our teenage sample to provide a more direct comparison to the study reported by Lee et al. (2007), who identified this effect in participants of this age range. The results confirmed the previously reported findings that gray matter density in teenagers was indeed significantly correlated with vocabulary score in both the left and the right pSMG (left: x = −40, y = −54, z = 52, Z = 3.6; right: x = 48, y = −54, z = 48, Z = 3.4). This result can be seen in Figure 2.
Figure 2.
Structural analysis of adolescence. Structural analysis results for the teenage group. Highlighted regions show an increase in gray matter density as a function of vocabulary score. When the statistical threshold was lowered to p < .001 uncorrected, a positive correlation with vocabulary was also observed in the left ventral precentral gyrus and the left angular gyrus. Both regions can be seen in this figure in addition to the left pSMG. The graph shows the effect of each of the four regressors on gray matter in the left pSMG labeled as follows on the x-axis: vocabulary (Voc), matrices (Mat), auditory memory for sentences (AM), and visual memory for sentences (VM). The y-axis shows effect sizes as the percentage increase relative to the global mean. The red bars are the 90% confidence intervals.
Structural Analysis 3: Region and Age Group Interactions on the Effect of Vocabulary
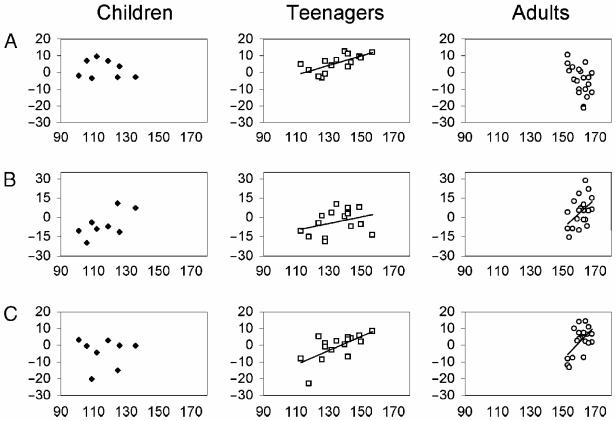
The results of Structural Analyses 1 and 2 indicate that the effect of vocabulary on gray matter differs across age. In the left temporal regions, the positive correlation with vocabulary score is continuous across all participants, whereas in the left pSMG, this relationship was observed only in the teenage years. The different trajectories of these three regions across age group are illustrated as scatter plots in Figure 3. Two ANCOVAs were carried out to compare the left pSMG with each temporal region (left pSTS and left pT-P). Both analyses confirmed a significant three-way Region × Group × Vocabulary Score interaction: (i) left pSMG versus left pSTS, F(1,32) = 11.4, p = .002, η2 = .26, and (ii) left pSMG versus left pT-P, F(1,32) = 16.0, p < .001, η2 = .334. Post hoc tests examining the Vocabulary × Group effect in each region explained these interactions by showing that the effect of vocabulary was stronger in teenagers than in adults in the left pSMG, F(1,32) = 19.1, p < .001, η2 = .37, but not in the left pT-P, F(1,32) = 2.875, p = .100, η2 = .082, or in the left pSTS, F(1,32) = 0.6, p = .46, η2 = .02.
Figure 3.
Scatter plots according to age group showing the relationship between vocabulary score (x-axis) and gray matter density (y-axis). Trajectories are plotted only where a statistically reliable relationship was observed. (A) Left pSMG [x = −40, y = −54, z = 52], where there is prominent trend for teenagers over and above all other age groups; (B) the left pSTS [x = −48, y = −36, z = 6], where the trajectory for adults appears steeper than those of children and teenagers (this positive correlation was marginally significant for children, p = .07). It should be noted that the trajectory for adults remains statistically reliable after the omission of two outliers; (C) the left pT-P [x = −50, y = −60, z = 16], where there is a consistent trend for both teenagers and adults.
In sum, the relationship between vocabulary score and gray matter density was present for teenagers but not for adults in left pSMG. By contrast, the relationships observed in left pSTS and pT-P were present for both teenagers and adults. The ability to detect relationships in the latter two regions in adults suggests that the failure to find the relationship for left pSMG did not result from a lack of vocabulary variance in the adult sample.
