Abstract
Knowledge of how valvular interstitial cells (VICs) interact with the extracellular matrix (ECM) would aid in not only better understanding the etiology of valvular disease, but also constructing appropriate environments for valve tissue engineering. In this work, the calcification of VICs cultured on ECM coatings (fibronectin, fibrin, collagen, laminin) or ECM-derived peptide sequences (RGDS, YIGSR, DGEA) was quantified via several techniques. Neutralizing antibodies to specific adhesion receptors were also applied, followed by quantification of phenotypic markers related to valve calcification. The calcification of VICs varied with the ECM component or peptide that was presented on the culture substrate. VICs calcified the most on RGDS and least on YIGSR and DGEA, while blocking specific receptors revealed that disruption of VIC binding via the α5β1 integrin or the 67kDa laminin receptor had a dramatic calcification-stimulating effect. Binding via the α2β1 integrin did not alter calcification or VIC phenotype. These findings were translated to 3-D peptide-modified scaffold environments that demonstrated varying levels of disease expression by VICs. Thus, specific adhesion receptors play a significant role in mediating the interactions between VICs and ECM that lead to calcification, which provides important information regarding the mechanisms of valvular disease and scaffold design for valve tissue engineering.
Keywords: heart valve, extracellular matrix, cell-material interactions, integrins
Introduction
The most prevalent cell in heart valve leaflets is the valvular interstitial cell (VIC), which acts to maintain the mechanical and physical properties of valves 1,2. VICs also synthesize extracellular matrix (ECM) molecules to repair or remodel their environment, which is crucial in not only maintaining proper valve function, but also in constructing a tissue-engineered heart valve 1–4. Several groups have demonstrated that the process of calcification is associated with VIC transdifferentiation or a phenotypic switch between normal and diseased states 4. During valvular disease, VICs are thought to differentiate into either myofibroblast-like cells, which are identified by markers of contractility such as α-smooth muscle actin, or into osteoblast-like cells, which are accompanied by an up-regulation of alkaline phosphatase and core binding factor alpha-1 (CBFα-1) in early stages and osteocalcin (OCN) in later stages of calcification 3,5.
Remodeling of the heart valve is associated with alterations in ECM structure and composition, as well as integrin expression 6. The ECM performs two major functions in valves: it maintains the valve’s physical and mechanical properties, and it interacts with VICs, thereby contributing to the regulation of VIC function and phenotype. This ECM-VIC interaction influences various cellular functions, such as migration, proliferation, adhesion, differentiation and apoptosis 6–8. Based on previous studies 9 and our current work 10,11, it is known that culture of VICs on different ECM components leads to variable levels of calcification. Specifically, the abundance of certain ECM components has been associated with increased VIC calcification, while other ECM components have demonstrated some anti-calcific properties.
The communication between cells and their ECM environment is mediated by integrin and non-integrin cell membrane receptors for distinct peptide sequences within ECM components, suggesting that the aforementioned ECM-influenced calcification events may be regulated by peptide-receptor interactions. Since the discovery of the adhesive RGD peptide on fibronectin 12, numerous peptide sequences derived from different ECM components have been described 13. It is well known that RGD is recognized by α5β1 and αvβ3 integrins 14, while YIGSR is a laminin-derived peptide motif that interacts with a 67kDa non-integrin protein receptor 15–17, and DGEA is a collagen-derived motif which associates with the α2β1 integrin 18. Each adhesion receptor can recognize specific peptide sequences on different ECM molecules, and this ligand-receptor interaction turns on downstream signaling pathways involving molecules such as Ras or MAPK, which, in turn, may affect processes such as calcification 19–21. For instance, several studies have documented that certain integrin-ligand binding events were required for osteoblast differentiation and mineralization to occur 8. Integrins such as αvβ3 and αvβ5 have been shown to mediate the progression of events leading to vascular disorders such as restenosis 22. In tissue engineering, isolated adhesive peptide sequences are frequently used in order to replicate the cell functions obtained when using an entire ECM molecule 23.
Remarkably, there are currently no approved treatments to stop the progression of valvular calcification, in part due to our incomplete understanding of valvular disease etiology. At present, heart valve disease is treated via total valve replacement, which is performed only when valve function has already significantly deteriorated. Although both mechanical and bioprosthetic valve replacements are generally associated with favorable clinical outcomes, they are also accompanied by serious limitations, such as their inability to grow and remodel, the required long-term use of anti-coagulants (for mechanical valves), and their rapid calcification (for bioprosthetic valves). Tissue-engineered heart valves have the potential to overcome many of the limitations associated with these synthetic valve replacements. However, numerous obstacles remain in constructing engineered tissues, including the identification of an appropriate scaffold environment. Characterization of VIC-peptide interactions can assist in the construction of valve tissue engineering scaffolds by identifying appropriate ECM or peptide components that will help to promote the specific cellular functions that are desired for this application.
In the current work, the aim is to characterize whether VIC interaction with specific peptide sequences influences calcification in VIC cultures. There are two main applications of this work: 1) to better understand the role of the ECM in regulating heart valve dysfunction, and whether this dysfunction is related to specific peptide-receptor interactions, and 2) to identify adhesive peptides that may be useful in constructing functional tissue-engineered heart valves.
Materials and Methods
All chemicals and cell culture solutions were obtained from Sigma-Aldrich (St. Louis, MO) unless noted otherwise.
VIC isolation and culture
Valvular interstitial cells were isolated from porcine aortic valves (Hormel, Inc., Austin, MN) by collagenase digestion as previously described 24 and cultured in growth medium (15% FBS, 2 mM L-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin in Medium 199) at 37°C, 5% CO2 for 2–4 passages.
Unless otherwise specified, VICs used in all experiments were seeded at a density of 50,000 cells/cm2 onto 24-well or 96-well plates. During the execution of experiments, the VICs were cultured in low-serum medium (1% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM L-glutamine, in Medium 199), and the medium was changed each day until Day 5.
ECM coatings
Tissue culture polystyrene (TCPS) plates (24-well or 96-well) were treated with solutions of type I collagen (Inamed Biomaterials, Fremont, CA; 2 μg/cm2), fibronectin (5 μg/cm2), laminin (BD Biosciences, San Jose, CA; 2 μg/cm2), fibrin, or left untreated as a control (TCPS). For the fibrin coating, fibrinogen (1 mg/ml) was first incubated in culture wells overnight at 4°C followed by three washes with PBST (0.05% Tween 20 in Phosphate Buffered Saline (PBS)) and one hour incubation with thrombin (0.6 mg/ml) at 37°C. All coatings were prepared in 50 mM bicarbonate coating buffer, pH 8.5, and rinsed three times with PBS prior to cell seeding. Protein adsorption was verified via measurements performed on separate plates using the BCA (bicinchoninic acid) Protein Assay (Pierce Biotechnology Inc., Rockford, IL) according to manufacturer’s instructions. The protein solution concentrations noted above were optimized such that the final, measured coating density for all conditions was approximately 1.2 μg/cm2.
