Abstract
LIM-homeodomain transcription factors (LIM-HDs) are essential in tissue patterning and differentiation. But their expression patterns in the inner ear are largely unknown. Here we report on a study of twelve LIM-HDs, by their tempo-spatial patterns that imply distinct yet overlapping roles, in the developing mouse inner ear. Expression of Lmx1a and Isl1 begins in the otocyst stage, with Lmx1a exclusively in the non-sensory and Isl1 in the prosensory epithelia. The second wave of expression at E12.5 includes Lhx3, 5, 9, Isl2 and Lmx1b in the differentiating sensory epithelia with cellular specificities. With the exception of Lmx1a and Lhx3, all LIM-HDs are expressed in ganglion neurons. Expression of multiple LIM-HDs within a cell type suggests their redundant function.
Keywords: LIM-HD, development, Inner ear, hair cells, differentiation
Introduction
Development of the mammalian inner ear begins with the otic placode formation, through thickening of the ectoderm adjacent to the developing hindbrain. Subsequently, the otic placode invaginates to form the otic vesicle, which further undergoes a series of morphogenetic changes, to ultimately give rise to a complex inner ear of six sensory organs consisting of three cristae, two vestibular maculae and a cochlea (Bryant et al., 2002; Barald and Kelley, 2004). Delaminating from the otocyst are neuroblasts that develop into auditory and vestibular neurons during later stages. Within each sensory organ, sensory patches form initially from a population of proliferating progenitors that exit the cell cycle as postmitotic committed precursors. Production of hair cells, the sensory cells of the inner ear, is determined by the function of Atoh1 (Math1), a bHLH transcription factor (Bermingham et al., 1999; Zheng and Gao, 2000; Kawamoto et al., 2003; Shou et al., 2003). Concomitantly, the Notch signaling pathway plays essential roles in the patterning of the inner ear sensory patch, establishing the arrayed configuration of hair cells separated by supporting cells (Kelley, 2006).
LIM-homeodomain transcription factors (LIM-HD) are characterized by two zinc finger motifs: the LIM domain for protein/protein interactions, and a homeodomain for binding to the DNA control elements of target genes. LIM-HDs have been studied extensively, in particular in spinal cord sub-type neuron specification and in pituitary gland development (Hobert and Westphal, 2000; Shirasaki and Pfaff, 2002). For example, in the mouse spinal cord, Lhx3 is necessary for the generation of motor neurons, whereas co-expression of Lhx3 and Isl1 results in production of interneurons (Thaler et al., 2002). Such combinatorial functions of multiple LIM-HDs within a particular cell population are widely used in spinal cord neuron sub-type specification (Allan and Thor, 2003). Increasingly, LIM-HDs are found to be essential in defining stem/progenitor cells properties, such as the role of Isl1 in the specification of heart progenitor cells and Lhx2 in determining the fate of hair follicle stem cells (Laugwitz et al., 2005; Moretti et al., 2006; Rhee et al., 2006). Twelve LIM-HD members have been described in the mouse genome, and can be divided into six sub-groups based on their sequence similarities (Hunter and Rhodes, 2005).
We and others have previously shown that Isl1, a LIM-HD member, is expressed in the proliferating progenitor pools that give rise to the inner ear sensory patch (Li et al., 2004; Radde-Gallwitz et al., 2004). Lhx3 expression has been found only in hair cells (Sage et al., 2005). A spontaneous mutation in the murine Lmx1a gene causes deafness due to the lack of inner ear formation, indicating an essential role of Lmx1a during early inner ear development (Millonig et al., 2000; Chizhikov et al., 2006). However, most LIM-HDs have not been examined for their expression profile and none has been linked to any pathway in the developing inner ear.
In this study, we systematically examined expression of all LIM-HDs in the developing mouse inner ear. We found that many LIM-HD genes are expressed during inner ear development, with expression patterns that encompass early progenitor cells, differentiating sensory epithelia and ganglion neurons. The expression patterns of LIM-HDs suggest that they play diverse roles in the development and function of multi-inner ear cell types.
Results
As the first step in elucidating potential roles for LIM-HDs during inner ear development, we systematically examined their expression patterns in developing mouse inner ears using RNA in situ hybridization. We chose stages from E10.5 to P6, encompassing otocyst development, inner ear compartmentalization, hair cell fate specification and cell differentiation. For early developmental stages, we used ISL1 and PAX2 immunostaining to mark the presumptive sensory epithelia (Li et al., 2004; Radde-Gallwitz et al., 2004). In addition, antibodies to ISL2 and LHX3 were used. For in situ hybridization, we validated the ribo-probes for each LIM-HD gene by distinct expression patterns in the brain. Lhx1, 2, 4, 5, 6, 9 and Lmx1b labeled different areas of the medulla oblongata, and Lhx2, 4 and Lmx1b also labeled areas of the retina. Lhx2 staining was strong in olfactory epithelium and Lhx8 was primarily expressed in the first branchial arch and notochord at E10.5 (data not shown). The expression patterns of the LIM-HD probes in brain matched the published results (Hunter and Rhodes, 2005). In the inner ear, fragments of all the LIM-HD gene-coding regions were amplified by RT-PCR (data not shown), yet Lhx1, 2, 4 and 8 were not detected by in situ hybridization. It is likely that the expression levels of the four LIM-HDs are below the threshold of detection sensitivity by in situ hybridization.
