Abstract
p21Waf1/Cip1/Sdi1 was originally identified as an inhibitor of cyclin-dependent kinases, a mediator of p53 in growth suppression and a marker of cellular senescence. p21 is required for proper cell cycle progression and plays a role in cell death, DNA repair, senescence and aging, and induced pluripotent stem cell reprogramming. Although transcriptional regulation is considered to be the initial control point for p21 expression, there is growing evidence that post-transcriptional and post-translational regulations play a critical role in p21 expression and activity. This review will briefly discuss the activity of p21 and focus on current knowledge of the determinants that control p21 transcription, mRNA stability and translation, and protein stability and activity.
Keywords: p53, p21Waf1/Cip1/Sdi, microRNA, RNA-binding proteins, mRNA stability, transcription
1. Introduction
Deregulated cell proliferation is a hallmark of carcinogenesis [1]. To develop cancer therapeutic strategies, intensive studies have been carried out to identify key factors regulating the cell cycle in the past two decades. Cell cycle progression is controlled by a set of cyclin-dependent kinases (CDKs), which are activated by their associated cyclins, but inhibited by two classes of CDK inhibitors. One group is the INK4 family, including p16INK4a, p15INK4b, p18INK4c, and p19INK4d, which specifically target CDK4 and CDK6 (Reviewed in [2–3]). Another group is the CIP/KIP family, including p21Cip, p27Kip1, and p57Kip2, which inhibit a broad spectrum of CDKs (Reviewed in [2–3]). p21, also named as Cip1, Waf1, Sdi1, and Cap20 [4–9], was discovered as a component of a quaternary complex consisting of cyclin D1, a CDK, the proliferating cell nuclear antigen (PCNA), and p21 in normal human fibroblasts [6]. Later studies found that p21 also associates with cyclin E-CDK2, cyclin A-CDK2, and cyclin B-CDK1 complexes, and has a universal inhibitory activity towards these CDKs [4, 7–8, 10]. In addition, p21 is a direct target of p53 tumor suppressor, and mediates p53-dependent cell cycle arrest in response to DNA damage [5]. When p21 was identified, the mechanism by which p53 suppresses cell growth was unclear. Therefore, the study provided first direct evidence that p53 exerts its activity through its downstream target genes. Consistent with the activity of p21 in growth suppression, p21 knockout mice are prone to accelerated tumor formation in response to genetoxic stresses, but the susceptibility to spontaneous tumors is delayed and mild [11–14]. However, p21 is more than an inhibitor of CDK. Indeed, p21 is implicated in cell death, DNA repair, cellular senescence, and aging [15–17]. Recent studies showed that p21 is found to be a barrier in reprogramming of induced pluripotent stem (iPS) cells [18]. Thus, the biological functions of p21 are far more complex than what we thought. As such a critical regulator of the cell cycle, p21 expression and activity are found to be tightly regulated by multiple mechanisms. In this review, we will discuss what is currently known about some of the key determinants in controlling p21 transcription and protein stability. We will also discuss the regulation of p21 by miRNA and RNA-binding proteins (RBMs), which has not been reviewed. The general information covering the function of p21 can be found in several recent reviews [19–21].
2. The biological functions of p21
2.1 p21 in cell cycle
P21 is accumulated in normal human fibroblasts arrested in G0, whereas depletion of p21 expression by anti-sense RNA promotes cell cycle reentry and DNA synthesis [22]. Thus, p21 is a negative regulator that maintains cells in G0 when the condition for cell cycle progression is not optimal [23]. Upon exposure to growth stimuli, a series of CDKs, including CDK4, CDK6, and CDK2, are sequentially activated during the cell cycle. In the late G1, phosphorylation of the retinoblastoma protein (pRb) is found to be essential for G1/S transition. pRb phosphorylation is initiated by cyclin D-CDK4/6 and phosphorylated pRb is separated from E2F transcription factors which transactivate genes necessary for the G1/S transition and S-phase, including cyclin E [24]. Activation of cyclin E-CDK2 complex further phosphorylates and completely releases pRb from interacting with E2Fs. However, association of p21 with cyclin D-CDK4/6 inhibits pRb phosphorylation and induces cell cycle arrest in G1 (Fig. 1). p21 also associates with and inactivates E2F, leading to cell cycle arrest and cellular senescence [25]. During S phase, PCNA, which is expressed as a ring-shaped trimeric complex, is necessary for the formation of DNA replication complex by interacting with replicative DNA polymerase δ (polδ) and replication factor C (RFC) [26–27]. However, the p21 binding site in PCNA overlaps with the polδ- and RFC-interaction sites [28], and therefore the association with p21 blocks the ability of PCNA to activate polδ, leading to DNA replication block and intra-S arrest [29–30].
Figure 1.
The role of p21 in cell survival and death. Under non-stressed conditions, p21 is expressed at low levels and promotes cell cycle progression. Under stress conditions, p21 expression is increased through p53-dependent and -independent pathways. Increased p21 interacts with and inhibits cyclin-CDKs activity. In most tumor cells, the cell cycle checkpoint function of p21 is lost due to p53 mutations. Akt1 phosphorylates and then stabilizes p21 protein for cell survival. P21 inhibits apoptosis by interacting with proapoptotic molecules such as caspase-3 and ASK1.
Upon exposure to γ-irradiation, cells deficient in p53 or p21 progress into mitosis but fail to undergo cytokinesis [31]. In addition, p21 inhibits both cyclin B1-Cdk1 and cyclin A-Cdk1/2 complexes, leading to permanent cell cycle exit in G2 in normal human fibroblasts [32]. Consistent with this, overexpression of p21 is capable of inducing both G1 and G2-arrest, and p21-induced G2 arrest appears to be more prominent in pRb-null cells [33]. Cyclin B-CDK1 complex, which phosphorylates and inhibits protein phosphatase Cdc25C, is a key regulatory factor for mitotic entry [34–35]. p21 cooperates with 14-3-3σ, a p53 target which normally sequesters cyclinB1-CDK1 complex in the cytoplasm, to control the G2/M transition [36]. Thus, p21 directly or indirectly maintains the G2/M checkpoint.
P21 is also present in active cyclin D/Cdk4 complexes [37]. MEFs lacking p21 and p27 fail to properly assemble cyclin D-Cdk complexes [38]. In fact, p21 stabilizes and promotes active cyclin-Cdk complex formation in a dose-dependent manner [14, 39–41]. This suggests that p21 is not solely a cyclin-Cdk inhibitor, and a certain amount of p21 is required for normal cell cycle progression.
2.2 p21 in cell death
In response to stress signals, p53 is capable of inducing cell cycle arrest and cell death. However, how the cell fate is determined is unclear. It is postulated that the cell type, the strength and type of the insults, and p53 target genes, including p21, control the biological outcome induced by activated p53 [42]. Indeed, it has been shown that Myc represses p53-dependent transcription of p21, and loss of p21 induction leads to p53-dependent apoptosis [43]. Upon DNA damage, caspase-3-mediated cleavage of p21 protein converts cancer cells from growth arrest to apoptosis [44]. Consistent with this, reports showed that cytoplasmic p21 inhibits apoptosis in response to multiple pro-apoptotic stress signals (Fig. 1). For examples, cytoplasmic p21 forms a complex with the apoptosis signal-regulating kinase 1 (ASK1) and inhibits activation of ASK1 and its substrate SAPK/JNK in the MAP kinase cascade [45]. In addition, Akt1/PKB enhances the stability and cytoplasmic localization of p21 and promotes cell survival by phosphorylation of p21 [46–47]. Moreover, several reports showed that p21 inhibits Fas-induced apoptosis by interacting with and then inhibiting the activity of caspase-3, which is dependent on p21 phosphorylation by protein kinase A (PKA) [48–50]. Furthermore, p21 acts as a mediator of the antiapoptotic effect of TNFα-induced NF-κB in Ewing tumor cells [51], and the switch from cell cycle arrest to apoptosis induced by TGF-β is dependent on caspase-3-mediated p21 degradation [52]. Conversely, induction of p21 is correlated with resistance to TGF-β-mediated apoptosis [51]. In contrast, p21 also promotes apoptosis in some cases [53]. Ectopic expression of p21 induces an array of genes, which trigger p53-independent apoptosis in human ovarian cancer cell lines [54]. In addition, Stat1 and p21, but not p53, are essential for oxysterol-induced apoptosis [55]. Moreover, expression of p21 along with HPV E7 protein in U2OS cells induces apoptosis by induction of cathepsin B, a mediator of apoptosis, but not activation of caspases [56]. Therefore, p21 may have pro- or anti-apoptotic functions in cell context- and stress-dependent manners. Nevertheless, the mechanism by which p21 induces either cell cycle arrest or cell death needs to be further explored in physiological systems.
2.3 p21 in DNA repair
PCNA is required for several DNA repair processes, including nucleotide excision repair (NER), base excision repair (BER), mismatch repair (MMR), and translesion DNA synthesis (TLS) [57–59]. As a binding partner of PCNA, p21 plays a role in these DNA repair processes. PCNA is required for MMR through binding to MLH1 and MLH2. However, this activity is inhibited by p21 or a p21 peptide containing the PCNA binding domain [60]. In addition, p21 inhibits long patch BER by inhibiting PCNA-directed stimulation of flap endonuclease 1 (FEN1), DNA ligase I, and polδ [61]. However, the role of p21 in NER is still uncertain. Studies showed that cells lacking p21 are not defective in NER in response to UV irradiation [62], and p21 does not inhibit NER in in vitro systems [29, 63–64]. A recent study showed that a p21 peptide containing the PCNA binding domain blocks both DNA replication and NER [65]. In addition, p21 expression is suppressed by DDB2, a protein involved in NER, whereas deletion or knockdown of p21 reverses NER-deficiency in DDB2-null cells [66]. Unlike most DNA damage stresses which induce p21 expression, UV irradiation triggers p21 proteolysis, which may be related with TLS (Reviewed in [58]). Indeed, a p21 peptide containing the PCNA-binding domain inhibits the assembly of the TLS polymerase, polη, with PCNA, whereas UV-induced degradation of p21 is correlated with efficient TLS [64]. Interestingly, it has been reported that upon exposure to UV irradiation, a p21 peptide containing the CDK-binding domain is able to promote ubiquitin-dependent degradation of PCNA [67], and conversely, p21 is degraded by DDB1 in a PCNA-dependent manner [68–70]. Taken together, these reports suggest that p21 functions as a general inhibitor of various DNA repair pathways by disrupting PCNA interaction with DNA repair molecules as well as promoting PCNA degradation.
