Abstract
Myofascial pain of the temporomandibular region (M-TMD) is a common, but poorly understood chronic disorder. It is unknown whether the condition is a peripheral problem, or a disorder of the central nervous system (CNS). To investigate possible CNS substrates of M-TMD, we compared the brain morphology of 15 women with M-TMD to 15 age- and gender-matched healthy controls. High-resolution structural brain and brainstem scans were carried out using magnetic resonance imaging (MRI), and data were analyzed using a voxel-based morphometry approach. The M-TMD group evidenced decreased or increased gray matter volume compared to controls in several areas of the trigeminothalamocortical pathway, including brainstem trigeminal sensory nuclei, the thalamus, and the primary somatosensory cortex. In addition, M-TMD individuals showed increased gray matter volume compared to controls in limbic regions such as the posterior putamen, globus pallidus, and anterior insula. Within the M-TMD group, jaw pain, pain tolerance, and pain duration were differentially associated with brain and brainstem gray matter volume. Self-reported pain severity was associated with increased gray matter in the rostral anterior cingulate cortex and posterior cingulate. Sensitivity to pressure algometry was associated with decreased gray matter in the pons, corresponding to the trigeminal sensory nuclei. Longer pain duration was associated with greater gray matter in the posterior cingulate, hippocampus, midbrain, and cerebellum. The pattern of gray matter abnormality found in M-TMD individuals suggests the involvement of trigeminal and limbic system dysregulation, as well as potential somatotopic reorganization in the putamen, thalamus, and somatosensory cortex.
Keywords: chronic pain, morphometry, trigeminal, neuroplasticity, imaging, somatotopy
Introduction
Temporomandibular disorders are a heterogeneous group of clinical problems that involve the masticatory muscles, the temporomandibular joint, and the associated structures. Myofascial pain of the masticatory region (M-TMD) is a common temporomandibular disorder that is characterized by a dull aching pain and hypersensitive regions of taut skeletal muscle fiber. The condition affects approximately 10.5% of American women [19], and is most commonly seen in women of child-bearing age. Clinical signs and symptoms involve jaw and face pain at rest that is exacerbated by function or palpation of the area.
While the etiology of M-TMD is not certain [1], there is evidence of central nervous system (CNS) sensitization. Individuals with M-TMD are often found to meet diagnostic criteria for fibromyalgia [25], a condition that may involve CNS sensitization. There is also evidence for hyperalgesia and temporal summation of heat pain, although it is debatable whether the increased pain sensitivity is specific to the masticatory area [37] or present throughout the body [47]. The latter case would suggest a facilitated processing of pain messages in the central nervous system, perhaps manifested by neural reorganization in the brain, brainstem, and spinal cord. The trigeminal pain system presents many sites for potential neural dysregulation. The classic trigeminothalamocortical pathway carries nociceptive information from trigeminal ganglion in the periphery, through spinal and brainstem nuclei, to the thalamus, and finally to the primary somatosensory cortex [31]. In particular, the trigeminal brainstem sensory nuclear complex is an important site for craniofacial nociceptive transmission [32] and may be a key region of neuroplasticity and central sensitization [45].
To our knowledge, no previous studies have been conducted that examine CNS abnormalities in M-TMD patients. In this study, we investigated possible CNS substrates of M-TMD by examining gray matter differences in 15 M-TMD individuals and 15 healthy controls. Participants completed a magnetic resonance imaging (MRI) session involving a high-resolution, T1-weighted, structural scan of the brain. Using voxel based morphometry (VBM), regional gray matter volume (GMV) differences between the M-TMD and control groups were assessed.
Materials and Methods
Subjects
Fifteen women with chronic, bilateral M-TMD were recruited from the Orofacial Clinic at the University of California, San Francisco (demographics in Table 1). The M-TMD group had an age range from 23 – 61 years, with a mean age of 38 (SD = 13.7) years. All 15 individuals were examined and diagnosed with M-TMD by a D.D.S. and board-certified TMD specialist, using standard diagnostic criteria [33]. Pain duration ranged from 1 year to 11 years (mean = 4.4 years, SD = 2.9). Inclusion criteria for the M-TMD group were: 1) at least 18 years of age, 2) diagnosis of chronic myofascial pain syndrome of the masticatory muscles, 3) pain at least 4 times a week in the jaw muscles for at least twelve weeks, 4) average pain severity of 4 on an 11-point scale for at least 1 hour per day, and 5) pain in the jaw, temples, face, pre-auricular area, or in the ear during rest or function. All M-TMD participants presented with bilateral jaw pain. The exclusionary criteria were: 1) pregnancy, 2) current opioid use, and 3) claustrophobia, 4) moderate or severe psychiatric disorder or current use of psychiatric medications, 5) presence of fibromyalgia or other chronic pain disorder, and 6) diagnosis of metabolic disease, coagulopathy, neurological disorder, vascular disease, or neoplasia. Participants taking non-steroidal anti-inflammatory drugs or acetaminophen were asked to stop those medications at least one day prior to their study appointment.
Table 1.
