Abstract
Despite mortality due to communicable diseases, poverty, and human conflicts, dementia incidence is destined to increase in the developing world in tandem with the ageing population. Current data from developing countries suggest that age-adjusted dementia prevalence estimates in 65 year olds are high (≥5%) in certain Asian and Latin American countries, but consistently low (1–3%) in India and sub-Saharan Africa; Alzheimer's disease accounts for 60% whereas vascular dementia accounts for ∼30% of the prevalence. Early-onset familial forms of dementia with single-gene defects occur in Latin America, Asia, and Africa. Illiteracy remains a risk factor for dementia. The APOE ε4 allele does not influence dementia progression in sub-Saharan Africans. Vascular factors, such as hypertension and type 2 diabetes, are likely to increase the burden of dementia. Use of traditional diets and medicinal plant extracts might aid prevention and treatment. Dementia costs in developing countries are estimated to be US$73 billion yearly, but care demands social protection, which seems scarce in these regions.
Introduction
Older people with dementia exist in nearly every country in the world. Dementia rates are predicted to increase at an alarming rate in the least developed and developing regions of the world despite mortality resulting from malnutrition, poverty, war, and infectious diseases. WHO projections suggest that by 2025, about three-quarters of the estimated 1.2 billion people aged 60 years and older will reside in developing countries.1 Thus, by 2040, if growth in the older population continues, and there are no changes in mortality or burden reduction by preventive measures, 71% of 81.1 million dementia cases will be in the developing world.2 About 4.6 million new cases of dementia are added every year, with the highest growth projections in China and its south Asian neighbours. These projections might be confounded by temporal changes due to shorter survival after dementia,3 lack of education and awareness, inadequate diagnostic assessment,4 and variability in costs of care of the elderly with dementia,5 all of which could lead to under-accounting of the dementia burden.6 In China, for example, 49% of patients with dementia were classified as normally ageing and only 21% had adequate access to diagnostic assessment,7 compared with 20% and more than 70%, respectively, in Europe.8
There are no known curative or preventive measures for most types of dementia. Diet and lifestyle could influence risk, and studies suggest that midlife history of disorders that affect the vascular system, such as hypertension, type 2 diabetes, and obesity, increase the risk for dementia including Alzheimer's disease (AD).9–12 Increased trends in demographic transition and urbanisation within many developing countries are predicted to lead to lifestyle changes.13 Delaying of onset, by modifying risk or lifestyle, decreases the prevalence and public health burden of dementia; a delay in onset of 1 year would translate to almost a million fewer prevalent cases in the USA.14 However, this in turn might increase demands on health services and costs for older populations.15
We review published prevalence estimates and modifying factors for brain ageing-related dementias in developing regions of the world, as defined by the United Nations.16 Our report is limited to ageing-related neurodegenerative and vascular dementias and does not address dementia secondary to retroviruses (eg, HIV) or other infectious agents, recognising that these might assume importance in younger adults or in specific regions. Other reviews have focussed on these issues,15,17,18 but we take particular note of genetic and environmental factors,18,19 in addition to the problems encountered in accounting for differences in dementia occurrence between developed and developing countries. Although more data from developing countries are needed, several comparative dementia prevalence and risk-factor assessment projects, which use similar designs, survey methods, and investigators, have been invaluable resources to allow examination of phenotypic variations in dementias in populations living in very different cultures and environments.18,20–22
Dementia screening
Neuropsychometric assessment seems to be the best method to screen individuals in most developing countries.23 At the outset, the lack of standardisation of screening tools has to be recognised as a major issue in the estimation of the true burden.20 Standardisation might not be readily achieved because of diversity of language, culture, and levels of literacy. In certain communities, more than 80% of elderly people do not read or write.24 The mini mental state examination (MMSE) has been translated into many languages, but its use might be limited even as an initial screening tool. Independent back translations and consistent informant assessments are therefore mandated. Neuropsychological test batteries with components relatively free of cultural and linguistic factors (eg, verbal tests of delayed recall and of language) have been developed,25 but experience suggests that assessments must be consistent with the culture and language of the population under study, and local normative data for test performance need to be compiled.26
To achieve universal standardisation and to chart the epidemiological transition and its effect on older people, the 10/66 Dementia Research Group centres have initiated evidence-based procedures for use in different catchment areas worldwide.27 These include cross-culturally validated assessments for dementia subtype diagnosis, other mental and physical health diagnoses, anthropometry, demographics, non-communicable disease risk factors, disability and functioning, health-service utilisation, care arrangements, and caregiver strain. Nested within the population-based studies is a randomised controlled trial of caregiver intervention for people with dementia and their families.27 The surveys include ascertainment of other mental disorders, vascular disease, chronic obstructive pulmonary disease, and arthritis.27 Documentation of specific functional decline can be a challenge, because in some cultures, elderly individuals might have a restricted range of activities available to them, or family members might take over these activities. However, a history of cognitive decline can generally be combined with psychometric testing to support the diagnosis. Determination of the correct age of individuals who do not possess formal documentation of birth is another factor that could hamper comparisons, although methods on how accuracy might be achieved have been recognised.28,29
Dementia prevalence and incidence
Since the Delphi study projections,2 several large-scale dementia prevalence studies have been done.30–39 Dementia prevalence estimates vary widely within developing countries (table 1). This variation might indicate differences in population age structure, genetics, and lifestyle, but could also be due to difficulty in standardising dementia assessment and reduced survival after diagnosis.15 The mean age-adjusted prevalence estimate for dementia among people aged 65 years and older living in developing countries, derived from data published within the past 10 years, was calculated to be 5.3% (95% CI 3.9–6.5; table 1). This estimate was obtained by determining the original sample sizes and numbers of dementia cases reported to be 65 years and older in individual studies per country, and re-calculating mean estimates and variation by use of SPSS 15.0, according to the method by Yang.59
Table 1. AD and VaD prevalence and key risk factors in developing countries.
| Year | Criteria | Sample size (n) | Age (years) | Prevalence (95% CI) | Causes of other dementias | |||
|---|---|---|---|---|---|---|---|---|
| All dementia | AD | VaD | ||||||
|
Asia | ||||||||
| China30* | 2007 | DSM-III, ICD-10 | 87 761 | >65 | 3.1% (2.8–3.5) | 2.0% (1.5–3.1) | 0.9% (0.7–1.1) | Mixed, PDD, DLB, FTD |
| China (Beijing, Xian, Shanghai, Chengdu)31 | 2005 | DSM-IV | 34 807 | >65 | 5.0% | 3.5% (3.0–3.9) | 1.1% (0.9–1.1) | Mixed, PDD, DLB |
| Taiwan40-43† | 1995-1998 | DSM-IIIR, DSM-IV | 7149 | >65 | 3.2% (1.5–4.9) | 1.9% (1.2–2.5) | 0.7% (0.1–1.3) | Mixed |
| South Korea3,32,44‡ | 1994-2005 | DSM-III, DSM-IV | 7096 | >65 | 10.1% (7.3–12.9) | 5.2% (3.5–6.8) | 2.1% (1.2–2.9) | Mixed |
| Thailand45 | 2001 | DSM-III | 4048 | >60 | 3.4% (2.8–4.0) | .. | .. | .. |
| India33,34,46-48§ | 1996-2006 | DSM-III, DSM-IV | 14 767 | >65 | 2.7% (1.4–4.0) | 1.3% (0.8–1.8) | 1.1% (0.2–1.9) | Mixed, PDD, DLB, PSD |
| Sri Lanka49 | 2003 | DSM-IV | 703 | >65 | 3.98% (2.6–5.7) | 2.85% | 0.6% | Mixed |
| Israel (Wadi Ara)50¶ | 2002 | DSM-IV | 823 | >65 | 21.1% | 20.5% | 6.0% | Mixed |
|
Africa | ||||||||
| Egypt51 | 1998 | DSM-IV | 1366 | >65 | 5.93% | 2.86% | 1.25% | Mixed |
| Nigeria52‖ | 1995 | DSM-III, ICD-10 | 2494 | >65 | 2.3% (1.2 -3.4) | 1.4% (0.62–2.2) | 0.72% | Mixed, DLB |
|
Latin America** | ||||||||
| Cuba53 | 1999 | DSM-IV | 799 | >60 | 8.2% (6.3–10.4) | 5.1% (3.6–6.6) | 1.9% (1.0–3.0) | Mixed, alcohol dementia |
| Argentina54 | 1999 | DSM-IV | 1900 | >65 | 11.5% | .. | .. | Age |
| Brazil35–37,55†† | 2002-2008 | DSM-IIIR, DSM-IV | 7513 | >65 | 5.3% (1.5–8.9) | 2.7% (0.1–5.2) | 0.9% (0.06–1.78) | Mixed, PDD |
| Chile56 | 1997 | DSM-IIIR | 2213 | >65 | 4.3% (3.5–5.3) | .. | .. | .. |
| Colombia57,58 | 2000 | DSM-IV | 1611 | >65 and >75 | 1.8% (1.2–2.7) and 3.4% (1.2–5.6) | .. | .. | .. |
| Peru38 | 2007 | DSM-IV | 1532 | >65 | 6.7% (5.5–8.0) | .. | .. | .. |
| Venezuela39 | 2002 | DSM-IV | 2438 | >55 and >65 | 8.0% (7.0–9.2) and 10.3% (8.3–13.0) | 4.0% (3.3–4.8) | 2.1% (1.6–2.7) | Mixed |
Developing countries defined according to United Nations definition.16 Age-adjusted prevalence estimates and variation for people aged 65 years and older were calculated from the original published sample sizes and numbers of cases using SPSS 15.0.59
Systematic analysis of 25 studies in 1980–2004 with sample range of 906–15 910 people.
Mean estimates for four studies from 1995 to 1998; incidence estimates substantiate the prevalence in these communities (see main text).
Mean estimates determined for seven studies from 1994 to 2005. Although South Korea and Taiwan are included here as developing UN Asian regions, the International Monetary Fund regard these countries as advanced economies.
Mean and variation analysis from six rural and urban studies.
Annual incidence of AD among cognitively impaired but not demented patients was 4.4%.
A small study needing confirmation, which used the cognitive screening interview for dementia to screen a hospital-based sample in Jos, revealed an overall dementia prevalence of 6.4% (95% CI 3.8–9.9%), with age, female sex, and body mass index (≤18.5 kg/m2) as major risk factors.60
In Uruguay, prevalence figures were 0.5% for 60–69 year olds and 4.4% for 70–79 year olds.61
Combined prevalence estimates from four Brazilian studies. DLB=Dementia with Lewy bodies. DSM=Diagnostic and Statistical Manual of Mental Disorders. FTD=frontotemporal dementia. ICD-10=International Classification of Diseases, 10th edition. Mixed=mixed AD and VaD. PDD=Parkinson's disease with dementia. PSD=post-stroke dementia. ..=not determined.
Surprisingly, countries in Latin America, such as Venezuela and Argentina, bear a higher burden of over 5% prevalence of dementia (figure). By contrast, a systematic analysis of six Indian studies suggests low prevalence (2–3%) of all dementias, with marginally fewer cases in urban compared with rural areas and in the northern versus southern states.33 Pooled analysis of 25 Chinese studies by Dong and colleagues,30 comprising a total population of more than 76 000, suggested that the overall prevalence of dementia was 3.1%, indicating a significant rise from 1980 to 2004. However, a recent survey of over 34 807 Han Chinese residents aged at least 55 years in 79 rural and 58 urban communities of four distant areas reported a crude prevalence estimate of 5.0%, and 6.8% after adjustment for negative screening.31 Higher prevalence was apparent in northern regions compared with the south, but no difference was evident among urban and rural Chinese residents.7 In the Upper Assiut region along the Nile, age-adjusted dementia prevalence in people aged 65 years and older was 5.9%.51 In the Yoruba (Niger-Kordofanian people) of Nigeria, dementia prevalence was low (2.3%) compared with an African American population in Indiana, USA (8.2%).52 Among Arabs living in Wadi Ara, a community south of Haifa in Israel, the crude prevalence estimate for all dementias was 21% in those aged over 60 years.50,74 Consanguinity among families was suggested as a reason for this high prevalence.74,75 Studies from developing countries in Eastern Europe have assessed some risk factors, but prevalence or incidence data in these communities are unknown.62
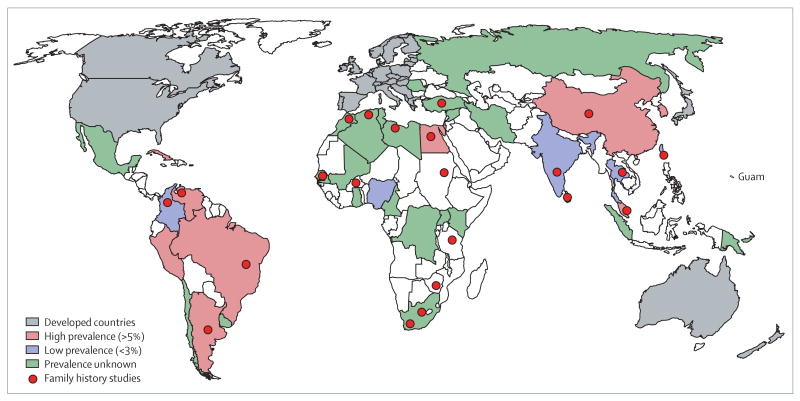
Figure. Sporadic and familial dementias in developing countries.