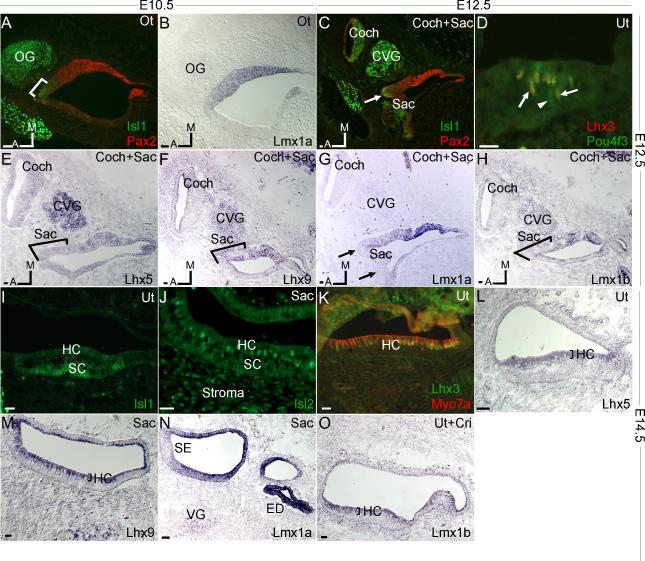
At E10.5, the otocyst is composed of progenitor cells destined to become sensory and non-sensory epithelial parts of the inner ear. Of the LIM-HDs examined, two of them, Isl1 and Lmx1a, were distinctly expressed at this stage (Fig.1A,B). As shown previously, labeling of ISL1 delineated the future sensory epithelium and otic ganglions (OG), whereas PAX2-positive domain defined the non-sensory and a portion of the sensory epithelium (overlapping partially with Isl1) (Fig. 1A, bracket) (Burton et al., 2004; Radde-Gallwitz et al., 2004). At this stage, expression of Lmx1a was prominently detected in the medial portion of the otocyst that overlapped almost completely with the PAX2-positive domain, indicating that Lmx1a likely marked the future non-sensory epithelium (Fig.1B). Unlike Isl1, Lmx1a expression was not detected in the otic ganglions. At E10.5, most otocyst cells are proliferating, suggesting that Isl1 and Lmx1a have roles in proliferating sensory and non-sensory progenitor cells, in addition to their functions in postmitotic cells (see below).
Figure. 1.
Expression of LIM-HDs in the developing inner ear: E10.5 (A,B), E12.5 (C-H) and E14.5 (I-O). Each stage was labeled with sidebars. A: ISL1 labeling in the region of future sensory epithelia (bracket) was distinct from primarily non-sensory PAX2 expression (red) in the medial portion of otocyst (Ot). OG, otic ganglions. B: In adjacent section, prominent Lmx1a expression was in a region overlapping with PAX2 expression. C: At E12.5, ISL1 expression was prominent in primordial cochlea (coch), cochleovestibular ganglions (CVG) and the developing saccular sensory epithelia, which overlapped slightly with PAX2 expression (arrow). D: Labeling with antibodies against LHX3 and POU4F3 showed immunoreactivities in nascent utricular hair cells. Most hair cells were LHX3 and POU4F3 double-positive (arrows to show example), whereas occasional early hair cells were only positive for POU4F3 (arrowhead). E-H: Expression of Lhx5, Lhx9, Lmx1a and Lmx1b in the inner ear at E12.5. Among them, only Lhx5 was expressed in the primordial cochlea (Fig.1E). Lmx1a was detected in the non-sensory epithelial region, but undetectable in the sensory epithelium positive for ISL1 (arrows, comparing to 1C) or in the cochleovestibular ganglions (Fig.1G). The sensory epithelial region of saccule is indicated by brackets, which correspond with ISL1 staining in adjacent section (data not shown). I: ISL1 expression in utricle at E14.5 was mainly in supporting cells (SC), with little expression in hair cells (HC). J: ISL2 expression was higher in saccular hair cells, weak in supporting cells and stroma. K: LHX3 expression was only in the utricular hair cells, coinciding with Myosin VIIa (MYO7A). L, M and O: Lhx5, Lhx9 and Lmx1b were detected in the sensory epithelial cell region. N: Robust Lmx1a expression was restricted to non-sensory epithelia including endolymphatic duct (ED), and was excluded in the vestibular ganglions (VG). Ut, utricle; Sac, saccule; Cri, crista; SE, sensory epithelia. Scale bar=20μm.
At E12.5, the otocyst undergoes morphological changes with the formation of the immature vestibular organs. The future cochlea is not yet developed and the primordial cochlear region is mainly comprised of progenitor cells exiting cell cycle (Chen et al., 2002). In the vestibular system, the sensory epithelium begins to differentiate into hair cells and supporting cells, by the appearance of nascent hair cells expressing Atoh1 and Pou4f3, two hair cell-specific transcription factors (Xiang et al., 1998; Bermingham et al., 1999). Prominent ISL1 labeling was restricted to a region of differentiating sensory epithelium, which partially overlapped with PAX2 labeling (Fig.1C, arrow). Expression of additional LIM-HDs became detectable at this stage including Lhx3, 5, 9 and Lmx1b (Fig.1D-F, H). Following hair cell specification by Atoh1, Pou4f3 is one of the earliest transcription factors expressed in all differentiating hair cells (Xiang et al., 1998). To delineate the timing between Lhx3 and Pou4f3 expression, double labeling was performed with anti-POU4F3 and anti-LHX3 antibodies. POU4F3 was detected in all hair cells yet only a subset of them was found to produce LHX3 (Fig.1D). Therefore, developmentally, the expression of Lhx3 was likely down-stream of Pou4f3. Weak expression of Lhx5 and Lhx9 was detected in the developing saccule outside the sensory epithelial region and cochleovestibular ganglions (CVG), with Lhx5 but not Lhx9 detected in primordial cochlea (Fig.1E,F). Consistent with E10.5, Lmx1a was present only in the non-sensory epithelial region of saccule, and absent in CVG or cochlea (Fig.1G). Comparison between Figures 1C and 1G showed that expression of Isl1 and Lmx1a is mutually exclusive. Lmx1b was weakly expressed in CVG and saccule, but not in the cochlea (Fig.1H). Among all the LIM-HDs expressed at this stage, only Isl1 and Lhx5 showed expression in the primordial cochlear region (Fig.1C,E).
