Abstract
Background:
Intravenous alteplase (rt-PA) remains the only approved treatment for acute ischemic stroke, but its use remains limited. In a previous pilot dose-escalation study, intravenous tenecteplase showed promise as a potentially safer alternative. Therefore, a Phase IIB clinical trial was begun to a) choose a best dose of tenecteplase to carry forward, and b) to provide evidence for either promise or futility of further testing of tenecteplase versus rt-PA. If promise was established, then the trial would continue as a Phase III efficacy trial comparing the selected tenecteplase dose to standard rt-PA.
Methods:
The trial began as a small, multi-center, randomized, double-blind, controlled clinical trial comparing 0.1, 0.25, and 0.4 mg/kg tenecteplase with standard 0.9 mg/kg rt-PA in patients with acute stroke within 3 hours of onset. An adaptive sequential design used an early (24 hour) assessment of major neurological improvement balanced against occurrence of symptomatic intracranial hemorrhage (ICH) to choose a “best” dose of tenecteplase to carry forward. Once a “best” dose was established, the trial was to continue until at least 100 pairs of the selected tenecteplase dose versus standard rt-PA could be compared by 3 month outcome using the modified Rankin Scale in an interim analysis. Decision rules were devised to yield a clear recommendation to either stop for futility or to continue into Phase III.
Results:
The trial was prematurely terminated for slow enrollment after only 112 patients had been randomized at 8 clinical centers between 2006 and 2008. The 0.4 mg/kg dose was discarded as inferior after only 73 patients were randomized, but the selection procedure was still unable to distinguish between 0.1 mg/kg and 0.25 mg/kg as a propitious dose at the time the trial was stopped. There were no statistically persuasive differences in 3 month outcomes between the remaining tenecteplase groups and rt-PA. Symptomatic ICH rates were highest in the discarded 0.4 mg/kg tenecteplase group and lowest (0/31) in the 0.1 mg/kg tenecteplase group. Neither promise nor futility could be established.
Conclusion:
This prematurely terminated trial has demonstrated the potential efficiency of a novel design in selecting a propitious dose for future study of a new thrombolytic agent for acute stroke. Given the truncation of the trial, no convincing conclusions can be made about the promise of future study of tenecteplase in acute stroke.
Keywords: acute ischemic stroke, tenecteplase, thrombolysis
Introduction
To date, the only approved treatment for acute ischemic stroke is intravenous recombinant tissue plasminogen activator (alteplase, rt-PA). Despite extensive efforts, implementation of this treatment has been limited, largely because of the narrow time limits within which treatment must be delivered, but also due to concerns regarding adverse bleeding risk1. Development of an alternative thrombolytic therapy that might be easier and safer to administer could lead to wider acceptance and use of thrombolytic therapy for stroke.
Tenecteplase is a modified version of rt-PA that is more fibrin-specific and has a longer half life, and can thus be administered as an intravenous bolus. It has been approved for use in myocardial infarction, where it is associated with fewer systemic bleeding complications than alteplase2. A dose-escalation safety study of tenecteplase in patients with acute ischemic stroke observed no symptomatic intracranial hemorrhages (ICHs) among 75 patients treated with doses ranging from 0.1 mg/kg to 0.4 mg/kg3. While three month outcomes were similar to patients treated with alteplase in the NINDS rt-PA Stroke Trial, the results at 24 hours indicated that there may be important differences in the clinical activity of the tested doses. The proportion of patients with major neurological improvement at 24 hours was an absolute 20% higher in the 0.1 mg/kg group than at the highest safe dose tested, 0.4 mg/kg, suggesting the possibility of an inverse dose-response. Further dose comparisons were considered prudent.
Based on these encouraging results, we designed an innovative, seamless, Phase IIB/III, randomized, multi-center, double-blind trial of intravenous tenecteplase versus standard-dose rt-PA in patients with acute ischemic stroke within 3 hours of onset. We report here the results of the trial, which was prematurely terminated during Phase IIB due to slower than expected enrollment.
Methods
Phase IIB of the trial had two goals: i) to use an efficient statistical strategy to select a “best” dose of tenecteplase for acute stroke using an early (24-hour) clinical outcome; and ii) to decide whether further comparison of these two interventions was promising or futile by comparing the selected tenecteplase dose to standard-dose rt-PA using safety and longer-term (3-month) efficacy outcomes. If tenecteplase proved promising, then Phase III provided for a pivotal randomized trial comparing the selected tenecteplase dose to rt-PA for 3-month clinical outcome.
