Abstract
The hypothalamus is a prominent target of nicotine action. We have previously shown that acute systemic nicotine treatment induces Fos expression in the lateral hypothalamus and perifornical area (LH/PFA), with orexin/hypocretin neurons being particularly responsive. However, the neurochemical correlates of acute nicotine treatment in the LH/PFA have not been described. Anatomical studies have revealed that this area receives afferents from cholinergic, glutamatergic and GABAergic telencephalic brain regions, suggesting a potential role for these neurotransmitters in mediating the hypothalamic component of nicotine effects on homeostatic phenomena such as arousal and appetite. Here, we used in vivo microdialysis to determine the effect of acute systemic or local nicotine on glutamate, acetylcholine and GABA efflux in the LH/PFA of rats. Local administration of nicotine significantly increased ACh and glutamate, but not GABA, in the LH/PFA. Thus, we further tested the role of afferent sources of glutamate and acetylcholine in mediating acute nicotine-induced activation of orexin neurons by unilaterally lesioning the prefrontal cortex or basal forebrain cholinergic regions. Lesioned animals showed reduced Fos-positive orexin neurons following nicotine treatment. These data suggest that both ACh and glutamate may mediate the effects of acute nicotine on the activity of hypothalamic neurons, including orexin/hypocretin cells. Changes in cholinergic or glutamatergic transmission in this region with chronic nicotine may contribute to long-term alterations in functions mediated by LH/PFA neurons, including feeding and arousal.
Keywords: nicotine, hypothalamus, microdialysis, acetylcholine, orexin, hypocretin
Introduction
The hypothalamus is a prominent central site of nicotine action that may mediate the effects of this drug on endocrine, circadian, reward and ingestive parameters (O'Hara et al. 1998; Miyata et al. 1999; Meguid et al. 2000; Harrison et al. 2002; Jo et al. 2002). Acute nicotine administration increases expression of the immediate-early gene product, Fos, in several hypothalamic regions (Ren and Sagar 1992), with the orexin/hypocretin population of neurons in the lateral hypothalamus and contiguous perifornical area (LH/PFA) appearing to be particularly sensitive to this drug (Pasumarthi et al. 2006). Furthermore, chronic nicotine treatment upregulates expression of orexin peptides and receptors (Kane et al. 2000). Combined with evidence that orexin transmission is required for the maintenance of nicotine self-administration (Hollander et al. 2008), these data suggest a prominent role for the orexin system in mediating many behavioral and physiological effects of systemic nicotine (Corrigall 2009).
Nicotine effects on neuronal populations in the lateral hypothalamus are likely mediated by stimulation of neurotransmitter release from axon terminals located in this area. Dopamine plays a crucial role in the reinforcing effects of nicotine (Corrigall 1991) and it has been reported that systemic nicotine increases dopamine release in the lateral hypothalamus (Zhang et al. 2001). However, dopamine is unlikely to mediate all effects of nicotine in the LH/PFA, particularly considering that few dopamine receptor-expressing neurons are found in this region (Bubser et al. 2005). Anatomical and functional studies suggest several other neurotransmitter candidates for mediating nicotine effects in the LH/PFA. For example, neurons in this area receive cholinergic inputs from magnocellular basal forebrain neurons (Jo et al. 2005; Sakurai et al. 2005; Yoshida et al. 2006) and ACh depolarizes orexin cells (Yamanaka et al. 2003). Extrinsic glutamatergic inputs to the LH/PFA originate from several sources, including the prefrontal cortex, the amygdala and the paraventricular nucleus of the thalamus (Sakurai et al. 2005). Nicotinic acetylcholine receptor (nAChR) expression has also been reported on glutamatergic terminals in the hypothalamus (Perez de la Mora et al. 1991; Yoshida et al. 2006). Furthermore, local circuit glutamate neurons in the LH/PFA may be essential for recruitment of networks of orexin neurons (Li et al. 2002). Finally, GABAergic inputs to the LH/PFA arise from several brain regions that may be important for nicotine effects on arousal and appetite, including the nucleus accumbens (Mogenson et al. 1983; Stratford and Kelley 1999) and central amygdala (Nakamura et al. 2009). Collectively, these three neurotransmitters are likely to mediate many of the effects of acute nicotine on arousal and appetite via the LH/PFA. Thus, the aim of the current study was to use in vivo microdialysis to provide the first description of the effect of systemic or local nicotine administration on ACh, glutamate and GABA levels in the LH/PFA. In addition, in order to test the extent to which nicotine effects on orexin neurons in this region are dependent on some major extrinsic sources of glutamatergic or cholinergic input to the LH/PFA, we lesioned the PFC and basal forebrain cholinergic system and measured nicotine-elicited Fos expression in orexin neurons.
Methods and Materials
Animals and surgery
Male Sprague-Dawley rats (Harlan Laboratories, Birmingham, AL), weighing approximately 250-300g (at the time of arrival) were used for all experiments. All animals were pair-housed in an environmentally controlled animal facility on a 12:12 h light: dark cycle with lights on at 07:00. Purina rat chow and water were available ad libitum. Animals were handled daily for at least one week prior to experimentation. All experiments were conducted during the light phase, beginning at least 2 hours after light phase onset and concluding at least 2 hours prior to the beginning of the dark phase. Animal care and use procedures were carried out in accordance with protocols written under the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institution Animal Care and Use Committee at the University of South Carolina.
All animals (n=6/treatment) were anesthetized using sodium pentobarbital and then a single microdialysis guide cannula (Bioanalytical Systems, Inc., West Lafayette, IN, USA) was placed into the LH/PFA using the following coordinates relative to bregma: AP -3.1 mm, L +1.4 mm, DV -7.2 mm from skull surface. The microdialysis cannula assembly was anchored to the skull with 2-3 jewelers' screws and dental cement. The hemisphere of cannula placement was counterbalanced so that each hemisphere was equally represented in the different treatment conditions. At the onset of emergence from anesthesia (as indicated by attempts at locomotion), each animal was given a single dose of analgesic (buprenorphine; 0.2 mg/kg, s.c.; Sigma, Inc., St. Louis, USA). Following surgery, all animals were allowed 3 to 4 recovery days, during which the animals were handled and habituated to the microdialysis environment.
Microdialysis
On the morning of each microdialysis session, the stylets were removed from the guide cannulas and replaced with microdialysis probes (Bioanalytical Systems) with a semipermeable membrane extending 2 mm beyond the ventral tip of the guide cannula. The probes were then continuously perfused at 2.0 μl/min with an artificial cerebrospinal fluid (aCSF; pH 6.9) composed of the following: NaCl 150 mM, KCl 3.0 mM, CaCl2 1.7 mM, MgCl2 0.9 mM, D-glucose 4.9 mM. The acetylcholinesterase inhibitor neostigmine bromide (100 nM; Sigma) was included in the perfusate to promote reliable recovery of detectable levels of ACh. Collection of dialysates (15 min each) began three hours following probe insertion to allow for stabilization of baseline ACh, glutamate and GABA efflux. Immediately upon collection, each dialysate was split (15 μl each) for separate analysis of ACh and amino acids and placed on dry ice and stored at -80°C until analysis. After the fourth baseline collection, each animal was treated with nicotine either locally or systemically. For local nicotine treatment the inlet line of the microdialysis probe was switched to another syringe containing one of the following: plain aCSF (VEH), 100 μM or 2.0 mM freebase nicotine (Sigma). These nicotine concentrations are within the range used in previous in vivo or in vitro studies on the effects of local administration of nicotine on ACh(Summers and Giacobini 1995), glutamate (Fedele et al. 1998)and GABA (Lu et al. 1998) release from other brain areas. Four additional dialysates were collected and the inlet line was switched back to plain aCSF. Again, four additional dialysates were collected.
For systemic nicotine treatment a single systemic (i.p.) injection of either vehicle (saline) or nicotine (0.4 mg/kg or 2.0 mg/kg; dose expressed as hydrogen tartrate salt; Sigma) was administered and seven additional dialysates were collected. We have previously shown that these systemic nicotine doses induce Fos expression in neurons (including orexin cells) of the LH/PFA (Pasumarthi et al. 2006).