Structural Analysis 4: The Effect of Region on the Relationship between Age and Gray Matter Density
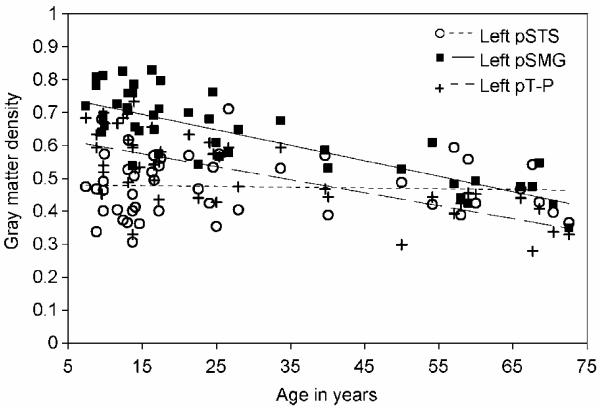
Finally, we considered whether the absence of the pSMG correlation in the adult group (as shown in Figure 3) might be explained by differences in the main effect of age on the pSMG versus the temporal regions. Figure 4 shows the main effect of age in the left pSMG, pSTS, and pT-P. These data indicate that there is a much shallower trajectory of decline in the left pSTS (β = −.051) in comparison to both the left pSMG (β = −.801) and the left pT-P region (β = −.708). The difference between the slopes of the left pSMG and the left pSTS was significant, F(1,45) = 30.460, p < .001, η2 = .404, consistent with previous reports that the rate of gray matter decline with age is steeper in parietal than in temporal regions. There was no significant difference between the slopes of the pSMG and the pT-P region, F(1,45) = 2.062, p = .158, η2 = .304. This is interesting given that our previous analysis (Structural Analysis 3) identified an effect of vocabulary that was dependent upon age group in the left pSMG relative to the left pT-P. This current result indicates that the disappearance of the adult correlation between vocabulary score and gray matter in the pSMG relative to the left pT-P cannot be explained by a lack of sensitivity to gray matter increases when there is a steep gray matter decline with age.
Figure 4.
Change in gray matter density over age. Scatter plot comparing change in gray matter density over age in the left pSTS, left pT-P, and left pSMG.
DISCUSSION
In this study, we investigated the brain regions where local gray matter varies with vocabulary knowledge. We hypothesized that these effects would be age dependent on the basis that strategies for acquiring vocabulary differ across lifespan. Vocabulary knowledge is generally acquired within the context of everyday experience of language through conversation and reading, although new words can also be learnt by forming an explicit link between a new word and an equivalent or a definition, a mode of learning taking place predominantly by instruction. Our specific predictions were that brain regions involved in learning vocabulary within the context of everyday language would show a positive correlation between vocabulary score and gray matter density, which would be continuous across lifespan, and that these regions would also be activated by reading and listening tasks. By contrast, we anticipated that for brain regions engaged in learning vocabulary by lexical or conceptual equivalents, the relationship between local brain structure and vocabulary knowledge would be discontinuous across lifespan and potentially restricted to age groups of participants engaged in formal education. Furthermore, these regions were not necessarily expected to be activated during reading and listening tasks for participants at any age. To test these hypotheses, we explored how the effect of vocabulary on gray matter interacted with age, and whether and how the affected regions were activated during auditory and written sentence processing.
The results of our structural and functional imaging analyses identified three different regions where vocabulary knowledge correlated with gray matter density. The first was the pSMG, consistent with the findings of Lee et al. (2007) and replicated with a different vocabulary measure and different participants. The second region was located deep in the left pSTS, and the third was in the left pT-P. The pSTS and the pT-P regions were not reported by Lee et al. (2007) and were only identified in the current study when we used ROIs, where vocabulary correlated with functional activation during written and auditory sentence processing.
