Abstract
Despite the enormous success of β-lactams as broad-spectrum antibacterials, they have never been widely used for the treatment of TB due to intrinsic resistance that is caused by the presence of a chromosomally-encoded gene (blaC) in Mycobacterium tuberculosis. Our previous studies of TB BlaC revealed that this enzyme is an extremely broad-spectrum β-lactamase hydrolyzing all β-lactam classes. Carbapenems are slow substrates that acylate the enzyme but are only slowly deacylated and can therefore act also as potent inhibitors of BlaC. We carried out the in vitro characterization of doripenem and ertapenem with BlaC. A steady-state kinetic burst was observed with both compounds with magnitudes proportional to the concentration of BlaC used. The results show apparent Km and kcat values of 0.18 µM and 0.016 min−1 for doripenem and 0.18 µM and 0.017 min−1 for ertapenem. FTICR mass spectrometry demonstrated that the doripenem and ertapenem acyl-enzyme complexes remain stable over a time period of 90 min. The BlaC-doripenem covalent complex obtained after 90 minutes of soaking was solved to 2.2 Å, while the BlaC-ertapenem complex obtained after a 90 minute soak was solved to 2.0 Å. The 1.3 Å diffraction data from a 10 minute ertapenem-soaked crystal revealed an isomerization occurring in the BlaC-ertapenem adduct in which the original Δ2 pyrroline ring was tautomerized to generate the Δ1 pyrroline ring. The isomerization leads to the flipping of the carbapenem-hydroxyethyl group to hydrogen bond to the carboxyl O2 of Glu166. The hydroxyethyl flip results in both decreased basicity of Glu166 and in a significant increase in the distance between the carboxyl O2 of Glu166 and the catalytic water molecule, slowing hydrolysis.
Tuberculosis (TB), caused by Mycobacterium tuberculosis, continues to be a worldwide health concern (1). There were an estimated 9.3 million new cases of TB in 2007 and approximately 1.3 million HIV-negative patient fatalities as well as nearly half a million deaths amongst HIV-positive populations (2). Even fifty years after the introduction of powerful antibiotics to treat TB, it has been estimated that one person is infected in the world every few seconds (3). The failure to control TB is due to the emergence of M. tuberculosis strains that are multiply drug resistant towards the front line antimycobacterial drugs such as isoniazid and rifampicin.
As one of the most important antibiotic families, β-lactams include a broad range of molecules including penicillin derivatives, cephalosporins, monobactams, carbapenems, and β-lactamase inhibitors. The carbapenems exhibit the broadest spectrum of activity among the β-lactam antimicrobials, providing safe and efficacious therapies in the treatment of serious infections caused by Gram-positive, Gram-negative, and anaerobic bacterial pathogens (4, 5). Carbapenem antibiotics were originally developed from thienamycin, a natural product identified in culture filtrates of Streptomyces cattleya (6). There are four carbapenems approved thus far for human use: imipenem, meropenem, ertapenem, and doripenem (5). Imipenem was the first carbapenem approved by the US Food and Drug Administration (FDA) in 1985, and is by far the most widely used carbapenem. The use of meropenem was approved in 1995, followed by ertapenem and doripenem in 2001 and 2007, respectively. Except for imipenem, all carbapenems are stable against the mammalian kidney dehydropeptidase (7). In clinical usage, imipenem and meropenem have to be given frequently to maintain high circulating levels. Also, weight-dosage adjustment of imipenem is required to minimize the chance of seizures (8). Ertapenem and doripenem can be given once per day due to their high target affinity and circulating stability (5, 9). The lower effective doses of these latter drugs reduces potential side effects, as well as the development of resistance (10). Currently, ertapenem and doripenem are used for complicated intra-abdominal, and urinary tract infections (11, 12).
Despite the general success of β-lactam antibiotics, they have not been widely used for the treatment of TB due to intrinsic resistance that is caused by the presence of a chromosomally-encoded gene (blaC) in M. tuberculosis for a Class A Ambler β-lactamase (BlaC). Like other Class A β-lactamases, BlaC catalyzes the opening of the β- lactam ring via nucleophilic attack by an active site serine residue to generate the acylenzyme, followed by the hydrolysis of the ester bond to generate the ring-opened, inactive product. Our previous studies of TB BlaC revealed that this enzyme is an extremely broad-spectrum β-lactamase hydrolyzing all β-lactam classes, including the carbapenems meropenem and imipenem (13). Being slow substrates that exhibit rapid acylation followed by a slow deacylation step, meropenem and imipenem also act as potent inhibitors of BlaC (14). FTICR mass spectrometry demonstrated that the acylated intermediate remains stable for many minutes (14). Such slow turnover rates allowed the determination of three-dimensional structure of BlaC in complex with meropenem at a resolution of 1.8 Å. In vivo studies showed that meropenem in combination with the β-lactamase inhibitor, clavulante, is bactericidal against clinical TB strains that are phenotypically exensively drug resistant (XDR-TB) (14). As an extension of our prior work, we carried out an in vitro characterization of doripenem and ertapenem with BlaC.
Materials and Methods
All chromatographic materials were purchased from Pharmacia. Meropenem and faropenem were from IKT Laboratories. Doripenem (as Doribax) was from Ortho-McNeil Pharmaceutical Inc (Raritan, NJ). Ertapenem (as Invanz) was from Merck & Co. Inc. The potassium salt of clavulanic acid was from Sigma Aldrich. All other chemicals were purchased from Sigma or Aldrich. Nitrocefin was purchased from Beckton Dickinson.
Purification of BlaC
Recombinant and truncated BlaC from M. tuberculosis expressed from plasmid pET28a(+) and purified to homogeneity as described by Hugonnet and Blanchard (13).
