Abstract
Ca2+ entry through store-operated Ca2+ channels involves the interaction at ER–PM (endoplasmic reticulum–plasma membrane) junctions of STIM (stromal interaction molecule) and Orai. STIM proteins are sensors of the luminal ER Ca2+ concentration and, following depletion of ER Ca2+, they oligomerize and translocate to ER–PM junctions where they form STIM puncta. Direct binding to Orai proteins activates their Ca2+ channel function. It has been suggested that an additional interaction of the C-terminal polybasic domain of STIM1 with PM phosphoinositides could contribute to STIM1 puncta formation prior to binding to Orai. In the present study, we investigated the role of phosphoinositides in the formation of STIM1 puncta and SOCE (store-operated Ca2+ entry) in response to store depletion. Treatment of HeLa cells with inhibitors of PI3K (phosphatidylinositol 3-kinase) and PI4K (phosphatidylinositol 4-kinase) (wortmannin and LY294002) partially inhibited formation of STIM1 puncta. Additional rapid depletion of PtdIns(4,5)P2 resulted in more substantial inhibition of the translocation of STIM1–EYFP (enhanced yellow fluorescent protein) into puncta. The inhibition was extensive at a concentration of LY294002 (50 μM) that should primarily inhibit PI3K, consistent with a major role for PtdIns(4,5)P2 and PtdIns(3,4,5)P3 in puncta formation. Depletion of phosphoinositides also inhibited SOCE based on measurement of the rise in intracellular Ca2+ concentration after store depletion. Overexpression of Orai1 resulted in a recovery of translocation of STMI1 into puncta following phosphoinositide depletion and, under these conditions, SOCE was increased to above control levels. These observations support the idea that phosphoinositides are not essential but contribute to STIM1 accumulation at ER–PM junctions with a second translocation mechanism involving direct STIM1–Orai interactions.
Keywords: calcium signal; endoplasmic reticulum calcium; Orai1; phosphoinositide; PtdIns(4,5)P2; PtdIns(3,4,5)P3; storeoperated calcium entry (SOCE); stromal interaction molecule 1 (STIM1)
Abbreviations: [Ca2+]i, intracellular free Ca2+ concentration; (E)CFP, (enhanced) cyan fluorescent protein; ER, endoplasmic reticulum; EYFP, enhanced yellow fluorescent protein; FKBP12, FK506-binding protein 12; FRB, fragment of mammalian target of rapamycin that binds FKBP12; fluo-4/AM, fluo-4 acetoxymethyl ester; GFP, green fluorescent protein; HEK-293, human embryonic kidney; ICRAC, Ca2+ release-activated Ca2+ current; NA, numerical aperture; PH, pleckstrin homology; PI3K, phosphatidylinositol 3-kinase; PI4K, phosphatidylinositol 4-kinase; PLC, phospholipase C; PM, plasma membrane; RFP, red fluorescent protein; RFP–ptase-dom, type IV 5-phosphatase domain fused to an RFP tag; SOC, store-operated Ca2+ channel; SOCE, store-operated Ca2+ entry; STIM, stromal interaction molecule
INTRODUCTION
SOCE (store-operated Ca2+ entry) is the major mechanism for receptor-induced Ca2+ influx in non-excitable cells and is important for Ca2+-dependent signalling events, including cell growth and differentiation, secretion, gene expression and Ca2+ homoeostasis [1]. Physiologically, activation of PLC (phospholipase C)-coupled receptors in the PM (plasma membrane) generates Ins(1,4,5)P3, resulting in the subsequent release of Ca2+ from the ER (endoplasmic reticulum) through Ins(1,4,5)P3 receptors. The depletion of Ca2+ from the ER store subsequently triggers Ca2+ influx through SOCs (store-operated Ca2+ channels) in the PM to elevate [Ca2+]i (intracellular free Ca2+ concentration) [1]. Importantly, the channels are activated not by the production of receptor-associated signalling molecules, but by the decrease in luminal ER Ca2+ content [2], involving a coupling between the ER and the PM.
Although SOCE and its associated current, ICRAC (Ca2+ release-activated Ca2+ current), was discovered a number of years ago [1], only more recently has the molecular mechanism responsible for this ER–PM coupling been uncovered. Previously, the ER Ca2+-sensing protein STIM1 (stromal interaction molecule 1) [3,4] and the PM channel Orai1 [5–7] were identified in high-throughput RNAi (RNA interference) screens as proteins which are essential for the activation of SOCs. STIM1 is normally distributed throughout the ER [4,8,9], where its N-terminal EF hand binds to Ca2+ within the ER lumen. Depletion of Ca2+ from within the ER lumen triggers the release of Ca2+ from the EF hand of STIM1, which induces the oligomerization of the EF-SAM domains of STIM1 within the ER [10–12] and the subsequent movement of STIM1 to ER–PM junctions where it forms aggregates known as ‘puncta’ [4,9]. STIM1 puncta at these sites interact with and induce the clustering of Orai1 channel subunits to form active SOCs [13,14]. Mutational analysis of the interacting domains of STIM1 revealed that the cytoplasmic domain of STIM1 is necessary and sufficient for the activation of SOCE [15]. Furthermore, several recent studies have identified a minimal region within the cytoplasmic domain of STIM1 which is essential for the clustering of Orai1 and the activation of SOCs [16–20]. However, the STIM1 protein also contains a short lysine-rich polybasic domain at its extreme C-terminus. Intriguingly, in some studies, mutation or deletion of the STIM1 polybasic domain has been shown to reduce puncta formation, SOCE and ICRAC [8,16,17,21], suggesting that the polybasic domain may be involved in STIM1 targeting to the PM, possibly through an interaction with some, as yet unidentified, PM component [8,16,21].
There are several lines of evidence to suggest that phosphorylated inositol lipids in the PM may regulate SOCE. First, depletion of phosphoinositides has been shown to block both SOCE and ICRAC in several cells types [22–24]. In addition, a previous study uncovered a requirement for both PtdIns(4,5)P2 and PtdIns(3,4,5)P3 to regulate the PM localization of many proteins which contain a cluster of polybasic amino acids within their C-termini [25]. The polybasic region of STIM1 would make it an excellent candidate as a membrane-targeting domain through binding to phosphoinositides [8], although other reports have argued against a role for phosphoinositides in STIM1 translocation [26]. The present study aimed to investigate the role of the phosphoinositides in the translocation of STIM1 and the activation of SOCE. Our results suggest that the phosphoinositides are PM components responsible for targeting STIM1 to ER–PM junctions and reveal a role for the phosphoinositides in the activity of SOCs.