Peptide coatings
Three peptide sequences: CGRGDS (Arg-Gly-Asp-Ser, RGD-containing peptide), CDPGYIGSR (Tyr-Ile-Gly-Ser-Arg, laminin-derived peptide) and CDDGEAG (Asp-Gly-Glu-Ala, collagen-derived peptide) were obtained from GenScript Corporation (Piscataway, NJ). The peptides and receptors explored in this study are presented in Table 1. Prior to cell seeding, TCPS plates were coated with specific peptide sequences (20 μg/cm2) overnight or left untreated as a control. All plates were washed three times with PBS prior to cell seeding. Final coating densities were determined by performing the BCA assay on the peptide solution before and after the coating process and were found to be approximately 1 μg/cm2 for all peptides.
Table 1.
| Peptide sequence | Receptor(s) | Derived from: |
|---|---|---|
| RGD | αvβ3, α5β1, α1β1 | fibronectin, fibrin, laminin, collagen |
| YIGSR | 67 kDa LN receptor | laminin |
| DGEA | α2β1 | collagen |
Nodule quantification and calcium assay
Alizarin Red S (ARS) staining was used to visualize and quantify the number of calcified nodules in each condition. Plates were fixed with 10% neutral buffered formalin, stored at 4°C overnight, and stained with a 2% solution of Alizarin Red S in PBS. All calcific nodules in each well were then manually counted under a microscope (Olympus IX51 with Hamamatsu 285 digital camera). Cetylpyridinium chloride (CPC) extraction of the ARS dye was then used for quantitative calcium measurements. Following fixation and ARS staining, cells were washed four times with PBS, followed by one wash with deionized water. The plates were then treated with 10% 1-hexadecetylpyridinium chloride monohydrate (Acros Organics, Morris Plains, NJ) in 10 mM sodium phosphate (pH 7.5), and incubated at room temperature for one hour with shaking. Samples of the resulting solution were transferred to a clean 96-well plate, and absorbance was read at 550 nm (Synergy™ HT Multi-Mode Microplate Reader, Biotek Instruments, Inc., Winooski, VT).
Function-blocking of adhesion receptors
In order to investigate the role of specific ligands and their receptors in regulating VIC calcification, the major receptors involved in cell adhesion to collagen, fibronectin, fibrin, and laminin substrates were blocked with appropriate function-blocking compounds. These compounds and their targeted receptors are listed in Table 2.
Table 2.
Compounds used to block the function of specific adhesion receptors
| Compound | Type/Function | Clone | [C] | Used on: |
|---|---|---|---|---|
| Anti-α2β1 integrin | monoclonal mouse anti-human; blocks α2β1 | BHA2.1 | 1 μg/ml | collagen |
| Anti-α5β1 integrin | monoclonal mouse anti-human; blocks α5β1 | P1D6 | 1 μg/ml | fibronectin, |
| Melibiose | antagonist for non-integrin 67 kDa elastin/laminin receptor | -- | 30 mM | fibrin laminin |
VICs were incubated with each function-blocking compound in low-serum medium (1% FBS) at 37°C for 15 minutes prior to seeding cells on the corresponding surface. For instance, 1 μg/ml anti-α2β1 was applied to VICs immediately prior to seeding on collagen-coated surfaces, while 30 mM melibiose was applied prior to seeding on laminin-coated surfaces. The blocking compounds were reapplied every other day until Day 5. Cross-blocking experiments were also performed as a control to ensure that the effect of the neutralization was specific to the receptor/ECM being targeted.
Immunostaining
Immunostaining for vinculin, an important marker of focal adhesion complexes, was performed on VICs cultured under the conditions described in previous sections. VICs were fixed in formalin and stored at 4°C overnight, permeabilized with 0.1% Triton X-100 for 10 minutes, and blocked with 5% bovine serum albumin (BSA) for one hour. A primary antibody to detect vinculin (monoclonal mouse anti-human, clone hVIN-1, 1 mg/ml; Southern Biotech, Birmingham, AL) was diluted 1:1000 in 1% BSA in PBS and applied to VIC samples for two and a half hours. All plates were then washed several times with PBS and incubated with HRP-conjugated goat anti-mouse (Pierce) diluted 1:5000 in 1% BSA in PBS and applied to all plates for 1 hour, followed by a 30 minute incubation with 1-Step Turbo TMB-ELISA (Thermo Fisher Scientific, Waltham, MA). Development of the colorimetric reaction was stopped with 1 N H2SO4 andabsorbance was read at 450 nm. After immunostaining, all plates were washed several times with PBS, stained with DAPI, and measured for fluorescence (excitation 340, emission 440) in order to normalize absorbance results to total DNA.
RNA isolation
Total RNA was isolated using TRI Reagent (Molecular Research Center, Cincinnati, OH) according to manufacturer’s instructions. VICs were lysed with 200 μl TRI reagent per well at 4°C with 50X protease inhibitor cocktail (BD Biosciences). The homogenate was stored at room temperature for 5 minutes to complete the dissociation of nucleoprotein complexes, at which point 0.15 ml chloroform per 600 μl TRI Reagent was added to the homogenate, followed by centrifugation at 13,000 × g for 15 minutes. After centrifugation, RNA was precipitated from the upper aqueous phase by adding 0.3 ml isopropanol per 600 μl TRI Reagent to the tubes and then centrifuging at 13,000 x g for 8 minutes. After this centrifugation step, the RNA pellet was washed with 75% ethanol and centrifuged at 8,000 × g for 5 minutes. The RNA pellet was air dried and dissolved in 75 μl H2O at 60°C for 15 minutes. RNA samples were stored at −20°C until subsequent use.
Real-time PCR analysis
Custom primers for various markers of myofibroblastic or osteogenic activity were obtained from Invitrogen (Carlsbad, CA) and are listed in Table 3. For cDNA construction, 250 ng of original RNA isolated from samples was reverse transcribed to a cDNA library using iScript (Bio-Rad Laboratories, Hercules, CA) according to manufacturer’s instructions. The absence of genomic DNA in the samples was verified by confirming that no gene amplification occurred in samples that had not been reverse-transcribed.
Table 3.