Kinetics
The steady state rate of hydrolysis of β-lactam ring was monitored as a decrease in the absorbance in the UV region, as described previously (13). Assays using doripenem, ertapenem, faropenem and meropenem were performed at 296 nm (ε = 7,540 M−1 cm−1), 295 nm (ε = 9,970 M−1 cm−1), 306 nm (ε = 3,445 M−1 cm−1), and 297 nm (ε = 6,152 M−1 cm−1), respectively. Assays using the chromogenic substrate nitrocefin were performed at 486 nm (ε = 20,500 M−1 cm−1). Assays were performed in 100 mM MES (pH 6.5). Reactions were initiated by the addition of enzyme at concentrations between 0.1–25 µM using 100 µM of the carbapenem substrate.
Inhibition Studies
Carbapenems at concentrations ranging from 0.1–10 µM were tested as inhibitors of 1.5 nM BlaC using 60 µM nitrocefin as substrate. Time courses were followed for 15 min. For slow onset inhibition, reaction velocities as a function of time were fitted to eq 1:
| (1) |
where [P] is the concentration of the product, vi and vs are the initial and final reaction velocities respectively for the reaction in the presence of inhibitor and kiso is the apparent first order rate constant for the inter-conversion between vi and vs, and t is time.
The general mechanism can be modeled as:
| (2) |
where k1 and k−1 represent the reversible binding to and dissociation from the carbapenem to BlaC, k2 represents the irreversible cleavage of the carbapenem β-lactam ring and k3 represents the hydrolysis of the BlaC-carbapenem adduct.
For this model, the rate constant that describes kiso is given by eq 3, where Kd equals k−1/k1.
| (3) |
In eq 4, the Km value can be expressed as:
| (4) |
In addition, from the determined k2 and k3 values, kcat is calculated from eq 7, assuming k2,k3≪k1,k−1.
| (5) |
Mass Spectrometry
All mass spectra were acquired on a 9.6 T Fourier Transform Ion Cyclotron Resonance (FTICR) mass spectrometer (Ionspec, Lake Forest, CA). To avoid salt interference, BlaC was dialyzed against 20 mM ammonium bicarbonate, pH 6.5. The molecular mass of each protein sample was determined for the 25+ charge state using the equation m = (m/z x 25)−25 on the isotopic centroid. To monitor the intermediate of steady state turnover or small molecular mass spectrometry, 51 µM of enzyme was incubated with 25 µM carbapenem in a total volume of 20 µL. An aliquot of 1 µL was withdrawn at desired time (0, 30, 60, and 90 min) and mixed with 9 µL of mixing solution (containing 50% acetonitrile and 0.1% formic acid). The resulting mixture was injected into the FTICR mass spectrometer.
Crystallization
BlaC was crystallized in the hanging drop vapor diffusion configuration over well conditions of 0.1 M HEPES, pH 7.5 and 2 M NH4H2PO4. The final pH of the well solution was 4.1. Protein at a concentration of 10 mg/ml was mixed 1:1 with the well solution and incubated at 18 °C. Initial crystals grew within a week but were small, sparse and amorphous. New wells were sealed and allowed to equilibrate overnight. Equilibrated drops were micro-seeded, which resulted in efficient crystal growth as well as improved morphology. Iterative seeding resulted in diffraction quality crystals of active enzyme.
Data collection and refinement
Crystals were soaked with either ~ 50 mM ertapenem or doripenem in mother liquor plus 20% glycerol as a cryo-protectant. Data were collected after 10 and 90 minute soaks with ertapenem and a 90 minute soak with doripenem at Brookhaven National Laboratory on beamlines X12C and X29, in which various resolutions of diffraction were obtained dependent on the soaking times and beamline. The data were processed using either HKL2000 (15) or Mosflm (16). Our previous structure of clavulanate bound M. tuberculosis β-lactamase (17) (PDB entry 3CG5) was used to phase all the data, using the CCP4 software suite (18). Iterative rounds of structural refinement and model building were performed in Refmac5 (19, 20) and Coot (21). Table 1 lists the data collection statistics for the structures as well as the final refinement statistics.
Table 1.
Data Collection and Refinement Statistics
| Data Collection | Doripenem Δ1-isomerζ |
Ertapenem Δ2-isomer |
Ertapenem Δ1-isomer |
|---|---|---|---|
| Resolution (Å) | 50.0-2.2 (2.32-2.20) |
50.0-1.30 (1.33-1.30) |
50.0-2.0 (2.07-2.00) |
| Completeness | 100% (100%) | 100.0% (100%) | 99.5 (99.9) |
| Redundancy | 7.6 (7.4) | 7.5 (5.7) | 4.4 (4.4) |
| I/sigma(I) | 3.8 (1.6) | 21.4 (1.8) | 9.8 (4.0) |
| Rmerge | 0.077 (0.47) | 0.057 (0.757) | 0.158 (0.373) |
| Space Group | P212121 | P212121 | P212121 |
| Unit cell (Å) |
a =49.989 b =68.068 c =75.792 α = β = γ = 90.0° |
a = 49.66 b = 67.92 c = 75.55 α = β = γ = 90.0° |
a =49.934 b =67.830 c =75.201 α = β = γ = 90.0° |
| Reflections | 13,695 (1,943) | 60,263 (4,388) | 17,920 (1,762) |
| Refinement Statistics | |||
| Rwork | 0.161 (0.176) | 0.147 (0.265) | 0.175 (0.191) |
| Rfree | 0.205 (0.237) | 0.176 (0.278) | 0.222 (0.281) |
| Average B-factors (Å2) | |||
| Protein | 6.97 | 10.49 | 6.09 |
| Adduct | 27.32 | 18.64 | 15.50 |
| Solvent | 17.51 | 32.64 | 14.36 |
| PO4 | 12.89 | 14.40 | 10.53 |
| RMS deviations | |||
| bonds (Å) | 0.010 | 0.010 | 0.012 |
| angles (°) | 1.204 | 1.428 | 1.386 |
| Ramachandra | Favored= 97.7% outliers= 0.0% |
Favored= 97.7% outliers= 0.0% |
Favored= 98.1% outliers= 0.0% |
| PDB accession code | 3IQA | 3M6B | 3M6H |
Values in parentheses are for the highest resolution bin.