By E14.5, the vestibular organs are further developed, with an increased number of differentiating hair cells. ISL1 was predominantly stained in supporting cells, with decreased production in hair cells (Fig.1I), suggesting that Isl1 expression may no longer be required for future hair cell differentiation. ISL2 labeling started to appear in hair cells, but was also found weakly in supporting cells and stroma (Fig.1J). Labeling of LHX3 was upregulated and confined to hair cells (Fig.1K), whereas Lhx5, 9 and Lmx1b were weakly expressed in the sensory epithelium, mainly in hair cells (Fig.1L,M,O). Lmx1a was significantly upregulated in the non-sensory epithelium region including the endolymphatic duct (Fig.1N).
At E16.5, LIM-HD expression patterns became more defined. In the vestibular system, ISL1 labeling was most prominent in the supporting cells, and remained weak in some hair cells (Fig.2A). ISL2 was detected at a level similar to E14.5, higher in hair cells than in supporting cells or stroma (Fig.2B). Prominent LHX3 labeling was confined to hair cells (Fig.2C). Lhx5 expression showed an interesting pattern: its expression alternated between hair cell and supporting cell regions, in which the expression in two discrete hair cell regions (below the lumen, brackets) was separated by expression in the supporting cell region (above the basal lamina, bracket) (Fig.2D). It is unclear about the structural significance of the expression pattern, as it does not entirely correlate with striola vs. extra-striola regions (data not shown). Lhx6, 9 and Lmx1b were weakly expressed in the sensory epithelium (Fig.2E,F,H). Expression of Lmx1a, however, was markedly reduced and restricted to the transitional epithelium and dark cells (Fig.2G, brackets).
Figure 2.
Expression of LIM-HDs in E16.5 vestibular system (A-H) and cochlea (I-P). A: ISL1 expression was mainly in utricular supporting cells. B: ISL2 expression was similar to E14.5, slightly stronger in hair cells than in supporting cells or stroma. C: LHX3 expression was upregulated in hair cells. D: In utricle, Lhx5 expression was in two regions associated with hair cells (brackets near the lumen), which was separated by a region with supporting cell expression (bracket close to the basal lamina). E, F and H: Lhx6, 9 and Lmx1b showed expression primarily in hair cell region. G: Lmx1a expression was greatly reduced and restricted to the transitional epithelium and dark cells of utricle and crista (brackets). I: ISL1 was expressed in all cochlear epithelial cells including hair cells and spiral ganglions (SG). J: ISL2 was widely expressed in the cochlea, with the labeling more prominent in hair cells. K: LHX3 was specifically expressed in hair cells. L,M,N,P: Lhx5, 6, 9 and Lmx1b were weakly expressed in cochlear hair cells, GER and SG. O: Lmx1a was expressed in the non-sensory epithelia (NSE), including primordial spiral prominence, stria vascularis and Reissner's membrane, as demarcated by the bracket. Scale bar=20μm.
In the E16.5 cochlea, hair cells have completed fate determination from the base to the apex, and are undergoing differentiation. Distinct supporting cell subtypes in the organ of Corti are clearly visible. ISL1 was prominently detectable in all cochlear epithelial cells (Fig.2I). This observation contrasted to a previous study in which Isl1 was no longer detectable at this stage (Radde-Gallwitz et al., 2004). In fact Isl1 expression was also detected at much later stages, including the P6 postnatal inner ear (Fig.3I,J). It is likely that the protocols we used for immunohistochemistry and in situ hybridization were more sensitive, allowing detection of Isl1 at later developmental stages. ISL2 was widely detected, with a slightly higher labeling level in hair cells than in other cells including supporting cells and the greater epithelial ridge (GER, Fig.2J). Similar to the vestibular system, LHX3 only appeared in the cochlear hair cells (Fig. 2K), while Lhx5, 6, 9 and Lmx1b were detected in hair cells and GER (Fig.2L,M,N,P). Lmx1a was exclusively expressed in the non-sensory epithelial cell (NSE) regions that will give rise to spiral prominence, primordial stria vascularis and Reissner's membrane (Fig.2O, bracket). All the LIM-HDs maintained their expression in the spiral ganglions, with the exception of Lmx1a and Lhx3 (data not shown).
Figure 3.