MATERIALS AND METHODS
Plasmids
The plasmids for the rapamycin-inducible phosphatase system were gifts from Dr Tamas Balla (National Institute for Child Health and Development, Bethesda, MD, U.S.A.). The PH (pleckstrin homology) domain of PLCδ1 cloned into GFP (green fluorescent protein)-C1 was a gift from Dr Matilda Katan (Institute of Cancer Research, London, U.K.). For EYFP (enhanced yellow fluorescent protein)-Rit and mCherry-Rit plasmid construction, total RNA was extracted from approx. 100×106 HeLa cells using TRIzol® reagent (Invitrogen) and was used as a template for reverse transcription using Improm-II reverse transcriptase (Promega) and oligo(dT)(15) priming. The resulting single-stranded cDNA was then used as a template in PCRs to amplify the full-length Rit protein. Full-length Rit was then used as a template to PCR-amplify the polybasic Rit tail (nucleotides 770–847) using specific primers containing restriction endonuclease sites to permit subsequent subcloning into the N-terminally tagging pEYFP-C1 vector (Clontech) or into the N-terminally tagging mCherry-C1 vector (a gift from Professor Roger Y. Tsien, Department of Pharmacology, University of California at San Diego, La Jolla, CA, U.S.A.). The primers for the Rit tail were based on the human sequence with GenBank® accession number NM_006912. For pcDNA-Orai1 plasmid construction, the mCherry-Orai1 plasmid described previously [27] was used as a template to amplify full-length Orai1 using specific primers containing restriction endonuclease sites, allowing for subsequent subcloning into the pcDNA3.1(−) vector (Invitrogen). For pCerulean-Orai1 plasmid construction, the Cerulean colour tag was excised from the pCerulean-C1 vector (a gift from Professor David W. Piston, Department of Molecular Physiology and Biophysics, Vanderbilt University Medical Centre, Nashville, TN, U.S.A.) by digestion using the AgeI and BsrGI restriction endonucleases and ligated into the mCherry-Orai1 vector to replace the mCherry tag. The STIM1-EYFP plasmid was described previously [27], and a STIM1-ECFP (enhanced cyan fluorescent protein) plasmid was constructed in a similar manner.
Chemicals and reagents
Cell culture reagents were purchased from Invitrogen, except for GeneJuice, which was purchased from Merck. Thapsigargin, oligomycin, wortmannin and rapamycin were purchased from Calbiochem. Fluo-4/AM (fluo-4 acetoxymethyl ester) was from Invitrogen/Molecular Probes. All other chemicals were from Sigma.
Cells and transfection
HeLa cells were maintained in DMEM (Dulbecco's modified Eagle's medium) supplemented with 10% (v/v) FBS (fetal bovine serum), 1% penicillin and streptomycin and 1% (v/v) MEM non-essential amino acids. Before transfection, cells were grown to 80–90% confluency in 35-mm-diameter Petri dishes with glass bottoms (MatTek). Plasmids and transfection reagents were added directly to the Petri dishes. Single transfection and co-transfections were carried out using 3 μg of plasmid(s) of interest in total per dish, supplemented with 8 μl of GeneJuice.
Solutions
The standard extracellular solution used during all experiments contained 140 mM NaCl, 4.7 mM KCl, 2 mM CaCl2, 1.13 mM MgCl2, 10 mM glucose and 10 mM Hepes (pH 7.2). For Ca2+ imaging experiments, cells were treated with 2 μM thapsigargin in Ca2+-free standard solution to deplete intracellular Ca2+ stores and were subsequently perfused with standard solution containing 5 mM Ca2+ to permit Ca2+ influx. All experiments were performed at room temperature (23 °C). Cells were washed three times in standard extracellular solution and mounted in a perfusion chamber on the stage of an inverted microscope prior to imaging.
Confocal microscopy and image analysis
Confocal images were acquired using a Leica AOBS SP2 microscope equipped with a ×63 water-immersion objective [NA (numerical aperture), 1.2] or a ×63 oil-immersion objective (NA, 1.4). Images of STIM1–EYFP were obtained with 496 nm excitation light and emission fluorescence selected with a 505–550 nm band-pass filter. For excitation of RFP (red fluorescent protein)-5-phosphatase and mCherry-Rit constructs, a 594 nm laser line was used and emission fluorescence was collected between 610 and 700 nm. For GFP (green fluorescent protein) imaging, cells were excited with the 488 nm laser line and emission was collected between 500 and 515 nm. For CFP (cyan fluorescent protein)- or Cerulean-tagged proteins, 405 nm excitation was used and emission fluorescence collected between 450 and 490 nm. Images were processed using the CorelDraw software package or ImageJ (National Institutes of Health). To determine the extent of fluorescence associated with the PM, regions of interest were drawn around the outside of each cell and immediately beneath the cell periphery. Peripheral fluorescence was calculated by subtraction of cytoplasmic fluorescence from the outer region of interest. Fluorescence values were calculated on the basis of the mean fluorescence per pixel in the respective regions rather than total fluorescence to avoid problems arising from variations in the size of the selected regions of interest. For quantification of STIM1–EYFP puncta formation, the puncta were selected as spots of high fluorescence intensity ranging from approx. 0.5 to 1.0 μm in diameter. Accuracy of puncta quantification was verified by independent blind counting.
Measurement of [Ca2+]i
HeLa cells were loaded with 5 μM fluo-4/AM for 30 min at room temperature to measure changes in [Ca2+]i and were imaged using a Leica AOBS SP2 confocal microscope. Fluo-4 was excited using a 488 nm laser and emission fluorescence was collected between 495 and 545 nm. To allow for comparisons between different cells from different experiments, fluo-4 fluorescence measurements for each cell were calculated as the ratio of the fluorescence at the first time point and, to eliminate variability between experiments in the resting fluorescence level, these values are shown as F/F0 normalized to the peak ratio for the control cells in each experiment.
RESULTS
STIM1 can translocate to puncta following ATP depletion and loss of the phosphoinositides PtdIns(4,5)P2 and PtdIns(3,4,5)P3
The phosphoinositides PtdIns(4,5)P2 and PtdIns(3,4,5)P3 have been suggested to bind the polybasic domain of STIM1 and target it to ER–PM junctions [8]. We have shown previously that STIM1 translocation to puncta is stimulated by ATP depletion which reduced the levels of PtdIns(4,5)P2 at the PM [27]. We investigated whether STIM1 puncta formation is dependent on PtdIns(3,4,5)P3 by treating cells with wortmannin prior to ATP depletion. We observed translocation into the cytosol of the polybasic tail from the small GTPase Rit linked to mCherry as a marker for PM PtdIns(4,5)P2 and PtdIns(3,4,5)P3 [25]. STIM1 puncta formation was, however, still observed after ATP depletion (results not shown).