Having identified the brain regions associated with vocabulary knowledge, we established regional differences in the effect due to age. In the pSMG, the relationship between vocabulary and gray matter was limited to the teenage years. By contrast, the positive correlations in both temporal regions were consistent across lifespan. This provides preliminary evidence that the effect of vocabulary in the pSMG may reflect learning through explicit instruction of lexical equivalents that occurs during formal education, whereas the effect of vocabulary in the left temporal regions may reflect the continuous acquisition of vocabulary that occurs from learning in context. The analysis of the functional activation data offered further evidence for this hypothesis because the temporal regions (pSTS and pT-P) were maximally activated by sentence processing, but in the pSMG there was no activation for either sentences or the words in scrambled sentences, irrespective of whether they were presented in the auditory or visual modality.
Our functional imaging results are consistent with previous studies of language comprehension carried out in both children and adults (Lindenberg & Scheef, 2007; Humphries, Binder, Medler, & Liebenthal, 2006; Spitsyna, Warren, Scott, Turkheimer, & Wise, 2006; Constable et al., 2004; Turkeltaub, Gareau, Flowers, Zeffiro, & Eden, 2003; Booth et al., 2002). Specifically, previous studies have shown that the left pT-P area is linked to syntactic and semantic processing (Mechelli, Josephs, Lambon Ralph, McClelland, & Price, 2007; Thierry, Giraud, & Price, 2003; Vandenberghe, Nobre, & Price, 2002), and the pSTS interfaces between semantic associations and speech production (see Binder et al., 1997, 2000, 2003;Hickok&Poeppel, 2004, 2007;Okada & Hickok, 2006; see also Humphries, Binder, Medler, & Liebenthal, 2007; Humphries et al., 2006; Scott, Leff & Wise, 2003; Price et al., 1997; Vandenberghe, Price, Wise, Josephs, & Frankowiak, 1996). Thus, the increased functional activation and gray matter density in the temporal regions for those with high vocabulary may reflect a larger number of associations between semantic processing and speech output (pSTS) and semantic processing and syntactic processing (pT-P). The stronger effect of vocabulary knowledge at the sentence level in these temporal areas may also indicate that our vocabulary measure is partially indexing additional sentence-level comprehension processes that are highly correlated with vocabulary score. Although it is difficult to separate the relative contribution of such highly correlated variables, future analyses using multiple behavioral measures may be able to determine the relative influence of a range of linguistic abilities on activation intensity and local brain structure. In addition, functional imaging studies that manipulate the contextual learning of new words (such as Mestres-Missé, Càmara, Rodriguez-Fornells, Rotte, & Münte, 2008) will also help to derive the role of temporal areas in integrating new lexical items into an existing language system.
The contrasting effects in the temporal and pSMG regions suggest that the respective correlations of vocabulary and gray matter density are influenced by different mechanisms. In the left pSMG, gray matter was only correlated with vocabulary in the teenage years, and this area was not activated during auditory or written sentence or word processing. However, this region has been found to be active during word learning tasks in which participants were taught the meaning of new words (Cornelissen et al., 2004). This finding and the positive correlation between vocabulary and gray matter density in the pSMG is therefore consistent with a vocabulary learning strategy that occurs predominantly in formal education; that is, the explicit instruction of lexical or conceptual equivalents for new words. After the teenage years, the relationship between vocabulary and gray matter density may be lost because vocabulary continues to increase by contextual learning, without using the pSMG. Nevertheless, greater gray matter in the pSMG in bilingual adults identified by Mechelli et al. (2004) indicates that increases in gray matter in this region can occur outside adolescence. Therefore, differences in gray matter in the pSMG in this instance may reflect the acquisition and the maintenance of two languages in the case of bilingualism or second language acquisition. Alternatively, increased pSMG gray matter in monolingual teenagers and bilingual adults may represent an active learning mechanism that facilitates language learning in a context of increased task demands—for instance, learning more complex and abstract vocabulary in secondary education or maintaining vocabulary knowledge across multiple languages.