A complete description of the design will be published elsewhere. Summarizing the major features, the dose-selection component of Phase IIB compared three tenecteplase doses: 0.1 mg/kg, 0.25 mg/kg, and 0.4 mg/kg. A rapid-response outcome score was assigned at 24 hours, as follows. Patient status was scored: 0 (worst), 1, or 2 (best) on a composite measure that balanced Major Neurological Improvement (MNI)4 against risk of symptomatic ICH. A symptomatic ICH was scored 0. MNI, defined as an 8 or more point improvement compared to baseline, or a score of 0, on the National Institutes of Health (NIH) Stroke Scale5 at 24 hours, was scored 2. A patient with neither symptomatic ICH nor MNI, or with both, was scored 1. Inferior dose arms were to be eliminated by a sequential selection procedure. Patients were randomized within sites to quadruplets – one of the three tenecteplase doses, or rt-PA – but only the triplet tenecteplase arms were involved in the selection procedure. (The rt-PA patients provided concurrent randomized controls for later comparisons of 3 month outcome with the selected tenecteplase dose.) Whenever a tenecteplase triplet completed 24-hour follow-up, the cumulative sum of the scores for each of the tenecteplase doses on the rapid-response outcome was calculated. A dose was eliminated when this cumulative score first fell 6 points behind the cumulative score of the leading dose. This criterion provided a probability of at least 80% of correctly selecting the best tenecteplase dose, if the true absolute difference between it and the two inferior doses was 10% or more on MNI, given a symptomatic ICH probability of 0.06 for each dose. Given the selection procedure, the sample size for Phase IIB was variable. The distribution of the number of patients needed was relatively narrow, with a mean of 278 and a standard deviation of 50. A maximum sample size of 600 patients for the Phase IIB portion was pre-established.
Once the tenecteplase dose was selected, randomization between that dose and rt-PA controls was to continue until at least 100 patients in each group had been randomized. At that point, an interim analysis would be performed. To avoid the low statistical power of a traditional hypothesis testing with 100 patients in each group, we pre-specified clinically meaningful decision rules that would lead to a clear recommendation to consider the preliminary results either sufficiently or insufficiently promising to continue. These rules combined symptomatic ICH rates at 24 hours and rates of Poor outcome at 3 months, using the primary outcome measure for Phase III. This was the modified Rankin Scale6, trichotomized into the ordered categories “Good” (Rankin = 0 or 1), “Intermediate” (Rankin = 2 or 3), or “Poor” (Rankin = 4, 5, or 6 [death]). For example, if the selected dose of tenecteplase showed a lower symptomatic ICH rate than rt-PA, defined as at least 2 fewer symptomatic ICHs, we would declare it promising if the observed proportion of patients with Poor 3-month outcome was less than or equal to that of rt-PA (Scenario 1). In Scenario 2, if the rate of symptomatic ICH within 24 hours for TNK was effectively the same (i.e., ± 1) as that for rt-PA, then the proportion of Poor outcomes on the 3 month Rankin scale would have needed to be at least 8 percentage points lower than that of patients with rt-PA for further study of tenecteplase to be declared promising. Additionally, if the proportion of Good outcomes for TNK was significantly less than the proportion of Good outcomes with rt-PA at the nominal two-tailed 0.001 level in either Scenario, then further study of tenecteplase would be declared futile. If the selected tenecteplase dose had 2 or more symptomatic ICHs than rt-PA at the interim analysis, then the research would stop. Simulations showed that the operating characteristics of these decision rules, taken together, may be regarded as a second “selection procedure.” That is, there was a calculated > 85% probability of correct selection (either continue or discontinue) using the design parameters.
If Phase IIB showed promise, Phase III would continue the trial, with additional clinical sites, to test two co-primary null hypotheses comparing tenecteplase and rt-PA on the trichotomized 3-month Rankin:
1) The proportion of Poor outcomes with tenecteplase treatment at the selected dose does not differ from the proportion of Poor outcomes with rt-PA treatment; and
2) The proportion of Good outcomes with tenecteplase treatment at the selected dose does not differ from the proportion of Good outcomes with rt-PA treatment.