All animals underwent three microdialysis sessions (with an off day between sessions) and received a different nicotine dose in each session. There was no crossover between systemic and local nicotine treatment groups. The order of the sessions (VEH; low nicotine; high nicotine) was counterbalanced across animals.
Neurochemical analysis
Acetylcholine dialysates were analyzed using HPLC with electrochemical detection as described earlier (Fadel et al. 2001; Fadel et al. 2005). Peaks corresponding to ACh were quantitated by comparison with a three point external standard curve bounding the expected range of ACh values. The detection limit for ACh by this method was approximately 10 fmol/injection. Basal ACh efflux for each animal in each session was defined as the median of the baseline (pre-drug) values and expressed as pmol ACh/15 μl dialysate.
Glutamate and GABA dialysates were also analyzed by HPLC with electrochemical detection using a pre-column o-phthaldialdehyde/sulfite derivatization procedure (Donzanti and Yamamoto 1988; Rowley et al. 1995; Burrows et al. 2000; Reznikov et al. 2007). Quantification was accomplished by comparison to a daily 3-point standard curve encompassing the expected range of microdialysate glutamate and GABA concentrations. Basal glutamate and GABA efflux for each animal in each session were calculated as the median of the baseline values. All efflux data was then expressed as a percent of basal efflux.
Lesion experiments
General surgical procedures (anesthesia, analgesia, etc.) were as described above. For lesioning of the medial PFC, 1.0 μl of either vehicle (aCSF; n=6/treatment) or ibotenic acid (0.5 μg/μl; n=9/treatment) was infused at a flow rate of 0.1 μl/min unilaterally into PFC at the following coordinates relative to bregma: AP +3.0 mm, ML +0.6 mm, DV -4.0 mm. Animals undergoing basal forebrain cholinergic lesions received unilateral infusions of 0.5 μl of vehicle (PBS; n=6/treatment) or the selective cholinotoxin 192 IgG-saporin (0.325μg/μl; n=9/treatment) into the basal forebrain at the coordinates: AP -0.6, ML -2.4, DV -7.6 mm.
Ten days later, each animal was injected (ip) with vehicle (saline; n = 6/treatment) or nicotine (2.0 mg/kg; n = 9/treatment) in a volume of 1.0 ml/kg. Two hours after the injection animals were anesthetized, transcardially perfused and their brains processed for double-label immunohistochemistry (Fos plus OxA) as described below.
Histology
Following the final microdialysis session, animals were deeply anesthetized using isofluorane and transcardially perfused with cold phosphate-buffered saline followed by 4% paraformaldehyde. The brains were collected, post-fixed overnight and transferred to 30% sucrose in 0.1 M phosphate buffer for cryoprotection. Coronal sections (45 μm each) through each region of interest were collected on a cryostat.
Given our interests in mechanisms of nicotine-induced activation of orexin neurons, we combined immunohistochemistry for OxA with an acetylcholinesterase background stain on serial coronal sections through the rostro-caudal extent of the hypothalamus to verify microdialysis probe placement within the orexin-containing region of the LH/PFA. Briefly, all the sections were incubated with a rabbit anti-OxA antibody (1:3000; Calbiochem) for 48 h at 4°C or overnight at room temperature, followed by another incubation in biotinylated donkey anti-rabbit secondary for 1.5 h at room temperature (1:1000; Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA) and then in horseradish peroxidase-conjugated streptavidin (1:1600; Jackson) for 1 h at room temperature. OxA-like immunoreactivity was visualized by developing the sections in a nickel sulfate-cobalt chloride intensified DAB solution with hydrogen peroxide, yielding blue-black OxA immunoreactive cell bodies and fibers. Sections were then mounted onto slides using 0.15% gelatin, dried overnight and processed for acetylcholinesterase background staining. After coverslipping and drying, sections were viewed with a Nikon E600 microscope equipped with a Photometrics color digital camera (Roper Scientific, Trenton, NJ, USA). Photomicrographic images were captured with IP Lab Software and imported into Adobe Photoshop where minor adjustments for contrast and brightness were made.
For lesioning studies, assessment of nicotine-elicited Fos expression in orexin neurons was performed by double-label immunohistochemistry (Fos plus OxA) on serial coronal sections through the rostro-caudal extent of the hypothalamus. Briefly, all sections were initially incubated with a goat anti-Fos antibody (1:3000; 48 h at 4°C; Santa Cruz Biotechnology, Santa Cruz, CA) followed by a biotinylated donkey anti-goat secondary (1:1000; 1.5 h at room temperature; Jackson ImmunoResearch Laboratories Inc., West Grove, PA) and horseradish peroxidase-conjugated streptavidin (1:1600; 1 h at room temperature; Jackson). Fos immunoreactivity was visualized by developing the sections in a nickel sulfate-cobalt chloride intensified DAB solution with 0.3% H2O2, yielding a blue-black reaction product confined to the nucleus of Fos-positive cells. Sections were then incubated in rabbit primary antisera directed against OxA (1:3000; at 4° C for 48 h Oncogene Research Products, Cambridge, MA), followed by unlabeled donkey anti-rabbit secondary antibody (1:100; at room temperature for 2 h; Jackson) and rabbit peroxidase anti-peroxidase (1:250; at room temperature for 1.5 h; Sternberger Monoclonals, Lutherville, MD). Orexin immunoreactivity was visualized by developing the sections in a plain DAB solution with 3.0% H2O2, resulting in light brown cytoplasmic staining of orexin-positive neurons.
Verification of lesion
Sections with PFC in either treatment group were subjected to Nissl staining to assess the location and extent of the ibotenic acid lesions, as indicated by morphology of pyramidal cells and a localized pattern of neuronal loss and infiltration of glial cells. Briefly, mounted sections were immersed in cresyl violet solution for approximately 2 min, dehydrated, cover slipped and viewed under a light microscope. Animals with lesions outside the boundaries of the medial PFC or that crossed the midline to the contralateral hemisphere were excluded from analysis. Animals included in the analysis had lesions centered primarily in the prelimbic cortex, with some involvement of adjacent anterior cingulate and infralimbic areas.
Basal forebrain cholinergic cell loss following 192 IgG-saporin was assessed by single-label choline acetyltransferase (ChAT) immunohistochemistry. Briefly, all sections were initially incubated with a goat anti-ChAT antibody (1:3000; 48 h at 4°C; Chemicon, Temecula, CA), followed by a biotinylated donkey anti-goat secondary (1:1000; 1.5 h at room temperature; Jackson ImmunoResearch Laboratories Inc., West Grove, PA) and horseradish peroxidase-conjugated streptavidin (1:1600; 1 h at room temperature; Jackson). ChAT immunoreactivity was visualized by developing the sections in a nickel sulfate/cobalt chloride-intensified DAB solution with 0.3% H2O2, yielding blue-black ChAT-positive somata and proximal dendrites.
Finally, the effect of both lesion manipulations on glutamatergic or cholinergic innervation of the LH/PFA was assessed in a subset of animals (N = 4/lesion condition) by double-label immunohistochemistry. For glutamatergic input from the PFC, hypothalamic sections from ibotenic acid-lesioned and sham-lesioned rats were first incubated with an antibody to the type-1 vesicular glutamate transporter (guinea pig anti-vGlut1; 1:1000; Millipore, Inc.) followed by a biotinylated donkey-anti-guinea pig secondary antibody and horseradish peroxidase/streptavidin as described above. Development with nickel/cobalt-enhanced DAB yielded blue-black vGlut1-immunoreactive varicosities and fibers. vGlut1 was chosen as a marker for PFC lesion effects on glutamatergic innervation of the LH/PFA because this appears to be the predominant vesicular glutamate transporter species expressed in cortical projection neurons (Omelchenko and Sesack 2007). For cholinergic input from the basal forebrain, hypothalamic sections from 192 IgG saporin-lesioned and sham-lesioned rats were first incubated with an antibody to the vesicular acetylcholine transporter (goat anti-vAChT; 1:6000; Phoenix Pharmaceuticals, Inc.) followed by a biotinylated donkey-anti-goat secondary antibody and horseradish peroxidase/streptavidin as described above. As with vGlut1, development with nickel/cobalt-enhanced DAB yielded blue-black vAChT-immunoreactive varicosities and fibers. Following development for vGlut1 or vAChT, all sections were double-labeled for orexin using the peroxidase anti-peroxidase method and plain DAB, yielding light-brown orexin-immunoreactive somata as described above.
