Abstract
Degradation of hybrid layers created in primary dentin occurs as early as 6 months in vivo. Biomimetic remineralization utilizes “bottom-up” nanotechnology principles for interfibrillar and intrafibrillar remineralization of collagen matrices. This study examined whether imperfect hybrid layers created in primary dentin can be remineralized. Coronal dentin surfaces were prepared from extracted primary molars and bonded using Adper Prompt L-Pop and a composite. One millimeter-thick specimen slabs of the resin-dentin interface were immersed in a Portland cement-based remineralization medium that contained two biomimetic analogs to mimic the sequestration and templating functions of dentin noncollagenous proteins. Specimens were retrieved after 1–6 months. Confocal laser scanning microscopy was employed for evaluating the permeability of hybrid layers to Rhodamine B. Transmission electron microscopy was used to examine the status of remineralization within hybrid layers. Remineralization at different locations of the hybrid layers corresponded with quenching of fluorescence within similar locations of those hybrid layers. Remineralization was predominantly intrafibrillar in nature as interfibrillar spaces were filled with adhesive resin. Biomimetic remineralization of imperfect hybrid layers in primary human dentin is a potential means for preserving bond integrity. The success of the current proof-of-concept, laterally-diffusing remineralization protocol warrants development of a clinically-applicable biomimetic remineralization delivery system.
Keywords: Biomimetic Remineralization, Primary Dentin, One-Step Adhesive, Intrafibrillar, Interfibrillar, Self-Etching
INTRODUCTION
Caries continues as the most prevalent problem in pediatric dentistry despite significant advances in prevention over the past few decades. According to the NIDCR Strategic Plan1, “dental caries begins early in life: 18% of preschoolers in the U.S. have already experienced tooth decay and by age 6–8, more than half have experienced this disease — making it 5–8 times more common than asthma. By age 17, more than 80% of the adolescent population is affected by caries.” Failure of dental restorations is a major concern in pediatric dental practices.2 Resin composites are more esthetic and lack undesirable metals, but have shorter lifetimes than amalgams, especially in molar teeth.3 The risk of secondary caries is 3.5 times higher in resin composite than in amalgam restorations.4 This declined performance is caused, in part, by the failure of adhesive resin-dentin bonds that are used to join the composites to dentin.
Resin-dentin bonds created in primary teeth by contemporary adhesives are imperfect.5 In vivo degradation of hybrid layers in primary dentin occurs as early as 6 months after intraoral function.6 Bond degradation occurs via water sorption, hydrolysis of ester linkages of methacrylate resins and activation of endogenous dentin matrix metalloproteinases (MMPs).7 Degradation of denuded collagen within hybrid layers may be prevented in vivo by the application of chlorhexidine as a MMP inhibitor.6,8 As chlorhexidine possesses only limited substantivity,9 the strategy of using chlorhexidine to prevent degradation of hybrid layers has been recently challenged. There is also concern regarding the presence of a resin-sparse, demineralized dentin zone which is susceptible to creep or cyclic fatigue during function. Recent studies, for example, demonstrated that mechanically damaged collagen is more susceptible to proetolysis10 and demonstrated a reduction in its thermal stability.11 Theoretically, the experimental use of “ethanol wet bonding” enables more hydrophobic resins to be applied to dentin for extending the longevity of resin-dentin bonds.12 As the technique is water-sensitive,13 it is dubious whether this protocol is clinically useful for bonding to deep, vital dentin.
Collagen fibrils that are stabilized by intrafibrillar and interfibrillar apatite crystallites in mineralized tissues do not degrade over time.14 Thus, remineralization of incompletely resin-infiltrated hybrid layers appears to be the logical approach for extending the longevity of resin-dentin bonds. Biomineralization studies based on classical “top-down” approaches15 could only provide evidence of extrafibrillar mineral precipitation.16 These precipitates are too large to fit into the gap zones of collagen fibrils to restore the mechanical properties of mineralized tissues.17,18 A nanotechnology-inspired biomimetic remineralization scheme has recently been developed.19 This biomimetic remineralization scheme involves the use of Portland cement and a simulated body fluid to produce apatite via a transient amorphous calcium phosphate20. In addition, it utilizes two polyanionic analogs to mimic the dual functions of dentin matrix proteins in sequestering amorphous calcium phosphate nanoprecursors, and acting as template molecules for guiding the intrafibrillar deposition of apatite crystallites within collagen fibrils of mineralized tissues.21 This remineralization scheme represents an example of the timely, non-classical “bottom-up”15 particle-mediated pathway of crystallization, wherein fluidic nanoprecursors stabilized by polymer molecules are transformed into mesocrystalline intermediates which eventually fuse to create single microscopic crystals.22
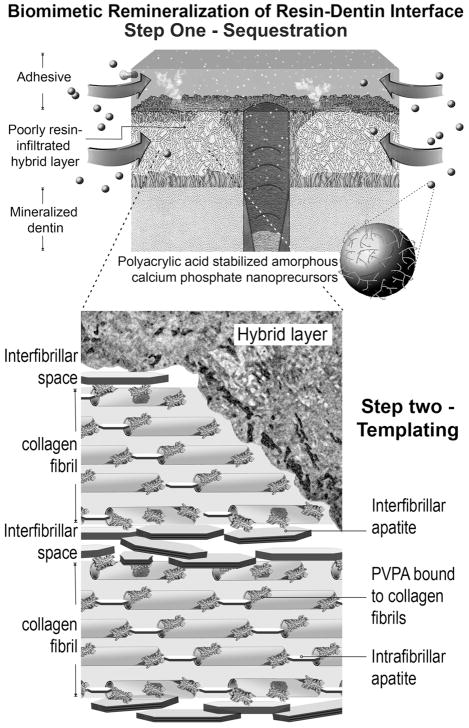
The aforementioned biomimetic remineralization scheme has been adopted for remineralization of hybrid layers created in dentin derived from the permanent dentition (Fig. 1).23 These data, which were based on lateral diffusion of remineralization components into sectioned specimens, provided the proof-of-concept of the viability of intrafibrillar and interfibrillar remineralization of incompletely resin-coated collagen fibrils within hybrid layers. Nevertheless, the sites of in vitro biomimetic remineralization within the hybrid layers were highly reminiscent of the sites of in vivo hybrid layer degradation observed in permanent teeth.8 To date, it is known that degradation of hybrid layers occurred in primary teeth in vivo.6 Primary dentin has been shown to differ from permanent dentin in both mechanical properties and mineral content.24,25 For example, the hardness and tensile strength of sound primary dentin are lower than those properties derived from sound permanent dentin. Differences in hardness and modulus of elasticity were also observed between bonded sound and bonded caries-affected dentin in primary teeth. Despite these differences, there is no data to support that imperfect hybrid layers created in primary dentin can likewise be remineralized using a biomimetic approach. Thus, the objective of the present study was to examine, with the complementary use of confocal laser scanning microscopy (CLSM) and transmission electron microscopy (TEM), whether hybrid layers created by an aggressive one-step self-etch adhesive in primary dentin can be remineralized at the proof-of-concept level using an open-face model of a dual biomimetic analog-containing remineralization scheme.