Red-shaded countries have prevalence or incidence estimates of all dementias that have been determined to be similar (>5%) to those in developed countries (grey-shaded countries). Blue-shaded countries have significantly lower prevalence (<3%) of dementia. Sample sizes for the estimates in the various studies were between 700 and 3200 individuals. Green-shaded areas show countries where there are published cases of dementia or subtypes (AD or VaD), where risk factors have been examined but prevalence or incidence are unknown. Reliable information was not available for countries without shading. Red spots show locations of families with neurodegenerative and vascular disorders causing dementia including AD, Parkinson's disease, Lewy body disease, frontotemporal lobar degeneration, Huntington's disease, amyotrophic lateral sclerosis, and CADASIL. Information on dementia prevalence and types was derived from many sources.22,29,31,35,37–39,41,45,46,49–52,58,62–73
The variations in prevalence within developing countries seem close to those found in the recently completed 10/66 survey of 14 960 residents aged over 65 years in 11 sites in seven low-income and middle-income countries (China, India, Cuba, Dominican Republic, Venezuela, Mexico, and Peru).20 Prevalence of dementia according to the Diagnostic and Statistical Manual of Mental Disorders (4th edition) varied widely, from less than 1% in the least developed countries, such as India and rural Peru, to 6.4% in Cuba. The 10/66 study also found that informants in the least developed countries were less likely to report cognitive decline and social impairment,20 suggesting possible underestimation of prevalence estimates in some locations.
Few incidence estimates are available to substantiate prevalence figures for those aged 65 years and older. Compared with developed countries, relatively lower annual incidence estimates of 1–2% are reported in certain countries, such as Brazil, Nigeria, India, and Taiwan.41,46,63,76 In a Brazilian community, incidence was determined to be 13.8 per 1000 person-years.76 In a comparative study, the Yoruba in Nigeria were found to be half as likely to develop dementia as African Americans in Indiana, USA; age-standardised annual incidence was 1.4% in the Yoruba versus 3.2% in African Americans.63 Among residents aged 60 years and older in Beijing, China, an incidence of 0.9% was determined at follow-up versus the original prevalence of 2.5%. AD was the most common type of dementia in both prevalent and incident cases.77
Subtypes of dementia
Alzheimer's disease
Late-onset AD is the most common subtype of age-related dementia, even in developing countries; 60% of all cases of dementia fulfilled the US National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (NINCDS–ADRDA) criteria.78 Total population projections suggest that 3.1 million people in China could have AD. Although unusually high prevalence was apparent in some countries, the mean AD prevalence was estimated to be 3.4% (95% CI 1.6–5.0), which is slightly lower than in developed countries. Age-adjusted low prevalence (<1.5%) was reported in sub-Saharan Africa (Nigeria) and India (table 1). The mean estimate was obtained by retrieving the original sample sizes and numbers of probable AD cases in individual studies per country and re-calculating the rate and variation by use of SPSS 15.0, according to the method by Yang.59 Autopsy studies done in some developing countries have confirmed that the neuropathological changes associated with AD are qualitatively similar to those in patients in developed countries;79–81 however, more work is needed, particularly given that reported AD cases could also have cerebrovascular changes.30,31,33
Consistent with the prevalence estimates, the incidence of AD for those aged 65 years and older was 7.7 per 1000 person-years in Brazil,76 and 3.24 per 1000 person-years in India.82 The annual incidence of AD in the Yoruba was determined to be 1.2%, substantially lower than the incidence of 2.5% in African Americans from Indiana.63
Vascular dementia
Vascular dementia (VaD) is recognised as the second most prevalent type of dementia. Neuroimaging is not routinely available in developing countries, which influences the accuracy of VaD detection and the confirmation of cases of mixed dementia. Analysis of data from 12 centres30–36,39,43,44 for which imaging findings were available indicates that 26% of cases of dementia fulfilled the US National Institute of Neurological Disorders and Stroke and Association Internationale pour la Recherché et l'Enseignement en Neurosciences (NINDS–AIREN) criteria for VaD.83 The mean estimate was obtained by determining the screened samples and numbers of reported VaD cases in individual studies per country and calculating the mean and variation in the same way as for AD prevalence. Prevalence estimates of VaD in developing countries range from 0.6% to 2.1% in those aged over 65 years (table 1). A third of 4.5 million Chinese patients with dementia are predicted to have VaD.31 With the exception of some Latin American and Asian countries,84 VaD prevalence in developing countries seems to be low. VaD might be more common among the Chinese and Malays, whereas AD is common in Indians and Eurasians.85 Subcortical VaD caused by small-artery disease,6 associated with hypertensive disease, seems to be a common (73%) cause of VaD.64 In several countries in Asia and Latin America, up to 10% of dementia cases are diagnosed with mixed dementia.30,40 Up to 30% of Chinese people in urban areas develop post-stroke cognitive impairment or delayed dementia after stroke.86–88 However, the prevalence of vascular cognitive impairment that involves all domains of cognitive function and causes of vascular injury is likely to be greater than that of VaD.89
Other subtypes of dementia
Prevalence data on other types of neurodegenerative dementia are limited. Single case reports and dementia prevalence studies do record causes of dementia other than AD (table 1). The first autopsy-confirmed case report of dementia with Lewy bodies in sub-Saharan Africa was reported in a Nigerian patient.90 Cases of dementia with Lewy bodies and Parkinson's disease with dementia have been reported in India,91 Sri Lanka,49 Taiwan,92 and China.30,93 Frontotemporal lobar degeneration, which involves a range of disorders associated with and without microtubule-associated Tau protein accumulation, does exist in developing countries but has rarely been described.30,92,94 Several cases of primary progressive aphasia with slow progressive deterioration of linguistic processes have been reported in Brazil.95 The Chamorros of Guam are affected by amyotrophic lateral sclerosis (ALS) and Parkinson's dementia complex (PDC), both of which are associated with pathological changes that resemble the neurofibrillary tangles found in AD.96 However, Guam has experienced rapid modernisation since World War II, and the incidence of ALS and PDC has declined.65 Recent studies indicate that the prevalence of dementia is approximately 12% among Chamorros aged 65 years or over (8.8% Guam dementia [clinically resembles AD], 1.5% PDC, and 1.3% VaD). Prion diseases, including sporadic, dominantly inherited, or transmitted cases of Creutzfeldt-Jakob disease, have also been described.66 The 129M susceptibility allele of the prion protein gene is found at high frequencies in Eurasian populations.67
Familial forms of dementia
Worldwide prevalence of early-onset dementias, generally defined as occurring before 65 years of age, is expected to be much higher than the global prevalence of early-onset AD at approximately 5.3 per 100 000 population.97 Most monogenic and complex disorders, including familial AD, Parkinson's disease with dementia, frontotemporal lobar degeneration, Huntington's disease, and small-vessel diseases of the brain, have been described in developing countries, but their frequencies are unknown (figure). Indigenous African and Asian families have been found with early-onset (33–45 years) AD caused by mutations in the amyloid precursor protein and presenilin genes.68,92,98,99 In Medellin, Colombia, the E280A mutation in the presenilin 1 gene (PSEN1) causes severe AD in a large kindred.100 Over 200 families with early-onset AD have also been identified among Caribbean Hispanics originating from the Dominican Republic and Puerto Rico.101 In 10% of these families, at least one family member had onset of dementia before the age of 55 years and almost half showed an association with a previously unreported presenilin mutation.101
Many families with early-onset VaD of small-vessel-disease type, in the form of cerebral autosomal dominant arteriopathy with subcortical infarcts and leucoencephalopathy (CADASIL),89 have been described in Asia, Africa, and Latin America.69,70,102 The trinucleotide repeat (CAG, GCC expansion) diseases, of which Huntington's disease is an example, are an important cause of disability and dementia in sub-Saharan Africa,103 Asia,92,104 and Latin America.105 In Maracaibo, Venezuela, the estimated prevalence of Huntington's disease in the state of Zulia is about 720 in 100 000 inhabitants, compared with 5–10 per 100 000 reported worldwide.71 Of note, environmental factors have been shown to influence the phenotypic expression of these dominant genes.72,106
Behavioural and psychological symptoms of dementia
Behavioural and psychological symptoms of dementia (BPSDs) are common among people with dementia in developing countries, although there seem to be marked regional variants.22,107 Several factors, including methods of reporting and cultural taboos, might account for the variations. However, at least one BPSD was reported in 70% of participants from 17 developing countries, and at least one case-level AGECAT psychiatric syndrome was shown by nearly half of those with dementia.27 Depression syndromes are most commonly followed by anxiety neurosis and schizophreniform or paranoid psychosis. Almost 80% of patients with AD in a Brazilian study had one or more BSPDs.108 Apathy was present in more than half (53%), followed by depression (38%), sleep alterations (38%), and anxiety (25%), whereas the most frequent neuropsychiatric symptoms in the cognitively impaired (but not demented) group were anxiety and sleep alterations, followed by depression.108 In India, patients with AD rather than VaD have significantly more delusions, hallucinations, anxieties, phobias, and caregiver distress with longitudinal patterns similar to those reported in developed countries.91 Poor cognitive performance was associated with significantly higher rates of depression in the Yoruba.109 Despite substantial socioeconomic and cultural differences between the Yoruba and African Americans, prevalence estimates of both mild and severe depression are generally similar in the two population samples.109 Although patterns of behavioural disturbances might vary,108,110 BPSDs seem to be common in developing and developed countries.
Early stages of dementia and mild cognitive impairment
The transition or prodromal stage between normal ageing and dementia or mild cognitive impairment (MCI) is a heterogeneous entity. Diagnostic criteria and standardisation of MCI are evolving, which makes direct comparisons among studies more difficult, possibly due to the stronger influence of illiteracy and socioeconomic factors, than in similar studies when dementia is diagnosed. Patients and families in developing countries are also less likely to admit or report cognitive difficulties because of prevailing cultural attitudes; identification of MCI in these countries therefore requires the presence of additional factors, such as infection and vascular comorbidites and poor nutrition. A small number of studies suggest that conversion rates of MCI or cognitive impairment with no dementia seem to be low in developing countries,111,112 but they report similar prevalences of impairment to those in developed countries. An Indian cross-sectional study reported that, in individuals aged 50 years and older, overall prevalence of MCI was 14.9% (95% CI 12.2–18.0) and that multiple-domain MCI was the most prevalent (8.85%) and was associated with increasing age, hypertension, and diabetes mellitus.113 In Brazil, prevalence of cognitive functional impairment was 16–19%.114 Higher age, low education, epilepsy, and depression were associated with increased risk, as were being female, widowhood, low social class, and head trauma. Stroke and diabetes were also associated with MCI within communities in Brazil, Puerto Rico, and Malaysia.114,115
Risk factors for dementia
Age and sex
Exposure early in life to deleterious conditions related to poverty, including infectious diseases, malnutrition, and prenatal stress, might influence the ageing process and reduce longevity for people in developing countries.116,117 Despite these realities, increasing age is the most consistent risk factor for dementia worldwide (table 2). Age was also a strong risk factor,30,51 with dementia prevalence of 2–11%, in those aged under 65 years. Nearly all studies in Latin America, Africa, and Asia confirm that women are marginally more likely to develop dementia and AD, particularly in very old age,76 on the basis of the greater expected numbers of ageing women,1 whereas VaD was slightly more prevalent in men (table 2).
Table 2. Comparison of risk factors for dementia, AD, and VaD, in developed and developing world regions.
| Developed regions (North America, Europe, Japan) | Asia (China, Guam, India, South Korea, Taiwan*) | Africa (Egypt, Nigeria, Kenya, South Africa) | Latin America (Argentina, Brazil, Venezuela) | |
|---|---|---|---|---|
| Increasing age | Positive | Positive | Positive | Positive |
| Female sex | Positive | Positive | Unclear | Unclear |
| Family history | Positive | Positive | .. | Positive |
| Head injury | Positive | .. | .. | Positive |
| Genes (APOE ε4 allele) | Positive | Positive | No risk | Unclear |
| Illiteracy or lack of education | Positive | Positive | Positive | Positive |
| MCI or cognitive impairment without dementia | Positive | Positive | .. | Positive |
| Urban living | Unclear | Unclear | Negative | Positive |
| Low socioeconomic status or poverty | Unclear | Positive | .. | Positive |
| Occupation as housewife | Negative | Positive | Unclear | Positive |
| Depressive illness | Positive | Positive | Positive | Positive |
| Vascular disease† | Positive | Positive | Positive | Unclear |
| Low fibre diet | Unclear | Positive | Positive | .. |
| Smoking | Positive | Positive | .. | Unclear |
In a 3-year incidence study, lower education, history of consistent unemployment, limited physical activity, and stroke history were identified as risk factors for dementia.84
Hypertension and diabetes were the most common risk factors associated with cases of AD and VaD. Studies in South Koreans and Jamaican Caribbeans established that smaller head circumference and shorter leg length were risk factors for dementia.118,119 Summary compiled from previously published studies.15,18,19,29–31,33–35,37–39,41,42,45–52,54,56,62,65,73,76,101,109,113,115,120–126 MCI=Mild cognitive impairment. ..=not determined.
