For the most accurate and reproducible hematological results, whole blood specimens should be analyzed as soon as possible after collection.3 With multi-center studies, samples may be drawn off-site and then transported at ambient temperatures to another laboratory.11 Transportation times may vary depending on distance and location of the laboratories.
Automated hematological analysis of specimens, particularly analysis of the differential count if delayed beyond 4 hours, has in the past yielded doubtful, and often invalid, results. Older technologies most commonly rely upon cell size or cell size together with scatter properties to differentiate white blood cell populations. Because the size and scatter properties of white cells change as a blood sample ages, automated analysis of the differential becomes more unreliable with time. New technologies for the performance of automated differentials have reduced many of the problems associated with automated differential counts dependent on scatter properties only.6,7,9 The properties of red blood cells (RBCs) also change with time in stored samples, most notably cell size and the red cell indices related to size.
The manufacturer recommends each laboratory establish its own standards of acceptability for results on samples held beyond the optimal time for analysis.12 This study examines the complete blood count (CBC) and differential (Diff) parameters as measured by the Sysmex XT-2000i to determine what changes occur and the validity of the results obtained with aged samples over time. The results will be used to determine what hematological parameters can be reported as clinically valid results if a sample cannot be analyzed in a timely manner. The stability over time of the manual Diff was also evaluated with a small group of samples. The purpose of the limited, additional study is to demonstrate what use, if any, the preparation of a smear and the performance of a manual Diff count could have in a sample 24 hours old or older. In some laboratories, samples may be drawn off site and may be transported to the laboratories by couriers.
Methods
The Sysmex XT-2000i uses the electric resistance detecting method (impedance technology) with hydro dynamic focusing to measure RBC, PLT, MPV, MCV write out on first reference, and Hematocrit (HCT). Fluorescence flow cytometry is used to measure WBC, Diff, the optical PLT count, and the reticulocyte count. The system employs a 633 nm semi-conductor laser for flow cytometry analysis. For the measurement by flow cytometry of the proportional count, expressed as percent of the total WBC, of neutrophils (NEUT), lymphocytes (LYMPH), monocytes (MONO), and eosinophils (EOS), white cells are stained with fluorescent dyes that bind to both DNA and RNA. Side Scatter (SSC) is employed to determine the internal complexity of the cell—the size, shape, and density of the nucleus and granules of the cell. Fluorescence and scatter measurements are combined to characterize white cell populations. Basophils (BASO) are measured separately using cell size and SSC properties. Hemoglobin (HGB) is measured photocolorimetrically using SLS-HGB, a cyanide-free method.
The reagents required for the operation of the Sysmex XT-2000i are supplied by Sysmex America (Mundelein, IL) and are listed as follows:
| Reagent | Function |
| Cellpack | RBC/PLT and HGB Diluent; rinsing of instrument; hydrodynamic focusing |
| Stromatolyser-4DL | Diff lysing reagent |
| Stromatolyser-4DS | Diff stain |
| Stromatolyser-FB | Diluent for WBC count and lyses all cells except BASO |
| Sulfolyser | Non-cyanide HGB lyse (sodium lauryl sulphate) |
| Ret-Search (II) | Dilutes sample for reticulocyte analysis |
| Ret-Search (II) Dye | Stains reticulocytes and platelets for analysis |
Hematocrit and MCV are direct measurements on the Sysmex XT-2000i. The MCV is an average of all RBC size measurements collected in the impedance counter. The HCT is the sum of all the RBC size measurements and reported in proportion to the total volume of the analysis sample. Calculated red cell indices are MCH write out first reference, MCHC write out first reference, and red cell distribution width (RDW). Red cell distribution width is reported on the Sysmex XT as both standard deviation from the mean red cell size (RDW-SD) and as coefficient of variation from the mean (RDW-CV).
The Sysmex XT-2000i provides 2 PLT counts. One is an impedance count that both enumerates the platelets (I-PLT) and estimates their size (mean platelet volume = MPV). The other is an optional optical count obtained in 1 of the flow analysis channels (O-PLT). The instrument also performs an optional reticulocyte count in 1 of the flow analysis channels. Red blood cells are stained, counted, and measured for size and fluorescence. Counts are expressed as percent of RBC (RET%).
This laboratory operates under Clinical Laboratory Improvement Act (CLIA) certification. Calibration of the instrument is confirmed each day using 2 levels of controls (Sysmex e-Check Hematology Control for Sysmex X-Series Analyzers, Sysmex America), according to the manufacturer’s recommendations.12 These recommendations are consistent with CLIA Interpretive Guidelines 493.1256(a)-(c) and 493.1256(d), Standard: Control procedures. Repeated analysis of a sample obtained from a healthy donor is used daily to confirm instrument precision, as per CLIA Interpretive Guideline 493.1253(b)(1)(i)(B), Precision (Reproducibility).
Samples
Whole blood from participants is drawn by venipuncture into dipotassium ethylenediamine-tetraacetate (K2-EDTA)-containing evacuated Vacutainer tubes (Becton Dickinson, Franklin Lakes, NJ) after informed consent was obtained. Samples were packaged according to CDC guidelines3 and transported by courier in insulated containers to this CLIA-certified laboratory. Most samples are delivered within 4 hours of collection. Samples held overnight before delivery are kept at room temperature (65–76°F).
The participants in the study included 56 healthy donors and 110 HIV-positive individuals. Healthy donors were age 18 or older and weighed at least 110 pounds. Donors were excluded from participation in the program if any of the following conditions were found:
history of chronic illness which might increase the risk associated with phlebotomy including, but not limited to, heart, lung, or kidney disease
current acute illness
history of clotting disorders
current medications which might increase the risk associated with phlebotomy
history of syncope or other difficulties with venipuncture
anemia, as determined by CBC or HCT
positive blood test for exposure to HIV-1, HIV-2, HTLV-1, HTLV-2, HIV p24 Ag, HBV sAg, or HCV sAG.
HIV-positive individuals were participants in a clinical study approved by the National Institute of Allergy and Infectious Diseases (NIAID) institutional review board.
A CBC and Diff count were performed on each sample within 4 hours of collection in open mode. Open mode is the reference mode for calibration in the Sysmex XT-2000i system; results in this mode are accepted as representative of the instrument’s performance in this study. The CBC includes WBC, RBC, HGB, HCT, MCV, MCH, MCHC, PLT, RDW-SD, RDW-CV, and MPV. Analysis of those same whole blood samples was repeated on the same instrument at 24 hours, 48 hours, and 72 hours. Samples remained capped at an ambient temperature throughout the study. According to CDC guidelines,3 an acceptable working temperature for the facility is 65°F to 76°F. Recorded temperatures during the period of the study were from 66°F to 72.5°F.
A small, additional study was included using a group of 20 samples also held at room temperature. These samples were analyzed in the same manner as all other samples in the larger study, but in addition, Wright-Geimsa stained smears were made from each sample at each time point. Manual Diff were performed according to Clinical and Laboratory Standards Institute document H20-A2,4 with the exception that, for the purposes of this study, smears were prepared from samples more than 4 hours old. These results were compared to the automated Diff at each time point.
In order to establish a baseline for measurement precision, 127 samples were analyzed twice within the same day as the samples were collected. Of the 127 samples, 54 were healthy donors. The results of these measurements were used to evaluate the stability of the samples stored at room temperature.
Statistical Analysis
To evaluate the stability of the CBC and the Diff count measurements from delayed samples at 24 hours, 48 hours, and 72 hours post collection, we computed the mean absolute difference, the mean percent difference, and the quotient of the measurements from the delayed samples relative to the measurements from samples within 4 hours of collection, together with the respective 95% confidence intervals. In addition, linear regressions were performed for the measurements from delayed samples versus the measurements from samples within 4 hours of collection. The intercepts and the slopes, as well as Pearson’s correlation coefficients, were reported with the respective 95% confidence intervals.
