Abstract
The selection of an appropriate photoinitiator system is critical for efficient polymerization of dental resins with satisfactory mechanical and physical properties. The purpose of this study was to evaluate the influence of adding an iodonium salt to two-component photoinitiator systems. Four photoinitiator systems were included in a model bisGMA/HEMA resin and used to prepare samples at different water contents; the dynamic mechanical properties and the final degree of conversion of the samples were then characterized. Addition of the iodonium salt to the two-component photoinitiator systems increased the final degree of conversion, glass transition temperature, rubbery modulus, and crosslink density. The photoinitiator system containing ethyl-4-(dimethylamino) benzoate as a co-initiator and the iodonium salt exhibited the highest rubbery modulus. The enhanced properties in the presence of the iodonium salt can be attributed to the production of an active phenyl radical with regeneration of the original camphorquinone, which may increase the compatibility between monomers and initiators, especially in the presence of water. The results support the hypothesis that a photoinitiator system containing an iodonium salt can increase both mechanical properties and final conversion of model resin polymerized in the presence of water.
Keywords: photoinitiator, dynamic mechanical properties, dentin adhesive, degree of conversion, iodonium salt
Introduction
The chemistry of the photoinitiators used in dental resins is critical to their efficient polymerization and to satisfactory mechanical and physical properties of the polymer. Most photoinitiators formulated for commercial dental resins consist of two-components: (i) the photoinitiator (typically a camphorquinone, CQ) which can absorb light directly and (ii) a co-initiator (typically an amine) that does not absorb light but interacts with the activated photoinitiator to generate a reactive free radical and initiates polymerization.1 Camphorquinone (CQ) is a typical visible light-activated free radical photoinitiator with an absorbance range between 400 and 500 nm. CQ requires a tertiary amine reducing agent, usually ethyl-4-(dimethylamino) benzoate (EDMAB) and/or 2-(dimethylamino)ethyl methacrylate (DMAEMA), for efficient polymerization to occur. Three-component systems, in which an iodonium salt is added to the above two-component systems, have recently emerged as efficient, visible-light-sensitive photoinitiators.2
Water present in the mouth is a major interfering factor when bonding adhesives and/or composites to the tooth.3 The water content of the dentin surface varies as a function of depth,4 the nature of the substrate (i.e. caries-affected or healthy),5 and the presence of residual rinse water. Under in vivo conditions, there is little control over the amount of water left on the tooth during dentin bonding. In the presence of water, methacrylate adhesives may undergo phase separation during photopolymerization, which leads to very limited infiltration of BisGMA6. Adhesive phase separation inhibits the formation of an impervious, structurally integrated bond at the composite/tooth interface7,8. Therefore, the behavior of photoinitiators in the presence of water becomes very critical for the success of a bonded restoration.
Although there have been numerous reports on the effect of water and photoinitiators on dental resins,1,9-13 there is little information regarding the effects of three-component photoinitiator system on the dynamic mechanical properties of dentin adhesives. The aim of this study was to evaluate the combined influence of a three-component photoinitiator system and water content on the dynamic mechanical properties and degree of conversion of a light-cured bisGMA/HEMA dental resin. The studies tested the hypothesis that a three-component photoinitiator system containing an iodonium salt (diphenyliodonium hexafluorophosphate (DPIHP)) increases both mechanical properties and final degree of conversion of a bisGMA/HEMA resin.
Materials & Methods
Materials
The model resin consisted of 2,2-bis[4-(2-hydroxy-3-methacryloxypropoxy) phenyl]-propane (BisGMA, Polysciences Inc., Washington, PA, USA) and hydroxyethylmethacrylate (HEMA, Acros Organics, NJ, USA) at 55/45 wt/wt ratio. The adhesives were formulated with 0 wt%, 8 wt% and 16 wt% water to simulate the moist environment of the mouth, and were selected on the basis of our previous work.14,15 The concentration of water was based on the total final weight of the model resin. When monitoring of the aliphatic C=C bond was employed to determine degree of conversion, heavy water (deuterium oxide, 99.9%, D2O) (Cambridge Isotope Laboratories, Inc. Andover, MA, USA) was used due to the absence of overlapping water peak at 1640 cm-1. Four photoinitiator systems (all from Aldrich, Milwaukee, WI) were used (Table 1). The two-component systems contained camphorquinone (CQ) as a hydrophobic photosensitizer and 2-(dimethylamino)ethyl methacrylate (DMAEMA) as a hydrophilic co-initiator or CQ and ethyl-4-(dimethylamino) benzoate (EDMAB) as a hydrophobic co-initiator. The three-component systems were prepared by adding diphenyliodonium hexafluorophosphate (DPIHP) as the iodonium salt to each of the two-component systems.