In order to obtain a quantitative assessment of the effect of the lesion manipulations on glutamatergic or cholinergic innervations of the LH/PFA, double-labeled sections from the injected hemisphere of sham or lesioned animals were viewed initially at low magnification (4×) and a random sampling of 20 orexin neurons from two different rostro-caudal levels of the LH/PFA was selected. At 40× magnification, points of apparent appositional contact between vGlut1- or vAChT-immunoreactive varicosities and orexin-immunoreactive somata or proximal dendrites were quantitated by systematically varying the stage-objective distance and counting points where both markers were present in the same focal plane with no visible space between them (see Figure 7).
Figure 7.
Effect of PFC ibotenic acid or basal forebrain 192 IgG-saporin lesions on vGlut1 or vAChT immunoreactivity in the LH/PFA. A. Ibotenic acid lesions of the PFC reduced the number of apparent appositions between vGlut1-immunoreactive varicosities and orexin neurons. B & C. Double-label immunohistochemistry for vGlut1 (dark puncta) and orexin A (light brown cell somata) from a sham (B) or ibotenic acid-treated rat (C). Arrows indicate points of apparent appositional contact. D. The cholinergic immunotoxin 192 IgG-saporin in the basal forebrain reduced the number of apparent appositions between vAChT-immunoreactive varicosities and orexin neurons. E & F. Double-label immunohistochemistry for vAChT (dark puncta) and orexin neurons (light brown somata) from a sham (E) or 192 IgG-saporin-treated (F) rat. Arrows indicate apparent appositional contacts. Scale bars, approximately 20 μm. *P < 0.05 vs. sham-lesioned group.
Statistics
Basal efflux data were expressed as pmol/15 μl dialysate (ACh) or μM (glutamate and GABA) and subjected to ANOVA (SPSS, Chicago, IL) with session number as a within-subjects factor to determine if basal neurotransmitter levels varied systematically across sessions. For analysis of the effects of nicotine, all data were expressed as percent of baseline (to control for individual differences in basal efflux) and subjected to ANOVA with treatment as a within-subjects factor and time as a repeated measure. The source of significant main effects or interactions was determined by Bonferroni comparisons. A significance criterion of P < 0.05 was used for all analyses.
For the lesion experiments, double (orexin + Fos) and single (orexin or Fos) - labeled cells were counted under 20× magnification in both medial and lateral sectors of the LH/PFA at two different rostro-caudal levels. Double-labeled data were expressed as a percentage of orexin neurons positive for Fos and analyzed by ANOVA followed by Bonferroni comparisons.
In addition, the number of single -label ChAT cell bodies were counted on the basal forebrain sections both on injection and non-injection side. Single-labeled ChAT data was also expressed as the percentage of ChAT-labeled cell bodies on non- injection side.
Results
Basal neurotransmitter efflux and probe placement
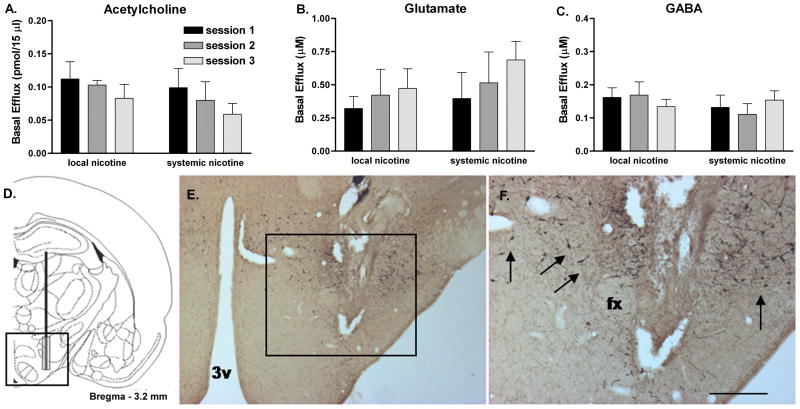
Basal efflux across all microdialysis sessions averaged 0.089 ± 0.008 pmol/15min for ACh, 0.468 ± 0.051 μM for glutamate and 0.144 ± 0.009 μM for GABA (Fig. 1A-C). Basal efflux of all of these neurotransmitters did not systematically change as a function of microdialysis session or route of nicotine treatment (systemic vs. local).
Figure 1.
Mean raw basal LH/PFA efflux values (uncorrected for probe recovery) for ACh (A), glutamate (B) and GABA (C) during the three systemic or local nicotine treatment sessions. Basal efflux was not significantly altered for any of the three neurotransmitters as a function of microdialysis session or route of nicotine treatment. D. Coronal hemisection schematic of probe placement (modified from Paxinos and Watson, 1997) in LH/PFA. E. Representative low-magnification photomicrograph of probe tract in the hypothalamus. F. Higher-magnification of the area outlined in (E) demonstrating the microdialysis probe tract surrounded by orexin-immunoreactive neurons (arrows). Abbreviations: 3v, third ventricle; fx, fornix; scale bar in (F) represents approximately 500μm. Error bars represent SEM.
Histological verification of microdialysis probe tracts revealed typical sampling regions near the lateral edge of the fornix, extending into the lateral hypothalamus proper (Fig. 1D). Orexin immunohistochemistry performed on acetylcholinesterase background-stained sections indicated that probe tracts were located in the region of orexin-immunoreactive neurons (Fig. 1E&F). Animals with probes located outside of the LH/PFA were excluded from analysis.
Nicotine effects on ACh efflux
Systemic administration of nicotine did not affect LH/PFA ACh efflux at either of the doses examined as indicated by a lack of significant main effects or interactions of DOSE and TIME (Fig. 2A).
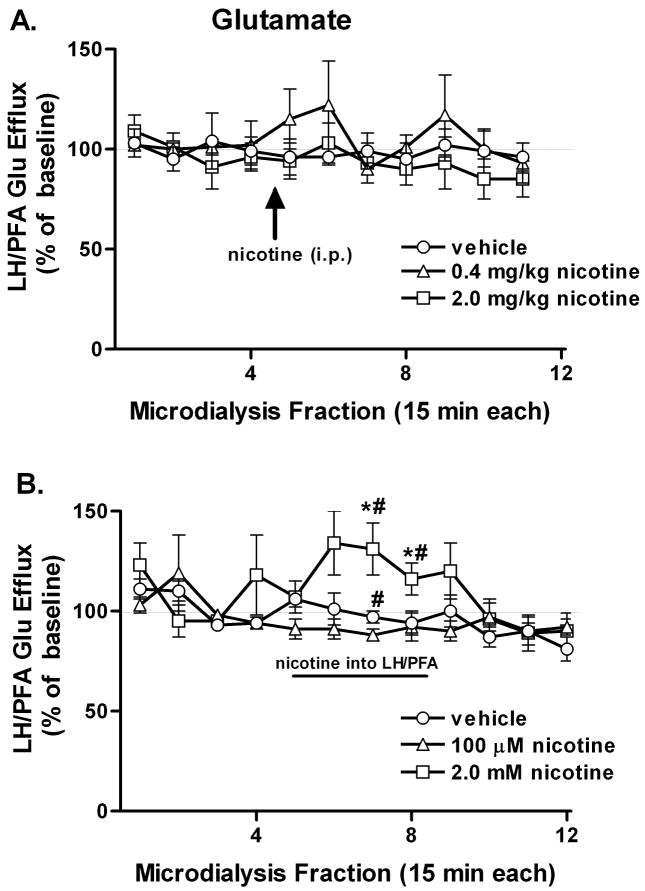
Figure 2.