This data processed using Mosflm
RESULTS and DISCUSSION
Kinetics
The accurate determination of the kinetic parameters for doripenem and ertapenem was severely hampered by apparent very low Km values, very low kcat values and the modest extinction coefficients accompanying hydrolysis. At the [BlaC] required to see any significant rate of reaction (~2 µM), variation of the [doripenem] or [ertapenem] at concentrations from 2–20 µM showed almost no difference in rate, suggesting their Km values were less than 2 µM. The steady-state kinetic parameters determined for faropenem, a structurally distinct penem, were Km = 55 ± 11 µM, and kcat = 0.65 ± 0.04 min−1 (data not shown). This Km value is ~17 times larger and the kcat value 8 times faster than those of meropenem (14).
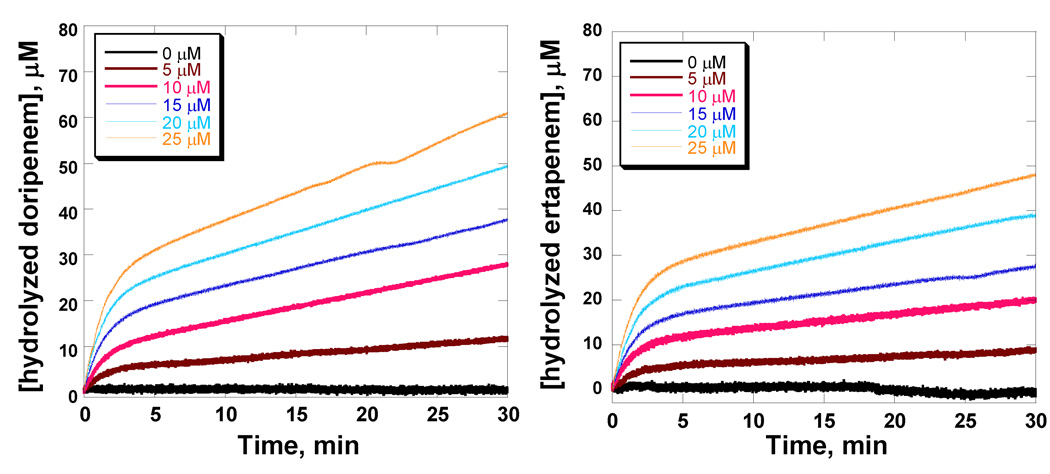
Detailed investigations of the kinetics of carbapenem hydrolysis under near stoichiometric enzyme concentrations were carried out over 30 minute time periods. As shown in Figure 1, a steady-state kinetic burst was observed with both compounds where the magnitudes of the burst are proportional to the concentration of BlaC used. Extrapolation of the rates of hydrolysis to the y-axis demonstrates that the acylation is stoichiometric with the concentration of enzyme.
Figure 1.
Time courses of doripenem (A) and ertapenem (B) hydrolysis with various concentrations of BlaC.
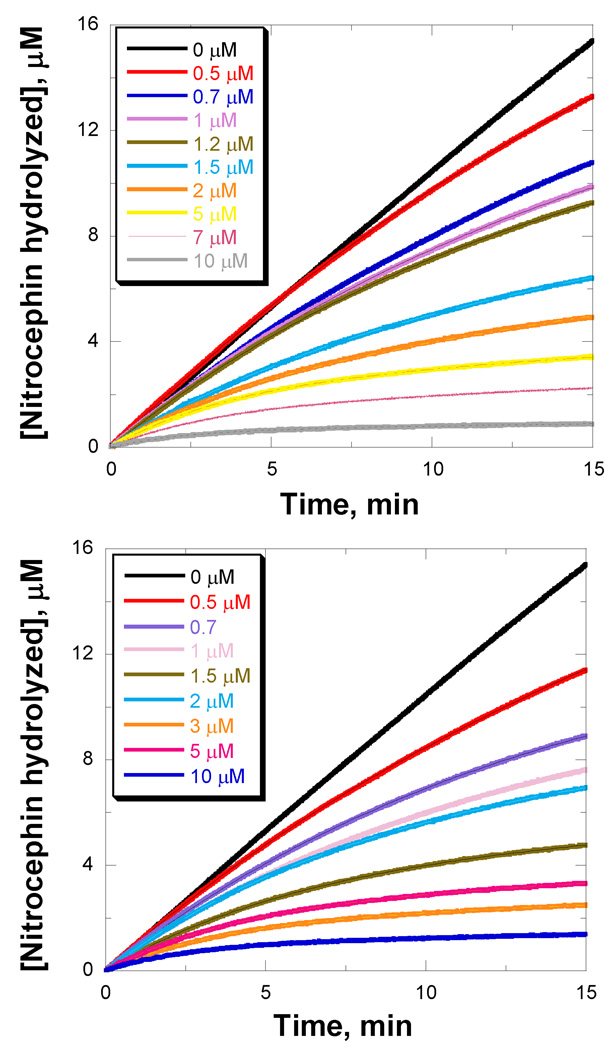
Due to the extremely feeble turnover rate, we further tested these carbapenems as inhibitors of the reaction of nitrocefin with BlaC. Nitrocefin is an extremely good substrate for BlaC and its β-lactam ring-opened form is extremely chromogenic. As shown in Figure 2, doripenem and ertapenem act as slow-onset, tight binding inhibitors of BlaC when the hydrolysis of nitrocefin was monitored. In contrast, faropenem exhibited standard, competitive inhibition with no time dependent component (data not shown). This type of time-dependent inhibition for a dead-end inhibitor is modeled as being due to the reversible formation of a non-covalent complex (E–I), followed by the reversible conversion to an isomerized complex (E–I*). However, in the case of a slow substrate for BlaC, the initially formed Michaelis complex reacts with the enzyme in an irreversible step to generate the BlaC-carbapenem covalent intermediate. This is then hydrolyzed slowly to regenerate the free enzyme that can react with nitrocefin. While the same equation is used to fit the two models, the kinetic constants that contribute to kiso (Figure S1) and Ki (or Kd) are different. Using the fits of the slow-onset data and eq 3 to calculate Kd, k2 and k3, we can then used eqs 4 and 5 to calculate the apparent Km and kcat values for doripenem (0.18 µM and 0.016 min−1, respectively), and for ertapenem (0.18 µM and 0.017 min−1, respectively). We have not corrected for the concentration of nitrocefin used in these experiments because of the large standard errors (>40%) associated with these kinetic parameters (the reported Km values are apparent values). However, the extremely tight binding and extremely low turnover of these carbapenems is evident from these rather imprecise kinetic data.
