Abstract
Autoimmune disease and/or autoantibodies have been reported in mood disorder patients. We screened for autoantibodies to Glutamic Acid Decarboxylase (GAD65), Thyroid Peroxidase (TPO), gastric H+/K+ ATPase (ATP4B), and Ro52 in a psychiatric patient cohort. A 24-year-old woman with Major Depressive Disorder (MDD) with reduced psychomotor activity was identified with unusually high serum GAD65 and Ro52 autoantibody titers. Anti-GAD65 and anti-Ro52 autoantibodies were also elevated in the CSF from this patient. Longitudinal examination revealed a four-fold increase in anti-GAD65 serum antibody titers which correlated with exacerbation of psychomotor symptomatology. These results suggest the possibility that CNS autoimmunity may be responsible for the psychomotor impairment in this MDD patient.
Keywords: GAD65, Ro52, Depression, Autoimmunity, Psychomotor Disturbance
1. Introduction
The relationship between the immune system and the pathogenesis of mood disorder has been the focus of numerous studies. Several groups observed an increased prevalence of autoimmune disease and/or autoantibodies in patients with bipolar disorder, including autoimmune thyroiditis and autoimmune atrophic gastritis (Kupka et al., 2002). Padmos et al. reported significant titers of autoantibodies to glutamic acid decarboxylase-65 (GAD65), thyroid peroxidase (TPO) and gastric H+/K+ ATPase (ATP4B) in a bipolar patient cohort (Padmos et al., 2004). Luciferase Immunoprecipitation Systems (LIPS) is a highly sensitive and specific assay which harnesses light-emitting recombinant antigen fusion proteins to quantitatively measure patient antibody titers (Burbelo et al., 2009a). Previously, LIPS has been shown to be as good as, or superior to existing immunoassays in the detection of antibodies in several autoimmune conditions, including Sjögren's Syndrome (Burbelo et al., 2009b), type I diabetes (Burbelo et al., 2010) and Stiff Person Syndrome (SPS) (Burbelo et al., 2008a). For detection of anti-GAD65 autoantibodies, LIPS demonstrated equal diagnostic performance to the established radiobinding assay for the diagnosis of type I diabetes (Burbelo et al., 2010). Using LIPS, we screened a cohort of 58 psychiatric patients and 44 healthy controls for autoantibodies to TPO, ATP4B, Ro52 and GAD65.
2. Materials and Methods
2.1 Patient Sera and CSF
Patients were evaluated under Institutional Review Board-approved protocols at the National Institute of Mental Health (Bethesda, MD), were medically healthy and had been unmedicated for at least three weeks prior to blood sampling. The cohort included 42 major depressive disorder patients (21 currently-depressed and 21 in full remission; DSM-IV criteria), 16 depressed bipolar disorder patients, and 44 healthy controls. The controls were recruited from the same community as the patients using advertisements in local media. All patients and controls underwent an identical screening process, which included both structured and unstructured psychiatric interviews, a medical history and physical examination conducted by a physician, electrocardiogram and a neuromorphological MRI scan to rule out gross brain pathology. Other clinical and laboratory tests were also performed, including complete blood count, electrolytes, liver and thyroid functions, urine analysis, pregnancy testing (for females) and HIV plus hepatitis viral screening. Sera and CSF were kept at - 80°C, thawed, aliquoted, then stored at 4°C prior to measurement.
2.2 Generation of Renilla luciferase fusion proteins
pREN2, a mammalian Renilla luciferase (Ruc) expression vector, was used to generate all plasmids. Human cDNA clones were amplified by PCR with specific linker-primer adapters as previously described, and a stop codon was included at the end of each coding sequence (Burbelo et al., 2005). In the case of the Ro52, a deletion mutant spanning amino acid residues 2-276 was used (Burbelo et al., 2009b). Unlike the full length Ro52, this deletion mutant did not require sera dilution for analysis (Burbelo et al., 2009b).
2.3 LIPS analysis
Following transfection of mammalian expression vectors, crude protein extracts were obtained as described in a detailed protocol of the LIPS assay, available along with a corresponding technical video from the Journal of Visualized Experiments (Burbelo et al., 2009a). For analysis, 1 μL equivalent of sera or 20 μL of CSF was used. The light unit (LU) data were obtained from the average of two independent experiments and corrected for background by subtracting the LU values of protein A/G beads alone.