To compare the I-PLT count with the O-PLT count, we applied Bland and Altman’s difference plot,1 where the paired difference of O-PLT measurement from I-PLT measurement was plotted against the average of the 2. If O-PLT was consistent with I-PLT, we would observe the points in the different plots randomly scattered around the horizontal line. Otherwise, the difference plot would indicate how the difference between O-PLT and I-PLT varies with the underlying platelet count.
Results
The range of results obtained on all parameters from healthy donors, HIV+ samples, and the combined results of all samples is shown in Table 1. The same-day precision results for all parameters of the CBC and Diff count are shown on Table 2, including the average absolute difference, average percent difference, quotient, intercept, slope, and correlation coefficient. The manufacturer’s suggested percent limits for reproducibility for all parameters of the CBC are given in Table 5. Also shown in Table 5 are the limits of variation and correlation coefficients for the accuracy of white blood cell types in a Diff count as provided by the manufacturer.12 For all parameters of the CBC in the same-day precision analysis, the average percent difference fell below the manufacturer’s percent limit for acceptable reproducibility. The upper limits of the confidence interval of the average percent difference fell below the manufacturer’s suggested percent limit as well. Similarly, the average percent difference, and also the correlation coefficient, of the same-day analysis of the RET% results were within the manufacturer’s suggested limits. This was also true when comparing the same-day analysis of the Diff counts to the 3 reproducibility criteria provided by the manufacturer for the Diff: average percent difference, absolute difference, and correlation coefficient. The results were all within those suggested limits and were also consistent with the results of the evaluation of the Sysmex-XT-2000i’s performance published in other studies.9 Because the results of the same-day precision analysis were in excellent agreement with the manufacturer’s recommendations for reproducibility on the Sysmex XT-2000i, these results were used to evaluate the performance of the instrument on the aged samples.
Table 1.
Results Ranges for Healthy Donors, HIV+ Patients, and Combined Results
| Healthy Donors | HIV+ Patients | Combined Results | |||||
|---|---|---|---|---|---|---|---|
| Units | Lowest | Highest | Lowest | Highest | Lowest | Highest | |
| WBC | 103/μl | 3.18 | 9.71 | 2.21 | 12 | 2.21 | 12 |
| RBC | 106/μl | 3.88 | 6.06 | 2.11 | 6.34 | 2.11 | 6.34 |
| HGB | g/dl | 11.6 | 18.6 | 8.5 | 18.6 | 8.5 | 18.6 |
| HCT | % | 34.8 | 52.4 | 25 | 52 | 25 | 52.4 |
| MCV | fL | 73.9 | 93.2 | 64.8 | 127.5 | 64.8 | 127.5 |
| MCH | pg | 24.2 | 32.9 | 21.1 | 45.5 | 21.1 | 45.5 |
| MCHC | g/dl | 31.7 | 36.5 | 32.4 | 38.6 | 31.7 | 38.6 |
| I-PLT | 103/μl | 163 | 429 | 93 | 547 | 93 | 547 |
| O-PLT | 103/μl | 174 | 420 | 98 | 514 | 98 | 514 |
| RDW-SD | fL | 37.2 | 54.2 | 35.4 | 65.4 | 35.4 | 65.4 |
| RDW-CV | % | 11.3 | 19.5 | 11.3 | 19.3 | 11.3 | 19.5 |
| MPV | fL | 9.1 | 12.6 | 8.9 | 13.6 | 8.9 | 13.6 |
| RET | % | 0.37 | 2.11 | 0.37 | 7.76 | 0.37 | 7.76 |
| NEUT | % | 26.5 | 71.7 | 27.3 | 88.3 | 26.5 | 88.3 |
| LYMPH | % | 15.9 | 60.7 | 8.1 | 61.7 | 8.1 | 61.7 |
| MONO | % | 4.1 | 14.7 | 2.7 | 19.1 | 2.7 | 19.1 |
| EOS | % | 0.8 | 10.4 | 0.1 | 10.3 | 0.1 | 10.4 |
| BASO | % | 0.1 | 2 | 0 | 1.2 | 0 | 2 |
Abbreviations: WBC, white blood cell; RBC, red blood cell; HGB, hemoglobin; HCT, Hematocrit; MCV, write out; MCH, write out; MCHC, write out; I-PLT, write out; O-PLT, write out; RDW-SD, standard deviation from the mean red cell size; RDW-CV, coefficient of variation from the mean; MPV, write out; RET, write out; NEUT, neutrophils; LYMPH; lymphocytes; MONO, monocytes; EOS, eosinophils, BASO, basophils.
Table 2.
Same-Day Precision Results: Absolute Difference, Average % Difference, Quotient, Intercept, Slope, Correlation Coefficient
| Absolute Difference (95% CI) | Ave % Difference (95% CI) | Quotient | Intercept (95% CI) | Slope (95% CI) | Correlation Coefficient (95% CI) | |
|---|---|---|---|---|---|---|
| WBC | 0.116 (0.097–0.134) | 2.097 (1.744–2.45) | 1.011 | −0.016 (−0.103–0.071) | 1.013 (0.999–1.028) | 0.997 |
| RBC | 0.029 (0.021–0.036) | 0.638 (0.458–0.818) | 1.001 | −0.046 (−0.114–0.021) | 1.011 (0.997–1.026) | 0.997 |
| HGB | 0.091 (0.077–0.104) | 0.638 (0.542–0.735) | 1.002 | −0.118 (−0.324–0.088) | 1.011 (0.996–1.025) | 0.997 |
| HCT | 0.429 (0.345–0.513) | 1.048 (0.84–1.256) | 1.008 | −0.143 (−1.301–1.015) | 1.011 (0.983–1.039) | 0.988 |
| MCV | 0.691 (0.625–0.756) | 0.75 (0.682–0.818) | 1.007 | 0.235 (−0.677–1.147) | 1.004 (0.994–1.014) | 0.998 |
| MCH | 0.294 (0.223–0.366) | 0.92 (0.714–1.126) | 1.002 | −0.362 (−1.134–0.411) | 1.013 (0.989–1.037) | 0.991 |
| MCHC | 0.392 (0.311–0.473) | 1.137 (0.897–1.376) | 0.994 | 2.126 (−1.145–5.397) | 0.933 (0.838–1.027) | 0.865 |
| I-PLT | 7.307 (4.318–10.296) | 2.995 (2.215–3.774) | 1.006 | 15.44 (3.474–27.414) | 0.937 (0.887–0.987) | 0.957 |
| O-PLT | 9.244 (7.939–10.549) | 4.155 (3.585–4.724) | 1.005 | 4.301 (−3.518–12.12) | 0.985 (0.952–1.018) | 0.982 |
| RDW-SD | 1.028 (0.936–1.12) | 2.339 (2.142–2.535) | 1.023 | −0.719 (−1.618–0.18) | 1.04 (1.019–1.06) | 0.994 |
| RDW-CV | 0.271 (0.224–0.318) | 2.022 (1.686–2.357) | 1.017 | 0.346 (−0.245–0.937) | 0.991 (0.947–1.035) | 0.969 |
| MPV | 0.306 (0.261–0.35) | 2.862 (2.437–3.287) | 1.027 | 0.51 (−0.065–1.084) | 0.979 (0.926–1.032) | 0.955 |
| RET | 0.105 (0.088–0.121) | 9.534 (8.184–10.884) | 1.001 | 0.118 (0.053–.0182) | 0.885 (0.832–0.937) | 0.948 |
| NEUT% | 1.101 (0.969–1.233) | 2.288 (1.925–2.651) | 1.007 | 1.895 (0.812–2.978) | 0.969 (0.949–0.989) | 0.993 |
| LYMPH% | 1.231 (1.029–1.434) | 3.678 (3.097–4.258) | 0.986 | 0.38 (−0.605–1.365) | 0.974 (0.947–1.001) | 0.988 |
| MONO% | 0.87 (0.758–0.982) | 10.978 (9.367–12-588) | 1.047 | 1.659 (1.088–2.23) | 0.834 (0.991–0.898) | 0.917 |
| EOS% | 0.285 (0.239–0.331) | 12.496 (10.341–14.652) | 1.022 | 0.199 (0.083–0.315) | 0.932 (0.894–0.971) | 0.974 |
| BASO% | 0.152 (0.124–0.18) | 32.231 (25.018–39.445) | 1.103 | 0.128 (0.063–0.192) | 0.766 (0.653–0.879) | 0.766 |
Abbreviations: WBC, white blood cell; RBC, red blood cell; HGB, hemoglobin; HCT, Hematocrit; MCV, write out; MCH, write out; MCHC, write out; I-PLT, write out; O-PLT, write out; RDW-SD, standard deviation from the mean red cell size; RDW-CV, coefficient of variation from the mean; MPV, write out; RET, write out; NEUT, neutrophils; LYMPH; lymphocytes; MONO, monocytes; EOS, eosinophils, BASO, basophils.
