Abstract
Biomaterial scaffolds capable of localized gene delivery are being investigated for numerous regenerative medicine applications and as model systems for fundamental studies of tissue formation. In this manuscript, we investigate the delivery of lentivirus from a tissue engineering scaffold using a surface immobilization strategy. PLG was employed as the biomaterial for delivery, which has been widely used for a number of tissue engineering applications. The virus was immobilized by freezing and subsequent lyophilization of the virus with the scaffold. The presence of sucrose during freezing and lyophilization maintained the activity of the lentivirus, and was similar to an adenovirus control. Collagen and fibronectin were investigated for their ability to enhance surface immobilization. Fibronectin modestly increased binding and transduction of the adenovirus, yet did not significantly impact the lentivirus delivery. Most of the immobilized lentivirus was released from the scaffold within 24 hours. In vivo implantation of the scaffolds yielded transgene expression that persisted for at least 4 weeks. These findings indicate the potential for delivering lentivirus from tissue engineering scaffolds using a surface immobilization strategy. To our knowledge, this report is the first to investigate lentivirus delivery from porous tissue engineering scaffolds. Delivery of lentiviral vectors from PLG scaffolds could provide an efficient and versatile gene delivery system for use with in vitro and in vivo models of tissue formation, and ultimately for therapeutic applications.
Keywords: PLG, lentivirus, tissue-engineering scaffold, surface immobilization, extracellular matrix
Introduction
Biomaterial scaffolds capable of localized gene delivery are being investigated for numerous regenerative medicine applications and as model systems for fundamental studies of tissue formation. In many instances, vectors delivered from the scaffold in vivo target cells within the host tissue, which can subsequently serve as bioreactors for the localized production of tissue inductive factors. Vectors delivered from the scaffold typically encode for secreted growth factors, such as angiogenic or neurotrophic factors, or bone morphogenetic proteins (BMP) [1-3]. Since these factors are secreted into the local microenvironment, a modest number of cells expressing the transgene can stimulate numerous cells locally. However, an increasing number of vectors are targeting factors that work intracellularly, such as transcription factors [4, 5] or shRNA [6]. Vectors that target intracellular processes will stimulate only those cells expressing the transgene, and will thus likely require more efficient delivery that can increase the number of cells that express the transgene.
The mechanisms of gene delivery from scaffolds have been described as a polymeric release or surface immobilization. For the polymeric release systems, the vector is typically encapsulated in the material with the objective of obtaining a sustained release. This approach requires that the vector be stable throughout the processing steps, and has been generally applied to non-viral approaches such as naked plasmid, polyplexes, and lipoplexes [1, 7-10]. The alternative approach involves surface immobilization in which the scaffold is pre-fabricated and the vector is subsequently immobilized. This approach avoids exposure of the vector to the biomaterials processing steps, and may thus retain the vector activity. This approach has been employed widely with non-viral vectors, including naked plasmid [11], polyplexes [12], lipoplexes [12-14], and viral vectors such as adenovirus [15-18]. The immobilization strategies have involved non-specific interactions between the vector and material [9, 12, 15], layer by layer deposition [19], biotin-avidin conjugation of the vectors to the material [16, 20], and antibody binding of vectors [21]. Lentiviral vectors are emerging as a prominent research tool due to the stable and long term expression, the high transduction efficiency and the low immunogenicity [22], yet have not been employed for delivery from biomaterials.
In this report, we investigate the delivery of lentiviral vectors from biomaterial scaffolds composed of poly(lactide-co-glycolide) (PLG) using a surface immobilization strategy. PLG scaffolds have been widely used for numerous tissue engineering applications, such as bone and nerve regeneration and islet transplantation [11, 23, 24]. We investigated immobilization through non-specific binding of the virus to PLG or to extracellular matrix (ECM) coated PLG. To maximize surface association of the virus, vectors were dried onto the scaffold following lyophilization or freezing in the presence or cryoprotectants. Binding and release of the virus were characterized on PLG disks in vitro, and the conditions that maximized gene transfer were subsequently translated to microporous scaffolds for in vitro and in vivo testing. Delivery of lentiviral vectors from PLG scaffolds could provide an efficient and versatile gene delivery system for use with in vitro and in vivo models of tissue formation, and ultimately for therapeutic applications.
