Abstract
A bovine acellular scaffold was found to facilitate tissue remodeling in a rat model of vocal fold injury, whereas hepatocyte growth factor (HGF) has been shown to have an anti-scarring effect in the larynx. This study examined the loading and release kinetics of HGF in vitro, and the potential of the acellular scaffold as a timed-release system for the delivery of HGF in vivo. Bilateral wounds were created in the posterior vocal folds of 20 rats, with HGF-loaded acellular scaffolds implanted into the wounds unilaterally, and scaffolds without HGF implanted into the contralateral vocal folds as control. The rats were humanely sacrificed after 3, 7, 30, and 90 days and their larynges were examined histologically and immunohistochemically. Expressions of key matrix proteins in the vocal fold coronal sections were quantified by digital image analysis. Results demonstrated a gradual, sustained release of HGF for at least 7 days in vitro, consistent with the detection of glycosaminoglycans inherent of the scaffold. In rat vocal folds implanted with HGF-loaded scaffolds, apparently fewer inflammatory cells were observed 3 days after surgery when compared to the control. The mean relative densities of collagen III and hyaluronic acid were significantly lower than those of the control 7 days after surgery. Scaffold implants were apparently degraded by 3 months in all animals, with no evidence of fibrosis or calcification. These data suggested that the bovine acellular scaffold could be promising for the exogenous delivery of select growth factors in vivo.
Keywords: larynx, biologic scaffold, decellularization, drug delivery, inflammatory response
INTRODUCTION
It was recently demonstrated that a biological extracellular matrix (ECM) scaffold fabricated by decellularization of the bovine vocal fold lamina propria could potentially become a promising surgical implant for vocal fold tissue repair and regeneration.1–3 The major constituents of acellular ECM scaffolds are various macromolecules including proteins, such as collagens, elastin, and adhesive glycoproteins, polysaccharides such as hyaluronic acid and glycosaminoglycans (GAGs), as well as hybrid peptide-sugar molecules known as proteoglycans, which consist of long-chain polysaccharides made of repeating disaccharide units synthesized as covalent, post-translational modifications of proteins.4, 5 All vertebrate cells synthesize proteoglycans and most of them are incorporated into the ECM. Given that many polypeptide growth factors strongly bind to these proteoglycans, the ECM is capable of concentrating circulating growth factors at specific locations and releasing them locally over a period of time, i.e., in a timed-release fashion.4 It has been shown that the incorporation of GAGs in various biomaterials, such as collagen and alginate, may result in the binding, modulation, and sustained release of biologically active growth factors.6, 7 Hence it is also likely that the GAGs inherent of acellular ECM scaffolds can serve as a reservoir for loading and releasing growth factors for specific biological functions in vivo.
Vocal fold scarring often occurs after injury of the lamina propria as part of the wound healing inflammatory response, resulting in severe dysphonia characterized by fibrosis, tissue stiffening and significantly impaired vocal fold vibration that is clinically very challenging.8 Hepatocyte growth factor (HGF), a multifunctional polypeptide involved in embryogenesis, angiogenesis, and tissue regeneration, has shown strong anti-fibrotic activity in different organ systems such as kidney, liver, and lung in animal studies.9 Recent studies have also supported its therapeutic potential in treating vocal fold scarring, as observed in cultures of human and canine fibroblasts as well as in a rabbit model of vocal fold injury.10–12 However, the local biological activity of exogenous HGF may be limited in vivo without a drug delivery system, since its half-life in circulation has been shown to be only approximately 5 min. due to its disposal by diffusion and rapid clearance by the liver.13–15
In our previous studies,1–3 an acellular ECM scaffold was developed and was shown to have favorable pore properties to promote host cell ingrowths and tissue remodeling. In the present study, the capability of the acellular scaffold for the timed-release of HGF was assessed, including the release kinetics of HGF in vitro. Through a rat model of vocal fold injury, acellular scaffolds loaded with HGF and control scaffolds without HGF were implanted into contralateral vocal folds. The subsequent laryngeal host response was examined histologically and immunohistochemically at different time points, with quantitative digital image analysis evaluating the expressions of key matrix proteins, including collagen I, collagen III, elastin, fibronectin, hyaluronic acid, and GAGs, in order to examine the therapeutic efficacy of the HGF-loaded bovine acellular scaffold in the vocal fold in vivo.
METHODS AND MATERIALS
Fabrication of bovine acellular scaffolds
Excised bovine larynges were obtained from healthy steers and heifers of approximately 30 months old, with body weight ranging from around 600 lbs. to 1300 lbs. Twelve larynges were procured from an abattoir immediately post-mortem following slaughtering of the animals. The animals were sacrificed for the purpose of meat packing instead of specifically for the present study. The animal euthanasia protocol was approved by the U.S. Department of Agriculture Meat and Poultry Inspection Program, and was consistent with the PHS Policy on Humane Care and Use of Laboratory Animals. The excised larynges were stored in phosphate-buffered saline (PBS) solution, the in situ length of each vocal fold was measured and a total of 24 vocal fold lamina propria specimens were dissected using phonomicrosurgical instruments. Acellular scaffolds were fabricated from the lamina propria using a novel decellularization method as described before.1, 3 Briefly, lamina propria specimens were first mounted on plastic frames to sustain an in situ tissue tension and then underwent sequential incubation in 3M sodium chloride solution, isotonic PBS solution with DNAse and RNAse, and 75% ethanol. The resultant scaffolds were sterilized using 70% ethanol followed by UV light and were stored in PBS at 4°C overnight. Finally the acellular scaffolds were freeze-dried under aseptic conditions and stored in liquid nitrogen.
Fabrication of HGF-loaded acellular scaffolds
To incorporate HGF, five lyophilized scaffolds were re-hydrated in a loading solution (filter-sterilized PBS solution) with 1µg /ml human recombinant HGF (Sigma, St. Louis, MO) at 4°C for 12 hours. Five other freeze-dried acellular scaffolds were also soaked in the loading solution without HGF, serving as control scaffolds. All scaffolds were immediately used for both in vitro and in vivo studies after this HGF loading procedure was completed.
Quantification of glycosaminoglycans in bovine acellular scaffolds
The content of GAGs in freeze-dried acellular scaffolds was quantified colorimetrically following the methods described by Rider.16 In brief, a working staining solution was prepared by dissolving 1, 9-dimethylmethylene blue (Sigma, St. Louis, MO) in deionized water with glycine, NaCl and HCl. Six freeze-dried acellular scaffold samples were weighted and then homogenized in 4ml PBS using a mechanical homogenizer (VWR, West Chester, PA). The resulting homogenates were subjected to centrifugation at 3000 rpm for 8 min. and the supernatant was collected. In a microtiter well plate, 30µl of heparan as a GAG standard (Acros, Morris Plains, NJ) or the homogenate samples were added to each well in triplicate. 200µl of dimethylmethylene blue working solution was then added to each well and the plate was read immediately at 513 nm, using a µQuant multi-well plate reader (BioTek Instruments, Winooski, VT).