Early-life negative events and physical attributes
Recent studies suggest that various genetic and environmental factors, including early-life brain development, body growth, socioeconomic conditions, environmental enrichment, head injury, and cognitive reserve, are likely to contribute to dementia risk.120,127 These factors have not been specifically investigated, but people in developing countries have a greater likelihood of early-life negative risks. Life expectancy at birth is much lower than in developed countries because of higher infant and maternal mortality and greater prevalence of infectious diseases. However, differences between developing and developed countries are substantially reduced in those who have reached the age of 65 years.128 In addition, older people in an area with high early-life mortality are not necessarily protected from dementia. In fact, such individuals continue to be at higher risk of death.129 Nevertheless, physical characteristics, such as leg length and head circumference, might be markers of early-life stressors,118,119,130 which result in reduced cognitive reserve. Significant negative early-life events might also increase the risk of AD among survivors.131
Literacy and education
On the one hand, illiteracy or low educational achievement has been shown to be a robust risk factor for dementia.120 On the other hand, intellectually stimulating, socially engaging, or physical activities might lower the risk of dementia.121 The situation is not different in developing countries, where surveys have consistently identified low education as a risk factor for dementia (table 2).85 However, in some communities, level of education, indexed by years of primary schooling, might not necessarily contribute to low prevalence.18 Low literacy is often linked to poverty or lower socioeconomic status, which is also associated with poorer health, lower access to health care, and increased risk of dementia (table 2).35,76,132
Genetic association studies and risk genes
Several groups in Asia and Latin America have done genetic association studies, spanning more than 127 polymorphisms across at least 69 different putative AD susceptibility genes.133 Genetic traits with autosomal recessive features are being explored in communities with high consanguinity. For example, studies in Wadi Ara have shown clustering of AD in families, and association with a new haplotype of the angiotensin-converting enzyme.134,135 The association of AD with at least two genes, apolipoprotein E (APOE) and neuronal sortilin-related receptor (SORL1),133,136,137 seems to be affected by ethnicity, age, sex, medical history, and geographical location. The APOE ε4 allele does not increase risk in sub-Saharan Africans and is only weakly associated with AD in Caribbean Hispanics and African Caribbean people of Jamaican origin.101,136,138 APOE ε4 is a risk factor for AD among women but not men in Venezuela.139 However, frequencies of the APOE ε4 allele are reported to be relatively increased in healthy Africans and some non-Africans: for example, 14–41% in indigenous people from Central African Republic, East Africa, Southern Africa, Malaysia, Australia, and Papua New Guinea,122,123,140 compared with 8–12% in Caucasians and Japanese.136 By contrast, certain groups have low frequencies of the APOE ε4 allele: 3–4% in the Wadi Ara Arabs in Israel, Oman, and Algeria,134 and 7% in North Indians and Taiwanese people.92,141
Comparative analysis showed that the APOE ε4 allele was a risk factor for AD in African Americans, but not in Yoruba Nigerians,124,142 or in population samples from the Vihiga and Nyeri districts of Kenya,29 or Kingston, Jamaica.143 There was also a lack of association of APOE genotypes with risk for dementia after adjusting for sex, age at diagnosis, and education,68,122,124 and in communities with high consangunity.134
Early-onset familial AD in developed countries has not been reported to be modified by the APOE ε4 allele. However, patients with early-onset AD carrying the APOE ε4 allele in the Colombian kindred with the E280A presenilin mutation were twice as likely to develop disease at an earlier age than those without the APOE ε4 allele.144 Low education and rural residence also influenced the age of onset in these patients.144 The APOE ε4 allele was strongly associated with late-onset familial AD among Caribbean Hispanics from the Dominican Republic and Puerto Rico,101 but not in Guamanians with dementia.65 The risk of ALS, PDC, and AD in Guam seems to be associated with genetic variants within the Tau gene, one of which increases the risk for progressive supranuclear palsy.145
The SORL1 gene, which might influence homoeostasis of the amyloid precursor protein, is thought to be the second most important gene to modify late-onset AD in multiple and ethnically diverse populations.137 Association of risk with this gene was found in Wadi Ara Arabs, among whom there is high consanguinity, as well as in Caribbean Hispanics137 and Han Chinese.146 Allelic heterogeneity in SORL1 is suggested by the novel single-nucleotide polymorphisms that have been found to be associated with the gene.133
Stroke and vascular disease risk factors
Stroke is an increasing burden in developing countries,147,148 and a major cause of mortality and long-term disability.149 Accumulating evidence suggests that stroke injury and vascular factors increase risk for AD and other dementias.10,89,150–155 Vascular factors, such as hypertension,9 dyslipidaemia,156,157 hyperinsulinaemia and type 2 diabetes,158,159 obesity,160 subclinical atherosclerosis,161 and arrhythmias,162 are associated with greater risk of cognitive impairment and dementia. Studies in Latin America also show that metabolic syndrome doubles the risk of cognitive impairment,163 and is significantly associated with functional dependence, depression, and low quality of life.164 Factors that decrease vascular function, such as tobacco use, which is common in countries such as China,165 might further influence cognition in old age.
The shift from infectious diseases to non-communicable but modifiable chronic disorders has resulted from gradual adoption of a Western lifestyle that includes excessive caloric intake, unhealthy diet, and decreased physical activity.160,165–170 This trend is expected to contribute to the global burden of AD.171,172 Vascular disease-controlling medications, such as antihypertensives and statins, might not be protective.173,174 The most cost-effective way to prevent dementia might be through dietary or lifestyle interventions in communities at variable risk of cardiovascular disease,175 such as the Yoruba,176 northern Indians,177 Venezuelans,178 and Wadi Ara Arabs.179
Dietary factors
Studies examining nutritional risk, which often rely on self-reports, are fraught with difficulties and should be cautiously interpreted. Observational data suggest that the low risk of dementia in some developing countries can be attributed to the type of diet.180 Diets rich in fruits, vegetables, and fibre improve human well-being and significantly reduce development of the pathological processes that are characteristic of neurodegenerative disorders.181 Chinese studies suggest that regular tea drinking might be protective against AD.182 The low incidence of dementia in the Yoruba Nigerians is consistent with their traditional low calorie and low fat diet consisting of grains, yam tubers (Dioscorea rotundata), vegetables, and some fish.176 Among Indonesians, there is a 30% lower risk of impairment with higher consumption of mucuna tempe,73 which has a high fibre content.183 By contrast, eating tofu has been associated with worsening memory, independent of age, sex, and education, among Indonesians,73 which concurs with the association of tofu consumption in midlife and cognitive impairment and brain atrophy in elderly Japanese Americans.184 Salivary phytooestrogens (genistein and daidizein) are associated with a higher risk of dementia, particularly in Javanese people aged over 68 years.73 The interaction between ageing and staple diets containing potential toxins might explain the dementia prevalence in certain locations such as Guam, where preparing or eating cycad fruit during young adulthood is associated with late-life dementia and PDC.145
Use of herbs and medicinal plants for dementia
Developing countries tend to retain traditional herbal medical practices and thus offer an invaluable resource for new anti-dementia therapies.185 However, the usefulness of such a resource relies on documented evidence of the effects. One of the largest long-term controlled clinical trials in progress on dementia prevention is based on the Asian traditional tree medicine Gingko biloba.186 Preliminary data have indicated significant effects on dementia progression,187 but the most recent Cochrane analysis concluded that evidence of predictable and clinically significant benefit of G biloba and standardised extract (EGb 761) for people with dementia is inconsistent and unconvincing.188 Huperzine A, originally isolated from Huperzia serrata, a type of moss used in traditional Chinese medicine (also known as qiang ceng ta),189 has been marketed in China as a new drug for AD treatment, and its derivative, ZT-1, is being developed as a new anti-AD drug.190,191 A plethora of pharmacognostic practices, including those for cognitive care, still exist in countries such as Africa, South America, India, and in other aboriginal cultures.185 Other relevant phytotherapeutics from developing countries, including combinations of traditional Chinese medicinal herbs (yi-gan san and ba wei di huang wan), sage (Salvia officinalis and Salvia lavandulaefolia), and lemon balm (Melissa officinalis), which have shown positive benefits on behavioural symptoms and cognition, need to be explored in wider studies.192,193
Several species of medicinal plants have activities in vitro or in vivo that are relevant to dementia (eg, anti-cholinesterase, anti-amyloid, anti-inflammatory, anti-oxidant, neuroprotective, and memory enhancing). The most frequently reported are blueberry, cannabis, club moss, curcumin, garlic, ginseng, green tea, pomegranate, and rhubarb.170,181,194,195 The dementia drug rivastigmine is a synthetic chemical analogue of physostigmine (from the Calabar bean, Physostigma venenosum), and galantamine is the main alkaloid in daffodil and snowdrop bulbs.196 Initiatives thus need to continue to protect, assess, and standardise traditional herbal medicines used in developing countries.
Mortality and dementia
Dementia modifies survival and increases the risk of death. A study among Shanghai residents indicated that the mortality risk ratios for AD and VaD, particularly in those over 75 years of age, were similar to the mortality risk ratio for cancer.125 In another Chinese study, the risk for death in patients with dementia was reported to be three times higher than in the whole cohort, although not related to a specific cause.84 In Brazilians, dementia was determined to be the most significant predictor of death, followed by age, history of stroke, complaints of visual impairment, heart failure, and severe arterial hypertension.197 In Ballabgarh, India, the median survival time after onset of dementia symptoms was determined to be 3.3 years for patients with dementia and 2.7 years for patients with AD compared with 5.0–9.3 years in developed countries.46 Dementia was also associated with increased mortality in Nigerians and African Americans (relative risk ratio, compared with the population studies, was 2.83 versus 2.05).198,199
Costs of dementia
Current projections indicate that the burden of disease, expressed as WHO-designated disability-adjusted life-years (DALYs), is unequally distributed between middle-income and low-income countries (table 3).16,200 However, if dementia prevalence in developing countries is assumed to increase substantially due to demographic transition, the DALYs per number of patients with dementia who are 65 years and older are similar between regions.5 To estimate the total costs, we modelled the societal worldwide costs as well as region-specific and country-specific costs by combining prevalence estimates,5 country-specific, and region-specific data on gross domestic product per person, and average wage with results from previously published cost-of-illness studies in several key countries from which detailed data about direct costs and informal care costs were available.201 From this model, total costs of dementia in developing countries are estimated to be US$72.6 billion yearly (table 3). By use of the lower Delphi estimates,2 costs for Africa would be US$2.9 billion.201 Cost estimates do not provide any information about the cost distribution among those who actually pay or how large a proportion of the total resources are required for a particular disorder.202 In developed countries, long-term institutional care constitutes the main cost,203,204 whereas in developing countries, informal care, usually at home,205 is invariably the only method of care.206,207
Table 3. Burden of AD and other types of dementia in terms of DALYs and cost of illness estimates in different world regions.
| DALYs | Costs (2005 US$) | ||||||
|---|---|---|---|---|---|---|---|
| Total (×103) | Per 100 000 persons | Per 1000 people with dementia* | Direct (×109) | Informal care (×109) | Illness (×109) | Per dementia patient | |
| Developed regions | 4741 | 395 | 350 | 168.1 | 74.7 | 242.8 | 17 964 |
| Developing regions | |||||||
| Middle income (less developed) | 5597 | 107 | 354 | 42.3 | 30.3 | 72.6 | 4588 |
| Low income (least developed) | 422 | 57 | 363 | 0.8 | 1.0 | 1.8 | 1521 |
Data on DALYs were derived from WHO.200
Prevalence and cost estimates were derived from previous estimates by creating a model that accounts for prevalence estimates, country-specific, and region-specific data on gross domestic product per person, and average wage with cost-of-illness for key countries within each region from which detailed data about direct costs and informal care costs were available.5,201 Regions are designated according to United Nations definitions.16
The cost model estimates that approximately 75% of the global costs occurred in middle-income countries, where 46% of patients with dementia worldwide reside,201 but informal care costs, which are increasing in developing countries,13,208 were proportionally greater (1.0 of 1.8 billion or 56% of total) in the least developed countries.201 For example, in Argentina and Brazil,209,210 the expenses (medical and non-medical) borne by families of people with dementia were considered to be very high. Similarly, in China, non-medical costs increased with severity of cognitive decline and increases in BPSDs, and daily 24-hour care was needed for those with a MMSE score below 11 and BPSDs.211 In Turkey, informal care was estimated (by use of a replacement cost approach) to be the major cost driver, although the amount of informal care time was lower than in other studies.206 The dominance of informal care was evident in South Korea, where these costs constituted 55% of societal costs,212 but in India, the amount of informal care was similar to that in middle-income countries.213 By contrast, the costs of home care (including informal care) with nursing care in Taiwan were less than for institutional care, particularly for patients with severe dementia if a replacement cost approach was used.214,215 The weakness of such projections is that they rely on extrapolation of data from middle-income countries,216 and require information about true prevalence, and the conceptualisation, quantification, and actual costs of informal care.201 Definitions of care activities, particularly in terms of instrumental activities of daily living and supervision versus normal family activities, also pose difficulties in the estimations of overall cost.