3. Results
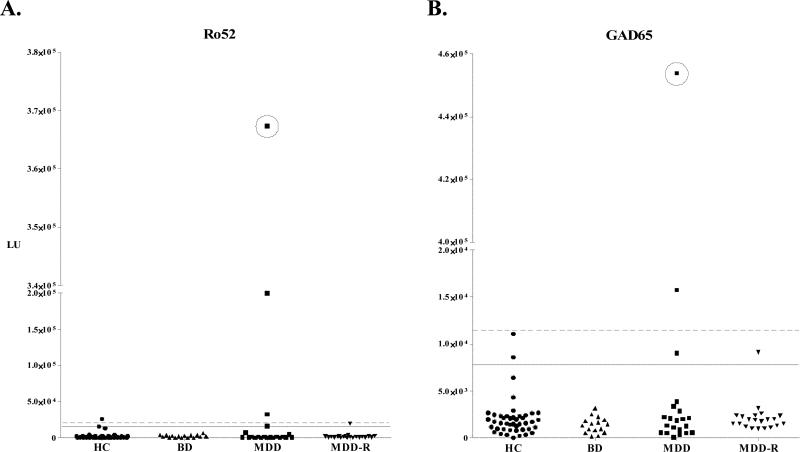
The LIPS assay format was used to screen a cohort of mood disorder patients and controls for autoantibodies to several targets including TPO, Ro52 and GAD65. For each antigen tested, we used a cutoff based on the average plus five standard deviations of the healthy controls. None of the psychiatric patients showed autoantibody titers to TPO, a known thyroid autoantigen, above the established cutoff (data not shown). One MDD patient and one control were seropositive for ATP4B (data not shown). One control and three MDD patients had significant autoantibody titers to Ro52 (Figure 1A). The highest anti-Ro52 autoantibody titer was MDD patient 225 (Figure 1A). Testing for anti-GAD65 autoantibodies revealed two MDD patients with markedly elevated anti-GAD65 antibody titers (Fig. 1B). Interestingly, it was also patient 225 who showed the highest anti-GAD65 autoantibodies, which were 200 standard deviations higher than the control mean. The anti-GAD65 autoantibody titer in patient 225 was comparable to the highest 10% of titers seen in type I diabetes patients and similar to patients with SPS (Burbelo et al., 2008a; Burbelo et al., 2008b). Based on these observations, patient 225 was studied in detail to understand the pathophysiological significance of these high autoantibody titers.
Fig. 1. Identification of a patient with significant autoantibody titers to both Ro52 and GAD65.
Forty-four healthy controls (HC; •), 16 patients with bipolar disorder (BD; ▲), 21 currently depressed patients with major depressive disorder (MDD; ■), and 21 patients with major depressive disorder in remission (MDD-R; ▼) were screened for autoantibodies to Ro52 (A) and GAD65 (B) by LIPS. The solid line represents the cut-off level derived from the mean plus 3 SD of the 44 healthy controls, while the dashed line is the cut-off for the mean plus 5 SD. Patient 225, who had the highest autoantibody titers to both antigens, is circled.
Patient 225, an African-American woman with MDD (whose family history included a first-degree relative with bipolar disorder), initially presented (March 2004) at age 24 with depressed mood of 12 months duration, anhedonia, guilt, self-depreciation, diminished libido, weight gain and initial insomnia. Particularly striking were her clinical manifestations of psychomotor disturbance, including restricted facial mobility and markedly reduced psychomotor activity; rating 6 on the retardation subscale of the CORE melancholia scale (Hadzi-Pavlovic et al., 1993; Parker and Hadzi-Pavlovic, 1996). There was no evidence of other autoimmune disease or diabetes, as additional testing for type I diabetes-associated autoantibodies (IA2 and Zinc Transporter-8) was negative.
Anti-GAD65 autoantibodies are associated with neurological disease, including SPS in which patients present with motor impairment including rigidity of axial and/or appendicular muscles and altered startle response in addition to showing high levels of anti-GAD65 autoantibodies in serum and cerebral spinal fluid (CSF) (Levy et al., 1999; Solimena et al., 1990). Thus, we next tested for autoantibodies in the CSF of patient 225 along with CSF from four random controls and one other MDD patient with available CSF. Only patient 225 showed titers of anti-GAD65 and anti-Ro52 autoantibodies in CSF that were above the normal range. These results establish that patient 225 displays high levels of anti-GAD65 and anti-Ro52 autoantibodies in serum and CSF and suggest that her psychomotor disturbance may be related to CNS autoimmunity.