Each hypothesis was tested at α=0.025, two-tailed, using the site-stratified Mantel-Haenszel on 1 degree of freedom procedure with ½ continuity correction. The planned sample size was 1,908 (954 per group). This provided 90% power to detect an 8% or greater reduction in Poor outcome without a reduction in Good outcome, or 89% power to detect an 8% increase in Good outcome without an increase in Poor outcome.
The premature termination of the trial precluded the planned comparisons of the selected dose of tenecteplase to rt-PA for promise or futility, as well as the full Phase III trial. A new “post-specified” analysis plan was developed by the investigators and approved by the trial Data and Safety Monitoring Board (DSMB), after the termination but before breaking the blind and before analysis of any efficacy data. The analysis plan compared the proportions of the three tenecteplase groups separately, and combined, with the rt-PA group, first on the Good outcome (Rankin 0-1) and then on the Poor outcome (Rankin 4-6), using Mantel-Haenszel tests. Given the fact that the original analysis plan was not followed and that the presented analysis is exploratory, nominal p-values were calculated without taking into account the multiple comparisons; no pre-specified level of significance was set. All outcome analyses are by treatment assignment (intent-to-treat).
The protocol and consent forms were reviewed and approved by the Institutional Review Board of each participating institution. Eligible patients were age 18 or over with serious neurological deficits believed to be on the basis of acute focal cerebral ischemia, and who were otherwise suitable for treatment with intravenous rt-PA within 3 hours of stroke onset using contemporary guidelines7. After informed consent was obtained, a web-based randomization method provided a treatment assignment to an unblinded investigative pharmacist at the site, who then prepared either 0.1 mg/kg, 0.25 mg/kg, or 0.4 mg/kg of tenecteplase to be administered in 10 ml of normal saline as a bolus, followed by 90 ml of normal saline administered over 1 hour; or 0.9 mg/kg of rt-PA with 10% of the total dose administered in 10 ml as a bolus followed by the remaining 90% in 90 ml of normal saline administered over 1 hour. Treating physicians, staff, and investigators, as well as trial patients, remained blinded to the identity of the study drug throughout. A baseline NIH Stroke Scale was performed immediately prior to initiation of study drug in each patient to confirm continued eligibility in the trial. Patients whose deficits had cleared or who had become otherwise ineligible in the interval between treatment assignment and actual treatment were considered to be “enrolled” but were not “randomized” or included in the analyses. Reasons for exclusion of enrolled but not randomized patients were recorded.
Randomized patients were managed in an intensive care or acute stroke unit for 24 hours following treatment using standard guidelines for post-thrombolytic stroke treatment. A follow-up NIH Stroke Scale examination was performed at 24 ± 2 hours following stroke onset, and a non-contrast head CT scan was performed at 48 ± 6 hours after treatment to assess for asymptomatic intracranial bleeding. If neurological worsening occurred, then a head CT scan was performed and the primary and contributing causes of the neurological worsening were recorded. Neurological worsening was defined as any clinically significant neurological change (appearance of a new deficit or worsening of previous deficits) that persisted for more than 8 hours. All CT scans (both baseline and follow-up) were sent to the Clinical Coordinating Center for interpretation blinded to treatment assignment by a study neuroradiologist who independently judged whether or not the scan depicted ICH. If hemorrhage was present on any follow up scan, the entire case was referred to an independent blinded clinical neurologist who adjudicated whether or not the hemorrhage was symptomatic or asymptomatic. A symptomatic ICH was defined as any clinically important neurological worsening (i.e., meeting neurological worsening criteria, see above) attributable to new hemorrhage seen on a follow-up head CT scan. Confluent hematoma occupying greater than 1/3 of the infarct volume and exerting space-occupying effects, and intraventricular or subarachnoid extension of blood were considered strongly, but the decisions of the neurological adjudicator were final. Symptomatic ICHs that became symptomatic within 24 hours of treatment were considered potentially attributable to study drug.
Safety was overseen by an independent Medical Monitor and DSMB. The DSMB was also charged with reviewing the progress of the selection procedure, protecting the integrity of the trial, and reviewing the results of the analysis for promise or futility.
Results
From March, 2006 through December, 2008, 112 patients were randomized into the trial at 10 hospitals in 8 clinical centers (see Appendix). One patient was randomized to rt-PA, but received 0.25 mg/kg tenecteplase, and one was randomized to 0.25 mg/kg tenecteplase, but received 0.7 mg/kg tenecteplase. The remaining 110 patients received the assigned medication and dose. Seventeen additional patients received a provisional treatment assignment but were excluded prior to final eligibility determination. Seven (7) became ineligible because of deficit resolution; drug was not available in time for 8; and 2 withdrew consent prior to treatment.
