Abstract
Background
p21-activated kinase 6 (PAK6) is a serine/threonine kinase belonging to the p21-activated kinase (PAK) family. We investigated the role of PAK6 in radiation-induced cell death in human prostate cancer cells.
Methods
We used a short hairpin RNA (shRNA) strategy to stably knock down PAK6 in PC3 and DU145 cells. Radiation sensitivities were compared in PAK6 stably knockdown cells versus the scrambled shRNA-expressing control cells.
Results
PAK6 mRNA and protein levels in PC3 and DU145 cells were upregulated upon exposure to 6 Gy of radiation. After irradiation, an increased percentage of apoptotic cells and cleaved caspase-3 levels were demonstrated in combination with a decrease in cell viability and a reduction in clonogenic survival in PAK6-knockdown cells. In addition, transfection with PAK6 shRNA blocked cells in a more radiosensitive G2-M phase and increased levels of DNA double-strand breaks. We further explored the potential mechanisms by which PAK6 mediates resistance to radiation-induced apoptosis. Inhibition of PAK6 caused a decrease in Ser112 phosphorylation of BAD, a proapoptotic member of the Bcl-2 family, which led to enhanced binding of BAD to Bcl-2 and Bcl-XL and release of cytochrome c culminating into caspase activation and cell apoptosis.
Conclusions
The combination of PAK6 inhibition and irradiation resulted in significantly decreased survival of prostate cancer cells. The underlying mechanisms by which targeting PAK6 may improve radiation response seem to be multifaceted, and involve alterations in cell cycle distribution and impaired DNA double-strand break repair as well as relieved BAD phosphorylation.
Keywords: PAK6, radiosensitization, apoptosis, BAD
Introduction
Prostate cancer (PCa) is one of the most common cancers and second leading cause of cancer death in Western European and American men, mostly because of the limitations of current therapeutic modalities, especially once the cancer has metastasized. Over 192,280 new cases and 27,360 deaths are expected for the year 2009 in the United States (1). Radiotherapy can be used as curative treatment for clinically localized PCa. However, local recurrences and radioresistance remain significant clinical problems. Using prostate-specific antigen (PSA) levels as a marker for tumor control, patients with organ-confined disease have a 5-year failure rate of 30-40% after ionizing radiation (2-4). Understanding the molecular mechanisms involved in the evolution of PCa cell survival following irradiation could help identify more specific target proteins and better design novel therapeutic agents that will help improve the outcome of radiotherapy.
p21-activated kinase 6 (PAK6) was first identified as an androgen receptor (AR) -interacting protein able to inhibit AR-mediated transcriptional responses (5). PAK6 is a serine/threonine kinase belonging to the p21-activated kinase (PAK) family. Currently, six members of the PAK family of protein kinases have been identified and can be classified into two groups based on their sequence homology and regulatory properties: Group I, including PAKs 1–3, and Group II, including PAKs 4–6 (6,7). PAKs are serine/threonine kinases that contain a Cdc42/Rac-interactive binding (CRIB) domain and a Ste20-related kinase domain. The binding of activated GTP-bound Cdc42 or Rac to group I PAKs dramatically stimulates their ability to phosphorylate exogenous substrates. In contrast, the group II PAKs, possess a substantial “basal” kinase activity that is not further stimulated by binding of activated GTPase. GTPase binding does mediate kinase relocalization: after binding Cdc42, PAK4 is relocalized to the Golgi (8), and PAK5 shuttles from the microtubule network to actin-rich structures (9). The PAK family members have been implicated in the regulation of multiple cellular functions, including actin reorganization, cell motility, gene transcription, cell transformation, apoptotic signaling, and more recently, steroid hormone receptor signaling.
After androgen stimulation, PAK6 was reported to interact with the ligand binding domain of AR and to translocate to the nucleus along with the AR, where PAK6 inhibits AR-mediated transcription (5,10). PAK6 has also been shown to bind the estrogen receptor (ER) and to inhibit ER-mediated gene transcription (11). PAK6 is regulated by Map kinase kinase 6 (MKK6) and p38 Mao kinase. The PAK6 activation loop has been shown to be regulated by both MKK6 and autophosphorylation (12). Northern blot analysis shows that PAK6 is mainly expressed in brain, testis, prostate, and breast tissues (5). By immunohistochemistry, PAK6 has been identified as being weakly expressed in normal prostate epithelium. Its expression was increased in primary and metastatic PCa, and was further increased in tumors that relapsed after androgen deprivation therapy (13).
In the present study, we sought to assess the effects of attenuation of PAK6 expression, accomplished through short-hairpin RNA (shRNA) - induced down-regulation of PAK6 mRNA and protein, on the radiation sensitivity of PCa cells, and to elucidate possible underlying mechanisms by which PAK6 may mediate radiation resistance.
Materials and methods
Cell Culture
PC3, DU145 and LNCaP (human prostate carcinoma cell lines) were obtained from the American Type Culture Collection (ATCC, Manassas, VA). PC3 and LNCaP cells were maintained in RPMI 1640, supplemented with 10% (v/v) FBS, and 1% penicillin/streptomycin at 37°C, 95% humidity, and 5% CO2. DU145 cells were maintained in DMEM, supplemented with 10% (v/v) FBS, and 1% penicillin/streptomycin at 37°C, 95% humidity, and 5% CO2.
Short Hairpin RNA-mediated Knockdown of PAK6
Commercial available short hairpin RNA (shRNA) constructs were obtained as bacterial glycerol stocks (Sigma-Aldrich Co, St. Louis, MO) and used to silence PAK6. PC3 and DU145 cells were transduced with 2.56 × 105 TU/ml virus and polybrene (8 μg/ml) (Chemicon, Pittsburgh, PA). Twenty-four hours later, antibiotic selection (10 μg/ml puromycin) was initiated for 10 days. Cells transduced with non-target shRNA (Sigma) served as control. Stable silencing of PAK6 was determined by Western blot and real-time RT-PCR.
Western Blot Analysis
Lysates were generated by placing PC3 and DU145 cells in RIPA lysis buffer. Bradford assays were performed to determine the total protein concentration and normalized to 1 ug/ul for all samples. Samples were prepared with a combination of loading dye and SDS Page and heated at 100°C for five minutes. All samples were run on 4-20% Tris-Glycine gels. 50 ul of protein lysate from each tissue was loaded within the wells. Gels were run constantly at 125V for three hours for maximum separation. Wet transfers were performed for an hour and a half at 25V using Millipore Immobolin transfer membranes that had been soaked in methanol for five minutes prior to use. Following the transfer the membranes were soaked for an hour in 5% milk in TBST. Membranes were incubated in primary antibody overnight targeting either PAK6 (Calbiochem, San Diego, CA), cleaved PARP, cleaved caspase-3, Phospho-BAD Ser 112, 136, and total BAD (Cell Signaling Technology, Danvers, MA), or β-actin (Sigma). Following overnight incubation in primary antibody membranes were washed again three times in TBST for 15 minutes apiece. The membranes were then incubated in secondary antibody in 5% milk in TBST for an hour. Following incubation in secondary antibody membranes were again washed for 45 minutes, and finally chemiluminescent detection was then used to detect targeted protein expression. β-actin levels served as loading controls.