Patient demographics. All participants were female and presented with bilateral jaw pain.
| Patient # | Age | TMD Duration (years) |
Pain Severity (0 – 10NRS) |
Algometry (lbs) |
Treatments |
|---|---|---|---|---|---|
| 1 | 26 | 3.5 | 5 | 2.3 | none reported |
| 2 | 29 | 3.5 | 6 | 1.9 | none |
| 3 | 48 | 2.5 | 6 | 1.7 | cyclobenzaprine |
| 4 | 47 | 11 | 8 | 1.9 | ibuprofen, nortriptyline, sulindac |
| 5 | 48 | 3.5 | 4 | 1.3 | none |
| 6 | 51 | 3 | 7 | 2.8 | none |
| 7 | 26 | 1 | 4 | 2.4 | naproxen, cyclobenzaprine |
| 8 | 23 | not reported | 1 | 2.8 | none |
| 9 | 53 | 2.5 | 0 | 2.7 | cyclobenzaprine, naproxen |
| 10 | 61 | 7.5 | 5 | 1.6 | gabapentin, celecoxib, clonazepam |
| 11 | 25 | 2.5 | 3 | 3.3 | amitriptyline, cyclobenzaprine |
| 12 | 25 | 8 | 2 | 3.8 | ibuprofen, cyclobenzaprine |
| 13 | 56 | 2 | 5 | 1.4 | gabapentin, ibuprofen, cyclobenzaprine |
| 14 | 26 | 6.5 | 4 | 2.0 | cyclobenzaprine |
Fifteen healthy controls were also recruited for the study. Controls were strictly age-matched to patients (within 6 months) on a case-by-case basis. Exclusionary criteria were the same as those for the M-TMD group, and controls were also excluded if they demonstrated any evidence of chronic pain or craniofacial pain disorder. No healthy controls were taking prescription medications. The final group of 30 women was 53% Caucasian, 23% Asian, 10% African-American, 10% Hispanic and 3% other (1 Arabic individual). One M-TMD individual was discovered to have corrupted MRI data due to scanner error, and was excluded from all analyses. Data from that person's matched control were retained for analysis. The final subject sample size of 29 exceeded that used in most VBM chronic pain studies, which average 21 subjects [27].
Procedures
After consenting, two measures of disease severity were collected from M-TMD patients. First, patients rated the severity of their present jaw pain on an 11-point numerical rating scale (NRS). Second, pressure algometry was used to obtain a behavioral measure of disease severity. Using an analog algometer, pressure was applied to the right masseter muscle at a rate of 1lb/sec until the participant indicated their maximum pain tolerance. Following the measures of disease severity, patients were prepared for scanning. Pressure algometry readings were not obtained from healthy controls.
MR data acquisition and processing
MRI data were collected using a GE Medical Systems 3.0 Tesla system with an 8-channel brain receiving coil. The protocol used a fast 3D-SPGR sequence with the following parameters: TR = 6.9 ms, TE = 1.6 ms, TI = 450ms, flip angle = 15°, matrix = 256 × 256, field of view = 25.6 × 25.6 cm, 156 axial slices with 1mm thickness, yielding a voxel size of 1 × 1 × 1 mm. The scanning parameters provided complete coverage of the brain, midbrain, pons, and cerebellum.
CNS abnormalities associated with M-TMD were assessed using VBM, a process that provides whole-brain maps of regional GMV differences between groups, and does not require a-priori hypotheses. VBM processing was conducted with SPM8 (Welcome Department of Cognitive Neurology, London, UK) in a MATLAB (Mathworks, Sherborn, MA) environment. Hi-resolution images for each participant were oriented to standard anterior and posterior commissure landmarks, and segmented into gray and white matter. Gray and white matter segments were then normalized across all participants into MNI stereotactic space with DARTEL [4]. Results were spatially smoothed with an 8mm Gaussian kernel. In order to more effectively localize gray matter abnormalities in the brainstem (where structures are small and closely positioned), that region was smoothed with a 3mm kernel.
Statistical analysis
Regional differences in GMV between M-TMD individuals and healthy controls were explored using independent samples t-tests on all gray matter voxels (using a mask to exclude non-gray matter regions such as white matter, dura, skull, and cerebrospinal fluid). The resulting brain and brainstem contrast map was thresholded at a voxel-level false discovery rate (FDR) of 0.05, yielding an adjusted critical T-score of 3.58 (uncorrected p < .0005). Furthermore, a cluster-extent, FDR-corrected significance threshold of 0.05 was applied. To accommodate their smaller size, brainstem regions were instead spatially thresholded with a minimum contiguous voxel count of 30. No region-of-interest or small-volume correction analyses were performed. To identify structures showing significant gray matter differences, we first translated MNI coordinates to the Talairach (TAL) coordinate system using the Nonlinear Yale MNI to Talairach Conversion Algorithm [24]. Resulting coordinates were then referenced with a Talairach atlas to provide structure names. Returns from the Talairach query were subsequently verified using structural landmarks from the 29-participant averaged brain, together with landmark-based atlases [17, 38].
A second set of analyses were performed on the patient group only. In those individuals, regional gray matter volume was regressed against markers of disease severity and chronicity. Separate regressions were performed for self-reported jaw pain, pressure algometry readings, and pain duration. Analyses were performed on the whole brain, masked for gray matter, with significant clusters exceeding a voxel- and cluster-level, FDR-corrected 0.05.
Results
Patients versus controls
Total (brain, brainstem, and cerebellar) GMV#x00027;s were computed for all individuals, and averaged separately for both the M-TMD and control groups. M-TMD individuals had an average GMV of 683.6 mL (SD = 81.3), while controls had 672.7 mL (SD = 55.2). A t-test revealed no overall difference in GMV between the two groups (t(27) = 0.43, p = 0.67). Age was significantly correlated with total GMV (r(29) = −0.68, p < .0005), with older individuals exhibiting less total GMV. Total GMV was regressed out in all subsequent analyses.