Sequences for real-time PCR
| Core Binding Factor alpha-1 (CBFα-1) | F-5′-GAG GAA CCG TTT CAG CTT ACT G-3′ R-5′-CGT TAA CCA ATG GCA CGA G-3′ |
| Heat Shock Protein 47 (HSP47) | F-5′-GCT GCT CGT CAA CGC CAT GT -3′ R-5′-CCA TCC AGG TCT TCA GCT GC-3′ |
| Matrix metalloproteinase 1 (MMP1) | F-5′-AAG CAG ACA TAA TGT ATC CTT TGT CA-3′ R-5′-TGA GCA TCC CCT CCA ATA CCT-3′ |
| Matrix metalloproteinase 13 (MMP13) | F-5′-ACA AGG GAT CCA GTC TCT CTA TGG T-3′ R-5′-TCC AGG GAT AAT GAA GGA TCA CA-3′ |
| Osteocalcin (OCN) | F-5′-TCA ACC CCG ACT GCG ACG AG -3′ R-5′-TTG GAG CAG CTG GGA TGA TGG -3′ |
| Transforming growth factor beta1 (TGF-β1) | F-5′-CGA GCC AGA GGC GGA CTA C -3′ R-5′-TTG GTT GCC GCT TTC CA -3′ |
| Beta Actin | F-5′-ATG GTG GGT ATG GGT CAG AA-3′ R-5′-CGG AGC TCG TTG TAG AAG GT-3′ |
Samples were processed for real-time PCR by mixing 0.5 μl of the cDNA construction, 5 μM of primers and SYBR Green SuperMix (Bio-Rad) in a 15 μl or 25 μl reaction as specified in the manufacturer’s protocol. For thermocycling, a standard protocol was used: PCR reactions were run over 40 cycles of denaturing at 95°C for 15 seconds, annealed at 60°C for 1 minute, and followed by melting curve analysis for 80 cycles of 55°C +0.5°C/cycle, 10 seconds per cycle, to further confirm the purity of the final PCR products (iCycler iQ Real-Time PCR Instrument, Bio-Rad). A standard comparative CT (or ΔΔ CT) method was used to analyze PCR data. The threshold cycles (CT) of all samples were first normalized to beta-actin as an internal control, and then the delta CT values for experimental samples was further normalized to the relevant control condition in each experiment.
VIC encapsulation in PEG hydrogels
In order to study the role of peptide sequences on the behavior of VICs in a 3-D environment, VICs were encapsulated in peptide-modified, PEG-based scaffolds. Poly(ethylene glycol) (PEG, 3.4 kDa) was diacrylated to form PEG-DA as previously described 25, and then further purified via dialysis for two days. Cysteine-terminated cell adhesion peptides (CGRGDS and CDPGYIGSR; Genscript) were covalently coupled to PEG-DA polymer chains via a thiol-ene reaction 26. To perform the cell encapsulation, VIC pellets were resuspended in a solution of 15% PEG-DA in PBS to achieve a final cell density of 5 ×106 cells/ml, and peptides were added to the solution at a final concentration of 1 mM. Then, the mixed suspension was combined with 1% Irgacure-2959 (Ciba Specialty Chemicals, Tarrytown, NY) and exposed to 3.9 mW/cm2 UV light for 5 min (UVP, LLC, Upland, CA). The control scaffolds did not contain any peptide modification. Cell-seeded hydrogel discs (1 mm tall, 8 mm diameter) were cut using a sterile cork-borer and placed into non-tissue culture treated 24-well polystyrene plates (n=9 samples per condition) and cultured for five days. RNA isolation from these hydrogels was performed as described above for 2-D samples, with the exception that the samples were mechanically homogenized in the TRI Reagent.
Statistics
All experiments were performed a minimum of three separate times, with n ≥ 3. Data were compared using ANOVA with Tukey’s HSD post-hoc test. P values less than orequal to 0.05 were considered statistically significant. Data are presented as mean ± standard deviation.
Results
ECM-dependence of VIC calcification
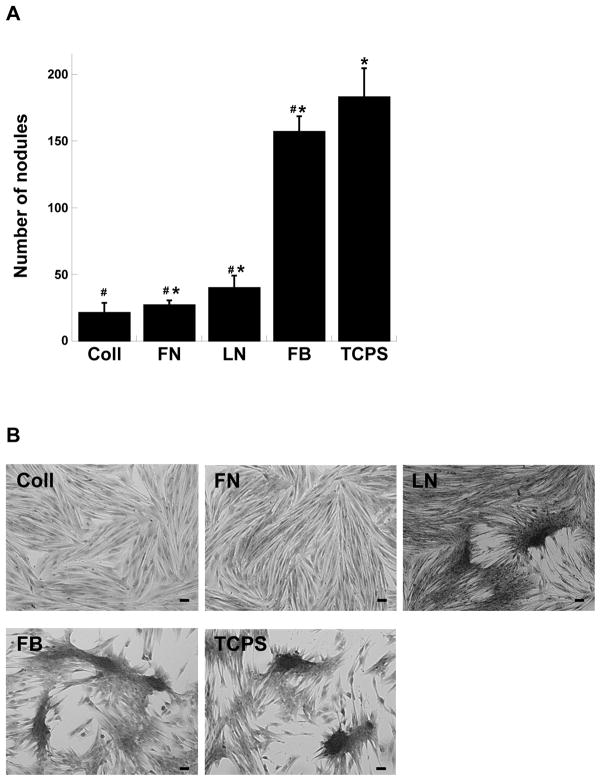
Porcine valvular interstitial cells (VICs) seeded on plates coated with different ECM molecules exhibited variable amounts of calcification by Day 5, as indicated by differences in the extent of calcific nodule formation. As shown by Figure 1 and our previous work 11, different ECM components exerted different effects on the level of VIC culture calcification. VICs formed relatively few nodules when cultured on collagen, fibronectin, or laminin, while nodule number was significantly increased when VICs were cultured on fibrin or the uncoated control, TCPS.
Figure 1.
Porcine valvular interstitial cells (VICs) were cultured on different ECM-coated plates and formalin-fixed on Day 5. (A) Calcific nodule number was counted following mineralization staining using Alizarin Red S. (B) Representative photomicrographs of VICs cultured on the different ECM-based substrates. Scale bar = 100 μm. FB = fibrin, FN = fibronectin, LN = laminin, Coll = collagen, TCPS = unmodified tissue culture polystyrene. #p<0.0001 compared to TCPS, *p<0.0001 compared to Coll, n=8 samples for each condition.