Expression of LIM-HDs in E18.5 cochlea (A-H) and P6 inner ear (I-P). Each stage was labeled with sidebars. A: ISL1 was down-regulated in hair cells at E18.5, while was maintained in all other cochlear cells. B: ISL2 showed the same expression pattern as in E16.5, with slightly higher expression in hair cells than in other cochlear cells. C: Isl2 in situ hybridization showed the same pattern as shown with immunostaining, including spiral ganglions (SG). D: LHX3 showed the same hair cell expression pattern as in E16.5. E, F and H: Expression of Lhx6, Lhx9 and Lmx1b was similar to their respective expression patterns in E16.5. G: Expression of Lmx1a was maintained in the spiral prominence (SP, arrow) and down-regulated in the marginal cells (MC, arrowhead). I: At P6, ISL1 expression was completely absent in hair cells and most supporting cells, while it was maintained in Hensen cells (HeC) and GER. J: Isl1 in situ hybridization showed the result that matched the immunostaining in 3I with the exception in Hensen cells, which may indicate that the immunostaining had higher sensitivity. K: ISL2 expression was up-regulated in hair cells while was maintained at lower level in supporting cells and GER. L: Isl2 in situ hybridization showed expression patterns nearly identical to that obtained from immunostaining study (3K). M,O: Expression of Lhx3 and 9 in P6 utricle and cochlea. N: Lhx5 expression was in the P6 cochlear hair cells, spiral ganglion neurons and very weakly in the GER. P: Lmx1a expression was significantly down-regulated in the transitional epithelium of utricle (bracket). Scale bar=20μm.
From E18.5 to P6, the vestibular system undergoes maturation with highly differentiated hair cells by P6. In the cochlea, the patterning of the organ of Corti and hair cell specification is complete by E18.5 (Chen et al., 2002), with hair cells expressing functional channel genes by P6 (Kros, 2007). Expression patterns of LIM-HDs at E18.5 were generally maintained as in E16.5, but with changed expression levels. In the vestibular system, ISL1 labeling was mainly in supporting cells and ISL2 was slightly up-regulated in hair cells (data not shown). The expression patterns of Lhx3, 5, 6, 9 and Lmx1b were consistent with that found in E16.5, with Lmx1a expression further reduced (data not shown). In E18.5 cochlea, ISL1 labeling was greatly reduced in hair cells but maintained in other cochlear cell types and GER (Fig.3A). ISL1 expression was subsequently down-regulated at P6 in all cochlear cells, with the exception of Hensen cells and GER (Fig.3I). In situ hybridization with an Isl1 ribo-probe showed an expression pattern at P6 that matched the antibody study (Fig.3J). In contrast, ISL2 level was maintained in hair cells, supporting cells and GER from E18.5 to P6 (Fig.3B,K). As ISL2 labeling was generally weaker in comparison to ISL1, we further performed in situ hybridization to verify the immunostaining results. For both E18.5 and P6, in situ hybridization of Isl2 showed patterns that matched the immunostaining obtained by using the antibody (Fig.3C,L). Prominent hair cell labeling of LHX3 was maintained in both E18.5 cochlea (Fig.3D) and P6 utricle (Fig.3M). Lhx5, 6, 9 and Lmx1b were continuously expressed in cochlear epithelium from E18.5 to P6 (Fig.3E,F,H,N,O). There was continuous down-regulation of Lmx1a in E18.5 cochlea, with expression maintained in the spiral prominence (Fig.3G, arrow), but down-regulated in marginal cells (Fig.3G, arrowhead). Little Lmx1a expression remained in the P6 utricular dark cells and transitional epithelium (Fig.3P, bracket). Table 2 summarizes LIM-HD expression patterns during inner ear development.
Table 2.
Summary of LIM-HD expression in developing inner ear. Level of expression is determined by observation of signal of a particular LIM-HD throughout the developmental stages.
| Lhx3 | Lhx5 | Lhx6 | Lhx9 | Isl1 | Isl2 | Lmx1a | Lmx1b | ||
|---|---|---|---|---|---|---|---|---|---|
| E10.5 | |||||||||
| Ot | – | – | – | – | + | – | + | – | |
| OG | – | – | – | – | + | – | – | – | |
| E12.5 | |||||||||
| Ves | +H | + | – | + | + | + | +NSE | + | |
| Coch | – | + | – | – | + | – | – | – | |
| CVG | – | + | – | + | + | – | –_ | + | |
| E14.5 | |||||||||
| Ves | +H | +SE | +SE | + | + | + | +NSE | +SE | |
| GN | – | + | + | + | + | + | – | + | |
| E16.5 | |||||||||
| Ves | +H | +SE | +SE | +SE | +S | +H,S | +NSE | +SE | |
| Coch | +H | + | + | + | + | + | +NSE | + | |
| GN | – | + | + | + | + | + | – | + | |
| E18.5 | |||||||||
| Ves | +H | +SE | +SE | +SE | +S | +H,S | +NSE | +SE | |
| Coch | +H | + | + | + | +S,GER | + | +SP | + | |
| GN | – | + | + | + | + | + | –_ | + | |
| P6 | |||||||||
| SE | +H | + | + | + | +HeC,GER | +H,S | +NSE | + | |
| GN | – | + | + | + | + | + | – | + |
The expression levels are classified +: detected; -: no expression. Ot- otocyst; OG- otic ganglions; Ves- vestibule; Coch- cochlea; CVG- cochleovestibular ganglions; GN- ganglion neurons; SE- sensory epithelia (include both hair cells and supporting cells); H- expression in hair cells; S- expression in supporting cells; GER- the greater epithelial ridge; NSE- non-sensory epithelia; HeC- Hensen cells.