Next, region-specific differences in GMV were assessed. The M-TMD group demonstrated several regions of greater GMV compared to healthy controls (Table 2). In cortical and limbic regions, M-TMD patients showed greater GMV (Figure 1) in the right inferior frontal gyrus (IFG), right anterior insula, right posterior putamen, left ventral posterior (VP) nucleus of the thalamus, right ventral lateral (VL) nucleus of the thalamus, and right globus pallidus. In the brainstem (pons), we found several additional regions of increased gray matter in M-TMD patients. First, on the level of the trigeminal nuclei, we observed bilateral clusters of increased gray matter approximately 7 mm anterior and lateral to the outside corners of the fourth ventricle, situated in regions consistent with the trigeminal sensory and/or motor nuclei (Figure 2). An additional significant cluster, located anterior and medial to the right trigeminal nuclei, was not consistent with known neuron body clusters, and instead was situated in the areas of white matter tracks (the medial lemniscus and or spinothalamic tract). More rostral in the pons, just caudal to the pons/midbrain junction, were two additional regions of increased gray matter in M-TMD individuals. The regions were situated bilaterally to the midline of the pons, on the ventral-most (base of the pons) surface, approximately in the region of pontine nuclei and the middle cerebellar peduncle.
Table 2.
Areas of significant gray matter volume differences between chronic myofascial temporomandibular pain patients and matched healthy controls. All regions survived a voxel-level, whole-brain, false discovery rate (FDR)-corrected threshold of 0.05 and a FDR-corrected cluster-extent threshold of 0.05. For smaller brainstem regions, a voxel-level FDR-corrected 0.05 was used, and a minimal contiguous voxel count of 30. Regions of lesser gray matter volume in the chronic pain group are listed first, followed by regions of greater gray matter volume.
| Region | MNI coordinates (mm) | T score | Voxel count | ||
|---|---|---|---|---|---|
| Direction of difference | |||||
| (M-TMD versus control) | (1 × 1 × 1 mm) | ||||
| X | y | z | |||
| R inferior frontal gyrus greater |
+44 | +38 | +4 | 5.54 | 487 |
| R anterior insula greater |
+43 | +14 | −2 | 4.72 | 347 |
| R posterior putamen greater |
+28 | −12 | +10 | 4.53 | 253 |
| L ventral posterior thalamus greater |
−20 | −16 | +3 | 4.91 | 379 |
| R ventral lateral thalamus greater |
+13 | −12 | +2 | 4.24 | 367 |
| R globus pallidus greater |
+19 | −8 | +2 | 5.36 | 166 |
| L trigeminal sensory/motor nucleus greater |
−12 | −35 | −34 | 6.14 | 40 |
| R trigeminal sensory/motor nucleus greater |
+14 | −35 | −35 | 5.91 | 36 |
| R medial lemniscus greater |
+9 | −30 | −34 | 5.91 | 182 |
| L middle cerebellar peduncle greater |
−9 | −17 | −28 | 6.57 | 63 |
| R middle cerebellar peduncle greater |
+7 | −17 | −25 | 6.29 | 76 |
| R primary somatosensory cortex lesser |
+38 | −27 | +59 | 4.77 | 162 |
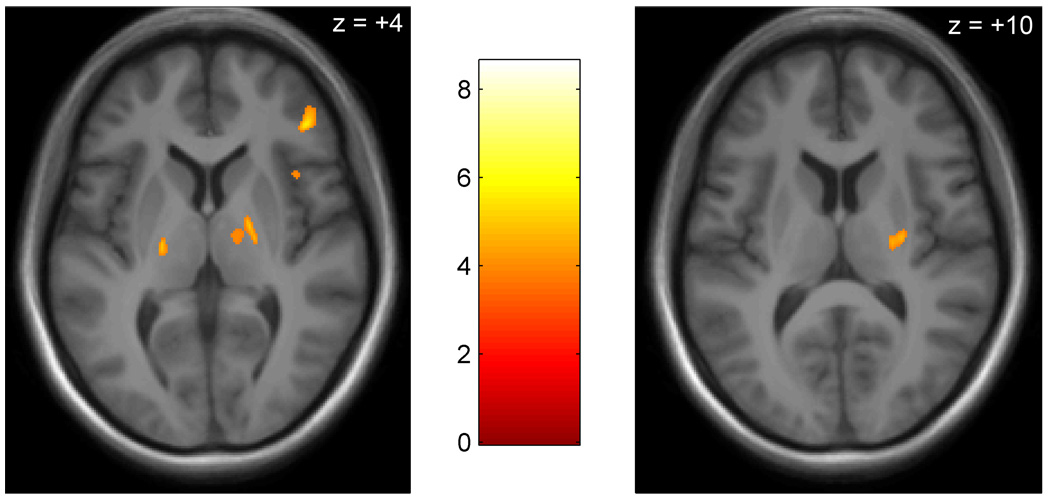
Figure 1. Regions of greater gray matter volume in the brains of individuals with chronic myofascial temporomandibular pain contrasted with controls.
Regions of greater gray matter volume (orange) are displayed on an MNI-normalized average of all student participant’s brain images (N = 29). All slices are axial; anterior is at top. Significant areas include (left pane, anterior to posterior) the right inferior frontal gyrus, right anterior insula, right globus pallidus, right thalamus, left thalamus, and (right pane) right posterior putamen.