Real-time RT-PCR
RNA was isolated from cell culture using an RNA extraction kit (Qiagen, Valencia, CA). After RNA was isolated, cDNA was produced for each sample using the Superscript III First-Strand Synthesis System for RT-PCR (Invitrogen, Carlsbad, CA). Quantification of genes of interest (PAK6) and an internal reference gene (GAPDH) was conducted using a fluorescence-based real time detection method (Stratagene Cloning Systems, La Jolla, CA). Primers and reagents were purchased from Applied Biosystems (Foster City, CA). All experiments were performed in triplicate.
Clonogenic Survival Assay
PC3 and DU145 cells were counted, diluted, and plated onto six well culture dishes with appropriate number of cells. Cells were allowed six hours to attach to the bottom of the culture dishes prior to irradiation. Cells were irradiated with single doses of 0, 2, 4, 6, or 8 Gy of gamma radiation. Following 14 days of incubation at 37°C the plates were stained with crystal violet (Sigma) for colony counting. Only colonies containing more than 50 cells were scored. Plating efficiency and surviving fractions were determined for each cell line. Each set of experiments was performed in triplicate.
MTT Assay
PC3 and DU145 were counted, diluted, and plated onto 24 well plates. Following an overnight incubation period, cells were irradiated with a single dose of 6 Gy radiation. Quantitative MTT Analysis was done using the MTT Cell Proliferation Assay Kit (ATCC). Quantitative analysis was done 24, 48, and 72 hours following irradiation. All MTT experiments were done in triplicate.
Apoptosis Assay
Apoptosis was measured by the detection of membrane externalization of phosphatidlyserine with Annexin V-FITC conjugate (Invitrogen). Floating and adherent cells were harvested 72 hours following a single does of 6 Gy radiation and washed with PBS. Cells were counted and resuspended in 1× Annexin-V binding buffer (containing 10 mM HEPES/NaOH, pH 7.4, 140 mM NaCl, 2.5 mM CaCl2). Next cells were aliquot at 100 μL/tube; and 5 μL of Annexin-V FITC and 10 μL of Propidium Iodide buffer were added to each tube. Following a 15 minute incubation period, 400 μL 1× Annexin-V binding buffer was added and cells were analyzed by flow cytometry.
Measurement of Cytochrome C Release
Mitochondrial versus cytosol fractions of cells were prepared using a Mitochondrial/Cytosol Fractionation Kit (Biovision, Mountain View, CA). Cells at 5 ×106 with or without different treatments were harvested by centrifugation at 700 g for 5 min. After washes with PBS, the cells were resuspended in a 250 μl extraction buffer containing a protease inhibitor mixture and dithiothreitol and incubated on ice for 30 min. The cells were then homogenized using a Dounce tissue grinder (Kimble/Kontes, Vineland, NJ) on ice. The homogenates were centrifuged at 700 g for 10 min at 4°C. The supernatant was collected and further centrifuged at 10,000 g for 30 min at 4°C. The resulting supernatants were harvested and designated as cytosolic fractions, and the pellets were resuspended in an appropriate buffer and designated as mitochondrial fractions. The cytochrome c was analyzed using Western blotting with cytochrome c monoclonal antibody or control antibody against β-actin.
Comet Assay
Comet assay slide preparation and analysis was done according to the Alkaline Electrophoresis Assay Kit protocol (Trevigen Inc., Gaithersburg, PA). Cells were treated with 6 Gy gamma irradiation and lysed four hours later following a single wash of PBS. Agar provided by the kit was heated and combined with 50 μL of cell solution at a concentration of 10K cells/mL. 75 μl of agar/cell solution was spread evenly on slides and allowed to cool at 4°C for 30 minutes. Following cooling, cells we submerged in Lysis Buffer, covered, and refrigerated for an hour. Excess buffer was removed and slides were then submersed in Alkaline Solution for 20 minutes. Following submersion the Alkaline solution was removed with two washes of TBE. Slides were then put in TBE solution and electrophoresis was performed for 10 minutes at 30 V. Following electrophoresis, slides removed from the TBE, washed twice in 70% Ethanol, and then air dried. SYBR Green in a 1:1000 dilution with PBS was used to stain each slide followed by cooling at 4°C. Slides from the assay were analyzed at 250× magnification using an epifluorescence microscope (Zeiss, Gottingen, Germany). A total of 100 randomly captured comets were examined from each slide.
Cell Cycle Analysis
Both adherent and detached PC3 and DU145 cells were collected by trypsinization and washed with PBS for 5 minutes by centrifugation at 1500 g. Cells were resuspended in a solution containing 1 μg/mL Propidium Iodide, 4 mmol/L sodium citrate, 1 mg/ml RNase A, and 0.1% Triton X-100. Fluorescence-activated cell sorting was done with a Coulter EPICS flow cytometer XL (Coulter, Hialeah, FL) and data was analyzed using SystemXL-II software (Coulter). Experiments with both cell lines were done in triplicate.
Statistical Analysis
All experiments were repeated thrice, and data were expressed as mean ± SD, with significance determined by Student's two tailed t test. A probability level of p < 0.05 was considered significant.