2.4 p21 in senescence and aging
Cellular senescence, one of the hallmarks of aging, can be induced by telomere dysfunction, DNA damage, chromatin instability, and oncogene activation. Senescence is a permanent cell cycle arrest controlled by two major pathways, the p16-pRb pathway and the p53-p21 pathway. In fact, p21 is first identified as an overexpressed marker in senescent cells [9] and later found to be capable of inducing premature senescence in both normal and tumor cells in a p53-independent manner [71–73]. In addition, studies showed that p21 is upregulated during oncogenic Ras-induced cellular senescence [74] and inhibits Ras-induced transformation [75–76]. Consistent with these observations, p21 levels are elevated in prematurely senescent fibroblasts from human or mice with premature aging syndromes [77–78]. Recently, the role of p21 in senescence and aging was examined in several premature aging mice models, including TERC-, Ku80-, and ATM-null mice (Reviewed in [79]). Loss of p21 in TERC-null mice with dysfunctional telomeres leads to improved stem cell function and increased lifespan without accelerating tumor formation [80], a phenotype also observed in TERC-p53 double knockout mice [81]. However, Ku80/p21 double knockout does not extend mouse lifespan although p21-deficiency rescues premature senescence in Ku80-null MEFs [78]. Moreover, knockout of p21 diminishes early onset of senescence in ATM-null MEFs, and ATM-p21 double knockout mice do not exhibit obvious early aging phenotype compared to ATM-null mice [82–84]. Furthermore, depletion of p21 in ATM-null mice increases the tolerance to tumor formation, particularly lymphomas, due to increased apoptosis in lymphoid cells [82–84]. Therefore, p21 regulates premature aging in some genetic backgrounds.
2.5 p21 in iPS cell reprogramming
Recent reports showed that the p53-p21 pathway is involved in induced pluripotent stem (iPS) cell generation. iPS is described as the process of reprogramming differentiated cells to pluripotent cells, such as adult somatic cells. A combination of four reprogramming factors, Oct4/Pou5f1, Sox2, Klf4, and c-Myc, is sufficient to induce pluripotent stem cells from adult mouse or human fibroblasts [85–88]. Successful reprogramming of differentiated human somatic cells into a pluripotent state would create patient- and disease-specific stem cells to investigate and provide cures for various diseases, such as Parkinson's disease, diabetes, and spinal cord injury [89]. However, the low efficiency and the tendency to induce malignant transformation compromise the clinical utility of this approach. It has been shown that p53 prevents the reprogramming of the cells carrying various types of DNA damage including uncapped telomere and DNA repair deficiencies, or exposed to extrinsic stresses [90]. Up to 10% MEFs lacking p53 become iPS cells even in the absence of c-Myc, and iPS cells can be generated from terminally differentiated T lymphocytes in which p53 is knocked out [91]. Moreover, knockdown of p53 or p21 in mouse embryo fibroblasts increases the reprogramming efficiency [91–92]. Thus, it is likely that the p53-p21 pathway functions as a barrier in iPS cell generation. However, Silencing p53 allows efficient reprogramming in spite of the presence of damaged DNA, and as a result, such iPS cells would carry aberrant chromosomes [90].
3. Transcriptional regulation of p21
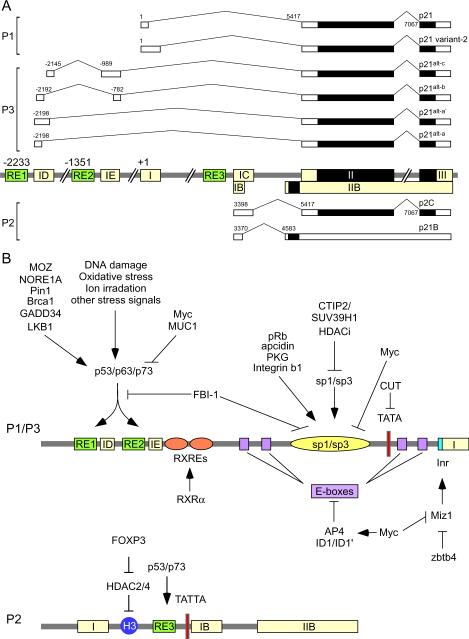
The p21 gene is localized on chromosome 6p21.2 and produces a polypeptide with 164 amino acids [5]. Due to the presence of alternative promoters and splicing (Fig. 2A), the p21 gene produces a smaller protein p21B with 132 amino acids [93] and several other transcripts that still encode p21 protein [93–95]. In addition, a novel p21 isoform is detected in canine cells [96]. However, it remains unclear whether the canine isoform is due to unique amino acid sequence or post-translational modifications.
Figure 2.
Human p21 locus. (A) Schematic presentation of various known p21 transcripts. The position of the first nucleotide of p21 transcript (GenBank Accession # Z85996) in the genomic DNA is assigned as the start site of the p21 locus. p21 is the classical p21Waf1/Cip1/Sdi1 transcript [5]. The alternate transcripts, p21variant-2, p21alt-a, p21alt-b, p21alt-c, and p21alt-a1, are presented as reported previously [95]. p21B and p21C are also presented as reported previously [93]. The protein coding regions are marked with solid black boxes. (B) Schematics of the p21 promoters along with various responsive elements, transcription factors, and upstream signals. Please see text for details.
p53 regulates gene expression by directly binding to a p53-responsive element (p53-RE) in the target gene [5]. Indeed, the p21 promoter contains two highly conserved p53-responsive elements (p53-RE) (Fig. 2B). In addition, a p53-RE is found in the P2 promoter of the p21 gene, through which p53 activates p21B transcription. Moreover, two p53 homologues, p63 and p73, transactivate p21 and p21B through binding to the p53-REs [97]. Since p53 is a sensor for various extrinsic stresses, including DNA damage and oxidative stress, these stress signals transcriptionally upregulate p21 via p53. Similarly, intrinsic and oncogenic stresses regulate p53 activity and subsequently p21 expression (reviewed in [98]). Furthermore, p53 is assisted and/or recruited by other cellular factors to regulate p21 expression. For examples, BRCA1 functions as p53-coactivator to activate the p21 promoter by recruiting p300/CBP, which in turn acetylates and stabilizes p53 [99–100]. In response to DNA damage, the prolyl isomerase Pin1 interacts with p53 and converts the cis and trans conformations of Ser-Pro motifs to facilitates p53 phosphorylation at Ser-33 and -46 [101–103]. As a result, enhanced p53 phosphorylation at Ser-33 and -46 leads to p53 activation and subsequent induction of p21 [101]. Consistent with this, Pin1 knockout MEFs are defective in p53 and p21 upregulation in response to DNA damage [101]. Similarly, GADD34, a member of growth arrest and DNA damage-inducible proteins, is able to induce p21 expression via enhancing p53 phosphorylation at Ser-15 [104]. The tumor suppressor LKB1, a serine/threonine kinase which associates with and phosphorylates p53 at Ser-15 and -392, promotes cell cycle arrest in G1 through p21 [105]. Moreover, cell division autoantigen 1 (CDA1) stabilizes p53 by inhibiting MDM2, an E3 ligase that ubiquitylates p53 and promotes p53 degradation, and hence transactivates p21 [106]. The monocytic leukemia zinc finger (MOZ), a MYST-type HAT, induces p21 transcription by interacting with p53 and acetylating histone on the p21 promoter [107]. Furthermore, NORE1A, a proapoptotic Ras effector, induces cell cycle arrest via p21 by promoting the nuclear localization of p53 [108].
In addition to p53, several transcription factors also activate p21 expression in a p53-independent manner. Indeed, DNA-binding elements for several transcription factors are present in the proximal p21 promoter, including six Sp1/Sp3 binding sites (Fig. 2B). These responsive elements are utilized to regulate p21 expression in response to various stimuli and stress signals, including NGF, butyrate, phorbol myristate acetate (PMA), and TGF-β (Reviewed in [98]). Studies showed that Sp1 and Sp3 activate p21 expression during differentiation in Caco-2 cells [109]. Sp1 also plays a role in DNA repair, cell growth, differentiation, and apoptosis by interacting with p53 to regulation p21 expression [110]. In addition, cGMP-dependent protein kinase G (PKG) activates several tumor suppressor proteins, such as p21 and p27 [111], by regulating Sp1 phosphorylation and transcriptional activity [111]. Moreover, the Rb protein is able to induce p21 through Sp1/Sp3 sites in epithelial cells [112], and overexpression of integrin β1 subunit upregulates p21 transcription by enhancing the recruitment of Sp1 to the p21 promoter [113]. Paradoxically, it has been reported that Sp1 inhibits proliferation and induces apoptosis in vascular smooth muscle cells by repressing p21 transcription and disrupting cyclinD1-CDK-p21 complex [114]. Therefore, regulation of p21 by Sp1 can be cell type-specific.
As a critical regulator of the cell cycle, it is not surprising that p21 is an effector of cancer therapeutic drugs, such as chemotherapeutic DNA damage agents and histone deacetylase (HDAC) inhibitors. Since DNA damage-induced p21 expression is mainly through the p53 pathway, thus we will discuss how HDAC inhibitors regulate p21 expression. Studies showed that HDAC inhibitors have anti-tumor effects by inducing cell cycle arrest, cellular senescence, and apoptosis [115]. HDACs regulate gene transcription by deacetylating histones and non-histone proteins such as transcription factors [116]. Previous studies have shown that several HDAC inhibitors strongly activate p21 expression either through enhancing histone acetylation on the p21 promoter or through the Sp1/Sp3 sites on the p21 promoter by releasing HDAC repressors (Reviewed in [117]). Indeed, treatment with HDAC inhibitor SAHA alters p21 expression by several mechanisms, including decreased HDAC1 activity, increased binding of RNA polymerase II to the p21 promoter [118], release of HDAC4 from the p21 promoter, and accelerated accumulation of acetylated H3 and H4 throughout the p21 promoter [119–120]. Similarly, HDAC inhibitor trichostatin A (TSA) can elevate H3 and H4 acetylation and p21 expression [116]. However, apicidin, another HDAC inhibitor, has been shown to induce p21 expression via promoting translocation of PKC from cytoplasm to nucleus, which is independent of histone acetylation [121]. Thus, although HDAC inhibitors have similar effect on histone remodeling, they utilize different mechanisms to regulated p21 expression.