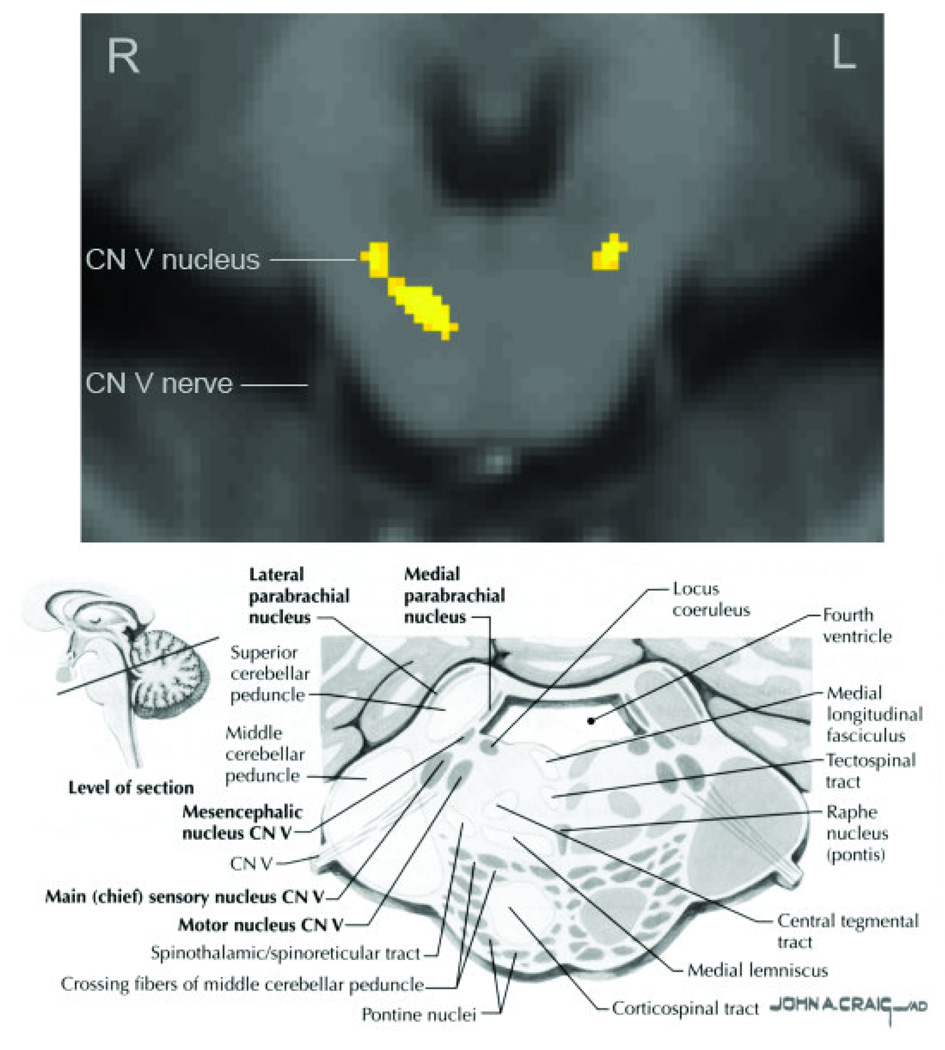
Figure 2. Regions of greater gray matter volume in the pons of individuals with chronic myofascial temporomandibular pain.
On the top pane, regions of greater gray matter volume (yellow) are displayed on an MNI-normalized average of all study participant’s brainstem images (N = 29). A single axial slice is shown at the level of the trigeminal nuclei (MNI plane −25 mm from the AC/PC line). The image has been rotated 180° to match the reference image (bottom pane), with the anterior (ventral) aspects of the pons at the bottom. Significant clusters are seen in the bilateral sensory or motor trigeminal nuclei. A third, unilateral cluster is seen, approximately in the area of the medial lemniscus. On the bottom pane, a reference image of the same slice and orientation is provided, with locations of major structures. (Image reprinted from Netter 2007, page 122).
When the reverse contrast (M-TMD < controls) was conducted, the M-TMD group was found to have only one area of lesser GMV compared to controls. The region was located just posterior to the central gyrus, in the primary somatosensory cortex.
Associations with pain severity and chronicity
Secondary analyses were then performed on the M-TMD group. These analyses were designed to determine if any regional GMV differences were associated with disease severity and chronicity. GMV was separately regressed against: 1) self-reported jaw pain, 2) pressure algometry, and 3) duration of illness. Four areas of GMV were negatively associated with greater jaw pain ratings (Table 3). Individuals with greater jaw pain showed less GMV in the right rostral anterior cingulate cortex (rACC; Figure 3), right Brodmann Area (BA) 8, left BA22, and the right posterior cingulate (PCC) with precuneus. No regional GMVs were positively associated with self-reported jaw pain. When regressed against pressure algometry, only one region showed a significant, positive relationship. That region was in the pons, in the same bilateral trigeminal nuclei regions found in the M-TMD versus control results. Greater GMV values in those regions were associated with higher pain tolerances (i.e. lower pain sensitivity). No regions showed a significant, negative relationship with pressure algometry. Duration of illness was positively correlated with GMV in the bilateral posterior cingulate, right hippocampus, bilateral midbrain (approximately located in the area of the substantia nigra), and right middle cerebellar peduncle. No significant negative correlations between duration of illness and GMV were observed.
Table 3.
Regional gray matter volume associations with M-TMD disease severity. Gray matter volume was regressed against a) self-reported jaw pain, b) pressure algometry of face pain tolerance, and c) illness duration. All regions survived a voxel-level and cluster-level, whole-brain, false discovery rate (FDR)-corrected threshold of 0.05 (except for brainstem structures that used a spatial extent threshold of 30 contiguous voxels).