Table I. Degree of Conversion Values and Curing Time of Adhesive Resins Containing Different Photoinitiator Systems and Different Water Content.
| Samples | Photoinitiator systema | Water content (%) | DC (%)b | CT (sec)c |
|---|---|---|---|---|
| CD-0 | CQ/DMAEMA | 0 | 74.7 (0.4) | 33 (0.7) |
| CD-8 | CQ/DMAEMA | 8 | 37.9 (3.4) | n/ad |
| CD-16 | CQ/DMAEMA | 16 | 37.8 (1.3) | n/a |
| CE-0 | CQ/ EDMAB | 0 | 84.9 (0.6) | 9 (0.5) |
| CE-8 | CQ/ EDMAB | 8 | 85.2 (0.4) | 19 (0.5) |
| CE-16 | CQ/ EDMAB | 16 | 89.7 (0.2) | 18 (0.5) |
| CDD-0 | CQ/DMAEMA/DPIHP | 0 | 88.0 (0.6) | 7 (0.4) |
| CDD-8 | CQ/DMAEMA/DPIHP | 8 | 93.9 (0.7) | 5 (0.4) |
| CDD-16 | CQ/DMAEMA/DPIHP | 16 | 95.7 (0.5) | 7 (0.4) |
| CED-0 | CQ/ EDMAB/DPIHP | 0 | 92.4 (1.4) | 5 (0.4) |
| CED-8 | CQ/ EDMAB/DPIHP | 8 | 97.2 (0.4) | 5 (0.4) |
| CED-16 | CQ/ EDMAB/DPIHP | 16 | 97.3 (0.7) | 5 (0.4) |
Abbreviations: CQ = Camphorquinone; DMAEMA = 2-(dimethylamino)ethyl methacrylate; EDMAB = ethyl-4-(dimethylamino) benzoate; DPIHP = diphenyliodonium hexafluorophosphate.
DC was determined by by using a LabRAM ARAMIS Raman spectrometer.
The curing time was taken as the period from which the light exposure was initiated to the moment at which the metal rod could not be moved by hand. The data are presented as mean values with standard deviations in parentheses, and the number of specimens tested for DC and CT is 4.
CT could not be measured because the cured polymer was gel-like due to its low DC.
Viscosities of the model adhesive resins
Rheological measurements for the liquid resin formulated with / without water were carried out in a TA Instruments AR2000 rheometer (New Castle, DE) in the controlled-rate mode. The measurements were made at 25 °C with 40 mm diameter and 2 ° cone angle in the shear rate range of 10/s to 100/s, at 10 points per decade to generate data on viscosity and shear rate.
Sample preparation, degree of conversion, and curing time
Rectangular beam specimens (1×1×11 mm3) cured in a glass-tubing mold (Wilmad Labglass, #LG-25001-100, Standard wall borosilicate tubing) were prepared for the determination of dynamic mechanical properties and degree of conversion (DC). The model adhesives were light-cured for 40 sec at room temperature with a commercial visible light-curing unit (Spectrum® 800, Dentsply, Milford, DE, USA) at an intensity of 550 mW cm-2 placed at a distance of 1 mm according to a protocol published previously.16 The polymerized samples were stored at room temperature for 2 days in a dark room, and then for 1 week in a vacuum oven in the presence of a drying agent.