Methods
Virus Production
Lentivirus and adenovirus were prepared for the studies using established techniques. Lentivirus was produced in HEK-293T cells grown in DMEM plus 10% FBS at 37°C, and 5% CO2. The lentiviral packaging vectors (pMDL-GagPol, pRSV-Rev, pIVS-VSV-G), previously described by Dull et al. [25], were co-transfected along with plenti-CMV-GFP, plenti-CMV-βgal, or plenti-CMV-luciferase into 293T cells using Lipofectamine 2000 (Roche Biosciences, Palo Alto, CA, USA) After 48 hours of transfection, the supernatant was collected and filtered (0.45 micron). Viruses were then concentrated using PEG-it (System Biosciences, Mountain, CA, USA), with the precipitated lentiviruses resuspended with PBS or 1M-scurose PBS. The virus titer was determined by HIV-1 p24 Antigen ELISA Kit (ZeptoMetrix Co., Buffalo, USA). Recombinant adenovirus carrying GFP (rAd-GFP) was constructed using Transpos-Ad™ method (Qbiogene, Carlsbad, CA, USA) according to the manufacturer's protocol. Viruses were produced in HEK-293A cells and purified by ViraBind™ Adenovirus Purification Kit (Cell Biolabs, San Diego, CA, USA). The adenoviral titer was determined by TCID50 assay on HEK-293A cells.
PLG disks for virus immobilization
Disks were fabricated for in vitro studies that investigate virus activity. For disk fabrication, PLG (75:25 mole ratio of lactide to glycolide i.v.=0.6–0.8 dL/g) (Boehringer Ingelheim Chemical, Petersburg, VA) pellets were heated to 82°C and pressed into a flat disk using a 5 kg weight. The temperature was slowly decreased from 82°C to 37°C and the disks were placed at 37 °C overnight. Disks had a radius of approximately 1.5 cm and were stored at room temperature until use. For protein coating of PLG, disks were incubated with collagen (10 μg or 50 μg in 30 μL) or fibronectin (2 μg or 10 μg in 30 μL) and dried overnight. PLG or ECM-coated PLG disks were covered by 10 μL of virus solution (4×109 LP/ml in 1M sucrose-PBS) for 1 hour at 4°C and lyophilized for 24 hours.
Assay of active viruses
The activity of the virus was determined to investigate the effect of virus processing. Active virus was measured by counting tranduced cells. Samples were serially diluted (from a dilution of 10-1 to 10-6) in PBS and mixed with HEK-293T cells growing in a monolayer. At 3 days after infection, GFP positive cells were counted under the fluorescence microscope. Bioactivity of the surface-lyophilized virus on the PLG disk was assessed by measuring the GFP positive cell area on the disk at 2 days after cell seeding. Captured image of GFP-positive area were analyzed by Scion Image software (Scion, Frederick, MD. USA). Color images were then converted to gray scale and thresholded to mark the pixel area. Pixel number was counted to indicate the level of cell infection.
3D PLG scaffolds with immobilized virus
Polymer scaffolds were fabricated using a gas foaming, particulate leaching process. PLG was dissolved in dichloromethane to 6% (w/w) solution, which was then emulsified in 1% poly(vinyl alcohol) to create microspheres. The scaffold was constructed by mixing 1.5 mg of microspheres with 50 mg of NaCl (250 μm < d < 425 μm), and then compression molding the mixture in a 5 mm KBr die at 1500 psi using a Carver press. The scaffold was then equilibrated with high pressure CO2 gas (800 psi) for 16 h in a custom-made pressure vessel. Afterwards, the pressure was released over a period of 25 min, which serves to fuse adjacent microspheres creating a continuous polymer structure. To remove the salt, each scaffold was leached in 5 mL of water for 4 h with fresh water replacement after 2 h. Similar to the PLG disks, collagen (100 μg) or fibronectin (20 μg) were mixed with PLG scaffolds and dried overnight. PLG or ECM-coated PLG scaffold were mixed with 10 μL of virus solution (1011 LP/ml in 1M sucrose-PBS) for 1 hour at 4 °C and lyophilized for 24 hours. Release studies were performed by washing the scaffolds with PBS and subsequent immersion in a 24 well plate, which contained 500 μL of 10% FBS DMEM medium at 37 °C. The medium was sampled (10 μL) at 1, 4, 24 hr time points, serially diluted (from a dilution of 0 to 10-4), and infected to the HEK293T cells. At 3 days after infection, GFP positive cells were counted.
In vitro cell transduction on the 3D PLG scaffold was analyzed by seeding and culturing cells on PLG scaffolds with immobilized virus. Lentivirus (10 μL) expressing beta-galactosidase (lenti-βgal, 1011 LP/ml in 1M sucrose-PBS) were mixed with ECM (50 μg of collagen or 10 μg of fibronectin; PLG-collagen or PLG-fibronectin) and lyophilized with 3D PLG scaffold. Scaffolds were washed with PBS and 5×105 of 293 cells were seeded on the top of the scaffolds. The expression of beta-galactosidase was visualized at 3 days after transduction by staining with X-gal [26].