Our conclusion that different vocabulary learning strategies are supported by left temporal and parietal neural systems rests on the exclusion of several potentially confounding factors. Specifically, the effect of vocabulary in the left pSMG might be greater in teenage years because of increased sensitivity in the correlation by virtue of (a) the wide range of vocabulary abilities in this age range and/or (b) the reduced error variance because the effect of age, or other unrelated factors, is smaller in the teenagers than the adult sample. In other words, even if we had been able to match the range of vocabulary in the teenage and adult groups, there would be an age-range confound because the age range is smaller in the teenage group (12–17 years) than in the adult group (21–73 years). The importance of matching age range is illustrated by the well-known effect of age on gray matter, which is characterized in terms of a preadolescent increase followed by a postadolescent decrease (Paus, 2005; Gogtay et al., 2004; Giedd et al., 1999). The rate of gray matter loss with age differs from region to region (as illustrated using our own data in Figure 4). The relevant point here is that, in the lifespan analysis, it will be more difficult to detect a small vocabulary-related increase in gray matter in the context of a steeper age-related decline in gray matter.
Fortunately, we were able to discount the influence of both vocabulary range and age range on the validity of our conclusions because we observed significantly different patterns of effect in three different brain regions. For instance, if the greater effect of vocabulary in teenage years was due to greater sensitivity from either a wider vocabulary range relative to adults or a narrower range of age-related gray matter decline relative to adults, then positive correlations with vocabulary should be stronger during the teenage years for all three regions. Instead, the increased effect of vocabulary in teenage years was only observed in pSMG, not in pSTS and pT-P. In addition, we were able to show that the trajectory of gray matter decline in the pSMG and pT-P was similar, although only pSMG demonstrated a differential relationship with vocabulary with age.
Finally, there are two remaining questions to consider. Firstly, why was no correlation between vocabulary score and gray matter density detected for young children? And secondly, why does pSMG gray matter correlate with second language proficiency in young adults (20–30 years)? In consideration of the first question, the sample of young children within our study was rather small (n = 9). Although these participants made a valuable contribution to our lifespan analysis, this sample size may not be sufficient as a group to detect an effect. However, a marginal trend was observed for pSTS, suggesting that there was some sensitivity to detect differences in this age group. This would require an additional explanation of why no similar trend was observed for pSMG given that these children were in formal education. Were this interaction to prove reliable in a larger sample, one could speculate that although vocabulary acquisition in the early years undoubtedly involves definitional learning, it may take place within the bounds of the gray matter already available to support this function in younger children. Regarding the role of bilingualism, learning a second language requires an individual to acquire words and link these with the vocabulary of their first language and with semantic knowledge. This process requires that links be made between lexical equivalents (Kroll & Potter, 1984), a process that will increase the demands on pSMG gray matter.
In conclusion, our study has identified contrasting effects of vocabulary knowledge on brain structure. We propose that the continuous positive correlation across lifespan observed in the left pSTS and the left pT-P reflects vocabulary learning within the context of everyday language experience with semantically and syntactically related words and sentences. Vocabulary knowledge is increased in this way through continued experience of language across lifespan, whereas the left pSMG is engaged in learning to link a new word with specific lexical equivalents, a mode of learning more typically exploited within formal education. In addition, the convergence of functional and structural correlations in the left temporal regions suggests that increased gray matter might be a consequence of increased neuronal activation or vice versa. Longitudinal studies are now required to ascertain how vocabulary learning changes over time within the same individuals at different points in the lifespan. This may indicate whether the effect of vocabulary correlates with learning rate and activation or to a genetic disposition to high gray matter in regions that enable vocabulary learning, that is, an inherited ability to acquire more knowledge.
Acknowledgments
This research was funded by the Wellcome Trust. Fiona Richardson was supported by an MRC Career Establishment Grant G0300188 and British Academy Grant SG-40400 awarded to Dr. Michael Thomas. Our thanks to Janice Glensman, Amanda Brennan, and David Bradbury for their assistance during scanning and neuropsychological testing.