Table 5.
Limits for acceptance of CBC and differential counts as recommended by the manufacturer.
| Percent Limits | Absolute Limits | Correlation Coefficient (r) | |
|---|---|---|---|
| WBC | ≤ 3% | ||
| RBC | ≤ 1.5% | ||
| HGB | ≤ 1.5% | ||
| HCT | ≤ 1.5% | ||
| MCV | ≤ 1.5% | ||
| MCH | ≤ 1.5% | ||
| MCHC | ≤ 2% | ||
| I-PLT | ≤ 4% | ||
| O-PLT | ≤ 6% | ||
| RDW-SD | ≤ 3% | ||
| RDW-CV | ≤ 3% | ||
| MPV | ≤ 4% | ||
| RET | ≤ 15% | ≥ 0.90 | |
| NEUT | ≤ 8% | ± 3.0 | ≥ 0.90 |
| LYMPH | ≤ 8% | ± 3.0 | ≥ 0.90 |
| MONO | ≤ 20% | ± 2.0 | ≥ 0.75 |
| EOS | ≤ 25% | ± 1.0 | ≥ 0.80 |
| BASO | ≤ 40% | ± 1.0 | ≥ 0.50 |
Abbreviations: WBC, white blood cell; RBC, red blood cell; HGB, hemoglobin; HCT, Hematocrit; MCV, write out; MCH, write out; MCHC, write out; I-PLT, write out; O-PLT, write out; RDW-SD, standard deviation from the mean red cell size; RDW-CV, coefficient of variation from the mean; MPV, write out; RET, write out; NEUT, neutrophils; LYMPH; lymphocytes; MONO, monocytes; EOS, eosinophils, BASO, basophils.
The mean, average absolute difference, average percent difference, and quotient between 4 hour samples and each time point for all parameters of the CBC are shown in Table 3A. The intercept, slope, and correlation coefficient between the results for all parameters of the CBC at each time point are shown in Table 3B. These results are shown for both population groups, healthy donors and HIV+ patients, and also for the combined population. When the results are compared to the results from the same-day precision measurements in Table 2, it is possible to assess the stability of the sample for a given measurement at each time point. The average percent differences with time for each parameter of the CBC are shown in the graphs of Figure 1.
Table 3.
CBC and Reticulocyte Results: Mean, Average Absolute Difference, Average Percent Difference, Quotient
| Units | 4 HR |
24 HRS |
48 HRS |
72 HRS |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Mean | Ave Abs Difference | Ave % Difference (95% CI) | Quotient | Mean | Ave Abs Difference | Ave % Difference (95% CI) | Quotient | Mean | Ave Abs Difference | Ave % Difference (95% CI) | Quotient | ||
| HEALTHY DONORS | ||||||||||||||
| WBC | 103/μl | 6.30 | 6.39 | 0.126 | 1.98 (0.96–3.01) | 1.012 | 6.41 | 0.148 | 2.66 (2.33–3.0) | 1.016 | 6.42 | 0.158 | 2.52 (1.99–3.05) | 1.018 |
| RBC | 106/μl | 4.76 | 4.76 | 0.027 | 0.58 (0.46–0.70) | 1 | 4.76 | 0.03 | 0.86 (0.74–0.99) | 0.999 | 4.77 | 0.03 | 0.65 (0.48–.82) | 1.002 |
| HGB | g/dl | 14.11 | 14.14 | 0.073 | 0.51 (0.36–0.66) | 1.002 | 14.14 | 0.086 | 0.71 (0.62–0.81) | 1.003 | 14.18 | 0.098 | 0.69 (0.54–0.85) | 1.005 |
| HCT | % | 41.35 | 43.17 | 1.82 | 4.39 (4.02–4.75) | 1.044 | 45.55 | 4.2 | 10.29 (9.93–10.66) | 1.101 | 47.26 | 5.91 | 14.27 (13.68–14.87) | 1.143 |
| MCV | fL | 87.01 | 90.86 | 3.85 | 4.43 (4.1–4.75) | 1.044 | 95.88 | 8.87 | 10.37 (10.09–10.66) | 1.102 | 99.20 | 12.19 | 14.02 (13.53–14.50) | 1.14 |
| MCH | pg | 29.67 | 29.74 | 0.24 | 0.81 (0.67–0.95) | 1.003 | 29.78 | 0.25 | 1.12 (0.97–1.27) | 1.004 | 29.76 | 0.29 | .99 (0.76–1.22) | 1.003 |
| MCHC | g/dl | 34.08 | 32.72 | 1.36 | 3.98 (3.64–4.32) | 0.96 | 31.05 | 3.04 | 8.87 (8.54–9.19) | 0.911 | 29.98 | 4.1 | 12.01 (11.51–12.51) | 0.88 |
| I-PLT | 103/μl | 257.09 | 257.34 | 6.89 | 2.66 (2.19–3.13) | 1.002 | 254.66 | 7.54 | 3.32 (2.86–3.78) | 0.991 | 250.54 | 8.73 | 3.39 (2.69–4.08) | 0.975 |
| O-PLT | 103/μl | 255.79 | 253.18 | 10.64 | 4.24 (3.44–5.04) | 0.992 | 242.73 | 18.41 | 6.72 (6.0–7.43) | 0.956 | 233.36 | 24.46 | 9.15 (7.9–10.41) | 0.918 |
| RDW-SD | fL | 42.22 | 46.01 | 3.79 | 9.01 (8.28–9.74) | 1.09 | 50.84 | 8.62 | 21.93 (21.19–22.66) | 1.205 | 52.93 | 10.71 | 25.43 (24.02–26.84) | 1.254 |
| RDW-CV | % | 13.50 | 14.14 | 0.65 | 4.79 (4.29–5.28) | 1.048 | 14.81 | 1.31 | 10.59 (10.04–11.15) | 1.097 | 14.85 | 1.36 | 10.09 (9.13–11.05) | 1.101 |
| MPV | fL | 10.74 | 11.43 | 0.7 | 6.51 (5.79–7.24) | 1.065 | 12.09 | 1.36 | 10.83 (10.16–11.51) | 1.128 | 12.71 | 1.98 | 18.53 (17.26–19.08) | 1.185 |
| RET | % | 1.085 | 1.0817 | 0.115 | 10.87 (8.82–12.92) | 1.007 | 0.907 | 0.198 | 16.27 (14.64–17.9) | 0.845 | 0.941 | 0.168 | 14.76 (12.07–17.45) | 0.879 |
| HIV+ PATIENTS | ||||||||||||||