Fig. 1.
A biomimetic remineralization scheme that has been adopted for remineralization of poorly resin-infiltrated hybrid layers created in permanent dentin. This remineralization scheme utilizes two biomimetic analogs of dentin noncollagenous proteins for: 1) sequestration of amorphous calcium phosphate nanoprecursors; and 2) acting as templates for guiding the deposition of apatite crystallites within (intrafibrillar remineralization) and around the collagen fibrils (intrafibrillar remineralization).
MATERIALS AND METHODS
Dentin Bonding
Twelve noncarious primary molars, extracted for orthodontic reasons, were used for the study. The teeth were collected after the parents’ informed consent was obtained under a protocol reviewed and approved by the Human Assurance Committee of the Medical College of Georgia. They were stored in 0.9% NaCl supplemented with 0.02% sodium azide solution to prevent bacterial growth and were used within a month after extraction. A flat dentin surface was prepared perpendicular to the longitudinal axis of each tooth using a low-speed Isomet diamond saw (Buehler Ltd., Lake Bluff, IL) under water-cooling. The occlusal dentin surface was polished with a 320-grit silicon carbide paper attached to an Ecomet III variable speed grinder-polisher (Buehler Ltd.) under running water to create a bonding surface that was devoid of enamel. Adper Prompt L-Pop (3M ESPE, St. Paul, MN,) was applied to the dentin surface and agitated for 15 sec after the two parts of adhesive was mixed according to manufacturer’s instruction. The adhesive was gently air-dried and light-cured for 40 sec using a quartz-tungsten-halogen light-curing unit with an output intensity of 600 mW/cm2. This was followed by incremental placement of two 2-mm thick layers of a resin composite. Each layer was light-cured separately for 40 sec each. The bonded teeth were stored in 37°C water for 24 h. Each tooth was then sectioned occluso-gingivally into 1-mm thick slabs, each containing the resin-dentin interface. Two central slabs from each tooth were selected for the study, one slab for the control group and the other slab for the experimental group (N=12).
Remineralization Medium
Type I white Portland cement (Lehigh Cement Company, Allentown, Pennsylvania, USA), was mixed with deionized water in a water-to-powder ratio of 0.35:1, placed in flexible silicone molds and allowed to set and aged at 100% relative humidity for one week before use. A simulated body fluid (SBF) was prepared by dissolving 136.8 mM NaCl, 4.2 mM NaHCO3, 3.0 mM KCl, 1.0 mM K2HPO4·3H2O, 1.5 mM MgCl2·6H2O, 2.5 mM CaCl2 and 0.5 mM Na2SO4 in deionized water26 and adding 3.08 mM sodium azide to prevent bacterial growth. This SBF also served as the control remineralization medium, which contained no biomimetic analogs, to For the preparation of the biomimetic remineralization medium, 500 μg/mL of polyacrylic acid (Mw = 1,800; Sigma-Aldrich, St. Louis, Illinois, USA) and 200 μg/mL of polyvinylphosphonic acid (Mw = 24,000; Sigma-Aldrich), were added to the SBF as dual biomimetic analogs. All solutions were buffered to pH 7.4 with 0.1 M Tris Base or 0.1 M HCl.
Biomimetic Remineralization
Each experimental specimen slab was placed over a set Portland cement block (ca. 1 g) inside a glass scintillation vial. The latter was filled with 15 mL of SBF containing the two biomimetic analogs. Each glass vial was capped to prevent evaporation of the solution and stored in an incubator at 37°C. The remineralization medium was changed every month, with its pH (after inclusion of Portland cement blocks) monitored weekly so that it was above 9.25. This ensured that apatite was formed instead of octacalcium phosphate.27 Experimental specimens were retrieved after 1–6 months (two slabs per month). Each specimen was first examined with CLSM for the presence of porous zones within the hybrid layers that were permeable to an aqueous fluorescent dye.28 After CLSM examination, the specimen was processed for TEM examination of the extent of remineralization within the hybrid layers. Control specimens were similarly placed over set Portland cement blocks in glass scintillation vials that were filled with 15 mL of SBF only. Two control specimens were also retrieved at the corresponding time intervals for CLSM and TEM examination.
Confocal Laser Scanning Microscopy
Control and experimental slabs were polished under running water with a wet 1200-grit silicon carbide paper. Each polished slab was ultrasonicated for 5 min and immersed in a 0.1 wt% Rhodamine B solution (Mw = 479, Sigma-Aldrich) dissolved in phosphate buffered saline (pH = 7.4).28 After 24 h, the dye-infiltrated slabs were rinsed with deionized water briefly and examined using a CLSM (LSM 510 META; Carl Zeiss, Thornwood, NY) that was coupled with a helium neon gas laser (80% of 543 nm excitation, 1.2 mW). A water immersion objective (C-Apochromat 63×/1.2 Carl Zeiss, Canada) was used for capturing images commencing at 5 μm beneath the polished surface to avoid superficial specimen preparation artifacts.