| Region with greater |
MNI coordinates (mm) | T score | relationship | ||
|---|---|---|---|---|---|
| X | y | z | disease | ||
| severity/duration | |||||
| Jaw pain | |||||
| R dorsal posterior cingulated | +10 | −68 | +15 | 8.00 | decreased GMV |
| R rostral anterior cingulated | +1 | +34 | +21 | 5.02 | decreased GMV |
| R superior frontal gyrus | +1 | +27 | +48 | 6.91 | decreased GMV |
| L superior temporal gyrus | −56 | −44 | +7 | 6.90 | decreased GMV |
| Pressure algometry | |||||
| L trigeminal nucleus | −14 | −39 | −35 | 5.58 | decreased GMV |
| R trigeminal nucleus | +14 | −38 | −35 | 5.93 | decreased GMV |
| Illness duration | |||||
| L ventral posterior cingulated | −11 | −47 | +15 | 4.83 | increased GMV |
| R ventral posterior cingulated | +6 | −47 | +15 | 5.25 | increased GMV |
| R hippocampus | +35 | −11 | −22 | 5.01 | increased GMV |
| L midbrain | −10 | −18 | −15 | 4.78 | increased GMV |
| R midbrain | +15 | −20 | −15 | 5.63 | increased GMV |
| R middle cerebellar penduncle | +35 | −11 | −22 | 5.01 | increased GMV |
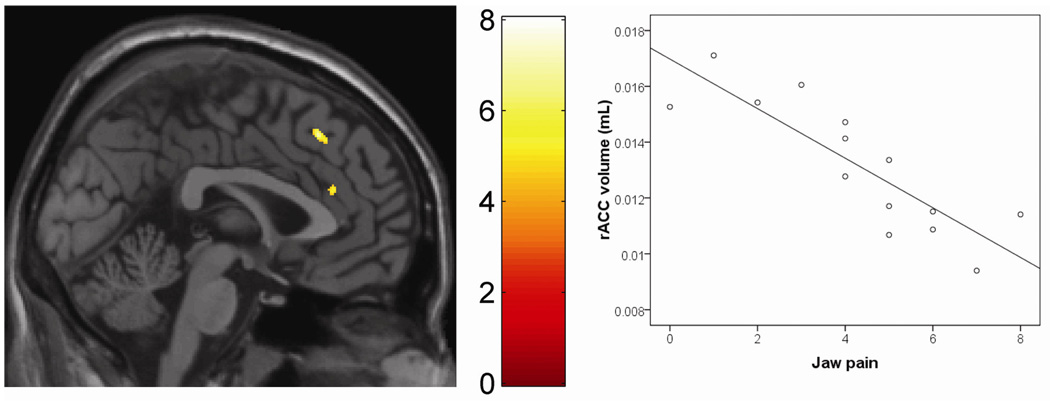
Figure 3. Self-reported jaw pain severity in M-TMD patients is associated with reduced regional gray matter volume.
The left pane shows a region of the rostral anterior cingulate cortex (inferior cluster) where gray matter volume is negatively associated with jaw pain (e.g., greater jaw pain associated with less gray matter volume). A single sagittal slice is shown (x = +2). Reduced volume is also seen in the superior frontal gyrus (superior cluster). In the right pane, a scatterplot shows the relationship between jaw pain, and gray matter volume in the rostral anterior cingulate of patients.
Discussion
We observed several regions of neural volume abnormality in the brain and brainstem of M-TMD individuals. The regions were found in areas associated with the sensory and affective components of pain processing, and comprise part of the trigeminothalamocortical and limbic systems.
Trigeminothalamocortical sensory system
Because M-TMD pain occurs in an area innervated by the trigeminal nervous system, supraspinal targets of that system are of special interest when exploring CNS substrates of M-TMD. The trigeminothalamocortical system involves inputs from spinal trigeminal nuclei, which then project through brainstem sensory nuclei, to the VP nucleus of the thalamus, and finally to the primary somatosensory cortex [31]. Human studies have confirmed the importance of these regions during the processing of acute facial pain in humans [13]. We observed gray matter abnormalities in several levels of the trigeminothalamocortical system.
First, we found bilateral GMV increases in the pons, corresponding to the chief sensory nucleus of the trigeminal system. The presence of neural abnormalities in the early trigeminal system is an important finding, as it may indicate spinal and/or peripheral nervous system dysregulations in M-TMD. Results from the pons must be viewed cautiously, however, given the compact nature of the region, small size of the structures, and need to normalize multiple individuals into a common space. To help mitigate some of the problems inherent to VBM of the pons, we utilized improved normalization algorithms under DARTEL, and maintained a higher spatial resolution.
Once leaving brainstem trigeminal nuclei, second-order projections continue to the VP thalamus, a chief relay in the trigeminal sensory pathway [36]. The VP distributes nonciceptive and other sensory messages originating from the body and the trigeminal system, and has been identified as a site of greater GMV in pain patients [40]. We found the M-TMD group to have significantly greater GMV than controls in the VP thalamus, perhaps suggesting enhanced facilitation of trigeminal sensory messages. We note, however, that the specific aspect of the VP identified in our analyses may be lateral to the region commonly innervated with facial sensory inputs. Research in nonhuman primates shows that the VP demonstrates somatotopic reorganization following experimentally-induced pain [9]. On the right side of the brain, the VL thalamus demonstrated increased GMV. The location of the bilaterally increased thalamic GMV may indicate somatotopic adaptation and reorganization associated with increased nonciceptive signaling from trigeminal nerves.
Once leaving the thalamus, third order neurons project to the primary somatosensory cortex. We found the M-TMD group to have less GMV than controls in the primary somatosensory cortex. Given the direct relationship between the thalamus and primary somatosensory cortex in processing facial pain, less GMV in this region is intriguing. However, we note that the primary somatosensory cortex is only rarely reported in pain VBM studies [40], and the region observed in our study seems to be located superior to the expected somatotopic region of the face [21, 29].
Limbic system
Chronic pain involves not only sensory, but also affective systems. We noted several limbic abnormalities in the M-TMD group, including the basal ganglia, as well as limbic-related cortical areas such as the anterior insula and inferior frontal gyrus.
First, GMV increases were observed in two basal ganglia nuclei: the right putamen and right globus pallidus. Both regions contain neurons responsive to nonciceptive input, and serve the function of preparing behavioral responses to noxious stimuli [8]. The putamen, in particular, has been found to be an important structure in the processing of pain, and is somatotopically organized for nociceptive information [5, 6, 18]. Gray matter increases in the putamen have been found in chronic back pain patients [40] and chronic vulvar pain patients [43], and PET research has indicated that chronic face pain (burning mouth syndrome) may be associated with decreased dopaminergic function in that region [20]. As in the thalamus, it is possible that gray matter increases observed in M-TMD individuals represents somatotopic reorganization associated with sustained, temporomandibular pain.