Activation of the rapamycin-inducible phosphatase results in rapid depletion of PM PtdIns(4,5)P2
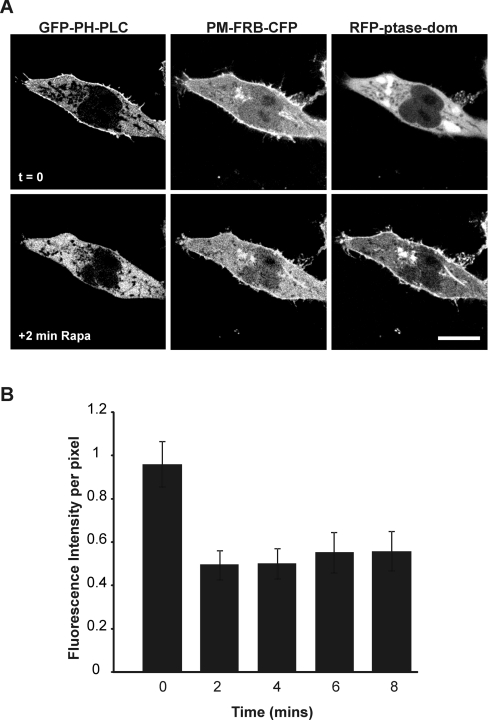
In the previous experiment, methods were used which would gradually decrease PtdIns(4,5)P2 levels over a period of time as ATP was depleted. The results could suggest that PtdIns(4,5)P2 and PtdIns(3,4,5)P3 are not essential for targeting STIM1 to the PM, but it is possible that depletion of PtdIns(4,5)P2 and PtdIns(3,4,5)P3 under these conditions was slow. We, therefore, investigated the potential role of phosphoinositides in STIM1 puncta formation using a more specific and more rapid approach to deplete the levels of PtdIns(4,5)P2 at the PM. To enable fast depletion of PtdIns(4,5)P2 we aimed to use a previously described inducible type IV 5-phosphatase to specifically hydrolyse PtdIns(4,5)P2 [25,29]. This system is based on the rapamycin-stimulated heterodimerization of two proteins: FRB [fragment of mTOR (mammalian target of rapamycin) that binds FKBP12 (FK506-binding protein 12)] and FKBP12 itself. In this system, FRB contains a PM-localization signal and also a CFP (PM-FRB–CFP) or RFP (PM-FRB–RFP) tag to monitor its localization. The FKBP12 protein is fused to a type IV 5-phosphatase and an RFP tag (RFP–ptase-dom) and is distributed throughout the cytosol where it is inactive in the absence of rapamycin. In order to test the ability of this phosphatase system to alter PtdIns(4,5)P2 levels in our hands, HeLa cells were co-transfected with the GFP-tagged PH domain of PLCδ1 (GFP-PH-PLC) to monitor PtdIns(4,5)P2 at the PM, along with the PM-FRB-CFP and RFP-ptase-dom constructs (Figure 1A). Perfusion with rapamycin (1 μM) resulted in the rapid translocation of the RFP–ptase-dom from the cytosol to the PM and the dissociation of GFP–PH-PLC from the PM (Figure 1B). Quantification of the fluorescence intensity at the PM showed that dissociation of GFP–PH-PLC is complete within 2 min with no further decrease in membrane fluorescence after 2 min (Figure 1B; n=6), suggesting that PtdIns(4,5)P2 depletion is rapid and complete within this time frame. This method therefore can be used as a robust means of specifically and rapidly depleting PtdIns(4,5)P2 at the PM.
Figure 1. Activation of the inducible phosphatase results in rapid and efficient depletion of PtdIns(4,5)P2.
HeLa cells were co-transfected with the GFP-PH-PLC, PM-FRB-CFP and RFP-ptase-dom constructs. At 24 h post-transfection, cells were treated with rapamycin (1 μM; Rapa). Rapamycin rapidly depleted PtdIns(4,5)P2 as shown by the removal of GFP–PH-PLC from the PM to the cytosol (A). Quantification of fluorescence at the PM reveals that depletion is complete within 2 min (B). Scale bar, 10 μm.
Neither PtdIns(4,5)P2 nor PtdIns4P/PtdIns(3,4,5)P3 alone are essential for targeting STIM1 to puntca
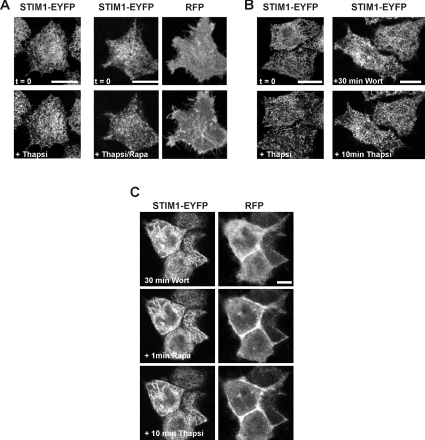
To test whether STIM1 accumulates in puncta via an interaction with PM PtdIns(4,5)P2, cells were transfected with the STIM1-EYFP construct along with RFP chimaeras of both the PM-FRB-RFP and RFP-ptase-dom constructs. In this case, there was some PM fluorescence from the PM-FRB-RFP construct prior to the addition of rapamycin (Figure 2A, upper right-hand panel), but a clear translocation of the RFP–ptase-dom from the cytosol to the PM was visible following rapamycin treatment (Figure 2A, lower right-hand panel). [Note that it was necessary to use the RFP-fusion proteins of both the PM-bound FRB and the 5-phosphatase domain so that in future experiments we could simultaneously monitor these proteins along with the localization of Cerulean–Orai1 and STIM1–EYFP proteins in the same cell.] Cells were perfused with rapamycin for 1 min to activate the phosphatase. Further perfusion with a combination of rapamycin and the SERCA (sarcoplasmic/endoplasmic reticulum Ca2+-ATPase) pump inhibitor thapsigargin (2 μM) to deplete intracellular stores failed to prevent formation of STIM1 puncta (Figure 2A, lower left-hand panel; n=8). Quantification of the number of puncta per cell indicated, however, a 40% reduction in the mean number of puncta per cell following PtdIns(4,5)P2 depletion (see Figure 5), indicating a partial contribution of this lipid. In cells transfected with STIM1-EYFP alone, thapsigargin-induced STIM1 translocation was observed after pre-incubation with 20 μM wortmannin to inhibit both PI3K (phosphatidylinositol 3-kinase) and PI4K (phosphatidylinositol 4-kinase) and deplete PtdIns4P/PtdIns(3,4,5)P3 (Figure 2B, right-hand panel; n=20), but the mean number of puncta was again reduced by approx. 40% (see Figure 5), arguing that PtdIns4P and PtdIns(3,4,5)P3 are not essential but also contribute to STIM1 puncta formation.
Figure 2. STIM1–EYFP translocation occurs in cells following depletion of either PtdIns(4,5)P2 or PtdIns4P/PtdIns(3,4,5)P3, but is inhibited by the depletion of multiple phosphoinositides.
HeLa cells were transfected with either STIM1-EYFP alone or co-transfected with the STIM1-EYFP, PM-FRB-RFP and RFP-ptase-dom constructs. (A) Control cells treated only with thapsigargin are shown on the left. In cells which overexpress all three proteins, depletion of PtdIns(4,5)P2 by the rapamycin-inducible phosphatase did not prevent the translocation of STIM1–EYFP stimulated by thapsigargin. (B) Control cells treated only with thapsigargin are shown on the left. Inhibition of PI3K and PI4K by wortmannin (20 μM) pre-treatment did not prevent thapsigargin-induced STIM1 translocation in cells expressing STIM1–EYFP alone. (C) Cells were co-transfected with the STIM1-EYFP, PM-FRB-RFP and RFP-ptase-dom constructs and perfused with 20 μM wortmannin for 30 min followed by addition of rapamycin. Addition of thapsigargin had little or no effect on the distribution of STIM1. Scale bars, 10 μm. Rapa, rapamycin; Thapsi, thapsigargin; Wort, wortmannin.
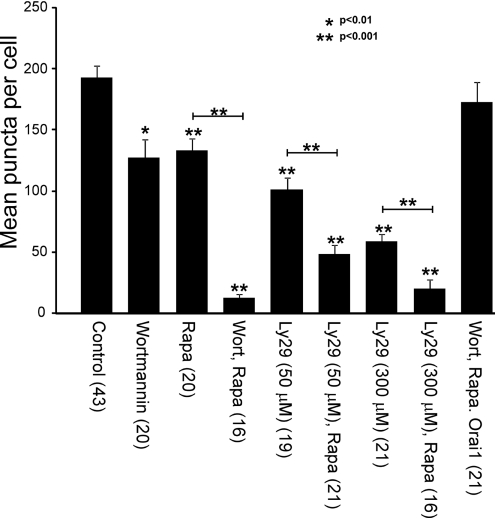
Figure 5. Quantification of puncta formed in response to thapsigargin treatment and the effect of depletion of PtdIns(4,5)P2 and inhibition of lipid kinases.