Effect of nicotine administration on ACh efflux in the LH/PFA. A. Systemic nicotine (0, 0.4 mg/kg or 2.0 mg/kg) did not affect ACh efflux in the LH/PFA. B. Local nicotine (100 μM) increased ACh efflux whereas the higher concentration of nicotine (2.0 mM) significantly decreased ACh efflux during the eighth collection interval. Nicotine reached the LH/PFA during the 6th-9th collection intervals. * P<0.05 vs. vehicle and 2.0 mM nicotine. # P<0.05 vs. 2.0 mM nicotine. Error bars represent SEM.
Local nicotine administration significantly increased ACh efflux in the LH/PFA (Fig. 2B) as indicated by a significant main effect of TIME (F11,66 = 4.952; P = 0.001) as well as a DOSE × TIME interaction (F22,132 = 1.869; P = 0.016). Bonferroni comparisons revealed that the lower concentration of nicotine significantly increased ACh efflux compared to VEH or high concentration of nicotine during the 8th and 9th collections (all t6 > 2.104; all P < 0.05). Elevations in ACh efflux at the low concentration of nicotine reached significance during the eighth collection and were maximal (about 240% of baseline) during the ninth collection (last nicotine perfusion fraction). The high concentration of nicotine did not increase ACh efflux; rather, a significant decrease (relative to VEH) was observed at the eighth collection.
Nicotine effects on glutamate efflux
Systemic administration of nicotine did not affect LH/PFA glutamate efflux at either of the doses examined as indicated by a lack of significant main effects or interactions of DOSE and TIME, although there was a trend for increased glutamate at the lower dose (Fig. 3A).
Figure 3.
Effect of nicotine administration on glutamate efflux in the LH/PFA. A. Systemic nicotine (0, 0.4 mg/kg or 2.0 mg/kg) did not affect glutamate efflux in the LH/PFA. B. Local nicotine (2.0 mM) increased glutamate efflux whereas the lower concentration of nicotine (100 μM) significantly decreased glutamate efflux during the seventh collection interval. * P<0.05 vs. vehicle. # P<0.05 vs. 100 μM nicotine. Error bars represent SEM.
Local nicotine administration significantly increased glutamate efflux in the LH/PFA (Fig. 3B) as indicated by significant main effects of DOSE (F2,10 = 16.389; P = 0.001) and TIME (F11,55 = 2.316; P = 0.020) as well as a DOSE × TIME interaction (F22,110 = 1.826; P = 0.022). Bonferroni comparisons revealed that the higher concentration of nicotine significantly increased glutamate levels compared to the vehicle or lower concentration of nicotine during the seventh and eighth collections (all t5>2.74; all P<0.05), with maximal effects exceeding 130% of baseline values. The low concentration of nicotine, conversely, was associated with a significant decrease in local glutamate levels during the seventh collection relative to both high nicotine and VEH (all t5>2.74; P < 0.05). Glutamate efflux rapidly returned to baseline levels upon cessation of nicotine perfusion through the microdialysis probe.
Nicotine effects on GABA efflux
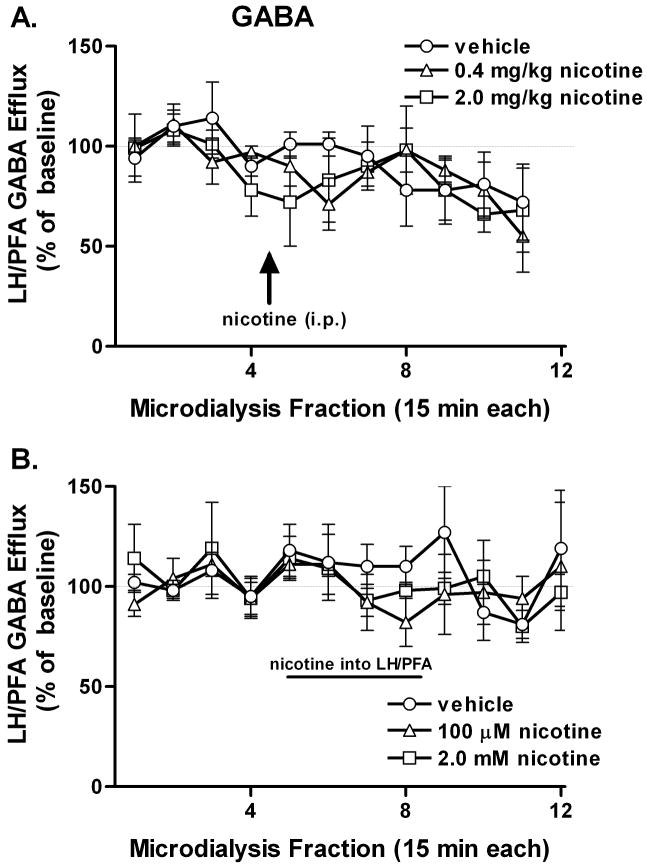
Neither systemic nor local nicotine administration significantly altered GABA efflux in the LH/PFA (Fig. 4).
Figure 4.
Effect of nicotine administration on GABA efflux in the LH/PFA: GABA efflux was not significantly altered by either systemic (A) or local (B) nicotine. Error bars represent SEM.
PFC lesions
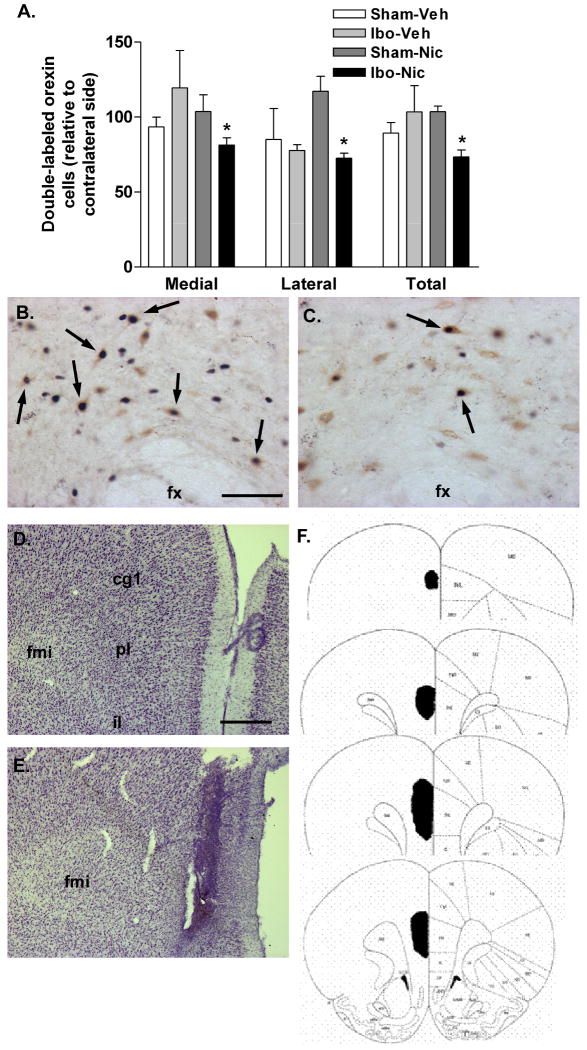
Ibotenic acid lesions of the medial prefrontal cortex resulted in attenuated nicotine-elicited Fos expression in orexin neurons (Fig. 5A&B). Two-way ANOVA revealed a significant interaction between nicotine treatment and prefrontal cortex lesion (F>3.640; P<0.05), with significant effects observed in medial and lateral sectors as well as the total LH/PFA (all t5>4.0; all P<0.05). This effect was particularly pronounced in the lateral bank of orexin neurons, where double-labeled cells were reduced by approximately 30%. No difference between lesioned and sham animals was observed following vehicle treatment. Post-mortem histological analysis indicated that ibotenic acid-induced loss of neurons was centered in the prelimbic cortex, extending dorsally into anterior cingulate and ventrally into infralimbic cortex (Fig. 5D-F). All cortical layers were affected and the lesioned area did not extend across the midline into the contralateral (non-injected) hemisphere.
Figure 5.