VIC-peptide interaction affects calcification
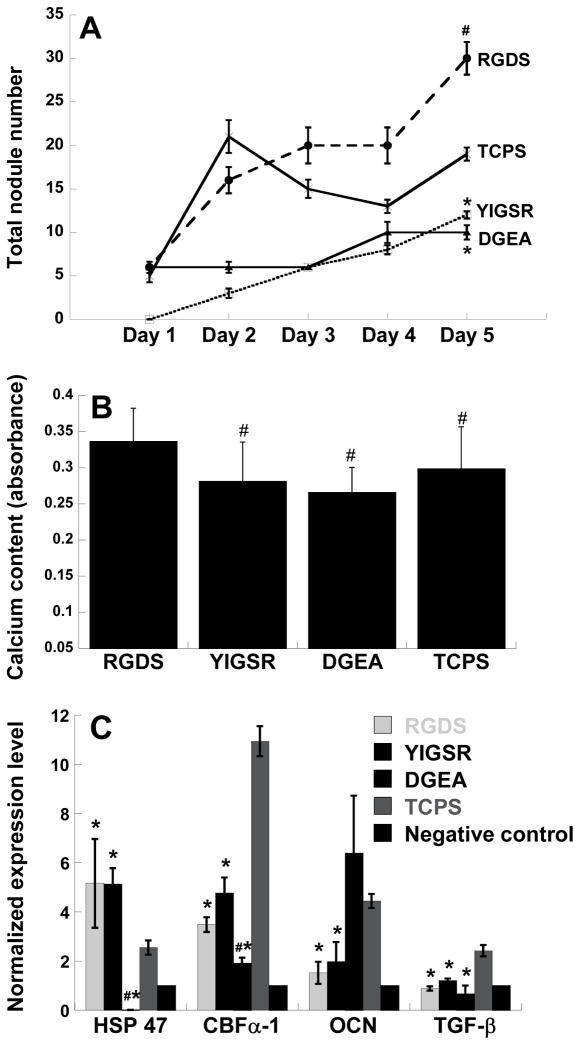
To examine whether the effects observed in Figure 1 could be explained by VIC interaction with isolated adhesive peptide sequences derived from the ECM molecules, VICs were seeded on plates coated with specific ECM-derived peptide sequences: RGDS, YIGSR and DGEA. On each day, six wells from each condition were stained for mineralization and total nodule number was counted manually. As seen in Figure 2A, different peptide conditions resulted in different nodule numbers at the final time point of Day 5, wherein culture on RGDS and TCPS was associated with the highest amount of calcification. In addition, the calcium concentration in the samples followed the same trend as nodule formation. As shown in Figure 2B, the highest amounts of calcium were found in the RGDS and TCPS culture conditions, correlating well with the nodule number data presented in Figure 2A.
Figure 2.
(A) Time course of calcific nodule formation on plates coated with adhesive peptides derived from different ECM components. *p<0.05 compared to TCPS, n=6 samples per condition. (B) Day 5 calcium content in VICs cultured on surfaces modified with different ECM-derived peptide sequences. #p<0.05 compared to RGDS, n=6 samples per condition. (C) Expression of HSP47, CBFα-1, OCN and TGF-β by VICs cultured for five days on peptide-coated plates as measured by qRT-PCR. Negative control refers to VICs cultured on collagen. *p<0.05 compared to TCPS, #p<0.05 compared to RGDS and YIGSR, n=3 samples per condition.
Culture of VICs on RGDS, YIGSR, or TCPS upregulated the expression of various disease markers relative to the non-calcifying control, as shown in Figure 2C. Because VICs on collagen tend to maintain a non-calcifying, relatively quiescent phenotype, this condition was used as the negative control. While culture on TCPS strongly upregulated the expression of all markers studied for these samples (HSP47, CBFα-1, OCN, and TGF-β1) in comparison to the negative control, culture on RGDS or YIGSR led to a much more modest upregulation in CBFα-1 and OCN but similarly strong upregulation of HSP47. With respect to gene expression, no significant differences between the RGDS and YIGSR conditions were observed. Although culture on DGEA was associated with expression levels of HSP47, CBFα-1, and TGF-β1 that were comparable to or lower than the negative (non-calcifying) control, the expression of OCN was significantly elevated and similar to that found on TCPS.
While these results confirmed the hypothesis that different peptide-cell interactions play a role in the process of calcification, they also show that this relationship is complex. For instance, RGD, one of the most commonly-shared peptide sequences among different ECM components, can be recognized by VICs when interacting with FN, FB, Coll or LN, and it is shown in Figure 2A that RGD permits extensive calcification. However, despite the fact that RGD is common to all of the ECM components tested, VICs on FN and Coll had few nodules, whereas they formed a large number of nodules on FB (Figure 1). These complex interactions motivated the integrin-blocking studies presented in the following section.
Integrin-mediated signals influence calcification in VIC cultures
RGD binds to both αVβ3 and α5β1 integrin receptors, while YIGSR’s main binding site is a 67kDa membrane protein, and DGEA binds to the α2β1 integrin receptor. To further elucidate the role of peptide-cell interactions in regulating calcification, VICs were combined with function-blocking antibodies to integrin receptors prior to seeding onto ECM-coated plates. As shown in Figure 3A, blocking specific receptor-ligand interactions significantly altered the levels of calcification compared to unblocked cultures, confirming the hypothesis that adhesion receptor-mediated signals influence calcification.
Figure 3.
(A) Number of calcific nodules formed in VIC cultures on each ECM-based substrate upon neutralization of specific adhesion receptors. X-axis labels denote which receptor is being blocked for each condition. *p<0.0001 compared to corresponding unblocked condition, n = 8 samples per condition. (B) Vinculin expression on each of the conditions in (A), n=12 samples per condition.
When VICs on either FN or FB were treated with anti-α5β1, the increase in nodule number was quite dramatic (p<0.0001 for FN, p<0.005 for FB). These results imply that the RGD-α5β1 interaction is important in maintaining a non-calcifying VIC phenotype. Meanwhile, treatment of VICs on LN with 30 mM melibiose, a potent antagonist for laminin’s major receptor (a non-integrin 67 kDa membrane receptor) 27, the nodule number increased significantly and was almost as high as that found on TCPS. This result correlates well with the data presented in Figure 2. Namely, if YIGSR provides a signal that suppresses calcification, then blocking it should cause negative result, such as the sharp increase in nodule number in Figure 3A. Lastly, blocking VICs on Coll with anti-α2β1 did not significantly alter the formation of nodules.
VIC production of vinculin, a key participant in focal adhesions and subsequent signal transduction, was significantly elevated above levels found on TCPS (p<0.0001) in all of the conditions shown in Figure 3B except LN. More importantly, Figure 3B indicates that the integrin-blocking experiments did not influence vinculin production (p>0.05 for all blocking experiments compared to their untreated controls), which suggests that blocking the individual receptors was not damaging the ability of cells to anchor to their substrates. Receptor blocking treatments did not have a significant effect on cell density at the final Day 5 time point (data not shown).