Results
Irradiation Induced Upregulation of PAK6 Expression
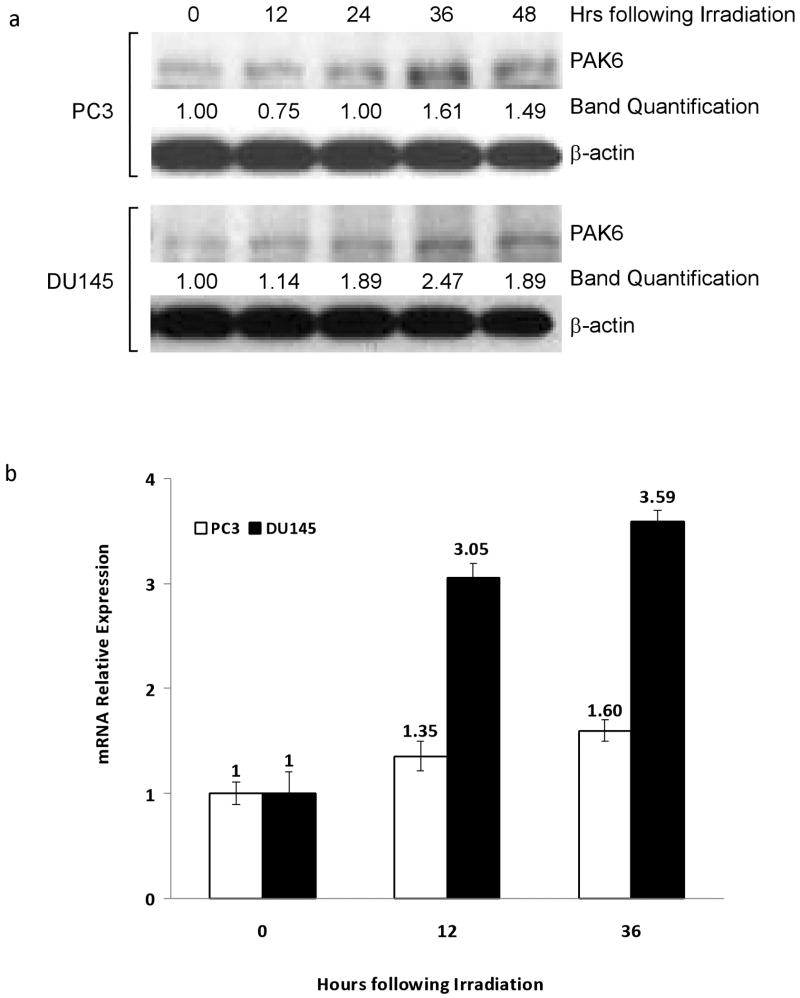
To investigate PAK6's role in mediating PCa radioresistance we used two human PCa cell lines PC3 and DU145, both of which are AR-negative and radioresistant. Western blot analysis showed both untreated cell lines expressed PAK6 protein. Following 6 Gy of irradiation there was a gradual increase in the protein level of PAK6 in both PC3 and DU145 cells; in both cases protein expression peaked 36 hours following irradiation (Figure 1a). To examine whether PAK6 mRNA level is altered after radiation treatment, we performed real-time reverse transcription-PCR (RT-PCR). As shown in Figure 1b, PAK6 mRNA expression increased 12 and 36 hours following irradiation in both cells lines mimicking our Western blot data. LNCaP cells, which are AR-positive and more radiosensitive compared to DU145 and PC3 cells (14), showed very weak expression of PAK6 before and after irradiation (data not shown). Therefore, PC3 and DU145 cells were used in our following studies.
Figure 1.
PAK6 expression is radiation-induced. (a) PC3 and DU145 cells were irradiated and lysed in twelve hour increments following a single dose of 6 Gy gamma irradiation. The protein levels of PAK6 were detected by Western blot. (b) Real-time RT-PCR analysis probing PAK6 mRNA levels in both cell lines at 12 and 36 hours following 6 Gy irradiation.
Inhibition of PAK6 Decreased Clonogenic Survival and Cell Viability
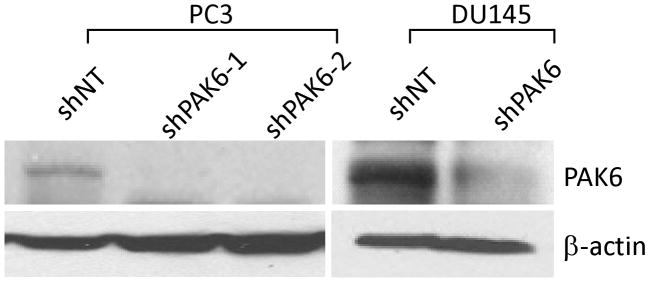
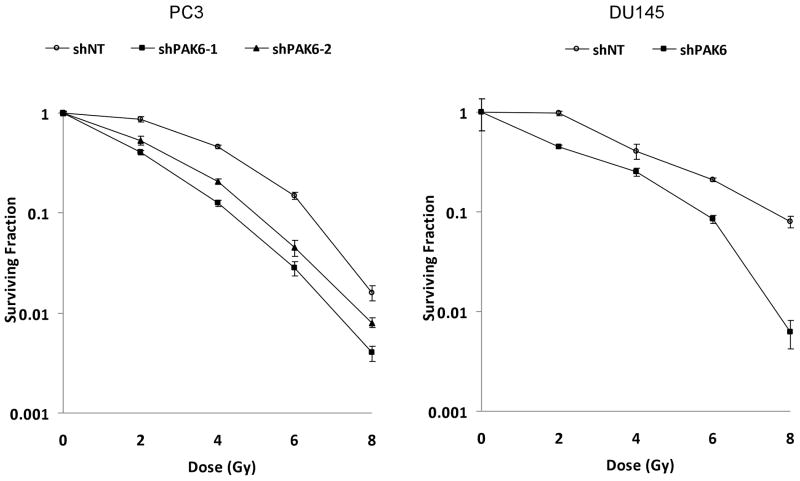
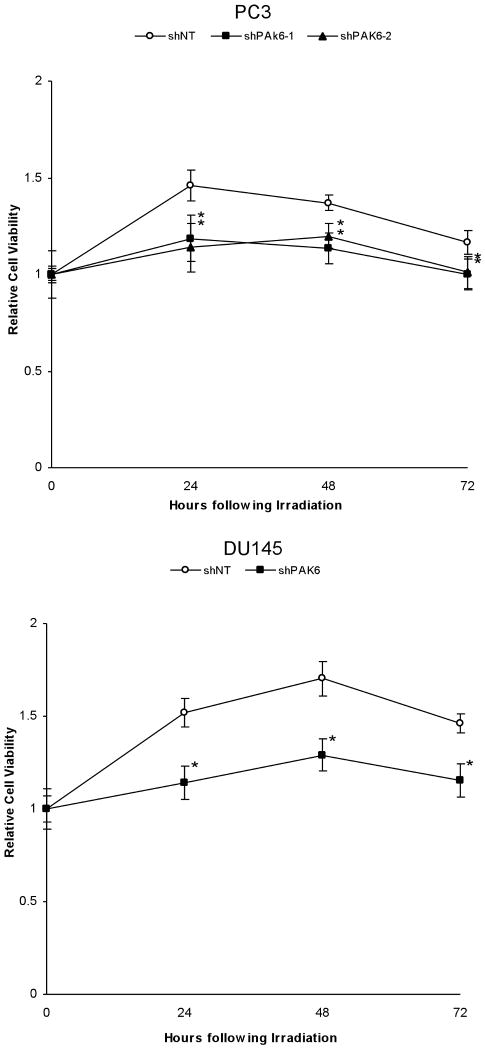
To establish the radiosensitizing ability of PAK6 inhibition, two PAK6-knockdown PC3 cell lines (PC3 shPAK6-1 and PC3 shPAK6-2) and one PAK6-knockdown DU145 cell line (DU145 shPAK6) were generated. Cells transfected with non-targeting shRNA (PC3 shNT and DU145 shNT) served as controls. Western blot confirmed the PAK6 knockdown efficiency (Figure 2). Clonogenic survival assay was performed following transfection with PAK6 shRNA in combination with radiation. As displayed in Figure 3, treatment with PAK6 shRNA shifted the survival curves downward for both PC3 and DU145 cells. To determine how PAK6 inhibition affects cell viability and proliferation, MTT assay was performed 24, 48, and 72 hours following irradiation. PAK6-deficient cell lines showed a significant reduction of cell viability at all three time points (24, 48, and 72 hours) following irradiation compared to control cells with normal PAK6 levels (Figure 4). These data indicated that PAK6 mediated radioresistance of PC3 and DU145 cells and targeted inhibition of PAK6 sensitized these cells to irradiation.
