Abstract
Several presynaptic proteins involved in neurotransmitter release in the CNS have been implicated in schizophrenia in human clinical genetic studies, in postmortem studies, and in studies of putative animal models of schizophrenia. The presynaptic protein RIM1α mediates presynaptic plasticity and cognitive function. We now demonstrate that mice deficient in RIM1α exhibit abnormalities in multiple schizophrenia-relevant behavioral tasks including prepulse inhibition, response to psychotomimetic drugs, and social interaction. These schizophrenia-relevant behavioral findings are relatively selective to RIM1α-deficient mice, as mice bearing mutations in the RIM1α binding partners Rab3A or synaptotagmin 1 only show decreased prepulse inhibition. In addition to RIM1α's involvement in multiple behavioral abnormalities, these data suggest that alterations in presynaptic forms of short-term plasticity are linked to alterations in prepulse inhibition, a measure of sensorimotor gating.
Introduction
Impaired glutamatergic synapse function has been implicated in schizophrenia (Carlsson et al., 1999, 2004; Lipska and Weinberger, 2000; Mechri et al., 2001; Coyle et al., 2003; Moghaddam and Jackson, 2003; Weinberger, 2005; Coyle, 2006; Javitt, 2007; Stone et al., 2007; Paz et al., 2008; Sodhi et al., 2008). In particular, pharmacologic blockade of NMDA receptors with phencyclidine, ketamine, or (+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine maleate (MK-801) can cause or exacerbate psychotic symptoms in humans (Moghaddam, 1994; Lahti et al., 1995; Braff et al., 2001; Gunduz-Bruce, 2009). Furthermore, such blockade leads to behavioral abnormalities in rodents that are often touted as relevant to human schizophrenia including social isolation, decreased cognitive function, and decreased prepulse inhibition (PPI) (Javitt and Zukin, 1991; Powell and Miyakawa, 2006; Large, 2007; Bubenikova-Valesova et al., 2008; Gunduz-Bruce, 2009; Noda et al., 2009).
Several studies in human postmortem tissues have identified altered expression of some presynaptic proteins in schizophrenia (Gabriel et al., 1997; Glantz and Lewis, 1997a,b; Blennow et al., 1999; Karson et al., 1999; Eastwood et al., 2001; Sawada et al., 2002, 2005; Vawter et al., 2002; Harrison et al., 2003; Eastwood and Harrison, 2005; Scarr et al., 2006; Bowden et al., 2008; Mudge et al., 2008; Roberts et al., 2008; Shen et al., 2009). Moreover, clinical genetic studies have identified genes encoding presynaptic proteins as risk factors for schizophrenia (Karson et al., 1999; Mirnics et al., 2000; Tachikawa et al., 2001; Chen et al., 2004; Lee et al., 2005; Muller et al., 2005; Verma et al., 2005; Kirov et al., 2008; Sudhof, 2008; Walsh et al., 2008; Rujescu et al., 2009). Consistent with these findings, mutation of genes encoding presynaptic proteins has resulted in mouse models relevant to schizophrenia (Drew et al., 2007; Dyck et al., 2007, 2009; Etherton et al., 2009). Thus, we hypothesized that deletion or mutation of the presynaptic protein RIM1α, and its binding partners Rab3A and synaptotagmin, would lead to schizophrenia-related behavioral abnormalities (Powell and Miyakawa, 2006).
Deficits in prepulse inhibition, a measure of sensorimotor gating, are associated with human schizophrenia and other neuropsychiatric disorders (Braff and Geyer, 1990; Braff et al., 2001; Ludewig et al., 2003; Geyer, 2006b). Although the anatomy and pharmacology of the startle response in the brainstem and the many higher brain regions that modify prepulse inhibition of the startle response have been well described (Braff and Geyer, 1990; Dulawa and Geyer, 1996; Geyer, 1998; Swerdlow et al., 2001; Geyer et al., 2002), the neuronal and synaptic mechanisms responsible for prepulse inhibition are less well characterized.
In the prepulse inhibition measure of sensorimotor gating, the interval between a prepulse and the startle pulse is on the order of 50–200 ms, approximately the same interval required for short-term, presynaptic plasticity (Zucker and Regehr, 2002; Abbott and Regehr, 2004). We therefore hypothesized that alterations in presynaptic short-term plasticity at central synapses may play a role in prepulse inhibition of startle. To test this hypothesis, we examined three presynaptic protein mutant mouse lines exhibiting increased paired-pulse facilitation (PPF) as well as one presynaptic mutant mouse line with no change in PPF.
Materials and Methods
Behavioral overview.
RIM1α−/−, Rab3A−/−, synaptotagmin 1 R233Q (Syt1R233Q) point mutant knock-in (KI), and RIM1αS413A KI mice were generated as described previously (Geppert et al., 1994a; Fernandez-Chacon et al., 2001; Schoch et al., 2002; Kaeser et al., 2008b). All mice were generated using SM1 129S6/SvEvTac or R1 129X1/SvJ embryonic stem-cell clones, and the resulting chimeric mice were bred with C57BL/6J mice to obtain F1 heterozygous mice. Each strain was backcrossed to C57BL/6J for at least three or four generations with the exception of RIM1αS413A KI mice, which were backcrossed only once. All mice used were sex-matched, littermate products of heterozygous matings mostly tested between 4–12 months of age. Only male mice were used in all studies, except for the Syt1R233Q KI mice, of which approximately equal numbers of males and females were used. No sex differences were observed in the Syt1R233Q KI mice, so data were pooled from both sexes.
Social interaction.
Interaction with a novel juvenile target mouse was performed essentially as described previously (Kwon et al., 2006; Tabuchi et al., 2007; Blundell et al., 2009).
Prepulse inhibition (sensorimotor gating).
We used a variation on the protocol of Dulawa and Geyer (1996, 2000). Startle chambers (San Diego Instruments) modified for mice were mounted atop a piezoelectric accelerometer that detects and transduces animal movements. Acoustic stimuli were delivered by high-frequency speakers mounted 33 cm above the cylinders. Animal movements were digitized and stored using computer software supplied by San Diego Instruments. From the onset of startle stimuli, 65 1 ms readings were recorded, and the amplitude of the startle responses was obtained in arbitrary units. Chambers were calibrated before each set of mice, and sound levels were monitored using a sound meter (Tandy).
Mice were subjected to five trial types in a 22 min session: pulse alone (40 ms, 120 dB, white noise pulse), three different prepulse/pulse trials (20 ms prepulse of 4, 8, or 16 dB above background noise level of 70 dB precedes the 120 dB pulse by 100 ms; onset to onset), and no stimulus. All trials were presented pseudorandomly with an average of 15 s (7–23 s) between the 62 trials. Testing began with a 5 min acclimation to the cylinders followed by four blocks of test trials. The first and last blocks consisted of six pulse-alone trials. Blocks 2 and 3 contained six pulse alone trials, five of each level of prepulse/pulse trials, and five no-stimulus trials. Data were analyzed for baseline startle amplitude (initial pulse-alone trials) and prepulse inhibition (percentage of decrease in startle amplitude for prepulse/pulse trials compared to pulse-alone trials).
Locomotor activity.
Mice were placed in a clean mouse cage with minimal bedding for 3 h. Horizontal activity was monitored using photobeams linked to computer data acquisition software (San Diego Instruments). Twelve mice were tested simultaneously under low-light conditions.
Locomotor response to psychotomimetics.
