Abstract
The cancer-preventive activity of vitamin E has been studied. Whereas some epidemiological studies have suggested a protective effect of vitamin E against cancer formation, many large-scale intervention studies with α-tocopherol (usually large doses) have not demonstrated a cancer-preventive effect. Studies on α-tocopherol in animal models also have not demonstrated robust cancer prevention effects. One possible explanation for the lack of demonstrable cancer-preventive effects is that high doses of α-tocopherol decrease the blood and tissue levels of δ-tocopherols. It has been suggested that γ-tocopherol, due to its strong anti-inflammatory and other activities, may be the more effective form of vitamin E in cancer prevention. Our recent results have demonstrated that a γ-tocopherol-rich mixture of tocopherols inhibits colon, prostate, mammary and lung tumorigenesis in animal models, suggesting that this mixture may have a high potential for applications in the prevention of human cancer. In this review, we discuss biochemical properties of tocopherols, results of possible cancer-preventive effects in humans and animal models and possible mechanisms involved in the inhibition of carcinogenesis. Based on this information, we propose that a γ-tocopherol-rich mixture of tocopherols is a very promising cancer-preventive agent and warrants extensive future research.
Introduction
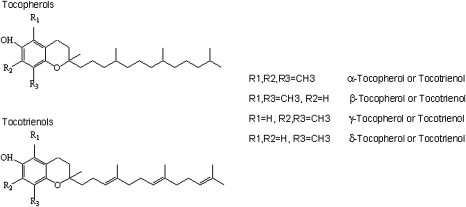
Vitamin E consists of a group of eight structurally related compounds: α-, β-, γ- and δ-tocopherols (α-, β-, γ- and δ-T) and α-, β-, γ- and δ-tocotrienols (α-, β-, γ- and δ-TT). All four tocopherols consist of a chromanol ring and a 16-carbon side chain, but they differ in the number and position of the methyl group on the ring (Figure 1). For example, α-T is tri-methylated (at the 5-, 7- and 8-positions of the chromanol ring), whereas γ-T is dimethylated (at the 7- and 8-positions). Tocotrienols have the same substitution pattern of methyl groups on the chromanol ring (for α-, β-, γ- and δ-form) as tocopherols, but they have an unsaturated 16-carbon side chain with double bonds at the 3′, 7′, and 11′ positions (1,2).
Fig. 1.
Chemical structure of tocopherols and tocotrienols.
Tocopherols cannot be synthesized in humans and animals; therefore, they need to be obtained from dietary sources. γ-T and α-T are the major dietary tocopherols present in the human diet. γ-T is the most consumed tocopherol, estimated to be consumed several times more than α-T (3). Tocopherols are plentiful in vegetable oils, such as oils from soybean, corn, sesame and cottonseeds, as well as nuts (4,5). Tocotrienols are present in trace amounts in oils derived from rice bran, barley, wheat germ and rye and are not consumed in large quantities in North America. Tocotrienols, however, are plentiful in palm oil (up to 800 mg/kg), mainly consisting of γ-TT and α-TT, and are consumed mostly in East-South Asia (6).
α-T has been traditionally recognized as ‘the’ vitamin E because of its superior activity in the classical fertility-restoration assay and its higher blood levels over other tocopherols and tocotrienols. For these reasons, most of the studies on vitamin E have focused on α-T; the distinct biological activities of other vitamin E molecules have not been studied to the same extent. Lately, it has been recognized that other tocopherols, such as γ-T and δ-T, as well as tocotrienols, have novel biological activities (7–9). As discussed in several reviews (7–9), γ-T has stronger anti-nitrative and anti-inflammatory activities than α-T and may be more effective in the prevention of cancer, as well as cardiovascular and neurodegenerative diseases. As will be discussed later, our recent results in animal models on the inhibition of colon, prostate and mammary carcinogenesis by a γ-T-rich mixture of tocopherols (γ-TmT, containing 59.3% γ-T, 25.4% δ-T, 13.5% α-T and 1.6% β-T) are very exciting (10–14). This review discusses our current understanding of the cancer-preventive and other activities of tocopherols and tocotrienols.
Absorption and metabolism of tocopherols
Dietary tocopherols are absorbed from the intestinal mucosa as the free phenolic form since esters are hydrolyzed by the pancreatic esterases prior to absorption. Tocopherols are incorporated into the chylomicrons and transported to the liver via the lymphatic system. Diet fat promotes transfer of vitamin E into the lymphatic system. The uptake of tocopherols into the liver is probably non-specific, but the transfer of tocopherols in the liver to very low-density lipoproteins is mediated by a specific α-T transfer protein (15,16). α-T transfer protein in the liver selectively transfers α-T to very low-density lipoproteins; α-T is, therefore, preferentially secreted into the circulation and transferred to non-hepatic tissues (2). Due to their low affinity for α-T transfer protein, hepatic γ-T and δ-T are less efficiently transferred to very low-density lipoproteins. Therefore, smaller portions of γ-T and δ-T are found in the blood and tissues, and most of them are excreted in the feces.
The major route of tocopherol metabolism is through side-chain degradation, initiated with hydroxylation of the ω-methyl group by cytochromes P450 4F or 3A and followed by five cycles of β-oxidation to cut off two-carbon units from the main chain in each cycle (1,17). A larger percentage of γ-T and δ-T than α-T is degraded through this pathway (18). The short side-chain metabolites, γ- and δ-carboxyethyl hydroxychroman (CEHC) (19,20), as well as lower levels of γ-carboxymethylbutyl hydroxychroman (21) were excreted in the urine in conjugated forms as glucuronides and sulfates. Metabolites of side-chain degradation of different chain lengths have been observed upon incubation of tocopherols and tocotrienols with HepG2 liver cancer cells (22). These metabolites have recently been characterized in mouse and human fecal and urine samples (Y.Zhao, M.J.Lee, C.Cheung, J.Ju, Y.K.Chen, B.Liu and C.S.Yang, submitted). These metabolites were secreted together with intact tocopherols and other lipids from the liver via bile into the intestine and excreted in the feces. These urinary metabolites may reflect dietary exposure of γ- and δ-T, vitamin E nutritional status and disease states, such as inflammation, or smoking that could affect tocopherol metabolism.
Anti-oxidative activities: trapping of reactive oxygen and nitrogen species
Vitamin E serves as antioxidants by preventing propagation of free radical reactions (2). Some in vitro studies have shown the superiority of α-T as an antioxidant over other tocopherols. Others, however, have found that antioxidant activities of γ-T are similar to or even greater than those of α-T (23). In addition to this direct antioxidant activity, tocopherols and their metabolites may serve as indirect antioxidants by activating NF-E2-related factor-2-related antioxidant enzymes. γ-Tocopheryl quinone, the terminal oxidation product of γ-T, has been shown to be more effective than α-tocopheryl quinone at increasing the transcription of activating transcription factor 4, a co-activator of NF-E2-related factor-2, as well as the levels of glutathione, a cellular antioxidant (24). It is worth noting that γ-tocopheryl quinone has been shown to induce endoplasmic reticulum stress, cytotoxicity and mutagenesis, whereas α-tocopheryl quinone has not (25,26).
Since results of in vitro studies are probably dependent on the assay systems used, it is important to determine antioxidant activities of vitamin E in vivo. F2-isoprostanes, isomers of prostaglandin F2, have been suggested as a reliable marker of in vivo free radical generation and oxidative lipid damage (27,28). 8-Isoprostane, an F2-isoprostane, is implicated as a causative mediator of pulmonary oxygen toxicity (29), and its level is elevated in heavy smokers (30). Recently, we observed that the plasma 8-isoprostane levels were increased during colon carcinogenesis in azoxymethane (AOM)-treated/dextran sulfate sodium (DSS)-treated mice. Dietary administration of γ-TmT resulted in significantly decreased plasma 8-isoprostane levels as well as reduced colon tumor formation (11). These results suggest that the increased oxidative stress during colon tumorigenesis was inhibited by γ-TmT.
γ-T may be nitrated at the five-carbon position to form 5-nitro-γ-T and is more effective than α-T at trapping reactive nitrogen species (31–35). 5-Nitro-γ-T is increased in the blood immediately after induction of acute inflammation in rats (36). Cooney et al. (32,33) have shown that γ-T, but not α-T, reduces nitrogen dioxide to nitric oxide in non-polar environments and forms 5-nitro-γ-T in more polar solvents. Nitrogen dioxide is a reactive free radical; if not reduced, it reacts with unsaturated fatty acid moieties to yield nitrite esters capable of nitrosating amines. Nitrogen dioxide can induce single-strand DNA breaks in V79 cells, and the reaction is optimally inhibited by γ-T in comparison with other lipid soluble antioxidants (37). Peroxynitrite-induced lipid peroxidation in liposomes is inhibited by both γ-T and α-T (31,38). Nitrotyrosine is a biomarker of NO-mediated protein modification and is commonly used to detect NO-mediated cellular damage. We observed that, in colon homogenates of AOM-treated/DSS-treated mice, the nitrotyrosine levels were much lower in the γ-TmT-treated group than in the control group, suggesting that the γ-TmT treatment reduces nitrosative stress (11).
Studies on tocopherols and human cancers
We have reviewed >70 publications on case–control, cohort and intervention studies examining the relationships between tocopherols and cancer risk at the four most common organ sites. The results are summarized in Table I and detailed information is provided in Supplementary Tables 1–4 (available at Carcinogenesis Online). In this section, we will first describe observational epidemiological studies on these four types of cancers and then the intervention studies, as they usually examined cancer risk at multiple organ sites.
Table I.
Number of studies on the risk of human cancers and the dietary intake or blood levels of total tocopherols
| Case–control studies |
Cohort studies |
Intervention studies |
||||
| Risk reduction | No association in risk | Risk reduction | No association in risk | Risk reduction | No association in risk | |
| Colon | 1 | 1 | 2 | 4 | 0 | 4 |
| Lung | 3 | 1 | 2 | 1 | 0 | 4 |
| Prostate | 7 | 7 | 3 | 6 | 1 | 3 |
| Breast | 7 | 8 | 0 | 9 | 0 | 0 |
Results based on a review of studies published since 1986.
Case–control and cohort studies
Colorectal cancer.
Since 1992, there have been two case–control studies (39,40) and six cohort studies (41–46) on the relationship between dietary intake or blood levels of tocopherols and risk of colorectal cancer (Table I, Supplementary Table 1 is available at Carcinogenesis Online). Of the two case–control studies reported, one found an inverse association between supplementary vitamin E intake and colorectal cancer risk (39), but the other did not find a protective effect of dietary or supplementary vitamin E against colorectal cancer (40). This study, however, found a significant inverse association between the plasma α-T:γ-T ratio and large adenoma (≥1 cm) occurrence; the odds ratio for the highest versus lowest quintile was 0.36 with a 95% confidence interval (CI) of 0.14–0.95 (P = 0.02) (40). The authors suggested that the plasma α-T:γ-T ratio is a more sensitive indicator of tocopherol intake and a better predictor for cancer risk than plasma α-T levels, but the molecular basis is unclear. Nevertheless, an early meta-analysis of five prospective, nested case–control studies including 289 cases of colorectal cancer and 1267 matched controls showed that high plasma levels of α-T were associated with a modest decrease in the incidence of colorectal cancer (odds ratio: 0.6; 95% CI: 0.4–1.0) (44).