P21 expression can be transcriptionally repressed by some factors [122]. One of the well-known factors is c-Myc, a bHLH transcription factor. Ectopic expression of c-Myc alleviates G1 cell cycle arrest by repressing p21 expression via sequestering Sp1 from the p21 promoter [123]. In addition, c-Myc associates with and inhibits Miz1, an initiator (Inr) sequence binding factor that can activate p21 expression during hematopoietic cell differentiation [124]. Moreover, c-Myc is capable of attenuating anti-estrogen-induced cell cycle arrest by repressing p21 [125]. Furthermore, c-Myc represses p21 expression via AP4, a bHLH-LZ transcription factor [126]. However, c-Myc also activates p21 transcription through p19ARF in a p53-dependent manner [127]. p19ARF stabilizes p53 by inhibiting MDM2 activity. Thus, p21 may play two opposing roles upon c-Myc activation. ID1, a bHLH transcription factor that lacks a DNA binding domain, is upregulated in multiple cancers and represses p21 expression to promote cell cycle progression [128]. Recently, we showed that an alternatively spliced isoform of ID1, ID1′, potently inhibits p21 expression [129]. Zbtb4, a POZ domain partner protein of Miz1 which is found to be downregulated in high grade neuroblastoma and other solid tumors, inhibits p21 expression by forming a heterodimer with Miz1 [130]. FBI-1, a proto-oncogene and a member of the POK family of transcription repressors, inhibits p21 expression by competing with Sp1 and p53 to bind to the p21 promoter [131]. CTIP2, a COUP-TF-interacting protein, is recruited to the Sp1 sites of the p21 promoter and inhibits p21 expression through interaction with HDACs and lysine methyltransferases [132].
In addition to the p53-RE, Sp1/Sp3 sites, and E-boxes, several other sites in the p21 promoter are targeted by various factors to regulate p21 expression (Fig. 2B). CUT, a homeodomain transcription factor, represses p21 expression via binding to a sequence that overlaps with the TATA box [133]. FOXP3, an X-linked tumor suppressor, is found to induce p21 expression via inhibiting the association of HDAC2 and HDAC4 to a site within intron 1 and thus increasing the local histone H3 acetylation [134]. Moreover, retinoid X receptor activates p21 expression through binding to two consensus retinoid X responsive elements in the p21 promoter [135]. Taken together, a large number of transcription factors are involved in p21 expression in response to intrinsic and extrinsic signals.
In general, transcriptional activators facilitate the assembly of transcriptionally competent initiation complexes and thus promote transcriptional initiation [136]. Interestingly, p21 expression is also regulated by transcriptional elongation. When cells are blocked in S phase, p53-dependent p21 mRNA elongation is ablated through dephosphorylation of RNA polymerase II (RNAP II) [137]. Chk1 is responsible for inhibiting p21 transcriptional elongation by disassembling elongation factors, such as DSIF (DRB sensitivity-inducing factor), CstF-64, and CPSF-100 [138]. In addition, other study showed that p53-dependent activation of p21 occurs through RNAP II phosphorylation and recruitment of the transcriptional elongation factors DSIF, P-TEFb (positive transcription elongation factor b), and FACT (faciliates chromatin transcription) to the downstream end of the p21 locus [139].
4. Post-transcriptional regulation of p21 mRNA
4.1. miRNA-mediated regulation of p21
miRNAs, a group of noncoding RNAs of ~22 nt long, regulate gene expression at the post-transcriptional level [140]. By binding to the 5′- and/or 3′-untranslated regions (UTRs) of a mRNA, miRNAs control the translational efficiency and stability of the target mRNA [141]. Recent miR Base (Release 14) annotates 1043 miRNA loci on the human genome [142] and approximately 30% of all human coding genes are regulated by miRNAs [143]. A number of miRNAs have been implicated in human pathologies, such as cancer, neurodegenerative disorders, and metabolic diseases [144–147]. miR-17-92, miR-106a-363, and miR-106b-25 clusters have been extensively investigated and were showed to down-regulate p21 expression (Fig. 3A). These miRNAs have identical nucleotide sequence within the seed region (Fig. 3B). Several reports showed that p21 is directly down-regulated by miR-17, miR-20a, miR-20b, miR-93, miR-106a, and miR-106b through binding to p21 3′-UTR [148–152]. As a result, overexpression of these miRNAs leads to increased cell proliferation and accelerated G1/S transition [148–150, 153]. In contrast, let-7a miRNA was found to increase p21 expression by targeting NIRF (Np95 ICBP90 ring finger) [154], but how NIRF controls p21 expression is not fully understood. Let-7a miRNA was originally found to modulate several transcription factors and regulate the temporal development in C. elegans [155–156]. Furthermore, several miRNAs were found to regulate p53 expression and consequently p21 expression. For example, miR-125a and miR-125b were found to repress p53 expression via binding to p53 3′-UTR, which leads to decreased levels of p21 [157–158]. Conversely, miR-29 family members upregulate p53 by targeting p85a and CDC42, and subsequently p21 expression [159].
Figure 3.
Regulation of p21 by various microRNAs (miRNAs). (A) Schematic diagram showing the genomic structure of five miRNA clusters. miR-106a and miR-17 clusters are a paralogue of miR-106b cluster. miR-19-92 cluster is related to miR-106a-92 cluster. P53 is also differentially regulated by miR-125 and miR-29 clusters. (B) Multiple sequence alignment of six miRNAs. All of these miRNAs share a core seed region, nt 2–8.
4.2. RNA-binding protein (RBP)-mediated regulation of p21
mRNA stability is a critical rate-limiting step in gene regulation, including p21 expression [160]. Most RNA-binding proteins which regulate p21 expression control p21 mRNA stability (Fig. 4). The first RBP found to regulate p21 mRNA stability is HuD [161]. HuD belongs to the Elav-like protein family, consisting of HuB (Hel-N1), HuC, HuD, and HuR [162–167]. The Elav-like RBPs contain three RNA recognition motifs (RRM) [168]. These proteins specifically bind to AU-rich elements (AREs) in the 3′-UTR of mRNAs, including p21, and increase their stability [165–166, 169–174]. HuR is predominantly a nuclear protein. In response to UV irradiation, treatment with prostaglandin A2 (PGA2), and accumulation of p16INK4a, HuR is translocated from the nucleus to the cytoplasm wherein HuR binds to and stabilizes p21 mRNA [173, 175–176].
Figure 4.

Regulation of p21 by RNA-binding proteins (RBPs). Several groups of RBPs are found to regulate p21 expression. Alternative names are included in parentheses. These RBPs contain one or more RNA-binding motifs, including RNA recognition domain (RRM), hnRNP K homology domain (KH), arginine/glycine-rich box (RGG), and double stranded RNA binding motif (dsRBD).
In addition to the Elav/Hu family of RNA-binding motif (RBM) proteins, RBM38 (also called RNPC1), a target of p53, is found to regulate p21 expression via stabilization of p21 mRNA in response to DNA damage [177]. Another study showed that RBM38 is highly expressed in muscle and regulates myogenesis by regulating p21 expression [178]. More recently, we showed that RBM38 and HuR, both of which can bind AU-rich elements (AREs) in p21 3'UTR, physically interact and preferentially bind the upstream and downstream AREs in p21 3'UTR, respectively [179]. In addition, we found that the RNA-binding activity of HuR to p21 transcript can be enhanced by RBM38 and the ability of RBM38 to regulate p21 mRNA stability is dependent on HuR [179]. RBM42, a binding partner of hnRNP K, coordinates with hnRNP K in the maintenance of cellular ATP level under stress conditions by blocking p21 mRNA degradation [180]. Musashi-1 (Msi-1), an evolutionary conserved RBM protein containing two RRMs, can bind to p21 3′-UTR and subsequently repress p21 translation [181]. As a result, MSI-1 is involved in the maintenance of stem cell state at least in part by repressing p21 translation [182]. Interestingly, CUGBP1 (CUG-binding protein 1) and calreticulin are found to regulate p21 translation by binding to the same region in p21 5′-UTR [183]. CUGBP1 competes with calreticulin to bind p21 5′-UTR and attenuates calreticultin-mediated translational repression of p21.
The PCBP family of RBPs contains three KH domains and is capable of binding poly(C) DNA and RNA sequences [184–185]. The first report by Giles et al. showed that poly(C)-binding protein 1 (PCBP1/CP1/hnRNP E1) binds to multiple elements in p21 3′-UTR and increases the stability of p21 mRNA upon treatment with epidermal growth factor (EGF) [186]. A recent study showed that co-depletion of PCBP1 and PCBP2 stabilizes p21 mRNA in a p53-independent manner, implying that p21 mRNA is destabilized by PCBPs [187]. Similarly, hnRNP K binds to the CU-rich elements in p21 3′-UTR and represses p21 translation [174], whereas AUF1 (hnRNP D) binds to p21 3′-UTR and decreases p21 mRNA stability [188]. These studies clearly indicated that PCBPs play a role in p21 expression via mRNA stability. However, further validation in a physiologically relevant condition is still needed.
NF90, a component of the splicesome which contains double stranded RNA binding motifs, is predominantly localized in the nucleus. Like HuR, NF90 is translocated from the nucleus to the cytoplasm and stabilizes p21 mRNA via binding to p21 3′-UTR [189]. Human T cell lymphotrophic virus type 1 (HTLV-1) encodes Tax, a 40 kDa transcription activator which is required for viral replication and cell transformation [190–191]. As a non-canonical RNA-binding protein, TAX regulates p21 transcription and mRNA stability at least in part via activation of APC/C (anaphase promoting complex/cyclosome) [192].