The apparatus described above was used. Mice were given a saline injection immediately before placement into the apparatus for 3 h. After the end of the first and the second hours, an intraperitoneal injection of MK-801 (0.1 then 0.2 mg/kg) was given, and mice remained in the chambers for 2 additional hours. MK-801 was prepared in sterile normal saline and given in volumes of ∼0.2 ± 0.1 cc. Normal saline injection volume was matched to that of the drug for each individual mouse. In RIM1α−/− mice, 4 d of locomotor activity with three saline injections was performed before the “drug day” with MK-801. This was to eliminate baseline locomotor differences on the testing day so that locomotor responses to psychotomimetics could be accurately measured. Similar 4 d habituation periods were given to Syt1R233Q KI mice, but not to Rab3A−/− or RIM1αS413A KI mice.
Statistics.
For social interaction data, Student's t test was used. PPI data were analyzed with two-way mixed ANOVAs with genotype as the between-subjects factor and PPI level as the within-subjects factor. For all locomotor activity data (with and without psychotomimetics), two-way mixed ANOVAs were conducted with genotype as a between-subjects factor and time as a within-subjects factor. Each hour of locomotor activity (i.e., the phase of locomotor activity after each intraperitoneal injection) was analyzed separately. For all nonreported ANOVA main effects and interactions, p > 0.05.
Results
Prepulse inhibition in mutant mice with altered presynaptic short-term plasticity
Consistent with the hypothesis that presynaptic short-term plasticity plays a role in prepulse inhibition, mice deficient in RIM1α, known to exhibit increased PPF and short-term presynaptic facilitation at excitatory synapses of area CA1 of the hippocampus (Schoch et al., 2002), were significantly impaired in prepulse inhibition of startle (Fig. 1a). In fact, RIM1α-deficient mice exhibit significantly decreased PPI at all three levels of prepulse tested (Fig. 1a) with no significant alteration in initial startle amplitude [two-way mixed ANOVA; main effect of genotype, F(1,26) = 14.35, p < 0.001; main effect of prepulse level, F(2,52) = 35.81, p < 0.001, no significant interaction; p values for post hoc planned comparisons at each prepulse level shown above bars (Fig. 1); N = 14]. Because RIM1α-deficient mice also exhibit increased locomotor activation in response to novel environments (Powell et al, 2004), we additionally tested PPI after 4 d of habituation to the startle chambers. Even after habituation to the chambers and background noise for 4 d, RIM1α−/− mice still exhibited the same significantly decreased PPI at all levels of prepulse (Fig. 1b) (two-way mixed ANOVA; main effect of genotype, F(1,26) = 12.45, p < 0.01; main effect of prepulse level, F(2,52) = 49.56, p < 0.001). Thus, this decreased PPI phenotype in RIM1α−/− mice was not caused by an altered response to novelty and is robust and repeatable.
Figure 1.

PPI deficits in presynaptic mutant mice with abnormal short-term, presynaptic facilitation (RIM1α−/−, Rab3A−/−, Syt1R233Q KI, but not RIM1αS413A KI). a, Significant decrease in prepulse inhibition of acoustic startle in RIM1α−/− mice versus wild-type (WT) littermate controls at all levels of prepulse (4, 8, and 16 dB above background of 70 dB; N = 14 in a–c; two-way mixed ANOVA, main effect of genotype, F(1,26) = 14.35, p < 0.001; main effect of prepulse level, F(2,52) = 35.81, p < 0.001; no significant interaction; p values for post hoc planned comparisons at each prepulse level shown above bars in all panels). b, PPI deficit in Rim1α−/− mice persists even after 4 preceding days of habituation to PPI chambers and background noise before repeat testing (two-way mixed ANOVA; main effect of genotype, F(1,26) = 12.45, p < 0.01; main effect of prepulse level, F(2,52) = 49.56, p < 0.001. c, Significant PPI deficits at all prepulse levels in Rab3A−/− mice versus WT littermate controls (N = 15; two-way mixed ANOVA; main effect of genotype, F(1,26) = 13.08, p < 0.01; main effect of prepulse level, F(2,52) = 43.55, p < 0.001; no significant interaction). d, Significant PPI deficit at prepulse of 8 dB above background noise in Syt1R233Q KI mice versus WT littermate controls (N = 14; two-way mixed ANOVA revealed no significant main effect of genotype, F(1,52) = 3.45, p = 0.07; main effect of prepulse level, F(1,52) = 28.38, p < 0.0001; no significant interaction). Planned comparisons revealed a significant difference only at the 8 dB above background prepulse level (p < 0.05). e, No change in PPI at any prepulse level or in baseline startle in RIM1αS413A KI mice versus WT littermate controls (N = 11; two-way mixed ANOVA, no main effect of genotype, F(1,21) = 0.01, p = 0.94; main effect of prepulse level, F(2,42) = 73.0, p < 0.001; no significant interaction). Error bars indicate mean ± SEM.
Mice deficient in the presynaptic protein Rab3A also exhibit a significant decrease in PPI (Fig. 1c) concomitant with a known increase in PPF in area CA1 of hippocampus (Geppert et al., 1997; Schoch et al., 2002). Again, although Rab3A knock-out (KO) mice showed no significant change in initial startle amplitude (two-way mixed ANOVA; main effect of genotype, F(1,26) = 13.08, p < 0.01; main effect of prepulse level, F(2,52) = 43.55, p < 0.001, no significant interaction; N = 15), PPI was reduced at all three levels of prepulse (Fig. 1c).
Similarly, Syt1R233Q KI mice had increased PPF (Fernandez-Chacon et al., 2001) and demonstrated significantly decreased PPI (Fig. 1d). The decrease in PPI in Syt1R233Q KI knock-in mice was significant at a single prepulse level (8 dB above background). No significant change in initial startle amplitude was observed in the Syt1R233Q KI mice (two-way mixed ANOVA revealed no significant main effect of genotype, F(1,52) = 3.45, p = 0.07; main effect of prepulse level, F(1,52) = 28.38, p < 0.0001, no significant interaction; p < 0.05, N = 14).
In an effort to demonstrate selectivity of the deficits in PPI, we examined another presynaptic protein mutant, the RIM1αS413A point mutant KI mice (RIM1αS413A KI), that does not exhibit any alteration in PPF or short-term facilitation (Kaeser et al., 2008b). The RIM1αS413A KI mice did not display a deficit in PPI at any level of prepulse tested (Fig. 1e), nor did they exhibit a change in initial startle response (two-way mixed ANOVA; no main effect of genotype, F(1,21) = 0.01, p = 0.94; main effect of prepulse level, F(2,42) = 73.0, p < 0.001, no significant interaction; N = 11).
Social interaction deficits selective to RIM1α−/− mice
It is possible that alterations in presynaptic short-term plasticity or in presynaptic proteins in general lead to broad behavioral abnormalities across multiple domains. To examine the selectivity of the PPI deficit and to determine whether additional schizophrenia-relevant behavioral abnormalities are present in these presynaptic mutant mice, we tested direct social interaction with a juvenile target mouse in each line.
Consistent with previously observed schizophrenia-related cognitive deficits (Powell et al., 2004) and decreased PPI (Fig. 1a–c) in RIM1α knock-out mice, these mice exhibited a dramatic decrease in social interaction with a juvenile (Fig. 2a). This finding indicates that RIM1α knock-out mice may exhibit a selective pattern of schizophrenia-relevant behavioral abnormalities (Powell and Miyakawa, 2006).
Figure 2.
