Preface
Neuronal synapses are important microstructures that underlie complex cognitive capacities. Recent studies, primarily in C. elegans and Drosophila, have revealed surprising similarities between these synapses and ‘sensory synapses’ residing at the tips of chemosensory cells that respond to environmental stimuli. Similarities in the structure, mechanisms, and specific molecules found at these sites extend to the pre-synaptic, post-synaptic, and glial entities composing each synapse type. In this article I propose that chemosensory synapses may serve as useful models of neuronal synapses, and consider the possibility that both synapse types derive from a common ancestral structure.
Neuronal synapses, the physical structures connecting neurons to each other, are major sites of information processing in the nervous system. These synapses are composed of pre-synaptic and post-synaptic neuronal termini1, and, in the case of excitatory synapses, are often ensheathed by glial extensions2,3. Information at neuronal synapses is conveyed by neurotransmitters released by presynaptic cells, and is processed at these sites by all three synapse-associated cells. Presynaptic neuronal termini control synaptic neurotransmitter activity by regulating release, reuptake, and neurotransmitter chemical structure. Postsynaptic termini control neurotransmitter efficacy by controlling recognition, through receptor choice, by inhibiting neurotransmitter activity (chemically or competitively), and by integrating multiple neurotransmitter signals from one or multiple presynaptic cells. Glial cells take up and release neurotransmitters, often in response to presynaptic neuronal cues, and also release neurotransmitter and neurotransmitter receptor inhibitors4 (Box 1). The synapse is, therefore, a complex and highly regulated information processing module.
Box 1. Glial functions at the synapse
Efforts to understand roles played by glia at the synapse have begun to reveal the developmental and functional importance for these cells at this information transfer site. Developmentally, glia-derived factors, including cholesterol74 and the secreted protein thrombospondin61 promote synapse formation; and phagocytic functions of glia seem to participate in synaptic remodeling at the neuromuscular junction75. Glia also seem to play important roles in defining and positioning synaptic sites during development in C. elegans76, and have been reported to regulate dendritic spine shape in the mouse through Eph signaling77. Although the functions of glia at synapses are not fully understood, they have been implicated in the regulation of a number of processes likely to affect synaptic efficacy. Glial cells express a variety of neurotransmitter transporters, including those for glutamate78, glycine79, and GABA80. Evidence that glia secrete neurotransmitters, including glutamate81, acetylcholine82, GABA83, and ATP84, has also been reported, suggesting possible direct effects of glia on postsynaptic targets. Glia also produce neurotransmission inhibitors, and these have been shown to have important functional consequences in specific settings. For example, a glia-derived acetylcholine receptor mimic attenuates cholinergic signaling in the fresh water snail, and D-serine, an NMDA receptor antagonist, is released by astrocytes, and may influence glutamatergic signaling in the CNS85.
Although the characterization of synaptic glia is well on its way, molecular studies of sensory organ glia have lagged behind. However, recent efforts to fill this gap by examining transcripts enriched in glia associated with sensory synapses in C. elegans have yielded intriguing gene lists32. In these lists are found genes encoding proteins containing thrombospondin type I domains, transporters related to GABA transporters, neurotransmitter-like peptides, glutamate receptors, and transporters involved in ion homeostasis. If validated, these similarities may further strengthen the idea that glia at sensory and neuronal synapses share common activities. Furthermore, studies of C. elegans sensory organ glia reveal key roles for these cells in regulating sensory neuron receptive ending morphology32, a phenomenon reminiscent of spine morphology control by astrocytes.
Sensory organs, like synapses, are also important sites of information processing. Environmental stimuli are encountered at these structures, and these signals are interpreted within sensory cells before passing on to higher levels of the nervous system. The dynamic range of sensory cells determines, in part, whether environmental signals can be distinguished from each other, and many sensory cells display adaptation aimed at blunting repetitive stimuli. Sensory capacities of modern day organisms are elaborate and diverse, and although it is unclear whether all sensory organs evolved from a common ancestral structure, some are likely to have evolved from a system whose primary task was the detection of environmental chemicals. Indeed, intriguing similarities between one class of animal and plant chemoreceptor and bacterial proteins tasked with detecting environmental chemicals5-7 have been described, suggesting that chemosensation is a very ancient sensory modality.
Recent studies, primarily in the nematode Caenorhabditis elegans and the fruitfly Drosophila melanogaster, have revealed intriguing commonalities in the logic, organization, and molecular machineries used by chemosensory receptive structures and neuronal synapses. In each system, small molecules- either metabolites present in the environment or neurotransmitters released by a presynaptic cell- bind to specific receptors localized to highly specialized cellular compartments on receptive neurons. Binding leads directly or indirectly to the opening or closing of ion channels, leading to neuronal activation or inhibition. Although receptor-ligand interactions occur widely in nature and control processes as diverse as cell-cell communication in the immune system, and endocrine signaling, the similarities uncovered in recent years between chemosensory organs and synapses go beyond generic parallels in signal transduction. Surprising molecular similarities have emerged between chemosensory structures and synapses at each level: the small molecule signals, the specialized receptive compartments, the identities of the receptors, and even the neighboring glia that surround each structure. These striking parallels suggest that ‘chemosensory synapses’ and perhaps ‘sensory synapses’ in general (not to be confused with the neuron-neuron synapses made by sensory neurons onto downstream interneurons) may be used to guide our understanding of neuron-neuron synapses. In this article, similarities between chemosensory receptive structures and neuron-neuron synapses are primarily explored, with additional parallels drawn from structures associated with other sensory modalities.
Presynaptic similarities
Although chemosensory-neuron termini are not associated with a physical presynaptic structure, there are parallels between the environmental food cues detected by chemosensory neurons, tuned to respond to essential food constituents, and presynaptically released neurotransmitters. For example, C. elegans, zebrafish, and mice can sense and chemotax towards amino acids, essential food constituents in their environment8-10. Likewise humans can taste amino acids (the Umami basic taste), and are particularly sensitive to the amino acid glutamate11. Many abundant and potent neurotransmitters are also amino acids12. These include glutamate, the major excitatory neurotransmitter in the mammalian brain, and glycine and GABA, two major inhibitory neurotransmitters. Other neurotransmitters, including serotonin, dopamine, epinephrine, norepinephrine, octopamine, and tyramine are derivatives of the amino acids tryptophan and tyrosine, and the neuromodulator histamine is a histidine derivative.