The DC of the methacrylate double bond was obtained using a LabRAM ARAMIS Raman spectrometer (LabRAM HORIBA Jobin Yvon, Edison, New Jersey) with a HeNe laser (λ=633 nm, a laser power of 17 mW) as an excitation source. The instrument conditions were: 200 μm confocal hole, 150 μm wide entrance slit, 600 gr/mm grating, and 10 × objective Olympus lens. Data processing was performed using LabSPEC 5 (HORIBA Jobin Yvon). The samples were mounted in a computer-controlled, high-precision x-y stage. To determine the DC, spectra of the uncured resins and beam samples were acquired over a range of 700 – 1800 cm-1. The change of the band height ratios of the aliphatic carbon-to-carbon double bond (C=C) peak at 1640 cm-1 and the aromatic C=C at 1610 cm-1 (phenyl) in both the cured and uncured states was monitored and DC17,18 was calculated by using the following equation based on the decrease in the intensity band ratios before and after light curing:
where R = band height at 1640 cm-1/band height at 1610 cm-1.
Curing time was evaluated by inserting a metal rod into the center of the adhesive resin immediately after placing the material into a two-end open glass tubing.19 The curing time was taken as the period from which the light exposure was initiated to the movement at which the metal rod could not be moved at all by hand, and reported as the average of four readings. The metal rod movement was approximately two times per second. The error in curing time is not more than half a second.
Dynamic mechanical analysis (DMA)
The viscoelastic properties of the polymerized dentin adhesives were characterized using DMA Q800 (TA Instruments, New Castle, USA) with a three-point bending clamp. In DMA, a sinusoidal stress is applied and the resultant strain is measured. The properties measured under this oscillating loading are storage modulus, loss modulus, and tan δ. The storage modulus (E′) represents the stiffness of a viscoelastic material and is proportional to the energy stored during a loading cycle. The loss modulus (E″) is related to the amount of energy lost due to viscous flow. The ratio of loss (E″) to storage modulus (E′) is referred to as the mechanical damping, or tan δ. For the DMA test, the temperature was varied from -20 to 200 °C with a ramping rate of 3 °C/min at a frequency of 1 Hz. No pre-heating cycle was applied, and the storage modulus and tan δ were recorded as a function of temperature. The tan δ value goes through a maximum as the polymer undergoes the transition from the glassy to the rubbery state. The glass transition temperature (Tg) was determined as the position of the maximum on the tan δ vs. temperature plot. Five specimens of each material were measured and the results averaged.
The results were analyzed statistically using analysis of variance (ANOVA), together with Tukey's test at α=0.05 (Microcal Origin Version 6.0, Microcal Software Inc., Northampton, MA).
Results
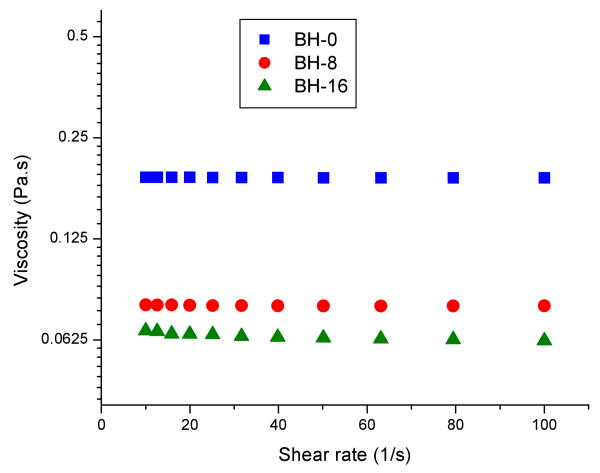
Figure 1 shows the measured viscosities of adhesive resins with different water content as a function of shear rate at 25 °C. Typical Newtonian behavior, with viscosity independent of shear rates, is observed. The viscosity of the resin solutions decreased in the order (Pa.s): BH-0 (0.191) > BH-8 (0.079) > BH-16 (0.065). As expected, the viscosities of the resin solutions decrease with increasing water content.
Figure 1.
The viscosities of adhesive resins containing different water content as a function of shear rate at 25 °C. Abbreviations: BH-0 = bisGMA/HEMA=55/45 + 0 wt% water; BH-8 = bisGMA/HEMA=55/45 + 8 wt% water; BH-16 = bisGMA/HEMA=55/45 + 16 wt% water.