Of the six cohort studies, two studies showed an inverse association between vitamin E intake and colorectal cancer risk (45,46). For example, the Iowa Women's Health Study (45) showed that a high intake of vitamin E was associated with a low risk of colon cancer (P for trend < 0.0001). This study also found that the protective effect was stronger in subjects under the age of 65 years than in subjects over the age of 65 (relative risk (RR): 0.16 for those 55–59 years old; 0.37 for those 60–64 years old and 0.93 for those 65–69 years old).
Lung cancer.
There have been four case–control studies (47–50) and three cohort studies (51–53) on the relationship between dietary or blood levels of tocopherols and risk of lung cancer since 1986 (Table I, Supplementary Table 2 is available at Carcinogenesis Online). Of the four case–control studies, three studies found lower serum α-T levels in lung cancer patients than those in matched controls (48–50). Two of these three studies found no difference in serum γ-T levels between lung cancer patients and the control subjects (48,49). Of the three cohort studies, two studies found a significant inverse association between dietary intake of vitamin E and risk of lung cancer (51,52). In both of these studies, the protective effects were found in current smokers, suggesting a preventive effect of dietary vitamin E against insult from cigarette smoking.
Prostate cancer.
There have been 14 case–control studies (49,54–66) and 9 cohort studies (53,67–74) on the relationship between dietary or blood levels of tocopherols and risk of prostate cancer since 1988 (Table I, Supplementary Table 3 is available at Carcinogenesis Online). Of the 14 case–control studies, seven showed an inverse association between dietary or blood levels of tocopherols and risk of prostate cancer (49,55,56,58,59,61,65). In two nested case–control studies (CLUE I and CLUE II), serum levels of γ-T, but not α-T, were significantly inversely associated with prostate cancer risk (56,75). In CLUE I, serum levels of γ-T were significantly lower in subjects who developed prostate cancers than control subjects (P = 0.02), but no dose–response trend was observed. A strong inverse association between γ-T and prostate cancer risk was observed in CLUE II (P = 0.0001) (56). Out of the nine cohort studies, six studies examined the association between dietary or supplementary vitamin E intake and prostate cancer risk, and all the studies did not find any significant association. In the National Institutes of Health-American Association of Retired Persons Diet and Health Study, dietary γ-T and δ-T were found to be significantly related to a reduced risk of advanced prostate cancer (RR: 0.68; 95% CI: 0.56–0.84 for γ-T and RR: 0.8; 95% CI: 0.67–0.96 for δ-T), but supplemental vitamin E (α-T) intake beyond dietary sources was not related to prostate cancer risk (67).
Breast cancer.
There have been 15 case–control studies since 1992 (Table I, Supplementary Table 4 is available at Carcinogenesis Online). Of the eight case–control studies examining an association between vitamin E intake and breast cancer risk (76–83), six studies found a significant inverse association (76–80,82). Out of the seven case–control studies examining an association between serum α-T and γ-T levels and breast cancer risk, only one study found a significant inverse association with both α-T and γ-T levels (84). The other six studies did not show such an association (85–90). All the nine reported cohort studies found no association between vitamin E intake and breast cancer risk (91–99).
Collectively, the results from human case–control and cohort studies are inconsistent. Some studies showed a clear inverse association between tocopherol intake and cancer risk, whereas others showed no such association.
Intervention studies
The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study was initially designed to investigate the prevention of lung cancer in male smokers with a daily supplement of 50 mg of all-racemic-α-tocopheryl acetate and 20 mg of β-carotene in a two-by-two design (100). The α-T supplementation for 5–8 years did not produce a significant effect on the incidence of lung cancer (100). It lowered the incidence of colorectal cancer, but the result was not statistically significant (101). Additional studies found no significant association between colorectal cancer risk and dietary vitamin E, dietary α-T, dietary γ-T or serum α-T levels (42). During the 6-year post-trial period, no post-intervention effect of the supplement on colon cancer risk was found (102).
The Alpha-Tocopherol, Beta-Carotene Cancer Prevention study showed that α-T supplementation (50 mg daily for 5–8 years) was significantly associated with the reduced incidence of prostate cancer and that higher serum α-T was associated with a reduced risk of prostate cancer (RR, 0.80; 95% CI: 0.66–0.96 for highest versus lowest quintile; P trend = 0.03) (68,102,103). These results encouraged the launching of the Selenium and Vitamin E Cancer Prevention Trial, a clinical trial to determine if one or both of these substances can help prevent prostate cancer when taken as dietary supplements. The recently published results indicated that selenium (200 μg/d from L-selenomethionine) and vitamin E (400 IU/d of all rac-α-tocopheryl acetate), taken alone or together for an average of 5 years, did not prevent prostate cancer (104). However, the α-T supplementation caused a 50% decrease in median plasma γ-T levels (104).
In the Women's Health Study with 39 876 healthy US women aged 45 years or older, the administration of 600 IU of natural-source vitamin E (α-T) on alternate days did not significantly affect the incidence of colon, lung or total cancers (105). In the recently published results from the Physicians’ Health Study II Randomized Control Trial, supplementation with vitamin E (400-IU synthetic α-T every other day) or vitamin C (500 mg synthetic ascorbic acid) to physicians for 8 years did not reduce the risk of prostate cancer or all other cancers (106). The results of these large, long-term trials with high doses of α-T are disappointing. There are at least two interpretations of the results: (i) supplementation of a nutrient to a population that is already adequate in this nutrient may not produce any beneficial effects and (ii) supplementation of a large quantity of α-T decreases the blood and tissue levels of γ-T and δ-T, which have been suggested to have unique cancer-preventive activities (7–9,23,107,108). Based on our results from animal models, we believe that a mixture of tocopherols may produce more beneficial effects than individual tocopherols.
Inhibition of tumorigenesis in animal models by tocopherols
Most of the animal studies that have been conducted used α-T and its synthetic analogs. Table II summarizes the results of 32 studies published since 1980. More detailed information is provided in Supplementary Tables 5–8 (available at Carcinogenesis Online). The following is a summary of studies on four common organ sites of carcinogenesis.
Table II.
Number of animal studies showing protective or no protective effects of tocopherols on tumor formation in different organs
| Site | α-Tocopherol or its analogs |
Other tocopherols |
||
| Protective effect | No protective effect | Protective effect | No protective effect | |
| Colon | 1 | 9 | 2a | 0 |
| Lung | (1)b | 0 | 0 | 0 |
| Prostate | 2 (1) | 1 (2) | 1a | 0 |
| Mammary gland | 4 (5) | 1 (0) | 2a | 0 |
Results based on a review of studies published since 1980. The number of xenograft studies is in parentheses.
Study with γ-tocopherol-enriched mixed tocopherols.
Study for the effect on metastasis to the lung.
Colon tumorigenesis
There have been a total of 12 studies on the effect of tocopherols on colon tumorigenesis and aberrant crypt foci formation (Table II, Supplementary Table 5 is available at Carcinogenesis Online). Ten studies were on α-T and its synthetic analogs; only one showed a protective effect (109). Eight studies showed no effect (110–117) and one showed an enhancement effect (118). On the other hand, γ-TmT, at 0.1% in the AIN76A diet, was demonstrated to inhibit AOM-induced colon aberrant crypt foci in rats (10). Recently, we also demonstrated that dietary γ-TmT treatment (0.3 and 0.17% in AIN93M diet) significantly inhibited inflammation and colon carcinogenesis in AOM-treated/DSS-treated mice (11). The inhibition was associated with the apoptosis-inducing, anti-inflammatory, anti-oxidative and reactive nitrogen species-trapping activities of tocopherols; γ-T and δ-T were present in higher concentrations than α-T in the colon and may play key roles in the inhibition of carcinogenesis (11).
Prostate tumorigenesis and transplanted prostate cancer cells
Of a total of six studies on α-T and its synthetic analogs on prostate cancer (Table II, Supplementary Table 6 is available at Carcinogenesis Online), three studies were on the effect on prostate carcinogenesis in rats and mice (119–121), and the other three studies were on their effects on the growth of human prostate cancer cells in nude mice (122–124); the results are inconsistent. One study found that treatment with α-tocopheryl succinate resulted in a significant reduction of prostate cancer incidence in a transgenic mouse model, but the diet used also contained other agents (800 IU of α-tocopheryl succinate, 200 μg of seleno-DL-methionine and 50 mg of lycopene). Our recent studies demonstrated that administration of 0.1% of γ-TmT in the diet of TRAMP mice significantly inhibited the development of palpable prostate tumors and prostate intraepithelial neoplasia. The treatment also upregulated NF-E2-related factor-2 and related detoxifying and anti-oxidative enzymes (12). As discussed previously, the induction of antioxidant enzymes may be due to the action of γ-tocopheryl quinone (24).
Mammary tumorigenesis
Of five studies on α-T and mammary tumorigenesis, four studies showed a protective effect (113,125–127) but one study showed no effect (128) (Table II, Supplementary Table 7 is available at Carcinogenesis Online). Recently, we demonstrated that dietary administration of γ-TmT (0.1% in the AIN76A diet) significantly inhibited N-methyl-N-nitrosourea-induced mammary tumorigenesis in rats (13). We found that mammary tumor growth and tumor multiplicity, as well as a proliferation marker, proliferating cell nuclear antigen, were markedly decreased by administration of γ-TmT. In a subsequent study with γ-TmT, administration of 0.1, 0.3 or 0.5% γ-TmT dose dependently suppressed mammary tumor development and growth. Tumor multiplicity was also significantly reduced by all three different doses of γ-TmT. The inhibition of mammary tumorigenesis was associated with increased expression of p21, p27, cleaved caspase-3 and peroxisome proliferator-activated receptor (PPAR)-γ, whereas Akt and the estrogen-dependent signaling pathways in mammary tumors were significantly decreased by γ-TmT treatment (14).
Lung cancer
There is only one publication on tocopherol and lung cancer reporting that supplementation with α-T failed to inhibit lung metastasis of intravenously inoculated murine colon adenocarcinoma cells in BALB/C mice (129) (Table II, Supplementary Table 8 is available at Carcinogenesis Online). Using γ-TmT at 0.3% in the AIN93M diet, we recently observed growth inhibition of CL-13 murine lung cancer cells growing syngeneically in A/J mice (130). We also demonstrated that dietary γ-TmT (0.3%) inhibited growth of H1299 human lung cells in xenografts in nude mice as well as inhibited lung tumorigenesis in A/J mice induced by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone or 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone plus benzo[a]pyrene (G.Lu, H.Xiao, G.Li, Y.K.Chen, J.Hao, S.Loy and C.S.Yang, submitted). The strongest inhibitory effect was observed with γ-TmT treatment starting at the beginning of the carcinogenesis experiment.