Table 1 shows the baseline characteristics and ischemic stroke subtypes (by TOAST criteria8 at 7-10 days following the entry stroke) by treatment group. The patients randomized to rt-PA were older and had more severe stroke deficits at baseline than patients in the tenecteplase groups. Four patients (2 in the 0.1 mg/kg tenecteplase group, and 2 in the rt-PA group) were determined to have had conversion disorders, hyperglycemia, or migraine as the cause of their acute neurological deficits. All four had complete resolution of their acute deficits.
TABLE 1.
Baseline Characteristics and Ischemic Stroke Subtypes by Treatment Group
| TNK 0.1 mg/kg N=31 |
TNK 0.25 mg/kg N=31 |
TNK 0.4 mg/kg N=19 |
rt-PA 0.9 mg/kg N=31 |
|
|---|---|---|---|---|
| Age (years, mean ± S.D.) | 67 (19) | 69 (15) | 68 (16) | 72 (16) |
| Sex (n, % male) | 12 (39%) | 16 (52%) | 13 (68%) | 17 (51%) |
| Race (n, % white) | 24 (77%) | 26 (84%) | 12 (63%) | 25 (81%) |
| Baseline NIH Stroke Scale Score (median, interquartile range) |
8 (5-11) | 10 (6-15) | 9 (5-17) | 13 (5-17) |
| Systolic BP (mm Hg, mean ± S.D.) | 156 (21) | 158 (31) | 152 (27) | 150 (23) |
| Diastolic BP (mm Hg, mean ± S.D.) | 86 (15) | 84 (14) | 82 (17) | 81 (13) |
| Pre-Stroke Rankin ≥ 2 (n, %) | 7 (23%) | 3 (10%) | 0 (0%) | 5 (16%) |
| Medical History | ||||
| Hypertension (n, %) | 25 (81%) | 25 (81%) | 17 (90%) | 22 (71%) |
| Diabetes (n, %) | 6 (19%) | 7 (23%) | 4 (21%) | 4 (13%) |
| Prior Stroke (n, %) | 6 (19%) | 10 (32%) | 5 (26%) | 4 (13%) |
| Heart Disease (n, %) | 20 (65%) | 14 (45%) | 11 (58%) | 24 (77%) |
| Hypercholesterolemia (n, %) | 16 (52%) | 15 (48%) | 8 (42%) | 17 (55%) |
| Active Smoker (n, %) | 2 (6.5%) | 7 (23%) | 0 (0%) | 7 (23%) |
| Ischemic Stroke Subtype | ||||
| Large Vessel Atherothromboembolic (n, %) | 3 (10%) | 9 (29%) | 5 (26%) | 2 (7%) |
| Cardioembolic (n, %) | 10 (32%) | 11 (36%) | 11 (58%) | 13 (42%) |
| Small Vessel (n, %) | 9 (29%) | 4 (13%) | 2 (11%) | 7 (23%) |
| Other Ischemic Stroke Cause (n, %) | 1 (3%) | 1 (3%) | 0 (0%) | 1 (3%) |
| Unknown Cause (n, %) | 6 (19%) | 6 (19%) | 1 (5%) | 6 (19%) |
| Not An Ischemic Stroke (n, %) | 2 (7%) | 0 (0%) | 0 (0%) | 2 (7%) |
TNK = tenecteplase
rt-PA = alteplase
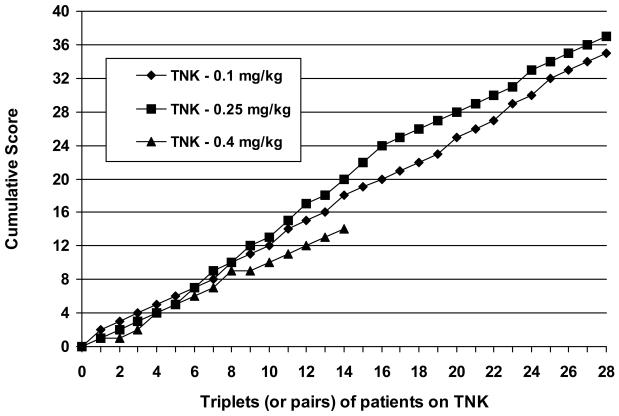
The results of the tenecteplase dose selection procedure are depicted in Figure 1. The 0.4 mg/kg dose fell 6 points behind the leading dose (0.25 mg/kg) after 14 triplets of tenecteplase patients completed 24-hour follow-up. The 0.4 mg/kg dose was therefore eliminated, and randomization to it discontinued. Randomization continued to tenecteplase 0.1 mg/kg, tenecteplase 0.25 mg/kg, or rt-PA 0.9 mg/kg. When the trial was terminated after 112 patients had been randomized, the cumulative difference between the two remaining tenecteplase doses had, at times, reached as many as 4 points, but not the 6 needed to reach the dose selection criterion.