Figure 2.
Knockdown of PAK6 in PC3 and DU145 cell lines. Two PAK6-knockdown PC3 cell lines (PC3 shPAK6-1 and PC3 shPAK6-2) and one PAK6-knockdown DU145 cell line (DU145 shPAK6) were generated using PAK6 shRNAs. Cells transfected with non-targeting shRNA (PC3 shNT and DU145 shNT) served as control. Western blot confirmed PAK6 knockdown efficiency.
Figure 3.
Clonogenic survival assay demonstrating inhibition of PAK6 increased the radiosensitivity of PC3 and DU145 cells.
Figure 4.
MTT assay illustrating the difference in cell growth between control and PAK6-deficient cell lines following 6 Gy irradiation. *, p < 0.05 versus control cells transfected with non-targeting shRNA.
PAK6 Knockdown Sensitizes Cells to Radiation-induced Apoptosis via the BAD Pathway
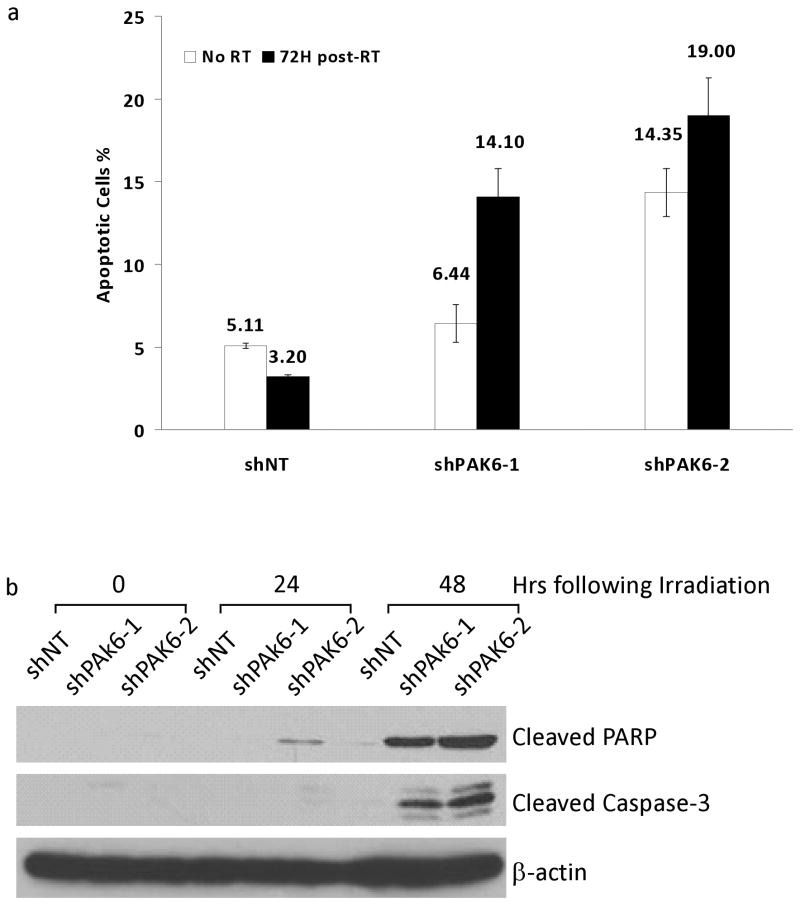
We compared the extent of radiation-induced apoptosis in PCa cells transfected with either PAK6 shRNA or scrambled shRNA. Annexin V analysis showed that the radiation-induced apoptotic cells were significantly higher in PAK6-knockdown PC3 cells compared with non-targeting cells (Figure 5a). Furthermore, PAK6 inhibition resulted in an increase of cleaved PARP and cleaved caspase-3 in PC3 cells (Figure 5b). Data in DU145 cells mirrored those in PC3 cells (data not shown). This suggested that a high level of PAK6 may confer resistance to radiation-induced apoptosis.
Figure 5.
PAK6-knockdown cells exhibit a greater degree of apoptosis compared to control cells following irradiation. (a) Annexin V Assay comparing the percentage of apoptotic cells in PAK6-deficient PC3 cells with control cells 72 hours following 6 Gy irradiation. (b) Cleaved PARP and cleaved caspase-3 levels in control and PAK6-knockdown PC3 cells at 24 and 48 hours following 6 Gy irradiation.