5. Post-translational regulation of p21 protein
5.1. Phosphorylation of p21
Several serine and threonine residues in p21 are phosphorylated by various protein kinases (Fig. 5). Among the sites is Thr-145, which is phosphorylated by Akt1/PKB, PKA, PKC, and Pim-1 [47, 49, 193–197]. Phosphorylation at Thr-145 regulates p21 translocation from the nucleus to the cytoplasm in breast tumor cells but not in endothelial cells [47, 193, 198–200], suggesting that p21 translocation can be context-dependent. Interestingly, Ser-146 can be phosphorylated by the same set of kinases for Thr-145, including Akt1, Pim-1, and PKC [193–195, 197–198]. Akt1-mediated phosphorylation at Ser-146 leads to p21 stabilization and enhanced tumor cell survival [193]. Overexpression of PKCζ or activation of endogenous PKCζ by PDK-1 phosphorylates p21 at Ser-146 and decreases the half life of p21 protein [195]. In contrast, the stability of p21 protein is increased by PKCδ-mediated phosphorylation at Ser-146 [194]. Thus, the effect of PKC-mediated phosphorylation at Ser-146 on p21 stability remains a matter of debate.
Figure 5.
Phosphorylation sites in p21 protein. P21 has six well-defined functional domains, including two cyclin-binding domains (Cy1 and Cy2), a kinase-binding domain (CDK), a Helix motif (Helix), a PCNA-binding domain (PCNA), and a nuclear localization signal (NLS). P21 polypeptide contains 20 serine/threonine residues, seven of which are found to be phosphorylated by various protein kinases indicated at the top of the figure. Five other serine and threonine residues are potential phosphorylation sites (S31, T97, S114, S123, and S137) as predicted by NetPhos 2.0 program (Technical University of Denmark).
Thr-57 and Ser-130 in p21 protein are located in front of a proline residue, which constitute a consensus site for proline-directed protein kinases, such as the CDK family, the MAPK family, and the GSK family. Indeed, GSKβ phosphorylates Thr-57 and subsequently decreases p21 protein stability [201]. Cyclin E-Cdk2 phosphorylates p21 at Ser-130 and decreases p21 stability via the ubiquitin-dependent degradation [202]. JNK and p38 phosphorylate p21 at Ser-130 and increase p21 stability [203]. Other C-terminal residues (Ser-98, Ser-153 and Ser-160) were found to be phosphorylated, but their effect on p21 protein stability and activity has not been well defined [196, 204–205].
5.2. Ubiquitin-dependent and -independent degradation of p21
Ubiquitin-dependent proteasomal degradation plays a critical role in the cell cycle control at least in part via p21 proteolysis. p21 inhibits cell cycle transition through its association with cyclin-Cdk complexes and PCNA [202, 206–209]. Several cell cycle-related E3 ligases, including SCFSKP2, CRL4CDT2 and APC/CCDC20, target p21 for degradation at various stages of the cell cycle [69, 202, 209–210]. Cdk2-mediated phosphorylation of p21 (Ser-130) has been detected at G1 and S phase, and phosphorylated p21 can be degraded in a proteasome-dependent manner [202, 207–208], suggesting that there is a regulatory link between p21 phosphorylation and its proteasome-mediated degradation. Consistent with this, suppression of p21 ubiquitylation and phosphorylation leads to increased stability of p21 protein [211–212].
P21 is also found to be degraded in an ubiquitin-independent manner [213–214]. First, like wild-type p21, lysine-less p21 mutant (p21K6R) has a short half life and rapidly degraded by proteasome [214]. Further supporting this idea is the observation that p21 can directly interact with C8 α-subunit of the 20S proteasome, which directs the initiation of ring formation and ubiquitin-independent 20S proteasome assembly [215–217]. Ras can stabilize p21 by promoting the formation of cyclin D1-p21 heterocomplex, which prevents p21 from 20S-proteasomal degradation [218]. Similarly, WISp39 specifically recruits Hsp90 to de novo synthesized p21 protein and subsequently prevents p21 from proteasomal degradation [219]. Interestingly, unlike conventional ubiquitin E3 ligase, Mdm2 promotes p21 turnover independent of its ubiquitin E3 ligase activity [220–221]. These data indicate that p21 is subject to ubiquitin-dependent and -independent degradation, but how these two pathways coordinately regulate p21 stability remains to be determined.
6. Concluding remarks
p21 is well-known as a key regulator of the cell cycle as well as cell death, DNA repair, senescence, aging, and iPS. Importantly, p21 can exert either positive or negative activities toward a specific cellular response in a context-dependent manner, including the cell type and the source of stress signals. In the future, generation of conditional p21 knockout in a specific tissue or developmental stage may help to understand the importance of p21 loss/stabilization in cancer and other disorders. Although many factors or mechanisms have been identified to regulate p21 expression, other factors yet to be identified may play a role and need to be further investigated. Moreover, studies are needed to examine how multiple pathways coordinately regulate p21 expression and its biological outcomes, which could provide a clue how to explore p21 for potential therapeutic applications.
Acknowledgements
This article is supported in part by NIH grants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- [1].Sherr CJ. Science. 1996;274(5293):1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- [2].Sherr CJ, Roberts JM. Genes Dev. 1995;9(10):1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- [3].Sherr CJ, Roberts JM. Genes Dev. 1999;13(12):1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- [4].Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. Cell. 1993;75(4):805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- [5].el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B. Cell. 1993;75(4):817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- [6].Xiong Y, Zhang H, Beach D. Cell. 1992;71(3):505–514. doi: 10.1016/0092-8674(92)90518-h. [DOI] [PubMed] [Google Scholar]
- [7].Gu Y, Turck CW, Morgan DO. Nature. 1993;366(6456):707–710. doi: 10.1038/366707a0. [DOI] [PubMed] [Google Scholar]
- [8].Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D. Nature. 1993;366(6456):701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- [9].Noda A, Ning Y, Venable SF, Pereira-Smith OM, Smith JR. Exp Cell Res. 1994;211(1):90–98. doi: 10.1006/excr.1994.1063. [DOI] [PubMed] [Google Scholar]
- [10].Harper JW, Elledge SJ, Keyomarsi K, Dynlacht B, Tsai LH, Zhang P, Dobrowolski S, Bai C, Connell-Crowley L, Swindell E, et al. Mol Biol Cell. 1995;6(4):387–400. doi: 10.1091/mbc.6.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Deng C, Zhang P, Harper JW, Elledge SJ, Leder P. Cell. 1995;82(4):675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- [12].Weinberg WC, Montano NE, Deng C. Oncogene. 1997;15(6):685–690. doi: 10.1038/sj.onc.1201230. [DOI] [PubMed] [Google Scholar]
- [13].Poole AJ, Heap D, Carroll RE, Tyner AL. Oncogene. 2004;23(49):8128–8134. doi: 10.1038/sj.onc.1207994. [DOI] [PubMed] [Google Scholar]
- [14].Philipp J, Vo K, Gurley KE, Seidel K, Kemp CJ. Oncogene. 1999;18(33):4689–4698. doi: 10.1038/sj.onc.1202840. [DOI] [PubMed] [Google Scholar]
- [15].Coqueret O. Trends Cell Biol. 2003;13(2):65–70. doi: 10.1016/s0962-8924(02)00043-0. [DOI] [PubMed] [Google Scholar]
- [16].Garner E, Raj K. Cell Cycle. 2008;7(3):277–282. doi: 10.4161/cc.7.3.5328. [DOI] [PubMed] [Google Scholar]
- [17].Gartel AL, Tyner AL. Mol Cancer Ther. 2002;1(8):639–649. [PubMed] [Google Scholar]
- [18].Li H, Collado M, Villasante A, Strati K, Ortega S, Canamero M, Blasco MA, Serrano M. Nature. 2009;460(7259):1136–1139. doi: 10.1038/nature08290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Abbas T, Dutta A. Nat Rev Cancer. 2009;9(6):400–414. doi: 10.1038/nrc2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Child ES, Mann DJ. Cell Cycle. 2006;5(12):1313–1319. doi: 10.4161/cc.5.12.2863. [DOI] [PubMed] [Google Scholar]
