Abstract
Endothelin-1 (ET-1) produced by various cancers is known to be responsible for inducing pain. While ET-1 binding to ETAR on peripheral nerves clearly mediates nociception, effects from binding to ETBR are less clear. The present study assessed the effects of ETBR activation and the role of endogenous opioid analgesia in carcinoma pain using an orthotopic cancer pain mouse model. mRNA expression analysis showed that ET-1 was nearly doubled while ETBR was significantly down-regulated in a human oral SCC cell line compared to normal oral keratinocytes (NOK). Squamous cell carcinoma (SCC) cell culture treated with an ETBR agonist (10−4M, 10−5 M, and 10−6 M BQ-3020) significantly increased production of β-endorphin without any effects on leu-enkephalin or dynorphin. Cancer inoculated in the hind paw of athymic mice with SCC induced significant pain, as indicated by reduction of paw withdrawal thresholds in response to mechanical stimulation, compared to sham-injected and NOK-injected groups. Intratumor administration of 3 mg/kg BQ-3020 attenuated cancer pain by approximately 50% up to 3 hours post-injection compared to PBS-vehicle and contralateral injection, while intratumor ETBR antagonist BQ-788 treatment (100 and 300 μg/kg and 3 mg/kg) had no effects. Local naloxone methiodide (500 μg/kg) or selective μ-opioid receptor antagonist (CTOP, 500 μg/kg) injection reversed ETBR agonist-induced antinociception in cancer animals. We propose that these results demonstrate that peripheral ETBR agonism attenuates carcinoma pain by modulating β-endorphins released from the SCC to act on peripheral opioid receptors found in the cancer microenvironment.
Keywords: endothelin, opioids, cancer, pain, cancer mouse model, oral cancer
Introduction
Pain is a frequent and disabling consequence of many types of cancers in humans. It is a primary determinant of a poor quality of life, especially in head and neck cancer patients. [11] Eighty-five percent of cancer patients experience severe pain in their final days of life [60] and up to ninety percent of those in terminal stages must cope with opiate-resistant pain related to tumor progression. [39,40,52,55] The etiology of cancer pain is unknown, but may involve mediator-dependent signaling by cancer cells to primary afferent sensory neurons in the cancer microenvironment. One candidate mediator is endothelin-1 (ET-1) [48,58,49], a vasoactive 21-amino acid peptide first isolated from porcine aortic endothelial cells. [66] ET-1 is a member of the endothelin family, which includes ET-2, ET-3, and the sarafotoxins [31,35], and is synthesized from its precursor, pre-pro ET-1, through a proteolytic cleavage by endothelin converting enzyme (ECE). [27] The physiological actions of endothelin are mediated by two G-protein coupled receptors (GPCRs), endothelin A (ETAR) and endothelin B (ETBR), which have been successfully cloned in mammals. [3,56]
ET-1 is synthesized by neurons and glial cells in the central and peripheral nervous systems [25,36] and may serve as an algogen to induce nociception. Studies in animals have demonstrated that injection of ET-1 evokes tactile allodynia [6], hyperalgesia from thermal [18] and mechanical [21] stimulation, and overt inflammatory nociception. [21,19,51] ET-1 also causes pain in humans through activation and sensitization of C nociceptors. [28,42] In addition to mediating nociception in noncancerous conditions, ET-1 also plays a role in cancer pain. ET-1 is highly expressed in different cancers, including bone [63,48], lung [1], colorectal [4], breast [2], prostate [43] and oral squamous cell carcinoma. [50] Furthermore, it has been demonstrated that treatment with selective ETAR antagonists attenuate ET-1 mediated pain associated with cancer [16,18,9,48,69,58,24,49], specifically by antagonizing ETARs present on primary afferent nociceptors. [17,26]
Whereas the role of ETAR in ET-1-induced pain is well characterized, the importance of ETBR for this pain is less clear. In rats, ETBR is believed to mediate mechanical hypernociception via cAMP formation and activation of a PKC-dependent phosphorylation cascade. [15] ETBR activation also elicits orofacial mechanical allodynia in rats with trigeminal neuralgia. [13] On the other hand, Khodorova and colleagues have demonstrated that selective activation of ETBR on normal skin keratinocytes stimulates release of β-endorphins [33] and further showed that ETBR agonism in rats inhibits ET-1-induced nociception in a naloxone-sensitive manner [32] involving an endogenous opioid-mediated analgesic cascade. Squamous cell carcinoma consists of malignant keratinocytes. Theoretically, the generation of peripheral opioids by carcinoma within the cancer microenvironment immediately adjacent to the sensitized afferent nociceptors is the ideal targeted approach for abrogating cancer pain. Since activation of ETBR on squamous cells modulates pain through endogenous opioid analgesia, we hypothesized that ETBR activation might modulate squamous cell carcinoma-induced pain. Therefore, in the present study, we investigated the effects of ETBR agonism on cancer-induced nociception, and whether these effects are dependent on opioid receptor functions.
Methods
2.1 Cell culture
HSC-3, an oral SCC cell line (ATCC, Manassas, VA) derived from a human tongue SCC, was cultivated in Dulbecco’s Modification of Eagle’s Medium (DMEM) with 4.5 g/L glucose, L-glutamine and sodium pyruvate, supplemented with 10% fetal bovine serum, 25 μg/mL fungizone, 100 μg/mL streptomycin sulfate, and 100 units/mL penicillin G. Primary normal oral keratinocytes (NOK) were harvested from normal gingival tissues using a modified technique described by Hybbinette et al. [30] Collection of oral epithelium was approved by the UCSF Committee on Human Research and consent was obtained from patients. Tissues were washed in 70% sterile ethanol, cut into 5 mm square sections, and incubated in 0.25 mg/mL dispase I at 37°C for approximately 3 hrs. The separated epidermis was minced and transferred to 0.25% trypsin for 5 min incubation at 37°C, then stopped with 0.25–0.5 mg/mL soybean trypsin inhibitor (Invitrogen, Carlsbad, CA) and centrifuged at 1300 rpm for 8 min. The sedimented cells were resuspended in Defined Keratinocyte Serum-free media (Invitrogen, Carlsbad, CA) supplemented with 50 μg/mL gentamicin and 25 μg/mL fungizone and cultivated at 37°C in 5% CO2.
2.2 Real-time quantitative RT-PCR in cell culture
Because ETBRs have not been previously quantified in oral SCC we used RT-PCR to quantify and compare transcript expression levels of ET-1 and ETBR in oral SCC and normal oral keratinocytes. ET-1 and ETBR mRNA expression levels were measured in the HSC-3 cell line relative to the normal oral keratinocyte control (NOK). 104 cells were cultivated in 300 μL of DMEM supplemented with 10% fetal bovine serum, 25 μg/mL fungizone, 100 μg/mL streptomycin sulfate, and 100 units/mL penicillin G on 96-well cell culture plates until 75%–80% confluent. Cells were then harvested and lysed for quantitative PCR analysis using the TaqMan® Gene Expression Cells-to-CT Kit (Applied Biosystems/Ambion, Austin, TX), performed at the Genome Analysis Core (University of California, San Francisco). Samples were run on an ABI 7700 Prism (PE Biosystems, Foster City, CA). Relative expressions of ET-1 and ETBR mRNA were calculated using the comparative Ct method as previously described. [14,58] Analysis was carried out using the software supplied with the ABI 7700 Prism. Overexpression was defined as expression >2.0 relative to the reference gene β-N-acetyl-glucosaminidase (β-Gus).
2.3 Immunofluorescence
Immunofluorescence was performed to validate the presence of ETBR in oral SCC. HSC-3 cells were seeded onto glass cover slips in 6-well plates overnight at 37°C with 5% CO2 in supplemented DMEM (see above). Cells were washed twice with PBS, fixed in ice-cold acetone for 5 min at room temperature (RT), permeabilized with 0.2% TritonX-100 for 15 min, washed three times with PBS then nonspecifically blocked with 3% bovine serum albumin (BSA) for 2 hrs. Incubation with primary rabbit polyclonal ETBR antibody (Abcam Inc., Cambridge, MA) diluted 1:100 in 3% BSA was performed at RT for 2 h followed by incubation with donkey anti-rabbit Texas Red-conjugated IgG secondary antibody (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) diluted 1:500 in 3% BSA for 1 h at RT. Nuclei were stained with 1:500 Hoechst stain (Invitrogen, Carlsbad, CA). The cover slips were washed and mounted on slides in Gel/Mount™ mounting medium (Biomeda Corp., Foster City, CA) and visualized on a Nikon Eclipse E600 microscope using epifluorescence. All images were captured and analyzed with RT Spot Software (Diagnostics Instruments, Inc., Sterling Heights, MI). Omission of the primary antibody was used as controls for the immunofluorescence.