Second, M-TMD individuals were found to have greater GMV than controls in the anterior insula. The anterior insula is a limbic-associated structure that receives inputs from the VP [34] and is involved in the integration of emotional and bodily states. The region appears to be critical in interoception, or the emotional awareness of internal states [10, 11], as well as the emotional aspects of the pain experience [46] and anticipation of sensation [26]. Greater GMV in the right anterior insula has been found to predict interoceptive accuracy of heartbeat, as well as subjective ratings of visceral awareness [12]. These results raise the intriguing possibility that greater GMV in M-TMD individuals may be related to a heightened sensory experience.
Third, we observed M-TMD individuals to have greater GMV in the right IFG. The IFG is known to considerable functional laterality, and the right side produces neural activations regardless of the location of noxious input [7, 48]. The region of greater volume observed in the M-TMD individuals was most closely aligned with the anterior and inferior aspects of Brodmann area 46.
Associations with pain severity and chronicity
Self-reported jaw pain severity was associated with decreased GMV in affect-processing regions such as the rACC and dPCC region. Reductions of GMV in the rACC are commonly reported in chronic pain studies [27] and has been found in other chronic, craniofacial pain disorders such as tension type headache [41] and migraine headache [50]. The region plays a key role in the affective aspects of pain sensation [35]. Because the rACC is also heavily involved many types of emotional processing [30], it has been posited as a bridge between chronic pain and often-observed psychological comorbidities [50]. The PCC/precuneus has also been identified as an important site in the affective experience of pain expectation [22], perhaps particularly involved in pain catastrophizing [44]. Interestingly, while the self-reported measure of disease severity was associated with affect-processing regions such as the rACC and PCC/precuneus, the behavioral marker of severity (pressure algometry) was instead associated with GMV variability in a sensory region (trigeminal nuclei in the pons). These findings suggest that the sensory and affective components of chronic pain may involve separate neurological substrates. Longer duration of disease was also associated with greater GMV in the PCC, albeit more ventrally. Individuals with a longer history of disease also showed greater GMV in the hippocampus and bilateral substantia nigra, perhaps evidencing compensatory analgesic adaptations.
General comments
Our results add to a small, but growing literature that identifies specific morphological differences between individuals with and without chronic pain conditions. Several reports now point to morphological abnormalities in the brains of individuals with chronic pain conditions such as migraine [39], chronic back pain [3], fibromyalgia [23], irritable bowel syndrome [14], and chronic tension headache [41]. Common areas of overlap include the cingulate cortex, thalamus, basal ganglia, insula, orbito-frontal cortex, and brainstem [27]. It is not known whether these observed brain abnormalities are causes or consequences of chronic pain [49]. One hypothesis is that gray matter abnormalities seen in chronic pain patients represent the neuroplastic “chronification” [51] or “learning” [2] of pain. It is also possible that the observed brain differences represent pre-existing vulnerabilities to chronic pain. A third possibility is that the abnormalities are not causal in the pain experience, and instead are simply CNS adaptations to aberrant peripheral input. Some studies suggest that changes in peripheral input can evoke neuroplastic changes in the brain. For example, human VBM data show that the loss of sensory input following limb amputation results in supraspinal adaptations in the form of regional gray matter atrophy in the contralateral posterolateral thalamus [15]. More relevant are recent results that show 8 days of painful, thermal stimulation can cause regional gray matter increases in the human brain [49]. Longitudinal studies are needed to determine what causal role CNS irregularities may play in the development, maintenance, and experience of chronic pain.
Our results diverge from previous studies in that we observed mostly GMV increases with chronic pain, instead of GMV decreases. One possible explanation for the different findings is that the pain duration of our participants (mean = 4.4 years) was shorter than that commonly reported in the literature. A sampling of pain durations in other VBM studies yielded averages of 20.6 years [50], 14.7 years [40], 8.5 years [41], and 14.4 years [42]. Limited evidence suggests that peripherally-induced pain can induce GMV increases in humans over a short duration of time [49], and it is possible that those regional increases may be followed by compensatory volume decreases over longer periods of time. The GMV increases we observed may also be a particular feature of TMD pain, and may identify a peripherally-mediated pain condition versus centrally-mediated ones. Currently, explanation of VBM findings is complicated by the uncertainty over what physiological substrates underlie the observed volumetric changes. VBM technology does not allow us to differentiate changes in neuropil, dendritic and axonal branching, cortical folding, gray matter ribbon thickness, or glia density [16, 28]. Despite these limitations, our results provide evidence for the presence of meaningful CNS abnormalities in individuals with M-TMD.
Acknowledgements
We would like to acknowledge the UCSF Osher Center for Alternative and Integrative Medicine for funding this study. We also acknowledge financial support from NIH NINDS NS053961, the John and Dodie Rosekrans Pain Research Endowment, and the Chris Redlich Pain Research Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to disclose.
Summary: M-TMD individuals evidence gray matter abnormalities in the trigeminal and limbic systems, as well as potential somatotopic reorganization in the putamen, thalamus, and somatosensory cortex.
Contributor Information
Jarred W Younger, Stanford University School of Medicine, Department of Anesthesia, Division of Pain Management; 780 Welch Rd., Suite 208E; Palo Alto, CA 94304; Phone: 650-721-1988; jarred.younger@stanford.edu.
Yoshi F. Shen, University of California San Francisco, San Francisco, CA.
Greg Goddard, University of California San Francisco, San Francisco, CA.
Sean C. Mackey, Stanford University School of Medicine, Stanford, CA.
