Abstract
Bright light can cause ocular discomfort and/or pain; however, the mechanism linking luminance to trigeminal nerve activity is not known. In this study we identify a novel reflex circuit necessary for bright light to excite nociceptive neurons in superficial laminae of trigeminal subnucleus caudalis (Vc/C1). Vc/C1 neurons encoded light intensity and displayed a long delay (>10 s) for activation. Microinjection of lidocaine into the eye or trigeminal root ganglion (TRG) inhibited light responses completely, whereas topical application onto the ocular surface had no effect. These findings indicated that light-evoked Vc/C1 activity was mediated by an intraocular mechanism and transmission through the TRG. Disrupting local vasomotor activity by intraocular microinjection of the vasoconstrictive agents, norepinephrine or phenylephrine, blocked light-evoked neural activity, whereas ocular surface or intra-TRG microinjection of norepinephrine had no effect. Pupillary muscle activity did not contribute since light-evoked responses were not altered by atropine. Microinjection of lidocaine into the superior salivatory nucleus diminished light-evoked Vc/C1 activity and lacrimation suggesting that increased parasympathetic outflow was critical for light-evoked responses. The reflex circuit also required input through accessory visual pathways since both Vc/C1 activity and lacrimation were prevented by local blockade of the olivary pretectal nucleus. These findings support the hypothesis that bright light activates trigeminal nerve activity through an intraocular mechanism driven by a luminance-responsive circuit and increased parasympathetic outflow to the eye.
Keywords: trigeminal subnucleus caudalis, photophobia, light, ocular pain
1. Introduction
Acute exposure to bright sunlight or artificial light is a common environmental stimulus that can cause ocular discomfort and pain [12,48]. Intolerance to bright light, commonly termed photophobia, is often associated with eye injury [9,35] or inflammation [8,41]. Photophobia also presents with several classes of headache [11,21,24] and in subjects with no apparent link to headache or eye injury such as trigeminal neuralgia [14] and blepharospasm [15]. Although it has long been held that an intact trigeminal nerve is necessary for photophobia, the mechanisms that link luminosity to activation of a somatic sensory nerve pathway have remained elusive. Early studies emphasized the importance of peripheral mechanisms and proposed that excessive eye blink or pupillary constriction due to bright light sensitized trigeminal nerves [27]. Central mechanisms also must play a role since spatial summation of the light stimulus increases the discomfort rating in control subjects [48], while unilateral pain lowers light-induced discomfort thresholds bilaterally in migraine patients [54]. Visual pattern perception is not necessary to experience photophobia [1]; however, some input through the optic nerve is required since intolerance to light due to eye injury is eliminated by enucleation of the eye [9].
The interior structures of the eye including the uveal tract, retina, and associated blood vessels receive a rich innervation from trigeminal sensory [4,51,52], sympathetic [50] and parasympathetic postganglionic efferent fibers [10,30]. Sensory, sympathetic and parasympathetic nerves form a close association with blood vessels of the head, an arrangement not commonly seen in other body regions [42], and may underlie the tight control of blood flow to craniofacial structures [23]. Although many intraocular trigeminal nerve fibers contain calcitonin gene-related peptide, a neuropeptide associated with peripheral nociceptors [44,52] and light sensitivity in animals [40], their function remains uncertain. Intraocular trigeminal nerves encode changes in ocular perfusion pressure, temperature and intraocular pressure [33,57] and are thought to serve mainly ocular homeostatic functions [47,53]. The notion that intraocular sensory nerves also mediate non-visual sensations has not been adequately tested.
Recently we reported that acute exposure to bright light increased the number of Fos-like immunoreactive neurons in superficial laminae of trigeminal subnucleus caudalis (Vc/C1) [39]. The Vc/C1 region shares many features with the spinal dorsal horn and is the main zone of termination for small diameter sensory nerves that supply craniofacial tissues including the eye [3]. The present study addressed three issues: what are the receptive field properties of light-responsive neurons in the Vc/C1 region; is light-induced activity linked to vasomotor mechanisms in the eye; and is parasympathetic outflow necessary to observe light-induced Vc/C1 neural activity? The data indicated that bright light activation of Vc/C1 neurons required input through the trigeminal ganglion, was prevented by intraocular microinjection of vasoconstrictive agents and markedly reduced by inhibition of the superior salivatory nucleus. Blockade of a major retinorecipient target, the olivary pretectal nucleus, prevented light-induced responses. These data support the hypothesis that intense light activates a trigeminal nociceptive pathway through an intraocular mechanism dependent on increased parasympathetic outflow to the eye.
2. Methods
The animal protocols were approved by the Institutional Animal Care and Use Committee of the University of Minnesota and conformed to the established guidelines set by The National Institutes of Health guide for the care the use of laboratory animals (PHS Law 99–158, revised 2002).
Animals and electrophysiology procedures
Male Sprague-Dawley rats (270–350 g, Harlan, Indianapolis, IN) were anesthetized initially with pentobarbital sodium (70 mg/kg ip). Catheters were positioned in the right femoral artery and jugular vein for monitoring blood pressure and drug infusion (gallamine triethiodide, 20 mg/kg/h, only during the recording session). After tracheotomy animals were respired artificially with oxygen-enriched room air and anesthesia was maintained with isoflurane (1.2–2.0%). Expiratory end-tidal CO2 (3.5–4.5%), mean arterial pressure (MAP, 100–120 mmHg) and body temperature (38°C) were monitored continuously and kept within normal range. Animals were placed in a stereotaxic frame and portions of the C1 vertebra were removed to expose the lower brainstem and upper cervical dorsal horn. The exposed surface was bathed in warm mineral oil. The spinomedullary junction region (Vc/C1), approximately 3–5 mm caudal to obex and ipsilateral to the stimulated eye, was explored with tungsten microelectrodes (5–9 Mohm, FHC, Bowdoinham, ME). Unit activity was collected through a DAQ interface board and LabVIEW software (National Instruments, Austen, TX), discriminated, stored and analyzed offline.
Characterization of light-responsive neurons
The search stimulus consisted of gently swiping a fine camelhair brush across the ocular surface (e.g., cornea surface and conjunctiva). Units were classified as cornea/conjunctiva (CC) units, activated by mechanical stimulation of the palpebral conjunctiva of the lower eyelid and cornea or conjunctiva only (CJ) units. All units received convergent cutaneous input from periorbital skin and were further classified as nociceptive specific (NS, pinch only) or wide dynamic range (WDR, pinch plus touch) as determined by use of calibrated von Frey filaments (1.2 g) and blunt forceps. The ocular surface was kept moist with artificial tears throughout the experiment.