Discussion
We comprehensively examined the expression patterns of all twelve known LIM-HDs in the developing mouse inner ear. The overlapping yet distinct expression profile of individual LIM-HDs, from the early proliferating progenitors (Isl1 and Lmx1a) to differentiating sensory epithelia (Lhx3, 5, 6, 9, Isl2 and Lmx1b), suggests that these proteins are involved in a number of developmental events in different cell types at different developmental stages. We used immunostaining and in situ hybridization to study Isl1 and Isl2 expression patterns, and observed the same pattern with the two methods, which further supported our results in general.
Over two thousand transcription factors have been identified in the mammalian genome, with only a handful studied in the inner ear for their expression patterns or functional roles (Gray et al., 2004). LIM-HDs are a well-studied protein family, particularly for their functions in the neuron sub-type specification, pituitary gland development and stem cell properties (Hobert and Westphal, 2000; Allan and Thor, 2003; Hunter and Rhodes, 2005; Andersson et al., 2006; Rhee et al., 2006). All LIM-HDs examined by in situ hybridization or immunostaining, with the exception of Lmx1a and Lhx3, are expressed in inner ear ganglion neurons at various stages, suggesting their roles in the development of cochleovestibular ganglions. Mouse knockout models have been produced for all the LIM-HDs (Hunter and Rhodes, 2005; Liodis et al., 2007). In all cases, LIM-HD knockouts showed discernable phenotype changes, with many showing abnormalities in neuronal development, demonstrating important functional roles of LIM-HDs in general. However, the effects on inner ear development in the LIM-HD knockout mice have not been investigated with the exception of Lmx1a. Our systematic analysis of LIM-HDs expression in the inner ear sets the stage for further investigation of their functional roles.
Lmx1a expression, which is present from E10.5 otocyst to later in inner ear development, has been consistent with its non-sensory epithelial origin. Its early expression largely overlaps with that of Pax2, which is involved in specification within the cochlear duct, in particular in the region between the stria vascularis and cochlear sensory epithelia (Burton et al., 2004). However, unlike Pax2 that is also expressed in prosensory patches and hair cells at later stages, Lmx1a expression is exclusively in the non-sensory epithelia, including the vestibular transitional epithelia, the saccular roof (data not shown), dark cells, strial vascularis and strial prominence. The expression pattern of Lmx1a makes it a good marker for lineage tracing study of non-sensory epithelial cell types. In a spontaneous homozygous Lmx1a mouse mutant, dreher, both cochlea and vestibular maculae fail to develop and the mice exhibit abnormalities including circling, head-tossing, and deafness (Millonig et al., 2000) (http://www.informatics.jax.org/javawi2/servlet/WIFetch?page=alleleDetail&key=51666), suggesting that normal development of non-sensory epithelia is disrupted in the absence of LMX1A, and Lmx1a is essential for the genesis of the inner ear. Future studies of the dreher mutant inner ear, based on the Lmx1a expression pattern, should help to elucidate the mechanism underlying the specification of non-sensory epithelium and its relationship to the development of the inner ear in general.
Early Isl1 expression in the otocyst suggests that it may have a role in cell lineage specification of the prosensory progenitors (Li et al., 2004; Radde-Gallwitz et al., 2004), and its subsequent downregulation in differentiating hair cells may be important for their proper differentiation. This is supported by a separate study in which Lhx3 overexpression results in suppression of Isl1 expression in the cochlea (ZY Chen, manuscript in preparation). The non-overlapping, sensory vs. non-sensory expression patterns of Isl1 and Lmx1a make them good candidates to label progenitors in the pro-sensory and pro-non-sensory regions respectively. The potential role of Isl1 in inner ear progenitor cells is consistent with recent studies demonstrating that Isl1 is associated with cardiac multipotent progenitor cells, which are capable of giving rise to all the major heart cell types accompanied by the reduction of Isl1 expression (Laugwitz et al., 2005; Moretti et al., 2006). Interestingly, ISL1 labeling is generally absent in late postnatal cochlea sensory epithelia yet is maintained in some utricular supporting cells even in the adult (data not shown). It is tempting to speculate that sustained Isl1 expression in adult utricles may contribute to the limited proliferation potential in supporting cells after hair cell death (Forge et al., 1993; Warchol et al., 1993; Lambert et al., 1997). Absence of Isl1 during early development may therefore result in failure for the inner ear to develop, whereas sustained Isl1 expression in hair cells may lead to impaired differentiation. These hypotheses can be addressed by conditional deletion of Isl1 in the otocyst, and by hair cell-specific activation of Isl1 in a transgenic mouse model.
Initiation of expression of Isl2, Lhx3, 5, 6, 9, and Lmx1b in differentiating sensory epithelia starts between E12.5-E13.5. Their expression is generally maintained throughout the developmental stages examined. ISL2 labeling is slightly up-regulated in hair cells after E12.5 but remains weak in supporting cells at later stages. Interestingly, the ISL2 staining pattern, to a certain degree, is inversely correlated with the expression pattern of ISL1 from E12.5 onwards. In the spinal cord motor neurons, ISL2 is expressed after expression of ISL1, and Isl2 mutant mice display defects in the migration and axonal projections of motor neurons (Thaler et al., 2004). In the inner ear, Isl2 may play a role in cells that initially express Isl1, to further define subsequent differentiation.