2.4 ELISA measurement of endogenous opioids
To evaluate the effect of ETBR agonism on opioid production and secretion in oral SCC, we used enzyme-linked immunosorbent assay (ELISA) for opioid peptide measurement. 105 HSC-3 cells were seeded onto 6-well tissue culture plates with 3 mL of DMEM supplemented with 10% fetal bovine serum, 25 μg/mL fungizone, 100 μg/mL streptomycin sulfate, and 100 units/mL penicillin G. HSC-3 cells were cultured for 24 h until the wells reached 70–80% confluence. Each well was washed once with PBS then incubated for 12 h in 1 mL of one of the following media: 1) DMEM alone, 2) DMEM with 10 ng/mL synthetic beta-endorphin (Sigma, St. Louis, MO) or leu-enkephalin (Phoenix Pharmaceuticals, Burlingame, CA), or 3) DMEM with one of ten-fold concentrations of BQ-3020 (10−4M to 10−9M, American Peptide Co., Sunnyvale, CA). Culture media were collected and treated with 1× HALT Protease Inhibitor Cocktail (Pierce, Rockford, IL) before performing ELISA to detect levels of β-endorphin (MD Biosciences, St. Paul, MN), leucine-enkephalin (leu-enk) and dynorphin (Phoenix Pharmaceuticals, Burlingame, CA). Opioid concentrations were calculated based on a calibration curve, with 55% supernatant recovery for β-endorphin and 12% recovery for leu-enk. Dynorphin concentrations were calculated with a 1:1 recovery ratio since no synthetic human dynorphin was readily available for recovery test.
2.5 SCC paw model
The cancer pain mouse model was produced as previously described. [58,49] Experiments were performed on 4 weeks-old adult female Foxn1nu, athymic, immunocompromised mice weighing 16–20 g at the time of SCC inoculation. Mice were housed in a temperature-controlled room on a 12:12 h light cycle (0700–1900 h light), with ad libitum access to food and water; estrous cycles were not monitored. All procedures were approved by the University of California, San Francisco Committee on Animal Research. Researchers were trained under the Animal Welfare Assurance Program. Mice were divided into three experimental groups: those receiving an injection of squamous carcinoma cells (SCC group), those receiving an injection of normal oral keratinocytes (negative control), and those receiving an injection of DMEM (sham operated). All injections were into the right hind paw. All groups were anesthetized by inhalation with 1–3% isofluorane throughout the inoculation procedure. Cell injections consisted of either 106 HSC-3 cells (SCC group) or NOK cells (negative control) in a vehicle consisting of 50 μL of DMEM into the plantar surface of the right hind paw. The sham-operated group received 50 μL of DMEM alone.
2.6 Behavioral testing for the SCC paw model
Behavioral testing was performed as described previously. [58] Testing was performed between 14:00 and 16:00 h (during the light phase). Mice were placed in a plastic cage with wire mesh floor which allowed access to the paws. Quantitative assay guidelines were used similar to a previously described technique. [11] 15 min were allowed for acclimation prior to testing. The probe was applied to the mid-plantar right hind paw, or the tumor-front on the hind paw toward the later stages of tumor development. Paw withdrawal thresholds were determined in response to pressure from an electronic von Frey anesthesiometer (2390 series, IITC Instruments, Woodland Hills, CA). The amount of pressure (g) needed to produce a paw withdrawal response was measured three times on each paw separated by 3 minute intervals to allow resolution of previous stimuli. The results of three tests were averaged for each paw for that day. The SCC, negative control and sham-injected groups were tested under this paradigm at 4, 7, 9, 11, 14, 16, and 18 days post-inoculation of SCC, NOK or vehicle.
2.7 Drug administration and pain behavioral testing
To determine whether agonism or antagonism of ETBR attenuates cancer-induced nociception, groups of tumor-inoculated animals were tested with either ETBR agonist (BQ-3020) or ETBR antagonist (BQ-788). Then to evaluate whether ETBR agonist-induced attenuation of carcinoma pain involves endogenous opioids, opioid receptor (OR) antagonists were administered following BQ-3020 injection. Drug testing was performed on days 18–25 following inoculation of oral SCC into the hind paw. Drugs were dissolved in a final volume of 20 μL PBS and injected subcutaneously into the mid-plantar hind paw at the site of greatest tumor development with a 30-gauge beveled needle. For single drug testing, either BQ-3020 (3mg/kg) or BQ-788 (100–3000 μg/kg) was injected after a 15 min baseline period and paw withdrawal thresholds were recorded at 5, 10, 15, 30, 45, 60, 90, 120, 180 min, and 24 h post-injection. For dual drug testing, BQ-3020 (3 mg/kg) was injected after a 15 min pre-injection reading and paw withdrawal thresholds were recorded at post-injection times 5, 10, 15, 30, 45, 60, and 90 min. Following the 90 min post-ETBR agonist reading, a second drug (PBS or OR antagonist) was injected and paw withdrawal testing was performed at 5, 10, 15, 30, 45, 60, 90, 120, 180 min, and 24 h. Mice groups were injected with one of the following drug combinations: 1) BQ-3020 (3 mg/kg) followed by PBS control, 2) BQ-3020 followed by nonspecific OR antagonist (500 μg/kg naloxone methiodide, Sigma, St. Louis, MO), 3) BQ-3020 followed by selective μ-OR antagonist (500 μg/kg Cys2-Tyr3-Orn5-Pen7-amide [CTOP], Sigma, St. Louis, MO), or 4) BQ-3020 followed by selective δ-OR antagonist (11 mg/kg naltrindole [NTI], Sigma, St. Louis, MO). κ-OR antagonist (2.5 mg/kg nor-binaltorphimine [nor-BNI], Sigma, St. Louis, MO) was injected 12 hr prior to injection with PBS control or BQ-3020. The investigators performing the injections and behavioral testing were blinded to the drugs administered.
2.8 Statistical Analysis
A one-way Analysis of Variance (ANOVA) with a Tukey Multiple Comparisons post-test was used to compare the withdrawal threshold of the cancer inoculated mice and sham over 18 days. The same test was used to compare the percent change in withdrawal threshold of the SCC inoculated mice before and after drug or control injection. ELISA protein measurements and mRNA gene expressions were also analyzed with one-way ANOVA. When an Equal Variance Test failed, one-way ANOVA with Dunn’s Method for Multiple Comparison post-test was performed. For all tests a p value of less than 0.05 was considered significant. Statistical analysis was performed using SigmaPlot for Windows (Version 11.0).
Results
3.1 ET-1 and ETBR expression
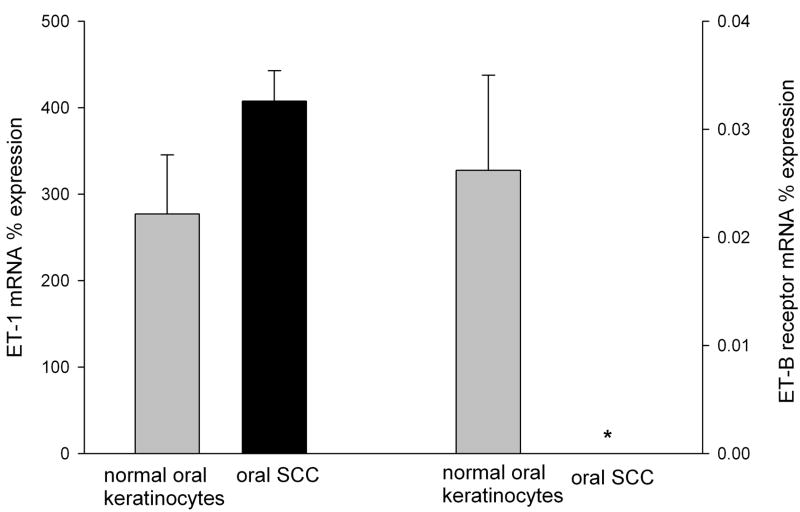
ET-1 and ETBR mRNA expression levels in oral SCC were compared to NOK controls (Fig. 1a) and ETBR localization was visualized in oral SCC with immunofluorescence (Fig. 1b). Expression levels were normalized to the expression of the housekeeping gene β-Gus. ET-1 mRNA expression in SCC cell culture (407.69 ± 68.02%) was nearly two-fold higher than NOK control (277.28 ± 35.09%). ETBR mRNA expression in SCC (0%) was significantly lower (p = 0.04, Tukey test) than in NOK control (0.026 ± 0.009%). Although normalized ETBR mRNA expression level was not increased, immunofluorescent labeling revealed ETBR expression on both cell membranes and dispersed in the cytoplasm of SCC cells (Fig. 1b).
Figure 1.
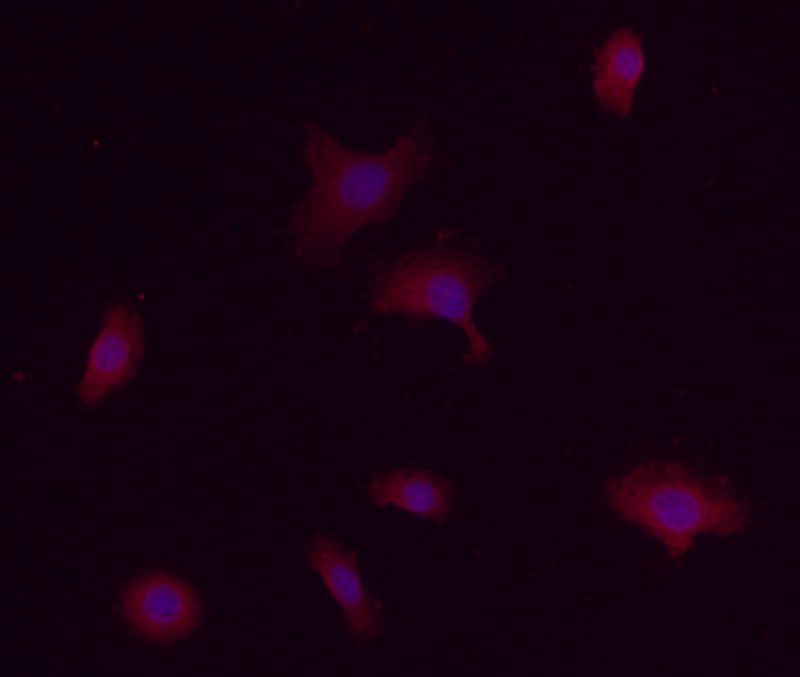
(a) Mean ET-1 and ETBR mRNA relative percent expression in NOK (n = 3) and oral SCC (n = 3) cell cultures normalized to expression of β-GUS mRNA levels. ETBR mRNA expression was undetectable in oral SCC compared to NOK control (p = 0.040, indicated with *). (b) Immunofluorescence staining of ETBR in oral SCC cell line (HSC-3). Rabbit polyclonal antibody against ETBR (1:100, Texas Red) shows dispersed staining in the cells. Nuclei are stained with Hoechst dye (1:500, blue).
