Abstract
Background
A 55-year-old woman with a 5-year history of osteoporosis treated for 4 years with an oral aminobisphosphonate presented with a recent vertebral fracture. A bone biopsy specimen revealed giant osteoclasts with more than 40 nuclear profiles.
Investigations
Bone mineral density determinations, measurement of serum 25-hydroxyvitamin D, intact parathyroid hormone, calcium, inorganic phosphorus, alkaline phosphatase and creatinine levels, urinary excretion levels of the N-telopeptide of type 1 collagen, and bone biopsy. Examination of the patient and review of the bone specimen.
Diagnosis
Giant osteoclasts after long-term bisphosphonate use, without evidence of malignancy.
Management
Interpretation of the bone biopsy specimen listed several bone disorders. The bone specimen was reviewed and the histological differential diagnosis was carefully considered. The giant osteoclasts were detached from bone and frequently apoptotic in a normal marrow stroma, with low-to-normal amounts of osteoid and osteoblasts. These features are typical of giant osteoclast formation after long-term aminobisphosphonate therapy. The patient was reassured that the bone findings were unlikely to be detrimental. Aminobisphosphonate treatment was reinstituted, and 1 year later the patient was asymptomatic.
Medscape® Continuing Medical Education online.
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education (CME) through the joint sponsorship of Medscape, LLC and Nature Publishing Group.
Medscape, LLC is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education for physicians.
Medscape, LLC designates this educational activity for a maximum of 0.5 AMA PRA Category 1 Credits™. Physicians should only claim credit commensurate with the extent of their participation in the activity. All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test and/or complete the evaluation at http://cme.medscape.com/public/naturereviews; and (4) view/print certificate.
Learning objectives
Upon completion of this activity, participants should be able to:
Describe the uses of bisphosphonates.
Identify potential causes of giant osteoclasts observed on bone biopsy.
Describe the likely effect of bisphosphonates on osteoclasts.
Describe distinguishing features of conditions causing giant, multinucleated osteoclasts in bone disorders.
Describe the clinical features of Paget’s disease of the bone.
The case
A 55-year-old white woman with a 5-year history of osteoporosis, treated for 4 years with an oral aminobisphosphonate, presented to a tertiary institution following a vertebral fracture and bone biopsy report of giant osteoclasts of unknown etiology. The patient was otherwise healthy with a normal BMI, and had menopausal symptoms 7 years earlier. She drank one to two cups of milk daily and ate cheese or yogurt two to three times per week. Smoking, more than one cup of coffee per day and previous fractures were denied. Her mother had experienced a hip fracture at age 70 years, and a maternal uncle developed a painful, bowed femur when he was 75 years old. Examination of the patient’s mouth revealed good dental hygiene without exposed bone. Skeletal percussion and compression elicited no discomfort. Deformity of the long bones was absent.
A bone mineral density (BMD) scan conducted 5 years previously showed T scores of −2.9 at the lumbar spine and −2.5 at the femoral neck. Laboratory data revealed normal values for 25-hydroxyvitamin D, intact parathyroid hormone, serum calcium, inorganic phosphorus and creatinine (Table 1). Urinary excretion of the N-telopeptide of type I collagen was elevated to 114 nM bone collagen equivalents (BCE) per nM creatinine (normal range 11–91 BCE/nM creatinine). A diagnosis of osteoporosis was made, and 70 mg of oral alendronate was administered once weekly. Supplemental calcium and vitamin D were also prescribed. After 3 months of treatment, the N-telopeptide excretion decreased to 30 nM BCE per nM creatinine, and 1 year later the patient gained BMD at both the spine (T-score −2.0) and the femoral neck (T-score −2.2). After 4 years of treatment, BMD values were within 1.5 SD of the normal young adult mean values (T-score −1.5), and alendronate treatment was discontinued.
Table 1.