Figure 2.
Time courses of nitrocefin hydrolysis by BlaC in the presence of doripenem (upper) and ertapenem (lower).
Mass Spectrometry
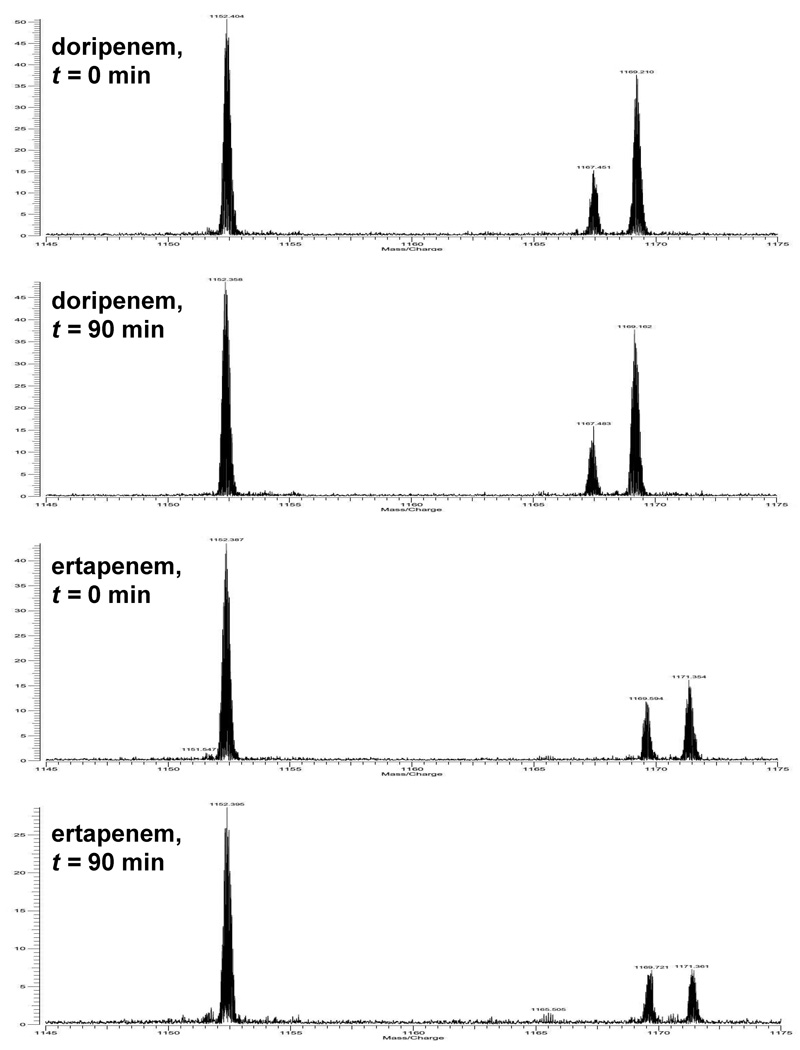
The rapid acylation and slow deacylation of BlaC by the carbapenems allows the observation of the covalently bound, acyl-enzyme intermediate by Fourier transform ion cyclotron resonance. A freshly prepared solution containing excess BlaC and doripenem displayed three peaks: the first peak corresponds to free BlaC with mass/charge ratio (m/z) = 28,785.0, a second peak corresponding to the covalently acylated BlaC-doripenem complex with mass/charge ratio (m/z) = 29, 204.1 and a third peak whose mass corresponds to the mass of the covalently acylated BlaC-doripenem complex minus 44 mass unit (m/z = 29, 161.0), as shown in Figure 3. With ertapenem, the two covalent acylated BlaC complex peaks observed had molecular masses of 29,260.0 and 29,217.1, corresponding to acylated BlaC-ertapenem complex and acylated BlaC-doripenem complete minus 44 mass units, respectively. This data demonstrates that both doripenem and ertapenem undergo the same chemical breakdown in the active site as meropenem (14). Once the acyl-enzyme forms, the carbapenems partition between hydrolysis and enzyme-catalyzed decomposition of the C6 hydroxyethyl substituent, via a retro-Aldol decomposition, which yields acetaldehyde (14). Intriguingly, the intensities of the acyl-enzyme complexes remain stable over the time period of 90 min for doripenem and ertapenem. This is in contrast with previous observations with meropenem, where the acylated forms of the enzyme started to diminish after several minutes. These data suggest that doripenem and ertapenem form more stable complexes with BlaC than meropenem, reinforcing the kinetic data.
Figure 3.
Mass spectra of enzyme-carbapenem species. The 25+ charge state ions are shown.
X-ray Crystallography
The 2.2 Å data from a 90 min doripenem-soaked crystal were refined to an Rwork of 0.161 and an Rfree of 0.205. The 1.3 Å diffraction data from a 10 minute ertapenem-soaked crystal refined to an Rwork of 0.147 and an Rfree of 0.176. The 2.0 Å diffraction data from a 90 min ertapenem-soaked crystal were refined to an Rwork of 0.175 and an Rfree of 0.222. In these three structures, the active site Ambler residue Ser70 has been covalently linked with the ring open form of these β-lactams in accordance with the acylation chemistry of the first half of the enzymatic reaction (Scheme 1). The quality of the electron density is displayed in Figure 4 and Figure 5 under a Fo-Fc omit calculated map contoured at 2.0 σ.