Transmission Electron Microscopy
Following CLSM examination, each slab was ultrasonicated in demineralized water for 5 min to remove the fluorescent dye. The control and experimental slabs were fixed in Karnovsky’s fixative and post-fixed in 1% osmium tetroxide. After fixation, each specimen slab was rinsed three times with sodium cacodylate buffer. The slab was dehydrated in an ascending ethanol series (50–100%), immersed in propylene oxide as a transitional medium and embedded in epoxy resin.29 For each monthly examination period, one 2 mm × 2 mm epoxy-resin infiltrated block was prepared from each control slab (i.e. 1 block × 2 slabs = 2 specimens), while two 2 mm × 2 mm epoxy-resin infiltrated blocks were prepared from each experimental slab (i.e. 2 block × 2 slabs = 4 specimens). Non-demineralized, 90 nm thick sections were prepared and examined without further staining using a JEM-1230 TEM (JEOL, Tokyo, Japan) at 110 kV.
RESULTS
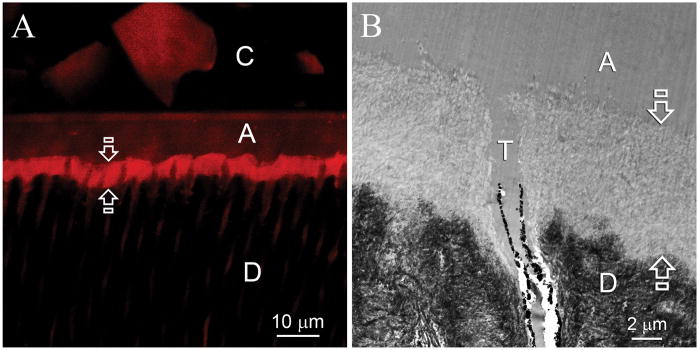
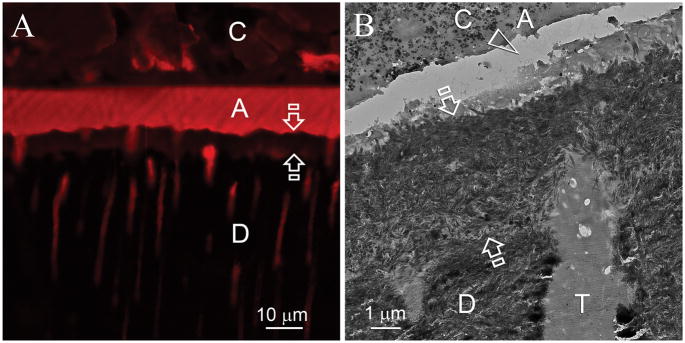
Control specimens examined after 1–6 months exhibited no remineralization in the hybrid layers over the entire examination period. Under CLSM, Rhodamine B was readily identified throughout the entire hybrid layer (Fig. 2A). Transmission electron microscopy of the same specimen revealed a 5–8 μm thick layer of completely demineralized dentin that was devoid of intrafibrillar and interfibrillar mineral crystallites (Fig. 2B).
Fig. 2.
Representative confocal laser scanning microscopy (CLSM) and transmission electron microscopy (TEM) images obtained from six months old specimens that had not been subjected to biomimetic remineralization. C: composite; A: adhesive; D: intertubular dentin. A. CLSM image showing that the entire hybrid layer (between open arrows) was permeable to Rhodamine B (Mw 479). B. TEM of the resin-dentin interface showed that the smear layer was completely dissolved. Etching of the underlying intact intertubular dentin by the aggressive one-step self-etch adhesive resulted in the formation of a 5–8 μm thick layer of completely demineralized collagen matrix (between open arrows) that was simultaneously infiltrated by the adhesive resin. Smear plugs were absent from the orifices of the dentinal tubules (T).
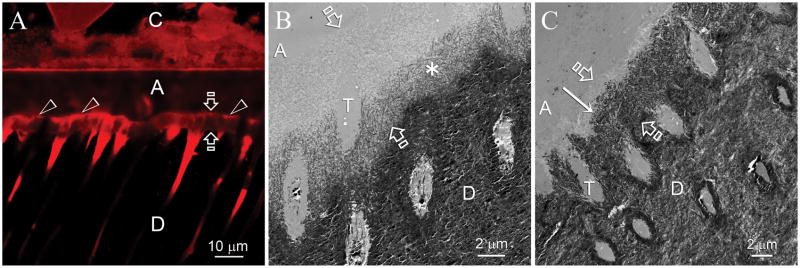
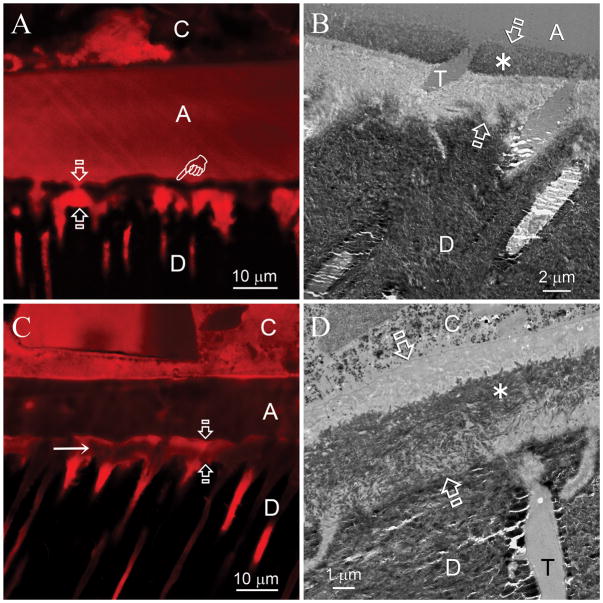
Remineralization of the hybrid layers in the experimental group became evident after 2 months. Heavy remineralization was seen between 3–4 months and did not improve further (i.e. self-limiting) after 4 months. Remineralization was most frequently observed along the base of the hybrid layers. Figure 3A is a CLSM view of a 6-month old specimen. Reduced fluorescence was predominantly identified within the hybrid layer as well as within resin tags in the dentinal tubules in the underlying mineralized dentin base. Figure 3B and 3C are experimental specimens that had undergone two and six months of biomimetic remineralization, respectively. Remineralization commenced from the base of the hybrid layer and did not proceed more than 4–5 μm from the mineralized base. In other experimental specimens, remineralization was observed either from the top (Figs. 4A and 4B) or the middle (Figs. 4C and 4D) of the hybrid layers. For some 4–6 month specimens, extensive remineralization that spanned across the entire thickness of the hybrid layers could be identified (Figs. 5A and 5B).