Bioluminescence imaging
Lentivirus expressing luciferase (lenti-Luc, 3×108 LP) was lyophilized on the surface of PLG scaffolds for 24 hours and implanted subcutaneously into male CD1 mice (20–22 g). In vivo luciferase expression was monitored using an IVIS imaging system (Xenogen Corp., Alameda, CA, USA), which includes a cooled CCD camera. For imaging, the animals were injected i.p. with D-luciferin (Molecular Therapeutics, Inc., MI, USA; 150 mg/kg body wt) using 28-gauge insulin syringes. The animals were placed in a light-tight chamber and bioluminescence images were acquired (every 5 min for a total of 20 min) until the peak light emission was confirmed. Gray-scale and bioluminescence images were superimposed using the Living Image software (Xenogen Corp.). A constant size region of interest (ROI) was drawn over the implantation site. The signal intensity was reported as an integrated light flux (photons/s), which was determined by IGOR software (WaveMetrics, OR, USA). Background photon fluxes were obtained using the same procedures prior to the injection of D-luciferin. Care and use of the laboratory animals followed the guidelines established by the Northwestern University Institutional Animal Care and Use Committee (IACUC).
Statistical analysis
The statistical significance of the differences between groups was analyzed by one-way ANOVA followed by Tukey's post hoc test for multiple comparisons. A level of P < 0.05 was accepted as significant.
Results
Lyophilization and viral activity
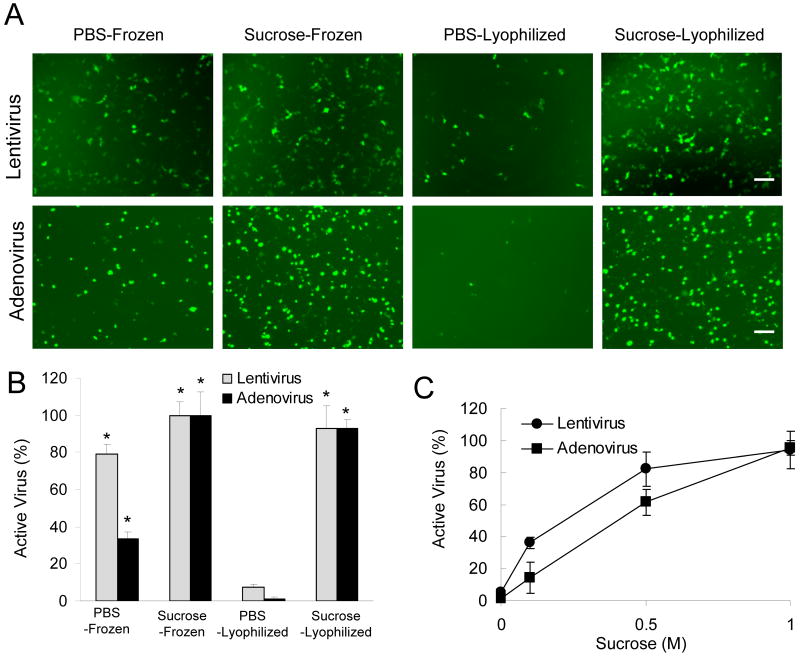
Initial studies investigated conditions to lyophilize viruses without loss of activity, which will be used in subsequent steps to promote association of the virus with the biomaterial surface. Sucrose was added to the solution as a cryoprotectant to maintain activity during the freezing and dehydration process. Viruses that express GFP (lenti-GFP, rAd-GFP) were frozen or lyophilized in PBS or 1M-sucrose PBS and the infectious activity were compared (Fig. 1A,B). The presence of sucrose during freezing relative to PBS alone significantly enhanced the virus activity for both lentivirus and adenovirus (p<0.01), though lentivirus retained approximately 80% activity without sucrose (Fig. 1B). Sucrose was essential during the lyophilization step, as less than 10% activity was maintained for lentivirus and adenovirus that was lyophilized without sucrose. Lyophilization in the presence of sucrose retained more than 90% of the activity for both adenovirus and lentivirus. The concentration of sucrose is critical to the virus stability (Fig. 1C), and the activity of adenovirus was more sensitive to the sucrose concentration compared to the lentivirus.
Fig. 1. Virus activity following lyophilization.
(A) Images of infected cells expressing GFP (lenti-GFP, rAd-GFP) for viruses that were frozen or lyophilized in 1M-sucrose. Bar represents 100 μm. The quantity of active virus expressed as a percentage of the titer of 1M-Sucrose PBS-Frozen control (B, C). The symbol * indicates statistical significance relative to PBS-Lyophilized group at p < 0.01.