In vitro release kinetics of HGF-loaded acellular scaffolds
Five HGF-loaded acellular scaffold samples and five control scaffold samples were used for determining the loading and release kinetics of HGF. The scaffold samples were first washed in 1.0ml PBS for 10 min. twice in order to remove all unbound HGF molecules. Each scaffold was incubated in a release medium (filter-sterilized PBS solution) at 4°C with continuous mechanical agitation. The release medium was replaced daily and its volume was measured. The concentration of HGF in the release medium was determined with direct enzyme-linked immunosorbent assay (ELISA). In brief, each well in a 96-well plate was coated with antigens in triplicate, by incubating 50µl of the samples or HGF standards (Sigma, St. Louis, MO) at 4°C overnight. Before coating, samples were centrifuged at 500g for 3 min. to remove any debris due to ECM scaffold degradation. The coated well plate was washed with washing buffer and incubated with 100µl of blocking buffer, and PBS with 1% bovine serum albumin for 2 hours at room temperature. A mouse anti-human HGF antibody (R&D systems, Minneapolis, MN) was added to the 96-well plate at room temperature for 3 hours, followed by extensive wash in a wash buffer. The well plate was incubated with HRP conjugated anti-mouse secondary antibody (Sigma, St. Louis, MO) for 2 hours at room temperature. An o-phenylenediamine dihydrochloride substrate (Sigma, St. Louis, MO) was added for visualization and sulfuric acid was added to stop the reaction after 10 min. The µQuant multi-well plate reader was used to detect the absorbance of the dyes at 492 nm.
Animal surgery
Twenty adult male Sprague-Dawley rats ranging from 400 to 500g were purchased from the Charles River Laboratories, Inc. (Wilmington, MA). The rats were housed in separate cages at room temperature and daily records were maintained. The experimental protocol was approved by the Institutional Animal Care and Use Committee of UT Southwestern Medical Center. The rat surgical procedure was similar to that of our previous study.3 Briefly, the rat was placed on a customized operating platform in supine position after anesthesia. An otoscope specula with a 4.0 mm orifice (Welch Allyn Inc., Skaneateles Falls, NY) was inserted through the mouth to aid in visualization of the vocal fold as well as to allow for insertion of phonomicrosurgical instruments. Topical anesthesia of 100µl 2% lidocaine (Lidoject, Butler Animal Health Supply, Dublin, OH) was applied to the vocal fold with a pipet, to reduce respiratory movement. The surgical incision sites were located about 0.8 mm lateral to the medial edges of the vocal folds and a 1-mm long longitudinal incision with a depth of 0.5–2.0 mm was made on each vocal fold. Before the implantation, each acellular scaffold was cut into a rectangular section of around 1.5mm by 0.5mm, with the thickness ranging from 0.5mm to 0.75mm. Microforceps were used to deliver the HGF-loaded scaffolds into the right vocal fold wounds and control scaffolds into the left vocal fold wounds. Each scaffold was secured and mechanically stable in the implant site since the scaffold was larger than that of the incision, with no suturing or surgical glue (e.g., fibrin glue) required to close the wounds.3 During the surgery, oxygen was supplied to the animal and its heart rate and oxygen saturation were also closely monitored by a veterinarian vital signs monitor (Model SurgiVet V9200, Smiths Medical PM, Waukesha, WI).
After surgery, the rats were returned to the housing facility after they were fully recovered. For the first 3 days after the surgery, the antibiotics sulfamethoxazole/trimethoprim (Hi-Tech Pharmacal Co., Amityville, NY) mixed in the soft diet Nutra-Gel (Bio-Serv, Frenchtown, NJ) was provided to facilitate their recovery as well as to prevent possible infection, given that the operation was conducted in the non-sterile airway. Rats were humanely sacrificed after 3, 7, 30, and 90 days (5 rats at each time point) by an intraperitoneal injection of Euthasol (Virbac AH, Fort Worth, TX), a solution of 390mg pentobarbitol sodium and 50mg phenytoin sodium per ml. Their larynges were excised and stored in 10% formalin for subsequent histological examination.
Histological examination with H&E and Alcian Blue staining
The host response to the scaffold implant was assessed by hematoxylin-eosin (H&E) staining, similar to Xu et al.3 In brief, the rat larynges were fixed overnight in 10% formalin, before paraffin embedding and sectioning using a microtome (RM 2255, Leica, Bannockburn, IL), at a thickness of 5.0 µm at the coronal plane of the larynx. Sample sections were stained with Harris hematoxylin and eosin Y solution (Sigma, St. Louis, MO) following standard protocol. The stained sections were protected by glass cover-slips after dehydrated with 95% and 100% alcohol, and cleared with xylene.
Glycosaminoglycans (GAGs) inherent of the acellular scaffolds as well as those synthesized by the host fibroblasts were detected by Alcian blue.3 In brief, acellular scaffold samples as well as paraffin-embedded sections of the laryngeal specimens were incubated in 1% Alcian blue solution (1.0% Alcian blue 8GX in 3.0% acetic acid solution, pH = 2.5) for 30 min. at room temperature, washed in tap water for 2 min., followed by rinsing in distilled water for 5 min.
Immunohistochemical examination
The immunohistochemical staining procedure for examining collagen I, collagen III, elastin and fibronectin was similar to that of our previous study.3 Antibodies were carefully selected to specifically bind the proteins synthesized by the host (rats) rather than those from the bovine scaffold. Briefly, laryngeal coronal section slides were deparaffinized and the sections were incubated in 20 µg/ml Proteinase K (Sigma, St. Louis, MO) epitope retrieval solution for 30 min. at 37°C to retrieve type I collagen, type III collagen, elastin, and fibronectin. The slides were subjected to incubation of a peroxidase suppressor (Pierce Biotechnology, Rockford, IL) for 30 min. and a blocking buffer (Pierce Biotechnology, Rockford, IL) for 30 min., so as to quench endogenous peroxidase activity and possible non-specific binding. Next, the primary antibody was applied overnight at 4°C, followed by a horseradish peroxidase (HRP)-conjugated secondary antibody for two hours at room temperature with washes in between. The binding of the antibody was located by a metal enhanced diaminobenzidine (DAB) substrate (Pierce Biotechnology, Rockford, IL) for 1 to 3 min.
For the detection of hyaluronic acid, the primary antibody was biotinylated hyaluronic acid binding protein (HABP) (Calbiochem, San Diego, CA), which binds specifically and strongly to hyaluronic acid.17 After incubation with HABP overnight at 4°C, deparaffinized sections were incubated with HRP-conjugated streptavidin (EMD, San Diego, CA) for 40 min. with washes in between. The streptavidin label was detected with the metal enhanced DAB peroxidase substrate working solution (Pierce Biotechnology, Rockford, IL). Epiglottic and tracheal tissues were used as positive control, whereas laryngeal sections with the primary antibody replaced by negative serum were used as negative control.3
Quantitative digital image analysis of histological sections
Images of the laryngeal section slides were captured using a Leica DM200 microscope (Bannockburn, IL) and a MicroFire microscope digital CCD camera (Optronics, Goleta, CA). The relative densities of collagen I, collagen III, elastin, fibronectin, hyaluronic acid and GAGs were estimated from their relative areas and staining intensities by digital image analysis using NIH Image J (Bethesda, MD), as described in Xu et al.3 Briefly, color images were first converted to 8-bit grayscale images, with the contrast improved by expanding the grayscale range of the histogram of pixel intensity to cover the full range from 0 (black) to 255 (white), using Photoshop (Adobe, San Jose, CA). The images were also processed to compensate for any non-uniform illumination of micrographs. For each image, thresholds were selected by visual comparisons with its corresponding H&E image such that pixels representing the areas of a molecule of interest in the grayscale image were clearly shown with a minimum background intensity. Pixels with intensities between the lower and the upper thresholds were deemed representative of the molecule of interest. All raters of the images were blinded to the specimen identity. For each larynx, the experimental (right) and the control (left) vocal folds were included in the same image, such that the same threshold intensity values were always applied to both vocal folds, avoiding any bias. The relative density of the molecule of interest expressed across the vocal fold area of each laryngeal coronal section was assessed. It was calculated as the fraction of the positively stained area to the total area of the implanted scaffold and the vocal fold tissues, including all soft tissues, i.e., muscles and connective tissues, but excluding cartilaginous and supraglottic tissues. The reliability of this image analysis procedure was determined previously, with errors lower than 4% in all cases.3
Statistical analysis
For results of the digital image analysis, paired Student’s t tests were conducted to determine if expressions of the ECM components in the left versus the right vocal folds were significantly different from one another, i.e., comparing their relative densities in the vocal fold implanted with the HGF-loaded scaffold vs. those in the vocal fold implanted with the control scaffolds without HGF. The level of significance (alpha) was set at 0.05.