Similarities between chemotactic signals that trigger signaling at chemosensory synapses and the neurotransmitter signals that activate canonical synapses also extend to other essential nutrients. For example, C. elegans can chemotax effectively towards choline13, a precursor of the essential membrane lipid phosphatidylcholine14, and the simple choline derivative acetylcholine (ACh) is a key neurotransmitter at the neuromuscular junction and at neuron-neuron synapses. Chemosensory neurons are also potent detectors of pH, as demonstrated by chemosensory-neuron-dependent avoidance of high proton concentrations in C. elegans15. Similarly, in C. elegans, protons can also serve as transmitters for muscle contraction16. Although in this case protons are released from intestinal cells, and not from a classical presynaptic terminus, they are nevertheless acting in the capacity of a transmitter. Thus, many important neurotransmitters are derivatives of or are themselves well-established sensory stimuli (Figure 1).
Figure 1. Similarities between sensory receptive endings and neuron-neuron synapses.
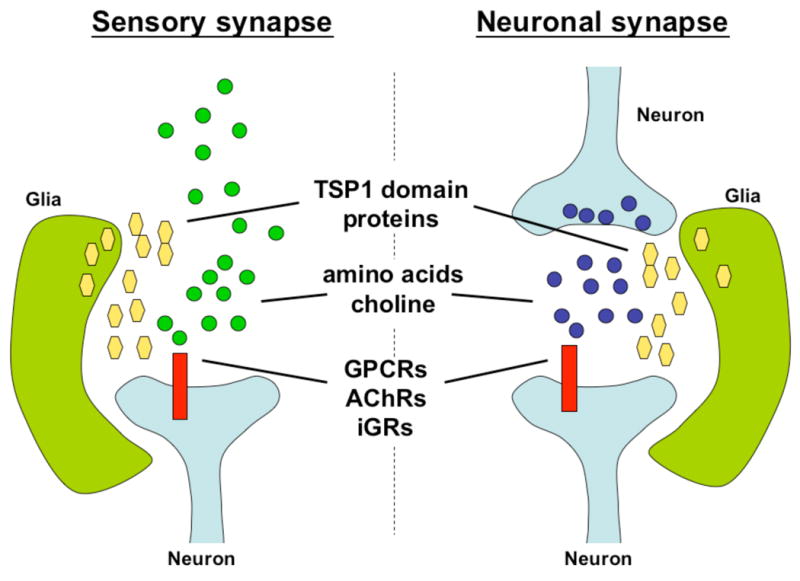
Structural and molecular similarities between sensory receptive endings and neuronal synapses are depicted. Both structures share commonalities in the ligands (amino acids, choline), in the receptors (GPCRs, AChrs, and iGRs), and in glial secreted proteins required for synaptic function (TSP1 domain proteins). Blue circles, neurotransmitters. Green circles, environmental nutrients. Yellow hexagons, thrombospondins and related proteins. Red rectangles, receptors for either neurotransmitters or environmental nutrients.
Postsynaptic similarities
Given that chemosensory synapses mirror canonical synapses in the cues they can detect, it may not be surprising to find that these similarities extend to the physical structures used for detection. At excitatory neuronal synapses, dendritic spines are the major components of postsynaptic neuronal architecture; whereas at chemosensory synapses most signal detection takes place within specialized chemosensory receptive endings residing at dendritic tips or at tips of specialized sensory cells. These structures are the major localization sites for neurotransmitter receptors and environmental receptors, respectively, and house signal transduction molecules and ion channels crucial for stimulus transmission.
Polarity and Trafficking
Both chemosensory and postsynaptic synaptic sites reside in apically polarized domains of their respective cells. It is presently unknown whether specification of these structures is guided by common polarity signals; however, axonal termini of at least some chemosensory neurons and central neurons are specified by shared mechanisms. For example, the kinase SAD-1 and its binding partner Neurabin are required for localizing presynaptic components to axons in both chemosensory and postsynaptic neurons17,18, and in C. elegans, sad-1 mutants inappropriately accrete presynaptic components within dendrites of both sensory neurons and motorneurons. These results suggest that formation of polarized structures that promote receptive ending formation, may also be under common control in these two cell types.
Trafficking of receptors and other signaling proteins is thought to be governed by pre-existing polarity cues. Some trafficking components seem to be used to deliver both chemosensory and neurotransmitter receptors to their respective dendritic compartments. For example, the C. elegans μ1 subunit of the clathrin adaptor complex AP1 is required for localizing both odorant receptors in sensory neurons19 and neurotransmitter receptors in postsynaptic neurons20. Similarly, the small GTPase Rab11 is required in Drosophila for rhodopsin localization to apical membranes21, and in rats for localization of AMPA receptors to postsynaptic membranes22. These similarities suggest not only relatedness in cargo, but also strengthen the possibility that polarity cues involved in generating sensory and postsynaptic signaling structures are related.
Signaling compartments
At first glance, chemosensory receptive endings and dendritic spines may appear only superficially similar: although both are cytoskeleton-rich membrane specializations, chemosensory receptive endings are often non-motile cilia that depend on microtubules for their structure23, while spine morphology is thought to be governed by actin24. However, several observations suggest these distinctions are not so clear-cut. Some chemosensory cells do not utilize cilia for signal detection. For example, taste receptor cells in mammals terminate in microvilli containing actin and actin binding Espin proteins25,26. Other sensory cells also use microvilli instead of cilia. For example, although sensory hair cells in the inner ear each contain a single microtubule-based kinocilium, mechanosensation is thought to take place primarily in the numerous stereocilia, which like spines are composed of actin27,28. A similar cellular architecture characterizes the C. elegans thermosensory neuron AFD, which possesses a single microtubule-based cilium surrounded by an array of microvilli-like protrusions lacking microtubules, and presumably supported by actin29,30. Loss of these microvilli but not of the cilium as a result of mutations in the gene ttx-1 or by ablation of neighboring glia is associated with thermosensory deficits31,32. Thus, actin is no stranger to sensory receptive endings. Conversely, microtubules may have a role to play at dendritic spines. A recent report suggests that microtubules are important regulators of dendritic spine morphology and interact through the microtubule-associated protein EB3 with the p140Cap/SNIP protein, which is enriched at the postsynaptic density (PSD)33. These observations suggest that the paradigm that dendritic spines use actin and sensory endings use microtubules is an oversimplification.