3.2 ELISA measurement of endogenous opioids
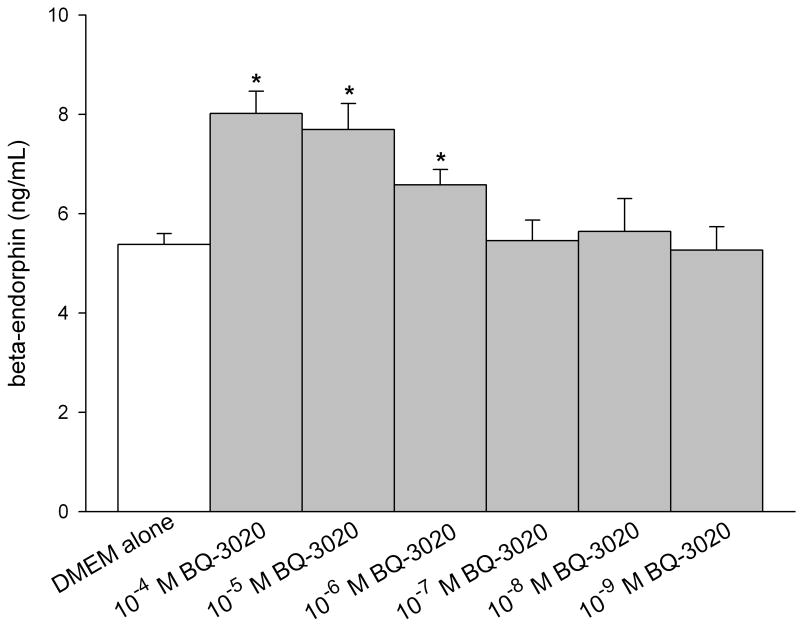
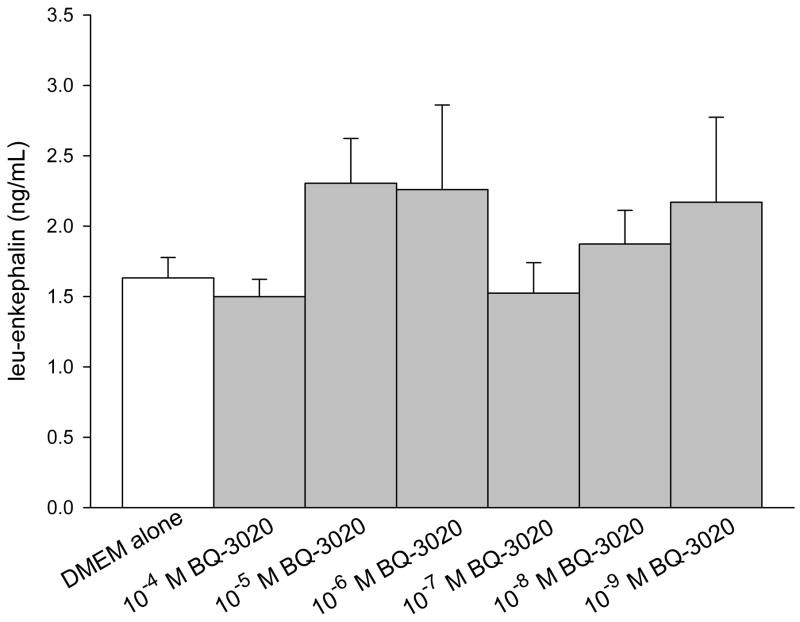
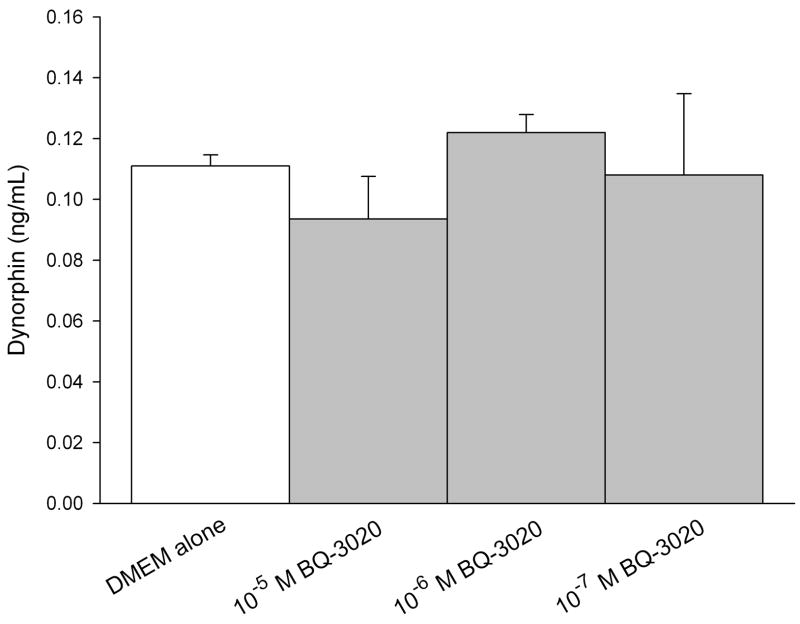
ELISA was performed on conditioned media of SCC cells to quantify production of endogenous opioids (β-endorphin, leu-enkephalin, and dynorphin). The concentration was calculated from a standard curve using a sigmoid logistics curve fitting program (Bio-Rad Laboratories, Inc., Hercules, CA) as appropriate for the opioid ELISA kits, followed by adjustments for percent recovery from synthetic peptide positive control treatments in culture. SCC cell culture treated with ETBR agonists significantly increased β-endorphin production compared to untreated SCC cultures at 5.38 ± 0.22 ng/mL (Fig. 2a). Treatment with 10−4 M BQ-3020 produced 8.02 ± 0.45 ng/mL of β-endorphin (p = 0.002, Tukey test); 10−5 M BQ-3020 produced 7.69 ± 0.53 ng/mL of β-endorphin (p = 0.007, Tukey test); and 10−6 M BQ-3020 produced 6.58 ± 0.31 ng/mL of β-endorphin (p = 0.019, Tukey test). ETBR agonist treatment had no effect on production of either leu-enkephalin or dynorphin (Fig. 2b–c).
Figure 2.
ELISA quantification of endogenous opioid concentrations in oral SCC conditioned media under different ETBR agonist (BQ-3020) treatment. (a) β-endorphin level is significantly increased when treated with 10−4 M, 10−5 M, and 10−6 M BQ-3020 compared to DMEM control (p = 0.002, 0.007, and 0.019, respectively), as indicated by an *. (b–c) Both leu-enkephalin and dynorphin levels are not affected by BQ-3020 incubation compared to DMEM control.
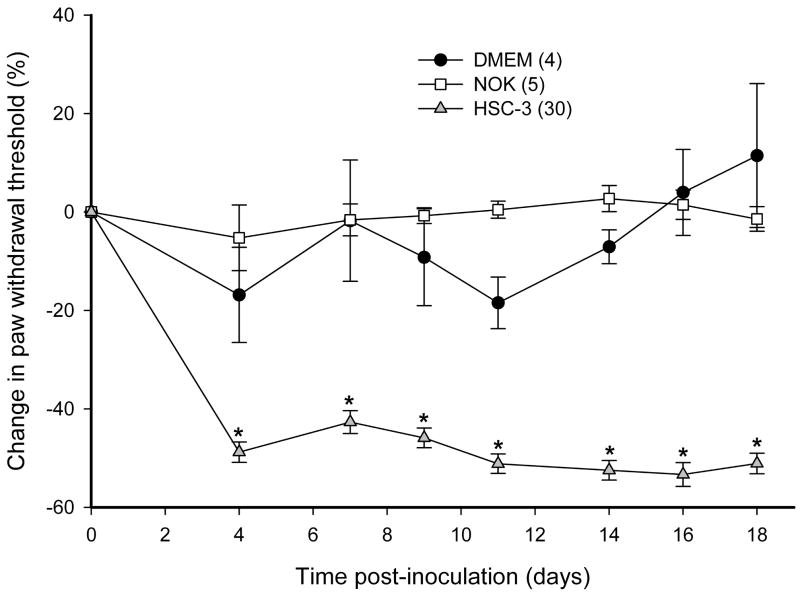
3.3 ETBR agonist/antagonist effects using the SCC mouse model
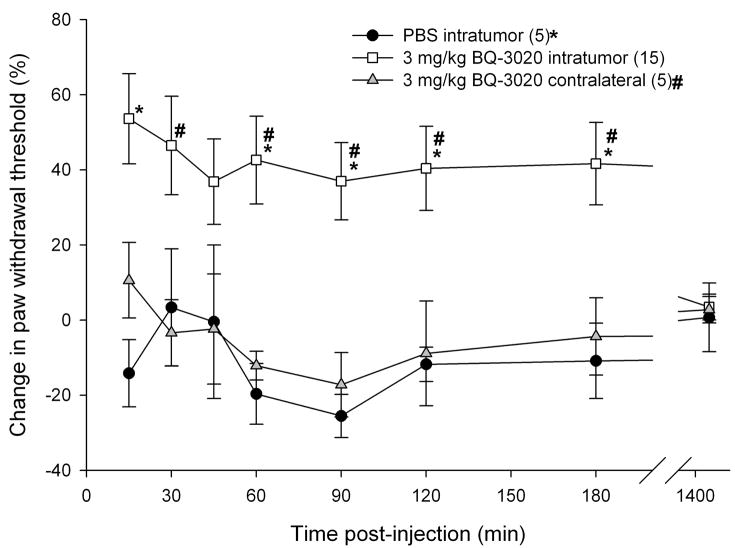
To determine whether SCC inoculation induced mechanical hyperalgesia in the mouse cancer model, paw withdrawal thresholds for the SCC (30 mice), NOK (5 mice), and DMEM sham (4 mice) groups were compared. Paw withdrawal thresholds for SCC animals (Fig. 3a) significantly dropped starting on post-inoculation day (PID) 4 and lasted up to PID18 as compared to both NOK and the sham-injected groups (p < 0.05, Tukey test or Dunn’s Method as appropriate). Either ETBR agonists or antagonists were administered to determine whether agonism or antagonism of the receptor affects carcinoma-induced nociception in the SCC mouse model. Intratumor injection of 3 mg/kg BQ-3020, an ETBR agonist, significantly increased paw withdrawal thresholds at 15 min post-injection and lasted up to 3 h compared to PBS-vehicle and contralateral drug injection (p < 0.05, Tukey test or Dunn’s Method as appropriate), indicating an attenuation of carcinoma-induced nociception (Fig. 3b). Intratumor injection with ETBR antagonist BQ-788 (100–3000 μg/kg) had no effect on paw withdrawal thresholds (Fig. 3c).