Light stimulation and experimental design
One neuron was recorded from each preparation and only neurons that displayed an increase in firing rate to light stimulation (Rmag value > 10, see below in Data Analysis) were included in experiments that involved drug application. Light stimulation was delivered from a thermal-neutral fiber optic halogen source (150W, Cole Parmer, Vernon Hills, IL) positioned 5 cm from the left ocular surface under dim ambient lighting conditions. Light intensity was measured at the ocular surface with a light meter (Control Co., Friendswood, TX). Light stimuli (30 s duration) were presented in a cumulative design at three intensities at 20 min interstimulus intervals: low (0.5 × 104), moderate (1 × 104) and high (2 × 104 lux).
Local drug microinjection into the eye (intravitreal, ivt) or trigeminal ganglion (TRG)
Light stimulation was presented before and 5 min after ivt lidocaine (2%), norepinephrine (1 or 10 mM, pH 7.4) or the selective alpha adrenergic receptor agonist, phenylephrine (1 or 10 mM, pH 7.4). Ivt injections were delivered from a 33 gauge needle inserted into the globe through the sclera posterior to the limbus. Intra-TRG injections of lidocaine (2%) or norepinephrine (10 mM) were delivered from a 33 gauge needle through a 26 gauge guide cannula positioned vertically above the ganglion: 3.1–3.3 mm caudal to bregma, 2.8–3.1 mm lateral and 9–10 mm ventral to the brain surface. All drugs were injected in a volume of 1 μl. Volume controls for ivt or TRG administration received an equivalent injection of artificial cerebral spinal fluid (aCSF, 1 μl, pH 7.4). To test the effect of ocular cholinergic activity atropine sulfate (10 mM, 20μl, pH 7.3) was applied to the ocular surface 10 min prior to light stimulation.
Superior salivatory nucleus (SSN) blockade
Lidocaine (2%, 100 nl) was microinjected ipsilateral to the stimulated eye via a glass micropipette positioned 10.4–10.6 mm posterior to bregma, 2.0–2.2 mm off the midline and 8.4–8.6 mm ventral to the cortical surface 10 min prior to light stimulation. Lidocaine was injected 10 min prior to light stimulation. Off-target control injections of lidocaine were placed 0.5 mm medial to SSN.
Superior salivatory nucleus (SSN) stimulation
To determine if direct activation of parasympathetic outflow affected light-responsive Vc/C1 neurons, the excitant DL-homocysteine (DLH, 50 mM, 100 nl, Sigma) [32] injected into the SSN ipsilateral to the stimulated eye via a glass micropipette at the same coordinates as noted above.
Olivary pretectal nucleus (OPN) blockade
Lidocaine (2%, 100 nl) was injected bilaterally in the OPN region from a dual glass micropipette assembly10 min prior to light stimulation. The pipette was positioned 4.8–5.2 mm posterior to bregma, 1.3–1.5 mm off the midline and 4.0–4.5 mm ventral to the cortical surface. Off-target control injections of lidocaine were placed 0.5 mm lateral to OPN.
Tear volume
Tear volume was measured after unilateral light stimulation (30 s duration) in a separate group of rats under isoflurane anesthesia (1.2–2.0%) and low ambient light conditions (< 1 lux). Tear volume samples were collected over 2 min and measured by the change in weight of a small filter paper strip (~5×8 mm) in contact with the cornea/conjunctiva [18]. In initial experiments ocular surface temperature was monitored during light stimulation by a needle thermistor placed laterally at the lower cornea/conjunctiva interface.
Data analysis
Neural recording data were acquired and displayed as peristimulus time histograms (PSTH) of spikes per 1 s bins, exported to a spreadsheet and analyzed off-line. Neural responses were analyzed as a response magnitude (Rmag) for each stimulus period defined as the cumulative sum of spikes for contiguous bins in which the spike count exceeded the mean+2SD of the background activity [17]. A total Rmag was calculated for each stimulus period and can be thought of as the “area under the curve”. Neurons were defined as light responsive if the total Rmag exceeded a value of 10 independent of light intensity. The high-threshold convergent cutaneous RF area of light-responsive units was mapped onto standardized drawings of the rat face with a small blunt forceps, digitized and quantified by a planimetric method using NIH software (ImageJ). Chi-square analysis determined if the light intensity threshold and proportion of CC and CJ units classified as NS and WDR were similar. Neural activity, tear volume, ocular surface temperature and MAP were assessed by ANOVA corrected for repeated measures. Significant treatment effects were further assessed by Newman-Keuls after ANOVA. The data were presented as mean ± SEM and significance set at P = 0.05.
Histology
At the end of the experiment, rats were given a bolus of barbiturate and perfused through the heart with saline followed by 10% formalin and the brain processed to recover the recording sites at the Vc/C1 region and injection sites in the SSN and OPN.
3. Results
Light intensity coding by trigeminal subnucleus caudalis (Vc/C1) neurons
In the initial series of experiments, 58 of 78 (74.4%) units recorded from superficial laminae at the spinomedullary junction region (Vc/C1) were excited by brief exposure to bright light. All recording sites were located within 200 μm of the dorsal brainstem surface in superficial laminae similar to our previous studies [17]. Light-responsive units had an ocular surface receptive field (RF) located on the cornea and conjunctiva (CC units, n = 46) or conjunctiva alone (CJ units, n = 12). Light-responsive units also received excitatory input from the adjacent periorbital skin and were further classified either as nociceptive specific (NS, n = 36) or wide dynamic range (WDR, n = 22). The mean cutaneous RF area of light-responsive and non-responsive neurons was similar (1.22 ± 0.11 versus 1.42 ± 0.14 cm2, respectively) and presentation of the light stimulation protocol did not affect the RF area (data not shown). Light-responsive and non-responsive units also displayed similar levels of background activity (1.91 ± 0.32 versus 1.97 ± 0.28 spikes/s) prior to presentation of the light stimulation protocol.
A similar percentage of CC (73%) and CJ units (80%) were light responsive (Fig 1a); however, the total response magnitude (Rmag) to increasing light intensity was greater for CC units (Fig 1b, F1,56 = 10.19, P < 0.005). CC units had lower thresholds for light activation than CJ units (X2df2 = 9.92, P < 0.007) as 30% of CC but only 17% of CJ units were excited by the lowest intensity. CC units also displayed longer response durations than CJ units (Fig 1c, F1.56 = 12.45, P < 0.001), while response latencies were similar (Fig 1d, F1,56 = 0.45, P > 0.10). The resting ocular surface temperature averaged 31.8 ± 0.25 °C (n = 8). Although high intensity light caused a small increase (33.7 ± 0.35 °C, F2,19 = 51.54, P < 0.001), this was well below the threshold temperature reported to activate corneal nociceptors or increase intraocular blood flow [2,38]. Together with the finding that application of lidocaine to the ocular surface did not affect light-evoked Vc/C1 neural activity (see below), it was not likely that thermal activation of nerve endings at the ocular surface caused this response.