Overall, results on the effect of α-T on animal carcinogenesis are inconsistent, with most studies showing no inhibition (e.g. in colon tumorigenesis) and some showing inhibition (e.g. in mammary tumorigenesis). On the other hand, recent studies with γ-TmT have consistently shown inhibitory effects against tumorigenesis in the colon, mammary gland, prostate and lung. Therefore, γ-TmT appears to be a promising agent for future investigation.
Possible cancer prevention mechanisms
Many studies have been conducted on the biological activities of tocopherols. The cancer prevention activity of tocopherols may be due to the following activities or a combination of these activities. The most commonly recognized are the anti-oxidative activities of tocopherols. The quenching of reactive nitrogen species by γ-T and δ-T, as well as the inhibitory activities of their metabolites against COX-2, make γ-T and δ-T stronger anti-inflammatory and anti-carcinogenic agents than α-T. γ-T and δ-T are also more effective at modulating the activities of certain receptors, signal transduction pathways and metabolic pathways that may contribute to the higher cancer-preventive activity of γ-T and δ-T. Some of the studies are described below.
Anti-oxidative activities and trapping of reactive nitrogen species
As was discussed in Anti-oxidative Activities: Trapping of Reactive Oxygen and Nitrogen Species, the anti-oxidative action is a common feature of all the forms of tocopherols, whereas γ-T and δ-T can effectively trap reactive nitrogen species. These activities have been demonstrated in our studies with γ-TmT in the AOM-induced/DSS-induced colon carcinogenesis model (11) and probably exist in other carcinogenesis systems.
Inhibition of COX-2 and anti-inflammatory activities
γ-T was shown to be more effective than α-T at inhibiting cyclooxygenase activity (131) and formation of pro-inflammatory eicosanoids (131–133). γ-T reduced prostaglandin E2 synthesis in both lipopolysaccharide-stimulated RAW264.7 macrophages and IL-1β-treated A549 human epithelial cells with an the concentration that causes 50% inhibition of 7.5 and 4 μM, respectively (131). The major metabolite of γ-T, γ-CEHC, also exhibited an inhibitory effect, with an the concentration that causes 50% inhibition of ∼30 μM in these cells. However, α-T, at 50 μM, only slightly reduced prostaglandin E2 formation in macrophages but had no effect in epithelial cells. The inhibitory effects of γ-T and γ-CEHC were due to the inhibition of COX-2 activity, rather than the protein expression or substrate availability. The inhibitory potency of γ-T and γ-CEHC was diminished by an increase in arachidonic acid concentration, suggesting that they compete with arachidonic acid at the active site of COX-2. Recent studies showed that long-chain carboxychromanol metabolites of vitamin E inhibited COX-2 more potently than shorter side-chain metabolites, whereas the sulfated carboxychromanols were ineffective (134). The long-chain metabolites in conditioned medium from γ-T, and even more so from δ-T, were more effective than conditioned medium from α-T, possibly because α-T was metabolized to long-chain metabolites to a lesser extent (135,136).
Some studies suggest that mixtures of tocopherols are superior to a single tocopherol at inhibiting inflammation. In subjects with metabolic syndrome (n = 20 per group), supplementation with a combination of γ-T and α-T each at 800 mg/day for 6 weeks resulted in more pronounced decreases in C-reactive protein, tumor necrosis factor-α and nitrotyrosine levels than supplementation with γ-T or α-T (800 mg/day) individually (137). We recently demonstrated that administration of γ-TmT to AOM-treated/DSS-treated mice reduced the colon inflammation index to 52% of the control and decreased levels of prostaglandin E2 and LTB4 in the colon and plasma (11).
Modulation of nuclear receptors
PPAR-γ, which belongs to the nuclear receptor family, is known to be important for inhibition of cell proliferation and induction of apoptosis in breast cancer. Upregulation of PPAR-γ may be one of the mechanisms for anti-carcinogenic action. Two studies have shown that γ-T is more effective than α-T at modulating the expression of PPAR-γ (138,139). Campbell et al. (138) showed that treatment of SW 480 colon cancer cells with α-T and γ-T (5–10 μM) increased the messenger RNA and protein levels of PPAR-γ, with a more pronounced effect produced by treatment with γ-T. De Pascale et al. (139) showed that all four natural tocopherols, α-T, β-T, γ-T and δ-T, increased transcriptional activity of PPAR-γ in NCTC 2544 human keratinocytes cell line, and γ-T displayed the strongest activity. Treatment with α-T, β-T, γ-T and δ-T also increased protein levels of PPAR-γ and transglutaminase-1, a downstream protein of PPAR-γ involved in terminal keratinocytes differentiation. Recently, we found that γ-TmT, γ-T and δ-T activated PPAR-γ transcription in estrogen receptor-positive breast cancer cell lines, MCF-7 and T47D cells; δ-T was more active than γ-T, whereas α-T was not active (14).
Pregnane X receptor is a nuclear receptor that recognizes xenobiotics, and it mediates the induction of genes involved in oxidation, conjugation and transportation of xenobiotics. In HepG2 cells, the transfected human pregnane X receptor was most strongly activated by α- and γ-TT followed by δ-, α- and γ-T. These results suggest a potential effect of individual forms of vitamin E on the metabolism of certain drugs and environmental chemicals (140). Prolonged treatment with α-T may induce CYP3A and enhance the side-chain degradation of tocopherols; this may lead to the lowering of blood levels of γ-T (2, 9, 104).
Mechanisms for inhibition of cell growth and induction of apoptosis in cell culture
γ-T has been shown to be more effective than α-T at inhibiting growth of colon, breast, prostate and lung cancer cells in culture (141–145). γ-T decreased the number of cells in S phase more effectively than α-T in human colon and prostate cells in culture by decreasing protein levels of cyclin D1 and cyclin E (key regulators of the G1–S transition) as well as p27kip1, p21cip1 and p16ink4a (142). Treatment of human glioma cells with γ-T and α-T inhibited cell growth, partially by increasing protein levels of integrin α5 and β1 (146). Overexpression of integrin α5 and β1 has been reported to inhibit cell cycle progression.
γ-T has also been shown to be effective at inducing apoptosis in cancer cells (141,144). γ-T (10–50 μM) or its combination with δ-T induced apoptosis in androgen-sensitive prostate LNCaP (but not in androgen-resistant PC-3 cells) by the induction of cytochrome c release, activation of caspase-9 and caspase-3, cleavage of poly-ADP-ribose polymerase and involvement of caspase-independent pathways (143). γ-T treatment also caused significant accumulation of dihydroceramide and dihydrosphingosine, and specific inhibitors of key enzymes of de novo synthesis of sphingolipids significantly protected cells from γ-T-induced apoptotic pathway (144). The study suggests that γ-T induced apoptosis by interrupting the de novo sphingolipid pathway in a prostate cancer cell line. Lyons et al. (147), however, reported that α-T (∼30 μM), but not γ-T, inhibited sterol-induced apoptosis in human monocytic U937 cells.
Our study with γ-TmT, as well as individual isoforms of tocopherols (10–100 μM concentration), demonstrated a dose-dependent inhibition of the estrogen-induced cell proliferation of the estrogen receptor-positive breast cancer cell line, MCF-7. α-T did not significantly inhibit the growth of estrogen receptor-positive human breast cancer cell line, MCF-7, whereas γ-T, and more strikingly δ-T, inhibited estrogen-induced cell proliferation in a dose-dependent manner (14).
Other possible mechanisms of action
α-T has been shown to exert anti-proliferative activity independent of its traditional antioxidant activity. α-T activated protein phosphatase 2A resulting in dephosphorylation and decreased protein kinase C activity (148,149). Additionally, α-T inhibited expression of the CD36 scavenger receptor which is a receptor involved in uptake of oxidized low-density lipoprotein and atherosclerosis progression (150). Short-term dietary supplementation with high doses of vitamin E was shown to increase T helper 1 cytokine production in patients with advanced colorectal cancer (151). In this study, supplementation of vitamin E (750 mg/day) for 2 weeks resulted in increased CD4:CD8 ratios and enhanced capacity of T cells for producing the T helper 1 cytokines interleukin 2 and interferon-γ.
α-Tocopheryl phosphate (α-TP) has recently been studied because of its potentially stronger anti-proliferative activity than that of α-T and its presence in food and animal tissues (152). α-TP was more effective than α-T at inhibiting cell proliferation (153). A mixture of α-TP and di-α-TP suppressed cell proliferation and CD36 levels in aortic smooth muscle and monocytic leukemia cells at a concentration lower than the effective concentration of α-T. It was reported that α-TP induced apoptosis in the osteosarcoma cell line MG-63 (154), whereas it was demonstrated to have cardioprotective and anti-apoptotic activity through the Akt survival pathway in a rat model of myocardial infarction (155). Although the physiological functions of the phosphorylated forms of tocopherol still remain to be established, the cancer-preventive activities of γ-TP and δ-TP are worth investigating. Since tocopherols are known to be embedded in lipid bilayers of cell membranes, it is interesting to consider that the phosphorylated form (tocopheryl phosphate) may be able to move to the cytosol and possibly the nucleus to trigger different biochemical reactions.
Studies on tocotrienols and cancer in humans, animals and cells
Tocotrienols, the vitamin E isomers with unsaturated side chains, have been shown to display stronger anticancer activities in vitro than tocopherols with γ- and δ-TT exhibiting more anticancer activities than α-TT (156–160). This subject has been reviewed recently (161). Although TTs possess antioxidant activity (162–164), the anticancer activity of TTs may be independent from its antioxidant activity because some redox-silent TT derivatives still exhibit anti-carcinogenic properties (165,166). For example, treatment of human lung adenocarcinoma cells with a redox-silent analog of α-TT led to accumulation of cells in the G1 phase of the cell cycle followed by apoptosis (165). This same redox-silent analog inhibited chemoresistant mesothelioma cell growth (167).
Recent results suggest that TTs affect many signaling pathways in cancer cells, including NF-kB-mediated pathways, phophatidylinositol-2 kinase/phosphoinositide-dependent/Akt, Raf/Erk and c-jun N-terminal kinase-related pathways (168–172). TTs also mediate many cellular processes including the reduction of DNA damage (173), activation of apoptosis (174), induction of cell cycle arrest (175), stabilization of the proteasome (176), and downregulation of telomerase activity (177). TT-induced apoptosis was observed in many different cancer cell lines (178–181), and usually involved proteins related to mitochondrial stress, such as alteration of Bcl-family proteins and caspases (182,183). However, the caspase activation induced by TTs may also involve mechanisms independent of death receptor and mitochondrial stress (174,184). In addition to apoptosis, γ- and δ-TTs also induced autophagy through a mitochondrial permeability transition pore opening-dependent, but caspase-independent, mechanism, suggesting the involvement of autophagy in TT-mediated cell death (185).