Dementia awareness, care, and services
Understanding the burden and costs of dementia is crucial to guide future health care and socioeconomic policy.27 Policymakers need evidence to prioritise and plan appropriately for the rapidly growing numbers of older people with dementia and other chronic diseases. Low public awareness, under-diagnosis, and under-treatment could be addressed by national mobilisation strategies to increase awareness and specialised training for heath professionals and authorities through mass media, scientific reports, and special activities, and by the setting up of open clinics in communities. For example, through such efforts, the mean proportion of patients with AD in China treated with acetylcholinesterase inhibitors and memantine increased from 12.1% (range 0.2–29.1%) in 2001 to 19.6% (range 2.3–41.1%) in 2007.217 The variation among the Chinese districts mainly depended on the levels of economic development and medical insurance cover.7
Social protection is hard to define, but is a major concern in most developing countries. This might be complicated by a lack of carers due to urban and economic migration, conflict, and HIV/AIDS. The circumstances of those with dementia in each centre of the 10/66 study, surveyed by use of an adapted version of the Client Service Inventory Report,27 highlight the vulnerabilities of dependent older people living in these regions.218 For people with dementia, the state does not provide long-term care; consequently, the family, particularly the patients' offspring, plays a vital part. An estimate of the worldwide costs of dementia used an average of 1.6 hours of informal personal care per day for all people with dementia.201 However, this figure is exceeded in most 10/66 study centres. In all Latin American centres other than Mexico, a sixth to a quarter of people with dementia have no children locally available to provide care. Even in rural China and in India, 5–10% lack this fundamental support.218 Children can provide food, shelter, personal care, and income for their parents through cash transfers (particularly important in India, Dominican Republic, rural Peru, Mexico, and China because of very low pension coverage). In all 10/66 centres, living with children is the norm, and three-generation households (including children under 16 years) are common.205 Nevertheless, around a fifth of people with dementia (10–37% by centre) live alone or with a spouse only, and hence can be considered vulnerable.207,218 Current research indicates that a worryingly high proportion of people with dementia lack the basic necessities for life (ie, food), particularly in parts of Latin America, and in India, where social protection is most insecure.4,205,207,209,210,218,219
International agreements, plans, and policy guidelines have called for an end to age discrimination and a focus on reducing disadvantage linked to poverty and the consequences of ill health.1 Ensuring social protection, allowing access to good quality age-appropriate health care, and addressing the problem of disability are key concerns. Thus, levels of caregiver strain, including that contributed by behavioural disturbances and stress, are as high as in developed countries despite extended family networks and home care.207 Moreover, dependency is strongly linked to poverty, and imposes additional economic strain on families.207 The Ibadan project in Nigeria has advocated periodic home visits, and empowerment of caregivers through regular meetings to make caring for individuals with dementia easier and more adaptable.220
Conclusions
The prevalence of dementia, particularly that of AD, is increasing in the developing countries of Asia and Latin America. However, reliable age-adjusted estimates indicate a low prevalence of dementia in India and sub-Saharan Africa. Difficulties in definition, ascertainment of decline in intellectual ability, and assessment of patients mean that meagre information on MCI is available in developing countries.20 Illiteracy and depressive illness remain strong risks for dementia. Further research is needed to examine why the APOE ε4 allele does not seem to influence AD progression in sub-Saharan Africa. Increasing frequency of vascular disease and global trends in modernisation will add to the burden of AD within developing countries. Harmonisation of screening methods worldwide could help to define risks and to devise novel approaches for dementia prevention. The impact of dementia in developing countries deserves further epidemiological and implementation research to enable early detection, widespread adequate treatment, and caregiver support. Such efforts will no doubt promote greater awareness, refine the policy agenda, and lead to a call for concerted action.
Search strategy and selection criteria
First-hand information on cognitive screening and several relevant references were provided by the World Federation of Neurology Dementia Research Group members and co-authors. A systematic literature search of PubMed and Medline was also done with combinations of search terms, including “developing countries” and “dementia”, with topic headings including “Alzheimer's disease”, “prevalence”, “incidence”, “cognitive impairment”, “mortality”, “risk factors”, “vascular dementia”, “Asia”, “Africa”, “Latin America”, “care”, and “costs”. PubMed was searched for relevant articles in any language (all understood by co-authors) until May, 2008. Searches were also done in the Cochrane database, EMBASE, Dare, NHS-EED, HTA, Applied Social Sciences Index and Abstracts, Social Services Abstracts, Sociological Abstracts, PsycINFO, and Social Sciences Citation Index with combinations of similar search terms. Some publications, particularly conference proceedings, were found through Google searches. The bibliography was derived from a total of 520 articles that were screened for relevance to this Review. The full list of search terms is available from the authors on request.
Acknowledgments
We thank Samantha Tannahill and Deborah Little for secretarial assistance. We are grateful to the discussants of the WHO–World Federation of Neurology–International Brain Research Organisation co-sponsored symposium on Brain Ageing and Dementia in Developing Countries held in Nairobi, Kenya, in April, 2007. We thank the Alzheimer's Research Trust (UK), the Medical Research Council (UK), the International Brain Research Organisation, and World Federation of Neurology for supporting the Dementia Research Group.
Footnotes
Members listed at end of review
Contributors: All the authors provided material and ideas on presentation, and contributed to the writing and editing of the Review at various stages of preparation. In addition, GEM, RA, RPF, AO, KH, FP, MP, RS, AW, ZXZ, and RNK provided key references and did the analyses presented in the tables. Most of the authors were also lead discussants at the World Federation of Neurology Dementia Research Group meeting in 2007.
World Federation of Neurology Dementia Research Group: Members and guests who contributed to the information compiled in this Review include Rufus Akinyemi (Nigeria), Belachew D Arasho (Ethiopia), Tarek Bellaj (Tunisia), José Bertelote (WHO), Santy Daya (South Africa), Wiesje M van der Flier (Netherlands), Catherine Dotchin (Tanzania), Angiola Fasanaro (Italy), Valery Feigin (New Zealand), Paul Francis (UK), Samuel Gatere (Kenya), Henry Houlden (UK), Eef Hogervorst (UK), Akira Homma (Japan), Paul Ince (UK), Jennifer Jones (USA), Ahmed Mussa Jusabani (Kenya), Zarina Kabir (Sweden-Bangladesh), Touré Kamadore (Senegal), Jean-Marie Kashama (Democratic Republic of Congo), Tharcisse Kayembe (Democratic Republic of Congo), Miia Kivipelto (Sweden), Girish J Kotwal (USA), Ennapadam S Krishnamoorthy (India), Debomoy Lahiri (USA), Donald Lehmann (UK), Mohamed Makrelouf (Algeria), Elizabeta Mukaetova-Ladinska (UK), Ken Nagata (Japan), Noeline K Nakasujja (Uganda), David Ndetei (Kenya), Arthur Oakley (UK), Ante Padjen (Canada), Robert Perry (UK), Stuart Pickering-Brown (UK), Mieczyslaw Pokorski (Poland), Dushyant Purohit (USA), Ingmar Skoog (Sweden), Manjari Tripathi (India), Susan van Rensburg (South Africa), Mathew Varghese (India), and Julie Williams (UK).
Conflicts of interest: RPF is a consultant to MIMvista, Inc. AW has been acting as a consultant to drug companies that are purchasing or developing drugs for treatment of Alzheimer's disease or other dementias (Pfizer, Janssen-Cilag, Novartis, Merz, Lundbeck, Forest, GlaxoSmithKline, Wyeth, Sanofi, Elan, Neurochem). All other authors have no conflicts of interest.
Contributor Information
Raj N Kalaria, Institute for Ageing and Health, Newcastle General Hospital, Newcastle upon Tyne, UK.
Gladys E Maestre, Institute for Biological Research, University of Zulia, Maracaibo, Venezuela and G H Sergievsky Center, Columbia University, New York, NY, USA.
Raul Arizaga, University of Buenos Aires, and Cognitive Neurology Unit, Neuraxis, Buenos Aires, Argentina.
Robert P Friedland, Department of Neurology, Case Western Reserve University School of Medicine, Cleveland, OH, USA.
Doug Galasko, Department of Neurosciences, University of California, San Diego, CA, USA.
Kathleen Hall, Department of Psychiatry, Indiana University School of Medicine, Indianapolis, IN, USA.
José A Luchsinger, Taub Institute for Research of Alzheimer's Disease and the Aging Brain, Columbia University, New York, NY, USA.
Adesola Ogunniyi, Departments of Medicine and Psychiatry, University College Hospital, Ibadan, Nigeria.
Elaine K Perry, Institute for Ageing and Health, Newcastle General Hospital, Newcastle upon Tyne, UK.
Felix Potocnik, Department of Psychiatry, University of Stellenbosch, Tygerberg, South Africa.
Martin Prince, Health Service and Population Research Department, Section of Epidemiology, King's College London, London, UK.
Robert Stewart, Health Service and Population Research Department, Section of Epidemiology, King's College London, London, UK.
Anders Wimo, Alzheimer's Disease Research Center, Department of Neurobiology, Care Sciences and Society, Karolinska Institute, Stockholm, Sweden.
Zhen-Xin Zhang, Memory and Movement Disorder Center, Department of Neurology, and Clinical Epidemiological Centre, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Beijing, China.
Piero Antuono, Dementia Research Center, Department of Neurology, Medical College of Wisconsin, Milwaukee, WI, USA.
References
- 1.WHO. Active ageing: a policy framework, 2002 health report. Geneva: World Health Organization; 2002. [Google Scholar]
- 2.Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366:2112–17. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suh GH, Shah A. A review of the epidemiological transition in dementia—cross-national comparisons of the indices related to Alzheimer's disease and vascular dementia. Acta Psychiatr Scand. 2001;104:4–11. doi: 10.1034/j.1600-0447.2001.00210.x. [DOI] [PubMed] [Google Scholar]
- 4.Raicher I, Shimizu MM, Takahashi DY, et al. Alzheimer's disease diagnosis disclosure in Brazil: a survey of specialized physicians' current practice and attitudes. Int Psychogeriatr. 2008;20:471–81. doi: 10.1017/S1041610207005819. [DOI] [PubMed] [Google Scholar]
- 5.Wimo A, Jonsson L, Winblad B. An estimate of the worldwide prevalence and direct costs of dementia in 2003. Dement Geriatr Cogn Disord. 2006;21:175–81. doi: 10.1159/000090733. [DOI] [PubMed] [Google Scholar]
- 6.Chen CP. Transcultural expression of subcortical vascular disease. J Neurol Sci. 2004;226:45–47. doi: 10.1016/j.jns.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Zhang ZX, Zahner GE, Roman GC, et al. Socio-demographic variation of dementia subtypes in China: methodology and results of a prevalence study in Beijing, Chengdu, Shanghai, and Xian. Neuroepidemiology. 2006;27:177–87. doi: 10.1159/000096131. [DOI] [PubMed] [Google Scholar]
- 8.Waldemar G, Phung KT, Burns A, et al. Access to diagnostic evaluation and treatment for dementia in Europe. Int J Geriatr Psychiatry. 2007;22:47–54. doi: 10.1002/gps.1652. [DOI] [PubMed] [Google Scholar]
- 9.Skoog I, Lernfelt B, Landahl S, et al. 15-year longitudinal study of blood pressure and dementia. Lancet. 1996;347:1141–45. doi: 10.1016/s0140-6736(96)90608-x. [DOI] [PubMed] [Google Scholar]
- 10.Luchsinger J, Mayeux R. Cardiovascular risk factors and Alzheimer's disease. Curr Atheroscler Rep. 2004;6:261–66. doi: 10.1007/s11883-004-0056-z. [DOI] [PubMed] [Google Scholar]
- 11.Whitmer RA, Gunderson EP, Barrett-Connor E, et al. Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. BMJ. 2005;330:1360. doi: 10.1136/bmj.38446.466238.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kivipelto M, Ngandu T, Laatikainen T, et al. Risk score for the prediction of dementia risk in 20 years among middle aged people: a longitudinal, population-based study. Lancet Neurol. 2006;5:735–41. doi: 10.1016/S1474-4422(06)70537-3. [DOI] [PubMed] [Google Scholar]
- 13.Ineichen B. Influences on the care of demented elderly people in the People's Republic of China. Int J Geriatr Psychiatry. 1998;13:122–26. doi: 10.1002/(sici)1099-1166(199802)13:2<122::aid-gps745>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 14.Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer's disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 1998;88:1337–42. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brayne C. The elephant in the room—healthy brains in later life, epidemiology and public health. Nat Rev Neurosci. 2007;8:233–39. doi: 10.1038/nrn2091. [DOI] [PubMed] [Google Scholar]
- 16.United Nations. Composition of macro geographical (continental) regions, geographical sub-regions, and selected economic and other groupings. [July 10, 2008]; http://unstats.un.org/unsd/methods/m49/m49regin.htm.