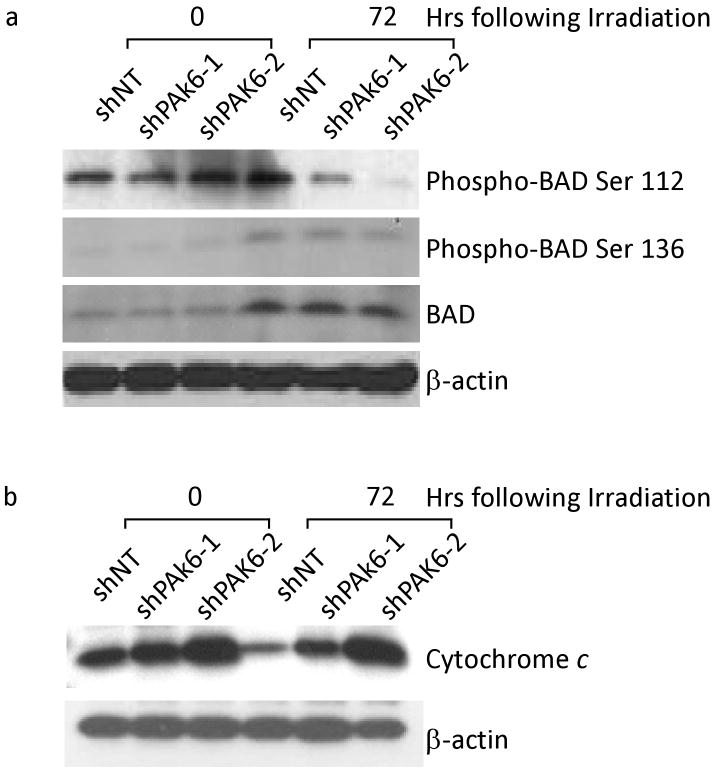
We further investigated the potential mechanisms by which PAK6 inhibition sensitized prostate cancer cells to radiation-induced apoptosis. Given that PAK4 and PAK5 have been reported to protect cells from apoptosis through phosphorylation of the apoptotic protein BAD (15,16), we turned our attention to the interaction between PAK6 and BAD. Figure 6a showed a significant decrease of phospho-BAD Ser112 protein levels in PAK6-knockdown PC-3 cells 72 hours following irradiation compared to control cells which showed a slight increase in protein expression after irradiation. Furthermore, we were able to show that PAK6 inhibition affects only Bad phosphorylation on Ser112 and does not alter BAD phosphorylation on Ser136 or total BAD levels. To verify that the mitochondrial apoptotic pathway was how PAK6 inhibition sensitized cells to radiation-induced apoptosis we looked at cytochrome c release in PAK6-deficient cells following irradiation. It has been reported that when BAD is phosphorylated, it can no longer interact with Bcl-2 or Bcl-xL, cytochrome c release is inhibited, and apoptosis is prevented (17). Western blot analysis on PAK6-deficient cells 72 hours following irradiation showed significantly higher levels of cytochrome c release compared to control cells (Figure 6b). Data in DU145 cells mirrored those in PC3 cells (data not shown). Taken together, our findings indicated that PAK6 may protect PCa cells from radiation-induced apoptosis by phosphorylating BAD on Ser 112.
Figure 6.
Changes within BAD pathway may elucidate a potential mechanism by which PAK6 mediates resistance to radiation-induced apoptosis. (a) Western blot analysis showing phosphorylation sites Ser112 and 136 on BAD as well as total BAD levels in both control and PAK6- knockdown PC3 cells. (b) Enhanced cytochrome c release in PAK6- knockdown PC3 cells compared to control cells 72 hours following 6 Gy irradiation.
Down-regulation of PAK6 Led to Persistence of Un-repaired Double Strand DNA Damage
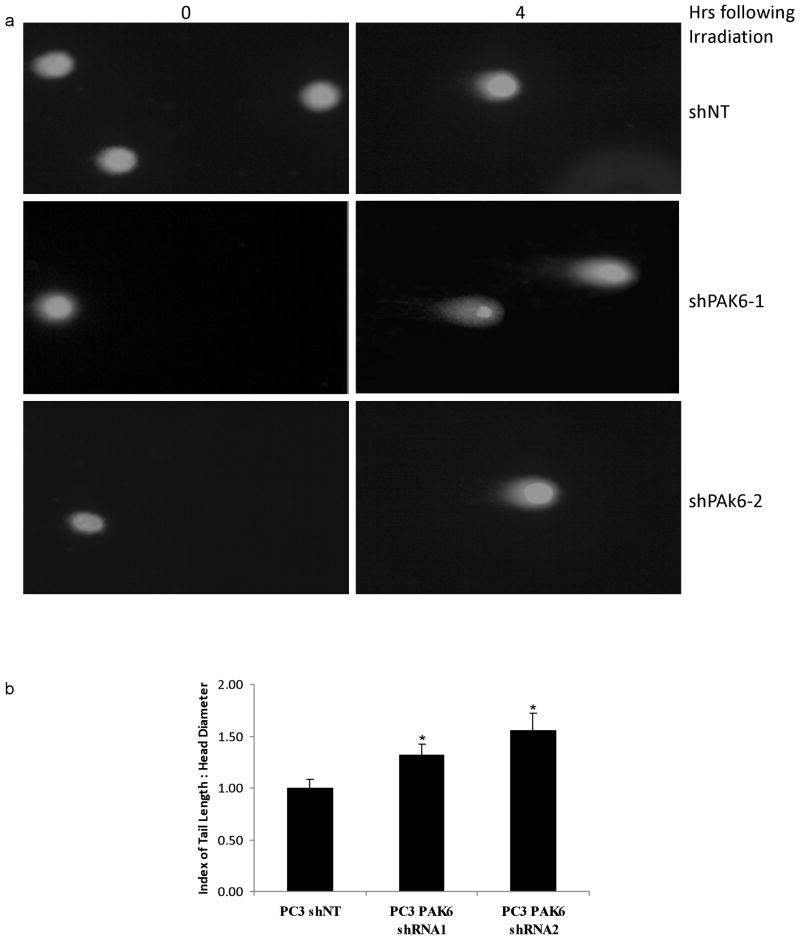
Since cell death following radiotherapy is commonly thought to be due to the extent of double stranded DNA damage, we investigated whether PAK6 inhibition enhanced dsDNA damage using the Comet assay. The degree of dsDNA breaks was indicated by the length of the Comet tail of migrating DNA. We found that knockdown of PAK6 resulted in a greater degree of double strand DNA damage in PC3 and DU145 (data not shown) cells. In contrast, control cells transfected with non-targeting shRNA showed a lesser degree of dsDNA damage as indicated by noticeably shorter Comet tail (Figure 7).
Figure 7.
(a) Comet assay showing enhanced double-strand DNA damage upon 6 Gy irradiation in PAK6-knockdown PC3 cells compared to control cells. (b) Quantification of Comet assay using the ratio of comet tail length/head diameter. *, p < 0.05 versus control cells transfected with non-targeting shRNA.