| WBC | 103/μl | 5.55 | 5.65 | 0.164 | 3.00 (2.53–3.48) | 1.017 | 5.58 | 0.154 | 2.81 (2.37–3.25) | 1.004 | 5.53 | 0.143 | 2.64 (2.27–3.01) | 0.997 |
| RBC | 106/μl | 4.50 | 4.50 | 0.034 | 0.8 (0.66–0.94) | 1 | 4.49 | 0.042 | 0.97 (0.82–1.12) | 0.999 | 4.49 | 0.044 | 1.02 (0.85–1.19) | 0.999 |
| HGB | g/dl | 14.45 | 14.50 | 0.092 | 0.64 (0.52–0.75) | 1.003 | 14.53 | 0.11 | 0.77 (0.64–0.9) | 1.006 | 14.54 | 0.117 | 0.82 (0.69–0.94) | 1.006 |
| HCT | % | 41.49 | 43.42 | 1.95 | 4.70 (4.33–5.08) | 1.046 | 45.82 | 4.33 | 10.39 (9.92–10.86) | 1.104 | 47.21 | 5.72 | 13.76 (13.26–14.26) | 1.138 |
| MCV | fL | 93.32 | 97.62 | 4.31 | 4.61 (4.32–4.9) | 1.046 | 103.10 | 9.78 | 10.47 (10.11–10.82) | 1.105 | 106.25 | 12.94 | 13.85 (13.47–14.23) | 1.138 |
| MCH | pg | 32.53 | 32.65 | 0.35 | 1.05 (0.89–1.21) | 1.003 | 32.76 | 0.42 | 1.26 (1.06–1.44) | 1.007 | 32.77 | 0.46 | 1.36 (1.12–1.59) | 1.007 |
| MCHC | g/dl | 34.83 | 33.41 | 1.44 | 4.13 (3.77–4.48) | 0.959 | 31.74 | 3.09 | 8.86 (8.44–9.27) | 0.911 | 30.81 | 4.01 | 11.5 (11.06–11.95) | 0.885 |
| I-PLT | 103/μl | 221.61 | 222.50 | 6.31 | 2.89 (2.39–3.4) | 1.005 | 219.37 | 7.62 | 3.49 (2.85–4.14) | 0.99 | 218.14 | 8.4 | 3.9 (3.1–4.71) | 0.985 |
| O-PLT | 103/μl | 215.14 | 212.73 | 9.36 | 4.32 (3.61–5.02) | 0.991 | 202.99 | 14.92 | 6.64 (5.75–7.53) | 0.952 | 197.99 | 19.51 | 8.62 (7.64–9.6) | 0.929 |
| RDW-SD | fL | 44.78 | 49.79 | 5.01 | 11.19 (10.46–11.92) | 1.112 | 54.93 | 10.16 | 22.66 (21.75–23.57) | 1.227 | 56.95 | 12.17 | 27.17 (26.12–28.21) | 1.272 |
| RDW-CV | % | 13.43 | 14.31 | 0.88 | 6.61 (6.01–7.12) | 1.066 | 14.91 | 1.48 | 11.04 (10.32–11.76) | 1.11 | 14.95 | 1.52 | 11.39 (10.63–12.14) | 1.114 |
| MPV | fL | 10.58 | 10.98 | 0.44 | 4.18 (3.72–4.63) | 1.039 | 11.57 | 1.02 | 9.81 (9.0–10.61) | 1.096 | 11.79 | 1.25 | 12.0 (11.24–12.75) | 1.117 |
| RET | % | 1.2045 | 1.131 | 0.131 | 11.00 (9.58–12.43) | 0.949 | 1.023 | 0.195 | 15.71 (13.75–17.67) | 0.857 | 1.0686 | 0.167 | 13.54 (11.7–15.83) | 0.901 |
| COMBINED RESULTS (HEALTHY DONORS & HIV+ PATIENTS) | ||||||||||||||
| WBC | 103/μl | 5.81 | 5.90 | 0.151 | 2.66 (2.2–3.13) | 1.015 | 5.86 | 0.152 | 2.66 (2.33–3.0) | 1.008 | 5.83 | 0.148 | 2.6 (2.3–2.9) | 1.004 |
| RBC | 106/μl | 4.59 | 4.59 | 0.032 | 0.73 (0.62–0.83) | 1 | 4.58 | 0.038 | 0.86 (0.74–0.99) | 0.999 | 4.59 | 0.04 | 0.9 (0.77–1.02) | 1 |
| HGB | g/dl | 14.34 | 14.38 | 0.086 | 0.59 (0.50–0.68) | 1.003 | 14.40 | 0.102 | 0.71 (0.62–0.81) | 1.005 | 14.42 | 0.111 | 0.78 (0.68–0.87) | 1.006 |
| HCT | % | 41.44 | 43.33 | 1.91 | 4.60 (4.32–4.87) | 1.046 | 45.73 | 4.28 | 10.29 (9.93–10.66) | 1.103 | 47.23 | 5.79 | 13.94 (13.55–14.32) | 1.139 |
| MCV | fL | 91.19 | 95.34 | 4.15 | 4.55 (4.33–4.77) | 1.045 | 100.67 | 9.48 | 10.37 (10.09–10.66) | 1.104 | 103.87 | 12.68 | 13.91 (13.61–14.2) | 1.139 |
| MCH | pg | 31.57 | 31.67 | 0.31 | 0.97 (0.85–1.09) | 1.003 | 31.75 | 0.36 | 1.12 (0.97–1.27) | 1.006 | 31.76 | 0.4 | 1.23 (1.06–1.41) | 1.006 |
| MCHC | g/dl | 34.58 | 33.18 | 1.41 | 4.08 (3.82–4.34) | 0.96 | 31.50 | 3.07 | 8.87 (8.54–9.19) | 0.911 | 30.53 | 4.04 | 11.68 (11.34–12.01) | 0.883 |
| I-PLT | 103/μl | 233.65 | 234.33 | 6.51 | 2.81 (2.44–3.18) | 1.004 | 231.35 | 7.59 | 3.32 (2.86–3.78) | 0.991 | 229.13 | 8.52 | 3.73 (3.15–4.31) | 0.982 |
| O-PLT | 103/μl | 228.93 | 226.46 | 9.79 | 4.29 (3.76–4.83) | 0.992 | 216.48 | 16.1 | 6.72 (6.0–7.43) | 0.953 | 209.99 | 21.19 | 8.8 (8.03–9.57) | 0.925 |
| RDW-SD | fL | 43.91 | 48.51 | 4.6 | 10.45 (9.89–11.02) | 1.105 | 53.55 | 9.64 | 21.93 (21.19–22.66) | 1.219 | 55.59 | 11.68 | 26.58 (25.74–27.42) | 1.266 |
| RDW-CV | % | 13.45 | 14.26 | 0.8 | 5.99 (5.59–6.39) | 1.06 | 14.87 | 1.42 | 10.59 (10.04–11.15) | 1.106 | 14.92 | 1.46 | 10.95 (10.35–11.55) | 1.109 |
| MPV | fL | 10.63 | 11.14 | 0.53 | 4.98 (4.56–5.41) | 1.048 | 11.75 | 1.14 | 10.83 (10.16–11.51) | 1.107 | 12.11 | 1.5 | 14.26 (13.44–15.07) | 1.141 |
| RET | % | 1.164 | 1.1145 | 0.125 | 10.96 (9.80–12.12) | 0.968 | 0.9839 | 0.196 | 16.27 (14.64–17.9) | 0.853 | 1.026 | 0.167 | 13.95 (12.45–15.46) | 0.893 |
Abbreviations: WBC, white blood cell; RBC, red blood cell; HGB, hemoglobin; HCT, Hematocrit; MCV, write out; MCH, write out; MCHC, write out; I-PLT, write out; O-PLT, write out; RDW-SD, standard deviation from the mean red cell size; RDW-CV, coefficient of variation from the mean; MPV, write out; RET, write out.
Figure 1.