APPENDIX
Sentence stimuli used during functional scanning consisted of the following grammatical constructions: actives, passives, subject-cleft, object-cleft, locatives, and datives. Examples of these sentences are shown below.
| Sentence Stimuli | |
|---|---|
| Sentence Type | Examples |
| Actives | The old dog bites the fox |
| The vase smashes the heavy clock | |
| The slim lady drinks the water | |
| The child chooses the yellow sweet | |
| Passives | The cow is chased by the fat horse |
| The movie is filmed by the famous actor | |
| The leopard is raced by the young lion | |
| The wise judge is delayed by the traffic | |
| Subject-cleft | It is the dancer that hugs the clown |
| It is the cook that loves the woman | |
| Object-cleft | It is the pig that the boy pushes |
| It is the bull that the donkey kicks | |
| Locatives | The red pencil is on the paper |
| The large cup is in the box | |
| Datives | Hold the party on the jungle island |
| Give the letter to the tall stranger | |
REFERENCES
- Allen JS, Bruss J, Kice Brown C, Damasio H. Normal neuroanatomical variation due to age: The major lobes and a parcellation of the temporal region. Neurobiology of Aging. 2005;26:1245–1260. doi: 10.1016/j.neurobiolaging.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke PSF, Bellgowan JA, Springer JA, Kaufman JN, et al. Human temporal lobe activation by speech and nonspeech sounds. Cerebral Cortex. 2000;10:512–518. doi: 10.1093/cercor/10.5.512. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Cox RW, Rao SM, Prieto T. Human brain language areas identified by functional magnetic resonance imaging. Journal of Neuroscience. 1997;17:353–362. doi: 10.1523/JNEUROSCI.17-01-00353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, McKieman KA, Parsons M, Westbury CF, Possing ET, Kaufman JN, et al. Neural correlates of lexical access during visual word recognition. Journal of Cognitive Neuroscience. 2003;15:372–393. doi: 10.1162/089892903321593108. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TB, Mesulam MM. Modality independence of word comprehension. Human Brain Mapping. 2002;16:251–261. doi: 10.1002/hbm.10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambati SM, Termin C, Ruffino M, Danna M, Lanzi G, Stella G, et al. Neuropsychological deficits and neural dysfunction in familial dyslexia. Brain Research. 2006;1113:174–185. doi: 10.1016/j.brainres.2006.06.099. [DOI] [PubMed] [Google Scholar]
- Brambati SM, Termin C, Ruffino M, Stella G, Fazio F, Cappa SF, et al. Regional reductions of gray matter volume in familial dyslexia. Neurology. 2004;63:742–745. doi: 10.1212/01.wnl.0000134673.95020.ee. [DOI] [PubMed] [Google Scholar]
- Chételat G, Desgranges B, Landeau B, Mézenge F, Poline JB, de la Sayette V, et al. Direct voxel-based comparison between grey matter metabolism and atrophy in Alzheimer's disease. Brain. 2008;131:60–71. doi: 10.1093/brain/awm288. [DOI] [PubMed] [Google Scholar]
- Constable RT, Pugh KR, Berroya E, Mencl WE, Westerveld M, Ni W, et al. Sentence complexity and input modality effects in sentence comprehension: An fMRI study. Neuroimage. 2004;22:11–21. doi: 10.1016/j.neuroimage.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Cornelissen K, Laine M, Renvall K, Saarinen T, Martin N, Salmelin R. Learning new names for new objects: Cortical effects as measured by magnetoencephalography. Brain and Language. 2004;89:617–622. doi: 10.1016/j.bandl.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Deichmann R, Schwarzbauer C, Turner R. Optimisation of the 3D MDEFT sequence for anatomical brain imaging: Technical implications at 1.5 and 3T. Neuroimage. 2004;21:757–767. doi: 10.1016/j.neuroimage.2003.09.062. [DOI] [PubMed] [Google Scholar]
- Demonet J-F, Chollet F, Ramsay S, Cardebat D, Nespoulos J-L, Wise R, et al. Anatomy of physiological and semantic processing in normal subjects. Brain. 1992;115:1753–1768. doi: 10.1093/brain/115.6.1753. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Matthews PM, Rushworth MFS. Semantic processing in the left inferior prefrontal cortex: A combined functional magnetic resonance imaging and transcranial magnetic stimulation study. Journal of Cognitive Neuroscience. 2003;15:71–84. doi: 10.1162/089892903321107837. [DOI] [PubMed] [Google Scholar]