The sequence of expression of some LIM-HDs in the inner ear is different from other tissues such as neurons. For instance, during spinal cord development of chicks and mice, motor neuron progenitors express Lhx3, and postmitotic motor neurons express Isl1 (Thaler et al., 2002). In contrast, in the developing inner ear, the opposite is true, with dividing sensory epithelia progenitors expressing Isl1 and postmitotic hair cells expressing Lhx3. The switch in timing and cell subtypes of Lhx3 and Isl1 expression in the inner ear may reflect the context-dependent functions of both LIM-HDs.
While Isl1 and Lmx1a are likely important in specification of progenitor cell populations of sensory and non-sensory epithelia, what is the functional significance of expression of other LIM-HDs in the differentiating hair cells and supporting cells? In the CNS, combinatorial functions of LIM-HDs play critical roles in sub-type neuron specifications (Thor et al., 1999; Shirasaki and Pfaff, 2002; Allan and Thor, 2003). The primary roles of LIM-HDs in early differentiation processes are consistent with recent studies showing that most LIM-HDs are bound by the Polycomb repressive complex subunit SUZ12, which suppresses genes involved in differentiation of embryonic stem cells (Lee et al., 2006). Within inner ear, combinatorial functions of LIM-HDs may be important in defining more cell subtypes beyond what is already known. Indeed, expression of a specific LIM-HD is far from uniform in a given inner ear cell type. Isl1, for instance, is predominantly expressed in the vestibular supporting cells, but can still be seen in occasional hair cells. Lhx3 is expressed in all hair cells, but with variable expression levels. The combination of subtle differential expression levels and patterns of LIM-HDs may stoichiometrically specify a particular cell subtype. Future experiments including expression profiling at single cell resolution and studies of multiple LIM-HD gene conditional knockout mice should reveal the functional roles of LIM-HDs and may identify novel inner ear cell types.
Experimental Procedure
RT-PCR and In situ hybridization
Specific primers corresponding to each LIM-HD gene were designed (Table 1). RT-PCR was performed using cDNA mixture prepared from mouse inner ears from E10.5 to P6, which included both utricle and cochleas. The conditions for RT-PCR were as follows: 94°C, 2 min; 94°C, 30 sec; 67°C, 1 min; 72°C, 1 min 30 sec for 35 cycles; 72°C, 10 min. All LIM-HDs were amplified by RT-PCR, indicating their expression in at least one of the stages examined. All the RT-PCR amplified the fragments of expected size (data not shown). RT-PCR fragments were further cloned into TOPO TA-Cloning vector (Invitrogen) and two independent clones for each LIM-HD RT-PCR were isolated and sequenced. The sequencing data showed all the LIM-HDs PCR products were proper clones (data not shown). For in situ hybridization, timed pregnant CD1 female mice were purchased from the Charles River Laboratories for embryo collection. Transverse sections of mouse inner ears, from E10.5 to P6, were prepared as described (Sage et al., 2006). Ribo-probes preparation and subsequent in situ hybridization follow the exact procedure as described (Sage et al., 2006).
Table 1.
Primers used to amplify individual LIM-HDs by RT-PCR.
| Lhx1-1438f | AAGTGCGTCCAGTGCTGTGAATGT |
| Lhx1-2308r | ATGATGGCACAAAGGGTAGGTCCA |
| Lhx2-1291f | ACCACCAGCTTCGGACAATGAAGT |
| Lhx2-2012r | TTCCTAAGGCACGTGGCAGTCTTT |
| Lhx4-605f | TGGGGCCAGTTCTACAAGAGTGTCA |
| Lhx4-1339r | AGCCAACAAGCCAGCATCCTTAGA |
| Lhx5-1401f | GATTCACCGACATGATCTCGCATC |
| Lhx5-2277r | AGGCCCCTCAGACTCAAAGTATGGA |
| Lhx6-239f | CAGCACACCGTCTGTCTGCTCG |
| Lhx6-999r | TGTCGTCGGACAGGGCAGAAGG |
| Lhx8-605f | CGGCCTGGAGATTGTGGACAAATA |
| Lhx8-1426r | ACTTATTGGCAGCTGGGTCATTGG |
| Lhx9-191f | GGAGGAGATGGAGCGCAGATCC |
| Lhx9-990r | CAAGCTGTTTGAGGTCCTTGGC |
| Lmx1a-2085f | TGGCAGGCAGCATGCTATAGTGAA |
| Lmx1a-2965r | TGCTTCCCCAGAAGGATCCTAACA |
| Lmx1b-212f | GCTACTTCCGGGATCGGAAACTGTA |
| Lmx1b-976r | AGATGGAGTCGTTCCCTGGCATTT |
| Isl1-218f | TTCTGAGGGTTTCTCCGGATTTGG |
| Isl1-1166r | CTGGCTACCATGCTGTTGGGTGTAT |
| Isl2-778f | ATCCGCGTGTGGTTTCAGAACAAG |
| Isl2-1761r | TCGTCCCACTATTCGCCTCACAAT |
Immunohistochemistry
Antibodies against POU4F3 (1:100) (Xiang et al., 1997), PAX2 (1:100, Covance, Princeton, NJ), LHX3, ISL1 (1:100, Developmental Studies Hybridoma Bank, University of Iowa), ISL2 (1:1000, Dr. Samuel Pfaff, Salk Institute) and Myosin VIIa (1:2000, Proteus BioSciences) were used for immunohistochemistry. Frozen sections of the inner ear tissues were dried for 15 minutes at 37°C, and re-hydrated in 1XPBS for 5 minutes. Then the sections were subjected to an antigen unmasking treatment using the Antigen Unmasking Solution (Vector laboratories) according to the manufacturer's protocol. The blocking, primary and secondary antibody incubation followed the standard protocol. The secondary antibodies were anti-rabbit Alexa Fluor 594 and/or anti-mouse Alexa Fluor 488 (Molecular probes) for fluorescent labeling.