Virus retention on PLG disks
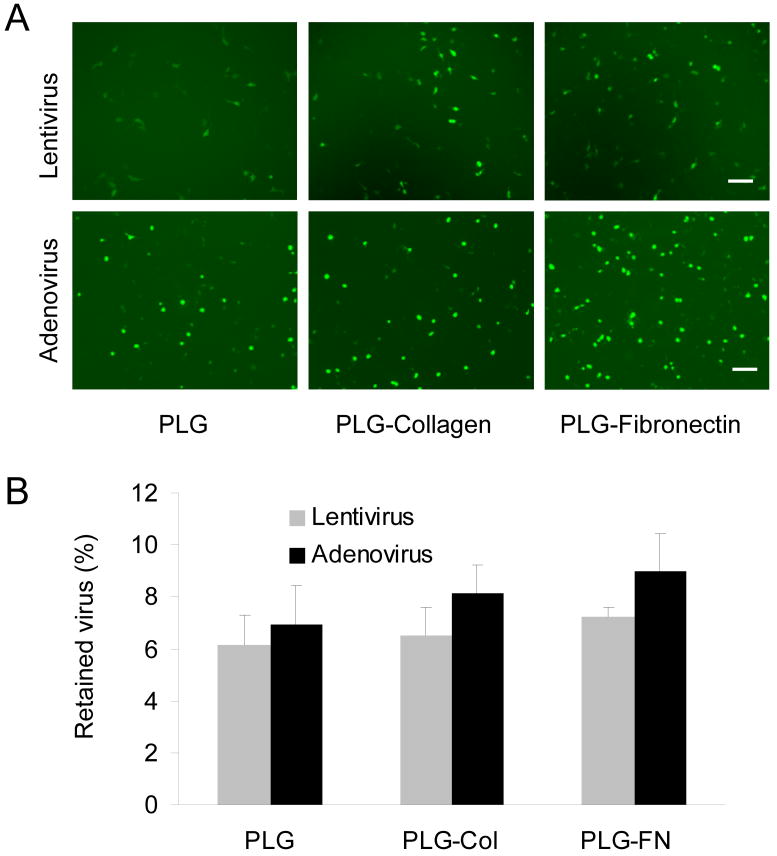
We subsequently investigated the lyophilization of virus onto PLG disks, with and without adsorbed extracellular matrix proteins, and determined the amount bound from the extent of GFP expression. Lyophilization onto the disks was performed with 1 M sucrose-PBS buffer, followed by cell seeding. The activity of virus on the surface was between 6% and 7% for the lentivirus and adenovirus (Fig. 2). PLG disks with collagen or fibronectin enhanced the amount of virus bound by approximately 1% to 2% respectively; however, these proteins did not significantly impact the binding of lentivirus.
Fig. 2. Virus retention 2D PLG disks.
(A) Image of infected cells expressing GFP for immobilization of Lenti-GFP (4×107 LP) and rAd-GFP (4×104 TCID50) in 1M sucrose-PBS buffer on PLG, with and without ECM modification. Bar represents 100 μm. (B) The amount of the retained virus was calculated by the GFP positive area. Values are mean ± SD.
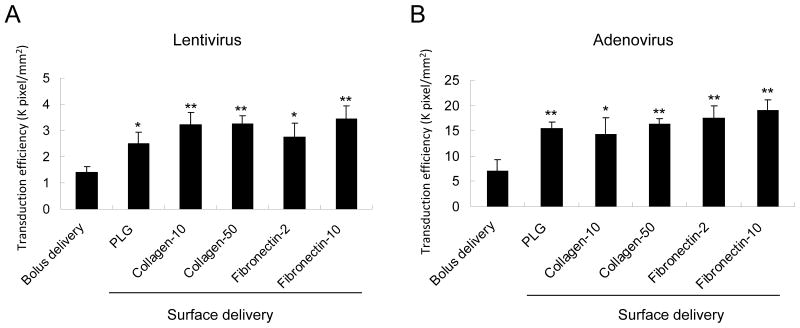
The transduction efficiency from the surface immobilized virus was subsequently compared to the transduction efficiency that can be obtained by the bolus addition of virus to the culture media, with the latter approach being the traditional method of transduction (Fig. 3). The transduced cell area of surface lyophilization was significantly increased by 1.8 times with lentivirus and 2.2 times with adenovirus compared to that of suspended virus (p<0.05). Different concentration and types of ECM coating has no significant effect on the transduction efficiency. These data suggest that lyophilized virus on PLG or ECM-coated PLG can enhance the transduction on the surface of PLG and effectively decrease the concentration of virus needed for transduction. The results with adenovirus are consistent with other reports for adenovirus immobilization to biomaterials [15]. The transduction efficiency achieved with lentivirus was approximately 5-fold less than that obtained with adenovirus; however, the results indicate that substantial activity of the lentivirus is retained, which has not been previously reported. All subsequent studies focus only on lentivirus delivery from PLG scaffolds.
Fig. 3. Transduction efficiency for surface and bolus delivery.
Transduction efficiency for PLG disks (0.32 cm2) coated with collagen (10 μg or 50 μg) or fibronectin (2 μg or 10 μg), with cells infected by bolus delivery (control) or following virus immobilization. Transduction efficiency was calculated by the pixels/mm2. The symbol * and ** indicate statistically significant differences relative to bolus viral delivery group at p < 0.05 and p< 0.01, respectively.