- [21].Liu G, Lozano G. Cancer Cell. 2005;7(2):113–114. doi: 10.1016/j.ccr.2005.01.019. [DOI] [PubMed] [Google Scholar]
- [22].Nakanishi M, Adami GR, Robetorye RS, Noda A, Venable SF, Dimitrov D, Pereira-Smith OM, Smith JR. Proc Natl Acad Sci U S A. 1995;92(10):4352–4356. doi: 10.1073/pnas.92.10.4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Li Y, Jenkins CW, Nichols MA, Xiong Y. Oncogene. 1994;9(8):2261–2268. [PubMed] [Google Scholar]
- [24].Geng Y, Eaton EN, Picon M, Roberts JM, Lundberg AS, Gifford A, Sardet C, Weinberg RA. Oncogene. 1996;12(6):1173–1180. [PubMed] [Google Scholar]
- [25].Afshari CA, Nichols MA, Xiong Y, Mudryj M. Cell Growth Differ. 1996;7(8):979–988. [PubMed] [Google Scholar]
- [26].Prelich G, Stillman B. Cell. 1988;53(1):117–126. doi: 10.1016/0092-8674(88)90493-x. [DOI] [PubMed] [Google Scholar]
- [27].Kelman Z. Oncogene. 1997;14(6):629–640. doi: 10.1038/sj.onc.1200886. [DOI] [PubMed] [Google Scholar]
- [28].Oku T, Ikeda S, Sasaki H, Fukuda K, Morioka H, Ohtsuka E, Yoshikawa H, Tsurimoto T. Genes Cells. 1998;3(6):357–369. doi: 10.1046/j.1365-2443.1998.00199.x. [DOI] [PubMed] [Google Scholar]
- [29].Li R, Waga S, Hannon GJ, Beach D, Stillman B. Nature. 1994;371(6497):534–537. doi: 10.1038/371534a0. [DOI] [PubMed] [Google Scholar]
- [30].Waga S, Hannon GJ, Beach D, Stillman B. Nature. 1994;369(6481):574–578. doi: 10.1038/369574a0. [DOI] [PubMed] [Google Scholar]
- [31].Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown JP, Sedivy JM, Kinzler KW, Vogelstein B. Science. 1998;282(5393):1497–1501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- [32].Baus F, Gire V, Fisher D, Piette J, Dulic V. EMBO J. 2003;22(15):3992–4002. doi: 10.1093/emboj/cdg387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Niculescu AB, 3rd, Chen X, Smeets M, Hengst L, Prives C, Reed SI. Mol Cell Biol. 1998;18(1):629–643. doi: 10.1128/mcb.18.1.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Peng CY, Graves PR, Thoma RS, Wu Z, Shaw AS, Piwnica-Worms H. Science. 1997;277(5331):1501–1505. doi: 10.1126/science.277.5331.1501. [DOI] [PubMed] [Google Scholar]
- [35].Nurse P. Cell. 1997;91(7):865–867. doi: 10.1016/s0092-8674(00)80476-6. [DOI] [PubMed] [Google Scholar]
- [36].Chan TA, Hwang PM, Hermeking H, Kinzler KW, Vogelstein B. Genes Dev. 2000;14(13):1584–1588. [PMC free article] [PubMed] [Google Scholar]
- [37].Zhang H, Hannon GJ, Beach D. Genes Dev. 1994;8(15):1750–1758. doi: 10.1101/gad.8.15.1750. [DOI] [PubMed] [Google Scholar]
- [38].Cheng M, Olivier P, Diehl JA, Fero M, Roussel MF, Roberts JM, Sherr CJ. EMBO J. 1999;18(6):1571–1583. doi: 10.1093/emboj/18.6.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].LaBaer J, Garrett MD, Stevenson LF, Slingerland JM, Sandhu C, Chou HS, Fattaey A, Harlow E. Genes Dev. 1997;11(7):847–862. doi: 10.1101/gad.11.7.847. [DOI] [PubMed] [Google Scholar]
- [40].Weiss RH, Joo A, Randour C. J Biol Chem. 2000;275(36):28340. [PubMed] [Google Scholar]
- [41].Besson A, Yong VW. Mol Cell Biol. 2000;20(13):4580–4590. doi: 10.1128/mcb.20.13.4580-4590.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Vousden KH, Prives C. Cell. 2009;137(3):413–431. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- [43].Seoane J, Le HV, Massague J. Nature. 2002;419(6908):729–734. doi: 10.1038/nature01119. [DOI] [PubMed] [Google Scholar]
- [44].Zhang Y, Fujita N, Tsuruo T. Oncogene. 1999;18(5):1131–1138. doi: 10.1038/sj.onc.1202426. [DOI] [PubMed] [Google Scholar]
- [45].Asada M, Yamada T, Ichijo H, Delia D, Miyazono K, Fukumuro K, Mizutani S. EMBO J. 1999;18(5):1223–1234. doi: 10.1093/emboj/18.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Willis AC, Chen X. Curr Mol Med. 2002;2(4):329–345. doi: 10.2174/1566524023362474. [DOI] [PubMed] [Google Scholar]
- [47].Zhou BP, Liao Y, Xia W, Spohn B, Lee MH, Hung MC. Nat Cell Biol. 2001;3(3):245–252. doi: 10.1038/35060032. [DOI] [PubMed] [Google Scholar]
- [48].Suzuki A, Tsutomi Y, Yamamoto N, Shibutani T, Akahane K. Mol Cell Biol. 1999;19(5):3842–3847. doi: 10.1128/mcb.19.5.3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Suzuki A, Kawano H, Hayashida M, Hayasaki Y, Tsutomi Y, Akahane K. Cell Death Differ. 2000;7(8):721–728. doi: 10.1038/sj.cdd.4400706. [DOI] [PubMed] [Google Scholar]
- [50].Suzuki A, Ito T, Kawano H, Hayashida M, Hayasaki Y, Tsutomi Y, Akahane K, Nakano T, Miura M, Shiraki K. Oncogene. 2000;19(10):1346–1353. doi: 10.1038/sj.onc.1203429. [DOI] [PubMed] [Google Scholar]
- [51].Javelaud D, Wietzerbin J, Delattre O, Besancon F. Oncogene. 2000;19(1):61–68. doi: 10.1038/sj.onc.1203246. [DOI] [PubMed] [Google Scholar]
- [52].Kim DK, Cho ES, Lee SJ, Um HD. Biochem Biophys Res Commun. 2001;289(1):34–38. doi: 10.1006/bbrc.2001.5928. [DOI] [PubMed] [Google Scholar]
- [53].Gartel AL. Leuk Res. 2005;29(11):1237–1238. doi: 10.1016/j.leukres.2005.04.023. [DOI] [PubMed] [Google Scholar]
- [54].Wu Q, Kirschmeier P, Hockenberry T, Yang TY, Brassard DL, Wang L, McClanahan T, Black S, Rizzi G, Musco ML, Mirza A, Liu S. J Biol Chem. 2002;277(39):36329–36337. doi: 10.1074/jbc.M204962200. [DOI] [PubMed] [Google Scholar]
- [55].Agrawal S, Agarwal ML, Chatterjee-Kishore M, Stark GR, Chisolm GM. Mol Cell Biol. 2002;22(7):1981–1992. doi: 10.1128/MCB.22.7.1981-1992.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Kaznelson DW, Bruun S, Monrad A, Gjerlov S, Birk J, Ropke C, Norrild B. Virology. 2004;320(2):301–312. doi: 10.1016/j.virol.2003.12.018. [DOI] [PubMed] [Google Scholar]
- [57].Jonsson ZO, Hubscher U. Bioessays. 1997;19(11):967–975. doi: 10.1002/bies.950191106. [DOI] [PubMed] [Google Scholar]
- [58].Prives C, Gottifredi V. Cell Cycle. 2008;7(24):3840–3846. doi: 10.4161/cc.7.24.7243. [DOI] [PubMed] [Google Scholar]
- [59].Moldovan GL, Pfander B, Jentsch S. Cell. 2007;129(4):665–679. doi: 10.1016/j.cell.2007.05.003. [DOI] [PubMed] [Google Scholar]
- [60].Umar A, Buermeyer AB, Simon JA, Thomas DC, Clark AB, Liskay RM, Kunkel TA. Cell. 1996;87(1):65–73. doi: 10.1016/s0092-8674(00)81323-9. [DOI] [PubMed] [Google Scholar]
- [61].Tom S, Ranalli TA, Podust VN, Bambara RA. J Biol Chem. 2001;276(52):48781–48789. doi: 10.1074/jbc.M109626200. [DOI] [PubMed] [Google Scholar]
- [62].Smith ML, Ford JM, Hollander MC, Bortnick RA, Amundson SA, Seo YR, Deng CX, Hanawalt PC, Fornace AJ., Jr Mol Cell Biol. 2000;20(10):3705–3714. doi: 10.1128/mcb.20.10.3705-3714.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Shivji MK, Ferrari E, Ball K, Hubscher U, Wood RD. Oncogene. 1998;17(22):2827–2838. doi: 10.1038/sj.onc.1202352. [DOI] [PubMed] [Google Scholar]
- [64].Soria G, Speroni J, Podhajcer OL, Prives C, Gottifredi V. J Cell Sci. 2008;121(Pt 19):3271–3282. doi: 10.1242/jcs.027730. [DOI] [PubMed] [Google Scholar]
- [65].Pan ZQ, Reardon JT, Li L, Flores-Rozas H, Legerski R, Sancar A, Hurwitz J. J Biol Chem. 1995;270(37):22008–22016. doi: 10.1074/jbc.270.37.22008. [DOI] [PubMed] [Google Scholar]
- [66].Stoyanova T, Yoon T, Kopanja D, Mokyr MB, Raychaudhuri P. Mol Cell Biol. 2008;28(1):177–187. doi: 10.1128/MCB.00880-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Soria G, Podhajcer O, Prives C, Gottifredi V. Oncogene. 2006;25(20):2829–2838. doi: 10.1038/sj.onc.1209315. [DOI] [PubMed] [Google Scholar]
- [68].Stuart SA, Wang JY. J Biol Chem. 2009;284(22):15061–15070. doi: 10.1074/jbc.M808810200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Abbas T, Sivaprasad U, Terai K, Amador V, Pagano M, Dutta A. Genes Dev. 2008;22(18):2496–2506. doi: 10.1101/gad.1676108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Nishitani H, Shiomi Y, Iida H, Michishita M, Takami T, Tsurimoto T. J Biol Chem. 2008;283(43):29045–29052. doi: 10.1074/jbc.M806045200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Fang L, Igarashi M, Leung J, Sugrue MM, Lee SW, Aaronson SA. Oncogene. 1999;18(18):2789–2797. doi: 10.1038/sj.onc.1202615. [DOI] [PubMed] [Google Scholar]
- [72].Wang Y, Blandino G, Givol D. Oncogene. 1999;18(16):2643–2649. doi: 10.1038/sj.onc.1202632. [DOI] [PubMed] [Google Scholar]
- [73].McConnell BB, Starborg M, Brookes S, Peters G. Curr Biol. 1998;8(6):351–354. doi: 10.1016/s0960-9822(98)70137-x. [DOI] [PubMed] [Google Scholar]