Other important anticancer properties of TTs are their anti-angiogenic activity and their ability to inhibit cancer invasion and metastasis. The anti-angiogenic effect of δ-TT is attributable to the regulation of phophatidylinositol-2 kinase/phosphoinositide-dependent kinase/Akt signaling and hypoxia-induced VEGF secretion as well as to the induction of a stress response in endothelial cells, partly associated with reactive oxygen species generated by δ-TT (186,187). γ-TT inhibited cancer cell invasion through downregulation of matrix metalloproteinase-2 and -9 and upregulation of tissue inhibitor of metalloproteinase-1 and -2 (188). γ-TT treatment also led to the suppression of mesenchymal markers and the restoration of epithelial markers, which are associated with inhibition of cell invasion (189).
The inhibition of tumor formation and growth has been studied in several mouse and rat models. In carcinogenesis models, oral administration of a 0.05% TT mixture in drinking water significantly suppressed spontaneous liver carcinogenesis in male C3H/He mice and glycerol-induced lung tumor promotion in 4NQO-initiated ddY mice (175). Other studies demonstrated that TTs inhibited the severity of cell damage in hepatocarcinogenesis (190,191). However, it was also reported that TTs did not have a significant effect on chemically induced rat mammary tumor latency and multiplicity (128). In a xenograft tumor model with B16 melanoma cells, γ-TT suppressed tumor growth and extended survival time of the host C57BL mice (159). Dietary γ-TT and δ-TT significantly delayed tumor growth in C3H/HeN mice implanted with murine hepatoma MH134 cells (192). The anticancer effect of TTs in animal studies requires further exploration.
Concluding remarks
The association of low vitamin E status with increased cancer risk as described above and observed in other human epidemiological studies (193–197) suggests the importance of these anti-oxidative nutrients in modulating cancer incidence. However, the results of most of the animal and human studies with α-T supplementation, as reviewed above, have not yielded supportive evidence. It is possible that tocopherols may reduce cancer risk when supplemented in populations with low vitamin E status. When given to humans and animals with adequate vitamin E nutrition, the cancer-preventive effects of tocopherols could be due to actions other than the anti-oxidative activity of α-T. In this aspect, as reviewed above, the most abundant γ-T is superior to α-T in the trapping of reactive nitrogen species, inhibition of COX-2 activity, activation of PPAR-γ and suppression of inflammation. δ-T, which is more abundant than α-T in some oils, also has some of these activities. We propose that a mixture of tocopherols, at ratios similar to those in our diet, could be a better cancer chemopreventive agent. This idea is supported by our recent results demonstrating that γ-TmT inhibited colon, mammary, prostate and lung carcinogenesis in rodent models as well as inhibiting growth of lung and prostate cancer xenograft tumors (10–14).
It has been suggested that γ-T is the major cancer-preventive form of vitamin E (8,107,198). However, the cancer-preventive activity of pure γ-T or δ-T still remains to be demonstrated. It is known that high levels of α-T intake can decrease the blood and tissue levels of γ-T. Whether high levels of dietary γ-T or δ-T can also decrease the blood and tissue levels of α-T remains to be investigated. It would be interesting to determine the contributions of each of the major forms of tocopherols (α-, γ- and δ-T) to cancer prevention and the possible interactions among these tocopherols as well as the mechanisms involved. In practical application, γ-TmT is probably the most promising agent to use. γ-TmT, a by-product in the refining of soybean oil, contains γ-T, α-T, δ-T and β-T in ratios approximate to those in dietary vegetable oils. Because it is readily available and inexpensive, γ-TmT and similar tocopherol preparations have a high potential for practical application and deserve further investigation in animal models and human trials.
In future epidemiological studies, more attention should be paid to dietary intake, blood and tissue levels of all major forms of tocopherols, as well as their ratios. Since γ-T and δ-T are more readily side-chain degraded, urinary levels of γ- and δ-CEHC may be explored as possible markers for the consumption of γ-T and δ-T and physiological conditions that affect their metabolism. Well-designed human intervention trials with γ-TmT may yield more definitive information on the cancer-preventive activities of tocopherols.
Supplementary material
Supplementary Tables 1–8 can be found at http://carcin.oxfordjournals.org/
Funding
National Institutes of Health (CA120915, CA122474, CA133021); National Institutes of Health training (CA108455-05) to S.C.P.
Supplementary Material
Acknowledgments
We thank Dr Harold L.Newmark for his inspiration in writing this review.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- α-, β-, γ- and δ-T
α-, β-, γ- and δ-tocopherol
- α-, β-, γ- and δ-TT
α-, β-, γ- and δ-tocotrienol
- AOM
azoxymethane
- CEHC
carboxyethyl hydroxychroman
- CI
confidence interval
- DSS
dextran sulfate sodium
- γ-TmT
γ-T-rich mixture of tocopherols
- PPAR-γ
peroxisome proliferator-activated receptor-γ
- RR
relative risk
- α-TP
α-tocopheryl phosphate
References
- 1.Traber MG. Vitamin E. In: Bowman BA, Russel RM, editors. Present Knowledge in Nutrition. Washington, DC: ILSI Press; 2006. pp. 211–219. [Google Scholar]
- 2.Traber MG. Vitamin E regulatory mechanisms. Annu. Rev. Nutr. 2007;27:347–362. doi: 10.1146/annurev.nutr.27.061406.093819. [DOI] [PubMed] [Google Scholar]
- 3.Ford ES, et al. Distribution of serum concentrations of alpha-tocopherol and gamma-tocopherol in the US population. Am. J. Clin. Nutr. 2006;84:375–383. doi: 10.1093/ajcn/84.1.375. [DOI] [PubMed] [Google Scholar]
- 4.U.S. Department of Commerce and U.S. Bureau of the Census. Fat and Oils: Production, Consumption, and Stocks 2004. 2005. [Google Scholar]
- 5.Eitenmiller T, et al. Vitamin E: Food Chemistry, Composition, and Analysis. New York: Marcel Dekker, Inc; 2004. [Google Scholar]
- 6.Sen CK, et al. Tocotrienols in health and disease: the other half of the natural vitamin E family. Mol. Aspects Med. 2007;28:692–728. doi: 10.1016/j.mam.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang Q, et al. Gamma-tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am. J. Clin. Nutr. 2001;74:714–722. doi: 10.1093/ajcn/74.6.714. [DOI] [PubMed] [Google Scholar]
- 8.Campbell S, et al. Development of gamma (gamma)-tocopherol as a colorectal cancer chemopreventive agent. Crit. Rev. Oncol. Hematol. 2003;47:249–259. doi: 10.1016/s1040-8428(03)00042-8. [DOI] [PubMed] [Google Scholar]
- 9.Hensley K, et al. New perspectives on vitamin E: gamma-tocopherol and carboxyelthylhydroxychroman metabolites in biology and medicine. Free Radic. Biol. Med. 2004;36:1–15. doi: 10.1016/j.freeradbiomed.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Newmark HL, et al. Mixed tocopherols inhibit azoxymethane-induced aberrant crypt foci in rats. Nutr. Cancer. 2006;56:82–85. doi: 10.1207/s15327914nc5601_11. [DOI] [PubMed] [Google Scholar]
- 11.Ju J, et al. A gamma-tocopherol-rich mixture of tocopherols inhibits colon inflammation and carcinogenesis in azoxymethane and dextran sulfate sodium-treated mice. Cancer Prev. Res. (Phila. Pa) 2009;2:143–152. doi: 10.1158/1940-6207.CAPR-08-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barve A, et al. Gamma-tocopherol-enriched mixed tocopherol diet inhibits prostate carcinogenesis in TRAMP mice. Int. J. Cancer. 2009;124:1693–1699. doi: 10.1002/ijc.24106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suh N, et al. Mixed tocopherols inhibit N-methyl-N-nitrosourea-induced mammary tumor growth in rats. Nutr. Cancer. 2007;59:76–81. doi: 10.1080/01635580701419022. [DOI] [PubMed] [Google Scholar]
- 14.Lee HJ, et al. Mixed tocopherols prevent mammary tumorigenesis by inhibiting estrogen action and activating PPAR-gamma. Clin. Cancer Res. 2009;15:4242–4249. doi: 10.1158/1078-0432.CCR-08-3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qian J, et al. Intracellular trafficking of vitamin E in hepatocytes: the role of tocopherol transfer protein. J. Lipid Res. 2005;46:2072–2082. doi: 10.1194/jlr.M500143-JLR200. [DOI] [PubMed] [Google Scholar]
- 16.Morley S, et al. Mechanisms of ligand transfer by the hepatic tocopherol transfer protein. J. Biol. Chem. 2008;283:17797–17804. doi: 10.1074/jbc.M800121200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sontag TJ, et al. Cytochrome P450 omega-hydroxylase pathway of tocopherol catabolism. Novel mechanism of regulation of vitamin E status. J. Biol. Chem. 2002;277:25290–25296. doi: 10.1074/jbc.M201466200. [DOI] [PubMed] [Google Scholar]
- 18.Sontag TJ, et al. Influence of major structural features of tocopherols and tocotrienols on their omega-oxidation by tocopherol-omega-hydroxylase. J. Lipid Res. 2007;48:1090–1098. doi: 10.1194/jlr.M600514-JLR200. [DOI] [PubMed] [Google Scholar]
- 19.Swanson JE, et al. Urinary excretion of 2,7, 8-trimethyl-2-(beta-carboxyethyl)-6-hydroxychroman is a major route of elimination of gamma-tocopherol in humans. J. Lipid Res. 1999;40:665–671. [PubMed] [Google Scholar]
- 20.Chiku S, et al. Novel urinary metabolite of d-delta-tocopherol in rats. J. Lipid Res. 1984;25:40–48. [PubMed] [Google Scholar]