Results of laboratory studies in a patient with osteoporosis who underwent 4 years of treatment with alendronate
| Laboratory test | Before alendronate therapy |
3 months after starting alendronate therapy |
4 years after starting alendronate therapy |
1 year after discontinuation of alendronate therapy |
Normal range |
|---|---|---|---|---|---|
| Lumbar spine BMD (T-score) |
−2.9 | ND | −1.5 | ND | ±2.0 |
| Femoral neck BMD (T-score) |
−2.5 | ND | −1.5 | ND | ±2.0 |
| Serum 25-hydroxyvitamin D (ng/ml) |
40 | ND | 38 | ND | 30–80 |
| Serum calcium (mg/dl) |
9.6 | ND | 9.3 | 9.6 | 8.8–10.2 |
| Serum intact PTH (pg/ml) |
32.8 | ND | 32.0 | 40.0 | 12.0–88.0 |
| Serum inorganic phosphorus (mg/dl) |
4.0 | ND | 4.1 | 3.8 | 2.5–4.5 |
| Serum creatinine (mg/dl) |
0.8 | ND | 0.7 | 0.8 | 0.6–1.1 |
| Serum alkaline phosphatase activity (IU/l) |
88 | 65 | 70 | 161 | 40–120 |
| Urinary N-telopeptide excretion (nM BCE per nM creatinine) |
114 | 30 | ND | 125 | 11–91 |
Abbreviations BCE, bone collagen equivalents; BMD, bone mineral density; ND, not determined; PTH, parathyroid hormone.
One year later, while bending forward to pick up her grandchild, the patient suffered a painful vertebral fracture. When measured 3 weeks after the fracture, urinary N-telopeptide excretion and serum alkaline phosphatase levels were elevated (125 nM BCE per nM creatinine and 161 IU/l, respectively) (Table 1). The family history of a bowed femur and the elevated biochemical markers of bone metabolism raised concern about a pathological fracture, and a bone biopsy was performed. The pathology report described giant osteoclasts of uncertain etiology with up to 40 nuclear profiles (two-dimensional images observed on a 5 μm-thick section taken through three-dimensional objects; Figure 1). It is important to appreciate that the number of nuclear profiles that can be enumerated from these thin sections is only a low estimate of the total number of nuclei present in osteoclasts, which measure from 80 μm to over 100 μm thick. There was no evidence of malignant or inflammatory cells.
Figure 1.

A photomicrograph of giant, detached, hypernucleated osteoclasts after long-term oral aminobisphosphonate therapy. Modified Masson stain, original magnification × 400.
Paget disease of bone, hyperparathyroidism, giant cell tumor and fibrous dysplasia were considered in the differential diagnosis. A review of the tissue specimen showed that excess osteoid was absent, osteoblast number was low-to-normal, and the marrow cavity had normal fat, blood vessels and hematopoietic cells. In addition, about one-third of the osteoclasts were apoptotic, as indicated by discretely condensed chromatin, nuclear fragmentation, nuclear peripheral beading and cell shrinkage. The biopsy findings were attributed to long-term treatment with alendronate, and were thought to have had no deleterious effects on the patient. Aminobisphosphonate treatment was reinstituted and, over the following 6–8 weeks, the elevated urinary N-telopeptide excretion and serum alkaline phosphatase levels returned to normal. When seen 1 year later, the patient was asymptomatic.
Discussion of diagnosis
Bisphosphonates are well established therapeutic inhibitors of bone resorption that have been used to treat Paget disease of bone, hypercalcemia of malignancy and, more extensively, to prevent osteoporotic fractures in postmenopausal women, men and patients with glucocorticoid-induced osteoporosis.1-6 The clinical efficacy of aminobisphosphonates is thought to result from their potent ability to reduce osteoclast numbers.7,8 However, the skeletal benefits of aminobisphosphonate treatment might not result from a reduction in the number of osteoclasts, as is currently thought.9
McClung et al.3 examined bone biopsy specimens obtained from postmenopausal women after a 3-year, double-blind, randomized, placebo-controlled, dose-ranging trial of oral alendronate to prevent bone resorption. In that trial, alendronate was shown to increase BMD from baseline at the lumbar spine, femoral neck, trochanter and total body, and decrease biochemical markers of bone turnover, as expected. Unexpectedly, a recent re-examination of those bone biopsy specimens showed that the number of osteoclasts was significantly higher in patients receiving 10 mg per day of alendronate for 3 years than in the placebo group, and the number of osteoclasts increased with the cumulative dose of the drug.10 More than one-quarter of these osteoclasts were giant cells with pyknotic nuclear morphology that were located adjacent to superficial resorption cavities. These giant cells had distorted or absent ruffled borders. Furthermore, giant, hypernucleated, detached osteoclasts with more than 40 nuclear profiles were found even after alendronate had been discontinued for 1 year. Over 30% of the giant cells were apoptotic, both by their morphological features and positive in situ end-labeling. Detection of human osteoclast apoptosis was noteworthy, because once osteoclasts become apoptotic, ingestion by marrow phagocytes normally occurs so quickly that apoptotic osteoclasts are rarely seen in human bone.