Figure 1.
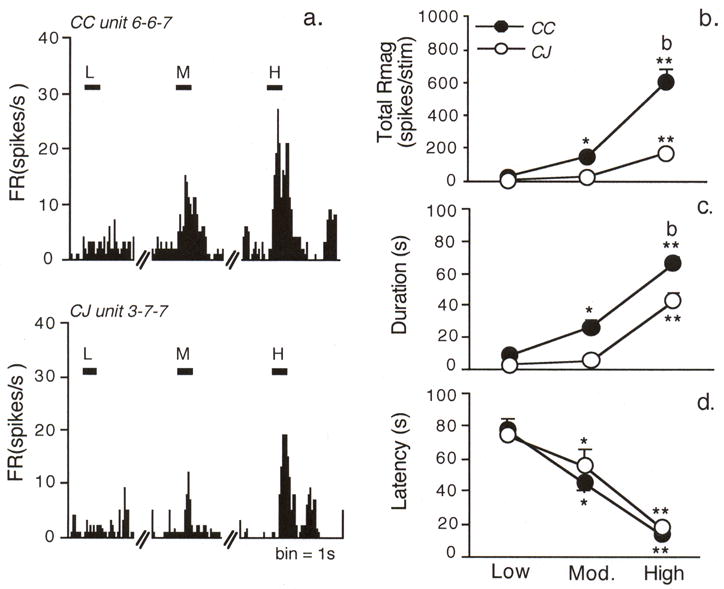
Trigeminal neurons in superficial laminae at the Vc/C1 region encode luminosity. Light stimuli were presented for 30 s duration at 20 min intervals at low, moderate and high intensity (0.5×104, 1×104, 2×104 lux, respectively). a. Light-evoked peristimulus time histograms for neurons classified as cornea/conjunctiva (CC, upper panel) or conjunctiva only (CJ, lower panel) units. b. Summary of the response magnitude (Rmag) for CC and CJ units. c. Summary of the response duration to light. Note the greater Rmag and response duration to light for CC compared to CJ units. d. Response latency decreases similarly with increasing light intensity for CC and CJ units. *P < 0.05, **P < 0.01 versus response to low intensity light; b = P < 0.01 versus CJ units.
Spontaneous tear volume was low and similar in both eyes (ipsilateral, 0.19 ± 0.04 mg/2 min; contralateral, 0.18 ± 0.04 mg/2 min, n = 8) prior to light stimulation. High intensity light increased tear volume more than 3-fold ipsilateral (0.70 ± 0.06 mg/2 min, P < 0.01) and 2-fold contralateral to the stimulus (0.36 ± 0.05 mg/2 min, P < 0.05). Low (~53% increase) and moderate light intensities (~40% increase) did not significantly alter tear volume ipsilateral to stimulation. Resting mean arterial pressure (MAP) averaged 113.4 ± 1.5 mmHg (n = 78) and did not change significantly (peak change = −1.8 ± 0.9 mmHg) after high intensity light stimulation.
Evidence for an intraocular origin of light-evoked Vc/C1 neural activity
Lidocaine injection into the trigeminal ganglion (TRG) or into the eye (ivt), ipsilateral to high intensity light stimulation, blocked completely the evoked neural response (~99% inhibition, Fig 2, F2,9 = 29.1, P < 0.001), while application on the ocular surface had little effect (~12% inhibition, n = 4). Light-evoked responses after intra-TRG (n = 4) or ivt (n = 4) injection recovered by 45 min after lidocaine in all cases (>70% of pre-lidocaine value). Intra-TRG lidocaine also eliminated completely the convergent input from periorbital skin for all units, whereas ivt or ocular surface lidocaine had little effect (<10% change in RF area). The decrease in light-evoked activity after intra-TRG or ivt lidocaine was not due to non-specific effects of volume injection, since in a separate group of rats (n = 10) an equal volume (1 μl) of aCSF had no effect (F1,10 = 0.18, P > 0.1). During light stimulation, prompt pupil constriction was seen, indicating that intra-TRG lidocaine did not compromise optic nerve function. These results indicated that input through the TRG was necessary for light-evoked Vc/C1 neural activity and the origin of this activity was sensory nerve terminals within the eye and not on the ocular surface.
Figure 2.

Light-evoked Vc/C1 activity requires input through the trigeminal ganglion. Intra-TRG (open circles) or intravitreal (ivt, solid squares) injection of lidocaine (2%, 1 μl) blocks completely the responses to high intensity light, whereas application to the ocular surface (open triangles) has no effect. **P < 0.01 versus pre-lidocaine value. b = P < 0.01 versus other groups.
Evidence for an intraocular adrenergic mechanism in light-evoked Vc/C1 unit activity
To determine if an adrenergic mechanism, such as vasomotor activity, within the eye influenced light-evoked Vc/C1 neural activity, norepinephrine or phenylephrine, potent vasoconstrictor agents, were given by ivt microinjection. The example seen in Fig 3a demonstrated that norepinephrine inhibited light-evoked activity by 10 min and lasted for at least 40 min. As summarized in Fig 3b, ivt injection of high dose (10 mM, 1 μl) norepinephrine (F2,40 = 22.9, P < 0.001, n = 7) and phenylephrine (F2,28 = 19.2, P < 0.001, n = 5) had similar inhibitory effects on light-evoked activity, whereas low dose (1 mM, 1 μl) norepinephrine (n = 5) or phenylephrine (n = 4), had no significant effect. The inhibitory effect of ivt norepinephrine was not likely due to a direct action on sensory neurons, since intra-TRG microinjection (pre-drug = 322 ± 142 versus post-drug = 483 ± 227 spikes per stimulus, n = 4) or ocular surface application of norepinephrine (pre-drug = 355 ± 94 versus post-drug = 377 ± 61 spikes per stimulus, n = 4) did not reduce light-evoked activity (P > 0.1). Ocular cholinergic or muscle activity also did not affect this response, since atropine (10 mM, 20 μl) applied to the ocular surface caused an immediate and persistent pupil dilatation, but did not reduce light-evoked neural activity (F2,36 = 16.3, P < 0.001, n = 10). The convergent cutaneous RF area of light-responsive neurons was not altered by norepinephrine regardless of the site of microinjection (data not shown). These results indicated that intraocular adrenergic mechanisms such as altered vasomotor function contributed to light-evoked activation of Vc/C1 neurons, whereas muscarinic cholinergic activity and iris muscle activity had no influence.
