Abstract
Studies of reconsolidation, in which retrieved memories are altered and restored, offer a novel approach for exploring the associative structure of fear memory. Here we show in rats that exposure to the unconditioned stimulus initiates an unconditioned stimulus–specific reconsolidation of learned fear that depends on the amygdala. Thus, specific features of the unconditioned stimulus appear to be encoded in the amygdala as part of fear memories stored there.
Protein synthesis-dependent memory reconsolidation has attracted much attention because of its possible application in treatment of mental disorders, such as anxiety or addictive disorders (1-3). Studies of reconsolidation also provide a novel way to explore the structure of memory traces. Many studies of reconsolidation have involved auditory fear conditioning, in which an acoustic conditioned stimulus is paired with an unconditioned stimulus, typically a mild electric shock to the feet or to the eyelids. Subsequent exposure to the conditioned stimulus alone then triggers fear responses, such as freezing (4). Considerable evidence suggests that the lateral amygdala is a key site required for fear memory acquisition, consolidation and reconsolidation, although other brain regions also contribute (4-10). Nevertheless, much remains unknown about how the lateral amygdala contributes to the organization of fear associations.
We have recently demonstrated that reconsolidation of auditory fear conditioning only occurs to the conditioned stimulus presented during reactivation and not to other conditioned stimuli paired during the same training session with the same unconditioned stimulus (9; see also: Supplementary Fig. 2). The unconditioned stimulus, however, is a powerful reminder capable of alleviating forgetting (11) or experimental amnesia (12) and has been shown to trigger reconsolidation (13). Here we examine the role of the unconditioned stimulus in reconsolidation processes in order to better understand the involvement of the lateral amygdala in maintaining cue conditioned associations.
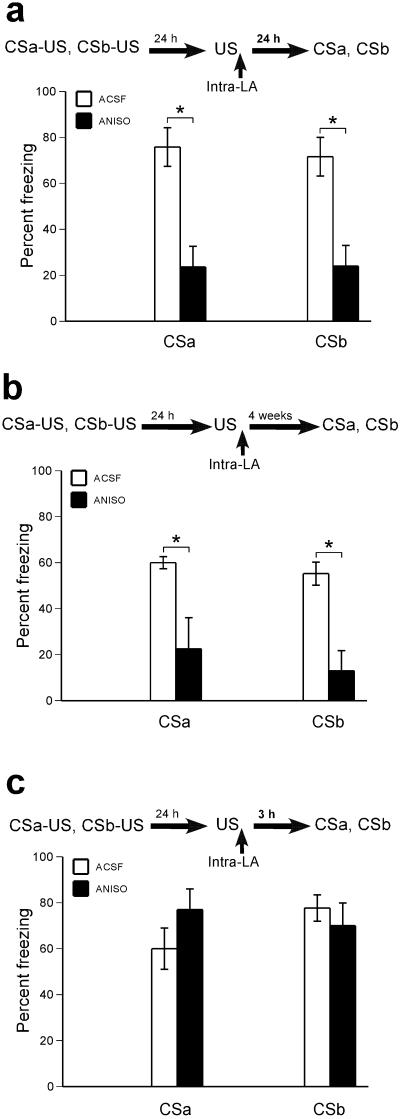
We first asked whether presentation of the unconditioned stimulus alone would render all conditioned stimulus associations to that unconditioned stimulus susceptible to disruption. Rats with cannulae implanted bilaterally in the lateral amygdala were conditioned to two distinct auditory stimuli: conditioned stimulus “a” (CSa) and conditioned stimulus “b” (CSb), each paired with the same footshock unconditioned stimulus (Supplementary Methods and Materials; all procedures were in accordance with the NIH Guide for the Care and Use of Experimental Animals, and were approved by the New York University Animal Care and Use Committee.). On the following day, rats were presented with a single unconditioned stimulus in order to reactivate the memory, followed immediately by intra–lateral amygdala infusions of protein synthesis inhibitor anisomycin or vehicle (artificial cerebrospinal fluid, ACSF). Twenty–four but not three hours later, when freezing to each tone was tested, rats treated with anisomycin showed a deficit in freezing to both conditioned stimuli as compared to the vehicle controls (this effect was also present at 4 weeks post-reactivation interval) at which the long-term memory was tested (Fig. 1a-c; for statistical results: see Fig. 1 Legend). This effect was not observed when the anisomycin was infused in areas outside of the lateral amygdala (Supplementary Fig. 3). Presenting an unconditioned stimulus previously associated with two distinct conditioned stimuli – whether directly (Fig. 1) or indirectly (Supplementary Fig. 4) – destabilizes all conditioned stimulus–unconditioned stimulus associations that are linked to the reactivated unconditioned stimulus.