Inhibition of PAK6 induced arrest of cells in G2-M phase
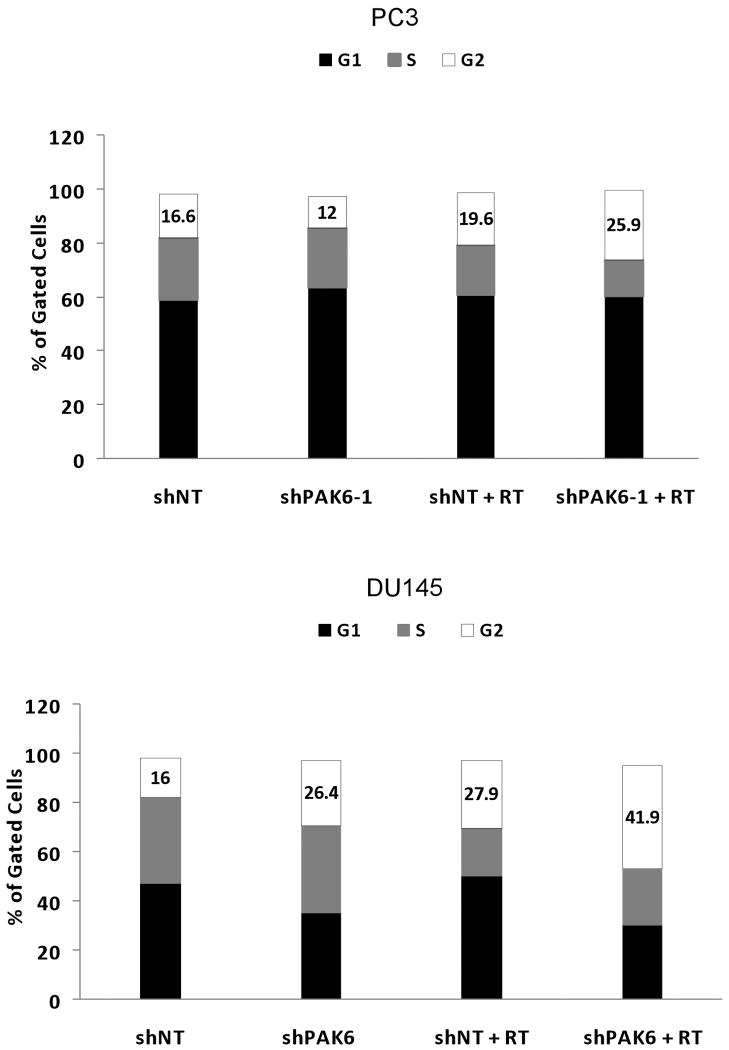
Cell cycle analysis before and after irradiation showed an increase in the G2-M fraction in both PC3 and DU145 cells treated with PAK6 shRNA, indicating a greater portion of both cell lines were blocked in a more radiosensitive stage of the cell cycle compared to cells treated with non-targeting shRNA (Figure 8).
Figure 8.
Cell cycle analysis identifying G2-M arrest in PAK6-deficient cell lines compared to control cells 24 hours following 6 Gy irradiation. Data were displayed as one representative out of three experiments.
Discussion
Radiotherapy is a promising, effective, and definitive treatment option for men with localized PCa. However, high local and systemic recurrence rates after radiation therapy illustrate that resistance and progression remain common events. >50% of men with high-risk characteristics (serum PSA > 20 ng/ml, > clinical T2, and Gleason score > 7) will have a >50% chance of failing radiation (18,19). Therefore, research has concentrated on two avenues, (a) delivering a higher dose of radiation and (b) sensitizing tumors to radiation. During the last few years, the implementation of three-dimensional conformal radiation techniques and the use of temporary androgen withdrawal have significantly improved long-term results of radiation therapy for locally advanced PCa (20-22). However, limitations of these techniques include increased risk of local complications when radiation doses exceed 70 Gy and side effects associated with androgen ablation. Thus, novel radiosensitizers targeting tumor-specific molecules are required to further improve radiation sensitivity of PCa.
Exposure to radiation results in DNA double-strand breaks and other cellular injuries. Cancer cells that are able to repair radiation-caused damages could become more aggressive and radioresistant than parental prostate cancer cells due to adaptive regulation of proteins potentially related to cytoprotective signaling pathways. Although the precise molecular mechanisms of radioresistance development in PCa is not fully understood, numerous studies have demonstrated that, in addition to known factors leading to reduced efficacy of radiotherapy (clinical stage, Gleason score, tumor hypoxia), radiation-induced activation of intracellular signaling events may partially explain the diversity in response. It is suggested that these intracellular events can mediate cell cycle progression, intensified cell motility, invasiveness, and DNA repair. One method to identify potential therapeutic targets associated with radiation resistance is to compare gene-expression in human PCa cells before and after irradiation. The present results show the PAK6 to be highly upregulated after irradiation in both PC3 and DU145 cells. Therefore, we hypothesized that PAK6 overexpression may influence the response of PCa cells to radiation.
To clarify the functional role of PAK6 in radiation, we used a shRNA strategy to stably knock down PAK6 in radioresistant PCa cells and then compared their radiosensitivity with scrambled shRNA-expressing cells. We showed that attenuation of PAK6 protein and mRNA expression by shRNA decreased cell viability and clonogenic activity. Knockdown of PAK6 resulted in an increase rate of radiation-induced apoptosis. Moreover, transfection with PAK6 shRNA blocked cells in a more radiosensitive G2-M stage and increased the DNA double-strand breaks induced by radiation. These results demonstrate for the first time that PAK6 mediates radiation resistance and targeted suppression of PAK6 increases the radiation sensitivity of PCa cells.
Apoptosis is one of the primary mechanisms that causes PCa cells to die when subjected to radiotherapy (23,24). One mechanism by which cancer cells become resistant to radiation is disruption of the pathways that lead to apoptosis. Primary or acquired resistance of PCa to current treatment protocol including radiotherapy has been associated with apoptosis resistance in cancer cells and linked to therapy failures (25). In this study, PAK6 inhibition significantly decreased the extent of radiation-induced apoptosis in prostate cancer cells, supporting a protective function of PAK6.
We further explored the mechanism by which PAK6 mediates radioresistance in prostate cancer cells. Both group I and II PAKs have been implicated in apoptosis, possessing either proapoptotic (PAK2 and PAK3) or antiapoptotic (PAK1, PAK4, and PAK5) properties. PAK2 and PAK3 get cleaved during apoptosis, most likely by caspase 3; this cleavage results in their activation, leading to morphological and membrane changes that occur during apoptosis (26-28). In contrast, PAK1, PAK4, and PAK5, which are not cleaved by caspases, have been reported to protect cells from apoptosis (15,16,29). The anti-apoptotic function of PAKs is thought to be related to phosphorylation of the proapoptotic protein BAD on Ser112 (PAK1, PAK4, and PAK5) and Ser136 (PAK1). BAD forms heterodimers with mitochondrial membrane-based partners such as Bcl-2 and Bcl-xL, antiapoptotic proteins that prevent the release of cytochrome c from mitochondria (30-32). This complex formation abrogates the antiapoptotic function of Bcl-2 and Bcl-xL, thereby facilitating apoptotic death via a cytochrome c-dependent pathway (33,34). Conversely, when BAD becomes phosphorylated and translocated into the cytoplasm through binding with the phosphoserine-binding protein 14-3-3τ, the uncomplexed Bcl-2 and Bcl-xL are then capable of suppressing cell death by blocking the release of mitochondrial cytochrome c (35,36). As a consequence, the dynamic interaction between Bcl-xL and BAD represents an alternative mechanism for cancer cells to evade apoptosis. Here we report that PAK6 can protect PCa cells from apoptosis in response to irradiation. As is the case for PAK4 and PAK5, one way that PAK6 may protect cells form apoptosis is by phosphorylating the proapoptotic protein BAD on Ser112. Consistent with this, we have found that both cytochrome c release and effector caspases such as caspase 3 increase in PCa cells treated with PAK6 shRNA and radiation.