Activation of orexin neurons by nicotine following prefrontal cortical lesions. A. Double-labeled (Fos/orexin) neurons on the injected side relative to the contralateral hemisphere in control (Sham) or ibotenic acid (Ibo) PFC-lesioned animals treated acutely with systemic vehicle (Veh) or nicotine (Nic). Ibotenic acid lesions resulted in a significant reduction in nicotine-activated orexin neurons ipsilateral to the lesion site. This attenuation was seen in both medial and lateral sectors of the LH/PFA. *P < 0.05 vs. Sham-Nic. B. Dual-label immunohistochemistry for orexin and Fos following nicotine (2 mg/kg) treatment in a sham-lesioned animal. Numerous double-labeled cells are seen in the perifornical area (arrows). C. Dual-label immunohistochemistry following acute nicotine in an ibotenic acid PFC-lesioned animal. Some double-labeling is observed (arrows) but less than in (B). D. Cresyl violet-stained hemisection from the medial PFC of a sham-lesioned animal. E. Cresyl violet staining in the medial PFC of an ibotenic acid-lesioned animal. The lesioned area is demarcated by extensive gliosis. F. Schematic indicating typical lesion extent following ibotenic acid infusions of the PFC. Abbreviations: fx, fornix; fmi, forceps minor of the corpus callosum; cg1, anterior cingulate cortex; pl, prelimbic cortex; il, infralimbic cortex. Scale bars: approximately 100 μm (B/C); approximately 500 μm (D/E).
vGlut1-immunoreactivity was of moderate density in the LH/PFA and consisted of finer-caliber fibers with less numerous varicosities and fewer points of apparent appositional contact on orexin neurons relative to vAChT immunoreactivity (Fig. 7B). Ibotenic acid lesions reduced the number of apparent appositions between vGlut1-positive varicosities and orexin cell bodies and proximal dendrites by about 35%, a statistically-signicant effect (t3 = 5.305; p < 0.05; Fig. 7A).
Basal forebrain cholinergic lesions
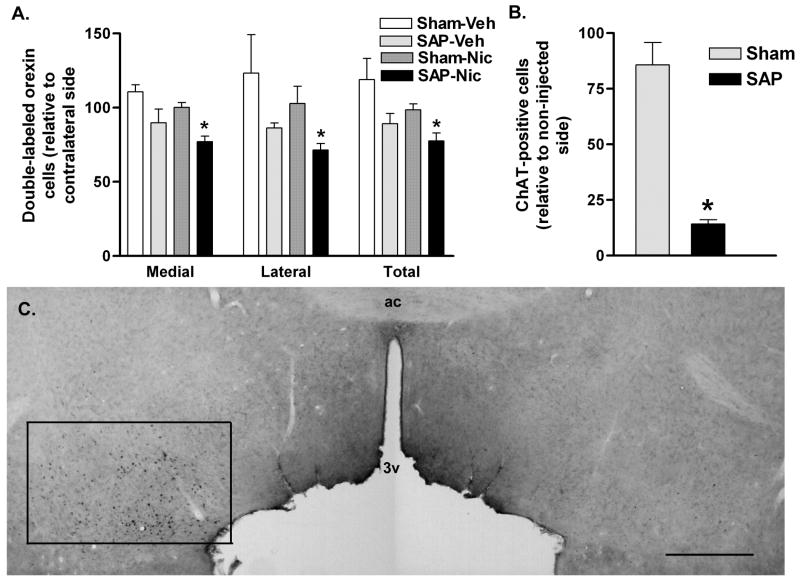
192 IgG-saporin lesions of the basal forebrain resulted in attenuated nicotine-elicited Fos expression in orexin neurons (Fig. 6A). Two-way ANOVA revealed a significant interaction between nicotine treatment and basal forebrain lesion (all F>2.348; all P<0.05), with significant effects observed in medial and lateral sectors as well as the total LH/PFA (all t5>3.7; all P<0.05). Histological verification of lesions indicated that 192 IgG-saporin caused a significant (t8=8.689; P<0.05) loss of more than 80% of ChAT-positive neurons in the ventral pallidum/substantia innominata portion of the basal forebrain, relative to the non-injected hemisphere (Fig. 6B&C). No significant loss of cholinergic neurons was seen in sham-lesioned animals.
Figure 6.
Activation of orexin neurons by nicotine following basal forebrain cholinergic lesions. A. Double-labeled (Fos/orexin) neurons on the injected side relative to the contralateral hemisphere in control (Sham) or 192 IgG-saporin (SAP) basal forebrain-lesioned animals treated acutely with systemic vehicle (Veh) or nicotine (Nic). 192 IgG-saporin lesions resulted in a significant reduction in nicotine-activated orexin neurons ipsilateral to the lesion site. This attenuation was seen in both medial and lateral sectors of the LH/PFA. *P < 0.05 vs. Sham-Nic. B. 192 IgG-saporin (SAP) treatment resulted in a greater than 80% loss of ChAT-immunoreactive cells in the basal forebrain relative to the non-lesioned hemisphere. No significant loss of cholinergic cells was seen in the sham-lesioned animals. *P < 0.05 vs. Sham. C. Immunohistochemistry for ChAT in the basal forebrain of a unilaterally-lesioned animal. The non-infused (left) hemisphere is characterized by the presence of numerous ChAT-immunoreactive cells in basal forebrain regions encompassing the substantia innominata, ventral pallidum and horizontal limb of the diagonal band of Broca, as indicated by the rectangle. The 192 IgG-saporin-infused (right) hemisphere is largely devoid of ChAT-immunoreactive neurons. Abbreviations: ac, anterior commissure; 3v, third ventricle. Scale bar equals approximately 1 mm.
vAChT-immunoreactivity was dense in the LH/PFA and consisted of large-caliber fibers with numerous varicosities. The typical orexin neuron from a control animal received numerous vAChT-immunoreactive apparent appositions (Fig. 7E). Basal forebrain 192 IgG-saporin lesions reduced these points of apparent appositional contact between vAChT-positive varicosities and orexin cell bodies and proximal dendrites by almost 40% (t3 = 4.189; p < 0.05; Fig. 7D).
Discussion
Collectively, the results of these experiments demonstrate that local nicotine administration increases ACh and glutamate, but not GABA, efflux in the LH/PFA. These neurochemical correlates of nicotine administration in the LH/PFA are likely to contribute to activation of orexin neurons, as nicotine-elicited Fos expression was significantly attenuated by lesioning major extrinsic sources of glutamatergic or cholinergic input to this area, the PFC and basal forebrain, respectively.
Microdialysis measurement of ACh, glutamate and GABA
As described above, cholinergic neurons of the basal forebrain appear to provide the predominant source of ACh in the rodent LH/PFA. Consistent with this model, 192 IgG-saporin lesions of the basal forebrain cholinergic system significantly reduced apparent appositional contacts between vAChT-immunoreactive varicosities and orexin neurons (Fig. 7). The incomplete nature of the cholinergic lesions is also consistent with the existence of cholinergic inputs to the hypothalamus from basal forebrain areas outside of the substantia innominata (targeted by the lesion surgery), such as the diagonal band of Broca (Sakurai et al. 2005).
There were (non-significant) trends for basal ACh efflux to decrease, and Glu efflux to increase, across microdialysis sessions (Fig. 1). The progressive gliosis that accompanies repeated microdialysis probe insertion is a likely mediator of both of these observations. Gliosis would cause an increase in the distance required from neuronally-released ACh to diffuse in order to be recovered by the dialysis probe. Conversely, the majority of basal glutamate measured with microdialysis is of non-vesicular, including glial, origin (Timmerman and Westerink 1997), thus accounting for the increase in this neurochemical with progressive gliosis. Importantly, however the order of nicotine dose administration was counterbalanced across animals—thus any interactions between basal efflux and drug effects would be evenly distributed across doses. Furthermore, while basal glutamate may have been largely of non-neuronal origin, it is likely that the nicotine-elicited component of glutamate efflux was largely calcium and tetrodotoxin-sensitive as has been shown previously in striatum and cortex (Toth et al. 1993; Gioanni et al. 1999).