Figure 1.
Graph depicting the results of the tenecteplase (TNK) dose selection procedure. The ordinate represents the cumulative score for each treatment group (see text for scoring procedure). The abscissa represents the triplets (or pairs) of patients stratified by enrollment site. After 14 triplets, the cumulative score for the 0.4 mg/kg tenecteplase dose fell 6 points behind the cumulative score for the leading dose, and therefore was eliminated. The selection procedure did not reach that criterion which would have selected between the 0.1 mg/kg and 0.25 mg/kg dose of tenecteplase before the trial was stopped.
Data were collected for five additional tenecteplase 0.4 mg/kg patients beyond the 14 used to eliminate that dose. Four were patients who had already been randomized to triplets that remained open, and therefore unanalyzed, when the 0.4 mg/kg dose was eliminated. The fifth was the only patient randomized at a site which was subsequently closed. The data from these patients did not contribute to the decision to eliminate the 0.4 mg/kg dose, but are included in all subsequent analyses.
Table 2 shows the 3 month outcomes and 24 hour MNI rates for the patients by treatment group. Four patients were either lost to follow up or voluntarily withdrew from the trial. Their 3 month Rankin categories were imputed using the last observation carried forward or the last recorded NIH Stroke Scale score using a pre-specified algorithm. The 0.1 mg/kg tenecteplase group had the lowest proportion of Poor outcomes (7/31, 22.6%), while the rt-PA group had 10/31 (32.3%) Poor outcomes. In terms of Good outcome, the 0.25 mg/kg tenecteplase group had the highest proportion (15/31, 48.4%), but the 0.1 mg/kg tenecteplase group was similar (14/31, 45.2%). By comparison, the rt-PA group had 13/31 (41.9%) Good outcomes. All P-values were > 0.3.
TABLE 2.
Outcomes at 3 Months (Rankin Good and Poor) and 24 Hours (Major Neurological Improvement) by Treatment Group
| TNK 0.1 mg/kg N=31 |
TNK 0.25 mg/kg N=31 |
TNK 0.4 mg/kg N=19 |
rt-PA 0.9 mg/kg N=31 |
|
|---|---|---|---|---|
| Rankin Good (n, %, 95% C.I.) |
14 (45.2%, 27.3-64.0) | 15 (48.4%, 30.2-66.9) | 7 (36.8%, 16.3-61.6) | 13 (41.9%, 24.6-60.9) |
| Rankin Poor (n, %, 95% C.I.) |
7 (22.6%, 9.6-41.1) | 11 (35.5%, 19.2-54.6) | 6 (31.6%, 12.6-56.6) | 10 (32.3%, 16.7-51.4) |
| MNI (n, %, 95% C.I.) |
7 (22.6%, 9.6-41.1) | 11 (35.5%, 19.2-54.6) | 4 (21.1%, 6.1-45.6) | 5 (16.1%, 5.5-33.7) |
C. I. = Confidence Interval
TNK = tenecteplase
rt-PA = alteplase
Table 3 shows selected safety measures. There were a total of 6 symptomatic ICHs; 3/19 (15.8%) in the 0.4 mg/kg group, 2/31 (6.5%) in the 0.25 mg/kg tenecteplase group, and none (0/31) in the 0.1 mg/kg tenecteplase group. By comparison, there was 1/31 (3.2%) symptomatic ICH in the rt-PA group. Additionally, there were 11 asymptomatic ICH's among the 4 treatment groups. There was 1 serious systemic hemorrhage in the 0.25 mg/kg group (a retroperitoneal hemorrhage) that resulted in life threatening hypotension and neurological worsening.
TABLE 3.