In addition to suppression of apoptosis-related cell death, most likely via inhibition of caspase activation, the underlying mechanisms by which PAK6 mediates radioresistance seem to be multifaceted. We found that PAK6 shRNA treatment altered the cell cycle distribution, resulting in an increased G2-M fraction 24 hours after transfection. Therefore, at the time of irradiation, the cells were blocked in a more radiosensitive stage of the cell cycle. Previous study has shown that PAK6 interacts with PP1B, a member of the PP2C family of Ser/Thr protein phosphatases (13). PP1B has been reported to dephosphorylate cyclin dependent kinases (37,38). It is possible that PAK6 may be involved in cell cycle control through PP1B/cyclin pathway. Furthermore, we observed a higher incidence of DNA double-strand breaks in PAK6 knockdown cells after irradiation, as measured by a comet assay. It remains unclear whether PAK6 plays a direct a direct or indirect role in the DNA repair process; however, it is an apparent that disrupting PAK6 disrupts double-strand DNA repair abilities of radiation-resist prostate cancer cells, thereby sensitizing these cells to radiation induced cell death. Both the PAK6 shRNA-induced arrest of cells in the more radiosensitive G2-M phase and the impaired post-irradiation DNA damage repair, may well have contributed to the induction of apoptosis through caspase -dependent or -independent pathways in otherwise resistant PCa cells against radiation.
In summary, our results suggest that the combination of PAK6 inhibition and irradiation resulted in significantly decreased survival of PCa cells. The underlying mechanisms involve alterations in cell cycle distribution and impaired DNA double-strand break repair as well as relieved BAD phosphorylation. These findings highlight the potential that PAK6 may be an effective target for radiation sensitization in prostate cancer. Additional studies are ongoing to validate PAK6 as a target for radiation sensitization using animal models as well as clinical samples.
Acknowledgments
Funding: This manuscript was supported by funds from NIH/NCI RO1 CA108633 (to AC) and funds from The Ohio State University/Arthur G. James Cancer Center (to AC).
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer Statistics, 2009. CA: a cancer journal for clinicians. 2009 doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Horwitz EM, Hanlon AL, Hanks GE. Update on the treatment of prostate cancer with external beam irradiation. Prostate. 1998;37(3):195–206. doi: 10.1002/(sici)1097-0045(19981101)37:3<195::aid-pros10>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 3.Kalkner KM, Wahlgren T, Ryberg M, Cohn-Cedermark G, Castellanos E, Zimmerman R, Nilsson J, Lundell M, Fowler J, Levitt S, Hellstrom M, Nilsson S. Clinical outcome in patients with prostate cancer treated with external beam radiotherapy and high dose-rate iridium 192 brachytherapy boost: a 6-year follow-up. Acta Oncol. 2007;46(7):909–917. doi: 10.1080/02841860601156140. [DOI] [PubMed] [Google Scholar]
- 4.Thompson I, Thrasher JB, Aus G, Burnett AL, Canby-Hagino ED, Cookson MS, D'Amico AV, Dmochowski RR, Eton DT, Forman JD, Goldenberg SL, Hernandez J, Higano CS, Kraus SR, Moul JW, Tangen CM. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2007;177(6):2106–2131. doi: 10.1016/j.juro.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Yang F, Li X, Sharma M, Zarnegar M, Lim B, Sun Z. Androgen receptor specifically interacts with a novel p21-activated kinase, PAK6. The Journal of biological chemistry. 2001;276(18):15345–15353. doi: 10.1074/jbc.M010311200. [DOI] [PubMed] [Google Scholar]
- 6.Arias-Romero LE, Chernoff J. A tale of two Paks. Biol Cell. 2008;100(2):97–108. doi: 10.1042/BC20070109. [DOI] [PubMed] [Google Scholar]
- 7.Kumar R, Gururaj AE, Barnes CJ. p21-activated kinases in cancer. Nat Rev Cancer. 2006;6(6):459–471. doi: 10.1038/nrc1892. [DOI] [PubMed] [Google Scholar]
- 8.Abo A, Qu J, Cammarano MS, Dan C, Fritsch A, Baud V, Belisle B, Minden A. PAK4, a novel effector for Cdc42Hs, is implicated in the reorganization of the actin cytoskeleton and in the formation of filopodia. Embo J. 1998;17(22):6527–6540. doi: 10.1093/emboj/17.22.6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cau J, Faure S, Comps M, Delsert C, Morin N. A novel p21-activated kinase binds the actin and microtubule networks and induces microtubule stabilization. J Cell Biol. 2001;155(6):1029–1042. doi: 10.1083/jcb.200104123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schrantz N, da Silva Correia J, Fowler B, Ge Q, Sun Z, Bokoch GM. Mechanism of p21-activated kinase 6-mediated inhibition of androgen receptor signaling. The Journal of biological chemistry. 2004;279(3):1922–1931. doi: 10.1074/jbc.M311145200. [DOI] [PubMed] [Google Scholar]
- 11.Lee SR, Ramos SM, Ko A, Masiello D, Swanson KD, Lu ML, Balk SP. AR and ER interaction with a p21-activated kinase (PAK6) Molecular endocrinology (Baltimore, Md. 2002;16(1):85–99. doi: 10.1210/mend.16.1.0753. [DOI] [PubMed] [Google Scholar]
- 12.Kaur R, Liu X, Gjoerup O, Zhang A, Yuan X, Balk SP, Schneider MC, Lu ML. Activation of p21-activated kinase 6 by MAP kinase kinase 6 and p38 MAP kinase. The Journal of biological chemistry. 2005;280(5):3323–3330. doi: 10.1074/jbc.M406701200. [DOI] [PubMed] [Google Scholar]
- 13.Kaur R, Yuan X, Lu ML, Balk SP. Increased PAK6 expression in prostate cancer and identification of PAK6 associated proteins. The Prostate. 2008;68(14):1510–1516. doi: 10.1002/pros.20787. [DOI] [PubMed] [Google Scholar]