Selective deficit in social interaction in RIM1α−/− mice. Time spent in social interaction with a juvenile target mouse, plotted normalized to respective WT littermate control, is significantly decreased in RIM1α−/− mice versus WT littermate controls (N = 11; p < 0.05). Normal time spent in social interaction was observed in Rab3A−/− (N = 13), Syt1R233Q KI (N = 13), and RIM1αS413A KI (N = 10) mice (p > 0.05). Error bars indicate mean ± SEM, *p < 0.05.
To examine the selectivity of this social deficit for RIM1α knock-out mice versus other presynaptic protein mutants with increased PPF, we tested social interaction in each of the other three presynaptic mutant lines. Rab3A−/−, Syt1R233Q KI, and RIM1αS413A KI mice all demonstrated normal direct juvenile social interaction (Fig. 2b–d). These findings are consistent with a selective pattern of schizophrenia-related behavioral abnormalities in RIM1α knock-out mice compared to the three other presynaptic protein mutants.
Altered response to MK-801 in RIM1α−/− mice
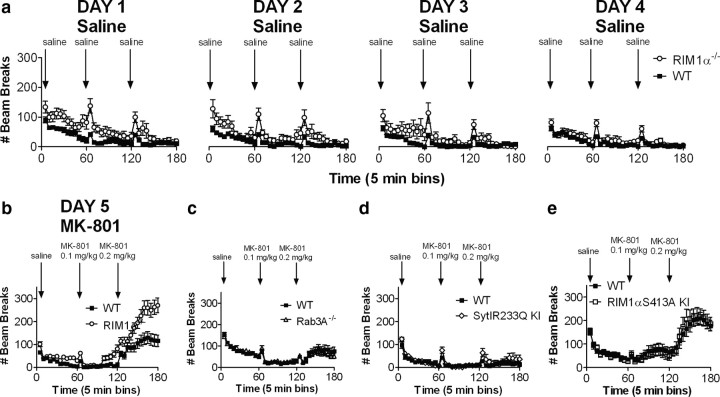
In addition to cognitive deficits (Powell et al., 2004), decreased PPI, and decreased social interaction, RIM1α knock-out mice demonstrated an increased locomotor response to novelty (Fig. 3a,b) and significantly enhanced locomotor activity after administration of the psychotomimetic, noncompetitive NMDA receptor antagonist MK-801 (Fig. 3b). To examine the RIM1α locomotor response to MK-801, we first habituated RIM1α knock-out mice to the locomotor testing apparatus for 4 d until there was no significant difference in locomotor activity in response to the chamber (Fig. 3a, day 4, final injection) (two-way ANOVA with repeated measures, main effect of genotype, F(1,22) = 2.92, p > 0.05). These data demonstrate that the RIM1α deletion-associated increase in locomotor response to novelty habituates over time as the novel context becomes familiar. On day 5, the initial locomotor response to saline injection was not significantly different in RIM1α knock-out mice (first saline injection, two-way ANOVA with repeated measures, main effect of genotype, F(1,22) = 4.12, p > 0.05; main effect of time, F(11,242) = 4.28, p < 0.05; interaction, F(11,242) = 1.31, p > 0.05). The locomotor response to 0.1 and 0.2 mg/kg MK-801, however, was significantly increased compared to wild-type littermate controls, as indicated by a significant interaction between genotype and time for both the 0.1 mg/kg injection (main effect of genotype, F(1,22) = 3.33, p = 0.08; main effect of time, F(11,242) = 4.28, p < 0.05; interaction, F(11,242) = 14.26, p < 0.05) and the 0.2 mg/kg injection (main effect of genotype, F(1,22) = 1.97, p > 0.05; main effect of time, F(11,242) = 14.26, p < 0.05; interaction, F(11,242) = 3.16, p < 0.05). Thus, RIM1α knock-out mice also exhibit increased locomotor response to novelty and increased sensitivity to the locomotor activating effects of the psychotomimetic drug MK-801.
Figure 3.
Exaggerated response to the psychotomimetic drug MK-801 selectively in RIM1α−/− mice. a, Locomotor activity is initially increased in RIM1α−/− mice and habituates over 4 d even with hourly normal saline injections. b, After 4 d of locomotor habituation, RIM1α−/− mice exhibit increased locomotor responses to escalating doses of MK-801 administered intraperitoneally at times indicated by arrows compared to WT littermate controls (N = 12). c–e, No difference in locomotor response to escalating doses of MK-801 in Rab3A−/− (N = 12), SytIR233Q KI (N = 12), or RIM1αS413A KI (N = 11) mutant mice. Higher doses of MK-801 (0.4 and 0.6 mg/kg) also had no significantly different effect on SytIR233Q KI mice (data not shown). Error bars indicate mean ± SEM.
Once again we tested whether the enhanced locomotor response to MK-801 in RIM1α knock-out mice might be a more general effect attributable to presynaptic protein mutation or altered presynaptic short-term plasticity. Rab3A knock-out mice showed no significant alteration in their response to MK-801 at either dose (Fig. 3c). Similarly, Syt1R233Q KI mice did not exhibit any significant alteration in locomotor response to these doses of MK-801 (Fig. 3d). Because the locomotor activation to 0.1 and 0.2 mg/kg of MK-801 appeared to be somewhat less robust in Syt1R233Q KI mice than in the other three mouse lines, we repeated this experiment using two higher doses of MK-801 (0.4 and 0.6 mg/kg), which produced a much more robust locomotor response in both wild-type littermate controls and Syt1R233Q KI mice, but no significant difference between the two groups (data not shown). Finally, RIM1αS413A KI mice also exhibited no significant difference in locomotor response to MK-801 at either dose (Fig. 3e). These data, along with previously published findings in these presynaptic mutants (Powell et al., 2004; Kaeser et al., 2008b), suggest a pattern of selective, schizophrenia-relevant behavioral abnormalities in RIM1α knock-out mice compared to each of the three other presynaptic mutant mouse models, with the notable exception of PPI deficits in the mouse lines with altered presynaptic short-term plasticity.
Discussion
Based on the correlation between the timescale of PPI and the timescale of the presynaptic plasticity phenotypes observed in the genetic mouse mutants, we suggest a model where presynaptic short-term plasticity may play a central role in mediating sensorimotor gating (Fig. 4). In our experiments, all three genetic manipulations that alter the magnitude of PPF, a common form of presynaptic short-term plasticity (Zucker and Regehr, 2002), changed PPI, a measure used to assess sensorimotor gating (Swerdlow et al., 2000), whereas one genetic manipulation that has no effect on presynaptic function does not change PPI. Furthermore, deletion of RIM1α also diminished social interactions and increased the locomotor response to the noncompetitive NMDA receptor antagonist MK-801. These schizophrenia-related behaviors likely operate at a much slower time scale, and are not affected in the genetic deletion of Rab3A and in the Syt1R233Q point mutant mice, suggesting that these deficits depend on RIM1α-mediated synaptic mechanisms that are not directly related to presynaptic short-term plasticity.
Figure 4.
Suggested mechanisms responsible for behavioral abnormalities. Alterations in short-term plasticity [PPF or paired-pulse depression (PPD)] are proposed to be responsible for altered sensorimotor gating or prepulse inhibition of startle (PPI) in the three presynaptic mutants (RIM1α−/−, Rab3A−/−, and SytIR233Q KI). Additional RIM1α−/− associated synaptic abnormalities are proposed to play a role in the broader schizophrenia-relevant phenotypes in these mice.