Long-term treatment with alendronate decreases osteoclast resorptive ability rather than number, probably by disrupting the cell’s ruffled border. Although these osteoclasts are no longer able to resorb bone, they continue to accumulate nuclei by fusion with their mononuclear macrophage precursors, and have a prolonged lifespan because they escape the death signal that normally results from the local high-calcium bath produced during bone resorption.10 Thereby, aminobisphosphonate treatment gives rise to a previously unrecognized cell phenotype: an apoptotic, excessively nucleated, colossal osteoclast, which is resistant to phagocytosis. Although these cells might be dysfunctional, and were of no direct danger to the patient described in this case, awareness of the condition is crucial because these giant osteoclasts could otherwise lead to unnecessary tests and referrals.
Differential diagnosis
The bone biopsy in this patient was obtained because the elevated biochemical markers of bone metabolism and the history of bowed leg in an elderly uncle suggested a pathological fracture. More likely, however, is that the elevated markers reflected the recent fracture and that the patient’s uncle had Paget disease of bone. Possible explanations for finding giant, hypernucleated osteoclasts in this patient include long-term aminobisphosphonate use, Paget disease, secondary hyperparathyroidism (as seen with renal insufficiency), giant cell tumor or fibrous dysplasia.11 Differentiation of these clinical conditions can be challenging, but is usually made based on the clinical history, radiography and characteristic histology (Table 2). However, in many cases, bone biopsy is not necessary to obtain the correct diagnosis.
Table 2.
Characteristic features of giant, multinucleated osteoclasts in bone disorders
| Condition | Detached osteoclasts | Osteoid | Osteoblasts | Abnormal marrow | Other features |
|---|---|---|---|---|---|
| Long-term therapy with aminobisphosphonates |
Increased; approximately 30% apoptotic |
Low-to-normal | Low-to-normal | No | Low-to-normal bone turnover |
| Hyperparathyroidism | No, but rare osteoclast apoptosis might be noted |
Increased | Increased | Peritrabecular fibrosis | Prominent, deep resorption cavities |
| Paget disease | Often, but no apoptosis unless treated with an aminobisphosphonate |
Increased | Increased | Fibrosis and increased blood vessels |
Mosaic architecture, woven bone |
| Giant cell tumor | Yes, but no apoptosis | Present in about one-third of cases |
Rare | Plump or ovoid-shaped cells interspersed with giant cells |
Cyst formation, hemorrhages, mitotic figures |
| Fibrous dysplasia | Yes, but no apoptosis unless treated with an aminobisphosphonate |
Might be excessive |
Retracted from the bone perimeter |
Long spindle-shaped fibroblasts and loose reticulin fibers |
Lesions resembling chinese characters, large osteocytic lacunae, woven bone |
Long-term treatment with alendronate
Diagnostic features found in a bone specimen exhibiting alendronate-induced giant osteoclasts include mononuclear cells interspersed between the detached osteoclasts and the bone surface (proof that the detachment is not due to tissue shrinkage during histological preparation), decreased or low-to-normal amounts of osteoid, low-to-normal numbers of osteoblasts and normal hematopoietic bone marrow (Figure 1). Giant osteoclasts are present in more than 50% of patients receiving long-term therapy with routinely used clinical doses of alendronate.10 In addition, as many as 30% of the osteoclasts could be apoptotic. Giant, detached and apoptotic osteoclasts can be found even after alendronate has been discontinued for 1 year. Giant osteoclasts have also been observed after oral and intravenous treatment with other aminobisphosphonates, including pamidronate and risedronate.12-15 These osteoclasts could have at least 40 nuclear profiles, compared with 2–8 profiles found in normal osteoclasts.