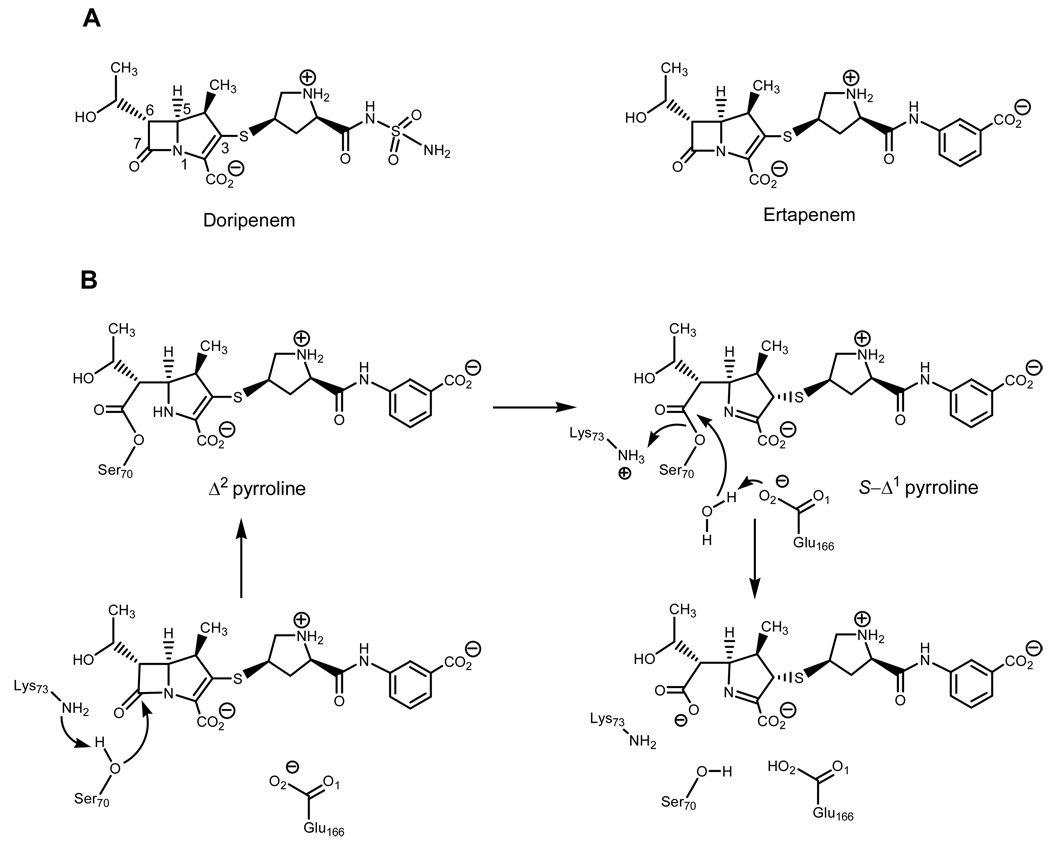
Scheme 1.
(A) the structures of doripenem and ertapenem. (B) The chemical mechanism of hydrolysis of ertapenem by the Mycobacterium tuberculosis BlaC.
Figure 4.
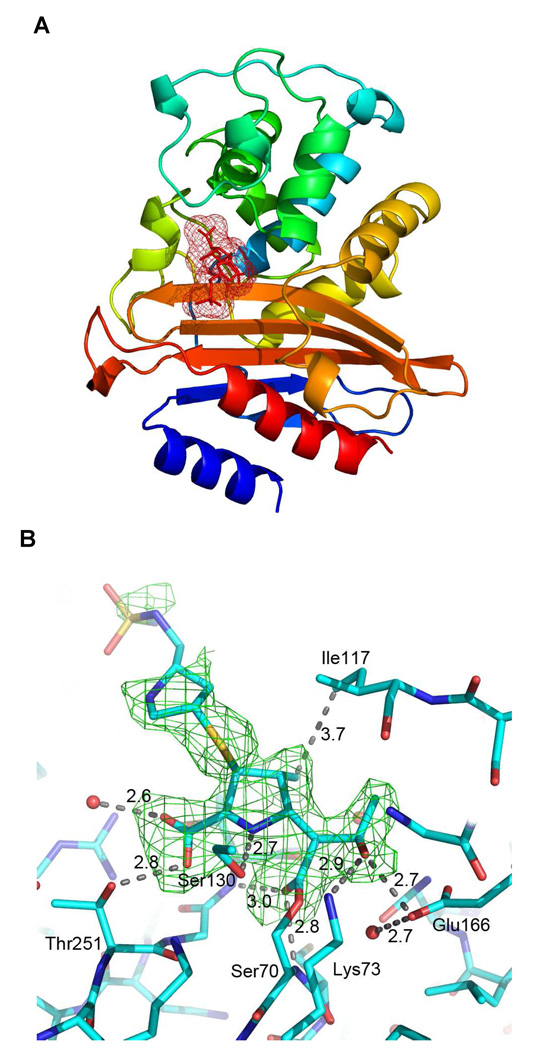
(A) Overall structure of BlaC displayed in rainbow from N term (blue) to the C term (red), with the doripenem adduct displayed in red surface mesh. (B) Fo-Fc omit density (green) contoured at 2.0 σ surrounds the covalent doripenem adduct formed at the Ambler active-site residue serine 70. All structure figures were produced using Pymol.
Figure 5.