RESULTS
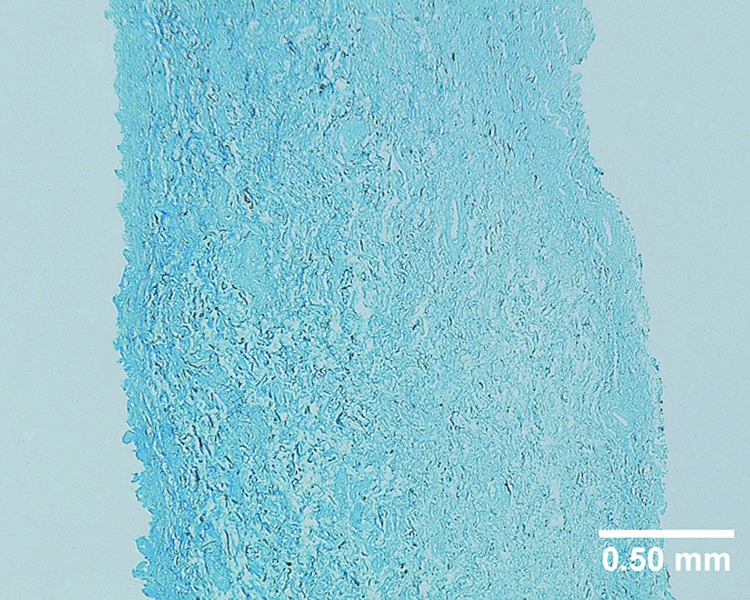
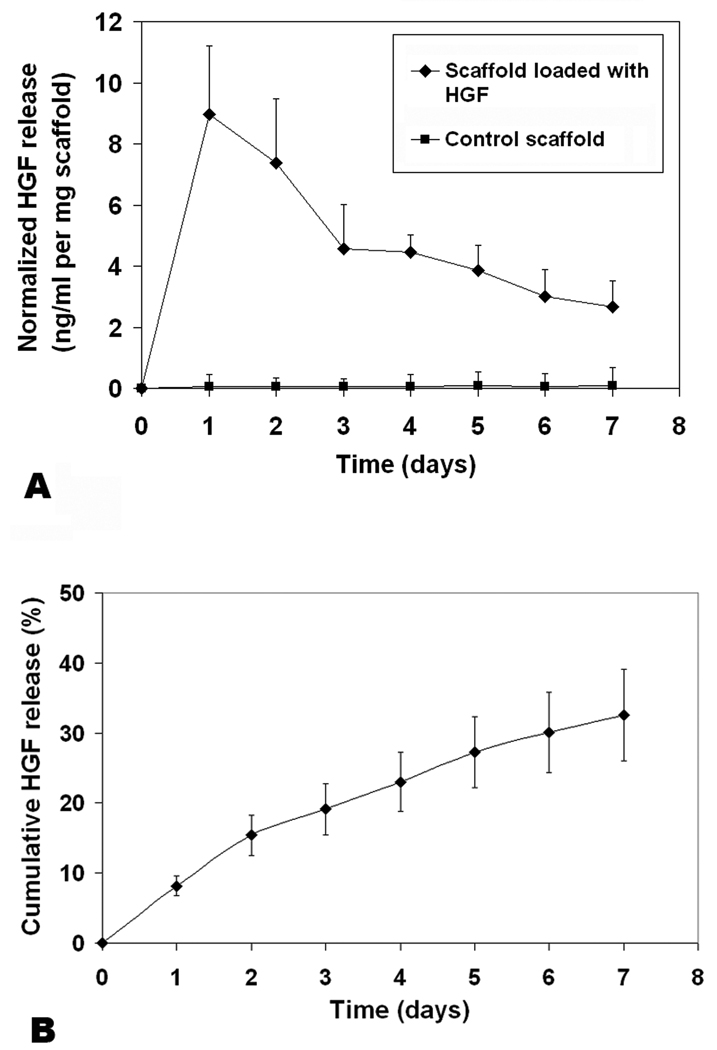
A uniform, intense color of light blue can be seen on the coronal section of the bovine acellular scaffold stained with Alcian blue (Figure 1), indicating the widespread distribution of glycosaminoglycans (GAGs) throughout the acellular scaffold. Results of the colorimetric assay with dimethylmethylene blue showed that 0.297% ± 0.07% of the dry weight of an acellular scaffold comprised of GAGs (n = 6), consistent with the level of GAGs reported in other acellular matrices.18 Since GAGs are the major components of proteoglycans, these findings indicated that proteoglycans inherent of the bovine vocal fold lamina propria were likely preserved following the saline-based decellularization process, despite the cycles of induced osmotic stresses. To assess the capability of the acellular scaffold for loading and releasing hepatocyte growth factor (HGF), in vitro HGF loading and release kinetics studies were conducted. Results of ELISA indicated that 9.01 ng ± 1.59 ng of HGF was loaded for each mg (dry weight) of the bovine acellular scaffold after an incubation in loading solution with 1.0 µg/ml HGF for 12 hours (n = 5). Figure 2 shows the rate of release of HGF from the acellular scaffold over a 7-day period. It was apparent that there was an initial release burst in the first 2–3 days, where HGF was released slightly more rapidly, when compared to the next five days, where HGF was gradually released in a sustained manner (Fig. 2). By 7 days, the cumulative amount of HGF released from the HGF-loaded scaffold was 32.6 % ± 6.5 % (n = 5) (Fig. 2B). As reported in Lai et al.,18 the amount of GAGs in an acellular scaffold is positively associated with the growth factor loading capacity and negatively correlated with the initial release burst as well as the cumulative release of the growth factor. Thus, the findings of a high HGF loading capacity, a less significant initial burst as well as a low cumulative amount of the growth factor being released were consistent with a relatively high level of GAGs inherent of the bovine acellular scaffold, supporting its potential as a reservoir of HGF and a vehicle for HGF delivery. Nonetheless, further studies are required to verify that the bioactivity of HGF would not be adversely affected by the processes of loading and release.
Figure 1.
Coronal section of a typical bovine acellular scaffold section stained with Alcian blue, demonstrating the widespread distribution of glycosaminoglycans (total magnification = 40 ×).
Figure 2.
(A) Profiles of hepatocyte growth factor (HGF) released from bovine acellular scaffolds loaded with HGF (9.01 ng HGF per mg scaffold) and from control scaffolds without HGF as a function of time (n = 5). Normalized concentration of HGF released (ng/ml per mg scaffold) is shown on the y-axis. (B) Cumulative levels of HGF released from the HGF-loaded scaffolds as a function of time (n = 5). Percentage of the HGF released with reference to the total HGF loaded is shown on the y-axis.