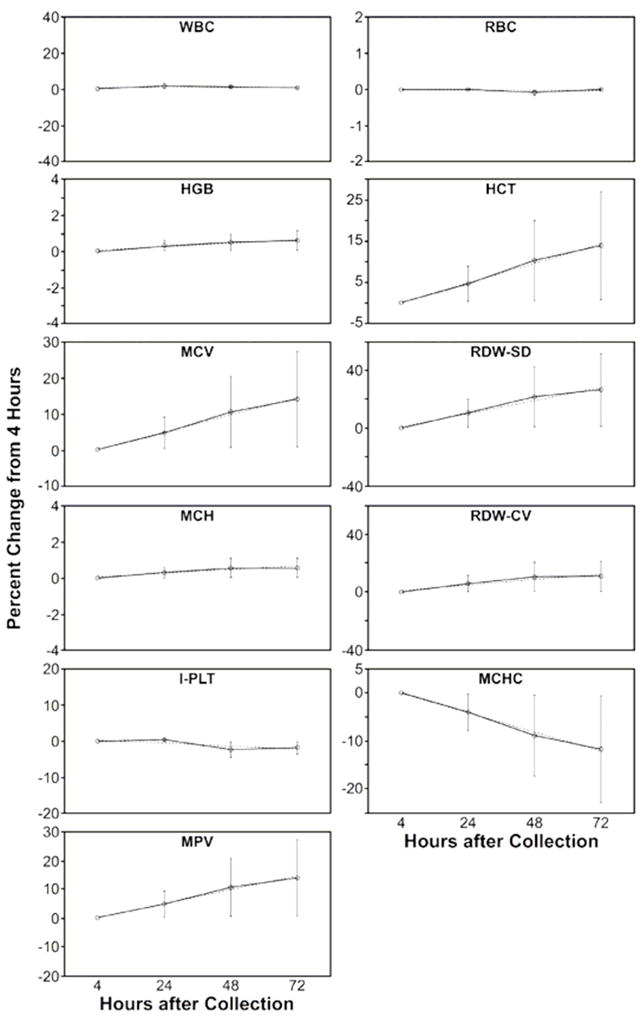
The WBC, RBC, and HGB remained very stable through 72 hours. There was somewhat more variability evident in the measurements for HIV+ patients compared to healthy donors at each time point. For example, the average percent difference of the HIV+ patients for these 3 parameters were higher than those of the healthy donors at each time point, but these results all compare well to the analysis of the same-day samples shown in Table 2. The results all fell within the confidence limits of the same-day samples, or the confidence limits of the result at a given time point overlapped with the confidence limits of the same-day samples.10
An increase in red cell size with time is apparent in both the HCT and MCV. Red cell indices dependent on cell size, the MCHC, RDW-SD, and RDW-CV, show the changes associated with increasing red cell volume. The MCH, dependent on the very stable RBC and HGB measurements, shows no significant change throughout the 72 hours.
The I-PLT measurement was stable through 72 hours, comparing well to the same-day precision measurements at each time point. The O-PLT count was less reproducible. It compared well to the same-day precision measurements at 24 hours, but successive measurements showed significant differences from the 4 hour measurements and from the same-day precision results. The slopes at 48 and 72 hours fell below 0.9, and the confidence limits of the average percent difference did not overlap with the same-day precision results. The MPV showed a marked increase within 24 hours and was not reproducible at any time point compared to the 4 hour measurement.
The RET% results are also shown in Table 3A and Table 3B. The mean counts dropped somewhat at 24 hours and continued to drop with time. Results for the 24-hour measurement compared well to the 4-hour measurements and to the same-day precision results, but the RET% was less stable after that time point. This is similar to results obtained on samples held at room temperature from a study comparing reticulocyte counts using ABX Pentra 120 Retic, the Sysmex R-2000, flow cytometry, and manual counts. The consistent decline in reticulocyte counts at room temperature was attributed to the maturation of the reticulocyte cells over time.8
The trends apparent in Table 3A and Table 3B are consistent with those seen by investigators with the Sysmex XE-2100,2 which is very similar to the Sysmex XT series instruments in technology and operation. These results are also consistent with limited unpublished studies on sample stability with the Sysmex XT-2000i available from Sysmex America.
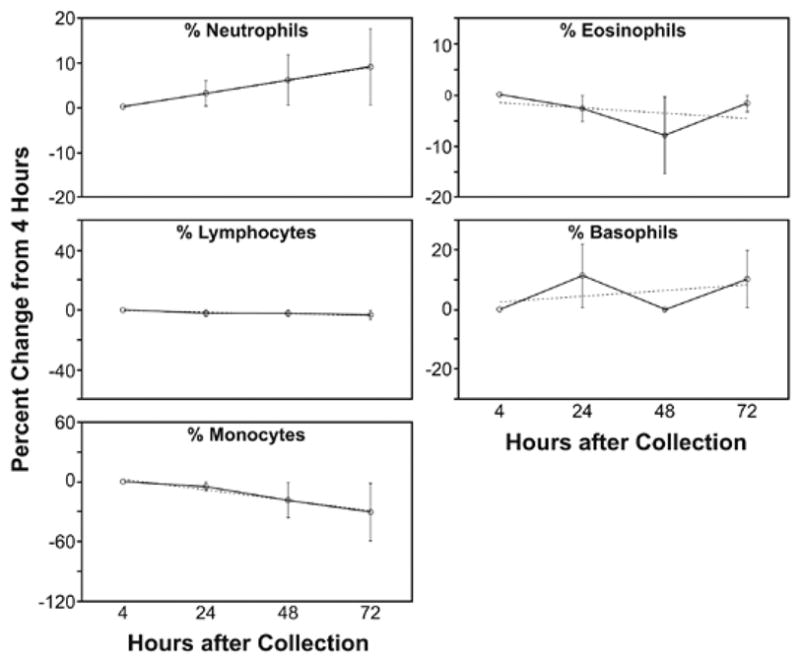
The Diff count performed with mixed results (Table 4A and Table 4B). The average percent difference with time for each parameter is shown in the graphs of Figure 2. The LYMPH, EOS, and BASO counts show almost no change with time, while NEUT counts rise slightly, and MONO counts decline significantly. After 24 hours, the MONO count is no longer stable compared to the 4-hour counts or the same-day precision results. NEUT counts, because they rise with time, show significant variability in average difference and average percent difference compared to the same-day precision results. However, slope and correlation coefficients at each time point are consistent with the slope and correlation of the same-day precision results. This indicates that despite the increase of the NEUT count with time, the increase is not clinically significant. The LYMPH counts show good stability to 48 hours, and the 72-hour measurements, though comparing well with the results at 4 hours, show a greater degree of variability. EOS counts also show good stability through 48 hours, with more variability apparent at 72 hours. And finally BASO counts show excellent stability through 72 hours.
Table 4.