Cells from the various treatments used in the study were analysed and the number of puncta formed following thapsigargin treatment counted and expressed as mean per cell±S.E.M. based on the number of cells indicated in parentheses. The statistical significance of values for all conditions are compared with control thapsigargin-treated cells, and indicated pairwise comparisons were determined using a two-tailed Student's t test. Statistically significant differences are indicated. Ly29, LY294002; Rapa, rapamycin, Thapsi, thapsigargin; Wort, wortmannin.
Phosphoinositides together regulate STIM1 translocation to puncta
Although the previous experiments suggest that PtdIns4P, PtdIns(4,5)P2 and PtdIns(3,4,5)P3 contribute to but are not the sole regulators of STIM1 clustering in puncta, they do not rule out the possibility that these lipids in combination can contribute to targeting STIM1 to ER–PM junctions. To test this, HeLa cells were co-transfected with the STIM1-EYFP, PM-FRB-RFP and RFP-ptase-dom constructs and pre-treated with 20 μM wortmannin for 30 min (Figure 2C) to inhibit PI3K and PI4K. Addition of rapamycin for 1 min induced translocation of the phosphatase to the membrane. Interestingly, additionally depleting PtdIns(4,5)P2 by this method appeared to essentially abolish thapsigargin-induced STIM1 translocation into puncta (Figure 2C; n=22). Quantification of STIM1 puncta (see Figure 5) indicated a 94% reduction in the mean number of puncta per cell under these conditions that was significantly larger than the inhibition due to wortmannin alone, suggesting that multiple phosphoinositides have a collective role in regulating STIM1 accumulation at ER–PM junctions.
PtdIns(4,5)P2 depletion further inhibits STIM1 translocation into puncta in cells treated with the PI3K/PI4K inhibitor LY294002
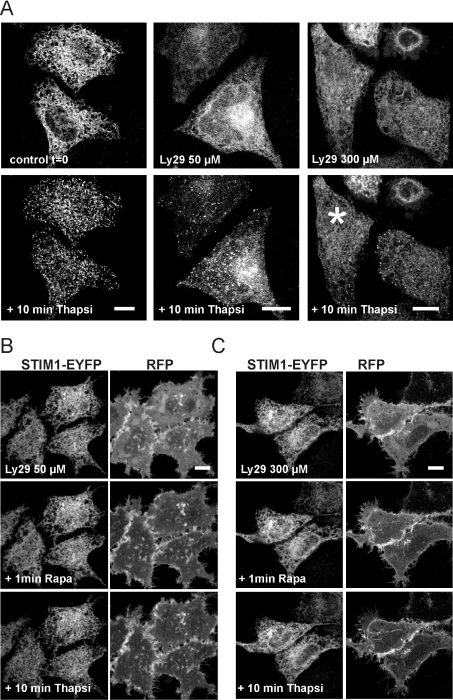
In order to confirm the results found with wortmannin, similar experiments were carried out with the alternative lipid kinase inhibitor LY294002 using HeLa cells co-transfected with STIM1-EYFP, PM-FRB-RFP and RFP-ptase-dom constructs. In these experiments LY294002 was used at two different concentrations at which it has been reported to inhibit PI3K alone (50 μM) or both PI3K and PI4K (300 μM) [28]. Following pre-treatment with the inhibitor alone, translocation into puncta was still observed in response to thapsigargin treatment, although the mean number of puncta per cell were reduced especially at the high concentration of LY294002 (Figure 3A; n=5). When cells were additionally treated with rapamycin to deplete PtdIns(4,5)P2 levels, STIM1 translocation to puncta was further inhibited and indeed was barely detectable especially at the higher concentration of LY29004 (Figures 3B and 3C). Quantification of the number of puncta formed per cell (see Figure 5) indicated a significantly larger effect after rapamycin treatment compared with LY294002 alone. There was a 75% reduction in the mean number of puncta per cell at the lower concentration of LY294002 after phosphatase activation with a further reduction at the higher concentration (90% inhibition). The large effect of the low concentration of LY294002 at which PI3K would be inhibited is consistent with major contributory roles for the lipids PtdIns(4,5)P2 and PtdIns(3,4,5)P3 in puncta formation.
Figure 3. Translocation of STIM1–EYFP is inhibited by the depletion of multiple phosphoinositides in cells treated with LY294002.
HeLa cells were co-transfected with the STIM1-EYFP, PM-FRB-RFP and RFP-ptase-dom constructs. (A) Cells were perfused with no additions (controls), or 50 or 300 μM LY294002 for 30 min followed by addition of thapsigargin, which resulted in some STIM1 translocation into puncta under all conditions. Note that after treatment with 300 μM LY294002, few ST1M1 puncta formed in some cells (e.g. the cell marked by an asterisk). (B) Cells were perfused with 50 μM LY294002 for 30 min followed by addition of rapamycin. Treatment with thapsigargin had little effect on the distribution of STIM1. (C) Cells were perfused with 300 μM LY294002 for 30 min followed by addition of rapamycin. Treatment with thapsigargin had little effect on the distribution of STIM1. Scale bar, 10 μm. Ly29, LY294002; Rapa, rapamycin; Thapsi, thapsigargin.
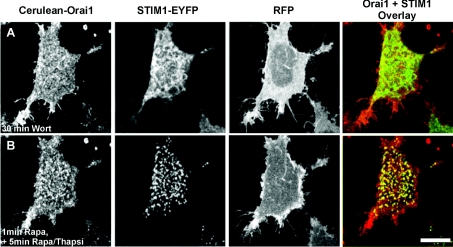
Overexpression of Orai1 rescues STIM1 puncta formation following phosphoinositide depletion
It is possible that STIM1 is targeted to phospholipids in the PM via its polybasic domain. Deletion or mutation of this domain has been reported in several studies to prevent STIM1 puncta formation and SOC activation [8,15]. It has recently been shown, however, that overexpression of Orai1 can rescue puncta formation and SOC activation under such conditions due to interaction of a separate domain within the C-terminus of STIM1 directly with Orai1 [16]. We therefore tested the prediction that STIM1 puncta formation would be restored in cells overexpressing Orai1 in addition to STIM1 under conditions where phosphoinositides are depleted. In order to do this, HeLa cells were co-transfected with the STIM1-EYFP, Cerulean-Orai1, PM-FRB-RFP and RFP-ptase-dom constructs and pre-incubated with wortmannin (Figure 4A). Translocation of the phosphatase was then stimulated by the addition of rapamycin, followed by the perfusion of cells with a combination of rapamycin and thapsigargin to deplete internal stores. Interestingly, translocation of STIM1–EYFP now occurred despite the depletion of phosphoinositides in cells where Cerulean–Orai1 was also overexpressed (Figure 4B; n=21) with the mean number of puncta per cell not significantly different from control untransfected cells treated with thapsigargin (Figure 5). These results agree with previous reports which support a role for the polybasic region of STIM1 in targeting STIM1 to the PM, but that a second domain within Orai1 can interact with STIM1 to independently target it to the PM for SOC activation [16–18].
Figure 4. Overexpression of Orai1 rescues STIM1 puncta formation following depletion of phosphoinositides.
HeLa cells were co-transfected with STIM1-EYFP, PM-FRB-RFP and RFP-ptase-dom constructs and Cerulean-Orai1. The cells were treated with 20 μM wortmannin (A) followed by addition of rapamycin to deplete PtdIns(4,5)P2. Overexpression of Orai1 rescued thapsigargin-stimulated translocation of STIM1 (B). Scale bar, 10 μm. Rapa, rapamycin, Thapsi, thapsigargin; Wort, wortmannin.