Fig. 3.
Remineralization was most frequently observed along the base of the hybrid layers. C: composite; A: adhesive; Between open arrows: hybrid layer; D: intertubular dentin, T: dentinal tubule. A. CLSM image of a specimen that had been immersed in the biomimetic remineralization medium for 6 months. Fluorescence was predominantly identified from the surface of the hybrid layer (open arrowheads) and from the dentinal tubules beneath the hybrid layer. Quenching of the fluorescence could be seen along the basal portion of the hybrid layer. B. TEM image taken from a specimen slab that had been immersed in the biomimetic remineralization medium for 2 months, showing an initial stage of remineralization that originated from the base of the hybrid layer. Remineralization was not very intense at this stage, as indicated by the less electron-dense nature of the remineralized part of the hybrid layer (asterisk). C. TEM image taken from the 6-month old specimen in Fig. 3A depicting a more advanced stage of remineralization. Remineralization stopped at approximately 1–2 μm from the dentin surface (arrow). The remineralized part of the hybrid layer exhibited a similar mineral density as the underlying unaltered mineralized intertubular dentin.
Fig. 4.
Biomimetic remineralization in primary dentin was also observed along the surface (A,B) and the middle portion (C,D) of the hybrid layers, albeit less frequently. C: composite; A: adhesive; Between open arrows: hybrid layer; D: intertubular dentin, T: dentinal tubule. A. CLSM image of a specimen that had been immersed in the biomimetic remineralization medium for 5 months. Quenching of the fluorescence was predominantly observed from the surface of the hybrid layer (pointer). B. A corresponding TEM image taken from the 5-month old specimen. A 2–3 μm thick zone of remineralization (asterisk) could be identified along the surface of the hybrid layer. The mineral density of this remineralized zone was similar to that of the intact intertubular dentin. C. CLSM image of a 4-month old specimen. Reduction in the intensity of the fluorescence could be seen within the middle portion of the hybrid layer (arrow). D. A corresponding TEM image taken from the 4-month old specimen showing remineralization of the middle portion of the hybrid layer (asterisk).
Fig. 5.
In some 4–6 month specimens, extensive remineralization that spanned across the entire thickness of the hybrid layers could be identified. C: composite; A: adhesive; Between open arrows: hybrid layer; D: intertubular dentin, T: dentinal tubule. A. CLSM image of a specimen that had been immersed in the biomimetic remineralization medium for 4 months. Quenching of the fluorescence derived from Rhodamine B was evident throughout the entire hybrid layer (between open arrows). B. TEM image of the same specimen. Heavy remineralization could be seen throughout the hybrid layer. The artifactual gap (open arrowhead) within the adhesive layer was created during ultramicrotomy.
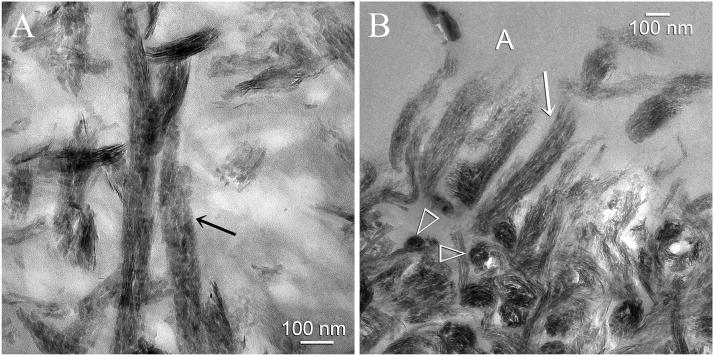
An example of intrafibrillar remineralization that occurred during the early stages of remineralization (2 months) is shown in Figure 6A. Some of the collagen fibrils along the base of the hybrid layer did not remineralize and remained electron-lucent. In stark contrast, adjacent collagen fibrils were heavily remineralized with intrafibrillar plate-like crystallites that were oriented along the longitudinal axis of the fibrils. An example of a more heavily remineralized status that was seen in the 4–6 month specimens is depicted in Figure 6B. Along the surface of the hybrid layer, a shag carpet-like appearance of tufted collagen fibrils30 could be seen in which there was partial unraveling of the fibrils along their severed ends. Heavy intrafibrillar remineralization was identified within these partially unraveled collagen fibrils.
Fig. 6.
High magnification TEM micrographs showing intrafibrillar remineralization of collagen fibrils within the hybrid layer. A. This view was taken from the base of the hybrid layer from a specimen that exhibited an early stage of remineralization. Those collagen fibrils that were better infiltrated by the adhesive did not remineralize and appeared electron-lucent. An ordered arrangement of apatite platelets could be identified within those collagen fibrils that remineralized (arrow). The rationale for the use of biomimetic analogs is to generate apatite nanocrystals that are small enough to fit into the gap zones of the collagen molecules (i.e. intrafibrillar remineralization). B. This view was taken from the dentin surface of a more heavily remineralized specimen. Interfibrillar spaces did not contain minerals as they were filled with adhesive resin. Cross-sections of the remineralized collagen fibrils (open arrowheads) clearly showed that they were fully packed with intrafibrillar minerals. Collagen fibrils along the dentin surface had a shag carpet-like appearance and were partially unraveled along their severed ends. Even these unraveled collagen fibrils were heavily remineralized with apatite nanocrystals (arrow).