We did not observe an effect of nicotine administration on GABA efflux in the hypothalamus. While direct, in vivo, neurochemical measurements of LH/PFA GABA in response to nicotine have not previously been reported, in vitro electrophysiological recordings from lateral hypothalamic slices suggest that nicotine increases GABAergic transmission in an α7-dependent manner (Jo et al. 2005). The discrepancy between our results and the previous observations may reflect the different time scales inherent in microdialysis and electrophysiology and/or the preservation of regulatory circuitry in the in vivo preparation.
Systemic vs. local nicotine effects on LH/PFA glutamate and ACh efflux
We observed that local, but not systemic, nicotine administration increased ACh and glutamate efflux. The doses of systemic nicotine were shown previously to induce Fos expression in the LH/PFA (Pasumarthi et al. 2006) and, in the current study, also increased locomotor activity (Supplemental Data), indicating that the failure of this route of administration to affect neurotransmitter efflux in the LH/PFA was not because the doses used were sub-threshold for behavioral activation. Local nicotine, meanwhile, did not affect locomotor behavior (Supplemental Data), demonstrating that increases in ACh and glutamate were not secondary to behavioral arousal.
The different response of neurotransmitter efflux in the LH/PFA to systemic vs. local nicotine administration suggests that direct postsynaptic effects of nicotine on orexin neurons may be the predominant determinant of Fos expression in these cells, whereas recruitment of glutamatergic and cholinergic inputs to the LH/PFA may be subject to rapid desensitization, precluding the ability to detect short-lived increases on the microdialysis time scale. Local nicotine, conversely, may produce longer-lasting increases in these neurotransmitters by sustained activation of terminally-expressed receptors (see below for further discussion). Additional factors, such as acute desensitization following repeated systemic nicotine administration, may also contribute. Ultrastructural determination of the anatomical relationship between nicotinic receptor-expressing terminals and post-synaptic cell types, as well as more thorough electrophysiological characterization of nicotine effects in this area, will allow for more empirically-based models of these speculative mechanisms.
The dose-response relationship of nicotine was qualitatively different for ACh and glutamate. Maximal increases in ACh efflux were produced by the low dose of nicotine, with the high dose eliciting a transient, but significant decrease in ACh. Conversely, glutamate efflux in the LH/PFA was increased by the high dose of nicotine but transiently decreased by the lower dose, suggestive of different nicotinic receptor mechanisms underlying effects on these two neurotransmitter systems.
What receptor mechanisms mediate the effects of local nicotine on ACh and glutamate efflux in the LH/PFA?
Both α4β2- and α7-containing nicotinic receptors appear to be expressed in the hypothalamus (Gotti et al. 2006). Studies mapping the regional distribution of individual nicotinic receptor subunits indicate the presence of α4 mRNA and protein in several hypothalamic areas (Wada et al. 1989; Shioda et al. 1997). The α7 subunit is also present in the hypothalamus, where it appears to be localized preferentially on glutamatergic terminals (Seguela et al. 1993; Nomikos et al. 2000), suggesting that local nicotine effects on glutamate efflux may be α7-mediated. The effects of systemic nicotine on ACh efflux in some basal forebrain target regions, such as the hippocampus, appear to be α4β2-mediated and follow a bell-shaped dose-response curve (Tani et al. 1998), consistent with our results. Furthermore, while α4β2 receptors possess higher affinity for ACh (Dani and Bertrand 2007), α7 receptors—in addition to promoting glutamate release—are also desensitized by the ACh metabolite/precursor, choline (Papke et al. 1996). Thus, the lesser effect of the high dose of nicotine on ACh efflux may have resulted in reduced choline-mediated desensitization of α7-receptors on glutamate terminals, contributing to the divergent dose-response and temporal profiles in the response of these neurotransmitters to local nicotine. Additional studies, including demonstrations of nicotinic receptor subunit colocalization with phenotypically characterized cell body and axon terminal populations within the lateral hypothalamus/perifornical area, will be required to definitively determine the receptor mechanisms underlying nicotine-elicited activation of orexin neurons.
We have previously shown that acute nicotine-elicited Fos expression in orexin neurons is blocked by mecamylamine or dihydro-beta-erythroidine, suggesting an effect mediated predominantly by β2-containing receptors (Pasumarthi et al. 2006). Thus, it is possible that the direct effects of local nicotine on ACh and glutamate efflux are driven largely by activation of presynaptic α7-containing receptors, whereas the systemic effects of nicotine on orexin neuron activation are mediated by β2-containing receptors, perhaps expressed directly on these neurons. Future studies exploring the effect of combined PFC and cholinergic lesions may provide further support for a synergistic interaction between these neurotransmitter systems in nicotine-elicited activation of hypothalamic neurons.
Attenuation of nicotine-elicited Fos expression by PFC or basal forebrain lesions
We observed that nicotine-elicited Fos expression was attenuated by lesions of either the medial PFC or the basal forebrain cholinergic system. In the case of the PFC lesions, this effect was particularly pronounced in the lateral bank of orexin neurons (Fig. 5). This subset of orexin neurons has been suggested to mediate orexin influences over drug-associated reward processing (Harris and Aston-Jones 2006). Selective activation of PFC-projecting lateral hypothalamic orexin neurons by atypical antipsychotic drugs has also been reported (Fadel et al. 2002), further underlying the importance of this circuitry in mediating some drug responses.
In the case of either PFC or basal forebrain cholinergic lesions, attenuation of nicotine-elicited Fos expression was significant, but incomplete; that is, some residual activation of these neurons by nicotine was spared. There are several possibilities for this result. First, these lesions were not total and it is possible that remaining inputs to the LH/PFA from spared regions of the PFC or basal forebrain cholinergic system were sufficient to mediate some degree of activation. Second, these lesions were unilateral, and while projections from the PFC or basal forebrain to the hypothalamus are thought to be primarily ipsilateral, there may be some contribution from spared glutamatergic or cholinergic inputs on the contralateral (non-lesioned) hemisphere. In addition, there are other afferent sources of these neurotransmitters in the LH/PFA, including glutamatergic inputs from the amygdala (Sakurai et al. 2005) and local interneurons (Acuna-Goycolea et al. 2004) and cholinergic inputs from basal forebrain regions outside our targeted zone in the substantia innominata, such as the medial septum and diagonal band of Broca (Sakurai et al. 2005). Consistent with all of these possibilities is the persistence of markers of glutamatergic and cholinergic innervation of the LH/PFA that we observed using vGlut1 and vAChT immunohistochemistry. Interestingly, the quantitative loss of these markers following PFC ibotenic acid or basal forebrain 192 IgG-saporin lesions closely paralleled the degree of reduced Fos expression in orexin neurons, indicating the importance of the corresponding neurotransmitters (irrespective of their anatomical source) in nicotine-elicited activation of orexin neurons. Finally, of course, is the likelihood that Fos expression in the hypothalamus represents merely one correlate of a complex cascade of nicotine-associated signaling events and neurotransmitter effects. Nonetheless, the ability of these lesions to significantly attenuate the Fos response demonstrates a potential physiologically-important role for the basal forebrain cholinergic system and the PFC to take part in nicotine influences over hypothalamic neurochemical, neuroendocrine and behavioral phenomena.
Significance of nicotine effects on hypothalamic glutamatergic and cholinergic neurotransmission
Behavioral, physiological and neurochemical data indicate that the basal forebrain cholinergic system plays a prominent role in nicotine effects (Linville et al. 1993; Riekkinen et al. 1993; Turchi et al. 1995; Arnold et al. 2003). In turn, nicotine administration activates orexin projections to the basal forebrain (Pasumarthi and Fadel 2008). This suggests that basal forebrain-lateral hypothalamic circuitry may represent a functional circuit important for integrating nicotine effects on arousal, attention and reward. In addition, self-stimulation of the PFC elicits LH Fos expression (Arvanitogiannis et al. 2000) and PFC projections to the LH are activated by cues associated with conditioned feeding (Petrovich et al. 2007). This circuitry may play a more general role in conditioned behavioral responses to appetive stimuli, including nicotine or other drugs of abuse.