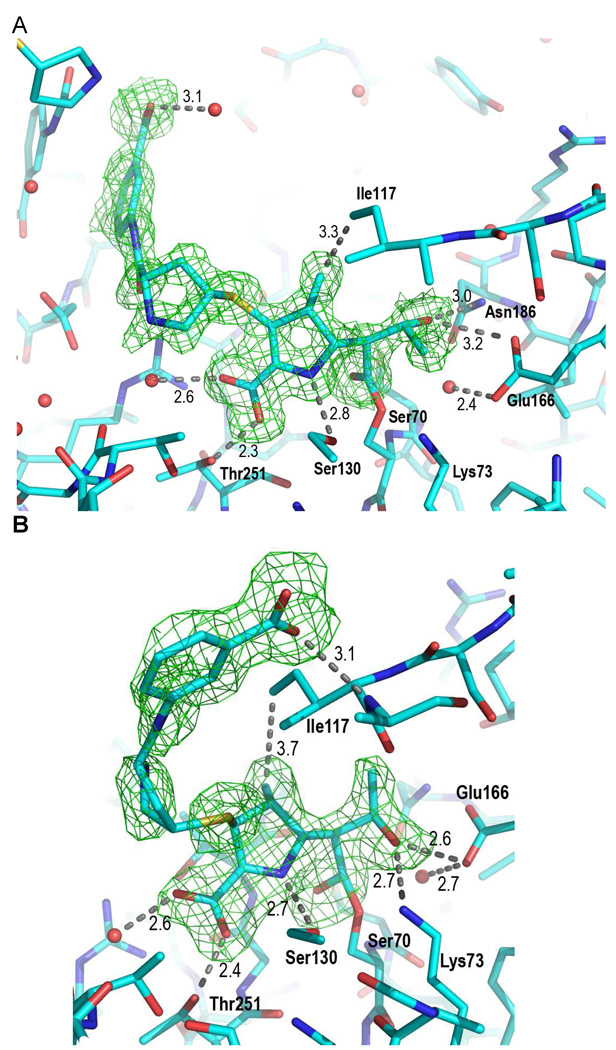
(A) Fo-Fc omit density (green) contoured at 2.0 σ surrounds the covalent ertapenem adduct formed at the Ambler active-site residue serine 70 in the pre-isomerization state. (B) Fo-Fc omit density (green) contoured at 2.0 σ surrounds the covalent ertapenem adduct formed at the Ambler active-site residue serine 70 in the post-isomerization state. The resolution of the densities unambiguously demonstrates the shift in stereochemistry with the change from sp2 to sp3 hybridization of the C3 carbapenem carbon atom with the change in the position of the density associated with the meta-amino-benzoate and the hydoxyethyl ertapenem moieties.
The C3 atom of the pyrroline rings of doripenem and ertapenem covalent adducts are sp3 hybridized in the 90 min soaks. These results require an isomerization event occurring in the BlaC-carbapenem adducts in which the original Δ2 pyrroline ring was tautomerized to generate the Δ1 pyrroline ring, evidenced by the collinear positioning of the C5, N1, C2, and C3 atoms. In addition, the BlaC-doripenem and ertapenem covalent adduct densities allow for the positioning of the thioether sulfur atom in the unambiguous assignment of the S configuration at C3, requiring protonation at the re face of the C2–C3 double bond. This is similar to our earlier findings with BlaC crystals soaked with meropenem on similar time scales (14), and represents the thermodynamically preferred product with the trans orientation of the C4 methyl and thioether substituents. Interestingly, in the recently reported structure of the Class D β-lactamase, OXA-1 covalent adduct with doripenem, revealed that while an identical isomerization had taken place, that the final Δ1 pyrroline ring product was of the opposite, R, stereochemistry (22).
In the structure determined after the shorter 10-minute soak, the BlaC-ertapenem adduct was covalently bound in the active site, but in a different geometry. On this shorter time scale, the BlaC-ertapenem adduct C3 atom is found in its original sp2 hybridization with the definitive collinear positioning of the thioether sulfer atom in line with the N1, C2, C3, and C4 bonds indicating the presence of the Δ2 pyrroline ring. This result requires that β-lactam ring cleavage and isomerization of the methyl pyrroline ring not be concerted.
The active site interactions vary in some subtle ways between the pre and post-isomerization ertapenem complexes, yet a number of common interactions are observed in all complexes. Both the BlaC-ertapenem and -doripenem adducts bind as covalent adducts with the active site Ser70 and position their lactam ring-opened ester carbonyl oxygen atom within the oxyanion hole formed from hydrogen bonding interactions with the Ser70 and Thr253 amide nitrogen atoms. All structures contain a hydrophobic interaction between the methyl group of the pyrroline ring and the sidechain of Ile117 and different forms of hydrogen bonding interactions between the C6 hydroxyethyl substituent of the carbapenem and Glu166. All three structures also show a conserved interaction between the sidechain hydroxyl of Ser130, which consistently hydrogen bonds the pyrroline ring nitrogen atom at a distance of 2.7–2.8 Å. The pyrroline C2 carboxylate group forms hydrogen bonds with the Thr251 hydroxyethyl side chain and an active site water molecule. In structures of other β-lactamase-carbapenem adducts, this carboxylate electrostatically interacts with a conserved arginine residue (R244 in TEM-1) (23), but this is not the case for BlaC. In the pre-isomerized ertapenem structure, there is an additional hydrogen bond between the C2 carboxylate of the pyrroline ring and Thr253, which is broken upon the repositioning of the meta-amino-benzoate ‘arm’ observed in the post-isomerized ertapenem structure. The isomerization and stereospecific protonation leads to a reorientation of the terminal portion of the molecule within the active site, allowing for the formation of the hydrogen bond between the terminal carboxylate group of the meta-amino-benzoic acid moiety and Ser118. A final difference between the initially formed Δ2-pyrroline isomer and the final Δ1-pyrroline form is the orientation of the C6 hydroxyethyl substituent. In the pre-isomerized complex it is oriented away from Lys73 and hydrogen bonds to the carboxyl O1 of Glu166 as well as the Asn186 nitrogen, but rotates upon isomerization to hydrogen bond with the carboxyl O2 of Glu166 and the ε-amino group of Lys73 in a manner similar to that observed for the doripenem complex.