DISCUSSION
The mineral content, modulus of elasticity and hardness of primary dentin are lower than those of permanent dentin.24,25 Conversely, dentinal tubular density is higher and the diameter of the dentinal tubules are wider in primary dentin.31 These inherent morphologic differences account for the disparity in mechanical properties between bonded primary dentin and bonded permanent dentin,32 as well as the lower bond strengths of resin restorations in primary dentin.33,34 These factors could also have contributed, in part, to the faster degradation of hybrid layers created in vivo in primary dentin,6 versus those produced in vivo in permanent dentin by the same adhesive.8 Mineralized collagen matrices are more stable than soft collagenous tissues in their ability to resist thermal denaturation35 and degradation by host matrix-derived MMPs.36 Thus, the rationale for examining the feasibility of replenishing poorly resin-infiltrated collagen fibrils in primary dentin hybrid layers with apatite minerals is well justified.
Previous studies on biomimetic remineralization were performed using TEM. This high resolution microscopic technique generates excellent information on crystallite shape and structure but samples very small tissue volumes. In the present study, CLSM was used prior to TEM examination to provide a non-destructive means of examining larger tissue volumes.25 Our CLSM and TEM of hybrid layers derived from the same specimens convincingly showed that those results were reciprocal. That is, regions with quenched fluorescence within the remineralized hybrid layers corresponded well with locations within the demineralized collagen matrices that became filled with intrafibrillar minerals. Earlier work by van der Veen et al.37 demonstrated that the intensity of fluorescent dye penetrating an artificial carious dentin lesion was proportional to the porosity or loss in mineral density within the lesion. More recent work by Toroian et al.38 provided additional data on the size exclusion characteristics of Type I collagen. In that study, water-soluble molecules smaller than 6 kDa were able to diffuse into the monolayer water compartments39 of nonmineralized or demineralized collagen fibrils. A molecular cut-off point was identified in which molecules larger than 40 kDa were excluded from the interior of the collagen fibrils and hence could not access the water compartments within the fibrils. Rhodamine B has a molecular mass of 0.48 kDa and can easily penetrate collagen fibrils that are not infiltrated by resin or are partially resin-encapsulated. In the same study, Toroian et al.38 demonstrated nicely that the internal water compartments in demineralized bone collagen were totally accessible to 14C-glucose (molecular mass = 0.18 kDa). Conversely, the passage of 14C-glucose was dramatically reduced when demineralized bone collagen was replaced by mineralized bone collagen. Moreover, the reduction in glucose passage volume matched the bone volume that was occupied by apatite minerals. This provides a more comprehensive rationale for why intrafibrillar remineralization resulted in quenching of dye fluorescence in the corresponding regions of hybrid layers.
An important aspect of the present study is that the remineralization process was non-uniform. The self-limiting remineralization process reflected the original status in which those hybrid layers were infiltrated by the one-step self-etch adhesive. Two levels of heterogeneity could be recognized, one at the CLSM/TEM level (Figs. 3–5) and the other only at high TEM magnifications (Fig. 6). The first level of heterogeneity is depicted by the different locations in which remineralization occurred within the hybrid layer. These variations may be the result of uneven distribution of hydrophobic and hydrophilic resin monomers within the hybrid layer. Adper Prompt L-Pop contains 2-hydroxymethyl methacrylate (HEMA) which prevents gross phase separation of the other mutually immiscible resin components into macroscopic droplets following evaporation of the adhesive solvent.40 Nevertheless, heterogeneous distribution of the hydrophobic and hydrophilic resins may still occur at the nanoscopic level,41,42 in the presence of osmotically-derived water from the underlying dentin.43 This may result in regional variations in composition that may affect the degree of conversion, the extent of collagen fibril penetration and/or encapsulation, and the water sorption characteristics of the resin-infiltrated zone. The more hydrophilic resin-infiltrated zones within hybrid layers act as pathways of water diffusion and hence are more amendable to biomimetic remineralization. Based on the previously observed similarity between in vitro remineralized regions23 and in vivo degradation sites8 in hybrid layers formed by etch-and-rinse adhesives, it is highly probable that the remineralized sites depicted in Figs. 3–5 represent regions that will undergo degradation after long-term storage.
The second level of heterogeneity occurs at the collagen fibrillar level. This is clearly exemplified by Fig. 6A. Self-etching adhesives are supposed to etch and infiltrate dentin simultaneously. Thus, it is rather surprising that within an area of less than 1 μm2, some of collagen fibrils were heavily remineralized and densely packed with mineral platelets, while others remained completely unmineralized. Collagen fibrils contain approximately 70 vol% water and 30 vol% collagen molecules at the physiologic level of hydration.44 During the process of natural mineralization, water from the collagen fibrils is replaced with minerals.45 Hence, the mineral contents of mineralized tissues are inversely proportional to their water contents.46,47 A recent study48 utilized magnetic response microscopy to monitor the mineralization of collagen sponges via a “bottom-up” calcium carbonate mineralization system.49 The study showed that there was a reduction in the mobility of the water molecules, as indicated by a reduction in the water proton transverse (T2) relaxation times,48 as water in the collagen fibrils was replaced by the amorphous calcium carbonate “polymer-induced liquid precursors”49 that eventually transformed into intrafibrillar calcite crystallites. Collagen fibrils contribute an important role in mineralization by providing the aqueous compartment for mineral growth. As water is replaced by minerals, the lateral spacing of the collagen fibrils becomes more compact while there is no change in the axial dimension.50 Thus mineralization of collagen fibrils may be perceived as a dehydration process.