- 21.Parker RS, et al. A novel 5′-carboxychroman metabolite of gamma-tocopherol secreted by HepG2 cells and excreted in human urine. Biochem. Biophys. Res. Commun. 2000;269:580–583. doi: 10.1006/bbrc.2000.2319. [DOI] [PubMed] [Google Scholar]
- 22.Birringer M, et al. Identities and differences in the metabolism of tocotrienols and tocopherols in HepG2 cells. J. Nutr. 2002;132:3113–3118. doi: 10.1093/jn/131.10.3113. [DOI] [PubMed] [Google Scholar]
- 23.Wagner KH, et al. Gamma-tocopherol—an underestimated vitamin? Ann. Nutr. Metab. 2004;48:169–188. doi: 10.1159/000079555. [DOI] [PubMed] [Google Scholar]
- 24.Ogawa Y, et al. Gamma-tocopheryl quinone, not alpha-tocopheryl quinone, induces adaptive response through up-regulation of cellular glutathione and cysteine availability via activation of ATF4. Free Radic. Res. 2008;42:674–687. doi: 10.1080/10715760802277396. [DOI] [PubMed] [Google Scholar]
- 25.Wang X, et al. Mechanism of arylating quinone toxicity involving Michael adduct formation and induction of endoplasmic reticulum stress. Proc. Natl Acad. Sci. USA. 2006;103:3604–3609. doi: 10.1073/pnas.0510962103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cornwell DG, et al. Mutagenicity of tocopheryl quinones: evolutionary advantage of selective accumulation of dietary alpha-tocopherol. Nutr. Cancer. 2002;43:111–118. doi: 10.1207/S15327914NC431_13. [DOI] [PubMed] [Google Scholar]
- 27.Morrow JD, et al. Formation of novel non-cyclooxygenase-derived prostanoids (F2-isoprostanes) in carbon tetrachloride hepatotoxicity. An animal model of lipid peroxidation. J. Clin. Invest. 1992;90:2502–2507. doi: 10.1172/JCI116143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Longmire AW, et al. Effect of oxygen tension on the generation of F2-isoprostanes and malondialdehyde in peroxidizing rat liver microsomes. Biochem. Pharmacol. 1994;47:1173–1177. doi: 10.1016/0006-2952(94)90389-1. [DOI] [PubMed] [Google Scholar]
- 29.Vacchiano CA, et al. Role of nonenzymatically generated prostanoid, 8-iso-PGF2 alpha, in pulmonary oxygen toxicity. J. Appl. Physiol. 1994;77:2912–2917. doi: 10.1152/jappl.1994.77.6.2912. [DOI] [PubMed] [Google Scholar]
- 30.Morrow JD, et al. Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers. Smoking as a cause of oxidative damage. N. Engl. J. Med. 1995;332:1198–1203. doi: 10.1056/NEJM199505043321804. [DOI] [PubMed] [Google Scholar]
- 31.Christen S, et al. Gamma-tocopherol traps mutagenic electrophiles such as NO(X) and complements alpha-tocopherol: physiological implications. Proc. Natl Acad. Sci. USA. 1997;94:3217–3222. doi: 10.1073/pnas.94.7.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cooney RV, et al. Gamma-tocopherol detoxification of nitrogen dioxide: superiority to alpha-tocopherol. Proc. Natl Acad. Sci. USA. 1993;90:1771–1775. doi: 10.1073/pnas.90.5.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cooney RV, et al. Products of gamma-tocopherol reaction with NO2 and their formation in rat insulinoma (RINm5F) cells. Free Radic. Biol. Med. 1995;19:259–269. doi: 10.1016/0891-5849(95)00019-t. [DOI] [PubMed] [Google Scholar]
- 34.Hoglen NC, et al. Reactions of peroxynitrite with gamma-tocopherol. Chem. Res. Toxicol. 1997;10:401–407. doi: 10.1021/tx960200h. [DOI] [PubMed] [Google Scholar]
- 35.Jiang Q, et al. Gamma-tocopherol supplementation inhibits protein nitration and ascorbate oxidation in rats with inflammation. Free Radic. Biol. Med. 2002;33:1534–1542. doi: 10.1016/s0891-5849(02)01091-2. [DOI] [PubMed] [Google Scholar]
- 36.Christen S, et al. Analysis of plasma tocopherols alpha, gamma, and 5-nitro-gamma in rats with inflammation by HPLC coulometric detection. J. Lipid Res. 2002;43:1978–1985. doi: 10.1194/jlr.d200023-jlr200. [DOI] [PubMed] [Google Scholar]
- 37.Bittrich H, et al. NO2-induced DNA single strand breaks are inhibited by antioxidative vitamins in V79 cells. Chem. Biol. Interact. 1993;86:199–211. doi: 10.1016/0009-2797(93)90098-j. [DOI] [PubMed] [Google Scholar]
- 38.Goss SP, et al. The effect of alpha-tocopherol on the nitration of gamma-tocopherol by peroxynitrite. Arch. Biochem. Biophys. 1999;363:333–340. doi: 10.1006/abbi.1998.1094. [DOI] [PubMed] [Google Scholar]
- 39.White E, et al. Relationship between vitamin and calcium supplement use and colon cancer. Cancer Epidemiol. Biomarkers Prev. 1997;6:769–774. [PubMed] [Google Scholar]
- 40.Ingles SA, et al. Plasma tocopherol and prevalence of colorectal adenomas in a multiethnic population. Cancer Res. 1998;58:661–666. [PubMed] [Google Scholar]
- 41.Wu K, et al. A prospective study on supplemental vitamin e intake and risk of colon cancer in women and men. Cancer Epidemiol. Biomarkers Prev. 2002;11:1298–1304. [PubMed] [Google Scholar]
- 42.Malila N, et al. Dietary and serum alpha-tocopherol, beta-carotene and retinol, and risk for colorectal cancer in male smokers. Eur. J. Clin. Nutr. 2002;56:615–621. doi: 10.1038/sj.ejcn.1601366. [DOI] [PubMed] [Google Scholar]
- 43.Slattery ML, et al. Vitamin E and colon cancer: is there an association? Nutr. Cancer. 1998;30:201–206. doi: 10.1080/01635589809514664. [DOI] [PubMed] [Google Scholar]
- 44.Longnecker MP, et al. Serum alpha-tocopherol concentration in relation to subsequent colorectal cancer: pooled data from five cohorts. J. Natl Cancer Inst. 1992;84:430–435. doi: 10.1093/jnci/84.6.430. [DOI] [PubMed] [Google Scholar]
- 45.Bostick RM, et al. Reduced risk of colon cancer with high intake of vitamin E: the Iowa Women's Health Study. Cancer Res. 1993;53:4230–4237. [PubMed] [Google Scholar]
- 46.Ghadirian P, et al. Nutritional factors and colon carcinoma: a case-control study involving French Canadians in Montreal, Quebec, Canada. Cancer. 1997;80:858–864. doi: 10.1002/(sici)1097-0142(19970901)80:5<858::aid-cncr5>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 47.Comstock GW, et al. The risk of developing lung cancer associated with antioxidants in the blood: ascorbic acid, carotenoids, alpha-tocopherol, selenium, and total peroxyl radical absorbing capacity. Cancer Epidemiol. Biomarkers Prev. 1997;6:907–916. [PubMed] [Google Scholar]
- 48.Ratnasinghe D, et al. Serum tocopherols, selenium and lung cancer risk among tin miners in China. Cancer Causes Control. 2000;11:129–135. doi: 10.1023/a:1008977320811. [DOI] [PubMed] [Google Scholar]
- 49.Goodman GE, et al. The association between lung and prostate cancer risk, and serum micronutrients: results and lessons learned from beta-carotene and retinol efficacy trial. Cancer Epidemiol. Biomarkers Prev. 2003;12:518–526. [PubMed] [Google Scholar]
- 50.Menkes MS, et al. Serum beta-carotene, vitamins A and E, selenium, and the risk of lung cancer. N. Engl J. Med. 1986;315:1250–1254. doi: 10.1056/NEJM198611133152003. [DOI] [PubMed] [Google Scholar]
- 51.Woodson K, et al. Association between alcohol and lung cancer in the alpha-tocopherol, beta-carotene cancer prevention study in Finland. Cancer Causes Control. 1999;10:219–226. doi: 10.1023/a:1008911624785. [DOI] [PubMed] [Google Scholar]
- 52.Yong LC, et al. Intake of vitamins E, C, and A and risk of lung cancer. The NHANES I epidemiologic followup study. First National Health and Nutrition Examination Survey. Am. J. Epidemiol. 1997;146:231–243. doi: 10.1093/oxfordjournals.aje.a009258. [DOI] [PubMed] [Google Scholar]
- 53.Shibata A, et al. Intake of vegetables, fruits, beta-carotene, vitamin C and vitamin supplements and cancer incidence among the elderly: a prospective study. Br. J. Cancer. 1992;66:673–679. doi: 10.1038/bjc.1992.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nomura AM, et al. Serum micronutrients and prostate cancer in Japanese Americans in Hawaii. Cancer Epidemiol. Biomarkers Prev. 1997;6:487–491. [PubMed] [Google Scholar]
- 55.Huang HY, et al. Supplementation of diets with alpha-tocopherol reduces serum concentrations of gamma- and delta-tocopherol in humans. J. Nutr. 2003;133:3137–3140. doi: 10.1093/jn/133.10.3137. [DOI] [PubMed] [Google Scholar]
- 56.Helzlsouer KJ, et al. Association between alpha-tocopherol, gamma-tocopherol, selenium, and subsequent prostate cancer. J. Natl Cancer Inst. 2000;92:2018–2023. doi: 10.1093/jnci/92.24.2018. [DOI] [PubMed] [Google Scholar]
- 57.Gann PH, et al. Lower prostate cancer risk in men with elevated plasma lycopene levels: results of a prospective analysis. Cancer Res. 1999;59:1225–1230. [PubMed] [Google Scholar]
- 58.Tzonou A, et al. Diet and cancer of the prostate: a case-control study in Greece. Int. J. Cancer. 1999;80:704–708. doi: 10.1002/(sici)1097-0215(19990301)80:5<704::aid-ijc13>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 59.Deneo-Pellegrini H, et al. Foods, nutrients and prostate cancer: a case-control study in Uruguay. Br. J. Cancer. 1999;80:591–597. doi: 10.1038/sj.bjc.6690396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kristal AR, et al. Vitamin and mineral supplement use is associated with reduced risk of prostate cancer. Cancer Epidemiol. Biomarkers Prev. 1999;8:887–892. [PubMed] [Google Scholar]
- 61.Vlajinac HD, et al. Diet and prostate cancer: a case-control study. Eur. J. Cancer. 1997;33:101–107. doi: 10.1016/s0959-8049(96)00373-5. [DOI] [PubMed] [Google Scholar]
- 62.Andersson SO, et al. Energy, nutrient intake and prostate cancer risk: a population-based case-control study in Sweden. Int. J. Cancer. 1996;68:716–722. doi: 10.1002/(SICI)1097-0215(19961211)68:6<716::AID-IJC4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 63.Rohan TE, et al. Dietary factors and risk of prostate cancer: a case-control study in Ontario, Canada. Cancer Causes Control. 1995;6:145–154. doi: 10.1007/BF00052775. [DOI] [PubMed] [Google Scholar]