Acknowledgements
We would like to thank Dr. Joe Adams for his constructive comments on the manuscript. The project was supported by NIH grants DC-04546, DC-06908 (Z.-Y.C.) and DC-6167 (S.H.). M.H. was supported in part by a grant from the DRF foundation. The research was conducted while Z.-Y.C. was a Pfizer/AFAR Innovation in Aging Research Grant recipient.
References
- Allan DW, Thor S. Together at last: bHLH and LIM-HD regulators cooperate to specify motor neurons. Neuron. 2003;38:675–677. doi: 10.1016/s0896-6273(03)00329-5. [DOI] [PubMed] [Google Scholar]
- Andersson E, Tryggvason U, Deng Q, Friling S, Alekseenko Z, Robert B, Perlmann T, Ericson J. Identification of intrinsic determinants of midbrain dopamine neurons. Cell. 2006:393–405. doi: 10.1016/j.cell.2005.10.037. [DOI] [PubMed] [Google Scholar]
- Barald KF, Kelley MW. From placode to polarization: new tunes in inner ear development. Development. 2004;131:4119–4130. doi: 10.1242/dev.01339. [DOI] [PubMed] [Google Scholar]
- Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, Zoghbi HY. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–1841. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- Bryant J, Goodyear RJ, Richardson GP. Sensory organ development in the inner ear: molecular and cellular mechanisms. Br Med Bull. 2002;63:39–57. doi: 10.1093/bmb/63.1.39. [DOI] [PubMed] [Google Scholar]
- Burton Q, Cole LK, Mulheisen M, Chang W, Wu DK. The role of Pax2 in mouse inner ear development. Dev Biol. 2004;272:161–175. doi: 10.1016/j.ydbio.2004.04.024. [DOI] [PubMed] [Google Scholar]
- Chen P, Johnson JE, Zoghbi HY, Segil N. The role of Math1 in inner ear development: Uncoupling the establishment of the sensory primordium from hair cell fate determination. Development. 2002;129:2495–2505. doi: 10.1242/dev.129.10.2495. [DOI] [PubMed] [Google Scholar]
- Chizhikov V, Steshina E, Roberts R, Ilkin Y, Washburn L, Millen KJ. Molecular definition of an allelic series of mutations disrupting the mouse Lmx1a (dreher) gene. Mamm Genome. 2006;17:1025–1032. doi: 10.1007/s00335-006-0033-7. [DOI] [PubMed] [Google Scholar]
- Forge A, Li L, Corwin JT, Nevill G. Ultrastructural evidence for hair cell regeneration in the mammalian inner ear. Science. 1993;259:1616–1619. doi: 10.1126/science.8456284. [DOI] [PubMed] [Google Scholar]
- Gray PA, Fu H, Luo P, Zhao Q, Yu J, Ferrari A, Tenzen T, Yuk DI, Tsung EF, Cai Z, Alberta JA, Cheng LP, Liu Y, Stenman JM, Valerius MT, Billings N, Kim HA, Greenberg ME, McMahon AP, Rowitch DH, Stiles CD, Ma Q. Mouse brain organization revealed through direct genome-scale TF expression analysis. Science. 2004;306:2255–2257. doi: 10.1126/science.1104935. [DOI] [PubMed] [Google Scholar]
- Hobert O, Westphal H. Functions of LIM-homeobox genes. Trends Genet. 2000;16:75–83. doi: 10.1016/s0168-9525(99)01883-1. [DOI] [PubMed] [Google Scholar]
- Hunter CS, Rhodes SJ. LIM-homeodomain genes in mammalian development and human disease. Mol Biol Rep. 2005;32:67–77. doi: 10.1007/s11033-004-7657-z. [DOI] [PubMed] [Google Scholar]
- Kawamoto K, Ishimoto S, Minoda R, Brough DE, Raphael Y. Math1 gene transfer generates new cochlear hair cells in mature guinea pigs in vivo. J Neurosci. 2003;23:4395–4400. doi: 10.1523/JNEUROSCI.23-11-04395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley MW. Regulation of cell fate in the sensory epithelia of the inner ear. Nat Rev Neurosci. 2006;7:837–849. doi: 10.1038/nrn1987. [DOI] [PubMed] [Google Scholar]
- Kros CJ. How to build an inner hair cell: challenges for regeneration. Hear Res. 2007;227:3–10. doi: 10.1016/j.heares.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Lambert PR, Gu R, Corwin JT. Analysis of small hair bundles in the utricles of mature guinea pigs. Am J Otol. 1997;18:637–643. [PubMed] [Google Scholar]
- Laugwitz KL, Moretti A, Lam J, Gruber P, Chen Y, Woodard S, Lin LZ, Cai CL, Lu MM, Reth M, Platoshyn O, Yuan JX, Evans S, Chien KR. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–653. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TI, Jenner RG, Boyer LA, Guenther MG, Levine SS, Kumar RM, Chevalier B, Johnstone SE, Cole MF, Isono K, Koseki H, Fuchikami T, Abe K, Murray HL, Zucker JP, Yuan B, Bell GW, Herbolsheimer E, Hannett NM, Sun K, Odom DT, Otte AP, Volkert TL, Bartel DP, Melton DA, Gifford DK, Jaenisch R, Young RA. Control of developmental regulators by Polycomb in human embryonic stem cells. Cell. 2006;125:301–313. doi: 10.1016/j.cell.2006.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Liu H, Sage C, Huang M, Chen ZY, Heller S. Islet-1 expression in the developing chicken inner ear. J Comp Neurol. 2004;477:1–10. doi: 10.1002/cne.20190. [DOI] [PubMed] [Google Scholar]