- 14.Scott SL, Gumerlock PH, Beckett L, Li Y, Goldberg Z. Survival and cell cycle kinetics of human prostate cancer cell lines after single- and multifraction exposures to ionizing radiation. International journal of radiation oncology, biology, physics. 2004;59(1):219–227. doi: 10.1016/j.ijrobp.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 15.Cotteret S, Jaffer ZM, Beeser A, Chernoff J. p21-Activated kinase 5 (Pak5) localizes to mitochondria and inhibits apoptosis by phosphorylating BAD. Molecular and cellular biology. 2003;23(16):5526–5539. doi: 10.1128/MCB.23.16.5526-5539.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gnesutta N, Qu J, Minden A. The serine/threonine kinase PAK4 prevents caspase activation and protects cells from apoptosis. The Journal of biological chemistry. 2001;276(17):14414–14419. doi: 10.1074/jbc.M011046200. [DOI] [PubMed] [Google Scholar]
- 17.Downward J. How BAD phosphorylation is good for survival. Nat Cell Biol. 1999;1(2):E33–35. doi: 10.1038/10026. [DOI] [PubMed] [Google Scholar]
- 18.D'Amico AV. Combined-modality staging for localized adenocarcinoma of the prostate. Oncology (Williston Park) 2001;15(8):1049–1059. discussion 1060-1042, 1064-1045, 1069-1070,1073-1045. [PubMed] [Google Scholar]
- 19.D'Amico AV, Cote K, Loffredo M, Renshaw AA, Schultz D. Determinants of prostate cancer-specific survival after radiation therapy for patients with clinically localized prostate cancer. J Clin Oncol. 2002;20(23):4567–4573. doi: 10.1200/JCO.2002.03.061. [DOI] [PubMed] [Google Scholar]
- 20.Leibel SA, Fuks Z, Zelefsky MJ, Hunt M, Burman CM, Mageras GS, Chui CS, Jackson A, Amols HI, Ling CC. Technological advances in external-beam radiation therapy for the treatment of localized prostate cancer. Seminars in oncology. 2003;30(5):596–615. doi: 10.1016/s0093-7754(03)00354-3. [DOI] [PubMed] [Google Scholar]
- 21.Mangar SA, Huddart RA, Parker CC, Dearnaley DP, Khoo VS, Horwich A. Technological advances in radiotherapy for the treatment of localised prostate cancer. Eur J Cancer. 2005;41(6):908–921. doi: 10.1016/j.ejca.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 22.Parker CC, Dearnaley DP. Radical radiotherapy for prostate cancer. Cancer treatment reviews. 2003;29(3):161–169. doi: 10.1016/s0305-7372(03)00070-7. [DOI] [PubMed] [Google Scholar]
- 23.Denmeade SR, Lin XS, Isaacs JT. Role of programmed (apoptotic) cell death during the progression and therapy for prostate cancer. Prostate. 1996;28(4):251–265. doi: 10.1002/(SICI)1097-0045(199604)28:4<251::AID-PROS6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 24.Szostak MJ, Kyprianou N. Radiation-induced apoptosis: predictive and therapeutic significance in radiotherapy of prostate cancer (review) Oncol Rep. 2000;7(4):699–706. doi: 10.3892/or.7.4.699. [DOI] [PubMed] [Google Scholar]
- 25.DiPaola RS, Patel J, Rafi MM. Targeting apoptosis in prostate cancer. Hematol Oncol Clin North Am. 2001;15(3):509–524. doi: 10.1016/s0889-8588(05)70229-x. [DOI] [PubMed] [Google Scholar]
- 26.Lee N, MacDonald H, Reinhard C, Halenbeck R, Roulston A, Shi T, Williams LT. Activation of hPAK65 by caspase cleavage induces some of the morphological and biochemical changes of apoptosis. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(25):13642–13647. doi: 10.1073/pnas.94.25.13642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rudel T, Bokoch GM. Membrane and morphological changes in apoptotic cells regulated by caspase-mediated activation of PAK2. Science (New York, NY. 1997;276(5318):1571–1574. doi: 10.1126/science.276.5318.1571. [DOI] [PubMed] [Google Scholar]
- 28.Walter BN, Huang Z, Jakobi R, Tuazon PT, Alnemri ES, Litwack G, Traugh JA. Cleavage and activation of p21-activated protein kinase gamma-PAK by CPP32 (caspase 3). Effects of autophosphorylation on activity. The Journal of biological chemistry. 1998;273(44):28733–28739. doi: 10.1074/jbc.273.44.28733. [DOI] [PubMed] [Google Scholar]
- 29.Schurmann A, Mooney AF, Sanders LC, Sells MA, Wang HG, Reed JC, Bokoch GM. p21-activated kinase 1 phosphorylates the death agonist bad and protects cells from apoptosis. Molecular and cellular biology. 2000;20(2):453–461. doi: 10.1128/mcb.20.2.453-461.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chipuk JE, Bhat M, Hsing AY, Ma J, Danielpour D. Bcl-xL blocks transforming growth factor-beta 1-induced apoptosis by inhibiting cytochrome c release and not by directly antagonizing Apaf-1-dependent caspase activation in prostate epithelial cells. The Journal of biological chemistry. 2001;276(28):26614–26621. doi: 10.1074/jbc.M100913200. [DOI] [PubMed] [Google Scholar]
- 31.Erhardt P, Cooper GM. Activation of the CPP32 apoptotic protease by distinct signaling pathways with differential sensitivity to Bcl-xL. The Journal of biological chemistry. 1996;271(30):17601–17604. doi: 10.1074/jbc.271.30.17601. [DOI] [PubMed] [Google Scholar]
- 32.Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes & development. 1999;13(15):1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 33.Green DR, Reed JC. Mitochondria and apoptosis. Science (New York, NY. 1998;281(5381):1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 34.Yang E, Korsmeyer SJ. Molecular thanatopsis: a discourse on the BCL2 family and cell death. Blood. 1996;88(2):386–401. [PubMed] [Google Scholar]
- 35.del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science (New York, NY. 1997;278(5338):687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- 36.Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L) Cell. 1996;87(4):619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- 37.Cheng A, Kaldis P, Solomon MJ. Dephosphorylation of human cyclin-dependent kinases by protein phosphatase type 2C alpha and beta 2 isoforms. The Journal of biological chemistry. 2000;275(44):34744–34749. doi: 10.1074/jbc.M006210200. [DOI] [PubMed] [Google Scholar]
- 38.Cheng A, Ross KE, Kaldis P, Solomon MJ. Dephosphorylation of cyclin-dependent kinases by type 2C protein phosphatases. Genes & development. 1999;13(22):2946–2957. doi: 10.1101/gad.13.22.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]