Figure 1. Exposure to the unconditioned stimulus alone triggers memory reconsolidation.
(a) Anisomycin (ANISO) infusions following an exposure to the footshock unconditioned stimulus alone disrupt the reconsolidation of auditory fear conditioning to both CSa and CSb [ANOVA: significant main effect of drug (F(1,14)=21.70, p<.001), n=7 and n= 9 for ACSF and ANISO, respectively]. (b) Amnesic effects of anisomycin do not reverse within 4 weeks [ANOVA: significant main effect of drug F(1,12)=36.06, p<.0001), n=7 and n= 7 for ACSF and ANISO, respectively]. (c) Short-term memory is not affected by anisomycin [n=6 ACSF; n=7 ANISO; No significant effects of drug (p=.7), conditioned stimulus (p=.5), or drug x conditioned stimulus (p=.5)]. Asterisks (*) indicate a significant difference between groups; error bars indicate standard error.
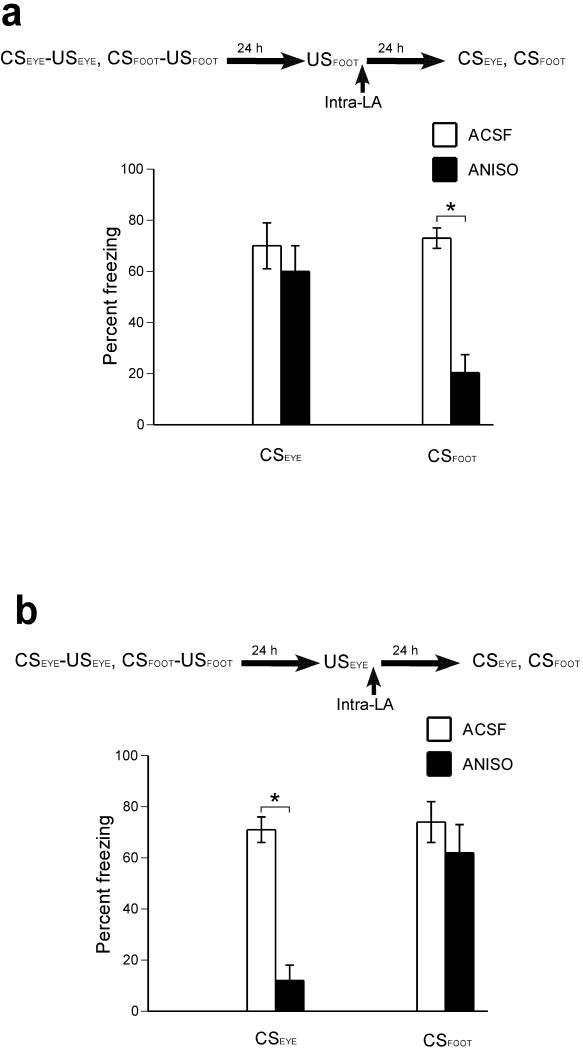
Prevailing models of auditory fear conditioning posit that the lateral amygdala is a site where a conditioned stimulus acquires affective properties during pairing with a noxious unconditioned stimulus via the formation of an association between neural representations of the conditioned stimulus with the general aversive properties of the unconditioned stimulus (4-8). According to these models, any experience of an aversive event should destabilize conditioned fear responses. We therefore asked whether the unconditioned stimulus – triggered reconsolidation is a selective process, or whether the exposure to an unconditioned stimulus eliciting fear renders any aversive memory labile. To this end, two distinct unconditioned stimuli, electrical shock to the feet (unconditioned stimulus applied to the foot or USFOOT) or to the eyelids (unconditioned stimulus applied to the eyelid or USEYE), were each paired with one of two distinct conditioned stimuli, CSFOOT (conditioned stimulus paired with USFOOT) and CSEYE (conditioned stimulus paired with USEYE) respectively (Supplementary Methods and Materials). Twenty–four hours later, rats were exposed either to USFOOT or to USEYE, followed by intra–lateral amygdala infusions of anisomycin or ACSF. On the following day, freezing behavior to CSEYE and CSFOOT was tested (Fig. 2a,b). Compared to the vehicle group, for which freezing to both conditioned stimuli was equivalent, the data from the drug group clearly show that anisomycin delivered to the lateral amygdala after exposure to the unconditioned stimulus disrupted fear responding only to the conditioned stimulus associated with the presented unconditioned stimulus, leaving intact freezing to the conditioned stimulus that had been paired with the other unconditioned stimulus (for statistical analysis see: Fig. 2 Legend). Thus, the disruption of reconsolidation was selective to the administered unconditioned stimulus.
Figure 2. Reconsolidation is selective to the reactivated unconditioned stimulus.