Table I shows the degree of conversion (DC) and the curing time (CT) of adhesive resins containing different photoinitiator systems. For the two-component systems, DC values of samples initiated by CQ/DMAEMA (i.e., CD-0, CD-8, CD-16) are lower than those for the other three photoinitiator systems. Significantly, DC values for samples initiated by CQ/DMAEMA are decreased dramatically by polymerization in the presence of water, from ∼75% in the absence of water (CD-0, Table I) to ∼38% with water (CD-8, CD-16, Table I). Including DPIHP significantly (p < 0.05) increased the DC for all photoinitiator systems, regardless of the presence of water (Table I). For example, incorporation of DPIHP into the CQ/DMAEMA system increased the DC from 75% (CD-0, Table I) to 88% (CDD-0, Table I) for the adhesives cured in the absence of water. In the presence of 8% water, the DC increased from 38% (CD-8, Table I) to 94% (CDD-8, Table I). Similarly, the incorporation of DPIHP into the CQ/EDMAB system increased the DC from 85% (CE-8, Table I) to 97% (CED-8, Table I) in the presence of 8 wt% water.
The curing time (CT) in the two-component systems was greater than in the three-component systems, and even longer in the presence of water (Table I). Interestingly, the CQ/DMAEMA system showed a CT of 33 seconds in the absence of water (CD-0, Table I), but, in the presence of water, metal rod movement continued freely as exposed to the light for over 5 minutes (CD-8, CD-16, Table I). The resulting resins have a low DC and gel-like consistency. The CT values in the three-component systems were on the order of 5 sec, and were unaffected by the presence of water.
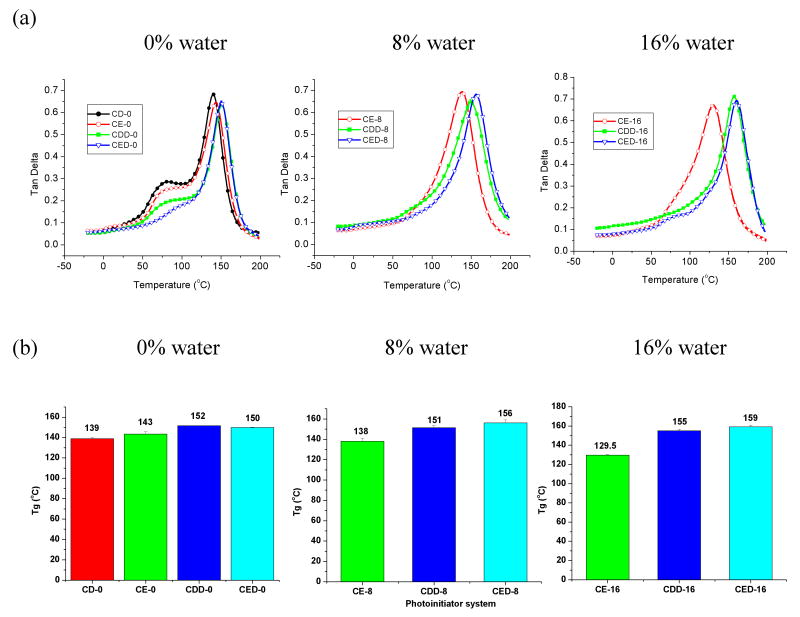
Figure 2 shows tan δ values as a function of temperature (Fig. 2a) and average glass transition temperatures (Tg) of model adhesives (Fig. 2b) cured in the absence and presence of water. The tan δ values gradually increased with increasing temperature for all samples tested, reaching a maximum in the glass transition region (Fig. 2a). Samples cured in the absence of water showed a shoulder at low temperature, a feature generally absent for samples cured in the presence of water (Fig. 2a). As mentioned above, the sample formulated with CQ/DMAEMA as two-component system and cured in the presence of water was gel-like, and thus, could not be tested by DMA. In addition, Tg values for samples polymerized with the CQ/EDMAB system were lower (130 to 143 °C) than those for the three-component systems (151 to 159 °C), regardless of the presence of water. Tg values for the two-component photoinitiator systems decreased slightly with increasing water content, while those for the three-component photoinitiator system showed no change or a slight increase with increasing water content (Fig. 2a, 2b).
Figure 2.
Representative tan δcurves (a) and average glass transition temperatures (Tg) (b) of dentin adhesives containing different water content and different photoinitiators as a function of temperature. N = 5 ± SD. See Table I for adhesive abbreviations.