- Dunn LM, Dunn LM, Whetton C, Burley J. The British Picture Vocabulary Scale. 2nd ed. NFER-Nelson; Windsor: 1997. [Google Scholar]
- Elliot CD, Smith P, McCulloch K. British Ability Scales Second Edition (BAS II) NFER-Nelson; London: 1997. [Google Scholar]
- Friston KJ, Ashburner J, Frith CD, Poline JB, Heather JD, Frankowiak RSJ. Spatial registration and normalization of images. Human Brain Mapping. 1995;2:1–25. [Google Scholar]
- Friston KJ, Holmes A, Worsley KJ, Poline JB, Frith CD, Frankowiak RSJ. Statistical parametric maps in functional imaging: A general linear approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Gathercole SE. Nonword repetition and word learning: The nature of the relationship. Applied Psycholinguistics. 2006;27:513–543. [Google Scholar]
- Gathercole SE, Hitch GJ, Service E, Martin AJ. Phonological short-term memory and vocabulary development: Further evidence from the nature of the relationship. Applied Cognitive Psychology. 1997;13:65–77. [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenboss A, et al. Brain development during childhood and adolescence: A longitudinal MRI study. Nature Neuroscience. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi K, Greenstein D, Vaituzis C, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences, U.S.A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golestani N, Pallier C. Anatomical correlates of foreign speech sound production. Cerebral Cortex. 2007;17:929–934. doi: 10.1093/cercor/bhl003. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Dorsal and ventral streams: A framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92:67–99. doi: 10.1016/j.cognition.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nature Reviews Neuroscience. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Hoeft F, Ueno T, Reiss AL, Meyler A, Whitfield-Gabrielli S, Glover GH, et al. Prediction of children's reading skills using behavioural, functional, and structural neuroimaging measures. Behavioural Neuroscience. 2007;121:602–613. doi: 10.1037/0735-7044.121.3.602. [DOI] [PubMed] [Google Scholar]
- Humphries C, Binder JR, Medler DA, Liebenthal E. Syntactic and semantic modulation of neural activity in auditory sentence comprehension. Journal of Cognitive Neuroscience. 2006;18:665–679. doi: 10.1162/jocn.2006.18.4.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries C, Binder JR, Medler DA, Liebenthal E. Time course of semantic processes during sentence comprehension: An fMRI study. Neuroimage. 2007;36:924–932. doi: 10.1016/j.neuroimage.2007.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde KL, Lerch JP, Griffiths TD, Evans AC, Peretz I. Cortical thickness in congenital amusia: When less is better than more. Journal of Neuroscience. 2007;27:13028–13032. doi: 10.1523/JNEUROSCI.3039-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde KL, Zatorre RJ, Griffiths TD, Lerch JP, Peretz I. Morphometry of the amusic brain: A two-site study. Brain. 2006;129:2562–2570. doi: 10.1093/brain/awl204. [DOI] [PubMed] [Google Scholar]
- Jesper LR, Andersson JLR, Hutton C, Ashburner J, Turner R, Friston KJ. Modelling geometric deformations in EPI time series. Neuroimage. 2001;13:903–919. doi: 10.1006/nimg.2001.0746. [DOI] [PubMed] [Google Scholar]
- Kilian AS, Nagy W, Pearson PD, Anderson RC, García GE. Learning vocabulary from context: Effects of focusing attention on individual words during reading (Tech. Rep. No. 619) Center for the Study of Reading; 1995. [Google Scholar]
- Kroll JF, Potter MC. Recognizing words, pictures, and concepts: A comparison of lexical, object, and reality decisions. Journal of Verbal Learning and Behavior. 1984;23:39–66. [Google Scholar]