Figure 3.
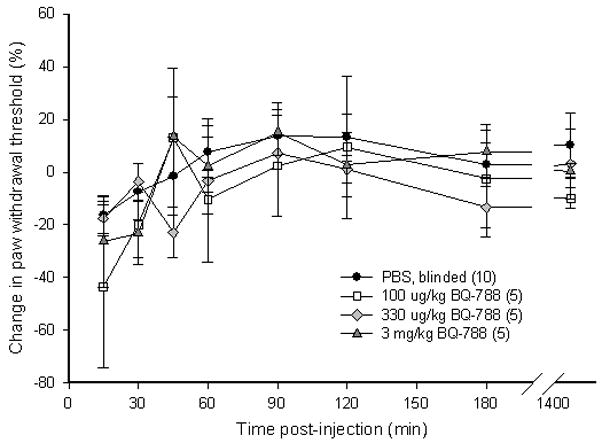
(a) Mean percent change in paw withdrawal threshold (PWT) of the right hind paws of the HSC-3 group (n = 30), NOK group (n = 5), and DMEM sham-injected group (n = 4). Mean PWT in the cancer group significantly decreases starting at PID4 and maintains throughout until PID18 (p < 0.05) compared to both NOK and DMEM groups. The NOK group is not significantly different from the DMEM group. (b) Mean PWT of cancer animals injected with PBS vehicle (n = 5) or BQ-3020 (3 mg/kg, n = 15) in the cancer, or BQ-3020 (3 mg/kg, n = 5) in the contralateral paw. Overall, BQ-3020 injection in the cancer significantly increases PWT compared to both PBS and contralateral BQ-3020 administrations (p < 0.05) for a minimum of 3 hrs, indicating the presence of antinociception with localized ETBR agonism. (c) Mean PWT of cancer animals injected with PBS vehicle (n = 10) or BQ-788 (100 μg, 330 μg, or 3 mg/kg, n = 5). BQ-788 has no significant effect on mean PWT. [* and # denote statistical significance compared to PBS vehicle and contralateral BQ-3020, respectively]
3.4 Non-specific opioid receptor antagonist dosing effect on carcinoma pain
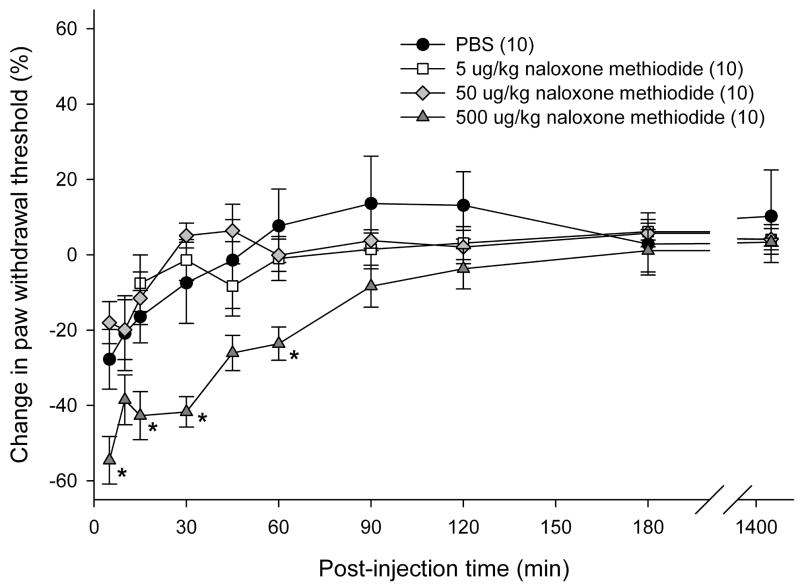
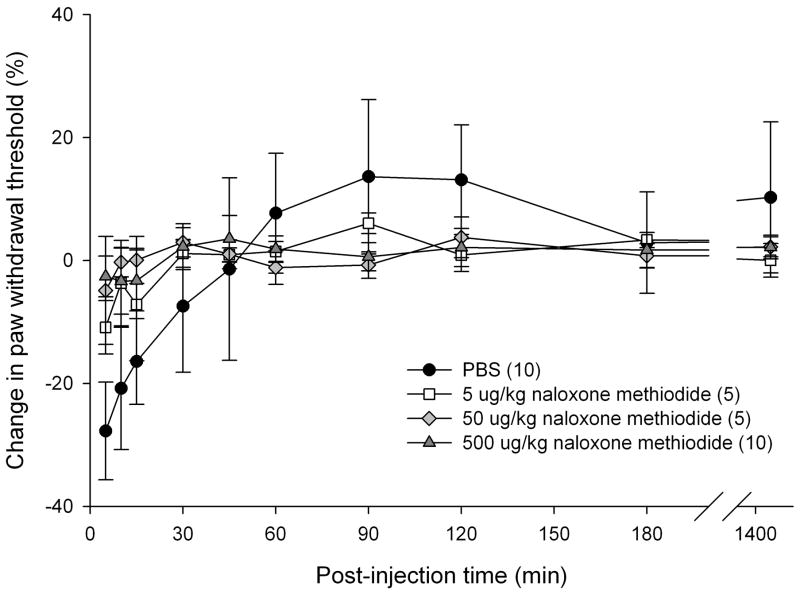
Since SCC is capable of producing endogenous opioids upon ETBR agonist treatment in vitro, the contribution of peripheral opioid receptors (OR) to attenuation of cancer pain was investigated in vivo. We previously reported that SCC produce elevated ET-1 peptides [50], which indicate that inoculated SCC tumors are capable of activating ETBR in cancer animals without administration of exogenous ETBR agonists. Relying on endogenous ETBR activation levels, various doses of non-specific peripheral OR antagonist (naloxone methiodide; 5, 50, and 500 μg/kg) were injected either intratumor or in the contralateral paw to evaluate the dosing effect of OR antagonist on cancer-induced nociception. 500 μg/kg naloxone methiodide injected intratumor most effectively decreased paw withdrawal thresholds in cancer animals for up to 60 min post-injection (p < 0.05, Tukey test) compared to PBS-vehicle and lower doses of naloxone methiodide (Fig. 4a). All doses of naloxone methiodide treatment in the contralateral paw had no effect on paw withdrawal threshold (Fig. 4b).
Figure 4.