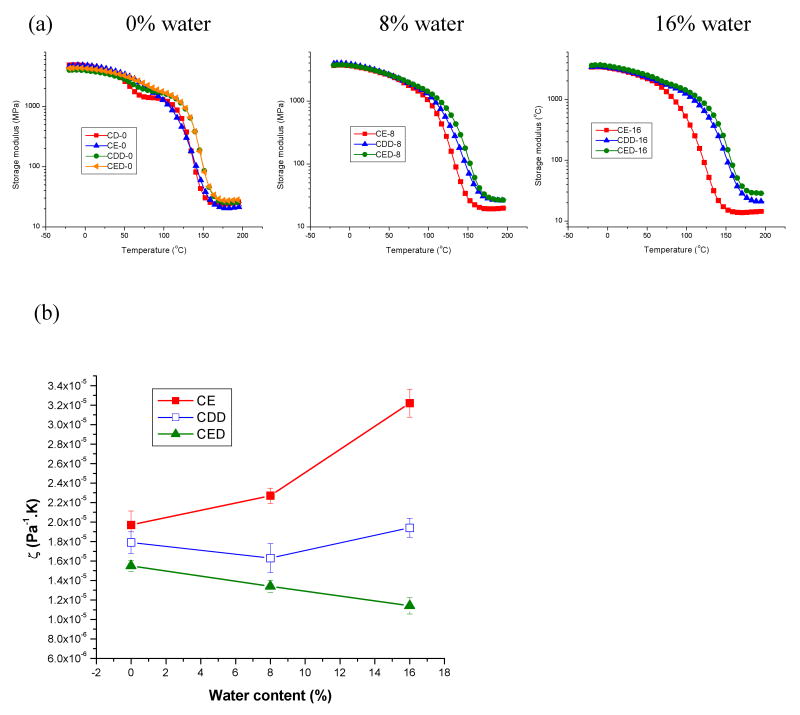
Figure 3a shows representative storage moduli of adhesives containing different photoinitiator systems and water content as a function of temperature. Figure 3b shows the inverse ratio (ζ) of the modulus in the rubbery region to the temperature at which the modulus was measured as a function of water content (%). Storage modulus values for polymer networks cured in the absence of water are in the range of 3.4 ∼ 3.7 GPa at 37 °C and in the range of 21 ∼ 26 MPa at temperatures in the rubbery plateau region. Similar values of storage modulus were obtained for all systems at 37 °C, regardless of water content. However, somewhat higher values were obtained for three-component systems in the rubbery region cured in the presence of water. The ζ value for the three-component system is less than that of two-component system (Fig. 3b). Interestingly, the ζ value for the CQ/EDMAB system increases with increasing water content, but generally decreases with increasing water content for the three-component systems. The lowest ζ values are observed for CQ/EDMAB/DPIHP photocured in the presence of 16% water, suggesting higher crosslink density in this resin. The difference between CQ/EDMAB and CQ/EDMAB/DPIHP is greatest at the highest water content (16%).
Figure 3.
Representative storage modulus of adhesives containing different photoinitiator systems and different water content as a function of temperature (a) and the inverse ratio (ζ) of the modulus in the rubbery region to temperature at which the modulus was measured plotted as a function of water content (%) in adhesives (b). ζ is inversely related to the crosslinking density of the copolymer.N = 5 +/- S.D. . See Table I for adhesive abbreviations.
Discussion
In dental resins, photopolymerization is catalyzed by mixed photoinitiator systems and occurs in the moist environment of the mouth. The model resin formulations used in this study are a mixture of a hydrophobic component (bisGMA) and a hydrophilic components (HEMA) and are based on conventional dentin adhesives.6,20 Commercial adhesives were not used, since differences in filler type and content, additives and processing conditions by the various manufacturers may influence results and adversely affect reproducibility. The model resins were formulated with water to simulate wet bonding conditions in the mouth and to allow for possible phase separation of the adhesive during photopolymerization.
Because water is present in the oral cavity, it is important to understand how the various properties of adhesives are affected by polymerization in the presence of water. Viscosity is also of practical importance for dental adhesives, since less viscous formulations are generally more desirable because of their ease of processing in various applications and their ability to infiltrate the demineralized dentin matrix. In the current study, the sharp decline in the viscosity of resins containing water can be attributed to the disruption of intermolecular associations caused by the presence of water (Fig. 1).