Paget disease
In western countries, Paget disease of bone is the second most common metabolic bone disorder after osteoporosis. Rarely diagnosed before the age of 50 years, the prevalence of Paget disease increases with advancing age.16 the disease can be monostotic or polyostotic, but is always focal with most bones remaining normal. Paget disease is characterized by localized, autonomous increases in bone resorption by osteoclasts followed by augmented and disorganized bone remodeling by osteoblasts.15 The normally sharp junction between cortical and cancellous bone becomes indistinct owing to expansion of the cancellous bone and increased resorption cavities in the cortical bone. The expanded bone is composed of a mosaic of small bone packets, some of which have woven collagen architecture whereas others are of lamellar construction. Osteoclasts are increased in number and size, with as many as 20–100 nuclear profiles (Figure 2). These giant, multinucleated osteoclasts were once considered to be pathognomonic for Paget disease, but evidence now suggests that this might no longer be true.10 They may be firmly attached to resorption cavities or completely detached from bone, and are accompanied by abundant osteoid and osteoblasts with a highly vascularized, fibrous marrow stroma. Osteoclast apoptosis occurs only after treatment with aminobisphosphonates.
Figure 2.

A photomicrograph showing more than 40 nuclear profiles in a giant detached osteoclast, a finding that was previously considered virtually pathognomonic of Paget disease of bone. Only when combined with the excess osteoid (stained red) and abundant osteoblasts (arrow), however, do the findings suggest Paget disease. Modified Masson stain, original magnification × 400.
Secondary hyperparathyroidism
Secondary hyperparathyroidism with accelerated bone formation is the most common form of renal osteodystrophy.17 Cortical bone shows reduced width, increased porosity and prominent intracortical osteoid, and intraosseous erosions. Cancellous bone is frequently augmented with wide and closely connected trabeculae. Often, the cortical–cancellous junction is indistinct. Subperiosteal cortical erosions containing osteoclasts, osteoid, and a loose fibrous stroma might be noted. In hyperparathyroidism, the osteoclasts are firmly attached to the bone and usually contain no more than 9–20 nuclear profiles (Figure 3). Osteoclast apoptosis is rare.
Figure 3.

A photomicrograph of secondary hyperparathyroidism resulting from renal insufficiency with a deep resorption cavity containing multinucleated osteoclasts. The osteoclasts are firmly attached to the bone and usually contain no more than 9–20 nuclear profiles. The bone marrow shows peritrabecular fibrous tissue. Modified Masson stain, original magnification × 400.
Giant cell tumor
Giant cell tumor of bone is a locally aggressive but benign tumor that afflicts young adults between the ages of 20 and 40 years. Most tumors occur in the long bones, often near joints, and are thought to arise from mesenchymal cells.11 These cells differentiate into both fibroblast-like stromal cells and multinucleated cells resembling osteoclasts. The basic microscopic pattern of a giant cell tumor is that of a moderately vascularized stroma associated with plump spindle-shaped or ovoid-shaped cells interspersed with detached giant cells with up to 50–100 nuclear profiles (Figure 4). Osteoclast apoptosis is absent.
Figure 4.
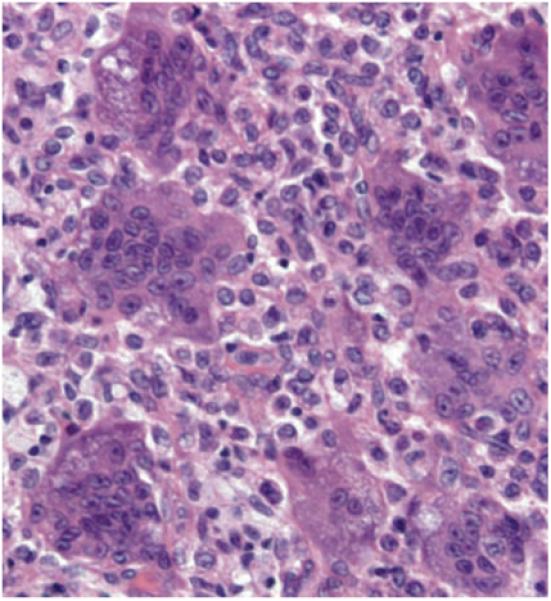
Multinucleated giant cells in a giant cell tumor of bone. The giant cells are in close contact with fibroblastic stromal cells, but are often detached from bone. Hematoxylin and eosin stain, original magnification × 400.