Regardless of whether postsynaptic and sensory structures employ actin or microtubules, a functional comparison suggests that both types of protrusions serve similar roles. Dendritic spines are chemically isolated compartments that are highly malleable in size and shape34. Where examined, spine volume correlates well with the degree of presynaptic activity35, and spine size and shape is responsive to developmental and homeostatic cues. For example, the number and density of dendritic spines of hippocampal neurons varies dramatically with estrogen levels in rats36,37. Likewise, chemosensory cilia and microvilli are chemically isolated compartments that are also morphologically malleable. In C. elegans, for example, the shape of the ciliated endings of the AWB chemosensory neurons is affected by the presence of chemosensory stimuli38, and mutations blocking sensory signal transduction promote alteration of cilia membrane shape, suggesting a feedback mechanism that may also operate in dendritic spines34. Furthermore, the shapes of the C. elegans AWC and AFD ciliated endings vary dramatically in response to pheromone-triggered developmental cues39.
Shared functions for sensory receptive endings and postsynaptic structures are also suggested by studies of the C. elegans gene daf-19. This gene has long been studied as a regulator of ciliogenesis, and its product, a conserved RFX transcription factor, binds directly upstream of many genes expressed in ciliated neurons40. A new study suggests that an alternatively-spliced form of the same gene is responsible for the resistance of daf-19 mutant animals to the paralytic effects of aldicarb, an ACh esterase inhibitor, and levamisole, an ACh receptor agonist. Resistance to these agents, specifically to levamisole, is a telltale sign of postsynaptic defects, suggesting that in addition to its roles in sensory neurons, daf-19 also functions in postsynaptic cells41.
Receptors
The architectural and functional parallels between sensory receptive endings and dendritic spines reflect the even more remarkable similarities between the chemosensory receptors and neurotransmitter receptors that decorate these structures (Figures 1 and 2). Neurotransmitter receptors are classified as slow (metabotropic) or fast (ionotropic) receptors1. Many of the metabotropic neurotransmitter receptors (including serotonin receptors, dopamine receptors, and the muscarinic acetylcholine receptor) are G-protein coupled receptors (GPCRs)42. Similarly, GPCRs serve as receptors for chemosensory stimuli including odorants and tastants in vertebrates and in C. elegans43,44, and light (rhodopsin)45 in many organisms. Importantly, GPCRs are used by mice and zebrafish to detect amino acids9,10, and it is likely that amino-acid detection in C. elegans also employs this receptor class.
Figure 2. Neurotransmitter receptor-related proteins are expressed at sensory receptive endings.
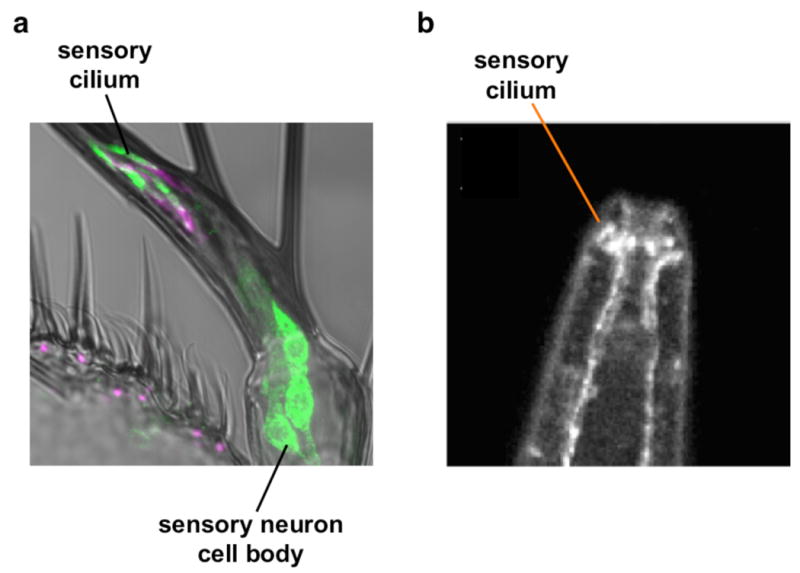
a, Immunostaining against the ionotropic glutamate receptor-like protein IR25a (green) demonstrating its localization to cilia and cell bodies of aristal sensory neurons in Drosophila. The ciliary base is marked with antibody mAb 21A6 (magenta). Figure generously provided by Dr. Richard Benton. Scale bar, 10 μm. b, Immunostaining against the C. elegans DEG-3 nACh receptor shows localization to sensory endings in the nose of the animal. Image reproduced with permission from ref. 13.
Ionotropic receptors fall into a number of classes, including those represented by glutamate and acetylcholine (ACh) receptors. C. elegans DEG-3, a member of the nicotinic ACh receptor family, is, surprisingly, not found at synapses, but is localized to sensory receptive endings of the IL2 sensory neurons, and is required for chemotaxis towards choline13. Similarly, a recent study in Drosophila revealed that proteins of the ionotropic glutamate receptor family are housed in sensory cilia of olfactory neuron dendrites, and respond to environmental stimuli5 (Figure 2).
Neurotransmitter receptors at postsynaptic sites are usually anchored by large molecular weight scaffolds. These scaffolds commonly contain proteins with PDZ (Postsynaptic density 95, Discs large, and Zonula occludens-1) domains46. Similarly, some sensory receptive endings also feature scaffolds that serve to hold signaling proteins in place. In Drosophila, phototransduction is mediated through a PDZ-containing scaffold called the INAD (inactivation-no-afterpotential D) macromolecular complex47. Although other components of this scaffold may differ from classic postsynaptic density components, the presence of a macromolecular assembly, and specifically of PDZ domains, suggest similar functions. PDZ proteins PSD95 and Veli-2 have also been found in association with the chemosensory transduction protein Ggamma13 in taste cells48; however, the functional significance of this interaction has not been established.
Together, these observations suggest that receptive endings on sensory neurons and postsynaptic dendritic spines share not only morphological and functional characteristics, but also possess similar molecular components.
Glial similarities
Of the cellular constituents of the neuronal synapse, perhaps the least is known about the glia that ensheath this structure. Nonetheless, recent studies are consistent with the idea that glia associated with neuron-neuron synapses and those associated with sensory receptive structures share functional and molecular characteristics49.