- [74].Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Cell. 1997;88(5):593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- [75].Michieli P, Li W, Lorenzi MV, Miki T, Zakut R, Givol D, Pierce JH. Oncogene. 1996;12(4):775–784. [PubMed] [Google Scholar]
- [76].Missero C, Di Cunto F, Kiyokawa H, Koff A, Dotto GP. Genes Dev. 1996;10(23):3065–3075. doi: 10.1101/gad.10.23.3065. [DOI] [PubMed] [Google Scholar]
- [77].Davis T, Singhrao SK, Wyllie FS, Haughton MF, Smith PJ, Wiltshire M, Wynford-Thomas D, Jones CJ, Faragher RG, Kipling D. J Cell Sci. 2003;116(Pt 7):1349–1357. doi: 10.1242/jcs.00331. [DOI] [PubMed] [Google Scholar]
- [78].Zhao B, Benson EK, Qiao R, Wang X, Kim S, Manfredi JJ, Lee SW, Aaronson SA. EMBO Rep. 2009;10(1):71–78. doi: 10.1038/embor.2008.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Benson EK, Zhao B, Sassoon DA, Lee SW, Aaronson SA. Cell Cycle. 2009;8(13):2002–2004. doi: 10.4161/cc.8.13.8997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Choudhury AR, Ju Z, Djojosubroto MW, Schienke A, Lechel A, Schaetzlein S, Jiang H, Stepczynska A, Wang C, Buer J, Lee HW, von Zglinicki T, Ganser A, Schirmacher P, Nakauchi H, Rudolph KL. Nat Genet. 2007;39(1):99–105. doi: 10.1038/ng1937. [DOI] [PubMed] [Google Scholar]
- [81].Chin L, Artandi SE, Shen Q, Tam A, Lee SL, Gottlieb GJ, Greider CW, DePinho RA. Cell. 1999;97(4):527–538. doi: 10.1016/s0092-8674(00)80762-x. [DOI] [PubMed] [Google Scholar]
- [82].Wang YA, Elson A, Leder P. Proc Natl Acad Sci U S A. 1997;94(26):14590–14595. doi: 10.1073/pnas.94.26.14590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Xu Y, Yang EM, Brugarolas J, Jacks T, Baltimore D. Mol Cell Biol. 1998;18(7):4385–4390. doi: 10.1128/mcb.18.7.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Shen KC, Heng H, Wang Y, Lu S, Liu G, Deng CX, Brooks SC, Wang YA. Cancer Res. 2005;65(19):8747–8753. doi: 10.1158/0008-5472.CAN-05-1471. [DOI] [PubMed] [Google Scholar]
- [85].Okita K, Ichisaka T, Yamanaka S. Nature. 2007;448(7151):313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- [86].Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- [87].Takahashi K, Yamanaka S. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- [88].Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, Bernstein BE, Jaenisch R. Nature. 2007;448(7151):318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- [89].Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Science. 1998;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- [90].Marion RM, Strati K, Li H, Murga M, Blanco R, Ortega S, Fernandez-Capetillo O, Serrano M, Blasco MA. Nature. 2009;460(7259):1149–1153. doi: 10.1038/nature08287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Hong H, Takahashi K, Ichisaka T, Aoi T, Kanagawa O, Nakagawa M, Okita K, Yamanaka S. Nature. 2009;460(7259):1132–1135. doi: 10.1038/nature08235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Kawamura T, Suzuki J, Wang YV, Menendez S, Morera LB, Raya A, Wahl GM, Belmonte JC. Nature. 2009;460(7259):1140–1144. doi: 10.1038/nature08311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Nozell S, Chen X. Oncogene. 2002;21(8):1285–1294. doi: 10.1038/sj.onc.1205191. [DOI] [PubMed] [Google Scholar]
- [94].Gartel AL, Radhakrishnan SK, Serfas MS, Kwon YH, Tyner AL. Oncogene. 2004;23(49):8154–8157. doi: 10.1038/sj.onc.1207820. [DOI] [PubMed] [Google Scholar]
- [95].Radhakrishnan SK, Gierut J, Gartel AL. Oncogene. 2006;25(12):1812–1815. doi: 10.1038/sj.onc.1209195. [DOI] [PubMed] [Google Scholar]
- [96].Zhang J, Chen X, Kent MS, Rodriguez CO. Mol Cancer Res. 2009;7(1):67–78. doi: 10.1158/1541-7786.MCR-08-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Harms K, Nozell S, Chen X. Cell Mol Life Sci. 2004;61(7–8):822–842. doi: 10.1007/s00018-003-3304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Gartel AL, Tyner AL. Exp Cell Res. 1999;246(2):280–289. doi: 10.1006/excr.1998.4319. [DOI] [PubMed] [Google Scholar]
- [99].Chai YL, Cui J, Shao N, Shyam E, Reddy P, Rao VN. Oncogene. 1999;18(1):263–268. doi: 10.1038/sj.onc.1202323. [DOI] [PubMed] [Google Scholar]
- [100].Lu M, Arrick BA. Oncogene. 2000;19(54):6351–6360. doi: 10.1038/sj.onc.1204025. [DOI] [PubMed] [Google Scholar]
- [101].Wulf GM, Liou YC, Ryo A, Lee SW, Lu KP. J Biol Chem. 2002;277(50):47976–47979. doi: 10.1074/jbc.C200538200. [DOI] [PubMed] [Google Scholar]
- [102].Zacchi P, Gostissa M, Uchida T, Salvagno C, Avolio F, Volinia S, Ronai Z, Blandino G, Schneider C, Del Sal G. Nature. 2002;419(6909):853–857. doi: 10.1038/nature01120. [DOI] [PubMed] [Google Scholar]
- [103].Zheng H, You H, Zhou XZ, Murray SA, Uchida T, Wulf G, Gu L, Tang X, Lu KP, Xiao ZX. Nature. 2002;419(6909):849–853. doi: 10.1038/nature01116. [DOI] [PubMed] [Google Scholar]
- [104].Yagi A, Hasegawa Y, Xiao H, Haneda M, Kojima E, Nishikimi A, Hasegawa T, Shimokata K, Isobe K. J Cell Biochem. 2003;90(6):1242–1249. doi: 10.1002/jcb.10711. [DOI] [PubMed] [Google Scholar]
- [105].Zeng PY, Berger SL. Cancer Res. 2006;66(22):10701–10708. doi: 10.1158/0008-5472.CAN-06-0999. [DOI] [PubMed] [Google Scholar]
- [106].Tu Y, Wu W, Wu T, Cao Z, Wilkins R, Toh BH, Cooper ME, Chai Z. J Biol Chem. 2007;282(16):11722–11731. doi: 10.1074/jbc.M609623200. [DOI] [PubMed] [Google Scholar]
- [107].Rokudai S, Aikawa Y, Tagata Y, Tsuchida N, Taya Y, Kitabayashi I. J Biol Chem. 2009;284(1):237–244. doi: 10.1074/jbc.M805101200. [DOI] [PubMed] [Google Scholar]
- [108].Calvisi DF, Donninger H, Vos MD, Birrer MJ, Gordon L, Leaner V, Clark GJ. Cancer Res. 2009;69(11):4629–4637. doi: 10.1158/0008-5472.CAN-08-3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Gartel AL, Goufman E, Najmabadi F, Tyner AL. Oncogene. 2000;19(45):5182–5188. doi: 10.1038/sj.onc.1203900. [DOI] [PubMed] [Google Scholar]
- [110].Koutsodontis G, Tentes I, Papakosta P, Moustakas A, Kardassis D. J Biol Chem. 2001;276(31):29116–29125. doi: 10.1074/jbc.M104130200. [DOI] [PubMed] [Google Scholar]
- [111].Cen B, Deguchi A, Weinstein IB. Cancer Res. 2008;68(13):5355–5362. doi: 10.1158/0008-5472.CAN-07-6869. [DOI] [PubMed] [Google Scholar]
- [112].Decesse JT, Medjkane S, Datto MB, Cremisi CE. Oncogene. 2001;20(8):962–971. doi: 10.1038/sj.onc.1204169. [DOI] [PubMed] [Google Scholar]
- [113].Fang Z, Fu Y, Liang Y, Li Z, Zhang W, Jin J, Yang Y, Zha X. J Cell Biochem. 2007;101(3):654–664. doi: 10.1002/jcb.21223. [DOI] [PubMed] [Google Scholar]
- [114].Kavurma MM, Khachigian LM. J Biol Chem. 2003;278(35):32537–32543. doi: 10.1074/jbc.M305650200. [DOI] [PubMed] [Google Scholar]
- [115].Minucci S, Pelicci PG. Nat Rev Cancer. 2006;6(1):38–51. doi: 10.1038/nrc1779. [DOI] [PubMed] [Google Scholar]
- [116].Haberland M, Montgomery RL, Olson EN. Nat Rev Genet. 2009;10(1):32–42. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Ocker M, Schneider-Stock R. Int J Biochem Cell Biol. 2007;39(7–8):1367–1374. doi: 10.1016/j.biocel.2007.03.001. [DOI] [PubMed] [Google Scholar]
- [118].Gui CY, Ngo L, Xu WS, Richon VM, Marks PA. Proc Natl Acad Sci U S A. 2004;101(5):1241–1246. doi: 10.1073/pnas.0307708100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Mottet D, Pirotte S, Lamour V, Hagedorn M, Javerzat S, Bikfalvi A, Bellahcene A, Verdin E, Castronovo V. Oncogene. 2009;28(2):243–256. doi: 10.1038/onc.2008.371. [DOI] [PubMed] [Google Scholar]
- [120].Richon VM, Sandhoff TW, Rifkind RA, Marks PA. Proc Natl Acad Sci U S A. 2000;97(18):10014–10019. doi: 10.1073/pnas.180316197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Han JW, Ahn SH, Kim YK, Bae GU, Yoon JW, Hong S, Lee HY, Lee YW, Lee HW. J Biol Chem. 2001;276(45):42084–42090. doi: 10.1074/jbc.M106688200. [DOI] [PubMed] [Google Scholar]
- [122].Gartel AL, Radhakrishnan SK. Cancer Res. 2005;65(10):3980–3985. doi: 10.1158/0008-5472.CAN-04-3995. [DOI] [PubMed] [Google Scholar]
- [123].Gartel AL, Ye X, Goufman E, Shianov P, Hay N, Najmabadi F, Tyner AL. Proc Natl Acad Sci U S A. 2001;98(8):4510–4515. doi: 10.1073/pnas.081074898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Wu S, Cetinkaya C, Munoz-Alonso MJ, von der Lehr N, Bahram F, Beuger V, Eilers M, Leon J, Larsson LG. Oncogene. 2003;22(3):351–360. doi: 10.1038/sj.onc.1206145. [DOI] [PubMed] [Google Scholar]