Fibrous dysplasia
Fibrous dysplasia results from an activating mutation of the gene encoding Gsα in bone that causes overactive cyclic AMP generation and proliferation of osteoblast progenitors in the bone marrow stroma, leading to increased interleuken-6 synthesis, high levels of c-fos proto-oncogene expression and increased numbers of osteoclasts. Skeletal lesions are usually diagnosed during childhood and adolescence. At the advancing margins of the lesions, the abnormally stimulated osteoclasts cause cortical expansion, pain and fractures. In the central areas of the lesions, the marrow is composed of loose reticulin fibers and immature mesenchymal cells that resemble long spindle-shaped fibroblasts.11 Cortical bone might be eroded by the increased numbers of osteoclasts that have an increased number of nuclear profiles. Frequently, the osteoclasts are detached from the bone perimeter (Figure 5). Cancellous bone in these lesions often resembles bold Chinese characters and shows enlarged osteocytic lacunae. Osteoclast apoptosis is absent unless the patient has been treated with aminobisphosphonates.
Figure 5.
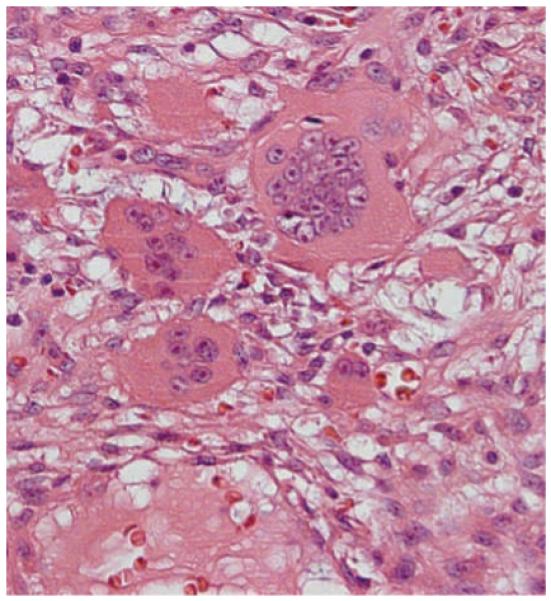
A focus of multinucleated giant cells in fibrous dysplasia. These cells are often detached from bone and located amidst a stroma that contains spindle-shaped fibroblasts and a loose pattern of reticulin fibers. Hematoxylin and eosin stain, original magnification × 400.
Treatment and management
Once the benign nature of alendronate-induced giant osteoclasts was recognized, treatment with bisphosphonates was resumed.
Conclusions
Aminobisphosphonates do not reduce the number of osteoclasts as previously thought. Instead, they increase the number of osteoclasts by prolonging their lifespan while interfering with their resorptive ability. Furthermore, the drugs induce the appearance of giant, hypernucleated, detached, apoptotic osteoclasts. Appreciation of these giant osteoclasts after long-term oral aminobisphosphonate therapy will avoid errors in diagnosis, as well as unnecessary tests and referrals.
Acknowledgments
The authors thank Dr Antoine Makdissi and Dr Vitaly Kantorovich for their careful reading of the manuscript. This work was supported by a VA Merit Review Grant from the Office of Research and Development, Department of Veterans Affairs and the National Institutes of Health (PO1-AG13918) and Tobacco Settlement Funds provided by the University of Arkansas for Medical Sciences, College of Medicine. Désirée Lie, University of California, Orange, CA, is the author of and is solely responsible for the content of the learning objectives, questions and answers of the Medscape-accredited continuing medical education activity associated with this article.