- 64.Hsing AW, et al. Diet, tobacco use, and fatal prostate cancer: results from the Lutheran Brotherhood Cohort Study. Cancer Res. 1990;50:6836–6840. [PubMed] [Google Scholar]
- 65.Knekt P, et al. Serum vitamin E and risk of cancer among Finnish men during a 10-year follow-up. Am. J. Epidemiol. 1988;127:28–41. doi: 10.1093/oxfordjournals.aje.a114788. [DOI] [PubMed] [Google Scholar]
- 66.Hayes RB, et al. Serum retinol and prostate cancer. Cancer. 1988;62:2021–2026. doi: 10.1002/1097-0142(19881101)62:9<2021::aid-cncr2820620925>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 67.Wright ME, et al. Supplemental and dietary vitamin E intakes and risk of prostate cancer in a large prospective study. Cancer Epidemiol. Biomarkers Prev. 2007;16:1128–1135. doi: 10.1158/1055-9965.EPI-06-1071. [DOI] [PubMed] [Google Scholar]
- 68.Weinstein SJ, et al. Serum and dietary vitamin E in relation to prostate cancer risk. Cancer Epidemiol. Biomarkers Prev. 2007;16:1253–1259. doi: 10.1158/1055-9965.EPI-06-1084. [DOI] [PubMed] [Google Scholar]
- 69.Stram DO, et al. Prostate cancer incidence and intake of fruits, vegetables and related micronutrients: the multiethnic cohort study* (United States) Cancer Causes Control. 2006;17:1193–1207. doi: 10.1007/s10552-006-0064-0. [DOI] [PubMed] [Google Scholar]
- 70.Kirsh VA, et al. Supplemental and dietary vitamin E, beta-carotene, and vitamin C intakes and prostate cancer risk. J. Natl Cancer Inst. 2006;98:245–254. doi: 10.1093/jnci/djj050. [DOI] [PubMed] [Google Scholar]
- 71.Rodriguez C, et al. Vitamin E supplements and risk of prostate cancer in U.S. men. Cancer Epidemiol. Biomarkers Prev. 2004;13:378–382. [PubMed] [Google Scholar]
- 72.Schuurman AG, et al. A prospective cohort study on intake of retinol, vitamins C and E, and carotenoids and prostate cancer risk (Netherlands) Cancer Causes Control. 2002;13:573–582. doi: 10.1023/a:1016332208339. [DOI] [PubMed] [Google Scholar]
- 73.Eichholzer M, et al. Smoking, plasma vitamins C, E, retinol, and carotene, and fatal prostate cancer: seventeen-year follow-up of the prospective basel study. Prostate. 1999;38:189–198. doi: 10.1002/(sici)1097-0045(19990215)38:3<189::aid-pros3>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 74.Chan JM, et al. Supplemental vitamin E intake and prostate cancer risk in a large cohort of men in the United States. Cancer Epidemiol. Biomarkers Prev. 1999;8:893–899. [PubMed] [Google Scholar]
- 75.Huang HY, et al. Prospective study of antioxidant micronutrients in the blood and the risk of developing prostate cancer. Am. J. Epidemiol. 2003;157:335–344. doi: 10.1093/aje/kwf210. [DOI] [PubMed] [Google Scholar]
- 76.Ronco A, et al. Vegetables, fruits, and related nutrients and risk of breast cancer: a case-control study in Uruguay. Nutr. Cancer. 1999;35:111–119. doi: 10.1207/S15327914NC352_3. [DOI] [PubMed] [Google Scholar]
- 77.Mannisto S, et al. Diet and the risk of breast cancer in a case-control study: does the threat of disease have an influence on recall bias? J. Clin. Epidemiol. 1999;52:429–439. doi: 10.1016/s0895-4356(99)00010-4. [DOI] [PubMed] [Google Scholar]
- 78.London SJ, et al. Carotenoids, retinol, and vitamin E and risk of proliferative benign breast disease and breast cancer. Cancer Causes Control. 1992;3:503–512. doi: 10.1007/BF00052746. [DOI] [PubMed] [Google Scholar]
- 79.Freudenheim JL, et al. Premenopausal breast cancer risk and intake of vegetables, fruits, and related nutrients. J. Natl Cancer Inst. 1996;88:340–348. doi: 10.1093/jnci/88.6.340. [DOI] [PubMed] [Google Scholar]
- 80.Braga C, et al. Intake of selected foods and nutrients and breast cancer risk: an age- and menopause-specific analysis. Nutr. Cancer. 1997;28:258–263. doi: 10.1080/01635589709514585. [DOI] [PubMed] [Google Scholar]
- 81.Bohlke K, et al. Vitamins A, C and E and the risk of breast cancer: results from a case-control study in Greece. Br. J. Cancer. 1999;79:23–29. doi: 10.1038/sj.bjc.6690006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ambrosone CB, et al. Interaction of family history of breast cancer and dietary antioxidants with breast cancer risk (New York, United States) Cancer Causes Control. 1995;6:407–415. doi: 10.1007/BF00052180. [DOI] [PubMed] [Google Scholar]
- 83.Mezzetti M, et al. Population attributable risk for breast cancer: diet, nutrition, and physical exercise. J. Natl Cancer Inst. 1998;90:389–394. doi: 10.1093/jnci/90.5.389. [DOI] [PubMed] [Google Scholar]
- 84.Ray G, et al. Role of lipids, lipoproteins and vitamins in women with breast cancer. Clin. Biochem. 2001;34:71–76. doi: 10.1016/s0009-9120(00)00200-9. [DOI] [PubMed] [Google Scholar]
- 85.Zaroukian S, et al. Correlation between nutritional biomarkers and breast cancer: a case-control study. Breast. 2005;14:209–223. doi: 10.1016/j.breast.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 86.Tamimi RM, et al. Plasma carotenoids, retinol, and tocopherols and risk of breast cancer. Am. J. Epidemiol. 2005;161:153–160. doi: 10.1093/aje/kwi030. [DOI] [PubMed] [Google Scholar]
- 87.Simon MS, et al. An evaluation of plasma antioxidant levels and the risk of breast cancer: a pilot case control study. Breast J. 2000;6:388–395. doi: 10.1046/j.1524-4741.2000.20067.x. [DOI] [PubMed] [Google Scholar]
- 88.Sato R, et al. Prospective study of carotenoids, tocopherols, and retinoid concentrations and the risk of breast cancer. Cancer Epidemiol. Biomarkers Prev. 2002;11:451–457. [PubMed] [Google Scholar]
- 89.Comstock GW, et al. The repeatability of serum carotenoid, retinoid, and tocopherol concentrations in specimens of blood collected 15 years apart. Cancer Epidemiol. Biomarkers Prev. 2001;10:65–68. [PubMed] [Google Scholar]
- 90.Dorgan JF, et al. Relationships of serum carotenoids, retinol, alpha-tocopherol, and selenium with breast cancer risk: results from a prospective study in Columbia, Missouri (United States) Cancer Causes Control. 1998;9:89–97. doi: 10.1023/a:1008857521992. [DOI] [PubMed] [Google Scholar]
- 91.Michels KB, et al. Dietary antioxidant vitamins, retinol, and breast cancer incidence in a cohort of Swedish women. Int. J. Cancer. 2001;91:563–567. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1079>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 92.Zhang S, et al. Dietary carotenoids and vitamins A, C, and E and risk of breast cancer. J. Natl Cancer Inst. 1999;91:547–556. doi: 10.1093/jnci/91.6.547. [DOI] [PubMed] [Google Scholar]
- 93.Verhoeven DT, et al. Vitamins C and E, retinol, beta-carotene and dietary fibre in relation to breast cancer risk: a prospective cohort study. Br. J. Cancer. 1997;75:149–155. doi: 10.1038/bjc.1997.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jarvinen R, et al. Diet and breast cancer risk in a cohort of Finnish women. Cancer Lett. 1997;114:251–253. doi: 10.1016/s0304-3835(97)04675-2. [DOI] [PubMed] [Google Scholar]
- 95.Kushi LH, et al. Intake of vitamins A, C, and E and postmenopausal breast cancer. The Iowa Women's Health Study. Am. J. Epidemiol. 1996;144:165–174. doi: 10.1093/oxfordjournals.aje.a008904. [DOI] [PubMed] [Google Scholar]
- 96.Hunter DJ, et al. A prospective study of the intake of vitamins C, E, and A and the risk of breast cancer. N. Engl. J. Med. 1993;329:234–240. doi: 10.1056/NEJM199307223290403. [DOI] [PubMed] [Google Scholar]
- 97.Rohan TE, et al. Dietary fiber, vitamins A, C, and E, and risk of breast cancer: a cohort study. Cancer Causes Control. 1993;4:29–37. doi: 10.1007/BF00051711. [DOI] [PubMed] [Google Scholar]
- 98.Friedenreich CM, et al. Recall bias in the association of micronutrient intake and breast cancer. J. Clin. Epidemiol. 1993;46:1009–1017. doi: 10.1016/0895-4356(93)90168-z. [DOI] [PubMed] [Google Scholar]
- 99.Graham S, et al. Diet in the epidemiology of postmenopausal breast cancer in the New York State Cohort. Am. J. Epidemiol. 1992;136:1327–1337. doi: 10.1093/oxfordjournals.aje.a116445. [DOI] [PubMed] [Google Scholar]
- 100.Albanes D, et al. Alpha-tocopherol and beta-carotene supplements and lung cancer incidence in the alpha-tocopherol, beta-carotene cancer prevention study: effects of base-line characteristics and study compliance. J. Natl Cancer Inst. 1996;88:1560–1570. doi: 10.1093/jnci/88.21.1560. [DOI] [PubMed] [Google Scholar]
- 101.Albanes D, et al. Effects of supplemental alpha-tocopherol and beta-carotene on colorectal cancer: results from a controlled trial (Finland) Cancer Causes Control. 2000;11:197–205. doi: 10.1023/a:1008936214087. [DOI] [PubMed] [Google Scholar]
- 102.Virtamo J, et al. Incidence of cancer and mortality following alpha-tocopherol and beta-carotene supplementation: a postintervention follow-up. JAMA. 2003;290:476–485. doi: 10.1001/jama.290.4.476. [DOI] [PubMed] [Google Scholar]
- 103.Heinonen OP, et al. Prostate cancer and supplementation with alpha-tocopherol and beta-carotene: incidence and mortality in a controlled trial. J. Natl Cancer Inst. 1998;90:440–446. doi: 10.1093/jnci/90.6.440. [DOI] [PubMed] [Google Scholar]
- 104.Lippman SM, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lee IM, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005;294:56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 106.Gaziano JM, et al. Vitamins E and C in the prevention of prostate and total cancer in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2009;301:52–62. doi: 10.1001/jama.2008.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wolf G. gamma-Tocopherol: an efficient protector of lipids against nitric oxide-initiated peroxidative damage. Nutr. Rev. 1997;55:376–378. doi: 10.1111/j.1753-4887.1997.tb01566.x. [DOI] [PubMed] [Google Scholar]