In vitro release and transduction from 3D microporous scaffold
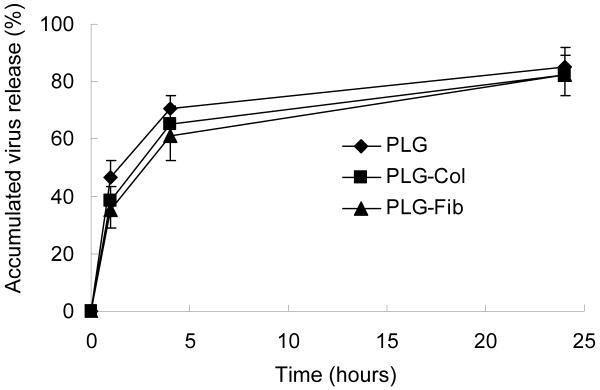
Lentivirus immobilization and transduction from 3D microporous scaffold was subsequent investigated. Lentivirus was lyophilized onto the scaffold, and the amount of virus released was characterized (Fig. 4). The percentage of virus release at 4 hours was 70.8%, 65.3%, and 61.3% for naked PLG, collagen-coated PLG, and fibronectin-coated PLG scaffold respectively. Within 24 hours, more than 80% of virus was released regardless of the scaffold condition. The rapid release from the scaffold was expected considering the relatively low retention efficiency on the 2D PLG disks.
Fig. 4. Virus release from 3D PLG scaffold.
Lentivirus encoding GFP(Lenti-GFP, 3 × 108 LP in 1M sucrose-PBS) were deposited alone, or following mixture with ECM (PLG-Col and PLG-Fib). Released virus was measured by GFP expression after incubation with 293 cells.
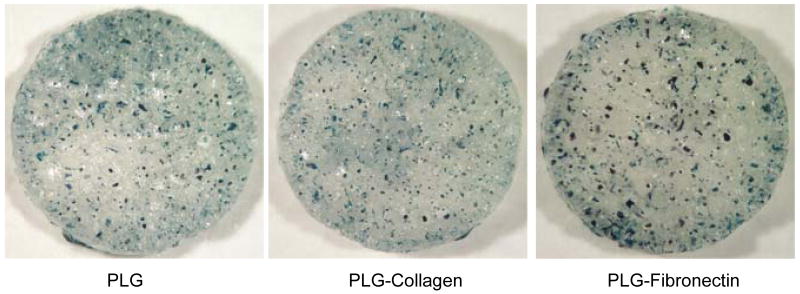
Cell transduction on the 3D PLG scaffold was investigated in vitro using lentiviruses expressing β-galactosidase. At 3 days after cell seeding, β-galactosidase expression was visualized to identify the distribution of transduced cells within the scaffold (Fig. 5). In PLG and ECM-coated PLG scaffold, cell transduction was observed throughout the PLG scaffold (Fig. 5). These data demonstrate that surface-lyophilized lentivirus provide effective and localized transgene expression on the surface of tissue engineering PLG scaffold.
Fig. 5. In vitro cell transduction on 3D PLG scaffold.
Transduction of 293 cells on scaffolds with deposited lentivirus enoding beta galactosdiase (Lenti-bgal, 3 × 108 LP in 1M sucrose-PBS). Lentivirus was deposited on (A) unmodified scaffold, and (B) collagen and (C) fibronectin modified scaffolds. X-gal staining was performed 3 days after cell seeding.
In vivo cell transduction on 3D PLG scaffold
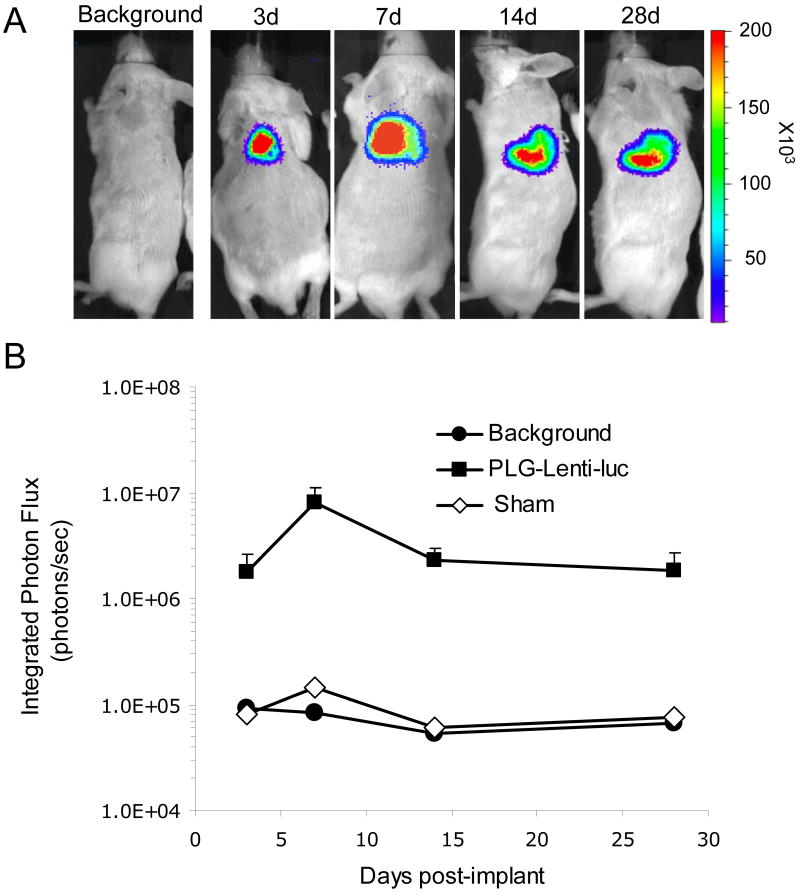
Lentivirus-lyophilized PLG scaffolds were then implanted to mice subcutaneously to investigate the ability to promote long term and localized expression in vivo. Bioluminescence imaging was employed to quantify luciferase expression following delivery of a lentivirus encoding for luciferase. Transgene expression was localized to the implantation site for all time points, indicating that expression at off-target sites was minimal (Fig. 6). Additionally, transgene expression persisted for at least 4 weeks in vivo and was consistent for all animals implanted. Taken together, these results indicate that lentivirus immobilized to microporous scaffolds may be a valuable tool to promote gene transfer in vivo.