- 17.Qiu C, De Ronchi D, Fratiglioni L. The epidemiology of the dementias: an update. Curr Opin Psychiatry. 2007;20:380–85. doi: 10.1097/YCO.0b013e32816ebc7b. [DOI] [PubMed] [Google Scholar]
- 18.Hendrie HC, Murrell J, Gao S, et al. International studies in dementia with particular emphasis on populations of African origin. Alzheimer Dis Assoc Disord. 2006;20:S42–46. doi: 10.1097/00002093-200607001-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brayne C. The EURODEM collaborative reanalysis of case-control studies of Alzheimer's disease: implications for public health. Int J Epidemiol. 1991;20:568–71. doi: 10.1093/ije/20.supplement_2.s68. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez JJL, Ferri CP, Acosta D, et al. The prevalence of dementia in Latin America, India and China. A 10/66 Dementia Research Group population-based survey. Lancet. 2008 July 28; doi: 10.1016/S0140-6736(08)61002-8. published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ganguli M, Chandra V, Gilby JE, et al. Cognitive test performance in a community-based nondemented elderly sample in rural India: the Indo-U.S. Cross-National Dementia Epidemiology Study. Int Psychogeriatr. 1996;8:507–24. doi: 10.1017/s1041610296002852. [DOI] [PubMed] [Google Scholar]
- 22.Prince M, Acosta D, Chiu H, et al. Dementia diagnosis in developing countries: a cross-cultural validation study. Lancet. 2003;361:909–17. doi: 10.1016/S0140-6736(03)12772-9. [DOI] [PubMed] [Google Scholar]
- 23.Chaves ML, Ilha D, Maia AL, et al. Diagnosing dementia and normal aging: clinical relevance of brain ratios and cognitive performance in a Brazilian sample. Braz J Med Biol Res. 1999;32:1133–43. doi: 10.1590/s0100-879x1999000900013. [DOI] [PubMed] [Google Scholar]
- 24.Hall KS, Ogunniyi AO, Hendrie HC, et al. A cross-cultural community based study of dementias: methods and performance of the survey instrument, Indianapolis, USA, and Ibadan, Nigeria. Int J Methods Psychiatr Res. 1996;6:129–42. [Google Scholar]
- 25.Maj M, Janssen R, Satz P, et al. The World Health Organization's cross-cultural study on neuropsychiatric aspects of infection with the human immunodeficiency virus 1 (HIV-1). Preparation and pilot phase. Br J Psychiatry. 1991;159:351–56. doi: 10.1192/bjp.159.3.351. [DOI] [PubMed] [Google Scholar]
- 26.Jorm AF, Jacomb PA. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): socio-demographic correlates, reliability, validity and some norms. Psychol Med. 1989;19:1015–22. doi: 10.1017/s0033291700005742. [DOI] [PubMed] [Google Scholar]
- 27.Prince M, Ferri CP, Acosta D, et al. The protocols for the 10/66 Dementia Research Group population-based research programme. BMC Public Health. 2007;7:165. doi: 10.1186/1471-2458-7-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hall KS, Gao S, Emsley CL, et al. Community screening interview for dementia (CSI ‘D’); performance in five disparate study sites. Int J Geriatr Psychiatry. 2000;15:521–31. doi: 10.1002/1099-1166(200006)15:6<521::aid-gps182>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 29.Chen CH, Mizuno T, Elston R, et al. A comparative study to screen dementia and APOE genotypes in an ageing East African population. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.06.014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong MJ, Peng B, Lin XT, et al. The prevalence of dementia in the People's Republic of China: a systematic analysis of 1980–2004 studies. Age Ageing. 2007;36:619–24. doi: 10.1093/ageing/afm128. [DOI] [PubMed] [Google Scholar]
- 31.Zhang ZX, Zahner GE, Roman GC, et al. Dementia subtypes in China: prevalence in Beijing, Xian, Shanghai, and Chengdu. Arch Neurol. 2005;62:447–53. doi: 10.1001/archneur.62.3.447. [DOI] [PubMed] [Google Scholar]
- 32.Shin HY, Chung EK, Rhee JA, et al. Prevalence and related factors of dementia in an urban elderly population using a new screening method [in Korean] J Prev Med Pub Health. 2005;38:351–58. [PubMed] [Google Scholar]
- 33.Das SK, Biswas A, Roy T, et al. A random sample survey for prevalence of major neurological disorders in Kolkata. Indian J Med Res. 2006;124:163–72. [PubMed] [Google Scholar]
- 34.Shaji S, Bose S, Verghese A. Prevalence of dementia in an urban population in Kerala, India. Br J Psychiatry. 2005;186:136–40. doi: 10.1192/bjp.186.2.136. [DOI] [PubMed] [Google Scholar]
- 35.Scazufca M, Menezes PR, Vallada HP, et al. High prevalence of dementia among older adults from poor socioeconomic backgrounds in Sao Paulo, Brazil. Int Psychogeriatr. 2008;20:394–405. doi: 10.1017/S1041610207005625. [DOI] [PubMed] [Google Scholar]
- 36.Ramos-Cerqueira AT, Torres AR, Crepaldi AL, et al. Identification of dementia cases in the community: a Brazilian experience. J Am Geriatr Soc. 2005;53:1738–42. doi: 10.1111/j.1532-5415.2005.53553.x. [DOI] [PubMed] [Google Scholar]
- 37.Bottino CM. PhD thesis. University of São Paulo; 2007. Prevalence of cognitive impairment and dementia in three districts of the municipality of Sao Paulo [in Portuguese] [Google Scholar]
- 38.Custodio N, Gutierrez C, García A. Prevalencia de demencia en una comunidad urbana de Lima: un estudio puerta a puerta [abstract]. Proceedings of the XII Pan-American Congress of Neurology; Santo Domingo, Dominican Republic. Oct 11–17 2007; p. 17. [Google Scholar]
- 39.Molero AE, Pino-Ramirez G, Maestre GE. High prevalence of dementia in a Caribbean population. Neuroepidemiology. 2007;29:107–12. doi: 10.1159/000109824. [DOI] [PubMed] [Google Scholar]
- 40.Lin RT, Lai CL, Tai CT, et al. Prevalence and subtypes of dementia in southern Taiwan: impact of age, sex, education, and urbanization. J Neurol Sci. 1998;160:67–75. doi: 10.1016/s0022-510x(98)00225-1. [DOI] [PubMed] [Google Scholar]
- 41.Liu HC, Fuh JL, Wang SJ, et al. Prevalence and subtypes of dementia in a rural Chinese population. Alzheimer Dis Assoc Disord. 1998;12:127–34. doi: 10.1097/00002093-199809000-00002. [DOI] [PubMed] [Google Scholar]
- 42.Liu HC, Lin KN, Teng EL, et al. Prevalence and subtypes of dementia in Taiwan: a community survey of 5297 individuals. J Am Geriatr Soc. 1995;43:144–49. doi: 10.1111/j.1532-5415.1995.tb06379.x. [DOI] [PubMed] [Google Scholar]
- 43.Liu HC, Wang SJ, Fuh JL, et al. The Kinmen Neurological Disorders Survey (KINDS): a study of a Chinese population. Neuroepidemiology. 1997;16:60–68. doi: 10.1159/000109672. [DOI] [PubMed] [Google Scholar]
- 44.Suh GH, Kim JK, Cho MJ. Community study of dementia in the older Korean rural population. Aust N Z J Psychiatry. 2003;37:606–12. doi: 10.1046/j.1440-1614.2003.01237.x. [DOI] [PubMed] [Google Scholar]
- 45.Jitapunkul S, Kunanusont C, Phoolcharoen W, Suriyawongpaisal P. Prevalence estimation of dementia among Thai elderly: a national survey. J Med Assoc Thai. 2001;84:461–67. [PubMed] [Google Scholar]
- 46.Chandra V, Ganguli M, Pandav R, et al. Prevalence of Alzheimer's disease and other dementias in rural India: the Indo-US study. Neurology. 1998;51:1000–08. doi: 10.1212/wnl.51.4.1000. [DOI] [PubMed] [Google Scholar]
- 47.Rajkumar S, Kumar S, Thara R. Prevalence of dementia in a rural setting: a report from India. Int J Geriatr Psychiatry. 1997;12:702–07. doi: 10.1002/(sici)1099-1166(199707)12:7<702::aid-gps489>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 48.Vas CJ, Pinto C, Panikker D, et al. Prevalence of dementia in an urban Indian population. Int Psychogeriatr. 2001;13:439–50. doi: 10.1017/s1041610201007852. [DOI] [PubMed] [Google Scholar]
- 49.de Silva HA, Gunatilake SB, Smith AD. Prevalence of dementia in a semi-urban population in Sri Lanka: report from a regional survey. Int J Geriatr Psychiatry. 2003;18:711–15. doi: 10.1002/gps.909. [DOI] [PubMed] [Google Scholar]
- 50.Bowirrat A, Friedland RP, Korczyn AD. Vascular dementia among elderly Arabs in Wadi Ara. J Neurol Sci. 2002;203-204:73–76. doi: 10.1016/s0022-510x(02)00269-1. [DOI] [PubMed] [Google Scholar]
- 51.Farrag A, Farwiz HM, Khedr EH, et al. Prevalence of Alzheimer's disease and other dementing disorders: Assiut-Upper Egypt study. Dement Geriatr Cogn Disord. 1998;9:323–28. doi: 10.1159/000017084. [DOI] [PubMed] [Google Scholar]
- 52.Hendrie HC, Osuntokun BO, Hall KS, et al. Prevalence of Alzheimer's disease and dementia in two communities: Nigerian Africans and African Americans. Am J Psychiatry. 1995;152:1485–92. doi: 10.1176/ajp.152.10.1485. [DOI] [PubMed] [Google Scholar]
- 53.Llibre JJ, Guerra MA, Perez-Cruz H, et al. Dementia syndrome and risk factors in adults older than 60 years old residing in Habana [in Spanish] Rev Neurol. 1999;29:908–11. [PubMed] [Google Scholar]
- 54.Pages-Larraya FP, Mari G. Prevalence of dementia of the Alzheimer's type, vascular dementia and other dementias in the city of Buenos Aires [in Portuguese] Acta Psiquiat Psicol Am Lat. 1999;45:122–141. [Google Scholar]
- 55.Herrera E, Jr, Caramelli P, Silveira AS, Nitrini R. Epidemiologic survey of dementia in a community-dwelling Brazilian population. Alzheimer Dis Assoc Disord. 2002;16:103–08. doi: 10.1097/00002093-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 56.Quiroga P, Calvo C, Albala C, et al. Apolipoprotein E polymorphism in elderly Chilean people with Alzheimer's disease. Neuroepidemiology. 1999;18:48–52. doi: 10.1159/000026195. [DOI] [PubMed] [Google Scholar]
- 57.Rosselli D, Ardila A, Pradilla G, et al. The Mini-Mental State Examination as a selected diagnostic test for dementia: a Colombian population study. GENECO [in Spanish] Rev Neurol. 2000;30:428–32. [PubMed] [Google Scholar]
- 58.Pradilla G, Vesga BE, Leon-Sarmiento FE, et al. Neuroepidemiology in the eastern region of Colombia [in Spanish] Rev Neurol. 2002;34:1035–43. [PubMed] [Google Scholar]
- 59.Yang B. Centre for Epidemiology and Research, NSW Department of Health (Australia); [July 10, 2008]. Meta prevalence estimates: generating combined prevalence estimates from separate population surveys. http://www.health.nsw.gov.au/pubs/2007/pooling_paper_final.html. [Google Scholar]
- 60.Ochayi B, Thacher TD. Risk factors for dementia in central Nigeria. Aging Ment Health. 2006;10:616–20. doi: 10.1080/13607860600736182. [DOI] [PubMed] [Google Scholar]
- 61.Ketzoian C, Rega I, Caceres R. Estudio de prevalencia de las principales enfermedades neurologicas en una poblacion del Uruguay. La Presna Medica Uruguaya. 1997;17:557–63. [Google Scholar]
- 62.Suhanov AV, Pilipenko PI, Korczyn AD, et al. Risk factors for Alzheimer's disease in Russia: a case-control study. Eur J Neurol. 2006;13:990–95. doi: 10.1111/j.1468-1331.2006.01391.x. [DOI] [PubMed] [Google Scholar]
- 63.Hendrie HC, Ogunniyi A, Hall KS, et al. Incidence of dementia and Alzheimer disease in 2 communities: Yoruba residing in Ibadan, Nigeria, and African Americans residing in Indianapolis, Indiana. JAMA. 2001;285:739–47. doi: 10.1001/jama.285.6.739. [DOI] [PubMed] [Google Scholar]
- 64.Alladi S, Kaul S, Meena AK, et al. Pattern of vascular dementia in India: study of clinical features, imaging, and vascular mechanisms from a hospital dementia registry. J Stroke Cerebrovasc Dis. 2006;15:49–56. doi: 10.1016/j.jstrokecerebrovasdis.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 65.Galasko D, Salmon D, Gamst A, et al. Prevalence of dementia in Chamorros on Guam: relationship to age, gender, education, and APOE. Neurology. 2007;68:1772–81. doi: 10.1212/01.wnl.0000262028.16738.64. [DOI] [PubMed] [Google Scholar]
- 66.Adam AM, Akuku O. Creutzfeldt-Jakob disease in Kenya. Trop Med Int Health. 2005;10:710–12. doi: 10.1111/j.1365-3156.2005.01435.x. [DOI] [PubMed] [Google Scholar]
- 67.Soldevila M, Calafell F, Andres AM, et al. Prion susceptibility and protective alleles exhibit marked geographic differences. Hum Mutat. 2003;22:104–05. doi: 10.1002/humu.9157. [DOI] [PubMed] [Google Scholar]
- 68.Heckmann JM, Low WC, de Villiers C, et al. Novel presenilin 1 mutation with profound neurofibrillary pathology in an indigenous Southern African family with early-onset Alzheimer's disease. Brain. 2004;127:133–42. doi: 10.1093/brain/awh009. [DOI] [PubMed] [Google Scholar]
- 69.Kalaria RN, Viitanen M, Kalimo H, et al. The pathogenesis of CADASIL: an update. J Neurol Sci. 2004;226:35–39. doi: 10.1016/j.jns.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 70.Gurumukhani JK, Ursekar M, Singhal BS. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leucoencephalopathy (CADASIL): a case report with review of literature. Neurol India. 2004;52:99–101. [PubMed] [Google Scholar]
- 71.Al-Jader LN, Harper PS, Krawczak M, Palmer SR. The frequency of inherited disorders database: prevalence of Huntington disease. Community Genet. 2001;4:148–57. doi: 10.1159/000051175. [DOI] [PubMed] [Google Scholar]