Prepulse inhibition of acoustic startle experimentally defines the phenomenon of sensorimotor gating, an evolutionarily conserved neuronal mechanism whereby an initial, lower amplitude noise pulse can decrease the response to a louder acoustic startle pulse with as little as a 50–200 ms delay between the two pulses (Braff and Geyer, 1990; Swerdlow et al., 1994, 2000, 2001; Dulawa and Geyer, 1996; Geyer et al., 2002). The activity of several brain regions is known to modify PPI, including limbic cortex, striatum, pallidum, or pontine tegmentum (Swerdlow et al., 2001). Indeed, several neuropsychiatric disorders, among them schizophrenia, bipolar mania, obsessive/compulsive disorder, and panic disorder and are associated with abnormal prepulse inhibition of startle in humans. Although much is known regarding the brain regions involved in modification of PPI, and a neuropharmacology has evolved around the alteration of PPI in a variety of models (Dulawa and Geyer, 1996; Brody et al., 2003, 2004; Swerdlow et al., 2005; Geyer, 2006a), the neurophysiological and synaptic basis of sensorimotor gating remains a mystery.
This manuscript implicates short-term presynaptic plasticity in PPI of acoustic startle and sensorimotor gating in general. Our data provide support for the hypothesis that short-term presynaptic plasticity that occurs on the same time scale as PPI plays a role in, or modulates, sensorimotor gating. Three different presynaptic protein mutant mouse lines with altered CNS short-term synaptic plasticity exhibit significant decreases in PPI. In the case of RIM1α−/− and Syt1R233Q KI mice, the short-term plasticity changes (increased PPF) are also associated with a decrease in initial probability of neurotransmitter release (Fernandez-Chacon et al., 2001; Schoch et al., 2002). In the case of Rab3A−/− mice, however, the increased PPF is not associated with altered release probability (Schoch et al., 2002). As an additional control, RIM1αS413A KI mice that do not show increased PPF also do not show alterations in PPI, indicating some degree of specificity for mouse lines with altered short-term plasticity. The RIM1αS413A KI mice were backcrossed to C57BL/6J only once before testing, although the other lines were backcrossed to C57BL/6J for three or four generations. This presents only a minor caveat to interpretation of this additional control mouse line; it is possible that RIM1αS413A mutant mice may lead to PPI changes after being further backcrossed to C57BL/6J, but these changes are somehow masked by additional 129Sv background remaining in this line. Despite this caveat, the correlation between PPI and PPF in all backgrounds, comparing mutant mice to their littermate controls of the same background, suggest that increased PPF can lead to decreased PPI of acoustic startle responses.
The alterations in PPI observed were not associated with significant alterations in initial startle response amplitude. In fact, in the Syt1R233Q KI and Rab3A−/− mice exhibit opposite trends toward alterations in initial startle response, increased in Rab3A−/− and decreased in Syt1R233Q KI mice. Despite these trends toward opposing effects on initial startle responses, both mice exhibit significant decreases in PPI, making initial startle changes an unlikely cause of the observed PPI deficits in these mouse lines.
Alteration of presynaptic genes linked to schizophrenia has resulted in additional mouse models relevant to the disorder and to PPI alterations. For example, neurexin 1 has been linked in many studies to both schizophrenia (Kirov et al., 2008; Sudhof, 2008; Walsh et al., 2008; Rujescu et al., 2009) and autism (Autism Genome Project Consortium et al., 2007; Sebat et al., 2007; Kim et al., 2008; Marshall et al., 2008; Yan et al., 2008; Zahir et al., 2008; Bucan et al., 2009; Glessner et al., 2009). We recently demonstrated altered PPI in neurexin-1α KO mice, along with increased repetitive grooming behavior and impaired nest building. These behavioral changes were associated with decreased glutamatergic synaptic transmission, but not alterations in PPF (Etherton et al., 2009). A recent synapsin II mouse model relevant to schizophrenia found several putative behavioral abnormalities relevant to schizophrenia including PPI (Dyck et al., 2009). Synapsin II KO mice, however, do not show altered PPF, although they do have enhanced posttetanic potentiation, a form of plasticity with a longer time course on the order of minutes rather than tens to hundreds of milliseconds (Rosahl et al., 1995; Silva et al., 1996). Thus, alterations in PPF are not the only mechanism whereby presynaptic protein mutations can lead to altered PPI.
We suggest that short-term plasticity of synaptic responses in the area of the brainstem startle circuitry or in modulatory regions plays a role in the effect of the prepulse on the startle pulse presented 50–200 ms later. Of course, it may be that alterations in presynaptic proteins and short-term plasticity lead to developmental circuit alterations in the acoustic startle areas or in any one of the many brain regions known to modify PPI. Future studies will focus on conditional regional or postdevelopmental deletions and mutations of presynaptic proteins in an effort to localize the synaptic plasticity deficits contributing to this alteration.
RIM1α appears to be particularly important for a wide variety of classic schizophrenia-relevant behavioral abnormalities (Powell et al., 2004; Powell, 2006; Powell and Miyakawa, 2006). Among the behaviors classically ascribed as schizophrenia relevant in RIM1α−/− mice, but not in RIM1αS413A KI, Rab3A−/−, or Syt1R233Q KI mice, are hippocampus-dependent cognitive function (Powell et al., 2004), locomotor response to novelty stress (Fig. 3) (Powell et al., 2004), social interaction (Fig. 2), locomotor response to psychotomimetics (Fig. 3), and PPI (Fig. 1). Several other behaviors are completely normal in the RIM1α−/− mice including motor coordination and motor learning on the rotarod, locomotor habituation, anxiety in the elevated plus maze and dark/light apparatuses, and nociception (Powell et al., 2004).
It is important to note that deletion of RIM1α in mice results in a relatively wide synaptic deficit in the hippocampus, with distinct phenotypes in different synapses (Castillo et al., 2002; Schoch et al., 2002; Kaeser et al., 2008a), probably because of overlapping functions of multiple RIM isoforms (Schoch et al., 2006; Kaeser et al., 2008a). These phenotypes include a reduction in synaptic strength at excitatory and inhibitory synapses in area CA1 (Schoch et al., 2002; Calakos et al., 2004; Kaeser et al., 2008a), multiple changes in short-term plasticity at excitatory and inhibitory synapses in area CA1, but not in mossy fiber terminal in area CA3 (Schoch et al., 2002; Calakos et al., 2004; Kaeser et al., 2008a), a reduction in the readily releasable pool of vesicles at excitatory hippocampal synapses (Calakos et al., 2004), and a lack of many forms of presynaptic long-term plasticity at multiple synapses in the hippocampus and in other brain areas (Castillo et al., 2002; Huang et al., 2005; Fourcaudot et al., 2006; Chevaleyre et al., 2007). Thus, it is difficult to attribute certain behavioral parameters to a specific from of synaptic plasticity at a defined synapse at this time. We consider the PPI abnormality likely to be mediated by paired-pulse ratios, because (1) the time scale of the behavior matches well with the time scale of the electrophysiological phenomenon, (2) because changes in paired-pulse ratios are the only electrophysiological deficit that is shared in all three lines (Geppert et al., 1994b; Fernandez-Chacon et al., 2001; Schoch et al., 2002), and (3) a related mouse line lacking altered PPF does not show altered PPI. For the other behavioral parameters measured, however, it is premature to draw more precise conclusions beyond the observation that they depend on RIM1α.