Most vertebrate excitatory neuronal synapses are ensheathed by astrocyte processes2,3,50, and most neuromuscular junctions are ensheathed by perisynaptic Schwann cells51. Likewise, invertebrate and vertebrate sensory receptive endings are generally associated with glia or glia-like cells: the retinal pigmented epithelium is associated with photoreceptor cells in the eye, sustentacular cells associate with olfactory neurons in the nose, and Deiters' cells surround the hair cells of the inner ear. All three cell types possess properties and express proteins suggestive of glial character. For example, like astrocytes, retinal pigmented epithelial cells and sustentacular cells have important phagocytic functions52,53. Sustentacular cells are electrically coupled and, like astrocytes, exhibit complex calcium ion dynamics when stimulated by ATP53, and like some astrocytes in adult animals, sustentacular cells can give rise to neurons54. Deiters' cells, like astrocytes, are thought to regulate extracellular potassium ion levels55, and express Glial Fibrillary Acidic Protein (GFAP), an intermediate filament protein that often serves as an astrocytic marker56. All glia in C. elegans associate with sensory neuron receptive endings, and some fully ensheath these endings30, an architecture also seen in the murine Grueneberg ganglion, which is thought to have sensory capacity and whose neuron-ensheathing glia are also GFAP positive57,58. Preliminary evidence suggests that all C. elegans glia express ion transporters, including a potassium chloride transporter (M. Katz and S. Shaham, unpublished), and may, like their vertebrate counterparts, control the ionic environment of sensory cilia.
An increasing body of evidence suggests that synaptic glia are essential for synaptic transmission and that glia modulate firing responses of postsynaptic neurons by secreting neurotransmitter inhibitors or receptor antagonists59,60 (Box 1). Similarly, in C. elegans, the glia associated with the amphid sensory organ are essential for sensory neuron function, as demonstrated by the profound sensory deficits exhibited by animals in which these glia have been ablated32. Vertebrate glia are also important for synaptogenesis, and recent studies have implicated astrocyte-released thrombospondin as a key mediator of this effect61. Intriguingly, in C. elegans, the glia associated with sensory neurons secrete a protein, FIG-1, containing thrombospondin type I and EGF-like type II domains, both of which are present in thrombospondin. Furthermore, FIG-1 modulates the physical and functional properties of sensory neurons32. Perhaps the most suggestive evidence for a functional correspondence between glia at sensory receptive endings and those at neuronal synapses is revealed by the anatomy of the C. elegans CEPsh glia (Figure 3). These bipolar cells send thin anterior processes towards the tip of the animal's nose, where they ensheath the receptive endings of the CEP neurons30 (as well as the CEM neurons in males). CEPsh glia play an important role in CEP neuron dendrite extension62, and are presumably important for CEP neuron sensory responses. From their posterior surfaces, CEPsh glia project large sheet-like processes that wrap around the nerve ring, the main neuropil of the animal, and extend processes that are in physical proximity to at least some neuron-neuron synapses63 (Figure 3). Although this arrangement- in which a single glial cell contacts both a sensory receptive ending and a canonical synapse- might result from anatomical happenstance, when taken together with the evidence described above, it is highly suggestive of functional similarities between these structures.
Figure 3. C. elegans CEPsh glia envelop sensory and neuron-neuron synapses.
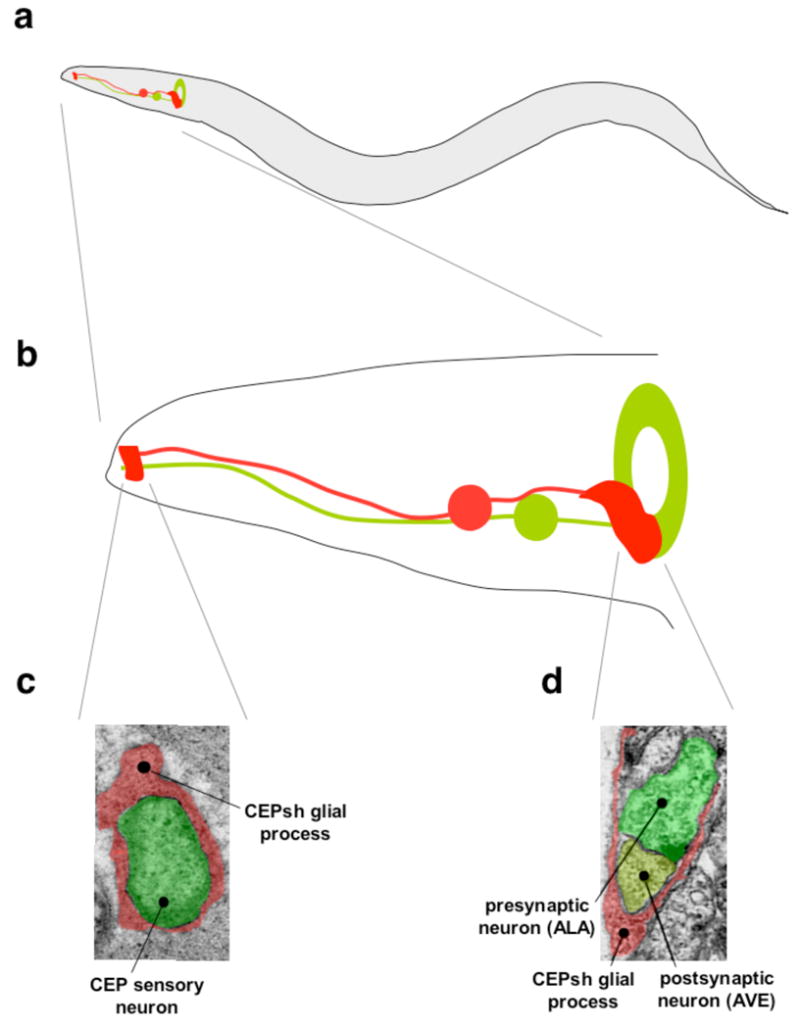
a, Schematic diagram of a C. elegans adult depicting the position of the nerve ring (green doughnut) where most synaptic contacts are located, one of the CEP sensory neurons (green line, neuronal processes, green circle, cell body), and the CEPsh glia (red). b, Magnified view of the head region depicted in a. c, An electron micrograph of a cross section of the C. elegans nose demonstrating the ensheathment of a CEP neuron sensory ending (green) by a CEPsh glial process (red) (Y. Lu and Shaham, unpublished). d, An electron micrograph depicting ensheathment of a neuronal synapse between the ALA (green) and AVE neurons (yellow-green) by CEPsh glia processes (red). Note the presence of a characteristic presynaptic density (black triangular spot) at the lower right portion of the ALA neuron. Image obtained with permission from ref. 63.