Fig. 6. In vivo tranduction by lentivirus-lyophilized PLG scaffold.
(A) Bioluminescence imaging and quantification of firefly luciferase expression for 4 weeks following subcutaneous implantation of lentivirus immobilized PLG scaffolds. Lentivirus expressing luciferase (Lenti-luc, 3 × 108 LP) was lyophilized onto the unmodified PLG scaffold. (B) Integrated light flux (photons/sec) as measured using constant-size regions of interest over the implant site (n = 4 for day 3, n = 7 for day 7, n = 3 for day 14 and n = 3 for day 28 for experimental and background data). Scaffolds lyophilized with lentivirus expressing luciferase (■), background, (●), and sham operation (◊, n = 1). Values are mean ± S.E.M.
Discussion
In this manuscript, we investigate the delivery of lentivirus and adenovirus from a tissue engineering scaffold using a surface immobilization strategy. Drying of viral and non-viral fectors onto biomaterial surfaces has been employed to maximize surface immobilization [9, 15, 27]. For lyophilization onto the surface, the freezing and dehydration processes can significantly decrease the activity of the vectors, and thus cryoprotectants are used. Sucrose is a commonly used stabilizer that is able to maintain the activity of proteins, and viral and non-viral vectors during freezing and dehydration. Lyophilization has been examined as an alternative pathogen inactivation procedure [28], but under the sucrose formulation, it has been used to preserve the viral activity of adenovirus and adeno-associated virus for a long period [29]. For both adenovirus and lentivirus, concentrations of 0.5 M retain approximately 80% of the virus activity, with 1 M retaining more than 95% of the activity. Sucrose concentrations on the order of 1M have been previously used with adenovirus [15].
Surface immobilization can be performed with retention of activity; however, the amount of immobilized vector is relatively low on PLG. The low amount of immobilized lentivirus indicates a relatively low affinity for the material, which is also consistent with near complete release from the scaffold within 24 hours. Adenovirus binding to naked PLG was similar to the lentivirus. Previous studies using adenovirus immobilization to hydroxyapatite disks (HA) indicated that greater than 30% of the virus remained on the material for up to 16 hours, indicating that the PLG surface is less efficient at binding adenovirus than HA. We investigated the inclusion of extracellular matrix proteins to increase binding of the virus, as some viruses have previously been shown to associate with specific ECM proteins [30]. Additionally, ECM proteins have enhanced the binding and delivery of several non-viral vectors through non-specific interactions [12, 31]. Adenovirus binding was enhanced by 50% in the presence of ECM proteins, though binding remained relatively low (12%), and lentivirus binding to the ECM modified surfaces was similar to the unmodified surfaces. Alternative strategies may facilitate virus immobilization to surfaces, such as biotinylation of the virus for binding to avidin modified surfaces [32] or the modification of the materials with antibodies to the virus [17]. These approaches have the potential to enhance surface binding and subsequent delivery, yet require more sophisticated modifications to either the vector or the material.
Although the quantity of immobilized vector is low, the high efficiency of the vector enables substantial transgene expression that persists for at least 4 weeks in vivo. The expression level and duration is consistent with previous reports of adenovirus delivery from biomaterial scaffolds [15]. Lentivirus is an integrating virus, thus expression is expected to persist for long-times; however, long term expression has been observed at this site without integration. Subcutaneous delivery of naked plasmid from the scaffolds has produced transgene expression that persisted for 105 days, which does not likely occur by integration [1]. However, the duration of expression observed with naked plasmid delivery at the subcutaneous site has not extended to other sites, such as the intraperitoneal fat or spinal cord [10, 24]. Interestingly, the expression levels with lentivirus delivery were between 2 and 10-fold higher than that obtained by delivering hundreds of micrograms of naked plasmid at the same site. Thus, the expression levels are similar to or greater with the lentivirus delivery using substantially less vector.