Figure 3.
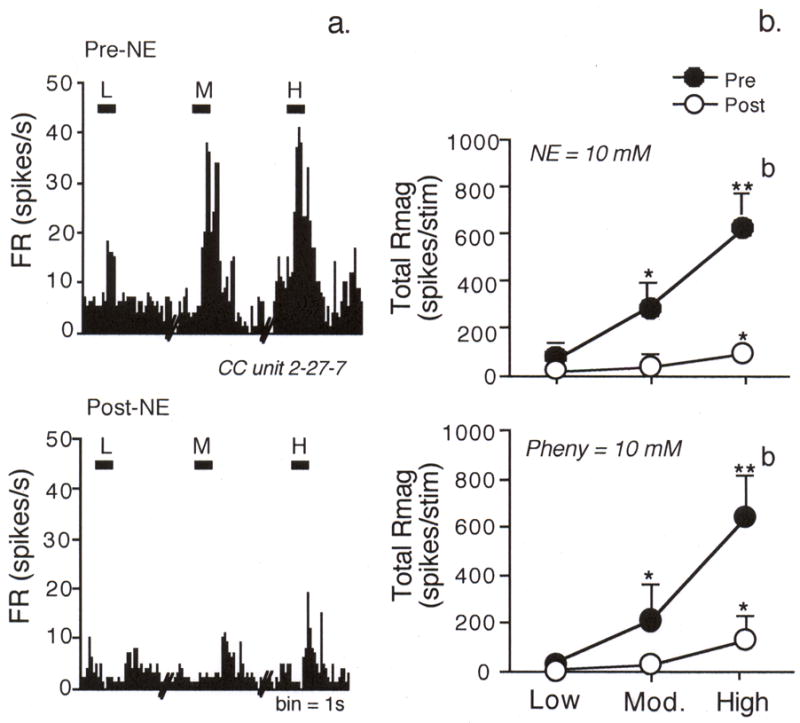
An intraocular adrenergic mechanism contributes to light-evoked Vc/C1 activity.
a. Peristimulus time histograms before (upper panel) and after (lower panel) ivt injection of norepinephrine (NE, 10 mM, 1 μl) on the responses to light of a CC neuron. b. Ivt injection of adrenergic agents inhibits light-evoked responses. High dose norepinephrine (upper panel, 10mM, 1 μl) or phenylephrine (lower panel, 10 mM, 1 μl) markedly inhibits the responses to moderate and high intensity light. *P < 0.05, **P < 0.01 versus response to low intensity light; b = P < 0.01 versus pre-drug value.
Evidence for superior salivatory nucleus (SSN) involvement in light-evoked responses
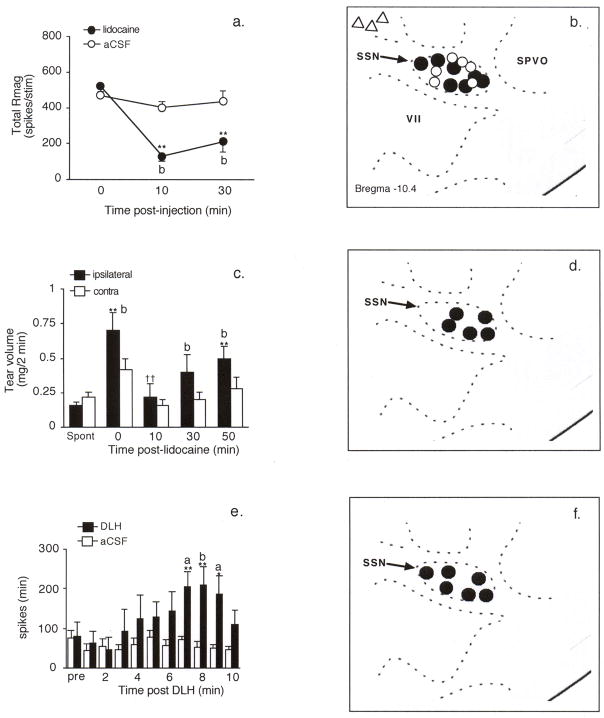
The SSN is the major source of parasympathetic preganglionic neurons to the blood vessels of the eye [10,30]. Lidocaine (2%, 100 nl, n = 8) injection into the SSN significantly inhibited light-evoked neural activity (Figs 4a, 4b) compared to vehicle (n = 5) injections (F1,11 = 7.3, P < 0.01). Off-target injections of lidocaine 0.5 mm medial to the SSN had no effect (< 10% change, n = 3). In a separate group of rats (n = 5) SSN lidocaine injection also prevented light-evoked increases in tear volume (Figs 4c, 4d; F1,4 = 8.9, P < 0.05). To determine if direct SSN activation influenced the activity of light-responsive Vc/C1 neurons, microinjection of the general excitant D,L-homocysteine (DLH, 50 mM, 100 nl) increased the firing rate of Vc/C1 neurons (Figs 4e, 4f; F10,90 = 6.75, P < 0.001, n = 6). Note that DLH-evoked increase in neural activity occurred after a delay of 5–8 min and was maintained for 3–5 min. The cutaneous RF area of light-responsive Vc/C1 neurons was not affected by SSN blockade or activation suggesting that the effect of SSN manipulation was selective for intraocular input. These data strongly suggested that the SSN played a key role in mediating light-evoked Vc/C1 neural activity.
Figure 4.
The superior salivatory nucleus (SSN) is critical for light-evoked responses by Vc/C1 neurons. a. Ipsilateral SSN injection of lidocaine (2%, 100 nl) blocks the Rmag to high intensity light stimulation. **P < 0.01 versus 0 min; b = P < 0.01 versus artificial CSF (aCSF) injected group. b. Lidocaine (solid circles) and vehicle (open circles) injection sites in SSN. Off-target injections of lidocaine are represented by open triangles. c. Light-evoked increase in tear volume is prevented by lidocaine blockade of the ipsilateral SSN. **P < 0.01 versus spontaneous tear value; b = P < 0.01 versus contralateral side; ††P < 0.01 versus light evoked tears at 0 min. d. Lidocaine injection sites in SSN ipsilateral to light stimulus and tear sample. e. Ipsilateral SSN injection of DL-homocysteine (DLH, 50 mM, 100 nl) increases the activity of light-responsive Vc/C1 neurons. **P < 0.01 versus 0 min; a = P < 0.05, b = P < 0.01 versus vehicle (aCSF) injected group. f. DLH injection sites in SSN. Abbreviations: SPVO, spinal trigeminal nucleus oralis; SSN, superior salivatory nucleus; VII, facial nucleus.