Incomplete polymerization can compromise the performance of resin-based dental restoratives. The presence of residual monomer can have a plasticizing effect on the polymer, thereby altering the physical and mechanical properties of the hardened materials.21 In addition, the presence of unreacted monomer can make the polymeric matrix more susceptible to oxidative and hydrolytic degradation reactions, leading to poor durability. It is important, therefore, to evaluate the final degree of conversion of monomer to polymer after polymerization. The studies reported here showed dramatic differences in DC and CT of bisGMA/HEMA resin between the two-component (CQ/DMAEMA and CQ/EDMAB) and three-component systems (CQ/DMAEMA/DPIHP and CQ/EDMAB/DPIHP) (Table I). Moreover, the resin formulated with the aliphatic amine (i.e., DMAEMA) showed significantly (p<0.05) lower DC and longer CT than the resin formulated with the aromatic amine(i.e., EDMAB), especially in the presence of water. This result is in agreement with previous findings that demonstrated a faster polymerization rate and higher DC with the CQ/aromatic amine initiator system.11,22 The addition of DPIHP to the two-component initiator systems thus increased DC and reduced CT dramatically.
Because DMA gives information on the relaxation of molecular motions which are sensitive to structure, it can be used to provide information on the properties of polymer networks, such as storage modulus, glass transition temperature and structural heterogeneity. DMA is particularly suitable for determining glass transitions because the change in modulus is much more pronounced in DMA than, for example, the heat capacity change in a DSC measurement. High Tg values are generally desirable for dental restoratives, since creep and distortion resulting from consuming hot fluids and foods are minimized with high Tg materials.12 The widths of the tan δ curves (Fig. 2) indicate that the glass transition occurs over a wide range of temperature rather than at a specific temperature. This broad glass transition can be attributed to the fact that the polymerization of multifunctional monomers produces heterogenous networks containing both highly crosslinked and less densely crosslinked regions.23 The main peaks of the tan δ curve correspond to polymer main chain relaxation. The shoulder at lower temperature for adhesives cured in the absence of water can be attributed to relaxation of chain segments for different crosslinked regions, which may be associated with the restriction of mobility of the propagating radicals with relatively high viscosity in a bulk solution without water. Interestingly, it can be seen that adhesives containing three-component photoinitiator systems showed Tg values that remained constant or increased with an increase in water content. In contrast, the polymer cured with the two-component CQ/DMAEMA system in the presence of water was gel-like and could not be tested mechanically, while the Tg of polymers cured with CQ/EDMAB system decreased with increasing water content. These results indicate that Tg is influenced by not only DC, but also by crosslink density; although CQ/EDMAB system gives higher DC as water content is increased, crosslink density may be different due to the poor compatibility of monomers with initiators and micro-phase separation in the presence of water.
The storage modulus indicates a change from a glassy state to the rubbery state over the temperature range. At very low temperatures, all the samples tested show a gradual decrease in storage moduli with increasing temperature. Near the glass transition temperature, storage moduli decrease drastically. As heating continues, all the samples reach the rubbery plateau, in which the storage modulus is insensitive to further increase in temperature. Among the photoinitiator systems tested, the three-component CQ/EDMAB/DPIHP system exhibited the highest rubbery modulus regardless of water content, while the comparable two-component system (i.e., CQ/EDMAB) showed significantly lower rubbery modulus in the presence of water. This rubbery modulus value has been related to the crosslink density of the material.24 The ratio of rubbery modulus to the absolute temperature at which that modulus was measured, ζ, is inversely related to the crosslink density of the polymer network and is directly proportional to the molecular weight between crosslinks.25,26 By this measure, the adhesives formulated with the aromatic amine (EDMAB) as a co-initiator and DPIHP as a third component showed higher crosslink density than those formulated with the aliphatic amine and a two-component initiator system, an implication consistent with the DC, Tg, and storage modulus results.