Conclusions
The similarities in functional logic, morphology, and molecular biology between neuronal synapses and sensory structures raise the possibility that these entities may have shared a common evolutionary origin. However, as with any speculative evolutionary argument, independent convergence of these structures as a result of common requirements for localized signaling cannot be ruled out. In many invertebrates, sensory-motor neurons, single neurons that respond to the environment and that also contact muscles directly, are common. For example, C. elegans inner labial neurons contain dendritic ciliated sensory endings and synapse through their axons onto head muscles63. Likewise, gastrodermal sensory neurons of hydra respond to ingested food cues with an apical cilium, and synapse onto muscle cells using an axonal protrusion64. These hybrid cells might represent an ancestral neuron class from which both sensory and postsynaptic neurons evolved. If indeed both structures did arise from a shared ancestral structure, studies of the demosponge Amphimedon hint at what such a preneural structure might have looked like. Genomic studies of this sponge, which lacks neurons, reveal that it possesses a large number of postsynaptic gene homologues, many of which resemble proteins associated with the vertebrate postsynaptic density65. These proteins are preferentially expressed in the larval flask cell65, which possesses a well-defined cilium, and is thought to have environmental sensing capacity66. Thus, an ancient cell akin to the flask cell might have served as the pre-neural ancestor of both postsynaptic and sensory neurons. Evidence that postsynaptic components in the flask cell are required for sensory transduction in this cell would bolster this hypothesis. Alternatively, it is also possible that an evolutionary precursor of chemosensory and postsynaptic neurons was a cell type designed to measure the internal environment of an animal. The recent description of olfactory receptor proteins on motile cilia in the airway of humans67 would be consistent with such a model.
Regardless of their evolutionary origins, however, the similarities between sensory receptive structures and neuronal synapses described here suggest the tantalizing possibility that our understanding of neuronal synapse biology may be greatly informed by an understanding of sensory organ function. It is even conceivable that the mechanistic underpinnings of memory acquisition and storage might be revealed at sensory receptive endings. In this respect it is of note that some learning paradigms in C. elegans seem to be associated with changes in sensory neurons, and not downstream interneurons. For example, prolonged exposure to some attractive sensory stimuli promotes behavioral adaptation to the stimulus that can persist for up to 24 hours. This response reflects a specific effect on sensory neuron output, as it is mediated by the cGMP-dependent kinase EGL-4, functioning within the relevant sensory neurons. Specifically, upon continuous exposure to stimulus, EGL-4 localization shifts from cilia to sensory-neuron nuclei to affect gene expression, a process that has been shown to be important for adaptation68-70. This adaptation phenomenon is reminiscent of synaptic phenomena including long-term potentiation71 (LTP) and depression (LTD) in which repetitive presynaptic stimulation, followed by neurotransmitter release, alters postsynaptic output over a time scale of hours or days. In the case of LTP, postsynaptic effects are also mediated by a cyclic nucleotide second messenger (cAMP)72, and translocation of nuclear import proteins, importins, carrying unknown cargo from synaptic sites to the nucleus correlates with LTP in hippocampal slices73. However, many details differ between these phenomena and at this point it is too early to tell whether they represent different facets of a common underlying mechanisms.
LTP and LTD may be correlates of memory formation, however, whether this is indeed the case remains highly debated. Although these phenomena can be induced at specific synapses, correlating synaptic changes with memory alteration at the level of the animal has been challenging. This reflects a general difficulty in correlating alterations at specific synapses with animal behavior. In this respect, sensory organs offer a distinct advantage. They are physically easy to engage, as they are generally exposed to the environment, and the effects of their manipulation on animal behavior are easy to assay. Furthermore, sensory neuron stimulation can be controlled using natural cues, and unlike many studies of neuron-neuron synapses, does not require non-native electrical stimulation of presynaptic cells.
Although the chemosensory synapse and the neuronal synapse are distinct entities in the nervous system, they possess similarities that go well beyond what one might expect from generic signaling platforms. Other signaling systems, such as hormone signaling pathways and immune signaling, use GPCRs, peptide ligands, and accessory cells; however, the striking conservation in the molecular details of these components between chemosensory and neuron-neuron synapses suggests a deeper relationship between these two structures. Both chemosensory and neuron-neuron synapses use similar ligands (e.g. amino acids), both structures use similar subclasses of receptors (e.g. ionotropic glutamate receptors), and both structures employ glia with similar properties as vital accessory cells. While many of the examples in this review are drawn from studies of C. elegans and Drosophila, the remarkable conservation of chemosensory organ morphology and molecular biology throughout the animal kingdom suggests that these similarities are likely broadly conserved. Consideration of these similarities might shed light on important synaptic processes such as signal integration, modulation of dendritic spine morphology, and glia-neuron communication, and might help to elucidate developmental programs that give rise to both structures to reveal the basic operating principles of all synapses.
Acknowledgments
I would like to thank Jim Darnell, Max Heiman, Maiken Nedergaard, and Leslie Vosshall for comments and discussions of the ideas presented here; and Dr. Richard Benton and Yun Lu for sharing images. This work was supported in part by NIH grant R01NS064273 to S.S.