During demineralization, these processes are reversed as the minerals within the collagen fibrils are replaced with water and the fibrils rehydrate. Ideally, during adhesive resin infiltration of a demineralized collagen matrix, the 6–7 Å thick water monolayer44 that separates the collagen molecules within the rehydrated collagen fibril is completely replaced by resin monomers. This should result in a co-continuous interfibrillar resin phase and intrafibrillar resin phase after polymerization. For more than a decade, there was no definitive proof on whether collagen fibrils are simply encapsulated by adhesive resins which form disconnected resin sheaths around the fibrils (interfacial model) or whether collagen molecules and microfibrils are penetrated and polymerized within the adhesive resin (interphasic model).51 Data from the present study offers pivotal evidence that both models can co-exist even within a very small region of the hybrid layer. The observation that remineralization did not occur in some fibrils suggests that water monolayers within the intrafibrillar spaces of those fibrils were completely replaced by resin monomers. As water is required for minerals to be deposited, residual water must be present within those adjacent fibrils that exhibited ultrastructural evidence of remineralization, with the corollary that the resins were merely encapsulating the fibrils to form resin sheaths. As water-soluble molecules below the molecular mass of 40 kDa readily diffuse into the water compartments of a collagen fibril,38 all water-miscible resin components of Adper Prompt L-Pop (2-HEMA, 0.18 kDa; Di-HEMA phosphate 0.32 kDa) should be able to infiltrate collagen fibrils and either replace or blend with the water monolayers around the collagen molecules. If blending occurs, it is possible that a hydrogel is formed within the fibrillar network. This provides a plausible explanation of the heavier remineralization of the hybrid layers along the base of the hybrid layers (Fig. 3B vs. Fig. 3C). Although Bis-GMA has a molecular mass of 0.51 kDa, the molecule is hydrophobic and is not readily miscible with water. Instead of diffusing into the internal water environment of the collagen fibril, phase separation of the Bis-GMA could have resulted in the monomer forming a resin sheath that traps water within collagen fibrils that are present in close proximity with those completely resin-infiltrated fibrils.
If water-filled collagen matrices in the hybrid layer are not remineralized, these water-containing hybrid layers will remain imperfect irrespective of the use of MMP inhibitors. The value of extending the longevity of “poor quality” hybrid layers is really questionable from a functional perspective, even for primary sound dentin. After caries removal, clinical bonding surface is more likely to be a combination of sound dentin and caries-affected dentin. Caries-affected dentin contains five times as much water as sound dentin because its mineral component is partially lost after exposure to acids produced by bacteria.52 This significantly larger amount of water makes its replacement by adhesive resins during bonding more difficult as water interferes with the polymerization of the adhesive. In addition, the depth of caries-affected dentin is highly variable but can extend hundreds of microns below the excavated dentin surface. Unlike bonding to sound dentin tested in the current study in which the extent of imperfect bonds is less than 5 μm deep, the incompletely-infiltrated zones in bonded caries-affected dentin may be more than 200 μm deep. Remineralization of incompletely-infiltrated zone in the caries-affected dentin is in progress to provide more convincing proof of our biomimetic remineralization scheme.
CONCLUSION
The use of CLSM in the current study provides a non-destructive means of examining larger tissue volumes to demonstrate the remineralized regions within hybrid layers. Non-uniform remineralization demonstrated by the complementary CLSM and TEM examination illustrates the heterogeneity in hybrid layers created in primary dentin that is infiltrated by an aggressive one-step self-etch adhesive. Biomimetic remineralization of imperfect hybrid layers in primary human dentin, mainly in the form of intrafibrillar remineralization, may be a potential means for preserving resin-dentin bond integrity. The feasibility of the current proof-of-concept, laterally-diffusing Portland cement-based remineralization protocol warrants the development of a clinically-applicable biomimetic remineralization delivery system through the incorporation of two biomimetic analogs into the adhesive or resin composite.
Supplementary Material
Acknowledgments
This study was supported by Grant R21 DE019213-01 from the National Institute of Dental and Craniofacial Research (PI. Franklin R. Tay). We thank Robert Smith for TEM technical assistance, Darren Baker for CLSM technical assistance, Thomas Bryan for epoxy resin embedding, as well as Michelle Barnes and Jane Flanders for secretarial support.
References
- 1.Diagnosis and management of dental caries throughout life. Bethesda, Maryland:National Institutes of Health Consensus Statement. 2001 March 26–28;18:1–24. [Google Scholar]
- 2.Soncini JA, Maserejian NN, Trachtenberg F, Tavares M, Hayes C. The longevity of amalgam versus compomer/composite restorations in posterior primary and permanent teeth: findings from the New England children’s amalgam trial. J Am Dent Assoc. 2007;138:763–772. doi: 10.14219/jada.archive.2007.0264. [DOI] [PubMed] [Google Scholar]