A recent study calls into question the relevance of increased locomotor response to novelty for schizophrenia (Perry et al., 2009). Based on findings from human clinical studies, it is possible that this behavioral abnormality may be more akin to bipolar mania in humans and not schizophrenia (Perry et al., 2009). Although assigning a specific neuropsychiatric disorder to a set of rodent behavioral abnormalities is fraught with difficulty, we feel the entire constellation of behavioral abnormalities in the RIM1α KO mice is more consistent with schizophrenia than bipolar mania or other disorders, given the cognitive abnormalities, response to psychotomimetic drugs, and social interaction deficits that are not typical of bipolar mania. That said, it is possible that the PPI alterations and locomotor response to novelty could be interpreted as relevant to other neuropsychiatric disorders such as bipolar mania. It will be of interest to see how these behavioral abnormalities respond to pharmacologic treatments used in humans for both schizophrenia and bipolar mania.
Overall, our data suggest that loss of RIM1α function can lead to selective abnormalities in behaviors typical of many other preclinical mouse models of schizophrenia, although this may also be interpreted as relevant for other neuropsychiatric disorders. More than one presynaptic protein has been implicated in schizophrenia, and the present results suggest the RIM gene family may embody potential candidate genes for human schizophrenia or other neuropsychiatric disorders. The subtleties of how some presynaptic protein abnormalities, but not others, lead to synaptic and circuit-level dysfunction resulting in selective behavioral symptoms will be critical to determine in future studies.
Footnotes
This work was supported by grants from the National Alliance for Research on Schizophrenia and Depression (C.M.P.; Constance and Stephen Lieber Investigator, P.S.K.), the National Institute of Mental Health (C.M.P., T.C.S.), the National Institute of Child Health and Human Development (C.M.P.), the Howard Hughes Medical Institute (T.C.S.), and the Hartwell Foundation (C.M.P.). T.C.S. and P.S.K. supplied the mice, C.M.P. conceived and designed the experiments, C.M.P. and J.B. performed the experiments, C.M.P. performed statistical analysis, and C.M.P. wrote the paper with input from J.B., P.S.K., and T.C.S. We thank current members of the Powell lab for critical reading of this manuscript.
References
- Abbott LF, Regehr WG. Synaptic computation. Nature. 2004;431:796–803. doi: 10.1038/nature03010. [DOI] [PubMed] [Google Scholar]
- Autism Genome Project Consortium. Szatmari P, Paterson AD, Zwaigenbaum L, Roberts W, Brian J, Liu XQ, Vincent JB, Skaug JL, Thompson AP, Senman L, Feuk L, Qian C, Bryson SE, Jones MB, Marshall CR, Scherer SW, Vieland VJ, Bartlett C, Mangin LV, et al. Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat Genet. 2007;39:319–328. doi: 10.1038/ng1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blennow K, Bogdanovic N, Gottfries CG, Davidsson P. The growth-associated protein GAP-43 is increased in the hippocampus and in the gyrus cinguli in schizophrenia. J Mol Neurosci. 1999;13:101–109. doi: 10.1385/JMN:13:1-2:101. [DOI] [PubMed] [Google Scholar]
- Blundell J, Tabuchi K, Bolliger MF, Blaiss CA, Brose N, Liu X, Sudhof TC, Powell CM. Increased anxiety-like behavior in mice lacking the inhibitory synapse cell adhesion molecule neuroligin 2. Genes Brain Behav. 2009;8:114–126. doi: 10.1111/j.1601-183X.2008.00455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden NA, Scott RJ, Tooney PA. Altered gene expression in the superior temporal gyrus in schizophrenia. BMC Genomics. 2008;9:199. doi: 10.1186/1471-2164-9-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braff DL, Geyer MA. Sensorimotor gating and schizophrenia. Human and animal model studies. Arch Gen Psychiatry. 1990;47:181–188. doi: 10.1001/archpsyc.1990.01810140081011. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Brody SA, Geyer MA, Large CH. Lamotrigine prevents ketamine but not amphetamine-induced deficits in prepulse inhibition in mice. Psychopharmacology (Berl) 2003;169:240–246. doi: 10.1007/s00213-003-1421-2. [DOI] [PubMed] [Google Scholar]
- Brody SA, Conquet F, Geyer MA. Effect of antipsychotic treatment on the prepulse inhibition deficit of mGluR5 knockout mice. Psychopharmacology (Berl) 2004;172:187–195. doi: 10.1007/s00213-003-1635-3. [DOI] [PubMed] [Google Scholar]
- Bubenikova-Valesova V, Horacek J, Vrajova M, Hoschl C. Models of schizophrenia in humans and animals based on inhibition of NMDA receptors. Neurosci Biobehav Rev. 2008;32:1014–1023. doi: 10.1016/j.neubiorev.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Bucan M, Abrahams BS, Wang K, Glessner JT, Herman EI, Sonnenblick LI, Alvarez Retuerto AI, Imielinski M, Hadley D, Bradfield JP, Kim C, Gidaya NB, Lindquist I, Hutman T, Sigman M, Kustanovich V, Lajonchere CM, Singleton A, Kim J, Wassink TH, et al. Genome-wide analyses of exonic copy number variants in a family-based study point to novel autism susceptibility genes. PLoS Genet. 2009;5:e1000536. doi: 10.1371/journal.pgen.1000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calakos N, Schoch S, Sudhof TC, Malenka RC. Multiple roles for the active zone protein RIM1alpha in late stages of neurotransmitter release. Neuron. 2004;42:889–896. doi: 10.1016/j.neuron.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Carlsson A, Hansson LO, Waters N, Carlsson ML. A glutamatergic deficiency model of schizophrenia. Br J Psychiatry Suppl. 1999;37:2–6. [PubMed] [Google Scholar]
- Carlsson ML, Carlsson A, Nilsson M. Schizophrenia: from dopamine to glutamate and back. Curr Med Chem. 2004;11:267–277. doi: 10.2174/0929867043456034. [DOI] [PubMed] [Google Scholar]
- Castillo PE, Schoch S, Schmitz F, Sudhof TC, Malenka RC. RIM1alpha is required for presynaptic long-term potentiation. Nature. 2002;415:327–330. doi: 10.1038/415327a. [DOI] [PubMed] [Google Scholar]
- Chen Q, He G, Wang XY, Chen QY, Liu XM, Gu ZZ, Liu J, Li KQ, Wang SJ, Zhu SM, Feng GY, He L. Positive association between synapsin II and schizophrenia. Biol Psychiatry. 2004;56:177–181. doi: 10.1016/j.biopsych.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Heifets BD, Kaeser PS, Sudhof TC, Castillo PE. Endocannabinoid-mediated long-term plasticity requires cAMP/PKA signaling and RIM1alpha. Neuron. 2007;54:801–812. doi: 10.1016/j.neuron.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle JT. Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell Mol Neurobiol. 2006;26:365–384. doi: 10.1007/s10571-006-9062-8. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Tsai G, Goff D. Converging evidence of NMDA receptor hypofunction in the pathophysiology of schizophrenia. Ann N Y Acad Sci. 2003;1003:318–327. doi: 10.1196/annals.1300.020. [DOI] [PubMed] [Google Scholar]