References
- 1.Kandel ER, Siegelbaum SA. In: Principles of Neural Science. Kandel ER, Schwartz JH, Jessell TM, editors. McGraw-Hill; 2000. pp. 175–186. [Google Scholar]
- 2.Spacek J. Three-dimensional analysis of dendritic spines. III. Glial sheath. Anat Embryol (Berl) 1985;171:245–252. doi: 10.1007/BF00341419. [DOI] [PubMed] [Google Scholar]
- 3.Ventura R, Harris KM. Three-dimensional relationships between hippocampal synapses and astrocytes. J Neurosci. 1999;19:6897–6906. doi: 10.1523/JNEUROSCI.19-16-06897.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaham S. Glia-neuron interactions in nervous system function and development. Curr Top Dev Biol. 2005;69:39–66. doi: 10.1016/S0070-2153(05)69003-5. [DOI] [PubMed] [Google Scholar]
- 5.Benton R, Vannice KS, Gomez-Diaz C, Vosshall LB. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell. 2009;136:149–162. doi: 10.1016/j.cell.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mayer ML, Armstrong N. Structure and function of glutamate receptor ion channels. Annu Rev Physiol. 2004;66:161–181. doi: 10.1146/annurev.physiol.66.050802.084104. [DOI] [PubMed] [Google Scholar]
- 7.Mayer ML. Glutamate receptors at atomic resolution. Nature. 2006;440:456–462. doi: 10.1038/nature04709. [DOI] [PubMed] [Google Scholar]
- 8.Bargmann CI, Horvitz HR. Chemosensory neurons with overlapping functions direct chemotaxis to multiple chemicals in C. elegans. Neuron. 1991;7:729–742. doi: 10.1016/0896-6273(91)90276-6. [DOI] [PubMed] [Google Scholar]
- 9.Nelson G, et al. An amino-acid taste receptor. Nature. 2002;416:199–202. doi: 10.1038/nature726. [DOI] [PubMed] [Google Scholar]
- 10.Alioto TS, Ngai J. The repertoire of olfactory C family G protein-coupled receptors in zebrafish: candidate chemosensory receptors for amino acids. BMC Genomics. 2006;7:309. doi: 10.1186/1471-2164-7-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chandrashekar J, Hoon MA, Ryba NJ, Zuker CS. The receptors and cells for mammalian taste. Nature. 2006;444:288–294. doi: 10.1038/nature05401. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz JH. In: Principles of Neural Science. Kandel ER, Schwartz JH, Jessell TM, editors. McGraw-Hill; 2000. pp. 280–297. [Google Scholar]
- 13.Yassin L, et al. Characterization of the deg-3/des-2 receptor: a nicotinic acetylcholine receptor that mutates to cause neuronal degeneration. Mol Cell Neurosci. 2001;17:589–599. doi: 10.1006/mcne.2000.0944. [DOI] [PubMed] [Google Scholar]
- 14.Alberts, B. et al. eds B. Alberts et al.) 619-620 (Garland Science, 2008).
- 15.Sambongi Y, et al. Caenorhabditis elegans senses protons through amphid chemosensory neurons: proton signals elicit avoidance behavior. Neuroreport. 2000;11:2229–2232. doi: 10.1097/00001756-200007140-00033. [DOI] [PubMed] [Google Scholar]
- 16.Beg AA, Ernstrom GG, Nix P, Davis MW, Jorgensen EM. Protons act as a transmitter for muscle contraction in C. elegans. Cell. 2008;132:149–160. doi: 10.1016/j.cell.2007.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crump JG, Zhen M, Jin Y, Bargmann CI. The SAD-1 kinase regulates presynaptic vesicle clustering and axon termination. Neuron. 2001;29:115–129. doi: 10.1016/s0896-6273(01)00184-2. [DOI] [PubMed] [Google Scholar]
- 18.Hung W, Hwang C, Po MD, Zhen M. Neuronal polarity is regulated by a direct interaction between a scaffolding protein, Neurabin, and a presynaptic SAD-1 kinase in Caenorhabditis elegans. Development. 2007;134:237–249. doi: 10.1242/dev.02725. [DOI] [PubMed] [Google Scholar]
- 19.Dwyer ND, Adler CE, Crump JG, L'Etoile ND, Bargmann CI. Polarized dendritic transport and the AP-1 mu1 clathrin adaptor UNC-101 localize odorant receptors to olfactory cilia. Neuron. 2001;31:277–287. doi: 10.1016/s0896-6273(01)00361-0. [DOI] [PubMed] [Google Scholar]
- 20.Margeta MA, Wang GJ, Shen K. Clathrin adaptor AP-1 complex excludes multiple postsynaptic receptors from axons in C. elegans. Proc Natl Acad Sci U S A. 2009;106:1632–1637. doi: 10.1073/pnas.0812078106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Satoh AK, O'Tousa JE, Ozaki K, Ready DF. Rab11 mediates post-Golgi trafficking of rhodopsin to the photosensitive apical membrane of Drosophila photoreceptors. Development. 2005;132:1487–1497. doi: 10.1242/dev.01704. [DOI] [PubMed] [Google Scholar]
- 22.Brown TC, Correia SS, Petrok CN, Esteban JA. Functional compartmentalization of endosomal trafficking for the synaptic delivery of AMPA receptors during long-term potentiation. J Neurosci. 2007;27:13311–13315. doi: 10.1523/JNEUROSCI.4258-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenbaum JL, Carlson K. Cilia regeneration in Tetrahymena and its inhibition by colchicine. J Cell Biol. 1969;40:415–425. doi: 10.1083/jcb.40.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matus A, Ackermann M, Pehling G, Byers HR, Fujiwara K. High actin concentrations in brain dendritic spines and postsynaptic densities. Proc Natl Acad Sci U S A. 1982;79:7590–7594. doi: 10.1073/pnas.79.23.7590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sekerkova G, Zheng L, Loomis PA, Mugnaini E, Bartles JR. Espins and the actin cytoskeleton of hair cell stereocilia and sensory cell microvilli. Cell Mol Life Sci. 2006;63:2329–2341. doi: 10.1007/s00018-006-6148-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sekerkova G, et al. Espins are multifunctional actin cytoskeletal regulatory proteins in the microvilli of chemosensory and mechanosensory cells. J Neurosci. 2004;24:5445–5456. doi: 10.1523/JNEUROSCI.1279-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flock A, Duvall AJ. The Ultrastructure of the Kinocilium of the Sensory Cells in the Inner Ear and Lateral Line Organs. J Cell Biol. (3rd) 1965;25:1–8. doi: 10.1083/jcb.25.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flock A, Cheung HC. Actin filaments in sensory hairs of inner ear receptor cells. J Cell Biol. 1977;75:339–343. doi: 10.1083/jcb.75.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perkins LA, Hedgecock EM, Thomson JN, Culotti JG. Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev Biol. 1986;117:456–487. doi: 10.1016/0012-1606(86)90314-3. [DOI] [PubMed] [Google Scholar]