| Table 4A. Differential Results: Mean, Avereage Absolute Difference, Average Percent Difference, Quotient | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 HR | 24 HRS | 48 HRS | 72 HRS | ||||||||||
| Mean | Mean | Ave Abs Difference | Ave % Difference (95% CI) | Quotient | Mean | Ave Abs Difference | Ave % Difference (95% CI) | Quotient | Mean | Ave Abs Difference | Ave % Difference (95% CI) | Quotient | |
| HEALTHY DONORS | |||||||||||||
| NEUT% | 57.42 | 58.20 | 1.33 | 2.36 (1.93–2.8) | 1.013 | 59.78 | 2.48 | 4.63 (3.62–5.65) | 1.044 | 60.42 | 3.33 | 6.34 (4.89–7.79) | 1.062 |
| LYMPH% | 31.07 | 30.59 | 1.15 | 3.82 (3.13–4.52) | 0.986 | 30.61 | 1.23 | 4.01 (3.16–4.87) | 0.988 | 30.57 | 1.29 | 4.24 (3.16–5.31) | 0.985 |
| MONO% | 8.01 | 7.81 | 1 | 12.98 (9.95–16.01) | 0.991 | 6.48 | 1.8 | 22.31 (18.03–26.59) | 0.823 | 5.63 | 2.65 | 31.85 (27.17–36.52) | 0.702 |
| EOS% | 2.93 | 2.82 | 0.36 | 13.24 (10.25–16.22) | 0.975 | 2.59 | 0.5 | 15.95 (11.38–20.52) | 0.924 | 2.81 | 0.48 | 16.96 (13.09–20.84) | 0.99 |
| BASO% | 0.57 | 0.58 | 0.13 | 25.51 (16.26–34.77) | 1.06 | 0.55 | 0.12 | 21.5 (14.52–28.47) | 0.98 | 0.57 | 0.14 | 27.75 (17.07–38.43) | 1.057 |
| HIV+ PATIENTS | |||||||||||||
| NEUT% | 51.73 | 53.57 | 1.92 | 3.97 (3.39–4.54) | 1.038 | 55.03 | 3.46 | 7.09 (6.16–8.03) | 1.067 | 56.65 | 4.94 | 10.17 (8.98–11.36) | 1.101 |
| LYMPH% | 35.07 | 34.16 | 1.24 | 3.76 (3.2–4.33) | 0.974 | 34.07 | 1.45 | 4.37 (3.65–5.08) | 0.971 | 33.58 | 1.92 | 5.8 (4.87–6.74) | 0.958 |
| MONO% | 9.61 | 8.83 | 1.19 | 12.11 (10.47–13.75) | 0.932 | 7.61 | 2.24 | 21.71 (18.81–24.62) | 0.811 | 6.44 | 3.23 | 32.68 (29.0–36.36) | 0.694 |
| EOS% | 3.14 | 2.98 | 0.41 | 16.27 (12.39–20.14) | 0.973 | 2.85 | 0.59 | 22.2 (17.53–26.86) | 0.92 | 2.87 | 0.6 | 25.67 (18.92–32.42) | 0.98 |
| BASO% | 0.45 | 0.45 | 0.12 | 30.65 (21.32–39.98) | 1.141 | 0.44 | 0.12 | 31.2 (23.49–38.9) | 1.082 | 0.46 | 0.13 | 31.54 (23.34–39.74) | 1.124 |
| COMBINED RESULTS (HEALTHY DONORS & HIV+ PATIENTS) | |||||||||||||
| NEUT% | 53.65 | 55.13 | 1.72 | 3.43 (3.0–3.85) | 1.03 | 56.63 | 3.13 | 6.26 (5.54–6.98) | 1.059 | 57.86 | 4.42 | 8.94 (7.98–9.91) | 1.089 |
| LYMPH% | 33.72 | 32.96 | 1.21 | 3.78 (3.35–4.22) | 0.978 | 32.90 | 1.37 | 4.25 (3.7–4.8) | 0.977 | 32.61 | 1.71 | 5.3 (4.57–6.02) | 0.967 |
| MONO% | 9.07 | 8.49 | 1.12 | 12.4 (10.93–13.88) | 0.952 | 7.23 | 2.09 | 21.91 (19.54–24.29) | 0.815 | 6.18 | 3.05 | 32.41 (29.53–35.3) | 0.696 |
| EOS% | 3.07 | 2.93 | 0.39 | 15.24 (12.49–17.99) | 0.974 | 2.76 | 0.56 | 20.09 (16.64–23.54) | 0.921 | 2.85 | 0.56 | 22.88 (18.11–27.64) | 0.983 |
| BASO% | 0.49 | 0.50 | 0.12 | 28.9 (22.04–35.76) | 1.114 | 0.48 | 0.12 | 27.89 (22.28–33.49) | 1.047 | 0.49 | 0.13 | 30.31 (23.83–36.78) | 1.102 |
| Table 4B. Differential Results: Intercept, Slope, and Correlation Coefficient | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 24 HRS |
48 HRS |
72 HRS |
|||||||||||
| Intercept | Slope (95% CI) | Correlation | Intercept | Slope (95% CI) | Correlation | Intercept | Slope (95% CI) | Correlation | |||||
| HEALTHY DONORS | |||||||||||||
| NEUT% | 0.008 | 1.013 (0.974–1.053) | 0.99 | 3.646 | 0.978 (0.927–1.028) | 0.982 | 6.571 | 0.942 (0.885–0.999) | 0.977 | ||||
| LYMPH% | 0.354 | 0.937 (0.936–1.01) | 0.99 | 1.167 | 0.947 (0.906–0.989) | 0.987 | 1.402 | 0.937 (0.893–0.981) | 0.986 | ||||
| MONO% | 0.643 | 0.896 (0.764–1.027) | 0.875 | 0.668 | 0.726 (0.564–0.888) | 0.767 | 0.46 | 0.634 (0.463–0.805) | 0.717 | ||||
| EOS% | 0.149 | 0.912 (0.836–0.988) | 0.954 | 0.393 | 0.75 (0.662–0.838) | 0.915 | 0.441 | 0.796 (0.721–0.871) | 0.947 | ||||
| BASO% | 0.006 | 1.015 (0.878–1.151) | 0.893 | 0.066 | 0.852 (0.714–0.99) | 0.855 | 0.088 | 0.834 (0.672–0.997) | 0.818 | ||||
| HIV+ PATIENTS | |||||||||||||
| NEUT% | 3.522 | 0.967 (0.941–0.993) | 0.99 | 5.234 | 0.963 (0.918–1.007) | 0.971 | 8.926 | 0.923 (0.875–0.97) | 0.964 | ||||
| LYMPH% | −0.174 | 0.979 (0.954–1.004) | 0.991 | −0.082 | 0.974 (0.944–1.003) | 0.987 | 0.213 | 0.951 (0.914–0.988) | 0.979 | ||||
| MONO% | 1.269 | 0.787 (0.713–0.861) | 0.896 | 2.367 | 0.546 (0.432–0.66) | 0.671 | 2.036 | 0.459 (0.346–0.571) | 0.61 | ||||
| EOS% | 0.011 | 0.948 (0.896–1.0) | 0.96 | −0.128 | 0.948 (0.867–1.03) | 0.91 | 0.123 | 0.876 (0.799–0.953) | 0.907 | ||||
| BASO% | 0.145 | 0.692 (0.565–0.818) | 0.718 | 0.123 | 0.715 (0.582–0.848) | 0.712 | 0.176 | 0.625 (0.502–0.747) | 0.693 | ||||
| COMBINED RESULTS (HEALTHY DONORS & HIV+ PATIENTS) | |||||||||||||
| NEUT% | 3.048 | 0.971 (0.949–0.993) | 0.989 | 5.211 | 0.958 (0.925–0.991) | 0.975 | 8.943 | 0.915 (0.878–0.952) | 0.968 | ||||
| LYMPH% | 0.115 | 0.974 (0.954–0.994) | 0.991 | 0.481 | 0.961 (0.938–0.985) | 0.987 | 0.833 | 0.94 (0.911–0.969) | 0.981 | ||||
| MONO% | 1.193 | 0.804 (0.741–0.868) | 0.89 | 1.759 | 0.603 (0.513–0.694) | 0.715 | 1.538 | 0.508 (0.417–0.599) | 0.653 | ||||
| EOS% | 0.055 | 0.937 (0.894–0.98) | 0.958 | 0.029 | 0.891 (0.827–0.954) | 0.907 | 0.222 | 0.852 (0.794–0.91) | 0.915 | ||||
| BASO% | 0.077 | 0.86 (0.767–0.953) | 0.816 | 0.095 | 0.787 (0.694–0.881) | 0.79 | 0.133 | 0.734 (0.639–0.829) | 0.766 | ||||
Abbreviations: NEUT, neutrophils; LYMPH; lymphocytes; MONO, monocytes; EOS, eosinophils, BASO, basophils.
Figure 2.