Phenotypic changes in response to receptor inhibition
The expression of various markers of myofibroblastic and osteogenic activity were quantified in order to determine whether the calcification changes induced by culture on isolated peptides or treatment with receptor-blocking compounds were due to stimulation of myofibroblastic or osteogenic cell phenotypes. Previous publications describing VIC calcification have shown that calcifying VICs express markers related to myofibroblast activity (i.e., α-SMA, HSP47, MMP-1, MMP-13) and/or markers related to osteogenic differentiation (i.e., alkaline phosphatase, osteocalcin, CBFα-1) 4,11,28–31. Expression of transforming growth factor-beta1 (TGF-β1) can be indicative of either a myofibroblastic or osteoblastic phenotype.
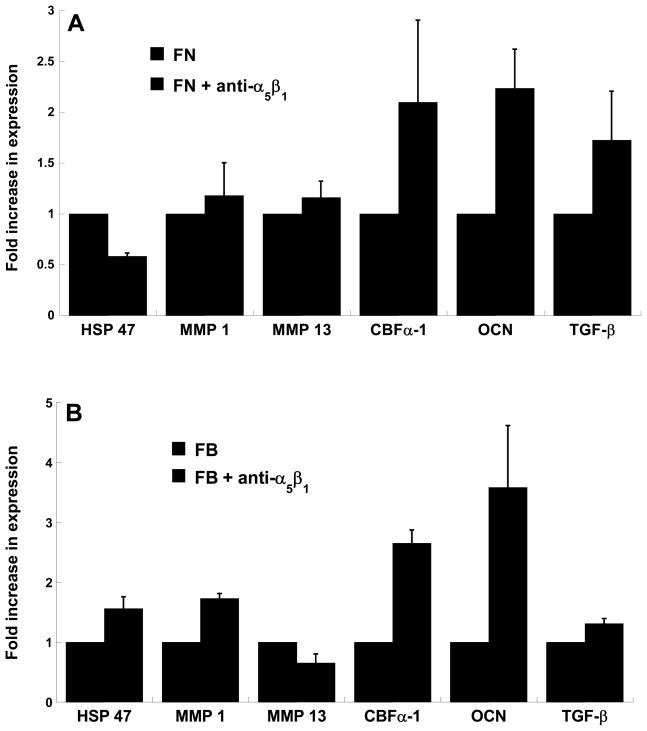
The observations detailed in Figure 3A – wherein blocking α5β1 on FN or FB surfaces resulted in increased nodule counts – were mirrored by changes in osteogenic gene expression by VICs, as measured by real-time PCR. Specifically, as shown in Figure 4, blocking α5β1 resulted in large increases in osteogenic activity, as indicated by increased expression levels of osteocalcin and the osteogenic transcription factor CBFα-1. Some of the markers for myofibroblast activity (HSP47, MMPs) were also increased by neutralizing the α5β1 receptor, but these increases were much more modest than those observed for the osteogenic markers.
Figure 4.
VICs were cultured on (A) fibronectin-coated or (B) fibrin-coated plates in the presence of absence of a neutralizing antibody to the α5β1 integrin receptor and then assayed on Day 5 for expression of HSP47, MMP1, MMP13, CBFα-1, OCN and TGF-β via qRT-PCR. n=3 samples per condition.
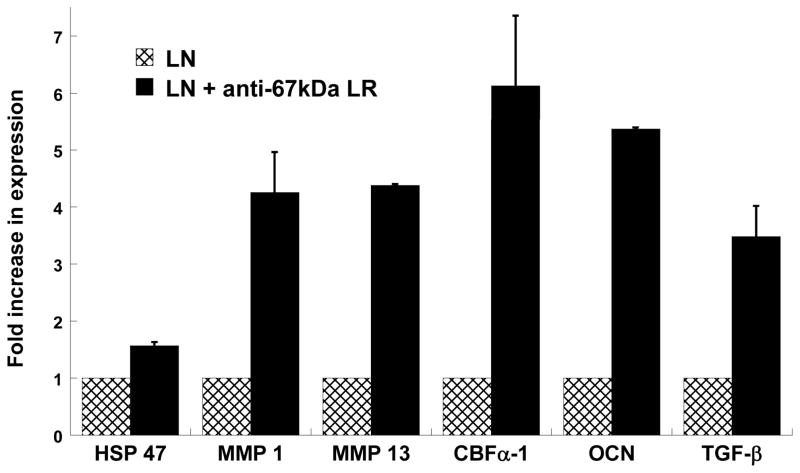
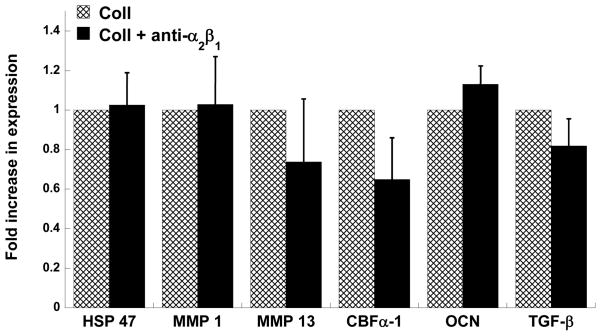
In the case of laminin, Figure 5 strongly reinforces earlier findings suggesting that disallowing YIGSR to bind to its 67 kDa non-integrin receptor dramatically increases calcification. The expression of all myofibroblast and osteoblast markers evaluated herein was greatly increased upon blocking the 67 kDa laminin receptor. The results for phenotypic expression by anti-α2β1-treated VICs on collagen were also highly consistent with the calcification results documented in Figure 3A. Namely, blocking the α2β1 integrin receptor of VICs cultured on collagen did not significantly alter the expression of any disease-related genes by the VICs (Figure 6).
Figure 5.
VICs were cultured on laminin-coated plates in the presence or absence of an antagonist for the 67 kDa membrane receptor and then assayed on Day 5 for expression of HSP47, MMP1, MMP13, CBFα-1, OCN and TGF-β via qRT-PCR. n=3 samples per condition.
Figure 6.
VICs were cultured on collagen-coated plates in the absence of presence of a neutralizing antibody to the α2β1 integrinreceptor and then assayed on Day 5 for expression of HSP47, MMP1, MMP13, CBFα-1, OCN and TGF-β via qRT-PCR. n=3 samples per condition.