The structures of the pre and post-isomerization states reveal the mechanistic basis for the relative stability of the carbapenems within the active site of BlaC and their ability to resist hydrolysis by the enzyme. As seen in Scheme1, deacylation-hydrolysis from the enzyme requires the activation of the conserved active site water by the side chain carboxyl O2 of Glu166. The probability of water activation by Glu166 decreases with the increased distance between the two. The distance between the carboxyl O2 of Glu166 and the active site water is significantly increased after isomerization from 2.4 Å to 2.7 Å, making water activation less probable. The isomerization event does not directly cause these changes but rather alters the positioning of the adduct such that the adduct-hydroxyethyl flips in the active site, breaking the hydrogen bond formed between the hydroxyl group with the side chain amide nitrogen of Asn186 and the adjacent carboxyl O1 of Glu166 oxygen. The hydroxyethyl substituent then rotates to generate a hydrogen bond network with Lys73 and the carboxyl O2 of Glu166, effectively ‘pulling’ this essential base away from the conserved active site water. These residues (Lys 73 and Glu166) are involved as general bases in the acylation and deacylation reactions, respectively. In addition, by hydrogen bonding carboxyl O2 of Glu166, the reoriented hydroxyethyl substituent reduced the basicity of Glu166. These two factors, introduced in the reorientation of the hydroxyethyl substituent in the post-isomerization complex, reduce water activation and thereby stabilize the acyl-intermediate in the active site. The studies reported here allow us to directly visualize the changes that occur between the pre- and post-isomerization adduct structures and are atomic level observations relevant to the biphasic kinetics previously reported for the reactions between carbapenems and the RTEM β-lactamase (24).
The Δ2 to Δ1-pyrroline isomerized forms of carbapenems have been known to form within the active sites of various β-lactamase enzymes (25). In confirmation of this, crystal structures of carbapenems bound within the active sites of the Class A β-lactamases TEM-1 (PDB entry 1BT5) (26) and SHV-1 (PDB entry 2ZD8) (27) as well as AmpC (PDB entry 1LL5) (28) a Class C β-lactamase all revealed the Δ2 form of the carbapenem bound in the active sites, while the Class D OXA-1 (PDB entry 3ISG) (22) and the Class A BlaC (PDB entry 3DWZ) (14) were both bound with carbapenems in the Δ1 isomerized forms with respective R and S-stereochemistries. Our findings are the first to show the structures of both the Δ2 and Δ1 forms of a carbapenem bound to a single β-lactamase. Interestingly, several of the structures of carbapenems bound as the Δ2 isomers show evidence for alternate conformations for the carbapenem-carbonyl oxygen position. This oxygen is found buried within the oxyanion-hole as well as bound in a position rotated by 180 degrees, usually facing an opposing serine residue (Ser130). In these instances it has been proposed that the flipping of the carbonyl oxygen from the oxyanion-hole blocks formation of the deacylation tetrahedral intermediate to inhibit the enzyme. In the cases of OXA-1 and BlaC, the carbapenem-carbonyl oxygen is only found bound tightly within the oxyanion-hole and no evidence of alternate conformers has been observed. In these cases inhibition by the carbapenem is likely due to disruption of water activation.
A second possible reason for the observed alternate conformers at the carbapenem-carbonyl is likely due to the position of the carbapenem-carboxylate moiety within those active sites. To date, those β-lactamases with alternate conformations for the carbapenem-carbonyl, have a highly conserved Arg244 reside which electrostatically interacts with the carbapenem-carboxylate moiety. The OXA-1 and BlaC active sites lack this arginine interaction and instead use a combination of threonine and/or serine residues coordinated with waters to bind the carboxylate moiety. These residues are located closer to the oxyanion hole and act to ‘clamp’ the carboxylate into a proximal position, as opposed to the Arg244 mechanism of carboxylate binding, where distance introduces flexibility, allowing for the alternate positioning of the pyrroline ring. This bonding pattern to the carbapenem allows for alternate “in/out” conformations of the carbapenem carbonyl in the oxyanion hole. In contrast, the carbapenem carbonyl is tightly bound in the oxyanion hole in BlaC in both the Δ2 and Δ1 forms reported here.
Supplementary Material
Acknowledgments
This work was supported in part by a grant from the National Institute of Health (AI33696 to J. S. B.) and in part by the Charles Revson Foundation (to L.W.T.)