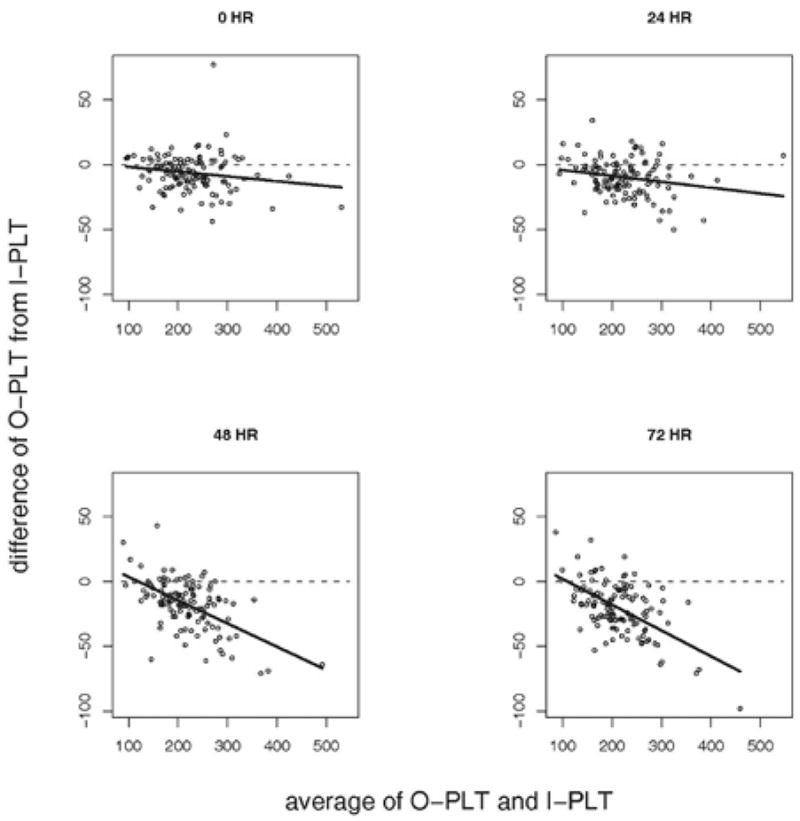
The results of the comparison of the I-PLT measurement to the O-PLT counts are summarized in Table 6. The mean of the O-PLT count is lower than the mean of the I-PLT at each time point, and the difference between the means increases with time. The Bland and Altman’s difference plots (Figure 3) show the differences between the 2 measurements increase with the underlying platelet counts. The solid lines in the plots represent the linear regression.
Table 6.
Comparison of Optical and Impedance Platelt Counts.
| 4 HR | 24 HRS | 48 HRS | 72 HRS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MEAN | AVE % DIFFERENCE (95% CI) | MEAN | AVE % DIFFERENCE (95% CI) | MEAN | DIFFERENCE (95% CI) | MEAN | AVE % DIFFERENCE (95% CI) | ||||
| I-PLT | O-PLT | I-PLT | O-PLT | I-PLT | O-PLT | I-PLT | O-PLT | ||||
| 227.42 | 221.13 | 5.01 (4.22–5.8) | 228.19 | 218.41 | 5.96 (5.19–6.73) | 224.85 | 207.53 | 8.6 (7.4–9.8) | 223.42 | 202.89 | 10.38 (9.11–11.65) |
Figure 3.
The summary of the comparison of automated and manual Diff counts over time is given in Table 7. The differences between the automated and manual counts on 4-hour samples are clinically insignificant. However, with time the automated counts are consistent with the results seen in the larger study, but the manual counts become increasingly divergent from the automated counts. The smears prepared at 72 hours are unusable; there are no data shown for manual counts at 72 hours.
Table 7.
Comparison of automated and manual diffs over time: average absolute difference, average percent difference, and correlation coefficient
| 4 HR |
24 HR |
48 HR |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean Automated Count |
Mean Manual Count |
Ave Difference (95% CI) |
Correlation Coefficient |
Mean Automated Count |
Mean Manual Count |
Ave Difference (95% CI) |
Ave % Difference (95% CI) |
Correlation Coefficient |
Mean Automated Count |
Mean Manual Count |
Ave Difference (95% CI) |
Ave % Difference (95% CI) |
Correlation Coefficient |
|
| NEUT | 52.4 | 53.2 | 3.1 (2.1–4.0) | 1 | 53 | 46.4 | 8.2 (5.8–10.6) | 18.3 (12.5–24.1) | 0.8 | 54.1 | 31.4 | 23.4 (17.1–29.8) | 102.1 (44.9–159.3) | 0.6 |
| LYMPH | 35.1 | 35.8 | 2.5 (1.7–3.3) | 1 | 34.3 | 42.1 | 8.1 (5.4–10.7) | 19.5 (13.3–25.8) | 0.9 | 34.4 | 55.9 | 22.1 (15.5–28.7) | 39.1 (29.6–48.5) | 0.4 |
| MONO | 8.6 | 7.3 | 2.1 (1.3–2.9) | 0.6 | 8.7 | 7.3 | 2.7 (2.0–3.4) | 48.5 (25.7–71.2) | 0.5 | 7.7 | 7.8 | 2 (1.3–2.6) | 28.5 (18.4–38.6) | 0.6 |
| EOS | 3.4 | 3.2 | 1.2 (0.9–1.6) | 0.9 | 3.5 | 3.3 | 1.5 (0.9–2.0) | 44.7 (29.3–60.2) | 0.8 | 3.4 | 3.8 | 1.7 (0.9–2.5) | 49.3 (29.5–69.1) | 0.7 |
| BASO | 0.5 | 0.7 | 0.5 (0.3–0.7) | 0.5 | 0.5 | 1.1 | 0.8 (0.5–1.2) | 60.2 (44.9–75.5) | 0.2 | 0.4 | 1.1 | 1 (0.2–1.7) | 66.3 (50.4–82.1) | 0.8 |
Abbreviations: NEUT, neutrophils; LYMPH; lymphocytes; MONO, monocytes; EOS, eosinophils, BASO, basophils.
Discussion
Results for WBC, RBC, HGB, I-PLT, and MCH show a high degree of stability when compared with results on 4-hour samples and with the results of the same-day precision analysis through 72 hours. While the HCT and MCV show acceptable stability to 48 hours when considering the slope and correlation coefficient, the average percent differences are significant at every time point. There is a consistent and significant rise in red cell size with time, indicating these data should be interpreted with caution. A consideration of nothing more than the mean MCV at each time point shows a normal MCV becoming an abnormal MCV (MCV ≥ 97) by 48 hours. This pattern is plainly borne out by examination of the raw data as well.
These same considerations apply when considering the results of the MCHC and RDW. The MCHC is calculated by dividing the HGB by the HCT. As the HCT rises, the MCHC is reduced; therefore a relatively small rise in the HCT creates a significant drop in MCHC. This accounts for the failure of the MCHC to make an acceptable comparison to the 4-hour results even at 24 hours, and the negative effect increases with time. The RDW is calculated with the standard deviation of the mean of the red cell volume (or the coefficient of variation of the mean of the red cell volume) divided by the MCV. The instability of the red cell size with time necessarily affects this parameter also, with the RDW-SD showing greater variability than the RDW-CV. Both parameters rise at each time point, and the results do not compare satisfactorily with those from 4-hour samples even at 24 hours.
The change in red cell size is also indicated in the Interpretive Program (IP) flags that appear with increasing frequency as the samples age. On 4-hour samples, a WBC IP flag is given on 12% of the samples, and 17% show an RBC IP flag. Of the flags given for the RBC analysis component, half (52%) are related to some variation in red cell size. The number for samples showing RBC IP flags increases with each time point: 27% at 24 hours, 44% at 48 hours, 47% at 72 hours. The proportion of those flags relating to RBC size also increases with each time point. By 72 hours, 88% of the RBC IP flags indicate either increased cell size or variation in cell size.
The WBC IP flags also increase with time, though not in the same high proportion as RBC flags. By 72 hours, 30% of samples show a WBC flag. The flag appearing with increasing frequency most predictably in the WBC analysis component is “NRBC?”, reaching 84% of WBC flags at 72 hours. The increasing incidence of this flag is consistent with the progressive aging and death of WBC over time. The presence of increasing numbers of pyknotic white cells would account for the appearance of this flag.