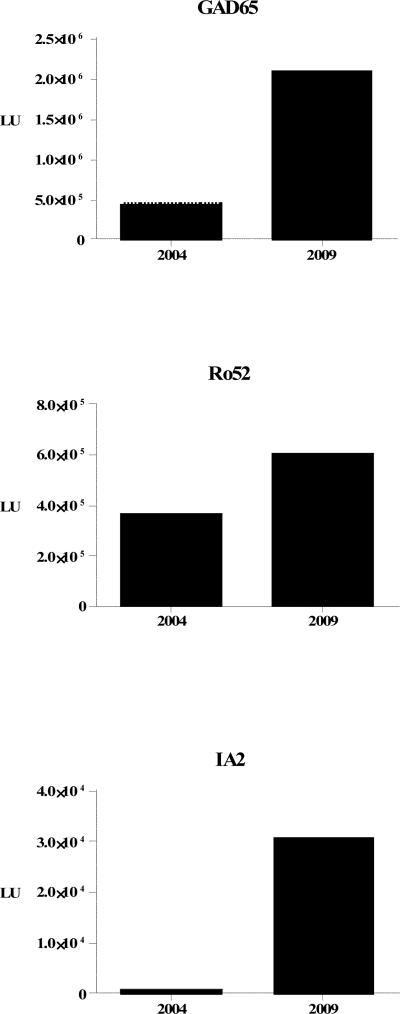
Due to these molecular and clinical findings, patient 225 was reevaluated in August, 2009 at which time her depressive symptoms had been in remission for over a year. However, her symptoms of psychomotor retardation had become more pronounced, and she now showed restricted range of facial expression, reduced gesticulation and fixed posture at the torso while seated (rating 8 on the CORE-retardation subscale). Reevaluation of serum autoantibodies revealed that anti-GAD65 autoantibody titers had increased four-fold, and her anti-Ro52 autoantibody titer doubled (Fig. 2). Testing also showed that her anti-IA2 antibody status, previously negative, was now positive. At this time, she displayed mild hyperglycemia and elevated C peptide. She showed evidence of hyperthyroidism (increased T4, reduced TSH, tachycardia, weight loss).
Fig. 2. Autoantibody titers in patient 225 increase over time.
Autoantibody titers to Ro52, GAD65 and IA2 were measured in patient 225 in April 2004 and again in August 2009. Note that she was seronegative for anti-IA2 autoantibodies in April 2004, but was above the established cutoff and seropositive in August 2009.
4. Discussion
From screening a cohort of psychiatric patients for autoantibodies, a female MDD patient with significant psychomotor disturbance, showed high levels of anti-GAD65 in both serum and CSF. These autoantibodies increased over time and correlated with the observed increase in psychomotor slowing in the patient. The presence of anti-GAD65 autoantibodies in the patient's serum and CSF is consistent with studies detecting these autoantibodies in other neurological diseases with movement disorders including SPS (Solimena et al., 1990), Batten Disease (Chattopadhyay et al., 2002) and cerebellar ataxia (Iwasaki et al., 2001). Nevertheless, anti-GAD65 autoantibodies are also detected before and after the onset of type I diabetes, and it is possible that this patient is developing type I diabetes. However, the patient also showed high levels of autoantibodies against Ro52 in serum and CSF, which increased over time and correlated with exacerbated psychomotor slowing. Although anti-Ro52 autoantibodies are typically found in several systemic autoimmune diseases, including Systemic Lupus Erythematosus (SLE) and Systemic Sclerosis (Franceschini and Cavazzana, 2005), the finding of high serum and CSF autoantibodies against both Ro52 and GAD65 is an unusual combination not commonly seen in type I diabetes or rheumatological disease. Since recent studies suggest that Ro52 may be an immunoglobin-binding protein (Keeble et al., 2008), the presence of these autoantibodies in this patient may reflect B-cell dysfunction. Taken together, these results suggest that the pronounced psychomotor impairment in the patient may be related to autoimmunity. Additional studies are warranted to examine the possibility that psychomotor slowing may have an autoimmune basis in a subset of psychiatric patients.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Burbelo PD, Ching KH, Klimavicz CM, Iadarola MJ. Antibody profiling by Luciferase Immunoprecipitation Systems (LIPS). J Vis Exp. 2009a doi: 10.3791/1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbelo PD, Goldman R, Mattson TL. A simplified immunoprecipitation method for quantitatively measuring antibody responses in clinical sera samples by using mammalian-produced Renilla luciferase-antigen fusion proteins. BMC Biotechnol. 2005;5:22. doi: 10.1186/1472-6750-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbelo PD, Groot S, Dalakas MC, Iadarola MJ. High definition profiling of autoantibodies to glutamic acid decarboxylases GAD65/GAD67 in stiff-person syndrome. Biochem Biophys Res Commun. 