Evidence for olivary pretectal nucleus (OPN) involvement in light-evoked responses
The OPN receives dense input from retinal ganglion neurons compared to surrounding pretectal regions [16,34] and is critical for the pupillary light reflex [56]. Lidocaine (2%, 100 nl, n = 5) microinjection into the OPN significantly inhibited light-evoked neural activity (Fig 5a,c) compared to vehicle (n = 3) injections (F1,11 = 6.79, P < 0.025). Off-target injections of lidocaine 0.5 mm lateral to the OPN had no effect (< 10% change, n = 3, Fig 5c). In a separate group of rats (n = 5) lidocaine blockade of OPN also prevented light-evoked increases in tear volume (Fig 5b, c; F1,5 = 8.91, P < 0.01). These data indicated that blockade of the OPN, a key retinoreceipient target, markedly reduced light-evoked Vc/C1 neural activity and tear formation.
Figure 5.
Inhibition of the olivary pretectal nucleus (OPN) prevents light-evoked Vc/C1 neural activity. a. Bilateral injection of lidocaine (2%, 100 nl) into the OPN blocks completely the Rmag to high intensity light stimulation. *P < 0.05, **P < 0.01 versus 0 min; a = P < 0.05, b = P < 0.01 versus vehicle (aCSF) group. b. Light-induced tear formation is prevented by OPN blockade. **P < 0.01 versus spontaneous tear value; a = P < 0.05, b = P < 0.01 versus contralateral side; † P< 0.05, ††P < 0.01 versus evoked tears at 0 min. c. Injection sites in OPN: solid circles = units, lidocaine injection; open circles = units, vehicle injection; solid triangles = tear volume, lidocaine injection. Off-target injections of lidocaine are represented by open triangles. d. An example of a OPN lidocaine injection site (asterisk). Abbreviations: APN, anterior pretectal nucleus; NOT, nucleus optic tract; OPN, olivary pretectal nucleus; PPN, posterior pretectal nucleus.
4. Discussion
These data provided for the first time strong evidence for a population of nociceptive neurons in the superficial laminae at the spinomedullary (Vc/C1) junction region that encoded the intensity of bright light. Remarkably, nearly 75% of Vc/C1 neurons identified by mechanical stimulation of the ocular surface also displayed increased firing rates to bright light suggesting this may be a common feature of trigeminal brainstem neurons that integrate sensory input from the eye. The threshold light intensity for activation of Vc/C1 neurons (~5000 lux) in the anesthetized rat was similar to the discomfort threshold in humans (~2900–6500 lux) [26,55] suggesting this preparation is a valid animal model to explore neural mechanisms for light intolerance in humans. It is not certain why further increases in luminance produced progressive increases in Vc/C1 activity; however, it is possible that additional neural circuits were recruited at high light intensities. The key features of the model included an intraocular transduction mechanism in the eye driven by increased parasympathetic outflow from the SSN resulting in excitation of TRG neurons. Activation of Vc/C1 neurons also required a relay of luminance information through accessory visual pathways since inhibition of the OPN completely blocked light-evoked Vc/C1 neural activity and lacrimation (Fig 6). Indeed, a wide spectrum of environmental stimuli reported to trigger migraine headache may involve increased parasympathetic nerve activity [5]. Conversely, acute exposure to intense light may have a general sensitizing influence since it may lower pain thresholds in headache patients [26] and evokes protective reflexes unrelated to visual sensation in healthy subjects [20].
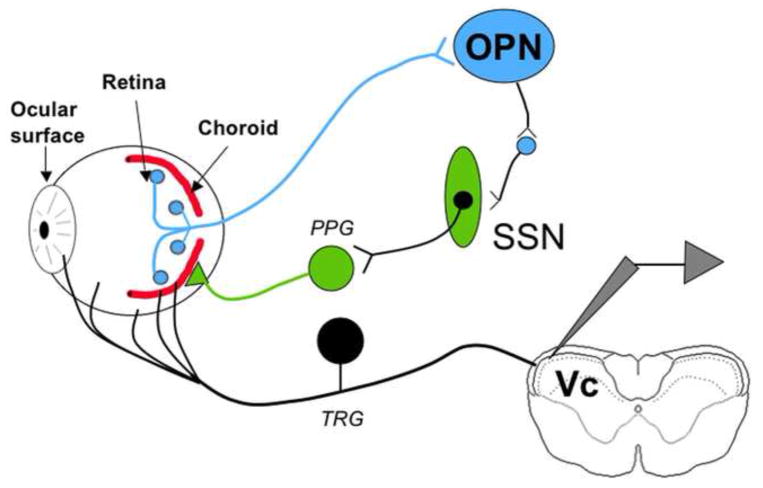
Figure 6.
The proposed model for light-induced activation of trigeminal neurons involves transduction of luminance in the eye and a relay of this information to the OPN. OPN activation results in increased parasympathetic outflow to the eye through the SSN. TRG neurons could be activated by transmitters released from parasympathetic postganglionic neurons or, for those fibers apposed to blood vessels, by mechanical deformation of ocular blood vessels due to changes in blood flow. Abbreviations. OPN, Olivary pretectal nucleus; SSN, Superior salivatory nucleus; PPG, Pterygopalatine ganglion; TRG, Trigeminal root ganglion.
Several features of the light-evoked neural response were consistent with an indirect intraocular mechanism linking luminosity with Vc/C1 activity. First, the onset of activity displayed a long delay (> 10 s) that far exceeded the latency expected for a photic-induced reflex such as eye blink [28] and could not be explained simply on the basis of calculated axonal conduction times and synaptic delay. Second, intra-TRG or ivt microinjection of lidocaine completely blocked light-evoked Vc/C1 neural activity confirming the importance of trigeminal sensory nerves and reduced the likelihood that such activity could derive from pathways that bypass the TRG. A recent imaging study revealed that bright light increased TRG activity in a photophobic patient [35]. Third, ivt microinjection of norepinephrine or phenylephrine prevented light-evoked Vc/C1 neural activation in a dose-related manner. This finding underscored the importance of an intraocular mechanism light-induced Vc/C1 activity. The exact mechanism for the norepinephrine effect was not determined; however, inhibition due to hypoxia seemed unlikely since corneal afferent nerves were markedly excited rather than inhibited by hypoxia [31]. However, since reduced retinal oxygen tension could have occurred after norepinephrine injection [29], it is possible that direct effects on retinal transduction could have contributed to the inhibition after injection of these vasoconstrictive agents. It was not likely that norepinephrine acted directly on TRG neurons [49], since injection into the TRG had no effect. Fourth, ocular surface application of atropine caused persistent pupillary dilatation but did not reduce light-evoked neural activity. Early studies on the nature of photophobia proposed that intense light enhanced iris or ciliary muscle contractions that sensitized trigeminal nerves and caused discomfort [27]. The present findings did not support this notion.