Mean percent change in PWT of cancer-inoculated animals injected with a ten-fold magnitude range of nonspecific opioid receptor (OR) antagonist, naloxone methiodide, either in the cancer or into the contralateral paw. (a) 500 μg/kg naloxone methiodide (n = 10) administered into the cancer paw of animals significantly reduced mean PWT compared to PBS (n = 10) and lower concentrations at 5 and 50 μg/kg (n = 10, each group) at t = 15 min after drug injection, lasting up to 1 hr post-injection (p < 0.05). [* denotes statistical significance] (b) 5 μg/kg (n = 5), 50 μ/kg (n = 5), and 500 μg/kg (n = 10) of naloxone methiodide administered into the contralateral hind paw has no effect on mean PWT compared to PBS (n = 10) injected into the cancer paw of animals
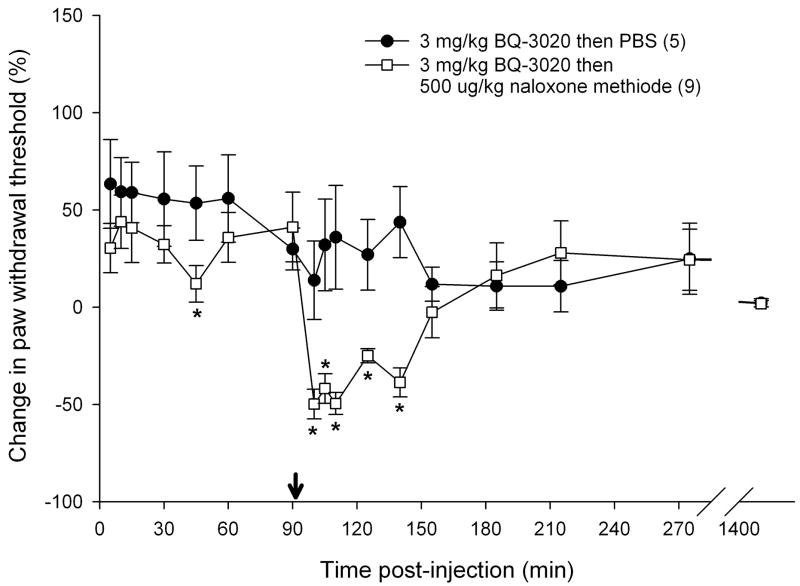
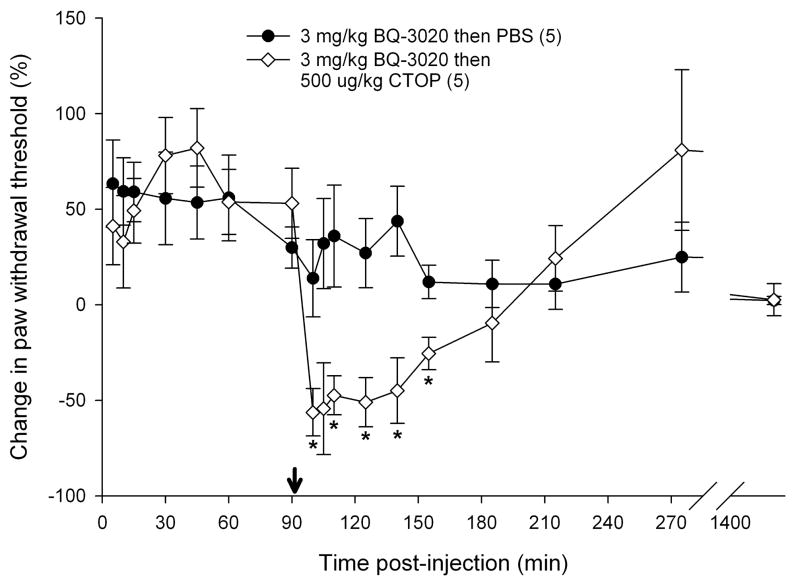
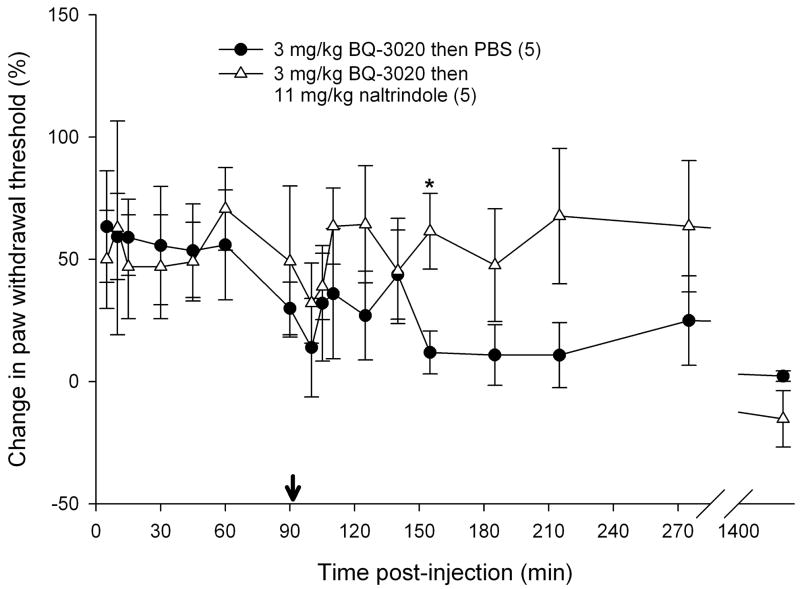
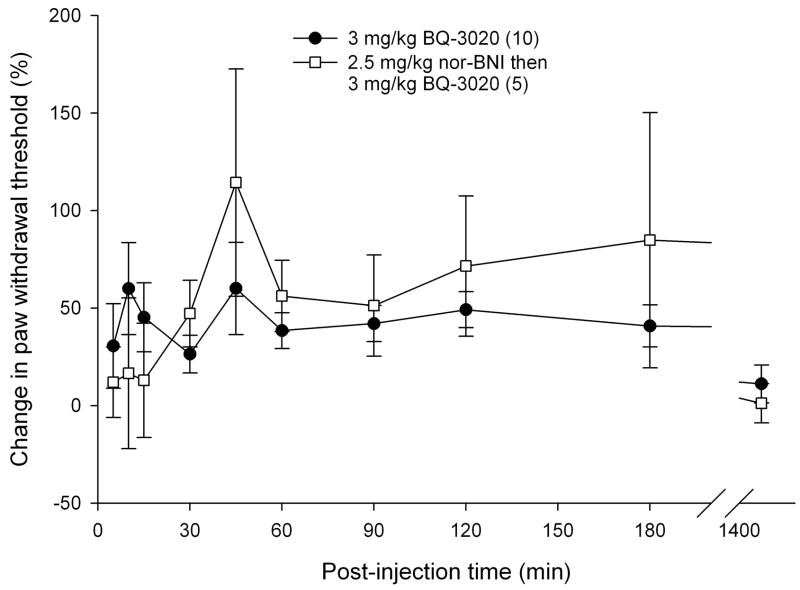
3.5 Reversal of attenuation with opioid receptor antagonists
To determine if endogenous opioids were involved in the attenuation of carcinoma nociception with ETBR agonists, we evaluated whether opioid receptor (OR) antagonists could reverse the antinociceptive effect. Since ETBR agonism attenuated carcinoma-induced nociception for up to 3 hours post-injection (Fig. 3b), OR antagonists were administered at the midpoint time, immediately following the 90 min recording, to evaluate its nociceptive effect on paw withdrawal thresholds. 3 mg/kg BQ-3020 was first injected to establish antinociceptive behavioral response to an ETBR agonist (Fig. 5). Administration of non-selective opioid receptor antagonist naloxone methiodide (500 μg/kg) decreased paw withdrawal threshold (Fig. 5a) immediately after injection relative to vehicle control (PBS), lasting about one hour (p < 0.05, Tukey test or Dunn’s Method as appropriate). Selective μ-opioid receptor antagonist CTOP (500 μg/kg) also reversed antinociception upon administration (Fig. 5b, p < 0.05, Tukey test) and lasted for approximately one hour when compared to PBS control. Specific δ-opioid receptor antagonist naltrindole (11 mg/kg) did not reverse ETBR agonist-induced antinociception (Fig. 5c). In fact, naltrindole appeared to slightly enhance antinociception at 65 min post-injection (p < 0.05, Tukey test) but quickly resolved at the next measurement 30 min later. In order to achieve selective κ-opioid receptor inhibition, tumor animals were pretreated with κ-OR antagonist nor-binaltorphimine (2.5 mg/kg nor-BNI) 12 hours before injection with BQ-3020. nor-BNI had no effect on paw withdrawal thresholds compared to BQ-3020 injection (Fig. 5d).
Figure 5.