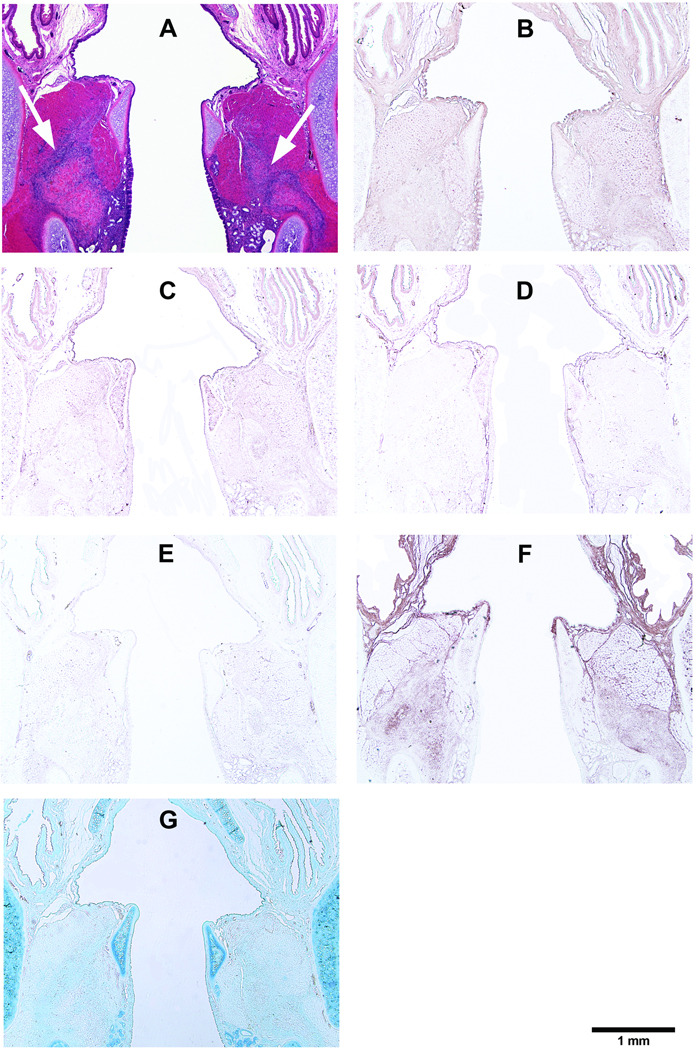
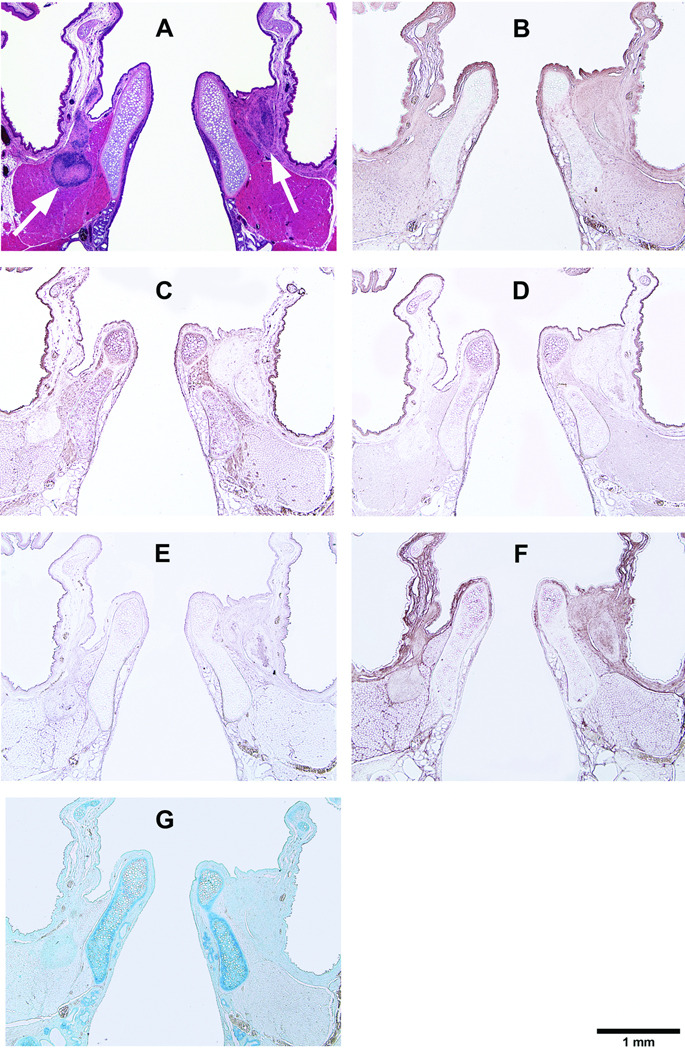
Corresponding to the location of the acellular scaffold implants, coronal sections of the rat laryngeal specimens were obtained at a plane approximately 1100 µm to 1350 µm posterior to the anterior commissure of the vocal folds. Figure 3 and Figure 4–Figure 7 show the typical histological sections of the laryngeal specimens obtained at the various time points (3 days, 7 days, 30 days, and 90 days, respectively, for Fig. 4–Fig. 7) following implantation of the acellular scaffolds. The host response to the HGF-loaded scaffolds and the control scaffolds without HGF is illustrated in the H&E sections (Fig. 3, Fig. 4A, Fig. 5A, Fig. 6A, Fig. 7A). Figure 3 shows the H&E sections at a higher magnification (with a total magnification of up to 400 ×) in order to better illustrate the laryngeal host response triggered by the HGF-loaded scaffolds and the control scaffolds. Three days after surgery, inflammatory cells including lymphocytes, eosinophils, polymorphonuclear neutrophils (PMNs) have infiltrated almost the entire control scaffold implant in the left vocal fold, whereas apparently fewer inflammatory cells can be observed in the HGF-loaded scaffold implant in the right vocal fold (Fig. 3A). Higher levels of proliferation and infiltration of inflammatory cells in the implants of both vocal folds were apparent in day 7 compared to those in day 3 (Fig. 3B), but there seemed to be no significant differences between the two vocal folds. Also, macrophages could be identified in both vocal folds by 7 days. After 30 days (Fig. 3C), both the HGF-loaded scaffold and the control scaffold implants were fully occupied by host inflammatory cells, as well as fibroblasts and foreign body giant cells, as the number of inflammatory cells decreased in the control scaffold and can be seen to accumulate around the edge of the implant. At the final time point (after 90 days), similar to our previous study,3 the scaffolds in both vocal folds were apparently fully degraded or resorbed without any evidence of calcification or fibrosis (Fig. 3D).
Figure 3.
Histological coronal sections of rat laryngeal specimens stained with H&E, showing the HGF-loaded acellular scaffolds implanted into the right vocal fold wounds and control scaffolds without HGF implanted into the left vocal fold wounds (A) 3 days, (B) 7 days, (C) 30 days, and (D) 90 days after surgery (total magnification = 40 ×). Arrows indicate the implants in the experimental (right) vocal folds and the control (left) vocal folds. Parts (1), (2) and (3) show the interfaces between the control scaffolds and the host tissue 3 days, 7 days and 30 days after surgery, respectively, at a higher magnification (400 ×). Parts (4), (5) and (6) show the interfaces between the HGF-loaded scaffolds and the host tissue 3 days, 7 days and 30 days after surgery, respectively, at a higher magnification (400 ×) (AC = arytenoid cartilage; TA = thyroarytenoid muscle; TC = thyroid cartilage; S = implanted acellular scaffold).