The present results indicated that increased parasympathetic outflow to the eye was required to activate Vc/C1 neurons by bright light. The SSN is a major source of parasympathetic preganglionic neurons to the eye, especially the choroid blood vessels [10,30]. Ocular blood flow to the eye is greater than other tissues of the body and the choroid receives more than 80% of that flow [7]. In the rat, inhibition of the SSN prevented increases in choroidal blood flow following noxious cutaneous stimulation [45], whereas direct activation of the SSN increased blood flow to the anterior choroid more than 3-fold [46]. Although we did not measure ocular blood flow, inhibition of SSN prevented light-evoked increases in tear volume, confirming an increase in parasympathetic activity. Conversely, stimulation of SSN by microinjection of DLH caused a progressive increase in the firing rate of light-responsive Vc/C1 neurons that peaked after 5–8 min at nearly 4-fold the resting discharge rate. These data indicated that the SSN was necessary for light-evoked neural activity and tear formation.
The OPN receives a dense direct input from retinal ganglion cells compared to adjacent pretectal regions [16,34] and is necessary for several photic-induced responses such as the pupillary light reflex, eye blink and circadian rhythms [13]. The OPN has extensive efferent projections to hypothalamic and brainstem regions associated with autonomic control [19,25]. We found that bilateral inhibition of the OPN blocked completely light-evoked Vc/C1 neural activity and tear formation. The magnitude of inhibition of light-evoked responses after OPN blockade was essentially 100%, whereas a small level of Vc/C1 neural activity remained after SSN blockade suggesting that the integration of luminance information by the OPN must be upstream of the parasympathetic outflow from the SSN.
This study identified a novel reflex circuit that may explain how intense light activates a somatic sensory nerve pathway resulting in discomfort or pain (Fig 6). Sensory convergence resulting in parasympathetic outflow is a key feature of some classes of headache [5]. Accordingly, SSN neurons were excited by stimulation of multiple branches of the trigeminal nerve [37], other cranial nerves [36] and cerebral cortex [22]. Light-responsive Vc/C1 neurons displayed a high degree of convergence as each was activated by low threshold mechanical stimulation of the ocular surface and pinch of periorbital skin. Since a high percentage of dura-responsive neurons in the Vc/C1 region are activated by periocular cutaneous stimulation [6,43], it will be important to determine if these neurons also are activated by bright light. A critical function for the trigeminal nerve is to maintain homeostasis for craniofacial tissues through the induction of protective reflexes [3]. The present data provide a mechanism whereby one such reflex, light-aversive behavior, is elicited to guard against retinal damage.
Summary (24 words)
Bright light activates neurons at the superficial laminae of trigeminal subnucleus caudalis through intraocular mechanisms driven by a luminance-responsive circuit and increased parasympathetic outflow.
Acknowledgments
The authors thank R. Thompson for excellent technical assistance and Dr. H. Hirata for early contributions to this project. The authors have no financial or other relationships to report that might lead to a conflict of interest. This work was supported by a grant from the NIH (NS26137).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Amini A, Digre K, Couldwell WT. Photophobia in a blind patient: An alternate visual pathway. Case report. J Neurosurg. 2006;105:765–768. doi: 10.3171/jns.2006.105.5.765. [DOI] [PubMed] [Google Scholar]
- 2.Belmonte C, Garcia-Hirschfeld J, Gallar J. Neurobiology of ocular pain. Progr Retinal Eye Res. 1997;16:117–156. [Google Scholar]
- 3.Bereiter DA, Hargreaves KM, Hu JW. Trigeminal mechanisms of nociception: peripheral and brainstem organization. In: Basbaum A, Bushnell MC, editors. Science of Pain. Vol. 5. New York City: Elsevier; 2009. pp. 435–460. [Google Scholar]
- 4.Bergua A, Schrodl F, Neuhuber WL. Vasoactive intestinal and calcitonin gene-related peptides, tyrosine hydroxylase and nitrergic markers in the innervation of the rat central retinal artery. Exp Eye Res. 2003;77:367–374. doi: 10.1016/s0014-4835(03)00121-0. [DOI] [PubMed] [Google Scholar]
- 5.Burstein R, Jakubowski M. Unitary hypothesis for multiple triggers of the pain and strain of migraine. J Comp Neurol. 2005;493:9–14. doi: 10.1002/cne.20688. [DOI] [PubMed] [Google Scholar]
- 6.Burstein R, Yamamura H, Malick A, Strassman AM. Chemical stimulation of the intracranial dura induces enhanced responses to facial stimulation in brain stem trigeminal neurons. J Neurophysiol. 1998;79:964–982. doi: 10.1152/jn.1998.79.2.964. [DOI] [PubMed] [Google Scholar]
- 7.Cioffi G, Granstam E, Alm A. Ocular circulation. In: Kaufman P, Alm A, editors. Alder’s Physiology of the Eye. St Louis: Mosby; 2003. pp. 747–784. [Google Scholar]
- 8.Cordero-Coma M, Anzaar F, Sobrin L, Foster CS. Systemic immunomodulatory therapy in severe dry eye secondary to inflammation. Ocul Immunol Inflamm. 2007;15:99–104. doi: 10.1080/09273940701299354. [DOI] [PubMed] [Google Scholar]