- Drew CJ, Kyd RJ, Morton AJ. Complexin 1 knockout mice exhibit marked deficits in social behaviours but appear to be cognitively normal. Hum Mol Genet. 2007;16:2288–2305. doi: 10.1093/hmg/ddm181. [DOI] [PubMed] [Google Scholar]
- Dulawa SC, Geyer MA. Psychopharmacology of prepulse inhibition in mice. Chin J Physiol. 1996;39:139–146. [PubMed] [Google Scholar]
- Dulawa SC, Geyer MA. Effects of strain and serotonergic agents on prepulse inhibition and habituation in mice. Neuropharmacology. 2000;39:2170–2179. doi: 10.1016/s0028-3908(00)00030-7. [DOI] [PubMed] [Google Scholar]
- Dyck BA, Skoblenick KJ, Castellano JM, Ki K, Thomas N, Mishra RK. Synapsin II knockout mice show sensorimotor gating and behavioural abnormalities similar to those in the phencyclidine-induced preclinical animal model of schizophrenia. Schizophr Res. 2007;97:292–293. doi: 10.1016/j.schres.2007.08.026. [DOI] [PubMed] [Google Scholar]
- Dyck BA, Skoblenick KJ, Castellano JM, Ki K, Thomas N, Mishra RK. Behavioral abnormalities in synapsin II knockout mice implicate a causal factor in schizophrenia. Synapse. 2009;63:662–672. doi: 10.1002/syn.20643. [DOI] [PubMed] [Google Scholar]
- Eastwood SL, Harrison PJ. Decreased expression of vesicular glutamate transporter 1 and complexin II mRNAs in schizophrenia: further evidence for a synaptic pathology affecting glutamate neurons. Schizophr Res. 2005;73:159–172. doi: 10.1016/j.schres.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Eastwood SL, Cotter D, Harrison PJ. Cerebellar synaptic protein expression in schizophrenia. Neuroscience. 2001;105:219–229. doi: 10.1016/s0306-4522(01)00141-5. [DOI] [PubMed] [Google Scholar]
- Etherton MR, Blaiss CA, Powell CM, Sudhof TC. Mouse neurexin-1alpha deletion causes correlated electrophysiological and behavioral changes consistent with cognitive impairments. Proc Natl Acad Sci U S A. 2009;106:17998–18003. doi: 10.1073/pnas.0910297106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Chacon R, Konigstorfer A, Gerber SH, Garcia J, Matos MF, Stevens CF, Brose N, Rizo J, Rosenmund C, Sudhof TC. Synaptotagmin I functions as a calcium regulator of release probability. Nature. 2001;410:41–49. doi: 10.1038/35065004. [DOI] [PubMed] [Google Scholar]
- Fourcaudot E, Humeau Y, Ehrlich ID, Luthi A. Mediation of presynaptic LTP at cortico-amygdala afferents by cAMP and RIM1alpha. Soc Neurosci Abstr. 2006;32:38–12. [Google Scholar]
- Gabriel SM, Haroutunian V, Powchik P, Honer WG, Davidson M, Davies P, Davis KL. Increased concentrations of presynaptic proteins in the cingulate cortex of subjects with schizophrenia. Arch Gen Psychiatry. 1997;54:559–566. doi: 10.1001/archpsyc.1997.01830180077010. [DOI] [PubMed] [Google Scholar]
- Geppert M, Bolshakov VY, Siegelbaum SA, Takei K, De Camilli P, Hammer RE, Sudhof TC. The role of Rab3A in neurotransmitter release. Nature. 1994a;369:493–497. doi: 10.1038/369493a0. [DOI] [PubMed] [Google Scholar]
- Geppert M, Goda Y, Hammer RE, Li C, Rosahl TW, Stevens CF, Sudhof TC. Synaptotagmin I: a major Ca2+ sensor for transmitter release at a central synapse. Cell. 1994b;79:717–727. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- Geppert M, Goda Y, Stevens CF, Sudhof TC. The small GTP-binding protein Rab3A regulates a late step in synaptic vesicle fusion. Nature. 1997;387:810–814. doi: 10.1038/42954. [DOI] [PubMed] [Google Scholar]
- Geyer MA. Behavioral studies of hallucinogenic drugs in animals: implications for schizophrenia research. Pharmacopsychiatry. 1998;31(Suppl 2):S73–S79. doi: 10.1055/s-2007-979350. [DOI] [PubMed] [Google Scholar]
- Geyer MA. Are cross-species measures of sensorimotor gating useful for the discovery of procognitive cotreatments for schizophrenia? Dialogues Clin Neurosci. 2006a;8:9–16. doi: 10.31887/DCNS.2006.8.1/mgeyer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA. The family of sensorimotor gating disorders: comorbidities or diagnostic overlaps? Neurotox Res. 2006b;10:211–220. doi: 10.1007/BF03033358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA, McIlwain KL, Paylor R. Mouse genetic models for prepulse inhibition: an early review. Mol Psychiatry. 2002;7:1039–1053. doi: 10.1038/sj.mp.4001159. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. Reduction of synaptophysin immunoreactivity in the prefrontal cortex of subjects with schizophrenia. Regional and diagnostic specificity. Arch Gen Psychiatry. 1997a;54:943–952. doi: 10.1001/archpsyc.1997.01830220065010. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. Reduction of synaptophysin immunoreactivity in the prefrontal cortex of subjects with schizophrenia. Regional and diagnostic specificity. Arch Gen Psychiatry. 1997b;54:660–669. doi: 10.1001/archpsyc.1997.01830190088009. [DOI] [PubMed] [Google Scholar]
- Glessner JT, Wang K, Cai G, Korvatska O, Kim CE, Wood S, Zhang H, Estes A, Brune CW, Bradfield JP, Imielinski M, Frackelton EC, Reichert J, Crawford EL, Munson J, Sleiman PM, Chiavacci R, Annaiah K, Thomas K, Hou C, et al. Autism genome-wide copy number variation reveals ubiquitin and neuronal genes. Nature. 2009;459:569–573. doi: 10.1038/nature07953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunduz-Bruce H. The acute effects of NMDA antagonism: from the rodent to the human brain. Brain Res Rev. 2009;60:279–286. doi: 10.1016/j.brainresrev.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Law AJ, Eastwood SL. Glutamate receptors and transporters in the hippocampus in schizophrenia. Ann N Y Acad Sci. 2003;1003:94–101. doi: 10.1196/annals.1300.006. [DOI] [PubMed] [Google Scholar]
- Huang YY, Zakharenko SS, Schoch S, Kaeser PS, Janz R, Sudhof TC, Siegelbaum SA, Kandel ER. Genetic evidence for a protein-kinase-A-mediated presynaptic component in NMDA-receptor-dependent forms of long-term synaptic potentiation. Proc Natl Acad Sci U S A. 2005;102:9365–9370. doi: 10.1073/pnas.0503777102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC. Glutamate and schizophrenia: phencyclidine, N-methyl-d-aspartate receptors, and dopamine-glutamate interactions. Int Rev Neurobiol. 2007;78:69–108. doi: 10.1016/S0074-7742(06)78003-5. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Kaeser PS, Kwon HB, Chiu CQ, Deng L, Castillo PE, Sudhof TC. RIM1α and RIM1β are synthesized from distinct promoters of the RIM1 gene to mediate differential but overlapping synaptic functions. J Neurosci. 2008a;28:13435–13447. doi: 10.1523/JNEUROSCI.3235-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeser PS, Kwon HB, Blundell J, Chevaleyre V, Morishita W, Malenka RC, Powell CM, Castillo PE, Sudhof TC. RIM1alpha phosphorylation at serine-413 by protein kinase A is not required for presynaptic long-term plasticity or learning. Proc Natl Acad Sci U S A. 2008b;105:14680–14685. doi: 10.1073/pnas.0806679105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karson CN, Mrak RE, Schluterman KO, Sturner WQ, Sheng JG, Griffin WS. Alterations in synaptic proteins and their encoding mRNAs in prefrontal cortex in schizophrenia: a possible neurochemical basis for “hypofrontality.”. Mol Psychiatry. 1999;4:39–45. doi: 10.1038/sj.mp.4000459. [DOI] [PubMed] [Google Scholar]