References
- 1.Alanen P. Occlusion and temporomandibular disorders (TMD): still unsolved question? J Dent Res. 2002;81:518–519. doi: 10.1177/154405910208100803. [DOI] [PubMed] [Google Scholar]
- 2.Apkarian AV, Baliki MN, Geha PY. Towards a theory of chronic pain. Prog Neurobiol. 2009;87:81–97. doi: 10.1016/j.pneurobio.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Apkarian AV, Sosa Y, Sonty S, Levy RM, Harden RN, Parrish TB, Gitelman DR. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004;24:10410–10415. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashburner J. A fast diffeomorphic image registration algorithms. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Bingel U, Gläscher J, Weiller C, Büchel C. Somatotopic representation of nociceptive information in the putamen: an event-related fMRI study. Cereb Cortex. 2004;14:1340–1345. doi: 10.1093/cercor/bhh094. [DOI] [PubMed] [Google Scholar]
- 6.Bingel U, Schoell E, Herken W, Büchel C, May A. Habituation to painful stimulation involves the antinociceptive system. Pain. 2007;131:21–30. doi: 10.1016/j.pain.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Brooks JCW, Nurmikko TJ, Bimson WE, Singh KD, Roberts N. fMRI of thermal pain: effects of stimulus laterality and attention. Neuroimage. 2002;15:293–301. doi: 10.1006/nimg.2001.0974. [DOI] [PubMed] [Google Scholar]
- 8.Chudler EH. Response properties of neurons in the caudate-putamen and globus pallidus to noxious and non-noxious thermal stimulation in anesthetized rats. Brain Res. 1998;812:283–288. doi: 10.1016/s0006-8993(98)00971-8. [DOI] [PubMed] [Google Scholar]
- 9.Churchill JD, Arnold LL, Garraghty PE. Somatotopic reorganization in the brainstem and thalamus following peripheral nerve injury in adult primates. Brain Res. 2001;910:142–152. doi: 10.1016/s0006-8993(01)02703-2. [DOI] [PubMed] [Google Scholar]
- 10.Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- 11.Craig ADB. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 12.Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- 13.DaSilva AFM, Becerra L, Makris N, Strassman AM, Gonzalez RG, Geatrakis N, Borsook D. Somatotopic activation in the human trigeminal pain pathway. J Neurosci. 2002;22:8183–8192. doi: 10.1523/JNEUROSCI.22-18-08183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis KD, Pope G, Chen J, Kwan CL, Crawley AP, Diamant NE. Cortical thinning in IBS: implications for homeostatic, attention, and pain processing. Neurology. 2008;70:153–154. doi: 10.1212/01.wnl.0000295509.30630.10. [DOI] [PubMed] [Google Scholar]
- 15.Draganski B, Moser T, Lummel N, Gänssbauer S, Bogdahn U, Haas F, May A. Decrease of thalamic gray matter following limb amputation. Neuroimage. 2006;31:951–957. doi: 10.1016/j.neuroimage.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 16.Eriksson SH, Free SL, Thom M, Symms MR, Martinian L, Duncan JS, Sisodiya SM. Quantitative grey matter histological measures do not correlate with grey matter probability values from in vivo MRI in the temporal lobe. J Neurosci Methods. 2009;181:111–118. doi: 10.1016/j.jneumeth.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haines D, editor. Neuroanatomy: An Atlas of Structures, Sections and Systems. Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 18.Iadarola MJ, Berman KF, Zeffiro TA, Byas-Smith MG, Gracely RH, Max MB, Bennett GJ. Neural activation during acute capsaicin-evoked pain and allodynia assessed with PET. Brain. 1998;121(Pt 5):931–947. doi: 10.1093/brain/121.5.931. [DOI] [PubMed] [Google Scholar]
- 19.Janal MN, Raphael KG, Nayak S, Klausner J. Prevalence of myofascial temporomandibular disorder in US community women. J Oral Rehabil. 2008;35:801–809. doi: 10.1111/j.1365-2842.2008.01854.x. [DOI] [PubMed] [Google Scholar]
- 20.Jääskeläinen SK, Rinne JO, Forssell H, Tenovuo O, Kaasinen V, Sonninen P, Bergman J. Role of the dopaminergic system in chronic pain -- a fluorodopa-PET study. Pain. 2001;90:257–260. doi: 10.1016/S0304-3959(00)00409-7. [DOI] [PubMed] [Google Scholar]
- 21.Kopietz R, Sakar V, Albrecht J, Kleemann A, Schopf V, Yousry I, Linn J, Fesl G, Wiesmann M. Activation of primary and secondary somatosensory regions following tactile stimulation of the face. Clinical Neuroradiology. 2009;19:135–144. doi: 10.1007/s00062-009-8022-3. [DOI] [PubMed] [Google Scholar]
- 22.Koyama T, McHaffie JG, Laurienti PJ, Coghill RC. The subjective experience of pain: where expectations become reality. Proc Natl Acad Sci U S A. 2005;102:12950–12955. doi: 10.1073/pnas.0408576102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuchinad A, Schweinhardt P, Seminowicz DA, Wood PB, Chizh BA, Bushnell MC. Accelerated brain gray matter loss in fibromyalgia patients: premature aging of the brain? J Neurosci. 2007;27:4004–4007. doi: 10.1523/JNEUROSCI.0098-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lacadie CM, Fulbright RK, Rajeevan N, Constable RT, Papademetris X. More accurate Talairach coordinates for neuroimaging using non-linear registration. Neuroimage. 2008;42:717–725. doi: 10.1016/j.neuroimage.2008.04.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leblebici B, Pektaş ZO, Ortancil O, Hürcan EC, Bagis S, Akman MN. Coexistence of fibromyalgia, temporomandibular disorder, and masticatory myofascial pain syndromes. Rheumatol Int. 2007;27:541–544. doi: 10.1007/s00296-006-0251-z. [DOI] [PubMed] [Google Scholar]