- 3.Abt E. The risk of failure is higher for composites than for amalgam restorations. J Evid Based Dent Pract. 2008;8:83–84. doi: 10.1016/j.jebdp.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Bernardo M, Luis H, Martin MD, Leroux BG, Rue T, Leitão J, DeRouen TA. Survival and reasons for failure of amalgam versus composite posterior restorations placed in a randomized clinical trial. J Am Dent Assoc. 2007;138:775–783. doi: 10.14219/jada.archive.2007.0265. [DOI] [PubMed] [Google Scholar]
- 5.Sanabe ME, Kantovitz KR, Costa CA, Hebling J. Effect of acid etching time on the degradation of resin-dentin bonds in primary teeth. Am J Dent. 2009;22:37–42. [PubMed] [Google Scholar]
- 6.Hebling J, Pashley DH, Tjäderhane L, Tay FR. Chlorhexidine arrests subclinical degradation of dentin hybrid layers in vivo. J Dent Res. 2005;84:741–746. doi: 10.1177/154405910508400811. [DOI] [PubMed] [Google Scholar]
- 7.Breschi L, Mazzoni A, Ruggeri A, Cadenaro M, Di Lenarda R, De Stefano Dorigo E. Dental adhesion review: aging and stability of the bonded interface. Dent Mater. 2008;24:90–101. doi: 10.1016/j.dental.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 8.Carrilho MRO, Geraldeli S, Tay F, de Goes MF, Carvalho RM, Tjäderhane L, Reis AF, Hebling J, Mazzoni A, Breschi L, Pashley D. In vivo preservation of the hybrid layer by chlorhexidine. J Dent Res. 2007;86:529–533. doi: 10.1177/154405910708600608. [DOI] [PubMed] [Google Scholar]
- 9.Caballero LG, Carmona IT, Gonzalez MC, Posse JL, Taboada JL, Dios PD. Evaluation of the substantivity in saliva of different forms of application of chlorhexidine. Quintessence Int. 2009;40:141–144. [PubMed] [Google Scholar]
- 10.Willett TL, Labow RS, Avery NC, Lee JM. Increased proteolysis of collagen in an in vitro tensile overload tendon model. Ann Biomed Eng. 2007;35:1961–1972. doi: 10.1007/s10439-007-9375-x. [DOI] [PubMed] [Google Scholar]
- 11.Willett TL, Labow RS, Lee JM. Mechanical overload decreases the thermal stability of collagen in an in vitro tensile overload tendon model. J Orthop Res. 2008;26:1605–1610. doi: 10.1002/jor.20672. [DOI] [PubMed] [Google Scholar]
- 12.Pashley DH, Tay FR, Carvalho RM, Rueggeberg FA, Agee KA, Carrilho M, Donnelly A, García-Godoy F. From dry bonding to water-wet bonding to ethanol-wet bonding. A review of the interactions between dentin matrix and solvated resins using a macromodel of the hybrid layer. Am J Dent. 2007;20:7–20. [PubMed] [Google Scholar]
- 13.Sadek FT, Pashley DH, Nishitani Y, Carrilho MR, Donnelly A, Ferrari M, Tay FR. Application of hydrophobic resin adhesives to acid-etched dentin with an alternative wet bonding technique. J Biomed Mater Res A. 2008;84:19–29. doi: 10.1002/jbm.a.31290. [DOI] [PubMed] [Google Scholar]
- 14.Trebacz H, Wójtowicz K. Thermal stabilization of collagen molecules in bone tissue. Int J Biol Macromol. 2005;37:257–262. doi: 10.1016/j.ijbiomac.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Zhang S. Fabrication of novel biomaterials through molecular self-assembly. Nat Biotechnol. 2003;21:1171–1178. doi: 10.1038/nbt874. [DOI] [PubMed] [Google Scholar]
- 16.Bradt J-H, Mertig M, Teresiak A, Pompe W. Biomimetic mineralization of collagen by combined fibril assembly and calcium phosphate formation. Chem Mater. 1999;11:2694–2701. [Google Scholar]
- 17.Katz JL, Misra A, Spencer P, Wang Y, Bumrerraj S, Nomura T, Eppell SJ, Tabib-Azar M. Multiscale mechanics of hierarchical structure/property relationships in calcified tissues and tissue/material interfaces. Mater Sci Eng A Struct Mater. 2007;27:450–468. doi: 10.1016/j.msec.2006.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bertassoni LE, Habelitz S, Kinney JH, Marshall SJ, Marshall GW., Jr Biomechanical perspective on the remineralization of dentin. Caries Res. 2009;43:70–77. doi: 10.1159/000201593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tay FR, Pashley DH. Guided tissue remineralisation of partially demineralised human dentine. Biomaterials. 2008;29:1127–1137. doi: 10.1016/j.biomaterials.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Tay FR, Pashley DH, Rueggeberg FA, Loushine RJ, Weller RN. Calcium phosphate phase transformation produced by interaction of the Portland cement component of white MTA with a phosphate-containing fluid. J Endod. 2007;33:1347–51. doi: 10.1016/j.joen.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 21.Gajjeraman S, Narayanan K, Hao J, Qin C, George A. Matrix macromolecules in hard tissues control the nucleation and hierarchical assembly of hydroxyapatite. J Biol Chem. 2007;282:1193–1204. doi: 10.1074/jbc.M604732200. [DOI] [PubMed] [Google Scholar]
- 22.Xu A-W, Ma Y, Cölfen H. Biomimetic mineralization. J Mater Chem. 2007;17:415–449. [Google Scholar]
- 23.Tay FR, Pashley DH. Biomimetic remineralization of resin-bonded dentin. J Dent Res. doi: 10.1177/0022034509341826. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Angker L, Swain MV, Kilpatrick N. Micro-mechanical characterisation of the properties of primary tooth dentine. J Dent. 2003;31:261–267. doi: 10.1016/s0300-5712(03)00045-9. [DOI] [PubMed] [Google Scholar]
- 25.Hosoya Y, Marshall GW., Jr The nano-hardness and elastic modulus of carious and sound primary canine dentin. Oper Dent. 2004;29:142–149. [PubMed] [Google Scholar]
- 26.Kokubo T, Kushitani H, Sakka S, Kitsugi T, Yamamuro T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W. J Biomed Mater Res. 1990;24:721–734. doi: 10.1002/jbm.820240607. [DOI] [PubMed] [Google Scholar]
- 27.Meyer JL, Eanes ED. A thermodynamic analysis of the secondary transition in the spontaneous precipitation of calcium phosphate. Calcif Tissue Res. 1978;25:209–216. doi: 10.1007/BF02010771. [DOI] [PubMed] [Google Scholar]