ABBREVIATIONS
- BlaC
Mycobacterium tuberculosis beta-lactamase
- TB
Tuberculosis
- XDR-TB
extensively drug resistant
Footnotes
Supporting Information Available
One figure showing the dependence of kburst on the [ertapenem] and [doripenem]. This material is available free of charge via the Internet at http://pubs.acs.org
References
- 1.Dye C, Floyd K, Uplekar M, Bierrenbach A, Bergstrom K, Blanc L, Grezmska M, Gunneberg C, Lonnroth K, Nunn P, Pantoja A, Raviglione S, Weyer K. Global tuberculosis control : surveillance, planning, financing. World Health Organization; WHO report 2008. 2008
- 2.Bauquerez R, Blanc L, Bierrenbach A, Brands A, Ciceri K, Falzon D, Floyd K, Glaziou P, Gunneberg C, Hiatt T, Hosseini M, Pantoja A, Uplekar M, Watt C, Wright A. Global Tuberculosis Control: Epidemiology, Strategy, Financing. World Health Organization; WHO Report 2009. 2009
- 3.Netto EM, Dye C, Raviglione MC. Progress in global tuberculosis control 1995–1996, with emphasis on 22 high-incidence countries. Global Monitoring and Surveillance Project. Int J Tuberc Lung Dis. 1999;3:310–320. [PubMed] [Google Scholar]
- 4.Mandell L. Doripenem: a new carbapenem in the treatment of nosocomial infection. Clin Infect Dis. 2009;49(Suppl 1):S1–S3. doi: 10.1086/599809. [DOI] [PubMed] [Google Scholar]
- 5.Baughman RP. The use of carbapenems in the treatment of serious infections. J Intensive Care Med. 2009;24:230–241. doi: 10.1177/0885066609335660. [DOI] [PubMed] [Google Scholar]
- 6.Birnbaum J, Kahan FM, Kropp H, MacDonald JS. Carbapenems, a new class of beta-lactam antibiotics. Discovery and development of imipenem/cilastatin. Am J Med. 1985;78:3–21. doi: 10.1016/0002-9343(85)90097-x. [DOI] [PubMed] [Google Scholar]
- 7.Livermore DM. Of Pseudomonas, porins, pumps and carbapenems. J Antimicrob Chemother. 2001;47:247–250. doi: 10.1093/jac/47.3.247. [DOI] [PubMed] [Google Scholar]
- 8.Calandra G, Lydick E, Carrigan J, Weiss L, Guess H. Factors predisposing to seizures in seriously ill infected patients receiving antibiotics: experience with imipenem/cilastatin. Am J Med. 1988;84:911–918. doi: 10.1016/0002-9343(88)90071-x. [DOI] [PubMed] [Google Scholar]
- 9.Bhavnani SM, Hammel JP, Cirincione BB, Wikler MA, Ambrose PG. Use of pharmacokinetic-pharmacodynamic target attainment analyses to support phase 2 and 3 dosing strategies for doripenem. Antimicrob Agents Chemother. 2005;49:3944–3947. doi: 10.1128/AAC.49.9.3944-3947.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lynch MJ, Drusano GL, Mobley HL. Emergence of resistance to imipenem in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1987;31:1892–1896. doi: 10.1128/aac.31.12.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Behera B, Mathur P, Das A, Kapil A. Ertapenem susceptibility of extended spectrum beta-lactamase-producing Enterobacteriaceae at a tertiary care centre in India. Singapore Med J. 2009;50:628–632. [PubMed] [Google Scholar]
- 12.Paterson DL, Depestel DD. Doripenem. Clin Infect Dis. 2009;49:291–298. doi: 10.1086/600036. [DOI] [PubMed] [Google Scholar]
- 13.Hugonnet JE, Blanchard JS. Irreversible inhibition of the Mycobacterium tuberculosis beta-lactamase by clavulanate. Biochemistry. 2007;46:11998–12004. doi: 10.1021/bi701506h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hugonnet JE, Tremblay LW, Boshoff HI, Barry CE, 3rd, Blanchard JS. Meropenem-clavulanate is effective against extensively drug-resistant Mycobacterium tuberculosis. Science. 2009;323:1215–1218. doi: 10.1126/science.1167498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Otwinowski Z, a MW. Processing of X-ray Diffraction Data Collected in Oscillation Mode. Methods in Enzymology. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 16.Leslie AGW. Mosflm. Joint CCP4 + ESF-EAMCB Newsletter on Protein Crystallography No. 26. 1992 [Google Scholar]
- 17.Tremblay LW, Hugonnet JE, Blanchard JS. Structure of the covalent adduct formed between Mycobacterium tuberculosis beta-lactamase and clavulanate. Biochemistry. 2008;47:5312–5316. doi: 10.1021/bi8001055. [DOI] [PubMed] [Google Scholar]
- 18.Potterton EBP, Turkenburg M, Dodson E. A graphical user interface to the CCP4 program suite. Acta. Cryst. 2003;D59:1131–1137. doi: 10.1107/s0907444903008126. [DOI] [PubMed] [Google Scholar]
- 19.Murshudov GN, Vagin AA, Dodson EJ. Refinement of Macromolecular Structures by the Maximum-Likelihood Method. Acta Cryst. 1997;D53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 20.Pannu NS, Murshudov GN, Dodson EJ, Read RJ. Incorporation of prior phase information strengthens maximum-likelihood structure refinement. Acta Crystallogr D Biol Crystallogr. 1998;54:1285–1294. doi: 10.1107/s0907444998004119. [DOI] [PubMed] [Google Scholar]
- 21.Emsley PCK. Coot: model-building tools for molecular graphics. Acta Crystallogr. 2004;D60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 22.Schneider KD, Karpen ME, Bonomo RA, Leonard DA, Powers RA. The 1.4 A crystal structure of the class D beta-lactamase OXA-1 complexed with doripenem. Biochemistry. 2009;48:11840–11847. doi: 10.1021/bi901690r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zafaralla G, Manavathu EK, Lerner SA, Mobashery S. Elucidation of the role of arginine-244 in the turnover processes of class A beta-lactamases. Biochemistry. 1992;31:3847–3852. doi: 10.1021/bi00130a016. [DOI] [PubMed] [Google Scholar]
- 24.Easton CJ, Knowles J. Inhibition of the RTEM beta-lactamase from Escherichia coli. Interaction of the enzyme with derivatives of olivanic acid. Biochemistry. 1982;21(12):2857–2862. doi: 10.1021/bi00541a008. [DOI] [PubMed] [Google Scholar]
- 25.Kalp M, Carey PR. Carbapenems and SHV-1 beta-lactamase form different acyl-enzyme populations in crystals and solution. Biochemistry. 2008;47:11830–11837. doi: 10.1021/bi800833u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maveyraud L, Mourey L, Kotra LP, Pedelac J, Guillet V, Mobashery S, Samama J. Structural Basis for Clinical Longevity of Carbapenem Antibiotics in the Face of Challenge by the Common Class A β-Lactamases from the Antibiotic-Resistant Bacteria. Journal of the American Chemical Society. 1998;120:9748–9752. [Google Scholar]
- 27.Nukaga M, Bethel CR, Thomson JM, Hujer AM, Distler A, Anderson VE, Knox JR, Bonomo RA. Inhibition of class A beta-lactamases by carbapenems: crystallographic observation of two conformations of meropenem in SHV-1. J Am Chem Soc. 2008;130:12656–12662. doi: 10.1021/ja7111146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beadle BM, Shoichet BK. Structural basis for imipenem inhibition of class C beta-lactamases. Antimicrob Agents Chemother. 2002;46:3978–3980. doi: 10.1128/AAC.46.12.3978-3980.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.