Mean percent change in PWT of the right hind paws of cancer-inoculated animals injected with ETBR agonist (3 mg/kg BQ-3020) followed by administration of different peripheral opioid receptor (OR) antagonist drugs or PBS alone. (OR antagonist injection indicated by ↓•) (a) Paw withdrawal for SCC group which received naloxone methiodide (500 μg/kg) at 90 min after BQ-3020 injection (n = 9) compared to PBS control group (n = 5). Mean PWT for the naloxone methiodide group has a significant negative change starting at 10 min following injection of the peripheral OR antagonist, with about 50 min duration (t = 100, 105, 110, 125, and 140 min), compared to the PBS control group (p < 0.05). (b) Mean PWT for SCC group which received μ-OR antagonist CTOP (500 μg/kg) at 90 min after BQ-3020 injection (n = 5). CTOP injection results in an immediate reduction of the mean PWT compared to the PBS control group (p < 0.05), starting at t = 100 min and lasting to t = 155 min. (c) Mean PWT for SCC group which received δ-OR receptor antagonist naltrindole (NTI, 11 mg/kg) at 90 min after BQ-3020 injection (n = 5). NTI injection has no overall effect on mean PWT compared to PBS controls, except for a single aberration at t = 155 min, where PWT is higher than the control group (p = 0.03). (d) Paw withdrawal for SCC group which received nor-binaltorphimine (2.5 mg/kg) 12 hrs prior to BQ-3020 treatment (n = 5). nor-BNI administration has no significant effect on mean PWT compared to the PBS control group. [* denotes statistical significance]
Discussion
These results are evidence that endogenous opioids are important modulators of ETBR agonist-mediated antinociception in a mouse model of cancer pain. Administration of non-selective opioid receptor antagonist naloxone methiodide or selective μ-opioid receptor antagonist CTOP prevented analgesia induced by ETBR agonism. Also our in vitro immunoassay demonstrated that the ETBR agonist BQ-3020 induced secretion of β-endorphin in oral SCC cell culture. ETBR activation in carcinoma cells stimulates endogenous opioid production in the tumor microenvironment to act on neighboring primary afferents and modulate cancer pain. Our results in combination with available studies on endothelin suggest dual control of cancer-induced pain, whereby ETAR on nerve terminals mediate nociception [16,18,9,48,69,58,24,49] while ETBR on neighboring cancer cells promote modulation of the nociceptive signal. The binary function of endothelin in nociception is much like its divergent actions in the vasculature, where ETAR mediates vasoconstriction and ETBR mediates vasodilatation, depending on their expression on different vascular cells. [54]
ET-B receptors are G-coupled protein receptors with seven transmembrane domain. [3] Unlike the G proteins associated with ETAR, ETBR predominantly interacts with Gαi1, Gαi2, and Gαq/11. [61] ETBR is expressed by various normal cell types [47], including dorsal root ganglion satellite cells, nonmyelinating ensheathing Schwann cells [51], skin keratinocytes [67,33], and human gingival keratinocytes. [23] ETBR is also found on different cancer cells, such as melanoma [34], breast carcinoma [2,65], and oral squamous carcinoma cell line SCC25. [5] In the current study, we localized ETBR proteins in cell culture with immunofluorescence but found that ETBR mRNA expression in our oral SCC cell line, HSC-3, was significantly lower than normal oral keratinocytes after normalizing to the housekeeping gene β-N-acetyl-glucosaminidase (β-Gus). EDNRB expression in SCC was undetected compared to normal oral keratinocytes. Note that expression levels were normalized to the expression of the housekeeping gene β-Gus to allow direct comparison between the cancer and normal cells. EDNRB is still expressed at the basal level similar to that of β-Gus. This may explain why ETBR proteins were detected our SCC cells under immunofluorescent imaging. Downregulation of the EDNRB gene in cancer is not uncommon, for previous findings in melanoma [34], hepatocellular carcinoma [29], and small cell lung carcinoma [12] have similarly reported EDNRB downregulation due to promoter hypermethylation. The epigenetic alteration of the tumor suppressor EDNRB gene plays an important role in cancer pathogenesis.
Regulation of EDNRB expression may also serve as a mechanism for modulating peripheral nociception. Mice with ETBR knockdown specifically in the sciatic nerves have been shown to have increased mechanical hyperalgesia and tactile allodynia. [7] Here, we demonstrated that mechanical allodynia is increased in mice inoculated with an oral SCC cell line (HSC-3) that is characterized with downregulated ETBR mRNA expression and increased ET-1 protein transcripts. [49] Paw withdrawal thresholds significantly dropped starting at PID4 and lasting to PID18. Furthermore, ETBR agonist BQ-3020 treatment in oral SCC increased secretion of β-endorphin peptides. A range of ten-fold concentrations of BQ-3020 were tested and higher doses at 10−4 to 10−6 M significantly induced β-endorphin levels compared to untreated cells. Endogenous opioids are commonly produced by peripheral neuronal cells to promote opioid analgesia, but other cell types are also capable of making opioids, such as leukocytes [8], visceral lining epithelial cells [20], skin keratinocytes [33], and even a number of cancers. Malignant melanoma [41], ovarian tumors [46], small cell lung carcinoma [38], and epidermoid carcinoma cells [57] have been reported to produce opioids, but our data indicating opioid production and secretion by an oral SCC is a novel finding. Opioids secreted by non-neuronal cells mediate analgesia in the same manner as their neural-derived counterparts. Studies have shown that β-endorphins produced by leukocytes are responsible for inhibiting inflammatory pain in both humans and animals. [37,53] Our in vitro and in vivo findings using an orthotopic cancer pain mouse model demonstrate that endogenous opioids are implicated in cancer pain attenuation induced by an ETBR agonist (BQ-3020). These findings also raise the question of whether peripherally-restricted opioid agonists or agents that increase the peripheral concentration of opioids can be used to treat cancer pain. Activation of peripheral μ-opioid receptors produces an antinociceptive effect in a rat neuropathic pain model. [45] In patients with inflammatory knee pain selective activation of peripheral opioid receptors produces analgesia. [62] Selective peripheral receptor activation is controlled by administering low, systemically inactive doses that are unable to cross the blood-brain barrier. These results with peripherally acting opioid agonists have been confirmed in a number of different randomized, controlled studies. [44] When low dose morphine is administered into an inflamed, sequestered anatomic site which can limit systemic redistribution (e.g. a joint) it is more effective (both magnitude and duration) than when a similar amount of the opiate is administered systemically. [44]
If peripherally-restricted opioid agonists are capable of producing analgesia there is the question of why peripherally restricted opioid receptor antagonists do not increase pain or decrease the analgesia produced by systemic opioid receptor agonists. Most studies that have looked at the effect of peripherally-restricted opioid receptor antagonists have evaluated the effect of these peripherally-restricted opioid receptor antagonists on gastrointestinal motility. Peripherally-restricted μ-opioid receptor antagonists, including alvimopan and methylnaltrexone, have been developed for the treatment of opioid-induced bowel dysfunction, a very common consequence of opioid analgesics in cancer pain. [22,68] Clinical data suggest that these peripherally-restricted opioid antagonists successfully treat opioid-induced bowel dysfunction but do not reduce the analgesia produced by the opioid agonists. [22,59,64,10] These drugs act at the gastrointestinal tract and likely have very low penetration into the diseased tissue causing pain. Moreover, systemic opioid receptor agonists continue to produce analgesia through central mechanisms.
Previous reports have indicated that β-endorphin is involved in ETBR agonist-mediated inhibition of ET-1-induced nociception in both rats and mice. [32,33] Our data further connects the mechanism to cancer-induced nociception since oral SCC is characterized with significantly elevated ET-1 peptides. [58,49] The analgesic effect of ETBR agonism is unlikely to be through sensory fibers, where ETBRs have not been detected [51], but instead is more likely the result of an indirect action mediated by β-endorphins secreted by oral SCC cells that are stimulated by increased ET-1 in the cancer microenvironment. In vitro ELISA data demonstrates that oral SCC is the source for the opioids. It is difficult to determine whether the opioid levels we measured in vitro occur in vivo. Regardless, the levels of secreted opioids are high enough to induce analgesia as demonstrated by our behavioral studies. Given that carcinoma pain is likely due to hypersensitivity of the nociceptive afferents within the cancer microenvironment the critical result of our study in terms of functional significance is that the carcinoma secretes opioids precisely where they are most likely to have a direct analgesic effect. Currently, there are no clinical trials evaluating the analgesic efficacy of ETBR agonists in patients. Drugs which target peripheral ETBRs within the cancer microenvironment and are free from the complications associated with systemic opioids have significant potential for improved pain management in cancer patients.
*Summary.
In the present study we demonstrate that peripheral endothelin B receptor (ETBR) agonism attenuates carcinoma pain by modulating β-endorphins released from the carcinoma to act on peripheral opioid receptors found in the cancer microenvironment.