- Lee HL, Devlin JT, Shakeshaft C, Stewart LH, Brennan A, Glensman J, et al. Anatomical traces of vocabulary acquisition in the adolescent brain. Journal of Neuroscience. 2007;27:1184–1189. doi: 10.1523/JNEUROSCI.4442-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. Neuroscience and Biobehavioral Reviews. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Lindenberg R, Scheef L. Supramodal language comprehension: Role of the left temporal lobe for listening and reading. Neuropsychologia. 2007;45:2407–2415. doi: 10.1016/j.neuropsychologia.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Crinion JT, Noppeney U, O'Doherty J, Ashburner J, Frackowiak RSJ, et al. Neurolinguistics: Structural plasticity in the bilingual brain. Nature. 2004;431:757. doi: 10.1038/431757a. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Josephs O, Lambon Ralph MA, McClelland JA, Price CJ. Dissociating stimulus-driven semantic and phonological effects during reading and naming. Human Brain Mapping. 2007;3:205–217. doi: 10.1002/hbm.20272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechelli A, Price CJ, Friston K, Ashburner J. Voxel-based morphometry of the human brain: Methods and applications. Current Medical Imaging Reviews. 2005;1:105–113. [Google Scholar]
- Mestres-Missé A, Càmara E, Rodriguez-Fornells A, Rotte M, Münte TE. Functional neuroanatomy of meaning acquisition from context. Journal of Cognitive Neuroscience. 2008;20:2153–2166. doi: 10.1162/jocn.2008.20150. [DOI] [PubMed] [Google Scholar]
- Nagy W, Herman PA, Anderson RC. Learning words from context. Reading Research Quarterly. 1985;20:233–253. [Google Scholar]
- Okada K, Hickok G. Identification of lexical-phonological networks in the superior temporal sulcus using fMRI. NeuroReport. 2006;17:1293–1296. doi: 10.1097/01.wnr.0000233091.82536.b2. [DOI] [PubMed] [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends in Cognitive Sciences. 2005;9:60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Price CJ, More CJ, Humphreys GW, Wise RJ. Segregating semantic from phonological processes during reading. Journal of Cognitive Neuroscience. 1997;9:3876–3883. doi: 10.1162/jocn.1997.9.6.727. [DOI] [PubMed] [Google Scholar]
- Scott SK, Leff A, Wise RJS. Going beyond the information given: A neural system supporting semantic interpretation. Neuroimage. 2003;19:870–876. doi: 10.1016/s1053-8119(03)00083-1. [DOI] [PubMed] [Google Scholar]
- Silani G, Frith U, Demonet JF, Fazio F, Perani D, Price C, et al. Brain abnormalities underlying altered activation in dyslexia: A voxel-based morphometry study. Brain. 2005;128:2453–2461. doi: 10.1093/brain/awh579. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across human life span. Nature Neuroscience. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Spitsyna G, Warren JE, Scott SK, Turkheimer FE, Wise RJS. Converging language streams in the human temporal lobe. Journal of Neuroscience. 2006;26:7328–7336. doi: 10.1523/JNEUROSCI.0559-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry G, Giraud AL, Price C. Hemispheric dissociation in access to the human semantic system. Neuron. 2003;38:499–506. doi: 10.1016/s0896-6273(03)00199-5. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Gareau L, Flowers LD, Zeffiro TA, Eden GF. Development of neural mechanisms for reading. Nature Neuroscience. 2003;6:767–773. doi: 10.1038/nn1065. [DOI] [PubMed] [Google Scholar]
- Vandenberghe R, Nobre AC, Price CJ. The response of the left temporal cortex to sentences. Journal of Cognitive Neuroscience. 2002;14:550–560. doi: 10.1162/08989290260045800. [DOI] [PubMed] [Google Scholar]
- Vandenberghe R, Price C, Wise R, Josephs O, Frankowiak RSJ. Functional anatomy of a common semantic system for words and pictures. Nature. 1996;383:254–256. doi: 10.1038/383254a0. [DOI] [PubMed] [Google Scholar]
- Wilke M, Krägeloh-Mann I, Holland SK. Global and local development of gray and white matter volume in normal children and adolescents. Experimental Brain Research. 2007;178:296–307. doi: 10.1007/s00221-006-0732-z. [DOI] [PMC free article] [PubMed] [Google Scholar]