Footnotes
Competing interests
The authors, the Journal Editor J. Buckland and the CME questions author D. Lie declare no competing interests.
References
- 1.Liberman U, et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. N. Engl. J. Med. 1995;333:1437–1443. doi: 10.1056/NEJM199511303332201. [DOI] [PubMed] [Google Scholar]
- 2.Bone HG, et al. Dose-response relationships for alendronate treatment in osteoporotic elderly women. J. Clin. Endocrinol. Metab. 1997;82:265–274. doi: 10.1210/jcem.82.1.3682. [DOI] [PubMed] [Google Scholar]
- 3.McClung M, et al. Alendronate prevents postmenopausal bone loss in women without osteoporosis. Ann. Intern. Med. 1998;128:253–261. doi: 10.7326/0003-4819-128-4-199802150-00001. [DOI] [PubMed] [Google Scholar]
- 4.Reid IR, et al. Alendronate in Paget’s Disease. Am. J. Med. 1996;101:341–348. doi: 10.1016/s0002-9343(96)00227-6. [DOI] [PubMed] [Google Scholar]
- 5.Saag KG, et al. Glucocorticoid-Induced Osteoporosis Intervention Study Group Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. N. Engl. J. Med. 1998;339:292–299. doi: 10.1056/NEJM199807303390502. [DOI] [PubMed] [Google Scholar]
- 6.Orwoll E, et al. Alendronate for the treatment of osteoporosis in men. N. Engl. J. Med. 2000;343:604–610. doi: 10.1056/NEJM200008313430902. [DOI] [PubMed] [Google Scholar]
- 7.Russell RG. Bisphosphonates: mode of action and pharmacology. Pediatrics. 2007;119(Suppl. 2):S150–S162. doi: 10.1542/peds.2006-2023H. [DOI] [PubMed] [Google Scholar]
- 8.Rogers MJ, et al. Cellular and molecular mechanisms of action of bisphosphonates. Cancer. 2000;88:2961–2978. doi: 10.1002/1097-0142(20000615)88:12+<2961::aid-cncr12>3.3.co;2-c. [DOI] [PubMed] [Google Scholar]
- 9.Fleisch H. Bisphosphonates in Bone Disease: From the Laboratory to the Patient. 4th edn Academic; San Diego: 2000. p. 42. [Google Scholar]
- 10.Weinstein RS, Roberson PK, Manolagas SC. Giant osteoclast formation and long-term oral bisphosphonate therapy. N.Engl. J. Med. 2009;360:53–62. doi: 10.1056/NEJMoa0802633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spjut HJ, Dorfman HD, Fechner RE, Ackermann LV. Atlas of Tumor Pathology, Second Aeries, Fascicle 5: Tumors of Bone and Cartilage. Armed Forces Institute of Pathology; Washington, DC: 1971. [Google Scholar]
- 12.Whyte MP, Wenkert D, Clements KL, McAlister WH, Mumm S. Bisphosphonate-induced osteopetrosis. N. Engl. J. Med. 2003;349:457–463. doi: 10.1056/NEJMoa023110. [DOI] [PubMed] [Google Scholar]
- 13.Rauch F, Travers R, Plotkin H, Glorieux FH. The effects of intravenous pamidronate on the bone tissue of children and adolescents with osteogenesis imperfecta. J. Clin. Invest. 2002;110:1293–1299. doi: 10.1172/JCI15952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dempster DW, et al. Unusual osteoclast morphology in teriparatide-treated patients who have been pretreated with alendronate. J. Bone Miner. Res. 2007;22(Suppl. 1):S29. [Google Scholar]
- 15.Muche B, et al. Bisphosphonates in male osteoporosis—long-term histomorphologic changes. J. Bone Miner. Res. 2003;18(Suppl. 2):S371. [Google Scholar]
- 16.Singer RS. Paget’s Disease of Bone. Plenum Medical Book Company; New York: 1977. [Google Scholar]
- 17.Weinstein RS. Chapter 20: The Clinical Use of Bone Biopsy. In: Coe FL, Favus MJ, editors. Disorders of Bone and Mineral Metabolism. 2nd edn Raven; New York: 2002. pp. 448–468. [Google Scholar]