2008a;366:1–7. doi: 10.1016/j.bbrc.2007.11.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbelo PD, Hirai H, Issa AT, Kingman A, Lernmark A, Ivarsson SA, Notkins AB, Iadarola MJ. Comparison of radioimmunoprecipitation with luciferase immunoprecipitation for autoantibodies to GAD65 and IA-2{beta}. Diabetes Care. 2010 doi: 10.2337/dc09-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbelo PD, Hirai H, Leahy H, Lernmark A, Ivarsson SA, Iadarola MJ, Notkins AL. A new luminescence assay for autoantibodies to mammalian cell-prepared insulinoma-associated protein 2. Diabetes Care. 2008b;31:1824–1826. doi: 10.2337/dc08-0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbelo PD, Leahy HP, Issa AT, Groot S, Baraniuk JN, Nikolov NP, Illei GG, Iadarola MJ. Sensitive and robust luminescent profiling of anti-La and other autoantibodies in Sjogren's syndrome. Autoimmunity. 2009b;42:515–524. doi: 10.1080/08916930902911738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S, Kriscenski-Perry E, Wenger DA, Pearce DA. An autoantibody to GAD65 in sera of patients with juvenile neuronal ceroid lipofuscinoses. Neurology. 2002;59:1816–1817. doi: 10.1212/01.wnl.0000041913.97883.8b. [DOI] [PubMed] [Google Scholar]
- Franceschini F, Cavazzana I. Anti-Ro/SSA and La/SSB antibodies. Autoimmunity. 2005;38:55–63. doi: 10.1080/08916930400022954. [DOI] [PubMed] [Google Scholar]
- Hadzi-Pavlovic D, Hickie I, Brodaty H, Boyce P, Mitchell P, Wilhelm K, Parker G. Inter-rater reliability of a refined index of melancholia: the CORE system. J Affect Disord. 1993;27:155–162. doi: 10.1016/0165-0327(93)90003-3. [DOI] [PubMed] [Google Scholar]
- Iwasaki H, Sato R, Shichiri M, Hirata Y. A patient with type 1 diabetes mellitus and cerebellar ataxia associated with high titer of circulating anti-glutamic acid decarboxylase antibodies. Endocr J. 2001;48:261–268. doi: 10.1507/endocrj.48.261. [DOI] [PubMed] [Google Scholar]
- Keeble AH, Khan Z, Forster A, James LC. TRIM21 is an IgG receptor that is structurally, thermodynamically, and kinetically conserved. Proc Natl Acad Sci U S A. 2008;105:6045–6050. doi: 10.1073/pnas.0800159105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupka RW, Nolen WA, Post RM, McElroy SL, Altshuler LL, Denicoff KD, Frye MA, Keck PE, Jr., Leverich GS, Rush AJ, Suppes T, Pollio C, Drexhage HA. High rate of autoimmune thyroiditis in bipolar disorder: lack of association with lithium exposure. Biol Psychiatry. 2002;51:305–311. doi: 10.1016/s0006-3223(01)01217-3. [DOI] [PubMed] [Google Scholar]
- Levy LM, Dalakas MC, Floeter MK. The stiff-person syndrome: an autoimmune disorder affecting neurotransmission of gamma-aminobutyric acid. Ann Intern Med. 1999;131:522–530. doi: 10.7326/0003-4819-131-7-199910050-00008. [DOI] [PubMed] [Google Scholar]
- Padmos RC, Bekris L, Knijff EM, Tiemeier H, Kupka RW, Cohen D, Nolen WA, Lernmark A, Drexhage HA. A high prevalence of organ-specific autoimmunity in patients with bipolar disorder. Biol Psychiatry. 2004;56:476–482. doi: 10.1016/j.biopsych.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Parker G, Hadzi-Pavlovic D. Development and Structure of the CORE System. In: Parker G, Hadzi-Pavlovic D, editors. Melancholia: A Disorder of Movement and Mood. Cambridge University Press; New York: 1996. pp. 82–129. [Google Scholar]
- Solimena M, Folli F, Aparisi R, Pozza G, De Camilli P. Autoantibodies to GABA-ergic neurons and pancreatic beta cells in stiffman syndrome. N Engl J Med. 1990;322:1555–1560. doi: 10.1056/NEJM199005313222202. [DOI] [PubMed] [Google Scholar]