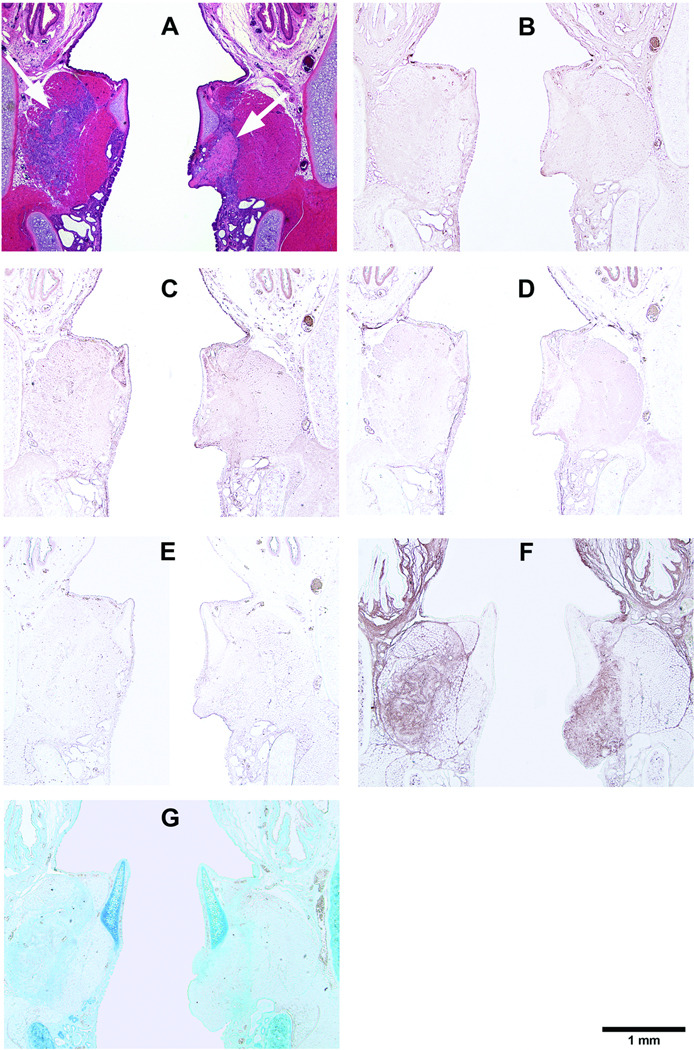
Figure 4.
Histological coronal sections of rat larynges showing HGF-loaded acellular scaffolds implanted into the right vocal fold wounds 3 days after surgery, with control scaffolds without HGF implanted into the left vocal fold wounds. (A) H&E (arrows indicating the implants on both sides); (B) Collagen type I; (C) Collagen type III; (D) Elastin; (E) Fibronectin; (F) Hyaluronic acid; and (G) Glycosaminoglycans.
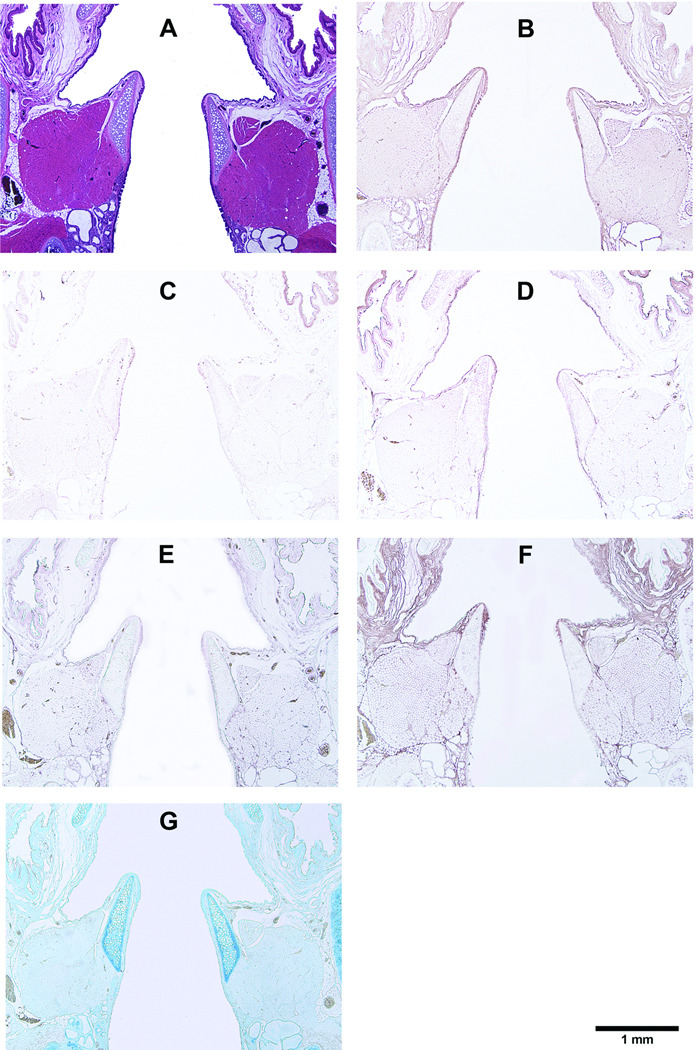
Figure 7.
Histological coronal sections of rat larynges showing HGF-loaded acellular scaffolds implanted into the right vocal fold wounds 90 days after surgery, with control scaffolds without HGF implanted into the left vocal fold wounds. (A) H&E (arrows indicating the implants on both sides); (B) Collagen type I; (C) Collagen type III; (D) Elastin; (E) Fibronectin; (F) Hyaluronic acid; and (G) Glycosaminoglycans.
Figure 5.
Histological coronal sections of rat larynges showing HGF-loaded acellular scaffolds implanted into the right vocal fold wounds 7 days after surgery, with control scaffolds without HGF implanted into the left vocal fold wounds. (A) H&E (arrows indicating the implants on both sides); (B) Collagen type I; (C) Collagen type III; (D) Elastin; (E) Fibronectin; (F) Hyaluronic acid; and (G) Glycosaminoglycans.
Figure 6.
Histological coronal sections of rat larynges showing HGF-loaded acellular scaffolds implanted into the right vocal fold wounds 30 days after surgery, with control scaffolds without HGF implanted into the left vocal fold wounds. (A) H&E (arrows indicating the implants on both sides); (B) Collagen type I; (C) Collagen type III; (D) Elastin; (E) Fibronectin; (F) Hyaluronic acid; and (G) Glycosaminoglycans.