Encapsulation of VICs in peptide-modified scaffolds
A preliminary experiment was performed to extend the study of VIC-peptide interactions to a 3-D environment. In this experiment, VICs were encapsulated within photocroslinked PEG-based hydrogels which were covalently modified with RGDS or YIGSR, or left unmodified. Because previous experiments failed to uncover any notable trends related to the DGEA peptide, its receptor (α2β1), and VIC calcification, the 3-D experiments were focused upon the more interesting cases of RGD and YIGSR. Figure 7 demonstrates that VIC interaction with either RGDS or YIGSR in this 3-D scaffold environment upregulated the expression of several disease-related markers. Consistent with 2-D calcification observations in earlier experiments, the greatest upregulation of disease markers was achieved via incorporation of RGDS into the scaffold structure. The expression of HSP47, OCN, and TGF-β1 by VICs in YIGSR-modified gels was significantly lower than that found in RGD-modified materials, while the unmodified hydrogels tended to be associated with the lowest disease marker expression overall.
Figure 7.
VICs were encapsulated within photocrosslinked PEG-based hydrogels covalently modified with RGDS, YIGSR, or no peptide. After five days of culture, VICs were assayed for expression of HSP47, CBFα-1, OCN and TGF-β via qRT-PCR. n=3 samples per condition. *p<0.05 compared to PEG-RGDS.
Discussion
This study explored the role of receptor-peptide interactions in the calcification of valvular interstitial cell cultures. This work was motivated by the observation that different levels of calcification are observed when VICs are cultured on different ECM coatings. Specifically, VICs exhibited very little calcification when cultured on fibronectin and collagen, yet showed severe calcification on either fibrin or uncoated tissue culture polystyrene (TCPS). We hypothesized that integrins and other receptors that recognize ECM-derived adhesion sequences might play an important role in mediating these signals. Tables 1 and 2 outline the different peptides and receptors explored in this study.
The integrin receptor family mediates various signaling transduction pathways and has been found to play a crucial role in cardiovascular disease 19,32. The integrin expression profile of VICs has only recently been identified 33 and relatively little remains known about VIC integrin expression in the context of valvular disease. The current investigation of receptor-peptide interactions yielded information about the role of these interactions in the in vitro calcification of VIC cultures; details of these trends are analyzed in subsequent paragraphs. For ease of explanation, the data presented in this paper have been summarized and condensed into Table 4.
Table 4.
Qualitative extent of calcification
| ECM | Peptide | Treatment |
|---|---|---|
| Laminin − |
YIGSR −− |
Block 67 kDa LR +++ |
| Collagen −−− |
DGEA −− |
Block α2β1 −−− |
| Fibronectin −−− |
RGD +++ |
Block α5β1 ++ |
| Fibrin ++ |
RGD +++ |
Block α5β1 +++ |
RGD is found in many ECM components, including all four ECM molecules tested in this paper, and this peptide has been used to promote mineralization in bone regeneration 34,35. When VICs were cultured on RGD-coated plates, the level of calcification significantly increased relative to other peptide conditions and the TCPS control. However, if RGD alone were the direct cause of this calcification, then the RGD-VIC interaction should have caused the same level of calcification across the four RGD-containing ECM components mentioned above, which was not the case. Thus, specific integrin (and non-integrin) receptors were targeted in function-blocking experiments in order to better understand the ECM-dependence of VIC calcification that was observed.
As noted in Table 1, RGD can bind to cells via multiple integrins, including α5β1 and αvβ3. The α5β1 integrin is strongly expressed by VICs 36, while less than 2% of VICs express αv, and β3 expression is similarly negligible 36. Thus, the experiments performed in this study focused on α5β1. In the present work, calcification of VICs cultured on FN and FB clearly increased upon blocking the α5β1 integrin. The consistent increase in calcification when blocking the α5β1 integrin implies that activation of this receptor is beneficial in reducing calcification. These findings are consistent with alterations in VIC phenotype revealed via real-time PCR, where neutralization of α5β1 resulted in strong upregulation of osteogenic markers. Our results complement those recently published by Fayet et al. 37, which demonstrated that VICs respond to wounding via upregulation of α5β1 expression. Together, these two studies suggest that blocking α5β1 may disrupt ‘normal’ VIC healing processes, leading to the calcification observed herein. Also, it appears that the calcification increases stimulated by the RGD peptide were not enacted via the α5β1 receptor; if this were the case, then blocking α5β1 should have decreased calcification, rather than increasing it.
The 67 kDa membrane receptor recognizes the YIGSR peptide sequence on laminin 15,16. In our work, laminin surfaces allowed a slightly higher level of calcification than collagen and fibronectin, but calcification on laminin was substantially lower than that obtained on TCPS or fibrin. It was found that the main laminin-specific adhesive peptide sequence, YIGSR, was generally not supportive of calcification. Blocking the 67 kDa laminin receptor confirmed that YIGSR binding via the 67 kDa receptor does have an attenuating effect on calcification. Upon blocking the 67 kDa laminin receptor, a significant increase in nodule formation occurred, together with a dramatic increase in expression of all disease markers that were measured. From these findings, it appears that the YIGSR-VIC interaction would normally signal the cell to not engage in calcification, and blocking this interaction results in increased calcification.
The two primary adhesion peptides in collagen are RGD and DGEA; RGD has been discussed above, while α2β1 is the major integrin that recognizes the DGEA peptide. According to Latif et al., VICs have relatively strong expression of both α2 and β1 integrin subunits 36. Throughout numerous experiments and treatments with various disease stimuli, VICs cultured on collagen appear to be fairly resistant to calcification 11. The peptide and receptor studies described herein were meant to help identify features or components of collagen that contribute to this anti-calcific property. Because of its ubiquity in ECM components that support a wide range of calcification in VIC cultures, it was doubtful that RGD-related events were responsible for collagen’s resistance to calcification. Thus, attention was turned toward DGEA and its receptor. However, neither culture on DGEA nor blocking the α2β1 integrin resulted in significant changes in calcific nodule formation. These data indicate that the ‘anti-calcific’ nature of collagen could not be undone by blocking VIC-ECM interactions with the α2β1 integrin receptor. Thus, unfortunately, the mechanism by which collagen inhibits VIC calcification continues to be poorly understood.
Vinculin expression by VICs was examined in order to confirm that the integrin-blocking conditions were not significantly altering the ability of VICs to adhere to and populate the cell culture surfaces. Vinculin is a common component of focal adhesions and an upstream initiator in signaling transduction related to actin polymerization 20. In this study, we found that vinculin expression by VICs cultured on the four ECM molecules tested in this study was not changed after administration of receptor-blocking compounds. Together with evidence that these treatments did not deleteriously affect cell number, it is likely that the changes in calcification observed upon administration of receptor-blocking compounds were not simply due to alterations in the fundamental ability of VICs to adhere to and survive on the surfaces.