- 72.Wexler NS, Lorimer J, Porter J, et al. Venezuelan kindreds reveal that genetic and environmental factors modulate Huntington's disease age of onset. Proc Natl Acad Sci USA. 2004;101:3498–503. doi: 10.1073/pnas.0308679101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hogervorst E, Yesufu A, Sadjimim T, et al. High tofu consumption and genistein levels are associated with an increased risk for dementia in Indonesian elderly. In: Hogervorst E, Henderson VW, Gibbs R, Brinton-Diaz R, editors. Hormones, Cognition and Dementia. Cambridge: Cambridge University Press; 2008. in press. [Google Scholar]
- 74.Bowirrat A, Treves TA, Friedland RP, Korczyn AD. Prevalence of Alzheimer's type dementia in an elderly Arab population. Eur J Neurol. 2001;8:119–23. doi: 10.1046/j.1468-1331.2001.00183.x. [DOI] [PubMed] [Google Scholar]
- 75.Farrer LA, Bowirrat A, Friedland RP, et al. Identification of multiple loci for Alzheimer disease in a consanguineous Israeli-Arab community. Hum Mol Genet. 2003;12:415–22. doi: 10.1093/hmg/ddg037. [DOI] [PubMed] [Google Scholar]
- 76.Nitrini R, Caramelli P, Herrera E, Jr, et al. Incidence of dementia in a community-dwelling Brazilian population. Alzheimer Dis Assoc Disord. 2004;18:241–46. [PubMed] [Google Scholar]
- 77.Li S, Yan F, Li G, et al. Is the dementia rate increasing in Beijing? Prevalence and incidence of dementia 10 years later in an urban elderly population. Acta Psychiatr Scand. 2007;115:73–79. doi: 10.1111/j.1600-0447.2006.00859.x. [DOI] [PubMed] [Google Scholar]
- 78.McKhann G, Drachman DA, Folstein M, Katzman R, Price DL, Stadlan EM. Clinical diagnosis of Alzheimer's disease—report of the NINCDS–ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 79.Ogeng'o JA, Cohen DL, Sayi JG, et al. Cerebral amyloid beta protein deposits and other Alzheimer lesions in non-demented elderly east Africans. Brain Pathol. 1996;6:101–07. doi: 10.1111/j.1750-3639.1996.tb00790.x. [DOI] [PubMed] [Google Scholar]
- 80.Yasha TC, Shankar L, Santosh V, et al. Histopathological and immunohistochemical evaluation of ageing changes in normal human brain. Indian J Med Res. 1997;105:141–50. [PubMed] [Google Scholar]
- 81.Shankar SK, Chandra PS, Rao TS. Alzheimer's disease— histological, ultrastructural, and immunochemical study of an autopsy-proven case. Indian J Psychiatry. 1988;30:291–98. [PMC free article] [PubMed] [Google Scholar]
- 82.Chandra V, Pandav R, Dodge HH, et al. Incidence of Alzheimer's disease in a rural community in India: the Indo-US study. Neurology. 2001;57:985–89. doi: 10.1212/wnl.57.6.985. [DOI] [PubMed] [Google Scholar]
- 83.Roman GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies: report of the NINDS-AIREN international workshop. Neurology. 1993;43:250–60. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 84.Li G, Shen YC, Chen CH, et al. A three-year follow-up study of age-related dementia in an urban area of Beijing. Acta Psychiatr Scand. 1991;83:99–104. doi: 10.1111/j.1600-0447.1991.tb07373.x. [DOI] [PubMed] [Google Scholar]
- 85.Ampil ER, Fook-Chong S, Sodagar SN, et al. Ethnic variability in dementia: results from Singapore. Alzheimer Dis Assoc Disord. 2005;19:184–85. doi: 10.1097/01.wad.0000189032.58450.81. [DOI] [PubMed] [Google Scholar]
- 86.Zhou DH, Wang JY, Li J, et al. Frequency and risk factors of vascular cognitive impairment three months after ischemic stroke in china: the Chongqing stroke study. Neuroepidemiology. 2005;24:87–95. doi: 10.1159/000081055. [DOI] [PubMed] [Google Scholar]
- 87.Tang WK, Chan SS, Chiu HF, et al. Frequency and clinical determinants of poststroke cognitive impairment in nondemented stroke patients. J Geriatr Psychiatry Neurol. 2006;19:65–71. doi: 10.1177/0891988706286230. [DOI] [PubMed] [Google Scholar]
- 88.Ballard CG, Morris CM, Rao H, et al. APOE ε4 and cognitive decline in older stroke patients with early cognitive impairment. Neurology. 2004;63:1399–402. doi: 10.1212/01.wnl.0000141851.93193.17. [DOI] [PubMed] [Google Scholar]
- 89.Hachinski V, Iadecola C, Petersen RC, et al. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006;37:2220–41. doi: 10.1161/01.STR.0000237236.88823.47. [DOI] [PubMed] [Google Scholar]
- 90.Ogunnyi A, Akang EE, Gureje O, et al. Dementia with Lewy bodies in a Nigerian: a case report. Int Psychogeriatr. 2002;14:211–18. doi: 10.1017/s1041610202008402. [DOI] [PubMed] [Google Scholar]
- 91.Pinto C, Seethalakshmi R. Behavioral and psychological symptoms of dementia in an Indian population: comparison between Alzheimer's disease and vascular dementia. Int Psychogeriatr. 2006;18:87–93. doi: 10.1017/S104161020500311X. [DOI] [PubMed] [Google Scholar]
- 92.Chen HH, Hu CJ. Genetic characteristics of dementia in Taiwan. Acta Neurol Taiwan. 2006;15:161–69. [PubMed] [Google Scholar]
- 93.Zhang ZX, Roman GC, Hong Z, et al. Parkinson's disease in China: prevalence in Beijing, Xian, and Shanghai. Lancet. 2005;365:595–97. doi: 10.1016/S0140-6736(05)17909-4. [DOI] [PubMed] [Google Scholar]
- 94.Momeni P, Rogaeva E, Van Deerlin V, et al. Genetic variability in CHMP2B and frontotemporal dementia. Neurodegener Dis. 2006;3:129–33. doi: 10.1159/000094771. [DOI] [PubMed] [Google Scholar]
- 95.Radanovic M, Senaha ML, Mansur LL, et al. Primary progressive aphasia: analysis of 16 cases. Arq Neuropsiquiatr. 2001;59:512–20. doi: 10.1590/s0004-282x2001000400006. [DOI] [PubMed] [Google Scholar]
- 96.Perl DP, Hof PR, Purohit DP, et al. Hippocampal and entorhinal cortex neurofibrillary tangle formation in Guamanian Chamorros free of overt neurologic dysfunction. J Neuropathol Exp Neurol. 2003;62:381–88. doi: 10.1093/jnen/62.4.381. [DOI] [PubMed] [Google Scholar]
- 97.Campion D, Dumanchin C, Hannequin D, et al. Early-onset autosomal dominant Alzheimer disease: prevalence, genetic heterogeneity, and mutation spectrum. Am J Hum Genet. 1999;65:664–70. doi: 10.1086/302553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Edwards-Lee T, Ringman JM, Chung J, et al. An African American family with early-onset Alzheimer disease and an APP (T714I) mutation. Neurology. 2005;64:377–79. doi: 10.1212/01.WNL.0000149761.70566.3E. [DOI] [PubMed] [Google Scholar]
- 99.Satishchandra P, Yasha TC, Shankar L, et al. Familial Alzheimer disease: first report from India. Alzheimer Dis Assoc Disord. 1997;11:107–09. doi: 10.1097/00002093-199706000-00007. [DOI] [PubMed] [Google Scholar]
- 100.Lopera F, Ardilla A, Martinez A, et al. Clinical features of early-onset Alzheimer disease in a large kindred with an E280A presenilin-1 mutation. JAMA. 1997;277:793–99. [PubMed] [Google Scholar]
- 101.Romas SN, Santana V, Williamson J, et al. Familial Alzheimer disease among Caribbean Hispanics: a reexamination of its association with APOE. Arch Neurol. 2002;59:87–91. doi: 10.1001/archneur.59.1.87. [DOI] [PubMed] [Google Scholar]
- 102.Bohlega S, Al Shubili A, Edris A, et al. CADASIL in Arabs: clinical and genetic findings. BMC Med Genet. 2007;8:67. doi: 10.1186/1471-2350-8-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Silber E, Kromberg J, Temlett JA, et al. Huntington's disease confirmed by genetic testing in five African families. Mov Disord. 1998;13:726–30. doi: 10.1002/mds.870130420. [DOI] [PubMed] [Google Scholar]
- 104.Saleem Q, Roy S, Murgood U, et al. Molecular analysis of Huntington's disease and linked polymorphisms in the Indian population. Acta Neurol Scand. 2003;108:281–86. doi: 10.1034/j.1600-0404.2003.00133.x. [DOI] [PubMed] [Google Scholar]
- 105.Paradisi I, Hernandez A, Arias S. Huntington disease mutation in Venezuela: age of onset, haplotype analyses and geographic aggregation. J Hum Genet. 2008;53:127–35. doi: 10.1007/s10038-007-0227-1. [DOI] [PubMed] [Google Scholar]
- 106.Mejia S, Giraldo M, Pineda D, et al. Nongenetic factors as modifiers of the age of onset of familial Alzheimer's disease. Int Psychogeriatr. 2003;15:337–49. doi: 10.1017/s1041610203009591. [DOI] [PubMed] [Google Scholar]
- 107.Ferri CP, Ames D, Prince M. Behavioral and psychological symptoms of dementia in developing countries. Int Psychogeriatr. 2004;16:441–59. doi: 10.1017/s1041610204000833. [DOI] [PubMed] [Google Scholar]
- 108.Tatsch MF, Bottino CM, Azevedo D, et al. Neuropsychiatric symptoms in Alzheimer disease and cognitively impaired, nondemented elderly from a community-based sample in Brazil: prevalence and relationship with dementia severity. Am J Geriatr Psychiatry. 2006;14:438–45. doi: 10.1097/01.JGP.0000218218.47279.db. [DOI] [PubMed] [Google Scholar]
- 109.Baiyewu O, Smith-Gamble V, Lane KA, et al. Prevalence estimates of depression in elderly community-dwelling African Americans in Indianapolis and Yoruba in Ibadan, Nigeria. Int Psychogeriatr. 2007;19:679–89. doi: 10.1017/S1041610207005480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Baiyewu O, Smith-Gamble V, Akinbiyi A, et al. Behavioral and caregiver reaction of dementia as measured by the neuropsychiatric inventory in Nigerian community residents. Int Psychogeriatr. 2003;15:399–409. doi: 10.1017/s1041610203009645. [DOI] [PubMed] [Google Scholar]
- 111.Baiyewu O, Unverzagt FW, Ogunniyi A, et al. Cognitive impairment in community-dwelling older Nigerians: clinical correlates and stability of diagnosis. Eur J Neurol. 2002;9:573–80. doi: 10.1046/j.1468-1331.2002.00434.x. [DOI] [PubMed] [Google Scholar]
- 112.Xu G, Meyer JS, Huang Y, et al. Cross-cultural comparison of mild cognitive impairment between China and USA. Curr Alzheimer Res. 2004;1:55–61. doi: 10.2174/1567205043480564. [DOI] [PubMed] [Google Scholar]
- 113.Das SK, Bose P, Biswas A, et al. An epidemiologic study of mild cognitive impairment in Kolkata, India. Neurology. 2007;68:2019–26. doi: 10.1212/01.wnl.0000264424.76759.e6. [DOI] [PubMed] [Google Scholar]
- 114.Lopes MA, Hototian SR, Bustamante SE, et al. Prevalence of cognitive and functional impairment in a community sample in Ribeirao Preto, Brazil. Int J Geriatr Psychiatry. 2007;22:770–76. doi: 10.1002/gps.1737. [DOI] [PubMed] [Google Scholar]
- 115.Hototian SR, Lopes MA, Azevedo D, et al. Prevalence of cognitive and functional impairment in a community sample from Sao Paulo, Brazil. Dement Geriatr Cogn Disord. 2008;25:135–43. doi: 10.1159/000112554. [DOI] [PubMed] [Google Scholar]
- 116.Wong R, Pelaez M, Palloni A, Markides K. Survey data for the study of aging in Latin America and the Caribbean: selected studies. J Aging Health. 2006;18:157–79. doi: 10.1177/0898264305285655. [DOI] [PubMed] [Google Scholar]
- 117.Reynolds RM, Godfrey KM, Barker M, et al. Stress responsiveness in adult life: influence of mother's diet in late pregnancy. J Clin Endocrinol Metab. 2007;92:2208–10. doi: 10.1210/jc.2007-0071. [DOI] [PubMed] [Google Scholar]
- 118.Kim JM, Stewart R, Shin IS, et al. Associations between head circumference, leg length and dementia in a Korean population. Int J Geriatr Psychiatry. 2008;23:41–48. doi: 10.1002/gps.1833. [DOI] [PubMed] [Google Scholar]
- 119.Kim JM, Stewart R, Shin IS, Yoon JS. Limb length and dementia in an older Korean population. J Neurol Neurosurg Psychiatry. 2003;74:427–32. doi: 10.1136/jnnp.74.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Borenstein AR, Copenhaver CI, Mortimer JA. Early-life risk factors for Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;2:63–72. doi: 10.1097/01.wad.0000201854.62116.d7. [DOI] [PubMed] [Google Scholar]
- 121.Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 2004;3:343–53. doi: 10.1016/S1474-4422(04)00767-7. [DOI] [PubMed] [Google Scholar]
- 122.Sayi JG, Patel NB, Premkumar DR, et al. Apolipoprotein E polymorphism in elderly east Africans. East Afr Med J. 1997;74:668–70. [PubMed] [Google Scholar]
- 123.Kalaria RN, Ogeng'o JA, Patel NB, et al. Evaluation of risk factors for Alzheimer's disease in elderly east Africans. Brain Res Bull. 1997;44:573–77. doi: 10.1016/s0361-9230(97)00310-9. [DOI] [PubMed] [Google Scholar]
- 124.Gureje O, Ogunniyi A, Baiyewu O, et al. APOE ε4 is not associated with Alzheimer's disease in elderly Nigerians. Ann Neurol. 2006;59:182–85. doi: 10.1002/ana.20694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Katzman R, Hill LR, Yu ES, et al. The malignancy of dementia. Predictors of mortality in clinically diagnosed dementia in a population survey of Shanghai, China. Arch Neurol. 1994;51:1220–25. doi: 10.1001/archneur.1994.00540240064017. [DOI] [PubMed] [Google Scholar]
- 126.Bazrgar M, Karimi M, Fathzadeh M, et al. Apolipoprotein E polymorphism in Southern Iran: E4 allele in the lowest reported amounts. Mol Biol Rep. 2007 June 27; doi: 10.1007/s11033-007-9113-3. published online. [DOI] [PubMed] [Google Scholar]
- 127.Borenstein AR, Wu Y, Mortimer JA, et al. Developmental and vascular risk factors for Alzheimer's disease. Neurobiol Aging. 2005;26:325–34. doi: 10.1016/j.neurobiolaging.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 128.WHO. WHO Statistical Information System (WHOSIS) Life tables for WHO member states. [July 10, 2008]; http://www.who.int/whosis/database/life_tables/life_tables.cfm.