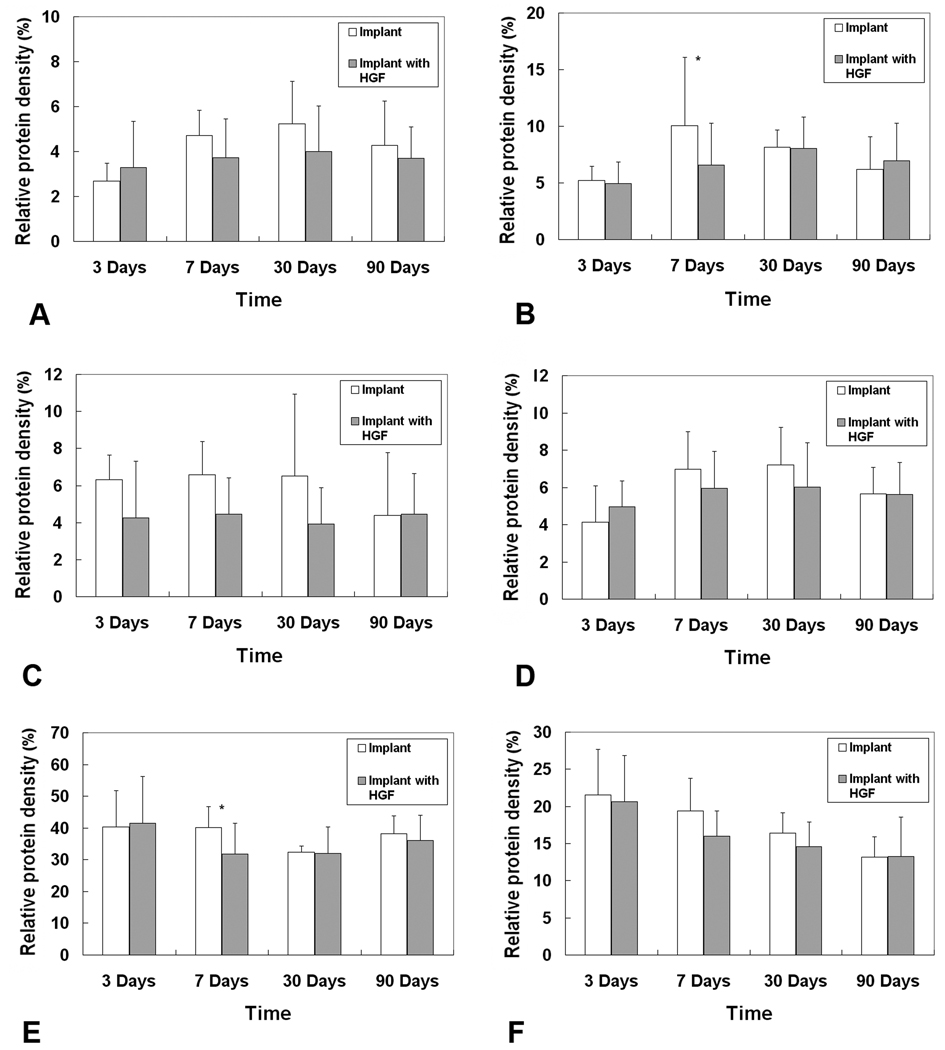
Results of digital image analysis showed that the synthesis and expressions of fibrous proteins (collagen I and III, elastin) were generally attenuated by HGF-loaded scaffolds as compared to control scaffolds (Figure 8). Although statistically significant differences were observed only for collagen III in day 7 (p = 0.047), marginally significant differences were also observed for collagen I in day 7 (p = 0.061) and day 30 (p = 0.062), and elastin in day 3 (p = 0.053) (Table 1). For other ECM components, the mean levels of fibronectin in the experimental vocal fold were lower than those in the control vocal fold, except for day 3 (Fig. 8D), but without statistically significant differences (Table 1). For hyaluronic acid (HA), its mean relative intensities in the experimental vocal folds were close to those of the control (Fig. 8E), except for day 7 where the HA level in the experimental group was significantly lower than the control (Table 1). Also, the expressions of GAGs seemed to be lowered by the HGF-loaded scaffolds as compared to the control scaffolds, although the differences were not statistically significant (Fig. 8F).
Figure 8.
Expressions of ECM proteins in the rat vocal fold implanted with the HGF-loaded acellular scaffold versus those in the vocal fold implanted with the control scaffold without HGF (n = 5): (A) Collagen type I; (B) Collagen type III; (C) Elastin; (D) Fibronectin; (E) Hyaluronic acid; (F) Glycosaminoglycans. * p < 0.05 for differences between the two groups.
Table 1.
Results of paired Student’s t tests (degree of freedom = 4) on the relative densities of ECM proteins in the vocal fold implanted with HGF-loaded scaffolds versus those in the vocal fold implanted with control scaffolds without HGF (n = 5).
| Time after implantation |
Collagen I | Collagen III | Elastin | Fibronectin | Hyaluronic Acid |
GAGsa | |
|---|---|---|---|---|---|---|---|
| 3 days |
t | 0.61 | −0.26 | −2.08 | 1.43 | 0.16 | −0.3 |
| p value | 0.287 | 0.403 | 0.053 | 0.113 | 0.439 | 0.389 | |
| 7 days |
t | −1.96 | −2.19 | −1.79 | −0.73 | −2.60 | −1.62 |
| p value | 0.061 | 0.047 | 0.074 | 0.252 | 0.030 | 0.090 | |
| 30 days |
t | −1.95 | −0.19 | −1.78 | −1.31 | −0.13 | −0.97 |
| p value | 0.062 | 0.428 | 0.074 | 0.131 | 0.452 | 0.194 | |
| 90 days |
t | −0.71 | 0.40 | 0.07 | −0.05 | −1.44 | 0.02 |
| p value | 0.259 | 0.355 | 0.474 | 0.481 | 0.112 | 0.492 | |
GAGs = glycosaminoglycans
DISCUSSION
Acellular scaffolds often demonstrate the capability to bind exogenous growth factors with their inherent glycosaminoglycans (GAGs), and with a controlled timed-release, they contribute to direct the matrix remodeling process by the host cells.18 In the present study, as GAGs in the bovine vocal fold lamina propria are water soluble, they could have been partially removed during the cycles of osmotic stresses induced during the saline-based decellularization process. Hence, the relative content of GAGs remaining in the bovine acellular scaffold could determine the levels of available growth factors and eventually the release and actions of the growth factors in vivo. Our results showed that GAGs were indeed found in the acellular scaffold despite having undergone decellularization (Fig. 1). The level of GAGs in the acellular scaffold (0.297% by weight) was similar to that of acellular matrices derived from the bovine pericardia (0.09 to 0.57% by weight).18 The GAGs inherent of the bovine acellular scaffold could facilitate the loading and the release of growth factors in a timed-release fashion. This was consistent with the observed in vitro HGF release profile (Fig. 2), which indicated that HGF could be loaded into the acellular scaffold and gradually released for over at least 7 days. Despite these promising results, further studies are required to determine the quantitative relationship between the level of HGF released, the degradation of HGF, and the activity of the HGF protein as a function of the GAG content inherent of the scaffold. Optimal methods for more accurate loading and delivery of growth factors through the modulation of GAGs in the acellular scaffold should be explored.
The H&E histological sections showed that on day 3, infiltration of acute inflammatory cells (Fig. 3 and Fig. 4A), including a large amount of lymphocytes, seemed to be delayed in the right vocal fold with the HGF-loaded scaffolds implanted. This finding was consistent with the observations of HGF attenuating the attachment of monocytes in vitro and the sequestration of macrophages in the rat kidney.19 However, by day 7, the intensity of the inflammatory reaction appeared to be similar in both vocal folds, with or without HGF loading. Indeed, in the present study, cells were only identified by their morphology and the photomicrographs were not evaluated at multiple levels of sectioning. Moreover, Badylak and Gilbert20 reported that xenografts developed from ECM scaffolds elicited mainly Th2 lymphocyte response and M2 macrophage response, which were believed to be associated with anti-inflammatory wound healing and graft acceptance. Hence, in order to further examine the immune response that would be triggered by bovine acellular scaffolds with or without HGF, and the effect of such immune response upon downstream remodeling events, quantitative assessment of the degree of inflammation at multiple levels of histological sectioning as well as accurate identification and quantification of inflammatory cells using specific phenotypic markers should be pursued in future studies.