- [125].Mukherjee S, Conrad SE. J Biol Chem. 2005;280(18):17617–17625. doi: 10.1074/jbc.M502278200. [DOI] [PubMed] [Google Scholar]
- [126].Jung P, Menssen A, Mayr D, Hermeking H. Proc Natl Acad Sci U S A. 2008;105(39):15046–15051. doi: 10.1073/pnas.0801773105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Felsher DW, Zetterberg A, Zhu J, Tlsty T, Bishop JM. Proc Natl Acad Sci U S A. 2000;97(19):10544–10548. doi: 10.1073/pnas.190327097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Prabhu S, Ignatova A, Park ST, Sun XH. Mol Cell Biol. 1997;17(10):5888–5896. doi: 10.1128/mcb.17.10.5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Qian Y, Chen X. J Biol Chem. 2008;283(33):22410–22416. doi: 10.1074/jbc.M800643200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Weber A, Marquardt J, Elzi D, Forster N, Starke S, Glaum A, Yamada D, Defossez PA, Delrow J, Eisenman RN, Christiansen H, Eilers M. EMBO J. 2008;27(11):1563–1574. doi: 10.1038/emboj.2008.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Choi WI, Jeon BN, Yun CO, Kim PH, Kim SE, Choi KY, Kim SH, Hur MW. J Biol Chem. 2009;284(19):12633–12644. doi: 10.1074/jbc.M809794200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Cherrier T, Suzanne S, Redel L, Calao M, Marban C, Samah B, Mukerjee R, Schwartz C, Gras G, Sawaya BE, Zeichner SL, Aunis D, Van Lint C, Rohr O. Oncogene. 2009;28(38):3380–3389. doi: 10.1038/onc.2009.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Coqueret O, Berube G, Nepveu A. EMBO J. 1998;17(16):4680–4694. doi: 10.1093/emboj/17.16.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Liu R, Wang L, Chen G, Katoh H, Chen C, Liu Y, Zheng P. Cancer Res. 2009;69(6):2252–2259. doi: 10.1158/0008-5472.CAN-08-3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Tanaka T, Suh KS, Lo AM, De Luca LM. J Biol Chem. 2007;282(41):29987–29997. doi: 10.1074/jbc.M701700200. [DOI] [PubMed] [Google Scholar]
- [136].Orphanides G, Lagrange T, Reinberg D. Genes Dev. 1996;10(21):2657–2683. doi: 10.1101/gad.10.21.2657. [DOI] [PubMed] [Google Scholar]
- [137].Mattia M, Gottifredi V, McKinney K, Prives C. Mol Cell Biol. 2007;27(4):1309–1320. doi: 10.1128/MCB.01520-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Beckerman R, Donner AJ, Mattia M, Peart MJ, Manley JL, Espinosa JM, Prives C. Genes Dev. 2009;23(11):1364–1377. doi: 10.1101/gad.1795709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Gomes NP, Bjerke G, Llorente B, Szostek SA, Emerson BM, Espinosa JM. Genes Dev. 2006;20(5):601–612. doi: 10.1101/gad.1398206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Bartel DP. Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- [141].Filipowicz W, Bhattacharyya SN, Sonenberg N. Nat Rev Genet. 2008;9(2):102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- [142].Griffiths-Jones S, Saini HK, van Dongen S, Enright AJ. Nucleic Acids Res. 2008;36(Database issue):D154–158. doi: 10.1093/nar/gkm952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Lewis BP, Burge CB, Bartel DP. Cell. 2005;120(1):15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- [144].Kloosterman WP, Plasterk RH. Dev Cell. 2006;11(4):441–450. doi: 10.1016/j.devcel.2006.09.009. [DOI] [PubMed] [Google Scholar]
- [145].Esquela-Kerscher A, Slack FJ. Nat Rev Cancer. 2006;6(4):259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- [146].Chang TC, Mendell JT. Annu Rev Genomics Hum Genet. 2007;8:215–239. doi: 10.1146/annurev.genom.8.080706.092351. [DOI] [PubMed] [Google Scholar]
- [147].Krutzfeldt J, Stoffel M. Cell Metab. 2006;4(1):9–12. doi: 10.1016/j.cmet.2006.05.009. [DOI] [PubMed] [Google Scholar]
- [148].Kim YK, Yu J, Han TS, Park SY, Namkoong B, Kim DH, Hur K, Yoo MW, Lee HJ, Yang HK, Kim VN. Nucleic Acids Res. 2009;37(5):1672–1681. doi: 10.1093/nar/gkp002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Ivanovska I, Ball AS, Diaz RL, Magnus JF, Kibukawa M, Schelter JM, Kobayashi SV, Lim L, Burchard J, Jackson AL, Linsley PS, Cleary MA. Mol Cell Biol. 2008;28(7):2167–2174. doi: 10.1128/MCB.01977-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Petrocca F, Visone R, Onelli MR, Shah MH, Nicoloso MS, de Martino I, Iliopoulos D, Pilozzi E, Liu CG, Negrini M, Cavazzini L, Volinia S, Alder H, Ruco LP, Baldassarre G, Croce CM, Vecchione A. Cancer Cell. 2008;13(3):272–286. doi: 10.1016/j.ccr.2008.02.013. [DOI] [PubMed] [Google Scholar]
- [151].Petrocca F, Vecchione A, Croce CM. Cancer Res. 2008;68(20):8191–8194. doi: 10.1158/0008-5472.CAN-08-1768. [DOI] [PubMed] [Google Scholar]
- [152].Fontana L, Fiori ME, Albini S, Cifaldi L, Giovinazzi S, Forloni M, Boldrini R, Donfrancesco A, Federici V, Giacomini P, Peschle C, Fruci D. PLoS One. 2008;3(5):e2236. doi: 10.1371/journal.pone.0002236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Li G, Luna C, Qiu J, Epstein DL, Gonzalez P. Mech Ageing Dev. 2009 doi: 10.1016/j.mad.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].He X, Duan C, Chen J, Ou-Yang X, Zhang Z, Li C, Peng H. FEBS Lett. 2009 doi: 10.1016/j.febslet.2009.10.007. [DOI] [PubMed] [Google Scholar]
- [155].Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR, Ruvkun G. Nature. 2000;403(6772):901–906. doi: 10.1038/35002607. [DOI] [PubMed] [Google Scholar]
- [156].Grosshans H, Johnson T, Reinert KL, Gerstein M, Slack FJ. Dev Cell. 2005;8(3):321–330. doi: 10.1016/j.devcel.2004.12.019. [DOI] [PubMed] [Google Scholar]
- [157].Le MT, Teh C, Shyh-Chang N, Xie H, Zhou B, Korzh V, Lodish HF, Lim B. Genes Dev. 2009;23(7):862–876. doi: 10.1101/gad.1767609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Zhang Y, Gao JS, Tang X, Tucker LD, Quesenberry P, Rigoutsos I, Ramratnam B. FEBS Lett. 2009;583(22):3725–3730. doi: 10.1016/j.febslet.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Park SY, Lee JH, Ha M, Nam JW, Kim VN. Nat Struct Mol Biol. 2009;16(1):23–29. doi: 10.1038/nsmb.1533. [DOI] [PubMed] [Google Scholar]
- [160].Schwaller J, Koeffler HP, Niklaus G, Loetscher P, Nagel S, Fey MF, Tobler A. J Clin Invest. 1995;95(3):973–979. doi: 10.1172/JCI117806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Joseph B, Orlian M, Furneaux H. J Biol Chem. 1998;273(32):20511–20516. doi: 10.1074/jbc.273.32.20511. [DOI] [PubMed] [Google Scholar]
- [162].King PH, Levine TD, Fremeau RT, Jr., Keene JD. J Neurosci. 1994;14(4):1943–1952. doi: 10.1523/JNEUROSCI.14-04-01943.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Sakai K, Gofuku M, Kitagawa Y, Ogasawara T, Hirose G, Yamazaki M, Koh CS, Yanagisawa N, Steinman L. Biochem Biophys Res Commun. 1994;199(3):1200–1208. doi: 10.1006/bbrc.1994.1358. [DOI] [PubMed] [Google Scholar]
- [164].Szabo A, Dalmau J, Manley G, Rosenfeld M, Wong E, Henson J, Posner JB, Furneaux HM. Cell. 1991;67(2):325–333. doi: 10.1016/0092-8674(91)90184-z. [DOI] [PubMed] [Google Scholar]
- [165].Ma WJ, Cheng S, Campbell C, Wright A, Furneaux H. J Biol Chem. 1996;271(14):8144–8151. doi: 10.1074/jbc.271.14.8144. [DOI] [PubMed] [Google Scholar]
- [166].Myer VE, Fan XC, Steitz JA. EMBO J. 1997;16(8):2130–2139. doi: 10.1093/emboj/16.8.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Good PJ. Proc Natl Acad Sci U S A. 1995;92(10):4557–4561. doi: 10.1073/pnas.92.10.4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Kenan DJ, Query CC, Keene JD. Trends Biochem Sci. 1991;16(6):214–220. doi: 10.1016/0968-0004(91)90088-d. [DOI] [PubMed] [Google Scholar]
- [169].Liu J, Dalmau J, Szabo A, Rosenfeld M, Huber J, Furneaux H. Neurology. 1995;45(3 Pt 1):544–550. doi: 10.1212/wnl.45.3.544. [DOI] [PubMed] [Google Scholar]
- [170].Levy NS, Chung S, Furneaux H, Levy AP. J Biol Chem. 1998;273(11):6417–6423. doi: 10.1074/jbc.273.11.6417. [DOI] [PubMed] [Google Scholar]