- Liodis P, Denaxa M, Grigoriou M, Akufo-Addo C, Yanagawa Y, Pachnis V. Lhx6 activity is required for the normal migration and specification of cortical interneuron subtypes. J Neurosci. 2007;27:3078–3089. doi: 10.1523/JNEUROSCI.3055-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millonig JH, Millen KJ, Hatten ME. The mouse Dreher gene Lmx1a controls formation of the roof plate in the vertebrate CNS. Nature. 2000;403:764–769. doi: 10.1038/35001573. [DOI] [PubMed] [Google Scholar]
- Moretti A, Caron L, Nakano A, Lam JT, Bernshausen A, Chen Y, Qyang Y, Bu L, Sasaki M, Martin-Puig S, Sun Y, Evans SM, Laugwitz KL, Chien KR. Multipotent Embryonic Isl1(+) Progenitor Cells Lead to Cardiac, Smooth Muscle, and Endothelial Cell Diversification. Cell. 2006;127:1151–1165. doi: 10.1016/j.cell.2006.10.029. [DOI] [PubMed] [Google Scholar]
- Radde-Gallwitz K, Pan L, Gan L, Lin X, Segil N, Chen P. Expression of Islet1 marks the sensory and neuronal lineages in the mammalian inner ear. J Comp Neurol. 2004;477:412–421. doi: 10.1002/cne.20257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee H, Polak L, Fuchs E. Lhx2 maintains stem cell character in hair follicles. Science. 2006;312:1946–1949. doi: 10.1126/science.1128004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage C, Huang M, Karimi K, Gutierrez G, Vollrath MA, Zhang DS, Garcia-Anoveros J, Hinds PW, Corwin JT, Corey DP, Chen ZY. Proliferation of functional hair cells in vivo in the absence of the retinoblastoma protein. Science. 2005;307:1114–1118. doi: 10.1126/science.1106642. [DOI] [PubMed] [Google Scholar]
- Sage C, Huang M, Vollrath MA, Brown MC, Hinds PW, Corey DP, Vetter DE, Chen ZY. Essential role of retinoblastoma protein in mammalian hair cell development and hearing. Proc Natl Acad Sci U S A. 2006;103:7345–7350. doi: 10.1073/pnas.0510631103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasaki R, Pfaff SL. Transcriptional codes and the control of neuronal identity. Annu Rev Neurosci. 2002;25:251–281. doi: 10.1146/annurev.neuro.25.112701.142916. [DOI] [PubMed] [Google Scholar]
- Shou J, Zheng JL, Gao WQ. Robust generation of new hair cells in the mature mammalian inner ear by adenoviral expression of Hath1. Mol Cell Neurosci. 2003;23:169–179. doi: 10.1016/s1044-7431(03)00066-6. [DOI] [PubMed] [Google Scholar]
- Thaler JP, Koo SJ, Kania A, Lettieri K, Andrews S, Cox C, Jessell TM, Pfaff SL. A postmitotic role for Isl-class LIM homeodomain proteins in the assignment of visceral spinal motor neuron identity. Neuron. 2004;41:337–350. doi: 10.1016/s0896-6273(04)00011-x. [DOI] [PubMed] [Google Scholar]
- Thaler JP, Lee SK, Jurata LW, Gill GN, Pfaff SL. LIM factor Lhx3 contributes to the specification of motor neuron and interneuron identity through cell-type-specific protein-protein interactions. Cell. 2002;110:237–249. doi: 10.1016/s0092-8674(02)00823-1. [DOI] [PubMed] [Google Scholar]
- Thor S, Andersson SG, Tomlinson A, Thomas JB. A LIM-homeodomain combinatorial code for motor-neuron pathway selection. Nature. 1999;397:76–80. doi: 10.1038/16275. [DOI] [PubMed] [Google Scholar]
- Warchol ME, Lambert PR, Goldstein BJ, Forge A, Corwin JT. Regenerative proliferation in inner ear sensory epithelia from adult guinea pigs and humans. Science. 1993;259:1619–1622. doi: 10.1126/science.8456285. [DOI] [PubMed] [Google Scholar]
- Xiang M, Gan L, Li D, Chen ZY, Zhou L, O'Malley BW, Jr., Klein W, Nathans J. Essential role of POU-domain factor Brn-3c in auditory and vestibular hair cell development. Proc Natl Acad Sci U S A. 1997;94:9445–9450. doi: 10.1073/pnas.94.17.9445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang M, Gao WQ, Hasson T, Shin JJ. Requirement for Brn-3c in maturation and survival, but not in fate determination of inner ear hair cells. Development. 1998;125:3935–3946. doi: 10.1242/dev.125.20.3935. [DOI] [PubMed] [Google Scholar]
- Zheng JL, Gao WQ. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat Neurosci. 2000;3:580–586. doi: 10.1038/75753. [DOI] [PubMed] [Google Scholar]