Thus, the results presented here suggest that DMAEMA is a less efficient photoreducer than EDMAB, leading to lower DC and dynamic mechanical properties. This behavior may be attributed to the fact that DMAEMA is more prone to combine with oxygen than aromatic amines.11,27 In addition, since DMAEMA carries a methacrylate group with a double bond, DMAEMA-dimer or oligomers may be formed in the presence of radicals.11,27 The addition of DPIHP to the two-component photoinitiator systems increased the final degree of conversion, Tg, storage modulus, and crosslink density, especially in the presence of water. The enhanced properties observed in the presence of the iodonium salt, DPIHP may be due in part to its ability to generate an active phenyl radical. As an electron acceptor, the iodonium salt abstracts an electron from the inactive CQ neutral radical, regenerating the original CQ and producing a diphenyliodonium radical. The diphenyliodonium radical rapidly fragments into a molecule of phenyl iodide and a phenyl radical that is very active in initiating the polymerization.28 In addition, since DPIHP is ionic in nature as a salt, it may increase the compatibility between amphiphilic monomers (i.e., having both hydrophilic and hydrophobic characteristics) and initiators, especially in the presence of water.13 The results indicate that the performance of photoinitiator systems can be quite sensitive to the presence of water, and thus these systems should be evaluated under both dry and wet conditions.
Acknowledgments
This investigation was supported by Research Grant: R01DE14392 (PI: Spencer) from the National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, MD 20892.
References
- 1.Ogunyinka A, Palin WM, Shortall AC, Marquis PM. Photoinitiation chemistry affects light transmission and degree of conversion of curing experimental dental resin composites. Dental Materials. 2007;23:807–813. doi: 10.1016/j.dental.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 2.Kim D, Scranton AB. The role of diphenyl iodonium salt (DPI) in three-component photoinitiator systems containing methylene blue (MB) and an electron donor. J Polym Sci A: Polym Chem. 2004;42:5863–5871. [Google Scholar]
- 3.Ferracane JL. Hygroscopic and hydrolytic effects in dental polymer networks. Dental Materials. 2006;22(3):211–222. doi: 10.1016/j.dental.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Spencer P, Hager C, Bohaty B. Comparison of interfacial characteristics of adhesive bonding to superficial versus deep dentin using SEM and staining techniques. J Dent. 2006;34:26–34. doi: 10.1016/j.jdent.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Ito S, Saito T, Tay FR, Carvalho RM, Yoshiyama M, Pashley DH. Water content and apparent stiffness of non-caries versus caries-affected human dentin. J Biomed Mater Res B Appl Biomater. 2005;72(1):109–16. doi: 10.1002/jbm.b.30130. [DOI] [PubMed] [Google Scholar]
- 6.Spencer P, Wang Y. Adhesive phase separation at the dentin interface under wet bonding conditions. J Biomed Mater Res. 2002;62:447–56. doi: 10.1002/jbm.10364. [DOI] [PubMed] [Google Scholar]
- 7.Spencer P, Wang Y, Bohaty B. Interfacial chemistry of moisture-aged class II composite restorations. J Biomed Mater Res B Appl Biomater. 2006;77:234–40. doi: 10.1002/jbm.b.30434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Spencer P. Interfacial chemistry of Class II composite restoration: Structure analysis. J Biomed Mat Res. 2005;75A:580–587. doi: 10.1002/jbm.a.30451. [DOI] [PubMed] [Google Scholar]
- 9.Sauro S, Watson TF, Tay FR, Chersoni S, Breschi L, Bernardi F, Prati C. Water uptake of bonding systems applied on root dentin surfaces: A SEM and confocal microscopic study. Dent Materials. 2006;22:671–80. doi: 10.1016/j.dental.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Fong H. Effects of Water Contents and Postcuring Conditions on Bis-GMA/TEGDMA Dental Restorative Composite Resins. Journal of Applied Polymer Science. 2004;94:492–502. [Google Scholar]