Lastly, encapsulation and culture of VICs within 3-D PEG scaffolds yielded further evidence for peptide-controlled regulation of VIC dysfunction. PEG-based materials provide a controlled environment for studying cell-peptide interactions, as unmodified PEG hydrogels are generally considered bioinert and do not permit protein adsorption 38. Thus, the impact of specific peptide sequences upon cell behavior can be studied in isolation from other non-specific stimuli, allowing changes in cell behavior to be directly attributed to changes in peptide presentation. In the current work, varying levels of disease marker expression were achieved via the incorporation of different peptide sequences, with RGDS stimulating the greatest expression of HSP47, osteocalcin, and TGF-β1. These 3-D results not only confirm the findings obtained in 2-D studies, but they also have significant implications for the use of tissue engineering in re-creating both diseased and healthy valve tissues. As noted above, by changing which peptide was included in the scaffold, we may achieve varying degrees of VIC dysfunction. This ability would be tremendously beneficial and important in creating models of valvular disease in order to study disease mechanisms and potential treatments. Conversely, these 3-D data also provide us with information about what attributes or modifications the scaffold environment should or should not possess in order to generate healthy tissue-engineered valve replacements.
Conclusions
The ECM, by interacting with VICs through different adhesive receptors, plays an important role in maintaining a certain cell phenotype. Specifically, the current study demonstrated that disruption of VIC-ECM binding that occurs via either the α5β1 integrin or 67 kDa laminin receptor can dramatically increase calcification. Understanding the mechanism by which VIC behavior is regulated by the ECM environment may shed light on the etiology of valve calcification and potential targets for its prevention. Meanwhile, ECM-derived peptide sequences are also useful in biomaterials and tissue engineering applications in order to encourage cell adhesion or elicit specific cellular responses 38,39. Appropriate selection of peptides or ECM components may enable control over cell phenotype to provide a bioactive environment for valve tissue regeneration. Thus, the results presented herein have applications to both achieving a better understanding of valve calcification and designing optimized environments for heart valve tissue engineering.
Acknowledgments
Funding for this work was provided by the NIH (1R01-HL093281) and an NSF CAREER Award (CBET-0547374) to Kristyn S. Masters and a Herb Fellowship to Xiaoxiao Gu.
Abbreviations (in alphabetical order)
- α-SMA
alpha smooth muscle actin
- ALP
alkaline phosphatase
- CBFa-1
core binding factor-alpha1
- Coll
collagen
- ECM
extracellular matrix
- ERK
extracellular-regulated kinase
- FN
fibronectin
- FB
Fibrin
- MAPK
mitogen activated protein kinase
- MMP
matrix metalloproteinase
- OCN
osteocalcin
- TCPS
tissue culture polystyrene
- TGF-β1
transforming growth factor-beta1
- VIC
valvular interstitial cell
Cited references
- 1.Butcher JT, Nerem RM. Porcine aortic valve interstitial cells in three-dimensional culture: comparison of phenotype with aortic smooth muscle cells. J Heart Valve Dis. 2004;13(3):478. [PubMed] [Google Scholar]
- 2.Mulholland DL, Gotlieb AI. Cell biology of valvular interstitial cells. Can J Cardiol. 1996;12(3):231–6. [PubMed] [Google Scholar]
- 3.Clark-Greuel JN, Connolly JM, Sorichillo E, Narula NR, Rapoport HS, Mohler ER, 3rd, Gorman JH, 3rd, Gorman RC, Levy RJ. Transforming growth factor-beta1 mechanisms in aortic valve calcification: increased alkaline phosphatase and related events. Ann Thorac Surg. 2007;83(3):946–53. doi: 10.1016/j.athoracsur.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 4.Rabkin-Aikawa E, Farber M, Aikawa M, Schoen FJ. Dynamic and reversible changes of interstitial cell phenotype during remodeling of cardiac valves. J Heart Valve Dis. 2004;13(5):841–7. [PubMed] [Google Scholar]
- 5.Rajamannan NM, Subramaniam M, Rickard D, Stock SR, Donovan J, Springett M, Orszulak T, Fullerton DA, Tajik AJ, Bonow RO, et al. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation. 2003;107(17):2181–4. doi: 10.1161/01.CIR.0000070591.21548.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin CQ, Bissell MJ. Multi-faceted regulation of cell differentiation by extracellular matrix. FASEB J. 1993;7(9):737–43. doi: 10.1096/fasebj.7.9.8330681. [DOI] [PubMed] [Google Scholar]
- 7.Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285(5430):1028–32. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 8.Schneider GB, Zaharias R, Stanford C. Osteoblast integrin adhesion and signaling regulate mineralization. J Dent Res. 2001;80(6):1540–4. doi: 10.1177/00220345010800061201. [DOI] [PubMed] [Google Scholar]
- 9.Butcher JT, Nerem RM. Porcine aortic valve interstitial cells in three-dimensional culture: comparison of phenotype with aortic smooth muscle cells. J Heart Valve Dis. 2004;13(3):478–85. discussion 485–6. [PubMed] [Google Scholar]
- 10.Monzack EL, Gu X, Masters KS. Efficacy of Simvastatin Treatment of Valvular Interstitial Cells Varies With the Extracellular Environment. Arterioscler Thromb Vasc Biol. 2009;29(2):246–253. doi: 10.1161/ATVBAHA.108.179218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodriguez KJ, Masters KS. Regulation of valvular interstitial cell calcification by components of the extracellular matrix. J Biomed Mater Res A. doi: 10.1002/jbm.a.32187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruoslahti E, Pierschbacher MD. New perspectives in cell adhesion: RGD and integrins. Science. 1987;238(4826):491–7. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- 13.Yamada KM. Adhesive recognition sequences. J Biol Chem. 1991;266(20):12809–12. [PubMed] [Google Scholar]
- 14.Pierschbacher MD, Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984;309(5963):30–3. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- 15.Iwamoto Y, Robey FA, Graf J, Sasaki M, Kleinman HK, Yamada Y, Martin GR. YIGSR, a synthetic laminin pentapeptide, inhibits experimental metastasis formation. Science. 1987;238(4830):1132–4. doi: 10.1126/science.2961059. [DOI] [PubMed] [Google Scholar]