Acknowledgments
Supported by NIH/NIDCR R21 DE018561
Footnotes
Conflict of Interest
There are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Ahmed SI, Thompson J, Coulson JM, Woll PJ. Studies on the expression of endothelin, its receptor subtypes, and converting enzymes in lung cancer and in human bronchial epithelium. Am J Respir Cell Mol Biol. 2000;22(4):422–431. doi: 10.1165/ajrcmb.22.4.3795. [DOI] [PubMed] [Google Scholar]
- 2.Alanen K, Deng DX, Chakrabarti S. Augmented expression of endothelin-1, endothelin-3 and the endothelin-B receptor in breast carcinoma. Histopathology. 2000;36(2):161–167. doi: 10.1046/j.1365-2559.2000.00795.x. [DOI] [PubMed] [Google Scholar]
- 3.Arai H, Hori S, Aramori I, Ohkubo H, Nakanishi S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature. 1990;348(6303):730–732. doi: 10.1038/348730a0. [DOI] [PubMed] [Google Scholar]
- 4.Asham E, Shankar A, Loizidou M, Fredericks S, Miller K, Boulos PB, Burnstock G, Taylor I. Increased endothelin-1 in colorectal cancer and reduction of tumour growth by ET(A) receptor antagonism. Br J Cancer. 2001;85(11):1759–1763. doi: 10.1054/bjoc.2001.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Awano S, Dawson LA, Hunter AR, Turner AJ, Usmani BA. Endothelin system in oral squamous carcinoma cells: specific siRNA targeting of ECE-1 blocks cell proliferation. Int J Cancer. 2006;118(7):1645–1652. doi: 10.1002/ijc.21525. [DOI] [PubMed] [Google Scholar]
- 6.Balonov K, Khodorova A, Strichartz GR. Tactile allodynia initiated by local subcutaneous endothelin-1 is prolonged by activation of TRPV-1 receptors. Exp Biol Med (Maywood) 2006;231(6):1165–1170. [PubMed] [Google Scholar]
- 7.Bertelli A, Clerico A, Chicca A, Giovannini L, Gorio A, Romano MA. Role of endothelin-1 in carrageenin-induced inflammation. Int J Tissue React. 1992;14(5):225–230. [PubMed] [Google Scholar]
- 8.Cabot PJ, Carter L, Gaiddon C, Zhang Q, Schafer M, Loeffler JP, Stein C. Immune cell-derived beta-endorphin. Production, release, and control of inflammatory pain in rats. J Clin Invest. 1997;100(1):142–148. doi: 10.1172/JCI119506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carducci MA, Nelson JB, Bowling MK, Rogers T, Eisenberger MA, Sinibaldi V, Donehower R, Leahy TL, Carr RA, Isaacson JD, Janus TJ, Andre A, Hosmane BS, Padley RJ. Atrasentan, an endothelin-receptor antagonist for refractory adenocarcinomas: safety and pharmacokinetics. J Clin Oncol. 2002;20(8):2171–2180. doi: 10.1200/JCO.2002.08.028. [DOI] [PubMed] [Google Scholar]
- 10.Chamberlain BH, Cross K, Winston JL, Thomas J, Wang W, Su C, Israel RJ. Methylnaltrexone treatment of opioid-induced constipation in patients with advanced illness. J Pain Symptom Manage. 2009;38(5):683–690. doi: 10.1016/j.jpainsymman.2009.02.234. [DOI] [PubMed] [Google Scholar]
- 11.Chaplin JM, Morton RP. A prospective, longitudinal study of pain in head and neck cancer patients. Head Neck. 1999;21(6):531–537. doi: 10.1002/(sici)1097-0347(199909)21:6<531::aid-hed6>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 12.Chen SC, Lin CY, Chen YH, Fang HY, Cheng CY, Chang CW, Chen RA, Tai HL, Lee CH, Chou MC, Lin TS, Hsu LS. Aberrant promoter methylation of EDNRB in lung cancer in Taiwan. Oncol Rep. 2006;15(1):167–172. [PubMed] [Google Scholar]
- 13.Chichorro JG, Zampronio AR, Rae GA. Endothelin ET(B) receptor antagonist reduces mechanical allodynia in rats with trigeminal neuropathic pain. Exp Biol Med (Maywood) 2006;231(6):1136–1140. [PubMed] [Google Scholar]
- 14.Connelly ST, Macabeo-Ong M, Dekker N, Jordan RC, Schmidt BL. Increased nitric oxide levels and iNOS over-expression in oral squamous cell carcinoma. Oral Oncol. 2005;41(3):261–267. doi: 10.1016/j.oraloncology.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 15.da Cunha JM, Rae GA, Ferreira SH, Cunha Fde Q. Endothelins induce ETB receptor-mediated mechanical hypernociception in rat hindpaw: roles of cAMP and protein kinase C. Eur J Pharmacol. 2004;501(1–3):87–94. doi: 10.1016/j.ejphar.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Dahlof B, Gustafsson D, Hedner T, Jern S, Hansson L. Regional haemodynamic effects of endothelin-1 in rat and man: unexpected adverse reaction. J Hypertens. 1990;8(9):811–817. doi: 10.1097/00004872-199009000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Davar G. Endothelin-1 and metastatic cancer pain. Pain Med. 2001;2(1):24–27. doi: 10.1046/j.1526-4637.2001.002001024.x. [DOI] [PubMed] [Google Scholar]
- 18.Davar G, Hans G, Fareed MU, Sinnott C, Strichartz G. Behavioral signs of acute pain produced by application of endothelin-1 to rat sciatic nerve. Neuroreport. 1998;9(10):2279–2283. doi: 10.1097/00001756-199807130-00025. [DOI] [PubMed] [Google Scholar]
- 19.De-Melo JD, Tonussi CR, D’Orleans-Juste P, Rae GA. Articular nociception induced by endothelin-1, carrageenan and LPS in naive and previously inflamed knee-joints in the rat: inhibition by endothelin receptor antagonists. Pain. 1998;77(3):261–269. doi: 10.1016/S0304-3959(98)00098-0. [DOI] [PubMed] [Google Scholar]
- 20.Denning GM, Ackermann LW, Barna TJ, Armstrong JG, Stoll LL, Weintraub NL, Dickson EW. Proenkephalin expression and enkephalin release are widely observed in non-neuronal tissues. Peptides. 2008;29(1):83–92. doi: 10.1016/j.peptides.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Ferreira SH, Romitelli M, de Nucci G. Endothelin-1 participation in overt and inflammatory pain. J Cardiovasc Pharmacol. 1989;13 (Suppl 5):S220–222. doi: 10.1097/00005344-198900135-00065. [DOI] [PubMed] [Google Scholar]
- 22.Foss JF. A review of the potential role of methylnaltrexone in opioid bowel dysfunction. Am J Surg. 2001;182(5A Suppl):19S–26S. doi: 10.1016/s0002-9610(01)00783-8. [DOI] [PubMed] [Google Scholar]
- 23.Fujioka D, Nakamura S, Yoshino H, Shinohara H, Shiba H, Mizuno N, Hasegawa N, Shindoh N, Uchida Y, Ogawa T, Kawaguchi H, Kurihara H. Expression of endothelins and their receptors in cells from human periodontal tissues. J Periodontal Res. 2003;38(3):269–275. doi: 10.1034/j.1600-0765.2003.00653.x. [DOI] [PubMed] [Google Scholar]
- 24.Fujita M, Andoh T, Saiki I, Kuraishi Y. Involvement of endothelin and ET(A) endothelin receptor in mechanical allodynia in mice given orthotopic melanoma inoculation. J Pharmacol Sci. 2008;106(2):257–263. doi: 10.1254/jphs.fp0072051. [DOI] [PubMed] [Google Scholar]
- 25.Giaid A, Gibson SJ, Ibrahim BN, Legon S, Bloom SR, Yanagisawa M, Masaki T, Varndell IM, Polak JM. Endothelin 1, an endothelium-derived peptide, is expressed in neurons of the human spinal cord and dorsal root ganglia. Proc Natl Acad Sci U S A. 1989;86(19):7634–7638. doi: 10.1073/pnas.86.19.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gokin AP, Fareed MU, Pan HL, Hans G, Strichartz GR, Davar G. Local injection of endothelin-1 produces pain-like behavior and excitation of nociceptors in rats. J Neurosci. 2001;21(14):5358–5366. doi: 10.1523/JNEUROSCI.21-14-05358.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grant K, Loizidou M, Taylor I. Endothelin-1: a multifunctional molecule in cancer. Br J Cancer. 2003;88(2):163–166. doi: 10.1038/sj.bjc.6700750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hans G, Deseure K, Robert D, De Hert S. Neurosensory changes in a human model of endothelin-1 induced pain: a behavioral study. Neurosci Lett. 2007;418(2):117–121. doi: 10.1016/j.neulet.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 29.Hsu LS, Lee HC, Chau GY, Yin PH, Chi CW, Lui WY. Aberrant methylation of EDNRB and p16 genes in hepatocellular carcinoma (HCC) in Taiwan. Oncol Rep. 2006;15(2):507–511. [PubMed] [Google Scholar]
- 30.Hybbinette S, Bostrom M, Lindberg K. Enzymatic dissociation of keratinocytes from human skin biopsies for in vitro cell propagation. Exp Dermatol. 1999;8(1):30–38. doi: 10.1111/j.1600-0625.1999.tb00345.x. [DOI] [PubMed] [Google Scholar]
- 31.Inoue A, Yanagisawa M, Kimura S, Kasuya Y, Miyauchi T, Goto K, Masaki T. The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc Natl Acad Sci U S A. 1989;86(8):2863–2867. doi: 10.1073/pnas.86.8.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khodorova A, Fareed MU, Gokin A, Strichartz GR, Davar G. Local injection of a selective endothelin-B receptor agonist inhibits endothelin-1-induced pain-like behavior and excitation of nociceptors in a naloxone-sensitive manner. J Neurosci. 2002;22(17):7788–7796. doi: 10.1523/JNEUROSCI.22-17-07788.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khodorova A, Navarro B, Jouaville LS, Murphy JE, Rice FL, Mazurkiewicz JE, Long-Woodward D, Stoffel M, Strichartz GR, Yukhananov R, Davar G. Endothelin-B receptor activation triggers an endogenous analgesic cascade at sites of peripheral injury. Nat Med. 2003;9(8):1055–1061. doi: 10.1038/nm885. [DOI] [PubMed] [Google Scholar]
- 34.Kikuchi K, Nakagawa H, Kadono T, Etoh T, Byers HR, Mihm MC, Tamaki K. Decreased ET(B) receptor expression in human metastatic melanoma cells. Biochem Biophys Res Commun. 1996;219(3):734–739. doi: 10.1006/bbrc.1996.0303. [DOI] [PubMed] [Google Scholar]