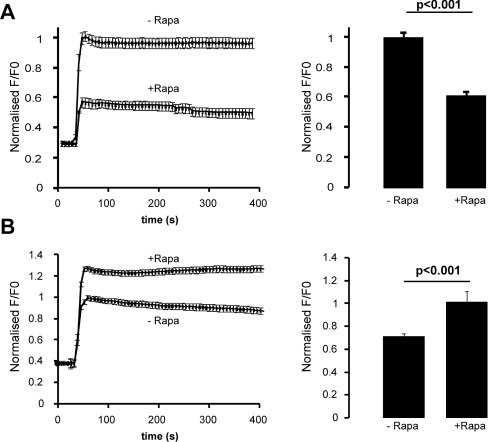
Phosphoinositides contribute to STIM1-mediated SOCE
A number of investigations point to a crucial role for phosphoinositides in the activity of SOCs [22,23,30]. To determine whether the inhibition of STIM1 puncta formation by phosphoinositide depletion affects SOCE, we monitored [Ca2+]i in HeLa cells expressing STIM1–ECFP and the PM-FRB-RFP and RFP-ptase-dom constructs by loading them with the Ca2+ indicator fluo-4/AM. The cells were pre-treated with wortmannin for 30 min. At the beginning of each recording, cells were perfused with either Ca2+-free solution or Ca2+-free solution containing rapamycin. Thapsigargin was subsequently added to deplete intracellular stores. SOCE could be activated by re-introducing Ca2+ to the solution and [Ca2+]i was then compared between cells which had been treated with and without rapamycin. Thapsigargin induced efficient SOCE leading to an increase in [Ca2+]i in control cells untreated with rapamycin (Figure 6A, upper trace; n=27). Influx was, however, greatly reduced in cells where phosphoinositides had been depleted (Figure 6A, lower trace; n=18). This significant reduction was also indicated by comparison of the peak fluorescence ratio values of each trace (Figure 6A), showing a 60% reduction after phosphoinositide depletion. These findings suggest that phosphoinositides at the PM contribute to SOCE under these conditions.
Figure 6. Phosphoinositide depletion reduces store-operated Ca2+ influx but this is reversed by the overexpression of Orai1.
(A) HeLa cells were transfected with the STIM1-EYFP, PM-FRB-RFP and RFP-ptase-dom constructs. At 48 h post-transfection, cells were loaded with fluo-4/AM (5 μM) and incubated in wortmannin (20 μM) for 30 min. Intracellular stores were depleted with thapsigargin (2 μM). Re-addition of Ca2+ to the external solution 420 s later allowed the measurement of Ca2+ influx in cells which had been treated with or without rapamycin (Rapa). Rapamycin treatment resulted in the inhibition of Ca2+ influx when compared with cells which had been incubated in the absence of rapamycin, as shown in the averaged traces. Quantification of the average peak values of Ca2+ influx from these traces revealed a significant decrease in peak [Ca2+]i values in rapamycin-treated cells. (B) In a similar experiment but with HeLa cells also overexpressing Orai from pcDNA3, Ca2+ influx was restored in rapamycin-treated cells to levels greater than that of cells which had not been treated with rapamycin, as shown in the averaged traces and the peak [Ca2+ ]i values.
Overexpression of Orai1 restores Ca2+ influx in cells depleted of phosphoinositides
In Figure 4 it was shown that overexpression of Orai1 recovers STIM1 puncta formation in cells depleted of phosphoinositides. To investigate whether SOCE is also restored under these conditions, cells were co-transfected with STIM-EYFP and the RFP phosphatase system as before but in this case untagged Orai1 was overexpressed in the same cells. Cells were loaded with fluo-4/AM and [Ca2+]i was recorded as described above. Surprisingly, the increase in [Ca2+]i due to Ca2+ influx in cells depleted of phosphoinositides was not only recovered by Orai1 overexpression, but was significantly higher in rapamycin-treated cells (Figure 6B, upper trace; n=15) than control cells untreated with rapamycin (Figure 6B, lower trace; n=18). These results suggest that, although phosphoinositides contribute to SOCE, they are not absolutely essential for Ca2+ influx and agree with previous reports that suggest that the interaction between STIM1 and Orai1 is the key for activation of SOCE [16–18]. In these conditions, Ca2+ influx appeared to be significantly greater following depletion of phosphoinositides, which may suggest an additional negative role for phosphoinositides in SOCE.
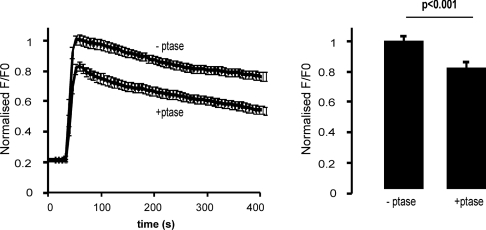
Phosphoinositides contribute to SOCE mediated by endogenous STIM1
The experiments described above on SOCE were carried out in cells overexpressing STIM1. In order to test whether SOCE mediated by endogenous STIM1 was affected by phosphoinositide depletion, cells were transfected with only the RFP phosphatase system and pre-treated with wortmannin. The response to Ca2+ re-addition after thapsigargin treatment was monitored after treatment with rapamycin. A direct comparison was made between cells in the same microscope fields that were non-transfected cells (−ptase) and acted as controls and transfected cells (+ptase). This experiment also rules out any effects of rapamycin itself as all cells were exposed to the drug. The results (Figure 7) indicated that phosphoinositide depletion also inhibited SOCE mediated by endogenous STIM1.
Figure 7. Phosphoinositide depletion reduces store-operated Ca2+ influx due to endogenous STIM1.
Cells were transfected with the PM-FRB-RFP and RFP-ptase-dom constructs. At 48 h post-transfection, cells were loaded with fluo-4/AM (5 μM) and incubated with wortmannin (20 μM) for 30 min and treated with rapamycin (1 μM) before thapsigargin treatment and re-addition of Ca2+ to the external solution after 620 s. In this experiment, [Ca2+]i was monitored in transfected (+ptase; n=22) and non-transfected cells (−ptase; n=18) in the same microscope fields and directly compared so that all cells had been treated with rapamycin.
DISCUSSION
Previous reports have established that the ER protein STIM1 regulates SOCE by translocating to ER–PM junctions following intracellular store depletion and that this can be visualized as the formation of STIM1 puncta [9,31,32]. The site of STIM1 puncta at the PM that is critical for SOCE and STIM1 clustering has been suggested to be in part determined by lipid raft domains which anchor it to the PM [33], but the constituent(s) within the PM responsible for this have not been identified. Phosphoinositides might be involved in STIM1 translocation as several studies have pointed to a role for PM phosphoinositides in the activity of SOCs [22–24].