- 129.Finch CE, Crimmins EM. Inflammatory exposure and historical changes in human life-spans. Science. 2004;305:1736–39. doi: 10.1126/science.1092556. [DOI] [PubMed] [Google Scholar]
- 130.Mak Z, Kim JM, Stewart R. Leg length, cognitive impairment and cognitive decline in an African-Caribbean population. Int J Geriatr Psychiatry. 2006;21:266–72. doi: 10.1002/gps.1458. [DOI] [PubMed] [Google Scholar]
- 131.Hong X, Zhang ZX, Li H, et al. Leisure activity and life events and Alzheimer's disease. Chin J Neurol. 2003;36:206–09. [Google Scholar]
- 132.Keskinoglu P, Giray H, Picakciefe M, et al. The prevalence and risk factors of dementia in the elderly population in a low socioeconomic region of Izmir, Turkey. Arch Gerontol Geriatr. 2006;43:93–100. doi: 10.1016/j.archger.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 133.Bertram L, McQueen MB, Mullin K, et al. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nat Genet. 2007;39:17–23. doi: 10.1038/ng1934. [DOI] [PubMed] [Google Scholar]
- 134.Farrer LA, Friedland RP, Bowirrat A, et al. Genetic and environmental epidemiology of Alzheimer's disease in Arabs residing in Israel. J Mol Neurosci. 2003;20:207–12. doi: 10.1385/JMN:20:3:207. [DOI] [PubMed] [Google Scholar]
- 135.Meng Y, Baldwin CT, Bowirrat A, et al. Association of polymorphisms in the angiotensin-converting enzyme gene with Alzheimer disease in an Israeli Arab community. Am J Hum Genet. 2006;78:871–77. doi: 10.1086/503687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278:1349–56. [PubMed] [Google Scholar]
- 137.Rogaeva E, Meng Y, Lee JH, et al. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat Genet. 2007;39:168–77. doi: 10.1038/ng1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Stewart R, Russ C, Richards M, et al. Apolipoprotein E genotype, vascular risk and early cognitive impairment in an African Caribbean population. Dement Geriatr Cogn Disord. 2001;12:251–56. doi: 10.1159/000051267. [DOI] [PubMed] [Google Scholar]
- 139.Molero AE, Pino-Ramirez G, Maestre GE. Modulation by age and gender of risk for Alzheimer's disease and vascular dementia associated with the apolipoprotein E-ε4 allele in Latin Americans: findings from the Maracaibo Aging Study. Neurosci Lett. 2001;307:5–8. doi: 10.1016/s0304-3940(01)01911-5. [DOI] [PubMed] [Google Scholar]
- 140.Corbo RM, Scacchi R. Apolipoprotein E (APOE) allele distribution in the world. Is APOE*4 a ‘thrifty’ allele? Ann Hum Genet. 1999;63:301–10. doi: 10.1046/j.1469-1809.1999.6340301.x. [DOI] [PubMed] [Google Scholar]
- 141.Ganguli M, Chandra V, Kamboh MI, et al. Apolipoprotein E polymorphism and Alzheimer disease: the Indo-US Cross-National Dementia Study. Arch Neurol. 2000;57:824–30. doi: 10.1001/archneur.57.6.824. [DOI] [PubMed] [Google Scholar]
- 142.Murrell JR, Price B, Lane KA, et al. Association of apolipoprotein E genotype and Alzheimer disease in African Americans. Arch Neurol. 2006;63:431–34. doi: 10.1001/archneur.63.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Morgan OS, Eldemire DA, Thesiger CH, et al. APOE allele frequencies in demented and nondemented elderly Jamaicans. Ann Neurol. 1998;43:545. doi: 10.1002/ana.410430423. [DOI] [PubMed] [Google Scholar]
- 144.Pastor P, Roe CM, Villegas A, et al. Apolipoprotein E ε4 modifies Alzheimer's disease onset in an E280A PS1 kindred. Ann Neurol. 2003;54:163–69. doi: 10.1002/ana.10636. [DOI] [PubMed] [Google Scholar]
- 145.Borenstein AR, Mortimer JA, Schofield E, et al. Cycad exposure and risk of dementia, MCI, and PDC in the Chamorro population of Guam. Neurology. 2007;68:1764–71. doi: 10.1212/01.wnl.0000262027.31623.b2. [DOI] [PubMed] [Google Scholar]
- 146.Tan EK, Lee J, Chen CP, et al. SORL1 haplotypes modulate risk of Alzheimer's disease in Chinese. Neurobiol Aging. 2007 Dec 4; doi: 10.1016/j.neurobiolaging.2007.10.013/ s0197-4580(07)00415-0. published online. [pii] [DOI] [PubMed] [Google Scholar]
- 147.Das SK, Banerjee TK, Biswas A, et al. A prospective community-based study of stroke in Kolkata, India. Stroke. 2007;38:906–10. doi: 10.1161/01.STR.0000258111.00319.58. [DOI] [PubMed] [Google Scholar]
- 148.Bhattacharya S, Saha SP, Basu A, Das SK. A 5 years prospective study of incidence, morbidity and mortality profile of stroke in a rural community of eastern India. J Indian Med Assoc. 2005;103:655–59. [PubMed] [Google Scholar]
- 149.Feigin VL, Lawes CM, Bennett DA, Anderson CS. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. 2003;2:43–53. doi: 10.1016/s1474-4422(03)00266-7. [DOI] [PubMed] [Google Scholar]
- 150.Richards SS, Emsley CL, Roberts J, et al. The association between vascular risk factor-mediating medications and cognition and dementia diagnosis in a community-based sample of African-Americans. J Am Geriatr Soc. 2000;48:1035–41. doi: 10.1111/j.1532-5415.2000.tb04777.x. [DOI] [PubMed] [Google Scholar]
- 151.Kalaria RN, Ballard C. Stroke and cognition. Curr Atheroscler Rep. 2001;3:334–39. doi: 10.1007/s11883-001-0028-5. [DOI] [PubMed] [Google Scholar]
- 152.Honig LS, Tang MX, Albert S, et al. Stroke and the risk of Alzheimer disease. Arch Neurol. 2003;60:1707–12. doi: 10.1001/archneur.60.12.1707. [DOI] [PubMed] [Google Scholar]
- 153.Vermeer SE, Prins ND, den Heijer T, et al. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–22. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 154.Snowdon DA, Greiner LH, Mortimer JA, et al. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study JAMA. 1997;277:813–17. [PubMed] [Google Scholar]
- 155.Craft S. Insulin resistance and Alzheimer's disease pathogenesis: potential mechanisms and implications for treatment. Curr Alzheimer Res. 2007;4:147–52. doi: 10.2174/156720507780362137. [DOI] [PubMed] [Google Scholar]
- 156.Tan ZS, Seshadri S, Beiser A, et al. Plasma total cholesterol level as a risk factor for Alzheimer disease: the Framingham Study. Arch Intern Med. 2003;163:1053–57. doi: 10.1001/archinte.163.9.1053. [DOI] [PubMed] [Google Scholar]
- 157.Evans RM, Emsley CL, Gao S, et al. Serum cholesterol, APOE genotype and the risk of Alzheimer disease in a population-based study of African Americans. Neurology. 2000;54:240–42. doi: 10.1212/wnl.54.1.240. [DOI] [PubMed] [Google Scholar]
- 158.Luchsinger JA, Tang MX, Stern Y, et al. Diabetes mellitus and risk of Alzheimer's disease and dementia with stroke in a multiethnic cohort. Am J Epidemiol. 2001;154:635–41. doi: 10.1093/aje/154.7.635. [DOI] [PubMed] [Google Scholar]
- 159.Biessels GJ, Staekenborg S, Brunner E, et al. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5:64–74. doi: 10.1016/S1474-4422(05)70284-2. [DOI] [PubMed] [Google Scholar]
- 160.Pi-Sunyer FX. The obesity epidemic: pathophysiology and consequences of obesity. Obes Res. 2002;10(suppl 2):97S–104S. doi: 10.1038/oby.2002.202. [DOI] [PubMed] [Google Scholar]
- 161.Hofman A, Ott A, Breteler MM, et al. Atherosclerosis, apolipoprotein E, and prevalence of dementia and Alzheimer's disease in the Rotterdam Study. Lancet. 1997;349:151–54. doi: 10.1016/S0140-6736(96)09328-2. [DOI] [PubMed] [Google Scholar]
- 162.Ott A, Breteler MM, de Bruyne MC, et al. Atrial fibrillation and dementia in a population-based study. The Rotterdam Study Stroke. 1997;28:316–21. doi: 10.1161/01.str.28.2.316. [DOI] [PubMed] [Google Scholar]
- 163.Roriz-Cruz M, Rosset I, Wada T, et al. Cognitive impairment and frontal-subcortical geriatric syndrome are associated with metabolic syndrome in a stroke-free population. Neurobiol Aging. 2007;28:1723–36. doi: 10.1016/j.neurobiolaging.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 164.Roriz-Cruz M, Rosset I, Wada T, et al. Stroke-independent association between metabolic syndrome and functional dependence, depression, and low quality of life in elderly community-dwelling Brazilian people. J Am Geriatr Soc. 2007;55:374–82. doi: 10.1111/j.1532-5415.2007.01068.x. [DOI] [PubMed] [Google Scholar]
- 165.Abdullah AS, Husten CG. Promotion of smoking cessation in developing countries: a framework for urgent public health interventions. Thorax. 2004;59:623–30. doi: 10.1136/thx.2003.018820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases: part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104:2746–53. doi: 10.1161/hc4601.099487. [DOI] [PubMed] [Google Scholar]
- 167.Fuentes R, Uusitalo T, Puska P, et al. Blood cholesterol level and prevalence of hypercholesterolaemia in developing countries: a review of population-based studies carried out from 1979 to 2002. Eur J Cardiovasc Prev Rehabil. 2003;10:411–19. doi: 10.1097/01.hjr.0000085247.65733.4f. [DOI] [PubMed] [Google Scholar]
- 168.Kearney PM, Whelton M, Reynolds K, et al. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–23. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 169.Dagogo-Jack S. Primary prevention of type-2 diabetes in developing countries. J Natl Med Assoc. 2006;98:415–19. [PMC free article] [PubMed] [Google Scholar]
- 170.Cooper RS, Amoah AGB, Mensa GA. High blood pressure: the foundation for epidemic cardiovascular disease in African populations. Ethn Dis. 2003;13:48–52. [PubMed] [Google Scholar]
- 171.Gaziano TA. Reducing the growing burden of cardiovascular disease in the developing world. Health Aff (Millwood) 2007;26:13–24. doi: 10.1377/hlthaff.26.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Prentice AM. The emerging epidemic of obesity in developing countries. Int J Epidemiol. 2006;35:93–99. doi: 10.1093/ije/dyi272. [DOI] [PubMed] [Google Scholar]
- 173.Murray MD, Lane KA, Gao S, et al. Preservation of cognitive function with antihypertensive medications: a longitudinal analysis of a community-based sample of African Americans. Arch Intern Med. 2002;162:2090–96. doi: 10.1001/archinte.162.18.2090. [DOI] [PubMed] [Google Scholar]