- 16.Massia SP, Rao SS, Hubbell JA. Covalently immobilized laminin peptide Tyr-Ile-Gly-Ser-Arg (YIGSR) supports cell spreading and co-localization of the 67-kilodalton laminin receptor with alpha-actinin and vinculin. J Biol Chem. 1993;268(11):8053–9. [PubMed] [Google Scholar]
- 17.Morais Freitas V, Nogueira da Gama de Souza L, Cyreno Oliveira E, Furuse C, Cavalcanti de Araujo V, Gastaldoni Jaeger R. Malignancy-related 67kDa laminin receptor in adenoid cystic carcinoma. Effect on migration and beta-catenin expression. Oral Oncol. 2007;43(10):987–98. doi: 10.1016/j.oraloncology.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Staatz WD, Fok KF, Zutter MM, Adams SP, Rodriguez BA, Santoro SA. Identification of a tetrapeptide recognition sequence for the alpha 2 beta 1 integrin in collagen. J Biol Chem. 1991;266(12):7363–7. [PubMed] [Google Scholar]
- 19.Brancaccio M, Hirsch E, Notte A, Selvetella G, Lembo G, Tarone G. Integrin signalling: the tug-of-war in heart hypertrophy. Cardiovasc Res. 2006;70(3):422–33. doi: 10.1016/j.cardiores.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 20.Giancotti FG, Tarone G. Positional control of cell fate through joint integrin/receptor protein kinase signaling. Annu Rev Cell Dev Biol. 2003;19:173–206. doi: 10.1146/annurev.cellbio.19.031103.133334. [DOI] [PubMed] [Google Scholar]
- 21.Lal H, Guleria RS, Foster DM, Lu G, Watson LE, Sanghi S, Smith M, Dostal DE. Integrins: novel therapeutic targets for cardiovascular diseases. Cardiovasc Hematol Agents Med Chem. 2007;5(2):109–32. doi: 10.2174/187152507780363223. [DOI] [PubMed] [Google Scholar]
- 22.Mousa SA. Vitronectin receptors in vascular disorders. Curr Opin Investig Drugs. 2002;3(8):1191–5. [PubMed] [Google Scholar]
- 23.Boateng SY, Lateef SS, Mosley W, Hartman TJ, Hanley L, Russell B. RGD and YIGSR synthetic peptides facilitate cellular adhesion identical to that of laminin and fibronectin but alter the physiology of neonatal cardiac myocytes. Am J Physiol Cell Physiol. 2005;288(1):C30–8. doi: 10.1152/ajpcell.00199.2004. [DOI] [PubMed] [Google Scholar]
- 24.Johnson CM, Hanson MN, Helgeson SC. Porcine cardiac valvular subendothelial cells in culture: cell isolation and growth characteristics. J Mol Cell Cardiol. 1987;19(12):1185–93. doi: 10.1016/s0022-2828(87)80529-1. [DOI] [PubMed] [Google Scholar]
- 25.Mann BK, West JL. Cell adhesion peptides alter smooth muscle cell adhesion, proliferation, migration, and matrix protein synthesis on modified surfaces and in polymer scaffolds. J Biomed Mater Res. 2002;60(1):86–93. doi: 10.1002/jbm.10042. [DOI] [PubMed] [Google Scholar]
- 26.Salinas CN, Cole BB, Kasko AM, Anseth KS. Chondrogenic differentiation potential of human mesenchymal stem cells photoencapsulated within poly(ethylene glycol)-arginine-glycine-aspartic acid-serine thiol-methacrylate mixed-mode networks. Tissue Eng. 2007;13(5):1025–34. doi: 10.1089/ten.2006.0126. [DOI] [PubMed] [Google Scholar]
- 27.Peterszegi G, Texier S, Robert L. Cell death by overload of the elastin-laminin receptor on human activated lymphocytes: protection by lactose and melibiose. Eur J Clin Invest. 1999;29(2):166–72. doi: 10.1046/j.1365-2362.1999.00423.x. [DOI] [PubMed] [Google Scholar]
- 28.Liu AC, Joag VR, Gotlieb AI. The emerging role of valve interstitial cell phenotypes in regulating heart valve pathobiology. Am J Pathol. 2007;171(5):1407–18. doi: 10.2353/ajpath.2007.070251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohler ER., 3rd Mechanisms of aortic valve calcification. Am J Cardiol. 2004;94(11):1396–402. A6. doi: 10.1016/j.amjcard.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 30.Rabkin E, Aikawa M, Stone JR, Fukumoto Y, Libby P, Schoen FJ. Activated interstitial myofibroblasts express catabolic enzymes and mediate matrix remodeling in myxomatous heart valves. Circulation. 2001;104(21):2525. doi: 10.1161/hc4601.099489. [DOI] [PubMed] [Google Scholar]
- 31.Taylor PM, Batten P, Brand NJ, Thomas PS, Yacoub MH. The cardiac valve interstitial cell. Int J Biochem Cell Biol. 2003;35(2):113–8. doi: 10.1016/s1357-2725(02)00100-0. [DOI] [PubMed] [Google Scholar]
- 32.Giancotti FG. Complexity and specificity of integrin signalling. Nat Cell Biol. 2000;2(1):E13–4. doi: 10.1038/71397. [DOI] [PubMed] [Google Scholar]
- 33.Latif N, Sarathchandra P, Taylor PM, Antoniw J, Yacoub MH. Localization and pattern of expression of extracellular matrix components in human heart valves. J Heart Valve Dis. 2005;14(2):218–27. [PubMed] [Google Scholar]
- 34.Ho MH, Hou LT, Tu CY, Hsieh HJ, Lai JY, Chen WJ, Wang DM. Promotion of cell affinity of porous PLLA scaffolds by immobilization of RGD peptides via plasma treatment. Macromol Biosci. 2006;6(1):90–8. doi: 10.1002/mabi.200500130. [DOI] [PubMed] [Google Scholar]
- 35.Ruoslahti E. RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- 36.Latif N, Sarathchandra P, Taylor PM, Antoniw J, Yacoub MH. Molecules mediating cell-ECM and cell-cell communication in human heart valves. Cell Biochem Biophys. 2005;43(2):275–87. doi: 10.1385/CBB:43:2:275. [DOI] [PubMed] [Google Scholar]
- 37.Fayet C, Bendeck MP, Gotlieb AI. Cardiac valve interstitial cells secrete fibronectin and form fibrillar adhesions in response to injury. Cardiovasc Pathol. 2007;16(4):203–11. doi: 10.1016/j.carpath.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 38.Hern DL, Hubbell JA. Incorporation of adhesion peptides into nonadhesive hydrogels useful for tissue resurfacing. J Biomed Mater Res. 1998;39(2):266–76. doi: 10.1002/(sici)1097-4636(199802)39:2<266::aid-jbm14>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 39.Burdick JA, Anseth KS. Photoencapsulation of osteoblasts in injectable RGD-modified PEG hydrogels for bone tissue engineering. Biomaterials. 2002;23(22):4315. doi: 10.1016/s0142-9612(02)00176-x. [DOI] [PubMed] [Google Scholar]
- 40.Mizuno M, Fujisawa R, Kuboki Y. Type I collagen-induced osteoblastic differentiation of bone-marrow cells mediated by collagen-alpha2beta1 integrin interaction. J Cell Physiol. 2000;184(2):207–13. doi: 10.1002/1097-4652(200008)184:2<207::AID-JCP8>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]