- [171].Shaw G, Kamen R. Cell. 1986;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- [172].Lafarga V, Cuadrado A, Lopez de Silanes I, Bengoechea R, Fernandez-Capetillo O, Nebreda AR. Mol Cell Biol. 2009;29(16):4341–4351. doi: 10.1128/MCB.00210-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Wang W, Furneaux H, Cheng H, Caldwell MC, Hutter D, Liu Y, Holbrook N, Gorospe M. Mol Cell Biol. 2000;20(3):760–769. doi: 10.1128/mcb.20.3.760-769.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Yano M, Okano HJ, Okano H. J Biol Chem. 2005;280(13):12690–12699. doi: 10.1074/jbc.M411119200. [DOI] [PubMed] [Google Scholar]
- [175].Al-Mohanna MA, Al-Khalaf HH, Al-Yousef N, Aboussekhra A. Nucleic Acids Res. 2007;35(1):223–233. doi: 10.1093/nar/gkl1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Yang X, Wang W, Fan J, Lal A, Yang D, Cheng H, Gorospe M. J Biol Chem. 2004;279(47):49298–49306. doi: 10.1074/jbc.M407535200. [DOI] [PubMed] [Google Scholar]
- [177].Shu L, Yan W, Chen X. Genes Dev. 2006;20(21):2961–2972. doi: 10.1101/gad.1463306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Miyamoto S, Hidaka K, Jin D, Morisaki T. Genes Cells. 2009 doi: 10.1111/j.1365-2443.2009.01347.x. [DOI] [PubMed] [Google Scholar]
- [179].Cho SJ, Zhang J, Chen X. Nucleic Acids Res. 2010 doi: 10.1093/nar/gkp1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Fukuda T, Naiki T, Saito M, Irie K. Genes Cells. 2009;14(2):113–128. doi: 10.1111/j.1365-2443.2008.01256.x. [DOI] [PubMed] [Google Scholar]
- [181].Battelli C, Nikopoulos GN, Mitchell JG, Verdi JM. Mol Cell Neurosci. 2006;31(1):85–96. doi: 10.1016/j.mcn.2005.09.003. [DOI] [PubMed] [Google Scholar]
- [182].Okano H, Kawahara H, Toriya M, Nakao K, Shibata S, Imai T. Exp Cell Res. 2005;306(2):349–356. doi: 10.1016/j.yexcr.2005.02.021. [DOI] [PubMed] [Google Scholar]
- [183].Iakova P, Wang GL, Timchenko L, Michalak M, Pereira-Smith OM, Smith JR, Timchenko NA. EMBO J. 2004;23(2):406–417. doi: 10.1038/sj.emboj.7600052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Gibson TJ, Thompson JD, Heringa J. FEBS Lett. 1993;324(3):361–366. doi: 10.1016/0014-5793(93)80152-k. [DOI] [PubMed] [Google Scholar]
- [185].Siomi H, Siomi MC, Nussbaum RL, Dreyfuss G. Cell. 1993;74(2):291–298. doi: 10.1016/0092-8674(93)90420-u. [DOI] [PubMed] [Google Scholar]
- [186].Giles KM, Daly JM, Beveridge DJ, Thomson AM, Voon DC, Furneaux HM, Jazayeri JA, Leedman PJ. J Biol Chem. 2003;278(5):2937–2946. doi: 10.1074/jbc.M208439200. [DOI] [PubMed] [Google Scholar]
- [187].Waggoner SA, Johannes GJ, Liebhaber SA. J Biol Chem. 2009;284(14):9039–9049. doi: 10.1074/jbc.M806986200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Lal A, Mazan-Mamczarz K, Kawai T, Yang X, Martindale JL, Gorospe M. EMBO J. 2004;23(15):3092–3102. doi: 10.1038/sj.emboj.7600305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Kao PN, Chen L, Brock G, Ng J, Kenny J, Smith AJ, Corthesy B. J Biol Chem. 1994;269(32):20691–20699. [PubMed] [Google Scholar]
- [190].Grassmann R, Aboud M, Jeang KT. Oncogene. 2005;24(39):5976–5985. doi: 10.1038/sj.onc.1208978. [DOI] [PubMed] [Google Scholar]
- [191].Matsumoto K, Shibata H, Fujisawa JI, Inoue H, Hakura A, Tsukahara T, Fujii M. J Virol. 1997;71(6):4445–4451. doi: 10.1128/jvi.71.6.4445-4451.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Zhang L, Zhi H, Liu M, Kuo YL, Giam CZ. Retrovirology. 2009;6:35. doi: 10.1186/1742-4690-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Li Y, Dowbenko D, Lasky LA. J Biol Chem. 2002;277(13):11352–11361. doi: 10.1074/jbc.M109062200. [DOI] [PubMed] [Google Scholar]
- [194].Oh YT, Chun KH, Park BD, Choi JS, Lee SK. Apoptosis. 2007;12(7):1339–1347. doi: 10.1007/s10495-007-0066-8. [DOI] [PubMed] [Google Scholar]
- [195].Scott MT, Ingram A, Ball KL. EMBO J. 2002;21(24):6771–6780. doi: 10.1093/emboj/cdf684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Scott MT, Morrice N, Ball KL. J Biol Chem. 2000;275(15):11529–11537. doi: 10.1074/jbc.275.15.11529. [DOI] [PubMed] [Google Scholar]
- [197].Zhang Y, Wang Z, Magnuson NS. Mol Cancer Res. 2007;5(9):909–922. doi: 10.1158/1541-7786.MCR-06-0388. [DOI] [PubMed] [Google Scholar]
- [198].Rossig L, Jadidi AS, Urbich C, Badorff C, Zeiher AM, Dimmeler S. Mol Cell Biol. 2001;21(16):5644–5657. doi: 10.1128/MCB.21.16.5644-5657.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Winters ZE, Leek RD, Bradburn MJ, Norbury CJ, Harris AL. Breast Cancer Res. 2003;5(6):R242–249. doi: 10.1186/bcr654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Xia W, Chen JS, Zhou X, Sun PR, Lee DF, Liao Y, Zhou BP, Hung MC. Clin Cancer Res. 2004;10(11):3815–3824. doi: 10.1158/1078-0432.CCR-03-0527. [DOI] [PubMed] [Google Scholar]
- [201].Rossig L, Badorff C, Holzmann Y, Zeiher AM, Dimmeler S. J Biol Chem. 2002;277(12):9684–9689. doi: 10.1074/jbc.M106157200. [DOI] [PubMed] [Google Scholar]
- [202].Bornstein G, Bloom J, Sitry-Shevah D, Nakayama K, Pagano M, Hershko A. J Biol Chem. 2003;278(28):25752–25757. doi: 10.1074/jbc.M301774200. [DOI] [PubMed] [Google Scholar]
- [203].Kim GY, Mercer SE, Ewton DZ, Yan Z, Jin K, Friedman E. J Biol Chem. 2002;277(33):29792–29802. doi: 10.1074/jbc.M201299200. [DOI] [PubMed] [Google Scholar]
- [204].Mercer SE, Ewton DZ, Deng X, Lim S, Mazur TR, Friedman E. J Biol Chem. 2005;280(27):25788–25801. doi: 10.1074/jbc.M413594200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Zhan J, Easton JB, Huang S, Mishra A, Xiao L, Lacy ER, Kriwacki RW, Houghton PJ. Mol Cell Biol. 2007;27(9):3530–3541. doi: 10.1128/MCB.00086-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Sarmento LM, Huang H, Limon A, Gordon W, Fernandes J, Tavares MJ, Miele L, Cardoso AA, Classon M, Carlesso N. J Exp Med. 2005;202(1):157–168. doi: 10.1084/jem.20050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Wang W, Nacusi L, Sheaff RJ, Liu X. Biochemistry. 2005;44(44):14553–14564. doi: 10.1021/bi051071j. [DOI] [PubMed] [Google Scholar]
- [208].Yu ZK, Gervais JL, Zhang H. Proc Natl Acad Sci U S A. 1998;95(19):11324–11329. doi: 10.1073/pnas.95.19.11324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Amador V, Ge S, Santamaria PG, Guardavaccaro D, Pagano M. Mol Cell. 2007;27(3):462–473. doi: 10.1016/j.molcel.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Kim Y, Starostina NG, Kipreos ET. Genes Dev. 2008;22(18):2507–2519. doi: 10.1101/gad.1703708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Bloom J, Amador V, Bartolini F, DeMartino G, Pagano M. Cell. 2003;115(1):71–82. doi: 10.1016/s0092-8674(03)00755-4. [DOI] [PubMed] [Google Scholar]
- [212].Coulombe P, Rodier G, Bonneil E, Thibault P, Meloche S. Mol Cell Biol. 2004;24(14):6140–6150. doi: 10.1128/MCB.24.14.6140-6150.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Chen X, Chi Y, Bloecher A, Aebersold R, Clurman BE, Roberts JM. Mol Cell. 2004;16(5):839–847. doi: 10.1016/j.molcel.2004.11.011. [DOI] [PubMed] [Google Scholar]
- [214].Sheaff RJ, Singer JD, Swanger J, Smitherman M, Roberts JM, Clurman BE. Mol Cell. 2000;5(2):403–410. doi: 10.1016/s1097-2765(00)80435-9. [DOI] [PubMed] [Google Scholar]
- [215].Touitou R, Richardson J, Bose S, Nakanishi M, Rivett J, Allday MJ. EMBO J. 2001;20(10):2367–2375. doi: 10.1093/emboj/20.10.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Gerards WL, de Jong WW, Bloemendal H, Boelens W. J Mol Biol. 1998;275(1):113–121. doi: 10.1006/jmbi.1997.1429. [DOI] [PubMed] [Google Scholar]
- [217].Gerards WL, de Jong WW, Boelens W, Bloemendal H. Cell Mol Life Sci. 1998;54(3):253–262. doi: 10.1007/s000180050147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Coleman ML, Marshall CJ, Olson MF. EMBO J. 2003;22(9):2036–2046. doi: 10.1093/emboj/cdg189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Jascur T, Brickner H, Salles-Passador I, Barbier V, El Khissiin A, Smith B, Fotedar R, Fotedar A. Mol Cell. 2005;17(2):237–249. doi: 10.1016/j.molcel.2004.11.049. [DOI] [PubMed] [Google Scholar]
- [220].Zhang Z, Wang H, Li M, Agrawal S, Chen X, Zhang R. J Biol Chem. 2004;279(16):16000–16006. doi: 10.1074/jbc.M312264200. [DOI] [PubMed] [Google Scholar]
- [221].Jin Y, Lee H, Zeng SX, Dai MS, Lu H. EMBO J. 2003;22(23):6365–6377. doi: 10.1093/emboj/cdg600. [DOI] [PMC free article] [PubMed] [Google Scholar]