Selected Safety Data by Treatment Group
| TNK 0.1 mg/kg N=31 |
TNK 0.25 mg/kg N=31 |
TNK 0.4 mg/kg N=19 |
rt-PA 0.9 mg/kg N=31 |
|
|---|---|---|---|---|
| Symptomatic ICH (n, %, 95% C.I.) |
0 (0%, 0-11.2) | 2* (6.5%, 0.8-21.4) | 3 (15.8%, 3.4-39.6) | 1 (3.2%, 0.1-16.7) |
| Asymptomatic ICH (n, %, 95% C.I.) |
3 (9.7%, 2.0-25.8) | 2 (6.5%, 0.8-21.4) | 2 (10.5%, 1.3-33.1) | 4 (12.9%, 3.6-29.8) |
| All ICH (n, %, 95% C.I.) | 3 (9.7%, 2.0-25.8) | 4 (12.9%, 3.6-29.8) | 5 (26.3%, 9.2-51.2) | 5 (16.1%, 5.5-33.7) |
| Major Systemic Bleeding (n, %, 95% C.I.) |
0 (0%, 0-11.2) | 1 (3.2%, 0.1-16.7) | 0 (0%, 0-17.6) | 0 (0%, 0-11.2) |
| Death within 3 months, All Causes (n, %, 95% C.I.) |
2 (6.5%, 0.8-21.4) | 7 (22.6%, 9.6-41.1) | 3 (15.8%, 3.4-39.6) | 8 (25.8%, 11.9-44.6) |
C.I. = Confidence Interval
ICH = Intracranial Hemorrhage
TNK = tenecteplase
rt-PA = alteplase
N.B.: Neither of these 2 ICH's is depicted in Figure 1 as a score of “0,” as one also had MNI (see text), and the second was re-adjudicated from asymptomatic to symptomatic by the independent adjudicator after the sequential score had been recorded, per protocol.
Discussion
This randomized, controlled, Phase IIB trial employed a number of novel design features in an attempt to answer efficiently several important clinical questions prior to escalating to a major Phase III efficacy trial. The first issue was to select an optimal dose of tenecteplase to carry forward into phase III from among 3 doses which had appeared safe in a previous study in acute stroke patients. We chose an adaptive, sequential dose selection procedure that used MNI at 24 hours balanced by risk, as measured by the incidence of symptomatic ICH, to choose among three different doses of tenecteplase. The selection procedure efficiently eliminated 0.4 mg/kg tenecteplase as “inferior” after only 73 patients had been randomized into the study (including patients concurrently randomized to rt-PA). The trial was stopped before a propitious dose of tenecteplase could be selected. Based upon the pre-specified criteria, we could not distinguish between the 0.1 mg/kg and 0.25 mg/kg doses after 28 pairs had been compared. As there may be as much as an absolute 10% true difference in 24 hour MNI rates between these two doses, further study would be required to make this distinction.
The second major purpose of the trial was to develop evidence for either promise or futility of further study of an optimal dose of tenecteplase compared to standard dose intravenous rt-PA. We planned to enroll at least 100 patients to either rt-PA or the optimal dose of tenecteplase and then to compare their 3 month outcomes in an interim analysis. Unfortunately, the premature termination of the trial preempted the planned assessment. With only 31 patients in each of the remaining treatment groups, there were major imbalances in several important baseline prognostic factors for outcome, and the uncertainty associated with the outcome proportions was so broad as to make our pre-specified decision rules for stopping or continuation substantially less reliable. The promising safety experience observed in the previous pilot dose-escalation study of tenecteplase was not duplicated in this trial. The observed symptomatic ICH rate in the 0.4 mg/kg tenectplase dose group was 15.8%, and contributed to its early relegation as an “inferior” tenecteplase dose. Only 1/31 (3.2%) symptomatic ICH was observed in the rt-PA group, but the confidence intervals include the widely reported 6% rate. The safest regimen appears to be the 0.1 mg/kg tenecteplase group in which no symptomatic intracranial hemorrhages were observed, and the point estimates suggest an absolute 9.7% reduction in Poor outcomes and 3.2% increase in Good outcomes in this group compared to rt-PA. None of these differences is statistically persuasive, and as noted above, the rt-PA group was older and had more severe stroke deficits at baseline.