In the present study, we investigated whether the phosphoinositides are required for STIM1 puncta formation. We used several methods to individually or simultaneously reduce PtdIns4P, PtdIns(4,5)P2 and PtdIns(3,4,5)P3 levels and studied the effects of phosphoinositide depletion on the formation of STIM1 puncta at ER–PM junctions triggered by Ca2+ store depletion. We also investigated the consequence of phosphoinositide depletion on STIM1-mediated SOCE. Initially, depletion of phosphoinositides was carried out using a combination of both high-dose wortmannin treatment at levels which have been shown to inhibit both PI3K and PI4K [28] and ATP depletion [27]. Under these conditions, STIM1 translocation still occurred even though levels of PtdIns(4,5)P2 and PtdIns(3,4,5)P3 were reduced as shown by dissociation of the mCherry-Rit reporter from the PM before the first signs of puncta formation (results not shown). Although this argues against a role for the phosphoinositides in the translocation of STIM1, it is possible that, since ATP depletion would alter the state of phosphorylation of many proteins, this treatment may have other effects on the localization of STIM1 such as uncovering an inhibitory mechanism which normally prevents the aggregation of STIM1 oligomers in puncta. Alternatively, ATP depletion may not deplete the phosphoinositides sufficiently or rapidly enough. It was clear that a more specific and more rapid method for the depletion particularly of PtdIns(4,5)P2 was required to investigate the specific roles of each of the phosphoinositides in STIM1 translocation. For this reason, we used an inducible phosphatase system, which has been reported to specifically and reliably deplete PtdIns(4,5)P2 levels [29,34]. The results shown in the present study reveal a contribution of phosphoinositides to the recruitment of STIM1, since puncta formation was inhibited under conditions that would deplete PtdIns4P, PtdIns(4,5)P2 and PtdIns(3,4,5)P3. Interestingly, puncta formation was partially affected by separate depletion of either PtdIns(4,5)P2 or PtdIns4P/PtdIns(3,4,5)P3, but depletion of all lipids was required for an essentially complete inhibition of puncta formation, suggesting that STIM1 can interact with multiple phosphoinositides in the process of puncta formation.
These results complement previous reports which have shown that the STIM1 polybasic domain plays a role in STIM1 function at the PM [8,16,17,21]. Several hypotheses have been put forward for the function of the polybasic domain in STIM1. Some have suggested that it interacts directly with Orai1 to regulate the activity of the channel [17], while others have proposed that it is targeted to the PM by an interaction with some constituent(s) within the PM itself [8]. It has been suggested that the polybasic domain may direct the recruitment of STIM1 to ER–PM junctions by binding directly to the phosphoinositides PtdIns(4,5)P2 and PtdIns(3,4,5)P3 via electrostatic interactions as is the case for small GTPases with polybasic tails [25]. Our studies are consistent with this hypothesis.
The results of the present study also show that the phosphoinositides contribute to SOCE since depletion of phosphoinositides significantly reduced thapsigargin-induced Ca2+ entry. Extensive phosphoinositide depletion resulted in a 94% reduction in STIM1 puncta formation, but only a 60% overall reduction in the peak fluo-4 fluorescence ratio. It should be noted, however, that the fluorescence ratio is not linearly related to [Ca2+]i leading to an underestimate of the degree of inhibition of SOCE and suggesting that phosphoinositides do play a crucial role in these conditions. The partial inhibitory effects of depletion of individual phosphoinositides on STIM1 puncta formation is consistent with previous findings on the effects of inhibition of PI3K [35] and PI4K [36,37] on agonist-induced Ca2+ influx. In addition, some studies have reported the inhibition of SOCE and ICRAC by the simultaneous inhibition of PI3K/PI4K [22,24]. Broad et al. [22] used high levels of wortmannin to inhibit both PI3K and PI4K and consequently blocked both Ca2+ influx and ICRAC in rat basophilic leukaemia cells. The authors observed that this effect was not due to a decrease in Ins(1,4,5)P3 and DAG (diacylglycerol) production but could not determine the precise mechanism for the phosphoinositides in SOC activity. Others [24] have used LY294002 to inhibit PI3K/PI4K activity in platelets and suggested that these kinases are involved in mediating Ca2+ entry through a mechanism which involves the re-organization of the actin cytoskeleton following store depletion. However, our present findings imply that it is probable that the phosphoinositides contribute to the regulation of Ca2+ influx through the recruitment of STIM1 to the PM following store depletion, since STIM1 puncta formation was substantially inhibited when PM phosphoinositides are depleted by treatment with either wortmannin or LY294002 and hydrolysis of PtdIns(4,5)P2 by an inducible phosphatase. The lower concentration of LY294002 that was used in the present study has been reported to have little effect on PI4K [28]. We established that at the low (50 μM) concentration of LY294002, dissociation from the Golgi complex of the PtdIns4P-specific PH domain reporter FAPPI [38], as a marker of PI4K inhibition, was 2.5-fold less that at the high (300μM) concentration (results not shown). The effectiveness of the lower concentration of LY294002 in inhibiting STIM1 puncta formation is consistent with key roles for the two lipids PtdIns(4,5)P2 and PtdIns(3,4,5)P3, although we cannot rule out an additional contribution of PtdIns4P.
A recent study [16] showed that a truncated STIM1 protein lacking the polybasic domain fails to form puncta when expressed alone in HEK-293 (human embryonic kidney) or HeLa cells but does redistribute into puncta and activate ICRAC when co-expressed with Orai1 in the same cells, reconciling different effects reported of deletion of this domain. This led to the proposal of two separate mechanisms for the recruitment of STIM1 to the PM. STIM1 can be anchored to the PM via a direct interaction of a region within its C-terminal ERM domain with Orai1 [16–20]. Alternatively, STIM1 can be recruited to ER–PM junctions via an Orai1-independent mechanism involving its polybasic domain [8,16]. It has been proposed that, under physiological conditions, the polybasic domain may initially direct STIM1 oligomers to ER–PM junctions and thereby facilitate its interaction with and activation of Orai1 [16]. This dual-targeting mechanism appears to be a vertebrate adaptation since the polybasic domain is present only in vertebrate STIM homologues. In the present study, we observed that STIM1 can effectively form puncta in cells depleted of PM phosphoinositides when Orai1 is overexpressed in the same cells, suggesting that the binding of STIM1 to the phosphoinositides is not essential for puncta formation. We also tested whether SOCE was recovered following phosphoinositide depletion by overexpression of Orai. Note that in our experiments overexpression of both STIM1 and Orai constructs in control cells did not produce an increase in SOCE above that seen with STIM1 alone as described in other studies [39–41]. Previous observations have, however, been derived from the study of HEK-293 cells in which there is little endogenous SOCE [39–41] and presumably low levels of endogenous Orai. Our results showed that Orai overexpression resulted in a recovery of SOCE in cells depleted of phosphoinositides.
Our present observations support the hypothesis that there are two mechanisms for STIM1 targeting to the PM. In addition, Ca2+ influx was restored to above that of control levels in these cells. These findings agree with several recent reports which suggest that the interaction between the ERM domain of STIM1 and Orai1 is necessary and sufficient for the activation of SOCs and does not require the polybasic domain [16–20]. Our observations also suggest two potential functions for the phosphoinositides in SOCE. Under physiological conditions, the phosphoinositides may contribute to the activation of SOCe by enhancing the recruitment of STIM1 to ER–PM junctions, thereby increasing the likelihood of an interaction between STIM1 and Orai1 for the activation of Ca2+ influx. Additionally, they might regulate the ability of STIM1 to activate SOCs when bound to Orai1, since Ca2+ influx is enhanced in the absence of the phosphoinositides in cells co-expressing Orai1. It is possible that, in the absence of the phosphoinositides, the ERM domain of STIM1 is exposed, resulting in enhanced binding of the ERM domain to Orai1 and consequently increased Ca2+ influx. Enhanced activation of SOCs by a STIM1 protein, which lacks a functional polybasic domain, has previously been observed in cells co-expressing Orai1 [17]. This regulatory mechanism may therefore be required under physiological conditions to prevent over-activation of the channels and may have evolved in vertebrates as an additional mechanism to protect cells against Ca2+ toxicity.