The O-PLT count, while showing acceptable stability at 24 hours, showed a significant drop in relation to the I-PLT over time. At 72 hours, the average O-PLT count was more than 20 × 103/μL lower than the I-PLT when looking at the combined results. A similar pattern of results in O-PLT and I-PLT measurement was observed in a stability study of the Abbott Cell-Dyn Sapphire,6 which suggests the differences between the 2 measurements with time are associated with the methodology, impedance counts, and counts by flow cytometry and not simply the technology of a given instrument. The Sysmex XT-2000i will select and report the O-PLT in preference to the I-PLT when an abnormal platelet distribution is detected in the impedance counter, notably when the platelet count is low. The results of this study suggest the O-PLT on aged samples should be used with reservation. It is possible the increasing difference between the optical and impedance platelet counts is related to the increasing number of IP flags for “PLT Clumps” given by the Sysmex XT-2000i as the samples age. On whole blood measured at 4 hours, 6% of the samples showed a “PLT Clumps” flag. At 24 hours, 24% of the samples were flagged for “PLT Clumps.” At 48 hours, the percentage increased to 79%, and then increased to 97% at 72 hours. This indicates progressive clumping of platelets with time may interfere with the optical platelet count, while apparently having less effect on the impedance count. Despite the increasing number of flags for clumped platelets, the average percent difference, slope, and correlation between and fresh and delayed samples for I-PLT measurements compares well with 4-hour and same-day measurements at all time points.
The Diff results in Table 4A and Table 4B show excellent stability out to 48 hours, and good stability to 72 hours, with the exception of the monocyte count. NEUT raises slightly with time, the average count of the combined results varying by 4.2 cells/100 from 4 hours to 72 hours. Average LYMPH dropped slightly overall with a change of 1.1 cells/100 in 72 hours in the combined results, but MONO changed by 2.9 cells/100 in 72 hours, or 32% compared to 4-hour samples. The absolute loss seems minimal, but the proportional loss is significant. The MONO count compares adequately to values on 4-hour samples only through 24 hours. Beyond 24 hours, the monocyte count becomes progressively lower. The reason for the change is difficult to determine. The cell size, structure, or degree of granulation may change enough with time to make it no longer recognizable as a monocyte to the system’s population analysis software.
The greater variability evident in the measurements for HIV+ patients compared to healthy donors at each time point is a consistent observation. This is strikingly obvious when comparing the slopes and average percent difference of a given parameter between the 2 populations. In terms of reproducibility, the effect is insignificant at 24 hours for most parameters but becomes more evident with time. This suggests the use of CBC or Diff results on samples from HIV+ patients after 24 hours should be limited to only the most stable measurements: WBC, RBC, HGB, and I-PLT. Because the MONO count is unstable after 24 hours, a Diff count should not be reported after that time.
The comparison of automated and manual Diffs with time demonstrates the degree to which Diff results are compromised when manual counts are attempted on aged bloods. No statistically or clinically usable manual counts were obtained on samples 24 hours or more old.
Conclusions
The receipt of day-old samples is a common occurrence in our laboratory. Because all hematology samples are also used for immunophenotyping by flow cytometry, they are stored at room temperature from the time of collection.3,5,11 Hematology instruments relying on white cell scatter properties for Diff analysis have not, in the past, allowed us to perform a reliable automated analysis of these bloods. This study demonstrates the Sysmex XT-2000i makes it possible to perform a clinically valid CBC and Diff on K2-EDTA anticoagulated blood 24 hours after collection when the sample is stored at room temperature. On a 24-hour old sample, the MPV and MCHC should be excluded from the report. Because the HCT, MCV, and both RDW parameters are consistently elevated due to the sample’s age, even at 24 hours, those results should also be excluded.
After 24 hours, a limited report could legitimately include WBC, RBC, HGB, and I-PLT for a sample out to 72 hours for all samples. A similar situation exists regarding the Diff count. The stability of NEUT, LYMPH, EOS, and BASO counts out to 72 hours is acceptable for all samples, while the MONO counts are not stable beyond 24 hours. The case could be made that because the average change in numbers of the monocytes is so small, a report of this result, with a comment to the effect that MONO may be reduced due to the sample’s age, could be appropriate. This is especially true considering that once 4 parts of a 5-part Diff are reported, the fifth part is easily calculated. Because of the greater variability of Diff measurements in samples from HIV+ patients, however, and because these samples account for the majority of our work, the best procedure would be to not report any results for an automated Diff count on a sample more than 24 hours old.
Our data indicate if an O-PLT result is selected by the instrument as the appropriate PLT measurement for a given sample, that result should not be reported if the sample is more than 24 hours old. This is apparent both from the comparison of the O-PLT from aged samples to 4-hour samples, and the comparison of the O-PLT to the I-PLT with increasing time.
The RET% counts are stable at 24 hours. After that, the counts are increasingly reduced with time, and should not be reported.
The failure to obtain useable manual Diff counts on aged bloods means that smears from those bloods cannot be used for the evaluation of anomalous automated CBC or Diff results or of flags the instrument may post on samples that are 24 hours old or older. Questionable results on aged samples that can only be evaluated or confirmed by the examination of a smear should not be reported.
The most reliable hematological results are obtained from samples analyzed the same day as they are collected, as soon after collection as possible. When immediate analysis is not possible, this study allows us to conclude that valid results from samples that are 24 hours old and older can be reported within the limitations discussed above.
Acknowledgments
This project has been funded in whole or in part with federal funds from the National Cancer Institute and National Institutes of Health under contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This research was supported (in part) by the National Institute of Allergy and Infectious Diseases.
References
- 1.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurements. Lancet. 1986;i:307–310. [PubMed] [Google Scholar]
- 2.de Baca M, Gulati G, Kocher W, et al. Effects of storage of blood at room temperature of hematologic parameters on Sysmex XE-2100. Lab Med. 2006;37:28–35. [Google Scholar]
- 3.Centers for Disease Control and Prevention. MMWR. RR-2. Vol. 46. CDC; 1997. Revised Guidelines for performing CD4+ T-Cell Determinations in Persons Infected with human immunodeficiency virus (HIV) pp. 1–29. [PubMed] [Google Scholar]
- 4.CLSI. Approved Standard. 2. Wayne, PA: CLSI; 2007. Reference Leukocyte (WBC) Differential Count (Proportional) and Evaluation of Instrumental Methods; pp. H20–A2. [Google Scholar]
- 5.Ekong T, Kupek E, Hill A, et al. Technical influences on immunophenotyping by flow cytometry. J Immunol Methods. 1993;164:263–273. doi: 10.1016/0022-1759(93)90319-3. [DOI] [PubMed] [Google Scholar]
- 6.Hedberg P, Lehto T. Aging stability of complete blood count and white blood cell differential parameters analyzed by Abbott CELL-DYN Sapphire hematology analyzer. Int J Lab Hematol. 2008;31:87–96. doi: 10.1111/j.1751-553X.2007.01009.x. [DOI] [PubMed] [Google Scholar]
- 7.Imeri F, Herklotz R, Risch L, et al. Stability of hematological analytes depends on the hematology analyzer used: A stability study with Bayer Advia 120, Beckman Coulter LH 750 and Sysmex XE 2100. Clinica Chimica Acta. 2008;397:68–71. doi: 10.1016/j.cca.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 8.Lacombe F, Lacoste L, Vial J-P, Briais A, et al. Automated reticulocyte counting and immature reticulocyte fraction measurement. Am J Clin Pathol. 1999;112:677–686. doi: 10.1093/ajcp/112.5.677. [DOI] [PubMed] [Google Scholar]
- 9.Langford K, Luchtman-Jones L, Miller R, et al. Performance evaluation of the Sysmex XT-2000i automated hematology analyzer. Lab Hematol. 2003;9:29–37. [PubMed] [Google Scholar]
- 10.Lewis S. Duality Assurance in Haematology. World Health Organization; 1998. WHO/LAB/98.4. [Google Scholar]
- 11.Shield C, Marlett P, Smith A, et al. Stability of human lymphocyte differentiation antigens when stored at room temperature. J Immunol Methods. 1993;62:347–352. doi: 10.1016/0022-1759(83)90179-5. [DOI] [PubMed] [Google Scholar]
- 12.Sysmex XT-2000i/XT-1800i Instructions for Use. Sysmex Corporation; Kobe, Japan: 2006. [Google Scholar]