- 28.Sauro S, Pashley DH, Mannocci F, Tay FR, Pilecki P, Sherriff M, Watson TF. Micropermeability of current self-etching and etch-and-rinse adhesives bonded to deep dentine: a comparison study using a double-staining/confocal microscopy technique. Eur J Oral Sci. 2008;116:184–193. doi: 10.1111/j.1600-0722.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- 29.Tay FR, Moulding KM, Pashley DH. Distribution of nanofillers from a simplified-step adhesive in acid-conditioned dentin. J Adhes Dent. 1999;1:103–117. [PubMed] [Google Scholar]
- 30.Sterrett JD, Murphy HJ. Citric acid burnishing of dentinal root surfaces. A scanning electron. doi: 10.1111/j.1600-051x.1989.tb01621.x. [DOI] [PubMed] [Google Scholar]
- 31.Sumikawa DA, Marshall GW, Gee L, Marshall SJ. Microstructure of primary tooth dentin. Pediatr Dent. 1999;21:439–444. [PubMed] [Google Scholar]
- 32.Hosoya Y, Tay FR. Hardness, elasticity, and ultrastructure of bonded sound and caries-affected primary tooth dentin. J Biomed Mater Res B Appl Biomater. 2007;81:135–141. doi: 10.1002/jbm.b.30646. [DOI] [PubMed] [Google Scholar]
- 33.Senawongse P, Harnirattisai C, Shimada Y, Tagami J. Effective bond strength of current adhesive systems on deciduous and permanent dentin. Oper Dent. 2004;29:196–202. [PubMed] [Google Scholar]
- 34.Uekusa S, Yamaguchi K, Miyazaki M, Tsubota K, Kurokawa H, Hosoya Y. Bonding efficacy of single-step self-etch systems to sound primary and permanent tooth dentin. Oper Dent. 2006;31:569–576. doi: 10.2341/05-102. [DOI] [PubMed] [Google Scholar]
- 35.Kronick PL, Cooke P. Thermal stabilization of collagen fibers by calcification. Connect Tissue Res. 1996;33:275–282. doi: 10.3109/03008209609028885. [DOI] [PubMed] [Google Scholar]
- 36.Sulkala M, Tervahartiala T, Sorsa T, Larmas M, Salo T, Tjäderhane L. Matrix metalloproteinase-8 (MMP-8) is the major collagenase in human dentin. Arch Oral Biol. 2007;52:121–127. doi: 10.1016/j.archoralbio.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 37.van der Veen MH, Tsuda H, Arends J, ten Bosch JJ. Evaluation of sodium fluorescein for quantitative diagnosis of root caries. J Dent Res. 1996;75:588–593. doi: 10.1177/00220345960750011201. [DOI] [PubMed] [Google Scholar]
- 38.Toroian D, Lim JE, Price PA. The size exclusion characteristics of type I collagen: implications for the role of noncollagenous bone constituents in mineralization. J Biol Chem. 2007;282:22437–22447. doi: 10.1074/jbc.M700591200. [DOI] [PubMed] [Google Scholar]
- 39.Cameron IL, Short NJ, Fullerton GD. Verification of simple hydration/dehydration methods to characterize multiple water compartments on tendon type 1 collagen. Cell Biol Int. 2007:531–539. doi: 10.1016/j.cellbi.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 40.Van Landuyt KL, Snauwaert J, Peumans M, De Munck J, Lambrechts P, Van Meerbeek B. The role of HEMA in one-step self-etch adhesives. Dent Mater. 2008;24:1412–1419. doi: 10.1016/j.dental.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 41.Eliades G, Vougiouklakis G, Palaghias G. Heterogeneous distribution of single-bottle adhesive monomers in the resin-dentin interdiffusion zone. Dent Mater. 2001;17:277–283. doi: 10.1016/s0109-5641(00)00082-8. [DOI] [PubMed] [Google Scholar]
- 42.Ye Q, Park JG, Topp E, Wang Y, Misra A, Spencer P. In vitro performance of nano-heterogeneous dentin adhesive. J Dent Res. 2008;87:829–833. doi: 10.1177/154405910808700911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hashimoto M, Fujita S, Kaga M, Yawaka Y. Effect of water on bonding of one-bottle self-etching adhesives. Dent Mater J. 2008;27:172–178. doi: 10.4012/dmj.27.172. [DOI] [PubMed] [Google Scholar]
- 44.Fullerton GD, Amurao MR. Evidence that collagen and tendon have monolayer water coverage in the native state. Cell Biol Int. 2006;30:56–65. doi: 10.1016/j.cellbi.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 45.Robertson RA, Elliot SP. The water content of bone: I. The mass of water, inorganic crystals, organic matrin and “CO2 space” components in a unit volume of dog bone. J Bone Joint Surg Am. 1957;39:167–188. [PubMed] [Google Scholar]
- 46.Eanes ED, Martin GN, Lundy DR. The distribution of water in calcified turkey leg tendon. Calcif Tissue Res. 1976;20:313–316. doi: 10.1007/BF02546418. [DOI] [PubMed] [Google Scholar]
- 47.Ito S, Saito T, Tay FR, Carvalho RM, Yoshiyama M, Pashley DH. Water content and apparent stiffness of non-caries versus caries-affected human dentin. J Biomed Mater Res B Appl Biomater. 2005;72:109–116. doi: 10.1002/jbm.b.30130. [DOI] [PubMed] [Google Scholar]
- 48.Chesnick IE, Mason JT, Giuseppetti AA, Eidelman N, Potter K. Magnetic resonance microscopy of collagen mineralization. Biophys J. 2008;95:2017–2026. doi: 10.1529/biophysj.107.120923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Olszta MJ, Odom DJ, Douglas EP, Gower LB. A new paradigm for biomineral formation: mineralization via an amorphous liquid-phase precursor. Connect Tissue Res. 2003;44(Suppl 1):326–334. [PubMed] [Google Scholar]
- 50.Fratzl P, Fratzl-Zelman N, Klaushofer K. Collagen packing and mineralization. An x-ray scattering investigation of turkey leg tendon. Biophys J. 1993;64:260–266. doi: 10.1016/S0006-3495(93)81362-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pashley DH, Nakabayashi N. Hybridization of dental hard tissues. Tokyo: Quintessence Publishing Co. Ltd; 1998. p. 66. [Google Scholar]
- 52.Ito S, Saito T, Tay FR, Carvalho RM, Yoshiyama M, Pashley DH. Water content and apparent stiffness of non-caries versus caries-affected human dentin. J Biomed Mater Res B Appl Biomater. 2005;72:109–116. doi: 10.1002/jbm.b.30130. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.