- 108.Reiter E, et al. Anti-inflammatory properties of alpha- and gamma-tocopherol. Mol. Aspects Med. 2007;28:668–691. doi: 10.1016/j.mam.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cook MG, et al. Effect of dietary vitamin E on dimethylhydrazine-induced colonic tumors in mice. Cancer Res. 1980;40:1329–1331. [PubMed] [Google Scholar]
- 110.Chester JF, et al. Augmentation of 1,2-dimethylhydrazine-induced colon cancer by experimental colitis in mice: role of dietary vitamin E. J. Natl Cancer Inst. 1986;76:939–942. [PubMed] [Google Scholar]
- 111.Chung H, et al. Vitamin E supplementation does not alter azoxymethane-induced colonic aberrant crypt foci formation in young or old mice. J. Nutr. 2003;133:528–532. doi: 10.1093/jn/133.2.528. [DOI] [PubMed] [Google Scholar]
- 112.Exon JH, et al. Chemopreventive effect of dietary d-alpha-tocopheryl succinate supplementation on precancer colon aberrant crypt formation and vitamin E analogue levels in young and old rats. Nutr. Cancer. 2004;49:72–80. doi: 10.1207/s15327914nc4901_10. [DOI] [PubMed] [Google Scholar]
- 113.Hagiwara A, et al. Organ-dependent modifying effects of caffeine, and two naturally occurring antioxidants alpha-tocopherol and n-tritriacontane-16,18-dione, on 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP)-induced mammary and colonic carcinogenesis in female F344 rats. Jpn. J. Cancer Res. 1999;90:399–405. doi: 10.1111/j.1349-7006.1999.tb00761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Maziere S, et al. Effect of resistant starch and/or fat-soluble vitamins A and E on the initiation stage of aberrant crypts in rat colon. Nutr. Cancer. 1998;31:168–177. doi: 10.1080/01635589809514699. [DOI] [PubMed] [Google Scholar]
- 115.Reddy BS, et al. Interactions of selenium deficiency, vitamin E, polyunsaturated fat, and saturated fat on azoxymethane-induced colon carcinogenesis in male F344 rats. J. Natl Cancer Inst. 1986;76:1157–1162. [PubMed] [Google Scholar]
- 116.Temple NJ, et al. Cabbage and vitamin E: their effect on colon tumor formation in mice. Cancer Lett. 1987;35:71–77. doi: 10.1016/0304-3835(87)90058-9. [DOI] [PubMed] [Google Scholar]
- 117.Yao K, et al. Modulation of colonic aberrant crypt foci and proliferative indexes in colon and prostate glands of rats by vitamin E. Nutr. Cancer. 1996;26:99–109. doi: 10.1080/01635589609514467. [DOI] [PubMed] [Google Scholar]
- 118.Toth B, et al. Enhancing effect of vitamin E on murine intestinal tumorigenesis by 1,2-dimethylhydrazine dihydrochloride. J. Natl Cancer Inst. 1983;70:1107–1111. [PubMed] [Google Scholar]
- 119.Venkateswaran V, et al. Antioxidants block prostate cancer in lady transgenic mice. Cancer Res. 2004;64:5891–5896. doi: 10.1158/0008-5472.CAN-04-0690. [DOI] [PubMed] [Google Scholar]
- 120.Nakamura A, et al. Lack of modification by naturally occurring antioxidants of 3,2′-dimethyl-4-aminobiphenyl-initiated rat prostate carcinogenesis. Cancer Lett. 1991;58:241–246. doi: 10.1016/0304-3835(91)90107-s. [DOI] [PubMed] [Google Scholar]
- 121.McCormick DL, et al. Chemoprevention of hormone-dependent prostate cancer in the Wistar-Unilever rat. Eur. Urol. 1999;35:464–467. doi: 10.1159/000019880. [DOI] [PubMed] [Google Scholar]
- 122.Limpens J, et al. Combined lycopene and vitamin E treatment suppresses the growth of PC-346C human prostate cancer cells in nude mice. J. Nutr. 2006;136:1287–1293. doi: 10.1093/jn/136.5.1287. [DOI] [PubMed] [Google Scholar]
- 123.Fleshner N, et al. Vitamin E inhibits the high-fat diet promoted growth of established human prostate LNCaP tumors in nude mice. J. Urol. 1999;161:1651–1654. [PubMed] [Google Scholar]
- 124.Basu A, et al. Alpha-tocopheryl succinate (alpha-TOS) modulates human prostate LNCaP xenograft growth and gene expression in BALB/c nude mice fed two levels of dietary soybean oil. Eur. J. Nutr. 2007;46:34–43. doi: 10.1007/s00394-006-0629-4. [DOI] [PubMed] [Google Scholar]
- 125.Dias MF, et al. Chemoprevention of DMBA-induced mammary tumors in rats by a combined regimen of alpha-tocopherol, selenium, and ascorbic acid. Breast J. 2000;6:14–19. doi: 10.1046/j.1524-4741.2000.98071.x. [DOI] [PubMed] [Google Scholar]
- 126.Hirose M, et al. Chemoprevention of heterocyclic amine-induced mammary carcinogenesis in rats. Environ. Mol. Mutagen. 2002;39:271–278. doi: 10.1002/em.10066. [DOI] [PubMed] [Google Scholar]
- 127.Hirose M, et al. Phenolics: blocking agents for heterocyclic amine-induced carcinogenesis. Food Chem. Toxicol. 1999;37:985–992. doi: 10.1016/s0278-6915(99)00092-7. [DOI] [PubMed] [Google Scholar]
- 128.Gould MN, et al. A comparison of tocopherol and tocotrienol for the chemoprevention of chemically induced rat mammary tumors. Am. J. Clin. Nutr. 1991;53:1068S–1070S. doi: 10.1093/ajcn/53.4.1068S. [DOI] [PubMed] [Google Scholar]
- 129.Ogasawara M, et al. Differential effects of antioxidants on the in vitro invasion, growth and lung metastasis of murine colon cancer cells. Biol. Pharm. Bull. 2007;30:200–204. doi: 10.1248/bpb.30.200. [DOI] [PubMed] [Google Scholar]
- 130.Lambert JD, et al. Inhibition of lung cancer growth in mice by dietary mixed tocopherols. Mol. Nutr. Food Res. 2009;8:1030–1035. doi: 10.1002/mnfr.200800438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jiang Q, et al. Gamma-tocopherol and its major metabolite, in contrast to alpha-tocopherol, inhibit cyclooxygenase activity in macrophages and epithelial cells. Proc. Natl Acad. Sci. USA. 2000;97:11494–11499. doi: 10.1073/pnas.200357097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jiang Q, et al. Gamma-tocopherol, but not alpha-tocopherol, decreases proinflammatory eicosanoids and inflammation damage in rats. FASEB J. 2003;17:816–822. doi: 10.1096/fj.02-0877com. [DOI] [PubMed] [Google Scholar]
- 133.O'Leary KA, et al. Effect of flavonoids and vitamin E on cyclooxygenase-2 (COX-2) transcription. Mutat. Res. 2004;551:245–254. doi: 10.1016/j.mrfmmm.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 134.Jiang Q, et al. Long-chain carboxychromanols, metabolites of vitamin E, are potent inhibitors of cyclooxygenases. Proc. Natl Acad. Sci. USA. 2008;105:20464–20469. doi: 10.1073/pnas.0810962106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jiang Q, et al. Identification and quantitation of novel vitamin E metabolites, sulfated long-chain carboxychromanols, in human A549 cells and in rats. J. Lipid Res. 2007;48:1221–1230. doi: 10.1194/jlr.D700001-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.You CS, et al. Long-chain carboxychromanols are the major metabolites of tocopherols and tocotrienols in A549 lung epithelial cells but not HepG2 cells. J. Nutr. 2005;135:227–232. doi: 10.1093/jn/135.2.227. [DOI] [PubMed] [Google Scholar]
- 137.Devaraj S, et al. Gamma-tocopherol supplementation alone and in combination with alpha-tocopherol alters biomarkers of oxidative stress and inflammation in subjects with metabolic syndrome. Free Radic. Biol. Med. 2008;44:1203–1208. doi: 10.1016/j.freeradbiomed.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Campbell SE, et al. Gamma (gamma) tocopherol upregulates peroxisome proliferator activated receptor (PPAR) gamma (gamma) expression in SW 480 human colon cancer cell lines. BMC Cancer. 2003;3:25. doi: 10.1186/1471-2407-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.De Pascale MC, et al. Increased expression of transglutaminase-1 and PPARgamma after vitamin E treatment in human keratinocytes. Arch. Biochem. Biophys. 2006;447:97–106. doi: 10.1016/j.abb.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 140.Landes N, et al. Vitamin E activates gene expression via the pregnane X receptor. Biochem. Pharmacol. 2003;65:269–273. doi: 10.1016/s0006-2952(02)01520-4. [DOI] [PubMed] [Google Scholar]
- 141.Campbell SE, et al. Comparative effects of RRR-alpha- and RRR-gamma-tocopherol on proliferation and apoptosis in human colon cancer cell lines. BMC Cancer. 2006;6:13. doi: 10.1186/1471-2407-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gysin R, et al. Gamma-tocopherol inhibits human cancer cell cycle progression and cell proliferation by down-regulation of cyclins. FASEB J. 2002;16:1952–1954. doi: 10.1096/fj.02-0362fje. [DOI] [PubMed] [Google Scholar]
- 143.Jiang Q, et al. Gamma-tocopherol induces apoptosis in androgen-responsive LNCaP prostate cancer cells via caspase-dependent and independent mechanisms. Ann. N. Y. Acad. Sci. 2004;1031:399–400. doi: 10.1196/annals.1331.056. [DOI] [PubMed] [Google Scholar]
- 144.Jiang Q, et al. gamma-Tocopherol or combinations of vitamin E forms induce cell death in human prostate cancer cells by interrupting sphingolipid synthesis. Proc. Natl Acad. Sci. USA. 2004;101:17825–17830. doi: 10.1073/pnas.0408340102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yu W. Evaluation of the Anticancer Actions of Natural Vitamin E Forms RRR-α-Tocopherol and γ-Tocopherol. 2006 Abstract for Poster #68 at the ACS International Research Conference on Food, Nutrition and Cancer in Washington, DC on 13–14 July 2006. [Google Scholar]
- 146.Samandari E, et al. The effect of gamma-tocopherol on proliferation, integrin expression, adhesion, and migration of human glioma cells. Biochem. Biophys. Res. Commun. 2006;342:1329–1333. doi: 10.1016/j.bbrc.2006.02.110. [DOI] [PubMed] [Google Scholar]
- 147.Lyons NM, et al. alpha-Tocopherol, but not gamma-tocopherol inhibits 7 beta-hydroxycholesterol-induced apoptosis in human U937 cells. Free Radic. Res. 2001;35:329–339. doi: 10.1080/10715760100300861. [DOI] [PubMed] [Google Scholar]