A study, which was published while this paper was in preparation, investigated the role of the phosphoinositides in STIM1- and Orai1-mediated Ca2+ entry and found only a small effect of phosphoinositide depletion on STIM1 puncta [42]. The experimental approach used in that study differed from that in the present one since STIM1 translocation and puncta formation were stimulated before subsequently depleting the phosphoinositides using a combination of the PtdIns(4,5)P2-specific inducible phosphatase system and high concentration of LY294002. It is possible that, in these experiments, the pre-formed STIM1 puncta might render PtdIns(4,5)P2 inaccessible to the phosphatase, thereby preventing its efficient hydrolysis. In the present study, stimulation of STIM1 puncta formation by store depletion was only carried out after the cells were treated with a combination of either wortmannin or LY294002 and the inducible phosphatase. This would, thereby, ensure a reduction in the levels of PM phosphoinositides prior to puncta formation. This method proved to be effective in preventing STIM1 puncta formation. Korzeniowski et al. [42] showed that PLC activation or PI3K/PI4K inhibition by LY294002 reduced both ICRAC and SOCE in COS-7 cells, even under conditions where PtdIns(4,5)P2 levels remained unchanged, suggesting that PtdIns(4)P rather than PtdIns(4,5)P2 is the major phosphoinositide required for activation of SOCs. Our studies suggest that PtdIns(4,5)P2 and PtdIns(3,4,5)P3 are the key lipids involved in targeting STIM1 to ER–PM junctions.
In summary, the present study confirms that there are two distinct modes of STIM1 translocation to the PM. One involves a direct interaction between STIM1 and Orai1, the other requiring PM phosphoinositides. We propose two functions for this interaction in the regulation of SOCE. Initially, the phosphoinositides may target STIM1 to ER–PM junctions to facilitate its subsequent interaction with Orai1 at these sites. In addition, the potential binding of the polybasic domain of STIM1 to phosphoinositides may regulate the activity of the Orai1 channel by determining the extent of STIM1–Orai1 binding. Further studies are required to confirm a direct interaction between STIM1 and the various phosphoinositides and to determine the extent to which each phosphoinositide species contributes to the activity of SOCs.
AUTHOR CONTRIBUTION
Ciara Walsh contributed to experimental design, carried out most of the experimental work and prepared the first draft of the paper. Michael Chvanov carried out initial experiments on the effect of phosphoinositide depletion on SOCE and discussed the experimental data. Lee Haynes prepared some of the plasmid constructs and provided advice on experimental design. Ole Petersen discussed the experimental data and contributed to experimental design. Alexei Tepikin contributed to the conception of the project, to the experimental design and edited the paper prior to submission. Robert Burgoyne contributed to the conception of the project, to the experimental design and the preparation of the final version of the paper.
ACKNOWLEGEMENTS
We are grateful to Tamas Balla, Matilda Katan, David W. Piston and Roger Y. Tsien for the gifts of plasmids used in the present work.
FUNDING
This work was supported by a Wellcome Trust Prize Ph.D. studentship [grant number 080910/Z/06/Z] to C.M.W., and a Medical Research Council programme grant [grant number G0700167] to A.V.T. and O.H.P.
References
- 1.Parekh A. B., Putney J. W., Jr Store-operated calcium channels. Physiol. Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 2.Putney J. W., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 3.Roos J., DiGregorio P. J., Yeromin A. V., Ohlsen K., Lioudyno M., Zhang S., Safrina O., Kozak J. A., Wagner S. L., Cahalan M. D., et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J. Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liou J., Kim M. L., Heo W. D., Jones J. T., Myers J. W., Ferrell J. E., Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr. Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feske S., Gwack Y., Prakriya M., Srikanth S., Puppel S. H., Tanasa B., Hogan P. G., Lewis R. S., Daly M., Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 6.Vig M., Peinelt C., Beck A., Koomoa D. L., Rabah D., Koblan-Huberson M., Kraft S., Turner H., Fleig A., Penner R., Kinet J. P. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang S. L., Yeromin A. V., Zhang X. H., Yu Y., Safrina O., Penna A., Roos J., Stauderman K. A., Cahalan M. D. Genome-wide RNAi screen of Ca2+ influx identifies genes that regulate Ca2+ release-activated Ca2+ channel activity. Proc. Natl. Acad. Sci. U.S.A. 2006;103:9357–9362. doi: 10.1073/pnas.0603161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liou J., Fivaz M., Inoue T., Meyer T. Live-cell imaging reveals sequential oligomerization and local plasma membrane targeting of stromal interaction molecule 1 after Ca2+ store depletion. Proc. Natl. Acad. Sci. U.S.A. 2007;104:9301–9306. doi: 10.1073/pnas.0702866104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu M. M., Buchanan J., Luik R. M., Lewis R. S. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J. Cell Biol. 2006;174:803–813. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stathopulos P. B., Li G. Y., Plevin M. J., Ames J. B., Ikura M. Stored Ca2+ depletion-induced oligomerization of stromal interaction molecule 1 (STIM1) via the EF-SAM region: an initiation mechanism for capacitive Ca2+ entry. J. Biol. Chem. 2006;281:35855–35862. doi: 10.1074/jbc.M608247200. [DOI] [PubMed] [Google Scholar]
- 11.Zheng L., Stathopulos P. B., Li G. Y., Ikura M. Biophysical characterization of the EF-hand and SAM domain containing Ca2+ sensory region of STIM1 and STIM2. Biochem. Biophys. Res. Commun. 2008;369:240–246. doi: 10.1016/j.bbrc.2007.12.129. [DOI] [PubMed] [Google Scholar]
- 12.Stathopulos P. B., Zheng L., Li G. Y., Plevin M. J., Ikura M. Structural and mechanistic insights into STIM1-mediated initiation of store-operated calcium entry. Cell. 2008;135:110–122. doi: 10.1016/j.cell.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Yeromin A. V., Zhang S. L., Jiang W., Yu Y., Safrina O., Cahalan M. D. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–229. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vig M., Beck A., Billingsley J. M., Lis A., Parvez S., Peinelt C., Koomoa D. L., Soboloff J., Gill D. L., Fleig A., Kinet J. P., Penner R. CRACM1 multimers form the ion-selective pore of the CRAC channel. Curr. Biol. 2006;16:2073–2079. doi: 10.1016/j.cub.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang G. N., Zeng W., Kim J. Y., Yuan J. P., Han L., Muallem S., Worley P. F. STIM1 carboxyl-terminus activates native SOC, Icrac and TRPC1 channels. Nat. Cell Biol. 2006;8:1003–1010. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- 16.Park C. Y., Hoover P. J., Mullins F. M., Bachhawat P., Covington E. D., Raunser S., Walz T., Garcia K. C., Dolmetsch R. E., Lewis R. S. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell. 2009;136:876–890. doi: 10.1016/j.cell.