- 30.Ward S, Thomson N, White JG, Brenner S. Electron microscopical reconstruction of the anterior sensory anatomy of the nematode Caenorhabditis elegans.?2UU. J Comp Neurol. 1975;160:313–337. doi: 10.1002/cne.901600305. [DOI] [PubMed] [Google Scholar]
- 31.Satterlee JS, et al. Specification of thermosensory neuron fate in C. elegans requires ttx-1, a homolog of otd/Otx. Neuron. 2001;31:943–956. doi: 10.1016/s0896-6273(01)00431-7. [DOI] [PubMed] [Google Scholar]
- 32.Bacaj T, Tevlin M, Lu Y, Shaham S. Glia are essential for sensory organ function in C. elegans. Science. 2008;322:744–747. doi: 10.1126/science.1163074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jaworski J, et al. Dynamic microtubules regulate dendritic spine morphology and synaptic plasticity. Neuron. 2009;61:85–100. doi: 10.1016/j.neuron.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 34.Nimchinsky EA, Sabatini BL, Svoboda K. Structure and function of dendritic spines. Annu Rev Physiol. 2002;64:313–353. doi: 10.1146/annurev.physiol.64.081501.160008. [DOI] [PubMed] [Google Scholar]
- 35.Yasumatsu N, Matsuzaki M, Miyazaki T, Noguchi J, Kasai H. Principles of long-term dynamics of dendritic spines. J Neurosci. 2008;28:13592–13608. doi: 10.1523/JNEUROSCI.0603-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McEwen BS, Milner TA. Hippocampal formation: shedding light on the influence of sex and stress on the brain. Brain Res Rev. 2007;55:343–355. doi: 10.1016/j.brainresrev.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li C, et al. Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proc Natl Acad Sci U S A. 2004;101:2185–2190. doi: 10.1073/pnas.0307313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mukhopadhyay S, Lu Y, Shaham S, Sengupta P. Sensory signaling-dependent remodeling of olfactory cilia architecture in C. elegans. Dev Cell. 2008;14:762–774. doi: 10.1016/j.devcel.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Albert PS, Riddle DL. Developmental alterations in sensory neuroanatomy of the Caenorhabditis elegans dauer larva. J Comp Neurol. 1983;219:461–481. doi: 10.1002/cne.902190407. [DOI] [PubMed] [Google Scholar]
- 40.Swoboda P, Adler HT, Thomas JH. The RFX-type transcription factor DAF-19 regulates sensory neuron cilium formation in C. elegans. Mol Cell. 2000;5:411–421. doi: 10.1016/s1097-2765(00)80436-0. [DOI] [PubMed] [Google Scholar]
- 41.Senti G, Swoboda P. Distinct isoforms of the RFX transcription factor DAF-19 regulate ciliogenesis and maintenance of synaptic activity. Mol Biol Cell. 2008;19:5517–5528. doi: 10.1091/mbc.E08-04-0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Siegelbaum SA, Schwartz JH, Kandel ER. In: Principles of Neural Science. Kandel ER, Schwartz JH, Jessell TM, editors. McGraw-Hill; 2000. pp. 229–252. [Google Scholar]
- 43.Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65:175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- 44.Troemel ER, Chou JH, Dwyer ND, Colbert HA, Bargmann CI. Divergent seven transmembrane receptors are candidate chemosensory receptors in C. elegans. Cell. 1995;83:207–218. doi: 10.1016/0092-8674(95)90162-0. [DOI] [PubMed] [Google Scholar]
- 45.Palczewski K, et al. Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- 46.Kim E, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci. 2004;5:771–781. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- 47.Tsunoda S, et al. A multivalent PDZ-domain protein assembles signalling complexes in a G-protein-coupled cascade. Nature. 1997;388:243–249. doi: 10.1038/40805. [DOI] [PubMed] [Google Scholar]
- 48.Li Z, Benard O, Margolskee RF. Ggamma13 interacts with PDZ domain-containing proteins. J Biol Chem. 2006;281:11066–11073. doi: 10.1074/jbc.M600113200. [DOI] [PubMed] [Google Scholar]
- 49.Heiman MG, Shaham S. Ancestral roles of glia suggested by the nervous system of Caenorhabditis elegans. Neuron Glia Biol. 2007;3:55–61. doi: 10.1017/S1740925X07000609. [DOI] [PubMed] [Google Scholar]
- 50.Peters A, palay SL, Webster HD. The Fine Structure of the Nervous System. Oxford University Press; 1991. pp. 273–311. [Google Scholar]
- 51.Todd KJ, Robitaille R. Neuron-glia interactions at the neuromuscular synapse. Novartis Found Symp. 2006;276:222–229. discussion 229-237, 275-281. [PubMed] [Google Scholar]
- 52.Finnemann SC. Focal adhesion kinase signaling promotes phagocytosis of integrin-bound photoreceptors. Embo J. 2003;22:4143–4154. doi: 10.1093/emboj/cdg416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suzuki Y, Takeda M, Farbman AI. Supporting cells as phagocytes in the olfactory epithelium after bulbectomy. J Comp Neurol. 1996;376:509–517. doi: 10.1002/(SICI)1096-9861(19961223)376:4<509::AID-CNE1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 54.Hansel DE, Eipper BA, Ronnett GV. Neuropeptide Y functions as a neuroproliferative factor. Nature. 2001;410:940–944. doi: 10.1038/35073601. [DOI] [PubMed] [Google Scholar]
- 55.Nenov AP, Chen C, Bobbin RP. Outward rectifying potassium currents are the dominant voltage activated currents present in Deiters' cells. Hear Res. 1998;123:168–182. doi: 10.1016/s0378-5955(98)00121-x. [DOI] [PubMed] [Google Scholar]
- 56.Rio C, Dikkes P, Liberman MC, Corfas G. Glial fibrillary acidic protein expression and promoter activity in the inner ear of developing and adult mice. J Comp Neurol. 2002;442:156–162. doi: 10.1002/cne.10085. [DOI] [PubMed] [Google Scholar]
- 57.Brechbuhl J, Klaey M, Broillet MC. Grueneberg ganglion cells mediate alarm pheromone detection in mice. Science. 2008;321:1092–1095. doi: 10.1126/science.1160770. [DOI] [PubMed] [Google Scholar]