LH/PFA orexin neurons are likely to play a special role in integrating nicotine effects on forebrain targets. There is a rapidly-accumulating body of literature documenting the involvement of this neuropeptide system in drug-associated arousal, reward and addiction (DiLeone et al. 2003; Aston-Jones et al. 2009). Chronic nicotine up-regulates expression of orexin peptides and receptors (Kane et al. 2000), and orexin neurotransmission within the insular cortex appears to be essential for maintenance of nicotine self-administration (Hollander et al. 2008). Collectively, these observations have led to the the hypothesis that orexins may mediate several aspects of nicotine-associated reward, arousal and addiction (Corrigall 2009). Our results indicate that glutamatergic and cholinergic mechanisms in the LH/PFA contribute to nicotine activation of the orexin system, and may thus influence orexin-nicotine interactions in brain regions as diverse as the PFC, basal forebrain and insular cortex.
Conclusion
In summary, we have shown that local nicotine administration significantly increases lateral hypothalamic ACh and glutamate efflux. Furthermore, lesioning major extrinsic sources of ACh and glutamate—the basal forebrain and PFC, respectively—attenuated nicotine-elicited activation of lateral hypothalamic orexin neurons. Thus, nicotine may influence hypothalamic regulation of arousal and feeding in part by local modulation of glutamate and ACh. Future studies investigating the extent to which hypothalamic nicotine is sufficient to activate orexin terminal regions such as the basal forebrain, PFC and insular cortex will shed further light on the functional significance of these neurochemical phenomena.
Supplementary Material
Acknowledgments
This work was supported in part by a grant from the National Institutes of Health (R01AG030646) to JF.
References
- Acuna-Goycolea C, Li Y, Van Den Pol AN. Group III metabotropic glutamate receptors maintain tonic inhibition of excitatory synaptic input to hypocretin/orexin neurons. J Neurosci. 2004;24:3013–3022. doi: 10.1523/JNEUROSCI.5416-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold HM, Nelson CL, Sarter M, Bruno JP. Sensitization of cortical acetylcholine release by repeated administration of nicotine in rats. Psychopharmacology (Berl) 2003;165:346–358. doi: 10.1007/s00213-002-1260-6. [DOI] [PubMed] [Google Scholar]
- Arvanitogiannis A, Tzschentke TM, Riscaldino L, Wise RA, Shizgal P. Fos expression following self-stimulation of the medial prefrontal cortex. Behav Brain Res. 2000;107:123–132. doi: 10.1016/s0166-4328(99)00120-5. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Smith RJ, Moorman DE, Richardson KA. Role of lateral hypothalamic orexin neurons in reward processing and addiction. Neuropharmacology. 2009;56 1:112–121. doi: 10.1016/j.neuropharm.2008.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubser M, Fadel JR, Jackson LL, Meador-Woodruff JH, Jing D, Deutch AY. Dopaminergic regulation of orexin neurons. Eur J Neurosci. 2005;21:2993–3001. doi: 10.1111/j.1460-9568.2005.04121.x. [DOI] [PubMed] [Google Scholar]
- Burrows KB, Nixdorf WL, Yamamoto BK. Central administration of methamphetamine synergizes with metabolic inhibition to deplete striatal monoamines. J Pharmacol Exp Ther. 2000;292:853–860. [PubMed] [Google Scholar]
- Corrigall WA. Understanding brain mechanisms in nicotine reinforcement. Br J Addict. 1991;86:507–510. doi: 10.1111/j.1360-0443.1991.tb01798.x. [DOI] [PubMed] [Google Scholar]
- Corrigall WA. Hypocretin mechanisms in nicotine addiction: evidence and speculation. Psychopharmacology (Berl) 2009;206:23–37. doi: 10.1007/s00213-009-1588-2. [DOI] [PubMed] [Google Scholar]
- Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- DiLeone RJ, Georgescu D, Nestler EJ. Lateral hypothalamic neuropeptides in reward and drug addiction. Life Sci. 2003;73:759–768. doi: 10.1016/s0024-3205(03)00408-9. [DOI] [PubMed] [Google Scholar]
- Donzanti BA, Yamamoto BK. An improved and rapid HPLC-EC method for the isocratic separation of amino acid neurotransmitters from brain tissue and microdialysis perfusates. Life Sci. 1988;43:913–922. doi: 10.1016/0024-3205(88)90267-6. [DOI] [PubMed] [Google Scholar]
- Fadel J, Sarter M, Bruno JP. Basal forebrain glutamatergic modulation of cortical acetylcholine release. Synapse. 2001;39:201–212. doi: 10.1002/1098-2396(20010301)39:3<201::AID-SYN1001>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Fadel J, Bubser M, Deutch AY. Differential activation of orexin neurons by antipsychotic drugs associated with weight gain. J Neurosci. 2002;22:6742–6746. doi: 10.1523/JNEUROSCI.22-15-06742.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel J, Pasumarthi R, Reznikov LR. Stimulation of cortical acetylcholine release by orexin A. Neuroscience. 2005;130:541–547. doi: 10.1016/j.neuroscience.2004.09.050. [DOI] [PubMed] [Google Scholar]
- Fedele E, Varnier G, Ansaldo MA, Raiteri M. Nicotine administration stimulates the in vivo N-methyl-D-aspartate receptor/nitric oxide/cyclic GMP pathway in rat hippocampus through glutamate release. Br J Pharmacol. 1998;125:1042–1048. doi: 10.1038/sj.bjp.0702130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioanni Y, Rougeot C, Clarke PB, Lepouse C, Thierry AM, Vidal C. Nicotinic receptors in the rat prefrontal cortex: increase in glutamate release and facilitation of mediodorsal thalamo-cortical transmission. Eur J Neurosci. 1999;11:18–30. doi: 10.1046/j.1460-9568.1999.00403.x. [DOI] [PubMed] [Google Scholar]
- Gotti C, Zoli M, Clementi F. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol Sci. 2006;27:482–491. doi: 10.1016/j.tips.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 2006;29:571–577. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Harrison AA, Gasparini F, Markou A. Nicotine potentiation of brain stimulation reward reversed by DH beta E and SCH 23390, but not by eticlopride, LY 314582 or MPEP in rats. Psychopharmacology (Berl) 2002;160:56–66. doi: 10.1007/s00213-001-0953-6. [DOI] [PubMed] [Google Scholar]
- Hollander JA, Lu Q, Cameron MD, Kamenecka TM, Kenny PJ. Insular hypocretin transmission regulates nicotine reward. Proc Natl Acad Sci U S A. 2008;105:19480–19485. doi: 10.1073/pnas.0808023105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo YH, Talmage DA, Role LW. Nicotinic receptor-mediated effects on appetite and food intake. J Neurobiol. 2002;53:618–632. doi: 10.1002/neu.10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo YH, Wiedl D, Role LW. Cholinergic modulation of appetite-related synapses in mouse lateral hypothalamic slice. J Neurosci. 2005;25:11133–11144. doi: 10.1523/JNEUROSCI.3638-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane JK, Parker SL, Matta SG, Fu Y, Sharp BM, Li MD. Nicotine up-regulates expression of orexin and its receptors in rat brain. Endocrinology. 2000;141:3623–3629. doi: 10.1210/endo.141.10.7707. [DOI] [PubMed] [Google Scholar]
- Li Y, Gao XB, Sakurai T, van den Pol AN. Hypocretin/Orexin excites hypocretin neurons via a local glutamate neuron-A potential mechanism for orchestrating the hypothalamic arousal system. Neuron. 2002;36:1169–1181. doi: 10.1016/s0896-6273(02)01132-7. [DOI] [PubMed] [Google Scholar]
- Linville DG, Williams S, Raszkiewicz JL, Arneric SP. Nicotinic agonists modulate basal forebrain control of cortical cerebral blood flow in anesthetized rats. J Pharmacol Exp Ther. 1993;267:440–448. [PubMed] [Google Scholar]