For implantation of porous scaffolds, we have previously demonstrated that transgene expression occurs immediately adjacent to the scaffold and within the scaffold [10]. Consistent with these results, the in vivo imaging studies indicate that expression is localized to the implant site. The cell types typically targeted by the gene delivery are those involved in the response to the injury created by implantation and the foreign body (i.e., the scaffold). Our previous studies with non-viral vectors indicated that macrophages were the primary cell type that expressed the transgene [10], whereas others delivering non-viral vectors have demonstrated that endothelial cells are transfected [33].
In conclusion, we demonstrate the ability to deliver lentivirus from porous PLG scaffolds in vitro and in vivo using a surface immobilization strategy. The binding efficiency of the lentivirus is relatively low and unaffected by the presence of either collagen or fibronectin. Though the quantity immobilized is low, the activity remains high and is able to transfect large number of cells in vitro and to produce significant and long-term expression in vivo. To our knowledge, this report is the first to investigate lentivirus delivery from a tissue engineering scaffold. Lentivirus is widely used as a research tool due to their efficiency and broad tropism. Additionally, libraries of genes and shRNA sequences in lentiviral constructs are commercially available, which in combination with the broad tropism and efficiency of the lentivirus, can facilitate studies of tissue formation that may ultimately provide therapeutic strategies.
Acknowledgments
Financial support for this research was provided by NIH RO1 EB005678, R21 EB006520, and RO1 EB003806.
References
- 1.Jang JH, Rives CB, Shea LD. Plasmid delivery in vivo from porous tissue-engineering scaffolds: transgene expression and cellular transfection. Mol Ther. 2005;12(3):475–83. doi: 10.1016/j.ymthe.2005.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Laporte L, Shea LD. Matrices and scaffolds for DNA delivery in tissue engineering. Adv Drug Deliv Rev. 2007;59(4-5):292–307. doi: 10.1016/j.addr.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang YC, et al. Bone regeneration in a rat cranial defect with delivery of PEI-condensed plasmid DNA encoding for bone morphogenetic protein-4 (BMP-4) Gene Ther. 2005;12(5):418–26. doi: 10.1038/sj.gt.3302439. [DOI] [PubMed] [Google Scholar]
- 4.Trentin D, et al. Peptide-matrix-mediated gene transfer of an oxygen-insensitive hypoxia-inducible factor-1alpha variant for local induction of angiogenesis. Proc Natl Acad Sci U S A. 2006;103(8):2506–11. doi: 10.1073/pnas.0505964102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phillips JE, et al. Engineering graded tissue interfaces. Proc Natl Acad Sci U S A. 2008;105(34):12170–5. doi: 10.1073/pnas.0801988105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheema SK, et al. Regulation and guidance of cell behavior for tissue regeneration via the siRNA mechanism. Wound Repair Regen. 2007;15(3):286–95. doi: 10.1111/j.1524-475X.2007.00228.x. [DOI] [PubMed] [Google Scholar]
- 7.Whittlesey KJ, Shea LD. Nerve growth factor expression by PLG-mediated lipofection. Biomaterials. 2006;27(11):2477–86. doi: 10.1016/j.biomaterials.2005.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang YC, et al. Long-term in vivo gene expression via delivery of PEI-DNA condensates from porous polymer scaffolds. Hum Gene Ther. 2005;16(5):609–17. doi: 10.1089/hum.2005.16.609. [DOI] [PubMed] [Google Scholar]
- 9.Jang JH, et al. Surface adsorption of DNA to tissue engineering scaffolds for efficient gene delivery. J Biomed Mater Res A. 2006;77(1):50–8. doi: 10.1002/jbm.a.30643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rives CB, et al. Layered PLG scaffolds for in vivo plasmid delivery. Biomaterials. 2009;30(3):394–401. doi: 10.1016/j.biomaterials.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Laporte L, Cruz Rea J, Shea LD. Design of modular non-viral gene therapy vectors. Biomaterials. 2006;27(7):947–54. doi: 10.1016/j.biomaterials.2005.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bengali Z, et al. Gene delivery through cell culture substrate adsorbed DNA complexes. Biotechnol Bioeng. 2005;90(3):290–302. doi: 10.1002/bit.20393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bengali Z, et al. Efficacy of immobilized polyplexes and lipoplexes for substrate-mediated gene delivery. Biotechnol Bioeng. 2008 doi: 10.1002/bit.22212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pannier AK, Anderson BC, Shea LD. Substrate-mediated delivery from self-assembled monolayers: effect of surface ionization, hydrophilicity, and patterning. Acta Biomater. 2005;1(5):511–22. doi: 10.1016/j.actbio.2005.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu WW, et al. Localized viral vector delivery to enhance in situ regenerative gene therapy. Gene Ther. 2007;14(11):891–901. doi: 10.1038/sj.gt.3302940. [DOI] [PubMed] [Google Scholar]