Results of the quantitative histological analysis indicated that the expressions of fibrous proteins including collagen I, collagen III and elastin synthesized by the host cells were generally reduced in the presence of HGF (Fig. 8). This finding was consistent with the well-documented anti-scarring properties of HGF.9 According to the manufacturer, the anti-type I collagen antibody could cross-react slightly with type III collagen but not vice versa. However, since the areas of sections stained by the anti-type I collagen antibody and by the anti-type III collagen antibody did not match with each other, any cross reaction of the anti-collagen type I antibody with collagen III was deemed negligible in the present study.3
The expression of fibronectin was elevated in the presence of HGF in the acute stage of injury (in day 3), agreeing with previous results observed in cultures of canine vocal fold fibroblasts.10 The fibronectin levels in vocal folds implanted with HGF-loaded scaffolds started to decrease afterwards and were below those of the control vocal folds at the subsequent time points. Given that fibronectin has shown chemoattractant properties for fibroblasts and other cells such as monocytes during wound healing,21 the decrease in fibronectin could be related to the observed attenuation of inflammatory cells infiltrating the HGF-loaded scaffolds (Fig. 3).
Although HGF has been reported to increase HA production by human vocal fold fibroblasts,11 the mean level of HA was only slightly increased in day 3 in vocal folds implanted with HGF-loaded scaffolds. By day 7, the level of HA in the experimental group was actually statistically lower than that of the control. A previous report on acute vocal fold scarring in a rat model showed a maximum increase in HA production 5 days post-surgery, followed by a substantial decrease by 7 days.22 No significant difference in HA level was observed between the scarred vocal folds and the control. Similar trends in HA changes were also reported in a rabbit vocal fold scarring model.23 Such transient changes in HA in early-stage wound healing were also observed in the present study in the vocal folds with HGF-loaded scaffold implants, but not in the control vocal folds. According to our previous study,3 the acellular scaffold alone could actually increase HA production, although not reaching statistical significance. Furthermore, even though HA is often considered as anti-fibrotic and it is a key to the maintenance of optimal viscoelasticity of the vocal folds,8, 24 HA has also been shown to be associated with xenograft rejection.25 Based on these somewhat conflicting findings in the literature, our findings highlighted the need for further studies to elucidate the effect of the acellular scaffold on HA production as a function of HGF loading and release. No difference in HA level was observed between the two groups after 30 days and 90 days.
Comparing to the results of our previous study in which acellular scaffolds were only implanted into one vocal fold in each rat,3 the relative levels of ECM proteins in the present study seemed to be higher by 90 days, except for GAGs. Furthermore, peak levels of collagen I, collagen III and elastin were reached in day 7 or day 30 in the present study, instead of at the earlier time point (day 3) in the previous study.3 This could be a consequence of the bilateral implantation, which might have increased the host response level, possibly affecting the overall level of protein synthesis as the wound healing progressed. Proteoglycans and GAGs including HA, decorin, biglycan, and fibromodulin contribute to regulate various important ECM functions and properties such as biocompatibility, fibrous protein formation, and viscoelastic properties.24 The observed lower levels of GAGs in vocal folds implanted with HGF-loaded scaffolds (Fig. 8) may be related to the delayed infiltration of host fibroblasts surrounding the implant, but further studies are required to examine the exact effect of HGF on different proteoglycans and GAGs individually. In future studies, more quantitative methods such as homogenization of vocal folds en bloc followed by enzyme-linked immunosorbent assays (ELISAs) could be used to better quantify the levels of the various ECM proteins as compared to the present study. Also, real-time polymerase chain reaction (RT-PCR) could be adopted to examine the gene expressions of host cells following the implantation.
Despite the general differences found between the experimental and the control vocal folds, it seemed that the HGF-loaded scaffold showed a rather weak effect in the current study. It is postulated that the effect of HGF on the host cells may have been attenuated by the cytokine signaling associated with the wound healing as well as the host reaction to the acellular scaffolds. Also, the sustained release, degradation, and activity of HGF in vivo were not known, which would likely significantly affect the therapeutic outcome of the HGF being delivered. Optimization of growth factor delivery with a sustained activity as well as optimal graft implantation are necessary for the success of tissue engineering approaches involving acellular scaffolds loaded with growth factors.
In the present study, the bovine acellular scaffolds were implanted into the more posterior, cartilaginous portion of the vocal fold instead of into the membranous vocal fold due to difficulties in the surgical procedure in the small rat vocal tract.3 Implantation of the acellular scaffolds into the membranous vocal fold should be targeted in future studies, in order to explore the potential of the acellular scaffold loaded with HGF or other growth factors for reconstruction of the vocal fold lamina propria. Moreover, more intensive, FDA-approved sterilization procedures instead of the use of only 70% ethanol and UV light could be conducted in future studies. Typical sterilization procedures (such as oxidation and alkylation), however, could have adverse effects on the structure and integrity of acellular ECM scaffolds and should be investigated cautiously.26, 27
There is also a potential concern on the use of HGF for the clinical treatment of voice disorders involving laryngeal cancer. In addition to its well documented anti-scarring effect, HGF has been known to increase the motility and scattering of some epithelial cells, such as tumor cells found in hypopharyngeal carcinoma.28, 29 Thus, it could be potentially unsafe to use HGF in patients with head and neck cancer and laryngeal cancer, which make up a significant portion of the clinical population with vocal fold scarring. The clinical applications and concerns of HGF are beyond the scope of the present study, but they should be addressed in further studies. Future studies should also examine the potential of the bovine acellular scaffold for the in vivo delivery of alternative growth factors and drugs.
CONCLUSIONS
A bovine acellular scaffold was shown to have the potential to facilitate tissue remodeling in a rat model of vocal fold injury in our previous study.3 In the present study, the potential of the acellular scaffold for the delivery and controlled release of hepatocyte growth factor (HGF) was evaluated both in vitro and in vivo in a rat model. The in vitro HGF release kinetics study revealed a gradual, sustained release profile similar to those reported for other acellular matrices.18 Differences were observed in the host inflammatory cell response and in the host ECM protein synthesis for acellular scaffold implants loaded with HGF versus those without HGF. These findings supported the hypothesis that the bovine acellular scaffold could be a vehicle for drug delivery in vivo. Further studies targeting the membranous vocal fold, optimal strategies for better controlled loading and delivery of HGF, as well as modifications in fabrication of the scaffolds are required to verify these findings pertaining to reconstruction of the lamina propria, and to optimize the delivery of HGF for more constructive tissue remodeling.
ACKNOWLEDGMENTS
This work was supported by NIH grant number R01 DC006101. The authors would like to thank Bao-Xi Qu, Paula Timmons, Jian Yang, Hao Xu, Jinhui Shen, Ling Zou and the staff of the Animal Resource Center of UT Southwestern Medical Center for their assistance.