- 35.Kloog Y, Sokolovsky M. Similarities in mode and sites of action of sarafotoxins and endothelins. Trends Pharmacol Sci. 1989;10(6):212–214. doi: 10.1016/0165-6147(89)90261-7. [DOI] [PubMed] [Google Scholar]
- 36.MacCumber MW, Ross CA, Snyder SH. Endothelin in brain: receptors, mitogenesis, and biosynthesis in glial cells. Proc Natl Acad Sci U S A. 1990;87(6):2359–2363. doi: 10.1073/pnas.87.6.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Machelska H, Stein C. Pain control by immune-derived opioids. Clin Exp Pharmacol Physiol. 2000;27(7):533–536. doi: 10.1046/j.1440-1681.2000.03287.x. [DOI] [PubMed] [Google Scholar]
- 38.Melzig MF, Nylander I, Vlaskovska M, Terenius L. Beta-endorphin stimulates proliferation of small cell lung carcinoma cells in vitro via nonopioid binding sites. Exp Cell Res. 1995;219(2):471–476. doi: 10.1006/excr.1995.1254. [DOI] [PubMed] [Google Scholar]
- 39.Mercadante S. Malignant bone pain: pathophysiology and treatment. Pain. 1997;69(1–2):1–18. doi: 10.1016/s0304-3959(96)03267-8. [DOI] [PubMed] [Google Scholar]
- 40.Mercadante S, Arcuri E. Breakthrough pain in cancer patients: pathophysiology and treatment. Cancer Treat Rev. 1998;24(6):425–432. doi: 10.1016/s0305-7372(98)90005-6. [DOI] [PubMed] [Google Scholar]
- 41.Nagahama M, Funasaka Y, Fernandez-Frez ML, Ohashi A, Chakraborty AK, Ueda M, Ichihashi M. Immunoreactivity of alpha-melanocyte-stimulating hormone, adrenocorticotrophic hormone and beta-endorphin in cutaneous malignant melanoma and benign melanocytic naevi. Br J Dermatol. 1998;138(6):981–985. doi: 10.1046/j.1365-2133.1998.02263.x. [DOI] [PubMed] [Google Scholar]
- 42.Namer B, Hilliges M, Orstavik K, Schmidt R, Weidner C, Torebjork E, Handwerker H, Schmelz M. Endothelin 1 activates and sensitizes human C-nociceptors. Pain. 2008;137(1):41–49. doi: 10.1016/j.pain.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 43.Nelson JB, Carducci MA. The role of endothelin-1 and endothelin receptor antagonists in prostate cancer. BJU Int. 2000;85 (Suppl 2):45–48. doi: 10.1046/j.1464-410x.2000.00063.x. [DOI] [PubMed] [Google Scholar]
- 44.Ness TJ. Pharmacology of peripheral analgesia. Pain Pract. 2001;1(3):243–254. doi: 10.1046/j.1533-2500.2001.01026.x. [DOI] [PubMed] [Google Scholar]
- 45.Obara I, Przewlocki R, Przewlocka B. Local peripheral effects of mu-opioid receptor agonists in neuropathic pain in rats. Neurosci Lett. 2004;360(1–2):85–89. doi: 10.1016/j.neulet.2004.01.056. [DOI] [PubMed] [Google Scholar]
- 46.Omar RA, el Tabbakh GH. Immunoreactive beta-endorphin in ovarian sex cord-stromal tumors. Arch Pathol Lab Med. 1987;111(5):436–439. [PubMed] [Google Scholar]
- 47.Peri S, Navarro JD, Kristiansen TZ, Amanchy R, Surendranath V, Muthusamy B, Gandhi TK, Chandrika KN, Deshpande N, Suresh S, Rashmi BP, Shanker K, Padma N, Niranjan V, Harsha HC, Talreja N, Vrushabendra BM, Ramya MA, Yatish AJ, Joy M, Shivashankar HN, Kavitha MP, Menezes M, Choudhury DR, Ghosh N, Saravana R, Chandran S, Mohan S, Jonnalagadda CK, Prasad CK, Kumar-Sinha C, Deshpande KS, Pandey A. Human protein reference database as a discovery resource for proteomics. Nucleic Acids Res. 2004;32(Database issue):D497–501. doi: 10.1093/nar/gkh070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peters CM, Lindsay TH, Pomonis JD, Luger NM, Ghilardi JR, Sevcik MA, Mantyh PW. Endothelin and the tumorigenic component of bone cancer pain. Neuroscience. 2004;126(4):1043–1052. doi: 10.1016/j.neuroscience.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 49.Pickering V, Jay Gupta R, Quang P, Jordan RC, Schmidt BL. Effect of peripheral endothelin-1 concentration on carcinoma-induced pain in mice. Eur J Pain. 2008;12(3):293–300. doi: 10.1016/j.ejpain.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pickering V, Jordan RC, Schmidt BL. Elevated salivary endothelin levels in oral cancer patients--a pilot study. Oral Oncol. 2007;43(1):37–41. doi: 10.1016/j.oraloncology.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 51.Pomonis JD, Rogers SD, Peters CM, Ghilardi JR, Mantyh PW. Expression and localization of endothelin receptors: implications for the involvement of peripheral glia in nociception. J Neurosci. 2001;21(3):999–1006. doi: 10.1523/JNEUROSCI.21-03-00999.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Portenoy RK, Lesage P. Management of cancer pain. Lancet. 1999;353(9165):1695–1700. doi: 10.1016/S0140-6736(99)01310-0. [DOI] [PubMed] [Google Scholar]
- 53.Rittner HL, Brack A, Machelska H, Mousa SA, Bauer M, Schafer M, Stein C. Opioid peptide-expressing leukocytes: identification, recruitment, and simultaneously increasing inhibition of inflammatory pain. Anesthesiology. 2001;95(2):500–508. doi: 10.1097/00000542-200108000-00036. [DOI] [PubMed] [Google Scholar]
- 54.Rubanyi GM, Polokoff MA. Endothelins: molecular biology, biochemistry, pharmacology, physiology, and pathophysiology. Pharmacol Rev. 1994;46(3):325–415. [PubMed] [Google Scholar]
- 55.Sabino MA, Luger NM, Mach DB, Rogers SD, Schwei MJ, Mantyh PW. Different tumors in bone each give rise to a distinct pattern of skeletal destruction, bone cancer-related pain behaviors and neurochemical changes in the central nervous system. Int J Cancer. 2003;104(5):550–558. doi: 10.1002/ijc.10999. [DOI] [PubMed] [Google Scholar]
- 56.Sakurai T, Yanagisawa M, Takuwa Y, Miyazaki H, Kimura S, Goto K, Masaki T. Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature. 1990;348(6303):732–735. doi: 10.1038/348732a0. [DOI] [PubMed] [Google Scholar]
- 57.Schauer E, Trautinger F, Kock A, Schwarz A, Bhardwaj R, Simon M, Ansel JC, Schwarz T, Luger TA. Proopiomelanocortin-derived peptides are synthesized and released by human keratinocytes. J Clin Invest. 1994;93(5):2258–2262. doi: 10.1172/JCI117224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schmidt BL, Pickering V, Liu S, Quang P, Dolan J, Connelly ST, Jordan RC. Peripheral endothelin A receptor antagonism attenuates carcinoma-induced pain. Eur J Pain. 2007;11(4):406–414. doi: 10.1016/j.ejpain.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 59.Schmidt WK. Alvimopan* (ADL 8-2698) is a novel peripheral opioid antagonist. Am J Surg. 2001;182(5A Suppl):27S–38S. doi: 10.1016/s0002-9610(01)00784-x. [DOI] [PubMed] [Google Scholar]
- 60.Shedd DP, Carl A, Shedd C. Problems of terminal head and neck cancer patients. Head Neck Surg. 1980;2(6):476–482. doi: 10.1002/hed.2890020606. [DOI] [PubMed] [Google Scholar]
- 61.Shraga-Levine Z, Sokolovsky M. Functional coupling of G proteins to endothelin receptors is ligand and receptor subtype specific. Cell Mol Neurobiol. 2000;20(3):305–317. doi: 10.1023/A:1007010125316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stein C, Hassan AH, Lehrberger K, Giefing J, Yassouridis A. Local analgesic effect of endogenous opioid peptides. Lancet. 1993;342(8867):321–324. doi: 10.1016/0140-6736(93)91471-w. [DOI] [PubMed] [Google Scholar]
- 63.Wacnik PW, Eikmeier LJ, Ruggles TR, Ramnaraine ML, Walcheck BK, Beitz AJ, Wilcox GL. Functional interactions between tumor and peripheral nerve: morphology, algogen identification, and behavioral characterization of a new murine model of cancer pain. J Neurosci. 2001;21(23):9355–9366. doi: 10.1523/JNEUROSCI.21-23-09355.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wolff BG, Michelassi F, Gerkin TM, Techner L, Gabriel K, Du W, Wallin BA. Alvimopan, a novel, peripherally acting mu opioid antagonist: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial of major abdominal surgery and postoperative ileus. Ann Surg. 2004;240(4):728–734. doi: 10.1097/01.sla.0000141158.27977.66. discussion 734–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wulfing P, Diallo R, Kersting C, Wulfing C, Poremba C, Rody A, Greb RR, Bocker W, Kiesel L. Expression of endothelin-1, endothelin-A, and endothelin-B receptor in human breast cancer and correlation with long-term follow-up. Clin Cancer Res. 2003;9(11):4125–4131. [PubMed] [Google Scholar]
- 66.Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 67.Yohn JJ, Smith C, Stevens T, Morelli JG, Shurnas LR, Walchak SJ, Hoffman TA, Kelley KK, Escobedo-Morse A, Yanagisawa M. Autoregulation of endothelin-1 secretion by cultured human keratinocytes via the endothelin B receptor. Biochim Biophys Acta. 1994;1224(3):454–458. doi: 10.1016/0167-4889(94)90282-8. [DOI] [PubMed] [Google Scholar]
- 68.Yuan CS. Clinical status of methylnaltrexone, a new agent to prevent and manage opioid-induced side effects. J Support Oncol. 2004;2(2):111–117. discussion 119–122. [PubMed] [Google Scholar]
- 69.Yuyama H, Koakutsu A, Fujiyasu N, Tanahashi M, Fujimori A, Sato S, Shibasaki K, Tanaka S, Sudoh K, Sasamata M, Miyata K. Effects of selective endothelin ET(A) receptor antagonists on endothelin-1-induced potentiation of cancer pain. Eur J Pharmacol. 2004;492(2–3):177–182. doi: 10.1016/j.ejphar.2004.04.016. [DOI] [PubMed] [Google Scholar]