- 26.Lovero KL, Simmons AN, Aron JL, Paulus MP. Anterior insular cortex anticipates impending stimulus significance. Neuroimage. 2009;45:976–983. doi: 10.1016/j.neuroimage.2008.12.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.May A. Chronic pain may change the structure of the brain. Pain. 2008;137:7–15. doi: 10.1016/j.pain.2008.02.034. [DOI] [PubMed] [Google Scholar]
- 28.Mechelli A, Friston KJ, Frackowiak RS, Price CJ. Structural covariance in the human cortex. J Neurosci. 2005;25:8303–8310. doi: 10.1523/JNEUROSCI.0357-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyamoto JJ, Honda M, Saito DN, Okada T, Ono T, Ohyama K, Sadato N. The representation of the human oral area in the somatosensory cortex: a functional MRI study. Cereb Cortex. 2006;16:669–675. doi: 10.1093/cercor/bhj012. [DOI] [PubMed] [Google Scholar]
- 30.Morgane PJ, Galler JR, Mokler DJ. A review of systems and networks of the limbic forebrain/limbic midbrain. Prog Neurobiol. 2005;75:143–160. doi: 10.1016/j.pneurobio.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 31.Moulton EA, Pendse G, Morris S, Strassman A, Aiello-Lammens M, Becerra L, Borsook D. Capsaicin-induced thermal hyperalgesia and sensitization in the human trigeminal nociceptive pathway: an fMRI study. Neuroimage. 2007;35:1586–1600. doi: 10.1016/j.neuroimage.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nash PG, Macefield VG, Klineberg IJ, Murray GM, Henderson LA. Differential activation of the human trigeminal nuclear complex by noxious and non-noxious orofacial stimulation. Hum Brain Mapp. 2009;583:3772–3782. doi: 10.1002/hbm.20805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okeson J, editor. Orofacial Pain: Guidelines for Assessment, Diagnosis, and Management. Chicago: Quintessence books; 1996. [Google Scholar]
- 34.Phillips ML, Young AW, Senior C, Brammer M, Andrew C, Calder AJ, Bullmore ET, Perrett DI, Rowland D, Williams SC, Gray JA, David AS. A specific neural substrate for perceiving facial expressions of disgust. Nature. 1997;389:495–498. doi: 10.1038/39051. [DOI] [PubMed] [Google Scholar]
- 35.Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288:1769–1772. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- 36.Purves D, Augustine G, Fitzpatrick D, Katz L, LaMantia A, McNamara J, Williams S, editors. Neuroscience. Sinauer; 2001. [Google Scholar]
- 37.Raphael KG. Temporal summation of heat pain in temporomandibular disorder patients. J Orofac Pain. 2009;23:54–64. [PMC free article] [PubMed] [Google Scholar]
- 38.Rubin M, Safdieh J, editors. Netter's Concise Neuroanatomy. Saunders; 2007. [Google Scholar]
- 39.Schmidt-Wilcke T, Gänssbauer S, Neuner T, Bogdahn U, May A. Subtle grey matter changes between migraine patients and healthy controls. Cephalalgia. 2008;28:1–4. doi: 10.1111/j.1468-2982.2007.01428.x. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt-Wilcke T, Leinisch E, Gänssbauer S, Draganski B, Bogdahn U, Altmeppen J, May A. Affective components and intensity of pain correlate with structural differences in gray matter in chronic back pain patients. Pain. 2006;125:89–97. doi: 10.1016/j.pain.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt-Wilcke T, Leinisch E, Straube A, Kämpfe N, Draganski B, Diener HC, Bogdahn U, May A. Gray matter decrease in patients with chronic tension type headache. Neurology. 2005;65:1483–1486. doi: 10.1212/01.wnl.0000183067.94400.80. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt-Wilcke T, Luerding R, Weigand T, Jürgens T, Schuierer G, Leinisch E, Bogdahn U. Striatal grey matter increase in patients suffering from fibromyalgia--a voxel-based morphometry study. Pain. 2007;132 Suppl 1:S109–S116. doi: 10.1016/j.pain.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 43.Schweinhardt P, Kuchinad A, Pukall CF, Bushnell MC. Increased gray matter density in young women with chronic vulvar pain. Pain. 2008;140:411–419. doi: 10.1016/j.pain.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 44.Seminowicz DA, Davis KD. Cortical responses to pain in healthy individuals depends on pain catastrophizing. Pain. 2006;120:297–306. doi: 10.1016/j.pain.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 45.Sessle BJ. Acute and chronic craniofacial pain: brainstem mechanisms of nociceptive transmission and neuroplasticity, and their clinical correlates. Crit Rev Oral Biol Med. 2000;11:57–91. doi: 10.1177/10454411000110010401. [DOI] [PubMed] [Google Scholar]
- 46.Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- 47.Svensson P, List T, Hector G. Analysis of stimulus-evoked pain in patients with myofascial temporomandibular pain disorders. Pain. 2001;92:399–409. doi: 10.1016/S0304-3959(01)00284-6. [DOI] [PubMed] [Google Scholar]
- 48.Symonds LL, Gordon NS, Bixby JC, Mande MM. Right-lateralized pain processing in the human cortex: an FMRI study. J Neurophysiol. 2006;95:3823–3830. doi: 10.1152/jn.01162.2005. [DOI] [PubMed] [Google Scholar]
- 49.Teutsch S, Herken W, Bingel U, Schoell E, May A. Changes in brain gray matter due to repetitive painful stimulation. Neuroimage. 2008;42:845–849. doi: 10.1016/j.neuroimage.2008.05.044. [DOI] [PubMed] [Google Scholar]
- 50.Valfrè W, Rainero I, Bergui M, Pinessi L. Voxel-based morphometry reveals gray matter abnormalities in migraine. Headache. 2008;48:109–117. doi: 10.1111/j.1526-4610.2007.00723.x. [DOI] [PubMed] [Google Scholar]
- 51.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]