- Kim HG, Kishikawa S, Higgins AW, Seong IS, Donovan DJ, Shen Y, Lally E, Weiss LA, Najm J, Kutsche K, Descartes M, Holt L, Braddock S, Troxell R, Kaplan L, Volkmar F, Klin A, Tsatsanis K, Harris DJ, Noens I, et al. Disruption of neurexin 1 associated with autism spectrum disorder. Am J Hum Genet. 2008;82:199–207. doi: 10.1016/j.ajhg.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirov G, Gumus D, Chen W, Norton N, Georgieva L, Sari M, O'Donovan MC, Erdogan F, Owen MJ, Ropers HH, Ullmann R. Comparative genome hybridization suggests a role for NRXN1 and APBA2 in schizophrenia. Hum Mol Genet. 2008;17:458–465. doi: 10.1093/hmg/ddm323. [DOI] [PubMed] [Google Scholar]
- Kwon CH, Luikart BW, Powell CM, Zhou J, Matheny SA, Zhang W, Li Y, Baker SJ, Parada LF. Pten regulates neuronal arborization and social interaction in mice. Neuron. 2006;50:377–388. doi: 10.1016/j.neuron.2006.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahti AC, Holcomb HH, Medoff DR, Tamminga CA. Ketamine activates psychosis and alters limbic blood flow in schizophrenia. NeuroReport. 1995;6:869–872. doi: 10.1097/00001756-199504190-00011. [DOI] [PubMed] [Google Scholar]
- Large CH. Do NMDA receptor antagonist models of schizophrenia predict the clinical efficacy of antipsychotic drugs? J Psychopharmacol. 2007;21:283–301. doi: 10.1177/0269881107077712. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Song JY, Kim JW, Jin SY, Hong MS, Park JK, Chung JH, Shibata H, Fukumaki Y. Association study of polymorphisms in synaptic vesicle-associated genes, SYN2 and CPLX2, with schizophrenia. Behav Brain Funct. 2005;1:15–21. doi: 10.1186/1744-9081-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipska BK, Weinberger DR. To model a psychiatric disorder in animals: schizophrenia as a reality test. Neuropsychopharmacology. 2000;23:223–239. doi: 10.1016/S0893-133X(00)00137-8. [DOI] [PubMed] [Google Scholar]
- Ludewig K, Geyer MA, Vollenweider FX. Deficits in prepulse inhibition and habituation in never-medicated, first-episode schizophrenia. Biol Psychiatry. 2003;54:121–128. doi: 10.1016/s0006-3223(02)01925-x. [DOI] [PubMed] [Google Scholar]
- Marshall CR, Noor A, Vincent JB, Lionel AC, Feuk L, Skaug J, Shago M, Moessner R, Pinto D, Ren Y, Thiruvahindrapduram B, Fiebig A, Schreiber S, Friedman J, Ketelaars CE, Vos YJ, Ficicioglu C, Kirkpatrick S, Nicolson R, Sloman L, et al. Structural variation of chromosomes in autism spectrum disorder. Am J Hum Genet. 2008;82:477–488. doi: 10.1016/j.ajhg.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechri A, Saoud M, Khiari G, d'Amato T, Dalery J, Gaha L. [Glutaminergic hypothesis of schizophrenia: clinical research studies with ketamine] Encephale. 2001;27:53–59. [PubMed] [Google Scholar]
- Mirnics K, Middleton FA, Marquez A, Lewis DA, Levitt P. Molecular characterization of schizophrenia viewed by microarray analysis of gene expression in prefrontal cortex. Neuron. 2000;28:53–67. doi: 10.1016/s0896-6273(00)00085-4. [DOI] [PubMed] [Google Scholar]
- Moghaddam B. Recent basic findings in support of excitatory amino acid hypotheses of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 1994;18:859–870. doi: 10.1016/0278-5846(94)90102-3. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Jackson ME. Glutamatergic animal models of schizophrenia. Ann N Y Acad Sci. 2003;1003:131–137. doi: 10.1196/annals.1300.065. [DOI] [PubMed] [Google Scholar]
- Mudge J, Miller NA, Khrebtukova I, Lindquist IE, May GD, Huntley JJ, Luo S, Zhang L, van Velkinburgh JC, Farmer AD, Lewis S, Beavis WD, Schilkey FD, Virk SM, Black CF, Myers MK, Mader LC, Langley RJ, Utsey JP, Kim RW, et al. Genomic convergence analysis of schizophrenia: mRNA sequencing reveals altered synaptic vesicular transport in post-mortem cerebellum. PLoS One. 2008;3:e3625. doi: 10.1371/journal.pone.0003625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller DJ, Klempan TA, De Luca V, Sicard T, Volavka J, Czobor P, Sheitman BB, Lindenmayer JP, Citrome L, McEvoy JP, Lieberman JA, Honer WG, Kennedy JL. The SNAP-25 gene may be associated with clinical response and weight gain in antipsychotic treatment of schizophrenia. Neurosci Lett. 2005;379:81–89. doi: 10.1016/j.neulet.2004.12.037. [DOI] [PubMed] [Google Scholar]
- Noda Y, Mouri A, Waki Y, Nabeshima T. [Development of animal models for schizophrenia based on clinical evidence: expectation for psychiatrists] Nihon Shinkei Seishin Yakurigaku Zasshi. 2009;29:47–53. [PubMed] [Google Scholar]
- Paz RD, Tardito S, Atzori M, Tseng KY. Glutamatergic dysfunction in schizophrenia: from basic neuroscience to clinical psychopharmacology. Eur Neuropsychopharmacol. 2008;18:773–786. doi: 10.1016/j.euroneuro.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry W, Minassian A, Paulus MP, Young JW, Kincaid MJ, Ferguson EJ, Henry BL, Zhuang X, Masten VL, Sharp RF, Geyer MA. A reverse-translational study of dysfunctional exploration in psychiatric disorders: from mice to men. Arch Gen Psychiatry. 2009;66:1072–1080. doi: 10.1001/archgenpsychiatry.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell CM. Gene targeting of presynaptic proteins in synaptic plasticity and memory: Across the great divide. Neurobiol Learn Mem. 2006;85:2–15. doi: 10.1016/j.nlm.2005.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell CM, Miyakawa T. Schizophrenia-relevant behavioral testing in rodent models: a uniquely human disorder? Biol Psychiatry. 2006;59:1198–1207. doi: 10.1016/j.biopsych.2006.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell CM, Schoch S, Monteggia L, Barrot M, Matos MF, Feldmann N, Sudhof TC, Nestler EJ. The presynaptic active zone protein RIM1alpha is critical for normal learning and memory. Neuron. 2004;42:143–153. doi: 10.1016/s0896-6273(04)00146-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RC, Roche JK, Conley RR. Differential synaptic changes in the striatum of subjects with undifferentiated versus paranoid schizophrenia. Synapse. 2008;62:616–627. doi: 10.1002/syn.20534. [DOI] [PubMed] [Google Scholar]