- 9.Custer PL, Reistad CE. Enucleation of blind, painful eyes. Ophthal Plast Reconstr Surg. 2000;16:326–329. doi: 10.1097/00002341-200009000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Cuthbertson S, LeDoux MS, Jones S, Jones J, Zhou Q, Gong S, Ryan P, Reiner A. Localization of preganglionic neurons that innervate choroidal neurons of pterygopalatine ganglion. Invest Ophthalmol Vis Sci. 2003;44:3713–3724. doi: 10.1167/iovs.02-1207. [DOI] [PubMed] [Google Scholar]
- 11.Drummond PD. A quantitative assessment of photophobia in migraine and tension headache. Headache. 1986;26:465–469. doi: 10.1111/j.1526-4610.1986.hed2609465.x. [DOI] [PubMed] [Google Scholar]
- 12.Fugate JM. Physiological basis for discomfort glare. Am J Optom Arch Am Acad Optom. 1957;34:377–387. doi: 10.1097/00006324-195707000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Gamlin PD. The pretectum: connections and oculomotor-related roles. Prog Brain Res. 2006;151:379–405. doi: 10.1016/S0079-6123(05)51012-4. [DOI] [PubMed] [Google Scholar]
- 14.Gutrecht JA, Lessell IM. Photophobia in trigeminal neuralgia. J Neuroophthalmol. 1994;14:122–123. [PubMed] [Google Scholar]
- 15.Hallett M, Evinger C, Jankovic J, Stacy M. Update on blepharospasm: report from the BEBRF International Workshop. Neurology. 2008;71:1275–1282. doi: 10.1212/01.wnl.0000327601.46315.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, Berson DM. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006;497:326–349. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirata H, Hu JW, Bereiter DA. Responses of medullary dorsal horn neurons to corneal stimulation by CO2 pulses in the rat. J Neurophysiol. 1999;82:2092–2107. doi: 10.1152/jn.1999.82.5.2092. [DOI] [PubMed] [Google Scholar]
- 18.Hirata H, Okamoto K, Tashiro A, Bereiter DA. A novel class of neurons at the trigeminal subnucleus interpolaris/caudalis transition region monitors ocular surface fluid status and modulates tear production. J Neurosci. 2004;24:4224–4232. doi: 10.1523/JNEUROSCI.0381-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hosoya Y, Sugiura Y, Ito R, Kohno K. Descending projections from the hypothalamic paraventricular nucleus to the A5 area, including the superior salivatory nucleus, in the rat. Exp Brain Res. 1990;82:513–518. doi: 10.1007/BF00228793. [DOI] [PubMed] [Google Scholar]
- 20.Hyden D, Arlinger S. On light-induced sneezing. Eye. 2009;23:2112–2114. doi: 10.1038/eye.2009.165. [DOI] [PubMed] [Google Scholar]
- 21.Irimia P, Cittadini E, Paemeleire K, Cohen AS, Goadsby PJ. Unilateral photophobia or phonophobia in migraine compared with trigeminal autonomic cephalalgias. Cephalalgia. 2008;28:626–630. doi: 10.1111/j.1468-2982.2008.01565.x. [DOI] [PubMed] [Google Scholar]
- 22.Ishizuka K, Murakami T. Cortically evoked responses of superior salivary nucleus neurons in the cat. Proc Finn Dent Soc. 1989;85:355–359. [PubMed] [Google Scholar]
- 23.Izumi H. Nervous control of blood flow in the orofacial region. Pharmacol Ther. 1999;81:141–161. doi: 10.1016/s0163-7258(98)00040-0. [DOI] [PubMed] [Google Scholar]
- 24.Kelman L. The triggers or precipitants of the acute migraine attack. Cephalalgia. 2007;27:394–402. doi: 10.1111/j.1468-2982.2007.01303.x. [DOI] [PubMed] [Google Scholar]
- 25.Klooster J, Vrensen GF. Anat Embryol. Vol. 198. Berl: 1998. New indirect pathways subserving the pupillary light reflex: projections of the accessory oculomotor nuclei and the periaqueductal gray to the Edinger-Westphal nucleus and the thoracic spinal cord in rats; pp. 123–132. [DOI] [PubMed] [Google Scholar]
- 26.Kowacs PA, Piovesan EJ, Werneck LC, Tatsui CE, Lange MC, Ribas LC, da Silva HP. Influence of intense light stimulation on trigeminal and cervical pain perception thresholds. Cephalalgia. 2001;21:184–188. doi: 10.1046/j.1468-2982.2001.00178.x. [DOI] [PubMed] [Google Scholar]
- 27.Lebensohn JE. Photophobia: mechanism and implications. Am J Ophthalmol. 1951;34:1294–1300. doi: 10.1016/0002-9394(51)91866-1. [DOI] [PubMed] [Google Scholar]
- 28.Lindquist DH, Vogel RW, Steinmetz JE. Associative and non-associative blinking in classically conditioned adult rats. Physiol Behav. 2009;96:399–411. doi: 10.1016/j.physbeh.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Linsenmeier RA. Electrophysiological consequences of retinal hypoxia. Graefes Arch Clin Exp Ophthalmol. 1990;228:143–150. doi: 10.1007/BF00935724. [DOI] [PubMed] [Google Scholar]
- 30.Loewy AD. Autonomic control of the eye. In: Loewy AD, Spyer KM, editors. Central regulation of autonomic functions. New York: Oxford; 1990. pp. 268–285. [Google Scholar]
- 31.MacIver MB, Tanelian DL. Activation of C fibers by metabolic perturbations associated with tourniquet ischemia. Anesthesiology. 1992;76:617–623. doi: 10.1097/00000542-199204000-00020. [DOI] [PubMed] [Google Scholar]
- 32.McMullan S, Lumb BM. Midbrain control of spinal nociception discriminates between responses evoked by myelinated and unmyelinated heat nociceptors in the rat. Pain. 2006;124:59–68. doi: 10.1016/j.pain.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 33.Mintenig GM, Sanchez-Vives MV, Martin C, Gual A, Belmonte C. Sensory receptors in the anterior uvea of the cat’s eye. An in vitro study. Invest Ophthalmol Vis Sci. 1995;36:1615–1624. [PubMed] [Google Scholar]
- 34.Morin LP, Blanchard JH, Provencio I. Retinal ganglion cell projections to the hamster suprachiasmatic nucleus, intergeniculate leaflet, and visual midbrain: bifurcation and melanopsin immunoreactivity. J Comp Neurol. 2003;465:401–416. doi: 10.1002/cne.10881. [DOI] [PubMed] [Google Scholar]