Finally, had the Phase IIB trial been completed, the plan was to continue seamlessly into a much larger Phase III efficacy trial comparing the 3 month outcomes between the selected dose of tenecteplase and standard rt-PA. The inclusion of the Phase IIB patients in the larger Phase III study has traditionally raised questions among statisticians and clinical trialists of potential bias and lack of control for Type I statistical error. However, simulations demonstrated beyond any reasonable doubt that, given the conservatism of the tests for promise or futility, and other features, the phase III trial as designed maintained excellent control of the type 1 error rate below 5% overall (results to be reported separately). Despite this, as of this writing the United States Food and Drug Administration has not approved this plan, and had the Phase IIB trial been allowed to continue to completion, a separate, independent Phase III trial might have been required.
Recently, Parsons and colleagues reported the results of a prospective pilot study of 15 patients selected by CT or MRI diffusion/perfusion mismatch and treated with intravenous tenecteplase at a dose of 0.1 mg/kg between 3 and 6 hours from onset of acute ischemic stroke9. Compared to a nonrandomized control group of 35 patients treated with standard rt-PA within the 3 hour time window, more tenecteplase-treated patients had major neurological improvement at 24 hours (66.7% vs. 20.0%), as well as improved reperfusion and large vessel recanalization compared to the rt-PA-treated group. These observations, along with the results of our trial, suggest that further study of tenecteplase as an alternative treatment for acute ischemic stroke may be warranted.
Acknowledgements and Funding
Supported by grants (R01-NS37666 and R01-NS45170) from the National Institute of Neurological Disorders and Stroke – National Institutes of Health
Genentech, Inc. supplied study drug (both tenecteplase and alteplase) for this clinical trial, but no other financial or other support.
The authors thank the patients and families who participated in this clinical trial.
This trial was registered with ClinicalTrials.gov (NCT00252239).
Appendix: The Tenecteplase in Stroke Investigators
University of California – San Diego (Sharp Memorial Hospital, Alvarado Hospital)
P.Lyden,
T. Hemmen,
B. Meyer
M.Chacon
M. Jenson
S.Yip
W.Brown
G. Tafreshi
P. Delaney
J. Sattin,
C. Fanale
S. Olson
K. Rapp
J. Werner
J. Bell
T. Rzesiewicz
M. Buda
T. Vu
Long Island Jewish Medical Center
T. Kwiatkowski
R. Libman
L. Schoenberg
J. Katz
A. Patil
R. Gonzaga-Camfield
M. Schaefer
M. Manlulu
Z. Faynblat
A. Johnson
A. Diamond
Colorado Neurological Instititute (Swedish Medical Center)
C. Fanale
R. Pratt
I. Chang
H. Monatt
C. Greenwald
K. Malleck
University of Texas – Houston (Memorial Hermann Hospital, Memorial Southwest Hospital)
J. Grotta (P.I.)
F. Yatsu
A. Alexandrov
M. Ribo
J. Choi
K. Illoh
E. Noser
N. Gonzales
R. Sugg
H. Shaltoni
A. Khaja
K. Albright
R. Martin
H. Hallevi
A. Barreto
S. Martin-Schild
A. Abraham
S. Savitz
I. Acosta
V. Misra
O. Chernyshev
D. Matherne
D. Wegner
R. Casey
M. Peck
N. Porche-Taylor
S. Shaw
D. Smith
M. Hess
L. Shen
A. Alderman
L. Nguyen
M. Olivares
T. Yeung
Johns Hopkins – Bayview Medical Center
R. Llinas (P.I.)
H. Chang
M. Frohler
C. Cronin
J. Berekely
X. Xiong
S. Zeiler
K. Thomas
R. Hoesch
C. Turtzo
J. Jordan
J. Alt
Mount Sinai Medical Center
S. Levine (P.I.)
S. Augustine
I. Cohen
S. Tuhrim
K. Sheinart
D. Horowitz.
C. Amory
D. Patterson
J. Weinberger
J. Bruns
Y. Chan
L. Blas
Univ. of Michigan Health Center
D. Brown (P.I.)
W. Barsan
T. Jacobs
J. Majersik
W. Meurer
L. Morgenstern.
V. Rajajee
P. Scott.
R. Silbergleit
L. Skolarus
M. Wang
D. Zahuranec
K. Maddox
A. Skyles
S. Weadock
University of Virginia Health Sciences Center
C. Haley (P.I.)
M. Davis
K. McCarthy
A. Adams
K. Johnston
B. Nathan
N. Solenski
B. Worrall
K. Barrett
R. Erwin
C. Domangue
M. Mauermann
Clinical Coordinating Center (University of Virginia)