REFERENCES
- 1.Xu CC, Chan RW, Tirunagari N. A biodegradable, acellular xenogeneic scaffold for regeneration of the vocal fold lamina propria. Tissue Eng. 2007;13:551–566. doi: 10.1089/ten.2006.0169. [DOI] [PubMed] [Google Scholar]
- 2.Xu CC, Chan RW. Pore architecture of a bovine acellular vocal fold scaffold. Tissue Eng Part A. 2008;14:1893–1903. doi: 10.1089/ten.tea.2007.0243. [DOI] [PubMed] [Google Scholar]
- 3.Xu CC, Chan RW, Weinberger DG, Efune G, Pawlowski KS. A bovine acellular scaffold for vocal fold reconstruction in a rat model. J Biomed Mater Res A. 2008 doi: 10.1002/jbm.a.32279. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pollard TD, Earnshaw WC. Cell biology. 1st ed. Philadelphia, PA: Elsevier Inc.; 2004. pp. 485–506. [Google Scholar]
- 5.Campbell MK, Farrell SO. Biochemistry. 5th ed. Belmont, CA: Thomson Brooks/Cole; 2006. pp. 434–462. [Google Scholar]
- 6.Pieper JS, Hafmans T, van Wachem PB, van Luyn MJA, Brouwer LA, Veerkamp JH, van Kuppevelt TH. Loading of collagen-heparan sulfate matrices with bFGF promotes angiogenesis and tissue generation in rats. J Biomed Mater Res. 2002;62:185–194. doi: 10.1002/jbm.10267. [DOI] [PubMed] [Google Scholar]
- 7.Ohta M, Suzuki Y, Chou H, Ishikawa N, Suzuki S, Tanihara M, Suzuki Y, Mizushima Y, Dezawa M, Ide C. Novel heparin/alginate gel combined with basic fibroblast growth factor promotes nerve regeneration in rat sciatic nerve. J Biomed Mater Res A. 2004;71:661–668. doi: 10.1002/jbm.a.30194. [DOI] [PubMed] [Google Scholar]
- 8.Hirano S. Current treatment of vocal fold scarring. Curr Opin Otolaryngol Head Neck Surg. 2005;13:143–147. doi: 10.1097/01.moo.0000162261.49739.b7. [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto K, Nakamura T. Hepatocyte growth factor (HGF) as a tissue organizer for organogenesis and regeneration. Biochem Biophys Res Commun. 1997;239:639–644. doi: 10.1006/bbrc.1997.7517. [DOI] [PubMed] [Google Scholar]
- 10.Hirano S, Bless D, Heisey D, Ford C. Roles of hepatocyte growth factor and transforming growth factor beta1 in production of extracellular matrix by canine vocal fold fibroblasts. Laryngoscope. 2003;113:144–148. doi: 10.1097/00005537-200301000-00027. [DOI] [PubMed] [Google Scholar]
- 11.Hirano S, Bless DM, Massey RJ, Hartig GK, Ford CN. Morphological and functional changes of human vocal fold fibroblasts with hepatocyte growth factor. Ann Otol Rhinol Laryngol. 2003;112:1026–1033. doi: 10.1177/000348940311201206. [DOI] [PubMed] [Google Scholar]
- 12.Hirano S, Bless DM, Rousseau B, Welham N, Montequin D, Chan RW, Ford CN. Prevention of vocal fold scarring by topical injection of hepatocyte growth factor in a rabbit model. Laryngoscope. 2004;114:548–556. doi: 10.1097/00005537-200403000-00030. [DOI] [PubMed] [Google Scholar]
- 13.Moreno E, Meneu JC, Calvo J, Pérez B, Sesma AG, Manrique A, Vegh I, Aragón AM, Grau M, Gimeno A, Jiménez C, Gómez R, Moreno A, Abradelo M, García I, de la Calle A. Modulation of hepatocyte growth factor plasma levels in relation to the dose of exogenous heparin administered: an experimental study in rats. Transplant Proc. 2005;37:3943–3947. doi: 10.1016/j.transproceed.2005.10.089. [DOI] [PubMed] [Google Scholar]
- 14.Liu ML, Mars WM, Zarnegar R, Michalopoulos GK. Uptake and distribution of hepatocyte growth factor in normal and regenerating adult rat liver. Am J Pathol. 1994;144:129–140. [PMC free article] [PubMed] [Google Scholar]
- 15.Ohno T, Hirano S, Kanemaru S, Yamashita M, Umeda H, Suehiro A, Tamura Y, Nakamura T, Ito J, Tabata Y. Drug delivery system of hepatocyte growth factor for the treatment of vocal fold scarring in a canine model. Ann Otol Rhinol Laryngol. 2007;116:762–769. doi: 10.1177/000348940711601008. [DOI] [PubMed] [Google Scholar]
- 16.Rider CC. Analysis of glycosaminoglycans and proteoglycans. Methods Mol Biol. 1998;76:131–143. doi: 10.1385/0-89603-355-4:131. [DOI] [PubMed] [Google Scholar]
- 17.Toole BP, Yu Q, Underhill CB. Hyaluronan and hyaluronan-binding proteins: Probes for specific detection. In: Iozzo RV, editor. Proteoglycan Protocols (Methods in Molecular Biology) Volume 171. New York, NY: Humana Press; 2001. pp. 479–485. [DOI] [PubMed] [Google Scholar]
- 18.Lai PH, Chang Y, Chen SC, Wang CC, Liang HC, Chang WC, Sung HW. Acellular biological tissues containing inherent glycosaminoglycans for loading basic fibroblast growth factor promote angiogenesis and tissue regeneration. Tissue Eng. 2006;12:2499–2508. doi: 10.1089/ten.2006.12.2499. [DOI] [PubMed] [Google Scholar]
- 19.Gong R, Rifai A, Dworkin LD. Anti-inflammatory effect of hepatocyte growth factor in chronic kidney disease: targeting the inflamed vascular endothelium. J Am Soc Nephrol. 2006;17:2464–2473. doi: 10.1681/ASN.2006020185. [DOI] [PubMed] [Google Scholar]
- 20.Badylak SF, Gilbert TW. Immune response to biologic scaffold materials. Semin Immunol. 2008;20:109–116. doi: 10.1016/j.smim.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirschi SD, Gray SD, Thibeault SL. Fibronectin: an interesting vocal fold protein. J Voice. 2002;16:310–316. doi: 10.1016/s0892-1997(02)00102-9. [DOI] [PubMed] [Google Scholar]
- 22.Tateya T, Tateya I, Sohn JH, Bless DM. Histological study of acute vocal fold injury in a rat model. Ann Otol Rhinol Laryngol. 2006;115:285–292. doi: 10.1177/000348940611500406. [DOI] [PubMed] [Google Scholar]
- 23.Thibeault SL, Rousseau B, Welham NV, Hirano S, Bless DM. Hyaluronan levels in acute vocal fold scar. Laryngoscope. 2004;114:760–764. doi: 10.1097/00005537-200404000-00031. [DOI] [PubMed] [Google Scholar]
- 24.Gray SD, Titze IR, Chan RW, Hammond TH. Vocal fold proteoglycans and their influence on biomechanics. Laryngoscope. 1999;109:845–854. doi: 10.1097/00005537-199906000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Lorant T, Tufveson G, Johnsson C. The graft content of hyaluronan is increased during xenograft rejection. Xenotransplantation. 2004;11:269–275. doi: 10.1111/j.1399-3089.2004.00132.x. [DOI] [PubMed] [Google Scholar]
- 26.Hodde J, Janis A, Hiles M. Effects of sterilization on an extracellular matrix scaffold: part II. Bioactivity and matrix interaction. J Mater Sci Mater Med. 2007;18:545–550. doi: 10.1007/s10856-007-2301-9. [DOI] [PubMed] [Google Scholar]
- 27.Hodde J, Janis A, Ernst D, Zopf D, Sherman D, Johnson C. Effects of sterilization on an extracellular matrix scaffold: part I. Composition and matrix architecture. J Mater Sci Mater Med. 2007;18:537–543. doi: 10.1007/s10856-007-2300-x. [DOI] [PubMed] [Google Scholar]
- 28.Jiang WG, Hallett MB, Puntis MC. Hepatocyte growth factor/scatter factor, liver regeneration and cancer metastasis. Br J Surg. 1993;80:368–373. doi: 10.1002/bjs.1800801104. [DOI] [PubMed] [Google Scholar]
- 29.Kim CH, Kim J, Kahng H, Choi EC. Change of E-cadherin by hepatocyte growth factor and effects on the prognosis of hypopharyngeal carcinoma. Ann Surg Oncol. 2007;14:1565–1574. doi: 10.1245/s10434-006-9320-5. [DOI] [PubMed] [Google Scholar]