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan J. P., Zeng W., Dorwart M. R., Choi Y. J., Worley P. F., Muallem S. SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nat. Cell Biol. 2009;11:337–343. doi: 10.1038/ncb1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawasaki T., Lange I., Feske S. A minimal regulatory domain in the C terminus of STIM1 binds to and activates ORAI1 CRAC channels. Biochem. Biophys. Res. Commun. 2009;385:49–54. doi: 10.1016/j.bbrc.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y., Deng X., Zhou Y., Hendron E., Mancarella S., Ritchie M. F., Tang X. D., Baba Y., Kurosaki T., Mori Y., et al. STIM protein coupling in the activation of Orai channels. Proc. Natl. Acad. Sci. U.S.A. 2009;106:7391–7396. doi: 10.1073/pnas.0900293106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muik M., Fahrner M., Derler I., Schindl R., Bergsmann J., Frischauf I., Groschner K., Romanin C. A cytosolic homomerization and a modulatory domain within STIM1 C terminus determine coupling to ORAI1 channels. J. Biol. Chem. 2009;284:8421–8426. doi: 10.1074/jbc.C800229200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Z., Lu J., Xu P., Xie X., Chen L., Xu T. Mapping the interacting domains of STIM1 and Orai1 in Ca2+ release-activated Ca2+ channel activation. J. Biol. Chem. 2007;282:29448–29456. doi: 10.1074/jbc.M703573200. [DOI] [PubMed] [Google Scholar]
- 22.Broad L. M., Braun F. J., Lievremont J. P., Bird G. S., Kurosaki T., Putney J. W., Jr Role of the phospholipase C-inositol 1,4,5-trisphosphate pathway in calcium release-activated calcium current and capacitative calcium entry. J. Biol. Chem. 2001;276:15945–15952. doi: 10.1074/jbc.M011571200. [DOI] [PubMed] [Google Scholar]
- 23.Jardin I., Redondo P. C., Salido G. M., Rosado J. A. Phosphatidylinositol 4,5-bisphosphate enhances store-operated calcium entry through hTRPC6 channel in human platelets. Biochim. Biophys. Acta. 2008;1783:84–97. doi: 10.1016/j.bbamcr.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Rosado J. A., Sage S. O. Phosphoinositides are required for store-mediated calcium entry in human platelets. J. Biol. Chem. 2000;275:9110–9113. doi: 10.1074/jbc.275.13.9110. [DOI] [PubMed] [Google Scholar]
- 25.Heo W. D., Inoue T., Park W. S., Kim M. L., Park B. O., Wandless T. J., Meyer T. PI(3,4,5)P3 and PI(4,5)P2 lipids target proteins with polybasic clusters to the plasma membrane. Science. 2006;314:1458–1461. doi: 10.1126/science.1134389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varnai P., Toth B., Toth D. J., Hunyady L., Balla T. Visualization and manipulation of plasma membrane-endoplasmic reticulum contact sites indicates the presence of additional molecular components within the STIM1-Orai1 complex. J. Biol. Chem. 2007;282:29678–29690. doi: 10.1074/jbc.M704339200. [DOI] [PubMed] [Google Scholar]
- 27.Chvanov M., Walsh C. M., Haynes L. P., Voronina S. G., Lur G., Gerasimenko O. V., Barraclough R., Rudland P. S., Petersen O. H., Burgoyne R. D., Tepikin A. V. ATP depletion induces translocation of STIM1 to puncta and formation of STIM1-ORAI1 clusters: translocation and re-translocation of STIM1 does not require ATP. Pflugers Arch. 2008;457:505–517. doi: 10.1007/s00424-008-0529-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Downing G. J., Kim S., Nakanishi S., Catt K. J., Balla T. Characterization of a soluble adrenal phosphatidylinositol 4-kinase reveals wortmannin sensitivity of type III phosphatidylinositol kinases. Biochemistry. 1996;35:3587–3594. doi: 10.1021/bi9517493. [DOI] [PubMed] [Google Scholar]
- 29.Varnai P., Thyagarajan B., Rohacs T., Balla T. Rapidly inducible changes in phosphatidylinositol 4,5-bisphosphate levels influence multiple regulatory functions of the lipid in intact living cells. J. Cell Biol. 2006;175:377–382. doi: 10.1083/jcb.200607116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trebak M., Lemonnier L., DeHaven W. I., Wedel B. J., Bird G. S., Putney J. W., Jr Complex functions of phosphatidylinositol 4,5-bisphosphate in regulation of TRPC5 cation channels. Pflugers Arch. 2009;457:757–769. doi: 10.1007/s00424-008-0550-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang S. L., Yu Y., Roos J., Kozak J. A., Deerinck T. J., Ellisman M. H., Stauderman K. A., Cahalan M. D. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu P., Lu J., Li Z., Yu X., Chen L., Xu T. Aggregation of STIM1 underneath the plasma membrane induces clustering of Orai1. Biochem. Biophys. Res. Commun. 2006;350:969–976. doi: 10.1016/j.bbrc.2006.09.134. [DOI] [PubMed] [Google Scholar]
- 33.Pani B., Ong H. L., Liu X., Rauser K., Ambudkar I. S., Singh B. B. Lipid rafts determine clustering of STIM1 in ER-plasma membrane junctions and regulation of SOCE. J. Biol. Chem. 2008;283:17333–17340. doi: 10.1074/jbc.M800107200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suh B. C., Inoue T., Meyer T., Hille B. Rapid chemically induced changes of PtdIns(4,5)P2 gate KCNQ ion channels. Science. 2006;314:1454–1457. doi: 10.1126/science.1131163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsu A. L., Ching T. T., Sen G., Wang D. S., Bondada S., Authi K. S., Chen C. S. Novel function of phosphoinositide 3-kinase in T cell Ca2+ signaling: a phosphatidylinositol 3,4,5-trisphosphate-mediated Ca2+ entry mechanism. J. Biol. Chem. 2000;275:16242–16250. doi: 10.1074/jbc.M002077200. [DOI] [PubMed] [Google Scholar]
- 36.Watanabe H., Takahashi R., Zhang X. X., Kakizawa H., Hayashi H., Ohno R. Inhibition of agonist-induced Ca2+ entry in endothelial cells by myosin light-chain kinase inhibitor. Biochem. Biophys. Res. Commun. 1996;225:777–784. doi: 10.1006/bbrc.1996.1250. [DOI] [PubMed] [Google Scholar]
- 37.Nakanishi S., Catt K. J., Balla T. Inhibition of agonist-stimulated inositol 1,4,5-trisphosphate production and calcium signaling by the myosin light chain kinase inhibitor, wortmannin. J. Biol. Chem. 1994;269:6528–6535. [PubMed] [Google Scholar]
- 38.Levine T. P., Munro S. Targeting of Golgi-specific pleckstrin homology domains involves both PtdIns 4-kinase-dependent and -independent components. Curr. Biol. 2002;12:695–704. doi: 10.1016/s0960-9822(02)00779-0. [DOI] [PubMed] [Google Scholar]
- 39.Peinelt C., Vig M., Koomoa D. L., Beck A., Nadler M. J., Koblan-Huberson M., Lis A., Fleig A., Penner R., Kinet J. P. Amplification of CRAC current by STIM1 and CRACM1 (Orai1) Nat. Cell Biol. 2006;8:771–773. doi: 10.1038/ncb1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mercer J. C., Dehaven W. I., Smyth J. T., Wedel B., Boyles R. R., Bird G. S., Putney J. W., Jr Large store-operated calcium selective currents due to co-expression of Orai1 or Orai2 with the intracellular calcium sensor, Stim1. J. Biol. Chem. 2006;281:24979–24990. doi: 10.1074/jbc.M604589200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soboloff J., Spassova M. A., Tang X. D., Hewavitharana T., Xu W., Gill D. L. Orai1 and STIM reconstitute store-operated calcium channel function. J. Biol. Chem. 2006;281:20661–20665. doi: 10.1074/jbc.C600126200. [DOI] [PubMed] [Google Scholar]
- 42.Korzeniowski M. K., Popovic M. A., Szentpetery Z., Varnai P., Stojilkovic S. S., Balla T. Dependence of stim1/orai1 mediated calcium entry on plasma membrane phosphoinositides. J. Biol. Chem. 2009;284:21027–21035. doi: 10.1074/jbc.M109.012252. [DOI] [PMC free article] [PubMed] [Google Scholar]