- 11.Schroeder WF, Vallo CI. Effect of different photoinitiator systems on conversion profiles of a model unfilled light-cured resin. Dental Materials. 2007;23:1313–1321. doi: 10.1016/j.dental.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 12.Rueggeberg FA, Ergle JW, Lockwood PE. Effect of photoinitiator level on properties of a light-cured and post-cure heated model resin system. Dental Materials. 1997;13:360–364. doi: 10.1016/s0109-5641(97)80107-8. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Spencer P, Yao X, Ye Q. Effect of co-Initiator and water on the photoreactivity and photopolymerization of HEMA/camphoroquinone-based reactant mixtures. J Biomed Mater Res. 2006;78A(3):721–728. doi: 10.1002/jbm.a.30733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ye Q, Wang Y, Spencer P. NanoPhase Separation in Polymers Exposed to Simulated Oral Environment. J Biomed Mater Res Appl Biomater, special issue. 2008;(special issue(early view)) doi: 10.1002/ibm.b.31047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye Q, Spencer P, Wang Y. Nanoscale patterning in crosslinked methacrylate copolymer networks: an atomic force microscopy study. J Appl Polym Sci. 2007;106:3843–3851. doi: 10.1002/app.27044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ye Q, Wang Y, Williams K, Spencer P. Characterization of photopolymerization of dentin adhesives as a function of light source and irradiance. J Biomed Mater Res Appl Biomater. 2007;80B(ye):440–446. doi: 10.1002/jbm.b.30615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shin WS, Li XF, Schwartz B, Wunder SL, Baran GR. Determination of the degree of cure of dental resins using Raman and FT-Raman spectroscopy. Dental Materials. 1993;9:317–324. doi: 10.1016/0109-5641(93)90050-z. [DOI] [PubMed] [Google Scholar]
- 18.Santis AD, Baldi M. Photo-polymerization of composite resins measured by micro-Raman spectroscopy. Polymer. 2004;45:3797–3804. [Google Scholar]
- 19.Xie D, Feng D, Chung ID, Eberhardt AW. A hybrid zinc–calcium–silicate polyalkenoate bone cement. Biomaterials. 2003;24(16):2749–2757. doi: 10.1016/s0142-9612(03)00090-5. [DOI] [PubMed] [Google Scholar]
- 20.Pashley EL, Zhang Y, Lockwood PE, Rueggeberg FA, Pashley DH. Effects of HEMA on Water Evaporation From Water-HEMA Mixtures. Dental Materials. 1998;14:6–10. doi: 10.1016/s0109-5641(98)00003-7. [DOI] [PubMed] [Google Scholar]
- 21.Vallo CI. Flexural strength distribution of a PMMA-based bone cement. J Biomed Mater Res Part B: Appl Biomater. 2002;63:226–236. doi: 10.1002/jbm.10129. [DOI] [PubMed] [Google Scholar]
- 22.Guo X, Wang Y, Spencer P, Ye Q, Yao X. Effects of water content and initiator composition on photopolymerization of a model BisGMA/HEMA resin. Dental Materials. 2008;24(6):824–831. doi: 10.1016/j.dental.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kannurpatti AR, Anderson KL, Anseth JW, Bowman CN. Use of “Living” radical polymerizations to study the structural evolution and properties of highly crosslinked polymer networks. J Polym Sci Part B: Polym Phys. 1997;35:2297–2307. [Google Scholar]
- 24.Kannurpatti A, Anseth J, Bowman C. A study of the evaluation of mechanical properties and structural heterogeneity of polymer networks formed by photopolymerizations of multifunctional (meth)acrylates. Polymer. 1998;39(12):2507–2513. [Google Scholar]
- 25.Charlesworth JM. Effect of crosslink density on molecular relaxations in diepoxide-diamine network polymers. Part 2. The rubbery plateau region. Polymer Engineering & Science. 1988;28(4):230–236. [Google Scholar]
- 26.Treloar LPG. The Physics of Rubber Elasticity. London: Oxford University Press; 1958. [Google Scholar]
- 27.Teshima W, Nomura Y, Tanaka N, Urabe H, Okazaki M, Nahara Y. ESR study of camphorquinone/amine photoinitiator systems using blue light-emitting diodes. Biomaterials. 2003;24:2097–2103. doi: 10.1016/s0142-9612(02)00636-1. [DOI] [PubMed] [Google Scholar]
- 28.Padon KS, Scranton AB. A Mechanistic Investigation of a Three-Component Radical Photoinitiator System Comprising Methylene Blue, N-Methyldiethanolamine, and Diphenyliodonium Chloride. Journal of Polymer Science: Part A: Polymer Chemistry. 2000;38:2057–2066. [Google Scholar]