(a) Anisomycin infusions following either footshock (USFOOT) or eyelidshock (USEYE) selectively disrupt fear memory reconsolidation for the conditioned stimulus associated with the reactivated unconditioned stimulus [Drug x CSTYPE x Reactivation-USTYPE interaction (F(1,24)=18.15, p<.001). Follow-up of the triple interaction with simple interaction effects indicated that anisomycin, following exposure to the USFOOT (n=8 and n=6 for ANISO and ACSF, respectively) impairs freezing responding to the USFOOT–paired conditioned stimulus (CSFOOT), but does not affect freezing to the USEYE–paired conditioned stimulus (CSEYE) [ANOVA: significant main effects of drug (F(1,12)=14.39, p<.01) and CSTYPE (F(1,12)=6.24, p<.05); and a significant CSTYPE x drug interaction (F(1,12)=8.60, p<.05)]. (b) Anisomycin infusions following an exposure to an USEYE (n=8 and n=6 for ANISO and ACSF, respectively) impair freezing responding to the USEYE–paired conditioned stimulus (CSEYE), but do not affect freezing to the USFOOT–paired conditioned stimulus (CSFOOT) [ANOVA: significant main effects of drug (F(1,12)=16.91, p<.01) and CSTYPE (F(1,12)=11.70, p<.01); and a significant CSTYPE x drug interaction (F(1,12)=9.57, p<.01)]. Asterisks (*) indicate a significant difference between groups; error bars indicate standard error.
Our findings demonstrate that independent fear memories are stored, retrieved, and reconsolidated in an amygdala–dependent manner according to their sensory features. Thus, sensory properties of the unconditioned stimulus seem to play a key role in fear associations in the lateral amygdala, a finding not predicted by current models of auditory fear conditioning. The selectivity of reconsolidation processes seems to protect the global integrity of memories. However, the mechanisms underlying this selectivity, whether they involve different populations of cells and/or distinct cellular and molecular processes, as well as the role of other brain structures in supporting this selectivity, remain to be studied. Better understanding how fear memories are organized is a critical step in developing reconsolidation-based therapeutic approaches to trauma (1-3).
Supplementary Material
Acknowledgments
The authors thank Claudia Farb and Dr. Jeffrey Erlich for their advice, and Sneh Kadakia for her technical assistance. This research was supported by following grants to J.E.L: R01 MH046516, R37 MH038774 and P50 MH058911, grants to V.D. and J.E.L: NSF-CNRS International Cooperative Grant, a CNRS PICS grant, and a CNRS-UPS-NYU collaboration grant EmoTime. L.D-M. was supported by a Fulbright/Spanish Ministry of Education postdoctoral fellowship.
Footnotes
Competing Interests Statement
The authors declare no conflicts of interests.
References
- 1.Sara SJ. Learn. Mem. 2000;7:73–84. doi: 10.1101/lm.7.2.73. [DOI] [PubMed] [Google Scholar]
- 2.Nader K, Schafe GE, Le Doux JE. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- 3.Tronson NC, Taylor JR. Nat. Rev. Neurosci. 2007;8:262–275. doi: 10.1038/nrn2090. [DOI] [PubMed] [Google Scholar]
- 4.LeDoux JE. Annu. Rev. Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 5.Fanselow MS, Poulos AM. Annu. Rev. Psychol. 2005;56:207–234. doi: 10.1146/annurev.psych.56.091103.070213. [DOI] [PubMed] [Google Scholar]
- 6.Davis M. Annu. Rev. Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- 7.Everitt BJ, Cardinal RN, Parkinson JA, Robbins TW. Ann. N. Y. Acad. Sci. 2003;985:233–250. [PubMed] [Google Scholar]
- 8.Maren S. Neuron. 2005;47:783–786. doi: 10.1016/j.neuron.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Doyère V, Dębiec J, Monfils MH, Schafe GE, LeDoux JE. Nat. Neurosci. 2007;10:414–416. doi: 10.1038/nn1871. [DOI] [PubMed] [Google Scholar]
- 10.Dębiec J, Doyère V, Nader K, Ledoux JE. Proc Natl Acad Sci U S A. 2006;103:3428–33. doi: 10.1073/pnas.0507168103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spear NE, Parsons P. Analysis of a reactivation treatment: Ontogeny and alleviated forgetting. In: Davies R, Roberts W, editors. Coding processes in animal memory. Erlbaum; Hillsdale, NJ: 1976. pp. 135–165. [Google Scholar]
- 12.Miller RR, Springer AD. Physiol. Behav. 1972;8:645–651. doi: 10.1016/0031-9384(72)90089-3. [DOI] [PubMed] [Google Scholar]
- 13.Milekic MH, Brown SD, Castellini C, Alberini CM. J. Neurosci. 2006;26:3010–3020. doi: 10.1523/JNEUROSCI.4818-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.