- 35.Moulton EA, Becerra L, Borsook D. An fMRI case report of photophobia: activation of the trigeminal nociceptive pathway. Pain. 2009;145:358–363. doi: 10.1016/j.pain.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murakami T, Ishizuka K, Uchiyama M. Convergence of excitatory inputs from the chorda tympani, glossopharyngeal and vagus nerves onto superior salivatory nucleus neurons in the cat. Neurosci Lett. 1989;105:96–100. doi: 10.1016/0304-3940(89)90018-9. [DOI] [PubMed] [Google Scholar]
- 37.Murakami T, Ishizuka K, Yoshihara M, Uchiyama M. Reflex responses of single salivatory neurons to stimulation of trigeminal sensory branches in the cat. Brain Res. 1983;280:233–237. doi: 10.1016/0006-8993(83)90053-7. [DOI] [PubMed] [Google Scholar]
- 38.Nagaoka T, Yoshida A. The effect of ocular warming on ocular circulation in healthy humans. Arch Ophthalmol. 2004;122:1477–1481. doi: 10.1001/archopht.122.10.1477. [DOI] [PubMed] [Google Scholar]
- 39.Okamoto K, Thompson R, Tashiro A, Chang Z, Bereiter DA. Bright light produces Fos-positive neurons in caudal trigeminal brainstem. Neuroscience. 2009;160:858–864. doi: 10.1016/j.neuroscience.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 40.Recober A, Kuburas A, Zhang Z, Wemmie JA, Anderson MG, Russo AF. Role of calcitonin gene-related peptide in light-aversive behavior: implications for migraine. J Neurosci. 2009;29:8798–8804. doi: 10.1523/JNEUROSCI.1727-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosenbaum JT, Nozik RA. Uveitis: many diseases, one diagnosis. Am J Med. 1985;79:545–547. doi: 10.1016/0002-9343(85)90049-x. [DOI] [PubMed] [Google Scholar]
- 42.Ruocco I, Cuello AC, Parent A, Ribeiro-da-Silva A. Skin blood vessels are simultaneously innervated by sensory, sympathetic, and parasympathetic fibers. J Comp Neurol. 2002;448:323–336. doi: 10.1002/cne.10241. [DOI] [PubMed] [Google Scholar]
- 43.Schepelmann K, Ebersberger A, Pawlak M, Oppmann M, Messlinger K. Response properties of trigeminal brain stem neurons with input from dura mater encephali in the rat. Neuroscience. 1999;90:543–554. doi: 10.1016/s0306-4522(98)00423-0. [DOI] [PubMed] [Google Scholar]
- 44.Schmid E, Leierer J, Doblinger A, Laslop A, Fischer-Colbrie R, Humpel C, Theodorsson E, Teuchner B, Lalehabbasi D, Dragosits E, Kunze C, Philipp W, Gottinger W, Troger J. Neurokinin a is a main constituent of sensory neurons innervating the anterior segment of the eye. Invest Ophthalmol Vis Sci. 2005;46:268–274. doi: 10.1167/iovs.04-0608. [DOI] [PubMed] [Google Scholar]
- 45.Shimura M, Uchida S, Suzuki A, Nakajima K, Aikawa Y. Reflex choroidal blood flow responses of the eyeball following somatic sensory stimulation in rats. Auton Neurosci. 2002;97:35–41. doi: 10.1016/s1566-0702(02)00013-9. [DOI] [PubMed] [Google Scholar]
- 46.Steinle JJ, Krizsan-Agbas D, Smith PG. Regional regulation of choroidal blood flow by autonomic innervation in the rat. Am J Physiol Regul Integr Comp Physiol. 2000;279:R202–209. doi: 10.1152/ajpregu.2000.279.1.R202. [DOI] [PubMed] [Google Scholar]
- 47.Stone RA, Kuwayama Y, Laties AM. Regulatory peptides in the eye. Experientia. 1987;43:791–800. doi: 10.1007/BF01945357. [DOI] [PubMed] [Google Scholar]
- 48.Stringham JM, Fuld K, Wenzel AJ. Spatial properties of photophobia. Invest Ophthalmol Vis Sci. 2004;45:3838–3848. doi: 10.1167/iovs.04-0038. [DOI] [PubMed] [Google Scholar]
- 49.Takeda M, Ikeda M, Tanimoto T, Lipski J, Matsumoto S. Changes of the excitability of rat trigeminal root ganglion neurons evoked by alpha(2)-adrenoreceptors. Neuroscience. 2002;115:731–741. doi: 10.1016/s0306-4522(02)00481-5. [DOI] [PubMed] [Google Scholar]
- 50.ten Tusscher MP, Klooster J, van der Want JJ, Lamers WP, Vrensen GF. The allocation of nerve fibres to the anterior eye segment and peripheral ganglia of rats. II. The sympathetic innervation. Brain Res. 1989a;494:105–113. doi: 10.1016/0006-8993(89)90148-0. [DOI] [PubMed] [Google Scholar]
- 51.ten Tusscher MP, Klooster J, Want JJvd, Lamers WP, Vrensen GF. The allocation of nerve fibres to the anterior eye segment and peripheral ganglia of rats. I. The sensory innervation. Brain Res. 1989b;494:95–104. doi: 10.1016/0006-8993(89)90147-9. [DOI] [PubMed] [Google Scholar]
- 52.Terenghi G, Polak JM, Ghatei MA, Mulderry PK, Butler JM, Unger WG, Bloom SR. Distribution and origin of calcitonin gene-related peptide (CGRP) immunoreactivity in the sensory innervation of the mammalian eye. J Comp Neurol. 1985;233:506–516. doi: 10.1002/cne.902330410. [DOI] [PubMed] [Google Scholar]
- 53.Troger J, Kieselbach G, Teuchner B, Kralinger M, Nguyen QA, Haas G, Yayan J, Gottinger W, Schmid E. Peptidergic nerves in the eye, their source and potential pathophysiological relevance. Brain Res Rev. 2007;53:39–62. doi: 10.1016/j.brainresrev.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 54.Vanagaite J, Pareja JA, Storen O, White LR, Sand T, Stovner LJ. Light-induced discomfort and pain in migraine. Cephalalgia. 1997;17:733–741. doi: 10.1046/j.1468-2982.1997.1707733.x. [DOI] [PubMed] [Google Scholar]
- 55.Woodhouse A, Drummond PD. Mechanisms of increased sensitivity to noise and light in migraine headache. Cephalalgia. 1993;13:417–421. doi: 10.1046/j.1468-2982.1993.1306417.x. [DOI] [PubMed] [Google Scholar]
- 56.Young MJ, Lund RD. The anatomical substrates subserving the pupillary light reflex in rats: origin of the consensual pupillary response. Neuroscience. 1994;62:481–496. doi: 10.1016/0306-4522(94)90381-6. [DOI] [PubMed] [Google Scholar]
- 57.Zuazo A, Ibanez J, Belmonte C. Sensory nerve responses elicited by experimental ocular hypertension. Exp Eye Res. 1986;43:759–769. doi: 10.1016/s0014-4835(86)80007-0. [DOI] [PubMed] [Google Scholar]