- 174.Szwast SJ, Hendrie HC, Lane KA, et al. Association of statin use with cognitive decline in elderly African Americans. Neurology. 2007;69:1873–80. doi: 10.1212/01.wnl.0000279333.77404.d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mendis S, Abegunde D, Yusuf S, et al. WHO study on Prevention of REcurrences of Myocardial Infarction and StrokE (WHO-PREMISE) Bull World Health Organ. 2005;83:820–29. [PMC free article] [PubMed] [Google Scholar]
- 176.Hall K, Murrell J, Ogunniyi A, et al. Cholesterol, APOE genotype, and Alzheimer disease: an epidemiologic study of Nigerian Yoruba. Neurology. 2006;66:223–27. doi: 10.1212/01.wnl.0000194507.39504.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Pandav RS, Chandra V, Dodge HH, et al. Hemoglobin levels and Alzheimer disease: an epidemiologic study in India. Am J Geriatr Psychiatry. 2004;12:523–26. doi: 10.1176/appi.ajgp.12.5.523. [DOI] [PubMed] [Google Scholar]
- 178.Molero AE, Altimari CC, Duran DA, et al. Total plasma homocysteine values among elderly subjects: findings from the Maracaibo Aging Study. Clin Biochem. 2006;39:1007–15. doi: 10.1016/j.clinbiochem.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 179.Mizrahi EH, Bowirrat A, Jacobsen DW, et al. Plasma homocysteine, vitamin B12 and folate in Alzheimer's patients and healthy Arabs in Israel. J Neurol Sci. 2004;227:109–13. doi: 10.1016/j.jns.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 180.Luchsinger JA, Noble JM, Scarmeas N. Diet and Alzheimer's disease. Curr Neurol Neurosci Rep. 2007;7:366–72. doi: 10.1007/s11910-007-0057-8. [DOI] [PubMed] [Google Scholar]
- 181.Martin A, Cherubini A, Andres-Lacueva C, et al. Effects of fruits and vegetables on levels of vitamins E and C in the brain and their association with cognitive performance. J Nutr Health Aging. 2002;6:392–404. [PubMed] [Google Scholar]
- 182.Wang QH, Zhang ZX, Tang MN, et al. Smoking, alcohol and tea drinking on Alzheimer's disease. Chin J Neurol. 2004;37:234–38. [Google Scholar]
- 183.Handajani S. Indigenous mucuna tempe as functional food. Asia Pac J Clin Nutr. 2001;10:222–25. doi: 10.1046/j.1440-6047.2001.00243.x. [DOI] [PubMed] [Google Scholar]
- 184.White LR, Petrovitch H, Ross GW, et al. Brain aging and midlife tofu consumption. J Am Coll Nutr. 2000;19:242–55. doi: 10.1080/07315724.2000.10718923. [DOI] [PubMed] [Google Scholar]
- 185.Perry E. Commentary: botanical potentials in Alzheimer's disease. J Altern Complement Med. 2007;13:345–46. doi: 10.1089/acm.2006.6405. [DOI] [PubMed] [Google Scholar]
- 186.DeKosky ST, Fitzpatrick A, Ives DG, et al. The Ginkgo Evaluation of Memory (GEM) study: design and baseline data of a randomized trial of Ginkgo biloba extract in prevention of dementia. Contemp Clin Trials. 2006;27:238–53. doi: 10.1016/j.cct.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 187.Dodge HH, Zitzelberger T, Oken BS, et al. A randomized placebo-controlled trial of Ginkgo biloba for the prevention of cognitive decline. Neurology. 2008;70:1809–17. doi: 10.1212/01.wnl.0000303814.13509.db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Birks J, Grimley Evans J. Ginkgo biloba for cognitive impairment and dementia. Cochrane Database Syst Rev. 2007;(2):CD003120. doi: 10.1002/14651858.CD003120.pub2. [DOI] [PubMed] [Google Scholar]
- 189.Ma X, Tan C, Zhu D, et al. Huperzine A from Huperzia species— an ethnopharmacolgical review. J Ethnopharmacol. 2007;113:15–34. doi: 10.1016/j.jep.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 190.Zhang HY, Tang XC. Neuroprotective effects of huperzine A: new therapeutic targets for neurodegenerative disease. Trends Pharmacol Sci. 2006;27:619–25. doi: 10.1016/j.tips.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 191.Little JT, Walsh S, Aisen PS. An update on huperzine A as a treatment for Alzheimer's disease. Expert Opin Investig Drugs. 2008;17:209–15. doi: 10.1517/13543784.17.2.209. [DOI] [PubMed] [Google Scholar]
- 192.Kennedy DO, Scholey AB. The psychopharmacology of European herbs with cognition-enhancing properties. Curr Pharm Des. 2006;12:4613–23. doi: 10.2174/138161206779010387. [DOI] [PubMed] [Google Scholar]
- 193.Dos Santos-Neto LL, de Vilhena Toledo MA, Medeiros-Souza P, de Souza GA. The use of herbal medicine in Alzheimer's disease: a systematic review. Evid Based Complement Alternat Med. 2006;3:441–45. doi: 10.1093/ecam/nel071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ramassamy C. Emerging role of polyphenolic compounds in the treatment of neurodegenerative diseases: a review of their intracellular targets. Eur J Pharmacol. 2006;545:51–64. doi: 10.1016/j.ejphar.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 195.Burgener SC, Buettner L, Coen Buckwalter K, et al. Evidence supporting nutritional interventions for persons in early stage Alzheimer's disease (AD) J Nutr Health Aging. 2008;12:18–21. doi: 10.1007/BF02982159. [DOI] [PubMed] [Google Scholar]
- 196.Olin J, Schneider L. Galantamine for Alzheimer's disease. Cochrane Database Syst Rev. 2002;(3):CD001747. doi: 10.1002/14651858.CD001747. [DOI] [PubMed] [Google Scholar]
- 197.Nitrini R, Caramelli P, Herrera E, Jr, et al. Mortality from dementia in a community-dwelling Brazilian population. Int J Geriatr Psychiatry. 2005;20:247–53. doi: 10.1002/gps.1274. [DOI] [PubMed] [Google Scholar]
- 198.Perkins AJ, Hui SL, Ogunniyi A, et al. Risk of mortality for dementia in a developing country: the Yoruba in Nigeria. Int J Geriatr Psychiatry. 2002;17:566–73. doi: 10.1002/gps.643. [DOI] [PubMed] [Google Scholar]
- 199.Lane KA, Gao S, Hui SL, et al. Apolipoprotein E and mortality in African-Americans and Yoruba. J Alzheimers Dis. 2003;5:383–90. doi: 10.3233/jad-2003-5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.WHO. Global burden of disease estimates. [July 10, 2008];2004 December; http://www.who.int/healthinfo/statistics/bodgbddeathdalyestimates.xls.
- 201.Wimo A, Jönsson L, Winblad B. An estimate of the total worldwide societal costs of dementia in 2005. Alzheimer Dement. 2007;3:81–91. doi: 10.1016/j.jalz.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 202.Andlin-Sobocki P, Jonsson B, Wittchen HU, Olesen J. Cost of disorders of the brain in Europe. Eur J Neurol. 2005;12(suppl 1):1–27. doi: 10.1111/j.1468-1331.2005.01202.x. [DOI] [PubMed] [Google Scholar]
- 203.Jacobzone S, Cambois E, Chaplain E, Robine J. Long term care services to older people, a perspective on future trends: the impact of an improved health of older persons Ageing working papers. Paris: OECD; 1998. [Google Scholar]
- 204.Moise P, Schwarzinger M, Um MY, et al. Dementia care in 9 OECD countries A comparative analysis. Paris: OECD; 2004. [Google Scholar]
- 205.Dias A, Dewey ME, D'Souza J, et al. The effectiveness of a home care program for supporting caregivers of persons with dementia in developing countries: a randomised controlled trial from Goa, India. PLoS ONE. 2008;3:e2333. doi: 10.1371/journal.pone.0002333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zencir M, Kuzu N, Beser NG, et al. Cost of Alzheimer's disease in a developing country setting. Int J Geriatr Psychiatry. 2005;20:616–22. doi: 10.1002/gps.1332. [DOI] [PubMed] [Google Scholar]
- 207.Prince M. Care arrangements for people with dementia in developing countries. Int J Geriatr Psychiatry. 2004;19:170–77. doi: 10.1002/gps.1046. [DOI] [PubMed] [Google Scholar]
- 208.Kabir ZN, Szebehely M, Tishelman C. Support in old age in the changing society of Bangladesh. Ageing Soc. 2002;22:615–36. [Google Scholar]
- 209.Allegri RF, Butman J, Arizaga RL, et al. Economic impact of dementia in developing countries: an evaluation of costs of Alzheimer-type dementia in Argentina. Int Psychogeriatr. 2007;19:705–18. doi: 10.1017/S1041610206003784. [DOI] [PubMed] [Google Scholar]
- 210.Veras RP, Caldas CP, Dantas SB, et al. Family care for demented elderly individuals: cost analysis. Rev Psiq Clín. 2007;34:5–12. [Google Scholar]
- 211.An CX, Yu X. A study on economic burden and correlated factors in patients with dementia. Chin Mental Health J. 2005;19:592–95. [Google Scholar]
- 212.Suh GH, Knapp M, Kang CJ. The economic costs of dementia in Korea, 2002. Int J Geriatr Psychiatry. 2006;21:722–28. doi: 10.1002/gps.1552. [DOI] [PubMed] [Google Scholar]
- 213.Dias A, Samuel R, Patel V, et al. The impact associated with caring for a person with dementia: a report from the 10/66 Dementia Research Group's Indian network. Int J Geriatr Psychiatry. 2004;19:182–84. doi: 10.1002/gps.1016. [DOI] [PubMed] [Google Scholar]
- 214.Chiu L, Shyu WC. Estimation of the family cost of private nursing home care versus home care for patients with dementia in Taiwan. Chang Gung Med J. 2001;24:608–14. [PubMed] [Google Scholar]
- 215.Chiu L, Tang KY, Liu YH, et al. Cost comparisons between family-based care and nursing home care for dementia. J Adv Nurs. 1999;29:1005–12. doi: 10.1046/j.1365-2648.1999.00969.x. [DOI] [PubMed] [Google Scholar]
- 216.Shah A, Murthy S, Suh GK. Is mental health economics important in geriatric psychiatry in developing countries? Int J Geriatr Psychiatry. 2002;17:758–64. doi: 10.1002/gps.696. [DOI] [PubMed] [Google Scholar]
- 217.Zhang XJ. Epidemiology of dementia in China. Neurobiol Aging. 2008;29(suppl 1):S27–28. [Google Scholar]
- 218.Prince M, Livingston G, Katona C. Mental health care for the elderly in low-income countries: a health systems approach. World Psychiatry. 2007;6:5–13. [PMC free article] [PubMed] [Google Scholar]
- 219.Jacob ME, Abraham VJ, Abraham S, Jacob KS. The effect of community based daycare on mental health and quality of life of elderly in rural south India: a community intervention study. Int J Geriatr Psychiatry. 2007;22:445–47. doi: 10.1002/gps.1706. [DOI] [PubMed] [Google Scholar]
- 220.Ogunniyi A, Hall KS, Baiyewu O, et al. Caring for individuals with dementia: the Nigerian experience. West Afr J Med. 2005;24:259–62. doi: 10.4314/wajm.v24i3.28211. [DOI] [PubMed] [Google Scholar]