- Rosahl TW, Spillane D, Missler M, Herz J, Selig DK, Wolff JR, Hammer RE, Malenka RC, Sudhof TC. Essential functions of synapsins I and II in synaptic vesicle regulation. Nature. 1995;375:488–493. doi: 10.1038/375488a0. [DOI] [PubMed] [Google Scholar]
- Rujescu D, Ingason A, Cichon S, Pietiläinen OP, Barnes MR, Toulopoulou T, Picchioni M, Vassos E, Ettinger U, Bramon E, Murray R, Ruggeri M, Tosato S, Bonetto C, Steinberg S, Sigurdsson E, Sigmundsson T, Petursson H, Gylfason A, Olason PI, et al. Disruption of the neurexin 1 gene is associated with schizophrenia. Hum Mol Genet. 2009;18:988–996. doi: 10.1093/hmg/ddn351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada K, Young CE, Barr AM, Longworth K, Takahashi S, Arango V, Mann JJ, Dwork AJ, Falkai P, Phillips AG, Honer WG. Altered immunoreactivity of complexin protein in prefrontal cortex in severe mental illness. Mol Psychiatry. 2002;7:484–492. doi: 10.1038/sj.mp.4000978. [DOI] [PubMed] [Google Scholar]
- Sawada K, Barr AM, Nakamura M, Arima K, Young CE, Dwork AJ, Falkai P, Phillips AG, Honer WG. Hippocampal complexin proteins and cognitive dysfunction in schizophrenia. Arch Gen Psychiatry. 2005;62:263–272. doi: 10.1001/archpsyc.62.3.263. [DOI] [PubMed] [Google Scholar]
- Scarr E, Gray L, Keriakous D, Robinson PJ, Dean B. Increased levels of SNAP-25 and synaptophysin in the dorsolateral prefrontal cortex in bipolar I disorder. Bipolar Disord. 2006;8:133–143. doi: 10.1111/j.1399-5618.2006.00300.x. [DOI] [PubMed] [Google Scholar]
- Schoch S, Castillo PE, Jo T, Mukherjee K, Geppert M, Wang Y, Schmitz F, Malenka RC, Sudhof TC. RIM1alpha forms a protein scaffold for regulating neurotransmitter release at the active zone. Nature. 2002;415:321–326. doi: 10.1038/415321a. [DOI] [PubMed] [Google Scholar]
- Schoch S, Mittelstaedt T, Kaeser PS, Padgett D, Feldmann N, Chevaleyre V, Castillo PE, Hammer RE, Han W, Schmitz F, Lin W, Sudhof TC. Redundant functions of RIM1alpha and RIM2alpha in Ca(2+)-triggered neurotransmitter release. EMBO J. 2006;25:5852–5863. doi: 10.1038/sj.emboj.7601425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebat J, Sebat J, Lakshmi B, Malhotra D, Troge J, Lese-Martin C, Walsh T, Yamrom B, Yoon S, Krasnitz A, Kendall J, Leotta A, Pai D, Zhang R, Lee YH, Hicks J, Spence SJ, Lee AT, Puura K, Lehtimäki T, et al. Strong association of de novo copy number mutations with autism. Science. 2007;316:445–449. doi: 10.1126/science.1138659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen YC, Liao DL, Chen JY, Wang YC, Lai IC, Liou YJ, Chen YJ, Luu SU, Chen CH. Resequencing and association study of vesicular glutamate transporter 1 gene (VGLUT1) with schizophrenia. Schizophr Res. 2009;115:254–260. doi: 10.1016/j.schres.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Rosahl TW, Chapman PF, Marowitz Z, Friedman E, Frankland PW, Cestari V, Cioffi D, Sudhof TC, Bourtchuladze R. Impaired learning in mice with abnormal short-lived plasticity. Curr Biol. 1996;6:1509–1518. doi: 10.1016/s0960-9822(96)00756-7. [DOI] [PubMed] [Google Scholar]
- Sodhi M, Wood KH, Meador-Woodruff J. Role of glutamate in schizophrenia: integrating excitatory avenues of research. Expert Rev Neurother. 2008;8:1389–1406. doi: 10.1586/14737175.8.9.1389. [DOI] [PubMed] [Google Scholar]
- Stone JM, Morrison PD, Pilowsky LS. Glutamate and dopamine dysregulation in schizophrenia—a synthesis and selective review. J Psychopharmacol. 2007;21:440–452. doi: 10.1177/0269881106073126. [DOI] [PubMed] [Google Scholar]
- Sudhof TC. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 2008;455:903–911. doi: 10.1038/nature07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Braff DL, Taaid N, Geyer MA. Assessing the validity of an animal model of deficient sensorimotor gating in schizophrenic patients. Arch Gen Psychiatry. 1994;51:139–154. doi: 10.1001/archpsyc.1994.03950020063007. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Braff DL, Geyer MA. Animal models of deficient sensorimotor gating: what we know, what we think we know, and what we hope to know soon. Behav Pharmacol. 2000;11:185–204. doi: 10.1097/00008877-200006000-00002. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology (Berl) 2001;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Shoemaker JM, Light GA, Braff DL, Stevens KE, Sharp R, Breier M, Neary A, Auerbach PP. Convergence and divergence in the neurochemical regulation of prepulse inhibition of startle and N40 suppression in rats. Neuropsychopharmacology. 2005;31:506–515. doi: 10.1038/sj.npp.1300841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuchi K, Blundell J, Etherton MR, Hammer RE, Liu X, Powell CM, Sudhof TC. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science. 2007;318:71–76. doi: 10.1126/science.1146221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachikawa H, Harada S, Kawanishi Y, Okubo T, Suzuki T. Polymorphism of the 5′-upstream region of the human SNAP-25 gene: an association analysis with schizophrenia. Neuropsychobiology. 2001;43:131–133. doi: 10.1159/000054880. [DOI] [PubMed] [Google Scholar]
- Vawter MP, Thatcher L, Usen N, Hyde TM, Kleinman JE, Freed WJ. Reduction of synapsin in the hippocampus of patients with bipolar disorder and schizophrenia. Mol Psychiatry. 2002;7:571–578. doi: 10.1038/sj.mp.4001158. [DOI] [PubMed] [Google Scholar]
- Verma R, Kubendran S, Das SK, Jain S, Brahmachari SK. SYNGR1 is associated with schizophrenia and bipolar disorder in southern India. J Hum Genet. 2005;50:635–640. doi: 10.1007/s10038-005-0307-z. [DOI] [PubMed] [Google Scholar]
- Walsh T, McClellan JM, McCarthy SE, Addington AM, Pierce SB, Cooper GM, Nord AS, Kusenda M, Malhotra D, Bhandari A, Stray SM, Rippey CF, Roccanova P, Makarov V, Lakshmi B, Findling RL, Sikich L, Stromberg T, Merriman B, Gogtay N, et al. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science. 2008;320:539–543. doi: 10.1126/science.1155174. [DOI] [PubMed] [Google Scholar]
- Weinberger DR. Genetic mechanisms of psychosis: in vivo and postmortem genomics. Clin Ther. 2005;27(Suppl A):S8–S15. doi: 10.1016/j.clinthera.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Yan J, Noltner K, Feng J, Li W, Schroer R, Skinner C, Zeng W, Schwartz CE, Sommer SS. Neurexin 1alpha structural variants associated with autism. Neurosci Lett. 2008;438:368–370. doi: 10.1016/j.neulet.2008.04.074. [DOI] [PubMed] [Google Scholar]
- Zahir FR, Baross A, Delaney AD, Eydoux P, Fernandes ND, Pugh T, Marra MA, Friedman JM. A patient with vertebral, cognitive and behavioural abnormalities and a de novo deletion of NRXN1alpha. J Med Genet. 2008;45:239–243. doi: 10.1136/jmg.2007.054437. [DOI] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]