- 16.Hu WW, Lang MW, Krebsbach PH. Development of adenovirus immobilization strategies for in situ gene therapy. J Gene Med. 2008;10(10):1102–12. doi: 10.1002/jgm.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levy RJ, et al. Localized adenovirus gene delivery using antiviral IgG complexation. Gene Ther. 2001;8(9):659–67. doi: 10.1038/sj.gt.3301452. [DOI] [PubMed] [Google Scholar]
- 18.Fishbein I, et al. Local delivery of gene vectors from bare-metal stents by use of a biodegradable synthetic complex inhibits in-stent restenosis in rat carotid arteries. Circulation. 2008;117(16):2096–103. doi: 10.1161/CIRCULATIONAHA.107.746412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jewell CM, Lynn DM. Multilayered polyelectrolyte assemblies as platforms for the delivery of DNA and other nucleic acid-based therapeutics. Adv Drug Deliv Rev. 2008;60(9):979–99. doi: 10.1016/j.addr.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Segura T, et al. Crosslinked hyaluronic acid hydrogels: a strategy to functionalize and pattern. Biomaterials. 2005;26(4):359–71. doi: 10.1016/j.biomaterials.2004.02.067. [DOI] [PubMed] [Google Scholar]
- 21.Jin X, et al. Immobilization of plasmid DNA on an anti-DNA antibody modified coronary stent for intravascular site-specific gene therapy. J Gene Med. 2008;10(4):421–9. doi: 10.1002/jgm.1165. [DOI] [PubMed] [Google Scholar]
- 22.Bennett J. Immune response following intraocular delivery of recombinant viral vectors. Gene Ther. 2003;10(11):977–82. doi: 10.1038/sj.gt.3302030. [DOI] [PubMed] [Google Scholar]
- 23.Salvay DM, et al. Extracellular matrix protein-coated scaffolds promote the reversal of diabetes after extrahepatic islet transplantation. Transplantation. 2008;85(10):1456–64. doi: 10.1097/TP.0b013e31816fc0ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laporte LD, et al. Plasmid Releasing Multiple Channel Bridges for Transgene Expression After Spinal Cord Injury. Mol Ther. 2008 doi: 10.1038/mt.2008.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dull T, et al. A third-generation lentivirus vector with a conditional packaging system. J Virol. 1998;72(11):8463–71. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bondi A, et al. The use of beta-galactosidase as a tracer in immunocytochemistry. Histochemistry. 1982;76(2):153–8. doi: 10.1007/BF00501918. [DOI] [PubMed] [Google Scholar]
- 27.De Laporte L, Lei Yan A, Shea LD. Local gene delivery from ECM-coated poly(lactide-co-glycolide) multiple channel bridges after spinal cord injury. Biomaterials. 2009 doi: 10.1016/j.biomaterials.2008.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uhlenhaut C, et al. Effects of lyophilization on the infectivity of enveloped and non-enveloped viruses in bone tissue. Biomaterials. 2005;26(33):6558–64. doi: 10.1016/j.biomaterials.2005.04.049. [DOI] [PubMed] [Google Scholar]
- 29.Croyle MA, Cheng X, Wilson JM. Development of formulations that enhance physical stability of viral vectors for gene therapy. Gene Ther. 2001;8(17):1281–90. doi: 10.1038/sj.gt.3301527. [DOI] [PubMed] [Google Scholar]
- 30.Bajaj B, Lei P, Andreadis ST. High efficiencies of gene transfer with immobilized recombinant retrovirus: kinetics and optimization. Biotechnol Prog. 2001;17(4):587–96. doi: 10.1021/bp010039n. [DOI] [PubMed] [Google Scholar]
- 31.Bengali Z, Rea JC, Shea LD. Gene expression and internalization following vector adsorption to immobilized proteins: dependence on protein identity and density. J Gene Med. 2007;9(8):668–78. doi: 10.1002/jgm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parrott MB, et al. Metabolically biotinylated adenovirus for cell targeting, ligand screening, and vector purification. Mol Ther. 2003;8(4):688–700. doi: 10.1016/s1525-0016(03)00213-2. [DOI] [PubMed] [Google Scholar]
- 33.Riddle KW, et al. Modifying the proliferative state of target cells to control DNA expression and identifying cell types transfected in vivo. Mol Ther. 2007;15(2):361–8. doi: 10.1038/sj.mt.6300017. [DOI] [PubMed] [Google Scholar]