- 58.Mamasuew K, Breer H, Fleischer J. Grueneberg ganglion neurons respond to cool ambient temperatures. Eur J Neurosci. 2008;28:1775–1785. doi: 10.1111/j.1460-9568.2008.06465.x. [DOI] [PubMed] [Google Scholar]
- 59.Smit AB, et al. A glia-derived acetylcholine-binding protein that modulates synaptic transmission. Nature. 2001;411:261–268. doi: 10.1038/35077000. [DOI] [PubMed] [Google Scholar]
- 60.Suh J, Jackson FR. Drosophila ebony activity is required in glia for the circadian regulation of locomotor activity. Neuron. 2007;55:435–447. doi: 10.1016/j.neuron.2007.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Christopherson KS, et al. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 62.Yoshimura S, Murray JI, Lu Y, Waterston RH, Shaham S. mls-2 and vab-3 Control glia development, hlh-17/Olig expression and glia-dependent neurite extension in C. elegans. Development. 2008;135:2263–2275. doi: 10.1242/dev.019547. [DOI] [PubMed] [Google Scholar]
- 63.White JG, Southgate E, Thomson JN, Brenner S. The Structure of the Nervous System of the Nematode Caenorhabditis elegans. Phil Trans R Soc Lond B. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- 64.Westfall JA, Wilson JD, Rogers RA, Kinnamon JC. Multifunctional features of a gastrodermal sensory cell in Hydra: three-dimensional study. J Neurocytol. 1991;20:251–261. doi: 10.1007/BF01235543. [DOI] [PubMed] [Google Scholar]
- 65.Sakarya O, et al. A post-synaptic scaffold at the origin of the animal kingdom. PLoS ONE. 2007;2:e506. doi: 10.1371/journal.pone.0000506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leys SP, Degnan BM. Cytological basis of photoresponsive behavior in a sponge larva. Biol Bull. 2001;201:323–338. doi: 10.2307/1543611. [DOI] [PubMed] [Google Scholar]
- 67.Shah AS, Ben-Shahar Y, Moninger TO, Kline JN, Welsh MJ. Motile cilia of human airway epithelia are chemosensory. Science. 2009;325:1131–1134. doi: 10.1126/science.1173869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.L'Etoile ND, et al. The cyclic GMP-dependent protein kinase EGL-4 regulates olfactory adaptation in C. elegans. Neuron. 2002;36:1079–1089. doi: 10.1016/s0896-6273(02)01066-8. [DOI] [PubMed] [Google Scholar]
- 69.Kaye JA, Rose NC, Goldsworthy B, Goga A, L'Etoile ND. A 3′UTR pumilio-binding element directs translational activation in olfactory sensory neurons. Neuron. 2009;61:57–70. doi: 10.1016/j.neuron.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.O'Halloran DM, Altshuler-Keylin S, Lee JI, L'Etoile ND. Regulators of AWC mediated olfactory plasticity in Caenorhabditis elegans. PLoS Genet. 2009 doi: 10.1371/journal.pgen.1000761. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Slack JR, Pockett S. Cyclic AMP induces long-term increase in synaptic efficacy in CA1 region of rat hippocampus. Neurosci Lett. 1991;130:69–72. doi: 10.1016/0304-3940(91)90229-m. [DOI] [PubMed] [Google Scholar]
- 73.Thompson KR, et al. Synapse to nucleus signaling during long-term synaptic plasticity; a role for the classical active nuclear import pathway. Neuron. 2004;44:997–1009. doi: 10.1016/j.neuron.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 74.Mauch DH, et al. CNS synaptogenesis promoted by glia-derived cholesterol. Science. 2001;294:1354–1357. doi: 10.1126/science.294.5545.1354. [DOI] [PubMed] [Google Scholar]
- 75.Fuentes-Medel Y, et al. Glia and muscle sculpt neuromuscular arbors by engulfing destabilized synaptic boutons and shed presynaptic debris. PLoS Biol. 2009 doi: 10.1371/journal.pbio.1000184. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Colon-Ramos DA, Margeta MA, Shen K. Glia promote local synaptogenesis through UNC-6 (netrin) signaling in C. elegans. Science. 2007;318:103–106. doi: 10.1126/science.1143762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Murai KK, Nguyen LN, Irie F, Yamaguchi Y, Pasquale EB. Control of hippocampal dendritic spine morphology through ephrin-A3/EphA4 signaling. Nat Neurosci. 2003;6:153–160. doi: 10.1038/nn994. [DOI] [PubMed] [Google Scholar]
- 78.Rothstein JD, et al. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 79.Adams RH, et al. Gene structure and glial expression of the glycine transporter GlyT1 in embryonic and adult rodents. J Neurosci. 1995;15:2524–2532. doi: 10.1523/JNEUROSCI.15-03-02524.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Minelli A, DeBiasi S, Brecha NC, Zuccarello LV, Conti F. GAT-3, a high-affinity GABA plasma membrane transporter, is localized to astrocytic processes, and it is not confined to the vicinity of GABAergic synapses in the cerebral cortex. J Neurosci. 1996;16:6255–6264. doi: 10.1523/JNEUROSCI.16-19-06255.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Araque A, Parpura V, Sanzgiri RP, Haydon PG. Glutamate-dependent astrocyte modulation of synaptic transmission between cultured hippocampal neurons. Eur J Neurosci. 1998;10:2129–2142. doi: 10.1046/j.1460-9568.1998.00221.x. [DOI] [PubMed] [Google Scholar]
- 82.Heumann R, Villegas J, Herzfeld DW. Acetylcholine synthesis in the Schwann cell and axon in the giant nerve fiber of the squid. J Neurochem. 1981;36:765–768. doi: 10.1111/j.1471-4159.1981.tb01654.x. [DOI] [PubMed] [Google Scholar]
- 83.Minchin MC, Iversen LL. Release of (3H)gamma-aminobutyric acid from glial cells in rat dorsal root ganglia. J Neurochem. 1974;23:533–540. doi: 10.1111/j.1471-4159.1974.tb06056.x. [DOI] [PubMed] [Google Scholar]
- 84.Newman EA. Glial cell inhibition of neurons by release of ATP. J Neurosci. 2003;23:1659–1666. doi: 10.1523/JNEUROSCI.23-05-01659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Oliet SH, Mothet JP. Regulation of N-methyl-D-aspartate receptors by astrocytic D-serine. Neuroscience. 2009;158:275–283. doi: 10.1016/j.neuroscience.2008.01.071. [DOI] [PubMed] [Google Scholar]