- Lu Y, Grady S, Marks MJ, Picciotto M, Changeux JP, Collins AC. Pharmacological characterization of nicotinic receptor-stimulated GABA release from mouse brain synaptosomes. J Pharmacol Exp Ther. 1998;287:648–657. [PubMed] [Google Scholar]
- Meguid MM, Fetissov SO, Varma M, Sato T, Zhang L, Laviano A, Rossi-Fanelli F. Hypothalamic dopamine and serotonin in the regulation of food intake. Nutrition. 2000;16:843–857. doi: 10.1016/s0899-9007(00)00449-4. [DOI] [PubMed] [Google Scholar]
- Miyata G, Meguid MM, Fetissov SO, Torelli GF, Kim HJ. Nicotine's effect on hypothalamic neurotransmitters and appetite regulation. Surgery. 1999;126:255–263. [PubMed] [Google Scholar]
- Mogenson GJ, Swanson LW, Wu M. Neural projections from nucleus accumbens to globus pallidus, substantia innominata, and lateral preoptic-lateral hypothalamic area: an anatomical and electrophysiological investigation in the rat. J Neurosci. 1983;3:189–202. doi: 10.1523/JNEUROSCI.03-01-00189.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S, Tsumori T, Yokota S, Oka T, Yasui Y. Amygdaloid axons innervate melanin-concentrating hormone- and orexin-containing neurons in the mouse lateral hypothalamus. Brain Res. 2009 doi: 10.1016/j.brainres.2009.04.049. [DOI] [PubMed] [Google Scholar]
- Nomikos GG, Schilstrom B, Hildebrand BE, Panagis G, Grenhoff J, Svensson TH. Role of alpha7 nicotinic receptors in nicotine dependence and implications for psychiatric illness. Behav Brain Res. 2000;113:97–103. doi: 10.1016/s0166-4328(00)00204-7. [DOI] [PubMed] [Google Scholar]
- O'Hara BF, Edgar DM, Cao VH, Wiler SW, Heller HC, Kilduff TS, Miller JD. Nicotine and nicotinic receptors in the circadian system. Psychoneuroendocrinology. 1998;23:161–173. doi: 10.1016/s0306-4530(97)00077-2. [DOI] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Glutamate synaptic inputs to ventral tegmental area neurons in the rat derive primarily from subcortical sources. Neuroscience. 2007;146:1259–1274. doi: 10.1016/j.neuroscience.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Bencherif M, Lippiello P. An evaluation of neuronal nicotinic acetylcholine receptor activation by quaternary nitrogen compounds indicates that choline is selective for the alpha 7 subtype. Neurosci Lett. 1996;213:201–204. doi: 10.1016/0304-3940(96)12889-5. [DOI] [PubMed] [Google Scholar]
- Pasumarthi RK, Fadel J. Activation of orexin/hypocretin projections to basal forebrain and paraventricular thalamus by acute nicotine. Brain Res Bull. 2008;77:367–373. doi: 10.1016/j.brainresbull.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasumarthi RK, Reznikov LR, Fadel J. Activation of orexin neurons by acute nicotine. Eur J Pharmacol. 2006;535:172–176. doi: 10.1016/j.ejphar.2006.02.021. [DOI] [PubMed] [Google Scholar]
- Perez de la Mora M, Mendez-Franco J, Salceda R, Aguirre JA, Fuxe K. Neurochemical effects of nicotine on glutamate and GABA mechanisms in the rat brain. Acta Physiol Scand. 1991;141:241–250. doi: 10.1111/j.1748-1716.1991.tb09074.x. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Ross CA, Holland PC, Gallagher M. Medial prefrontal cortex is necessary for an appetitive contextual conditioned stimulus to promote eating in sated rats. J Neurosci. 2007;27:6436–6441. doi: 10.1523/JNEUROSCI.5001-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren T, Sagar SM. Induction of c-fos immunostaining in the rat brain after the systemic administration of nicotine. Brain Res Bull. 1992;29:589–597. doi: 10.1016/0361-9230(92)90127-j. [DOI] [PubMed] [Google Scholar]
- Reznikov LR, Grillo CA, Piroli GG, Pasumarthi RK, Reagan LP, Fadel J. Acute stress-mediated increases in extracellular glutamate levels in the rat amygdala: differential effects of antidepressant treatment. Eur J Neurosci. 2007;25:3109–3114. doi: 10.1111/j.1460-9568.2007.05560.x. [DOI] [PubMed] [Google Scholar]
- Riekkinen P, Jr, Riekkinen M, Sirvio J. Effects of nicotine on neocortical electrical activity in rats. J Pharmacol Exp Ther. 1993;267:776–784. [PubMed] [Google Scholar]
- Rowley HL, Martin KF, Marsden CA. Determination of in vivo amino acid neurotransmitters by high-performance liquid chromatography with o-phthalaldehyde-sulphite derivatisation. J Neurosci Methods. 1995;57:93–99. doi: 10.1016/0165-0270(94)00132-z. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Nagata R, Yamanaka A, Kawamura H, Tsujino N, Muraki Y, Kageyama H, Kunita S, Takahashi S, Goto K, Koyama Y, Shioda S, Yanagisawa M. Input of orexin/hypocretin neurons revealed by a genetically encoded tracer in mice. Neuron. 2005;46:297–308. doi: 10.1016/j.neuron.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Seguela P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW. Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. J Neurosci. 1993;13:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioda S, Nakajo S, Hirabayashi T, Nakayama H, Nakaya K, Matsuda K, Nakai Y. Neuronal nicotinic acetylcholine receptor in the hypothalamus: morphological diversity and neuroendocrine regulations. Brain Res Mol Brain Res. 1997;49:45–54. doi: 10.1016/s0169-328x(97)00122-8. [DOI] [PubMed] [Google Scholar]
- Stratford TR, Kelley AE. Evidence of a functional relationship between the nucleus accumbens shell and lateral hypothalamus subserving the control of feeding behavior. J Neurosci. 1999;19:11040–11048. doi: 10.1523/JNEUROSCI.19-24-11040.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers KL, Giacobini E. Effects of local and repeated systemic administration of (-)nicotine on extracellular levels of acetylcholine, norepinephrine, dopamine, and serotonin in rat cortex. Neurochem Res. 1995;20:753–759. doi: 10.1007/BF01705545. [DOI] [PubMed] [Google Scholar]
- Tani Y, Saito K, Imoto M, Ohno T. Pharmacological characterization of nicotinic receptor-mediated acetylcholine release in rat brain--an in vivo microdialysis study. Eur J Pharmacol. 1998;351:181–188. doi: 10.1016/s0014-2999(98)00314-8. [DOI] [PubMed] [Google Scholar]
- Timmerman W, Westerink BH. Brain microdialysis of GABA and glutamate: what does it signify? Synapse. 1997;27:242–261. doi: 10.1002/(SICI)1098-2396(199711)27:3<242::AID-SYN9>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Toth E, Vizi ES, Lajtha A. Effect of nicotine on levels of extracellular amino acids in regions of the rat brain in vivo. Neuropharmacology. 1993;32:827–832. doi: 10.1016/0028-3908(93)90192-6. [DOI] [PubMed] [Google Scholar]
- Turchi J, Holley LA, Sarter M. Effects of nicotinic acetylcholine receptor ligands on behavioral vigilance in rats. Psychopharmacology (Berl) 1995;118:195–205. doi: 10.1007/BF02245840. [DOI] [PubMed] [Google Scholar]
- Wada E, Wada K, Boulter J, Deneris E, Heinemann S, Patrick J, Swanson LW. Distribution of alpha 2, alpha 3, alpha 4, and beta 2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J Comp Neurol. 1989;284:314–335. doi: 10.1002/cne.902840212. [DOI] [PubMed] [Google Scholar]
- Yamanaka A, Muraki Y, Tsujino N, Goto K, Sakurai T. Regulation of orexin neurons by the monoaminergic and cholinergic systems. Biochem Biophys Res Commun. 2003;303:120–129. doi: 10.1016/s0006-291x(03)00299-7. [DOI] [PubMed] [Google Scholar]
- Yoshida K, McCormack S, Espana RA, Crocker A, Scammell TE. Afferents to the orexin neurons of the rat brain. J Comp Neurol. 2006;494:845–861. doi: 10.1002/cne.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Meguid MM, Miyata G, Varma M, Fetissov SO. Role of hypothalamic monoamines in nicotine-induced anorexia in menopausal rats. Surgery. 2001;130:133–142. doi: 10.1067/msy.2001.115513. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.