C. Haley (P.I.)
M. Davis
C. Beebe
K. McCarthy
C. Hicks
D. Phillips
Statistical Analysis Center (Columbia University)
J. Thompson (P.I.)
B. Levin
G. Levy
R. Buchsbaum
R. Arbing
A. Tierney
R. MacArthur
R. Prodhan
Medical Monitor
T. Bleck
Intracranial Hemorrhage Clinical Adjudicator
G. Albers
Data and Safety Monitoring Board
M. Walker (Chair)
J. Hallenbeck
D. Stump
T. Cook
National Institute of Neurological Disorders and Stroke
S. Janis
P. Gilbert
Footnotes
Conflicts of Interest:
Dr. Haley reports serving as a consultant to GlaxoSmithKline. Dr. Grotta reports holding a patent on the experimental compound, caffeinol, and serving as a consultant for Lundbeck. Dr. Lyden reports receiving research support from Photothera, consultancies with Photothera, Mitsubishi Pharma, and Benechill, serving on an advisory board for CoAxia, and receiving honoraria from Mitsubishi Pharma. Dr. Brown reports receiving research support from CVR Global, Inc. Dr. Fanale reports serving on a Speaker's Bureau for Genentech. Dr. Llinas reports receiving research support from Diogenix. Dr. Levine reports having served on an advisory board for Astra Zeneca. Dr. Johnston reports serving as a consultant to Diffusion Pharmaceuticals Inc., Remedy Pharmaceutical, and OnoPharma USA; and serving on advisory boards for Astra Zeneca. Drs. Thompson, Hemmen, Libman, Kwiatkowski, Levy, Levin, and Mr. Buchsbaum report nothing to disclose.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brown DL, Barsan WG, Lisabeth LD, Gallery ME, Morgenstern LB. Survey of emergency physicians about recombinant tissue plasminogen activator for acute ischemic stroke. Ann Emerg Med. 2005;46:56–60. doi: 10.1016/j.annemergmed.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 2.Assessment of the Safety and Efficacy of a New Thrombolytic (ASSENT-2) Investigators Single bolus tenecteplase compared with front-loaded alteplase in acute myocardial infarction: the ASSENT-2 double-blind randomized trial. Lancet. 1999;354:716–722. doi: 10.1016/s0140-6736(99)07403-6. [DOI] [PubMed] [Google Scholar]
- 3.Haley EC, Lyden PD, Johnston KC, Hemmen TM, the TNK in Stroke Investigators A pilot dose-escalation safety study of tenecteplase in acute ischemic stroke. Stroke. 2005;36:607–612. doi: 10.1161/01.STR.0000154872.73240.e9. [DOI] [PubMed] [Google Scholar]
- 4.Brown DL, Johnston KC, Wagner DP, Haley EC. Predicting major neurological improvement with intravenous tissue plasminogen activator treatment of stroke. Stroke. 2004;35:147–150. doi: 10.1161/01.STR.0000105396.93273.72. [DOI] [PubMed] [Google Scholar]
- 5.Lyden P, Raman R, Liu L, Grotta J, Broderick J, Olson S, Shaw S, Spilker J, Meyer B, Emr M, Warren M, Marler J. NIHSS traning and certification using a new digital video disk is reliable. Stroke. 2005;36:2446–2449. doi: 10.1161/01.STR.0000185725.42768.92. [DOI] [PubMed] [Google Scholar]
- 6.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJA, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–607. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- 7.Adams HP, del Zoppo G, Alberts MJ, Bhatt D, Brass L, Furlan A, Grubb RL, Hiagshida RT, Jauch EC, Kidwell C, Lyden PD, Morgenstern LB, Qureshi AI, Rosenwasser RH, Scott PA, Wijdiks EFM. Guidelines for the early management of adults with ischemic stroke. A guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups. Stroke. 2007;38:1655–1711. doi: 10.1161/STROKEAHA.107.181486. [DOI] [PubMed] [Google Scholar]
- 8.Adams HP, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DS, Marsh EE. Classification subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 9.Parsons MW, Miteff F, Spratt N, Loisell A, Attia J, Levi CR. Acute ischemic stroke. Imaging guided tenecteplase treatment in an extended time window. Neurology. 2009;72:915–921. doi: 10.1212/01.wnl.0000344168.05315.9d. [DOI] [PubMed] [Google Scholar]