- 148.Ricciarelli R, et al. alpha-Tocopherol specifically inactivates cellular protein kinase C alpha by changing its phosphorylation state. Biochem. J. 1998;334 doi: 10.1042/bj3340243. (Pt 1), 243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Clement S, et al. The effect of alpha-tocopherol on the synthesis, phosphorylation and activity of protein kinase C in smooth muscle cells after phorbol 12-myristate 13-acetate down-regulation. Eur. J. Biochem. 1997;246:745–749. doi: 10.1111/j.1432-1033.1997.t01-2-00745.x. [DOI] [PubMed] [Google Scholar]
- 150.Ricciarelli R, et al. Vitamin E reduces the uptake of oxidized LDL by inhibiting CD36 scavenger receptor expression in cultured aortic smooth muscle cells. Circulation. 2000;102:82–87. doi: 10.1161/01.cir.102.1.82. [DOI] [PubMed] [Google Scholar]
- 151.Malmberg KJ, et al. A short-term dietary supplementation of high doses of vitamin E increases T helper 1 cytokine production in patients with advanced colorectal cancer. Clin. Cancer Res. 2002;8:1772–1778. [PubMed] [Google Scholar]
- 152.Gianello R, et al. Alpha-tocopheryl phosphate: a novel, natural form of vitamin E. Free Radic. Biol. Med. 2005;39:970–976. doi: 10.1016/j.freeradbiomed.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 153.Ogru E, et al. Modulation of cell proliferation and gene expression by alpha-tocopheryl phosphates: relevance to atherosclerosis and inflammation. Ann. N. Y. Acad. Sci. 2004;1031:405–411. doi: 10.1196/annals.1331.058. [DOI] [PubMed] [Google Scholar]
- 154.Rezk BM, et al. Alpha-tocopheryl phosphate is a novel apoptotic agent. Front. Biosci. 2007;12:2013–2019. doi: 10.2741/2206. [DOI] [PubMed] [Google Scholar]
- 155.Mukherjee S, et al. Cardioprotection with alpha-tocopheryl phosphate: amelioration of myocardial ischemia reperfusion injury is linked with its ability to generate a survival signal through Akt activation. Biochim. Biophys. Acta. 2008;1782:498–503. doi: 10.1016/j.bbadis.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Nesaretnam K, et al. Effect of tocotrienols on the growth of a human breast cancer cell line in culture. Lipids. 1995;30:1139–1143. doi: 10.1007/BF02536615. [DOI] [PubMed] [Google Scholar]
- 157.Goh SH, et al. Inhibition of tumour promotion by various palm-oil tocotrienols. Int. J. Cancer. 1994;57:529–531. doi: 10.1002/ijc.2910570415. [DOI] [PubMed] [Google Scholar]
- 158.Yu W, et al. Induction of apoptosis in human breast cancer cells by tocopherols and tocotrienols. Nutr. Cancer. 1999;33:26–32. doi: 10.1080/01635589909514744. [DOI] [PubMed] [Google Scholar]
- 159.He L, et al. Isoprenoids suppress the growth of murine B16 melanomas in vitro and in vivo. J. Nutr. 1997;127:668–674. doi: 10.1093/jn/127.5.668. [DOI] [PubMed] [Google Scholar]
- 160.Guthrie N, et al. Inhibition of proliferation of estrogen receptor-negative MDA-MB-435 and -positive MCF-7 human breast cancer cells by palm oil tocotrienols and tamoxifen, alone and in combination. J. Nutr. 1997;127:544S–548S. doi: 10.1093/jn/127.3.544S. [DOI] [PubMed] [Google Scholar]
- 161.Constantinou C, et al. Vitamin E and cancer: an insight into the anticancer activities of vitamin E isomers and analogs. Int. J. Cancer. 2008;123:739–752. doi: 10.1002/ijc.23689. [DOI] [PubMed] [Google Scholar]
- 162.Ahmad NS, et al. Tocotrienol offers better protection than tocopherol from free radical-induced damage of rat bone. Clin. Exp. Pharmacol. Physiol. 2005;32:761–770. doi: 10.1111/j.1440-1681.2005.04264.x. [DOI] [PubMed] [Google Scholar]
- 163.Mazlan M, et al. Comparative effects of alpha-tocopherol and gamma-tocotrienol against hydrogen peroxide induced apoptosis on primary-cultured astrocytes. J. Neurol. Sci. 2006;243:5–12. doi: 10.1016/j.jns.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 164.Maniam S, et al. Palm tocotrienol exerted better antioxidant activities in bone than alpha-tocopherol. Basic Clin. Pharmacol. Toxicol. 2008;103:55–60. doi: 10.1111/j.1742-7843.2008.00241.x. [DOI] [PubMed] [Google Scholar]
- 165.Yano Y, et al. Induction of cytotoxicity in human lung adenocarcinoma cells by 6-O-carboxypropyl-alpha-tocotrienol, a redox-silent derivative of alpha-tocotrienol. Int. J. Cancer. 2005;115:839–846. doi: 10.1002/ijc.20809. [DOI] [PubMed] [Google Scholar]
- 166.Kashiwagi K, et al. A redox-silent analogue of tocotrienol inhibits hypoxic adaptation of lung cancer cells. Biochem. Biophys. Res. Commun. 2008;365:875–881. doi: 10.1016/j.bbrc.2007.11.085. [DOI] [PubMed] [Google Scholar]
- 167.Kashiwagi K, et al. A redox-silent analogue of tocotrienol acts as a potential cytotoxic agent against human mesothelioma cells. Life Sci. 2009;84:650–656. doi: 10.1016/j.lfs.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 168.Ahn KS, et al. Gamma-tocotrienol inhibits nuclear factor-kappaB signaling pathway through inhibition of receptor-interacting protein and TAK1 leading to suppression of antiapoptotic gene products and potentiation of apoptosis. J. Biol. Chem. 2007;282:809–820. doi: 10.1074/jbc.M610028200. [DOI] [PubMed] [Google Scholar]
- 169.Shah SJ, et al. Gamma-Tocotrienol inhibits neoplastic mammary epithelial cell proliferation by decreasing Akt and nuclear factor kappaB activity. Exp. Biol. Med. 2005;230:235–241. doi: 10.1177/153537020523000402. [DOI] [PubMed] [Google Scholar]
- 170.Samant GV, et al. gamma-Tocotrienol inhibits ErbB3-dependent PI3K/Akt mitogenic signalling in neoplastic mammary epithelial cells. Cell Prolif. 2006;39:563–574. doi: 10.1111/j.1365-2184.2006.00412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sun W, et al. Gamma-tocotrienol-induced apoptosis in human gastric cancer SGC-7901 cells is associated with a suppression in mitogen-activated protein kinase signalling. Br. J. Nutr. 2008;99:1247–1254. doi: 10.1017/S0007114507879128. [DOI] [PubMed] [Google Scholar]
- 172.Sylvester PW, et al. Vitamin E inhibition of normal mammary epithelial cell growth is associated with a reduction in protein kinase C(alpha) activation. Cell Prolif. 2001;34:347. doi: 10.1046/j.1365-2184.2001.00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chin SF, et al. Reduction of DNA damage in older healthy adults by Tri E Tocotrienol supplementation. Nutrition (Burbank, Los Angeles County, Calif.) 2008;24:1–10. doi: 10.1016/j.nut.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 174.Shah S, et al. Tocotrienol-induced caspase-8 activation is unrelated to death receptor apoptotic signaling in neoplastic mammary epithelial cells. Exp. Biol. Med. 2004;229:745–755. doi: 10.1177/153537020422900806. [DOI] [PubMed] [Google Scholar]
- 175.Wada S, et al. Tumor suppressive effects of tocotrienol in vivo and in vitro. Cancer Lett. 2005;229:181–191. doi: 10.1016/j.canlet.2005.06.036. [DOI] [PubMed] [Google Scholar]
- 176.Das S, et al. Cardioprotection with palm tocotrienol: antioxidant activity of tocotrienol is linked with its ability to stabilize proteasomes. Am. J. Physiol. Heart Circ. Physiol. 2005;289:H361–H367. doi: 10.1152/ajpheart.01285.2004. [DOI] [PubMed] [Google Scholar]
- 177.Eitsuka T, et al. Down-regulation of telomerase activity in DLD-1 human colorectal adenocarcinoma cells by tocotrienol. Biochem. Biophys. Res. Commun. 2006;348:170–175. doi: 10.1016/j.bbrc.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 178.Srivastava JK, et al. Tocotrienol-rich fraction of palm oil induces cell cycle arrest and apoptosis selectively in human prostate cancer cells. Biochem. Biophys. Res. Commun. 2006;346:447–453. doi: 10.1016/j.bbrc.2006.05.147. [DOI] [PubMed] [Google Scholar]
- 179.Sakai M, et al. Apoptosis induction by [gamma]-tocotrienol in human hepatoma Hep3B cells. J. Nutr. Biochem. 2006;17:672–676. doi: 10.1016/j.jnutbio.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 180.Shun MC, et al. Pro-apoptotic mechanisms of action of a novel vitamin E analog (alpha-TEA) and a naturally occurring form of vitamin E (delta-tocotrienol) in MDA-MB-435 human breast cancer cells. Nutr. Cancer. 2004;48:95–105. doi: 10.1207/s15327914nc4801_13. [DOI] [PubMed] [Google Scholar]
- 181.Nesaretnam K, et al. Tocotrienols inhibit growth of ZR-75-1 breast cancer cells. Int. J. Food Sci. Nutr. 2000;51(suppl.):S95–S103. [PubMed] [Google Scholar]
- 182.Agarwal MK, et al. Tocotrienol-rich fraction of palm oil activates p53, modulates Bax/Bcl2 ratio and induces apoptosis independent of cell cycle association. Cell Cycle. 2004;3:205–211. doi: 10.4161/cc.3.2.654. [DOI] [PubMed] [Google Scholar]
- 183.Sun W, et al. gamma-Tocotrienol induces mitochondria-mediated apoptosis in human gastric adenocarcinoma SGC-7901 cells. J. Nutr. Biochem. 2009;20:276–284. doi: 10.1016/j.jnutbio.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 184.Shah SJ, et al. Tocotrienol-induced cytotoxicity is unrelated to mitochondrial stress apoptotic signaling in neoplastic mammary epithelial cells. Biochem. Cell Biol. 2005;83:86–95. doi: 10.1139/o04-127. [DOI] [PubMed] [Google Scholar]
- 185.Rickmann M, et al. Tocotrienols induce apoptosis and autophagy in rat pancreatic stellate cells through the mitochondrial death pathway. Gastroenterology. 2007;132:2518–2532. doi: 10.1053/j.gastro.2007.03.107. [DOI] [PubMed] [Google Scholar]
- 186.Shibata A, et al. Tocotrienol inhibits secretion of angiogenic factors from human colorectal adenocarcinoma cells by suppressing hypoxia-inducible factor-1{alpha} J. Nutr. 2008;138:2136–2142. doi: 10.3945/jn.108.093237. [DOI] [PubMed] [Google Scholar]
- 187.Shibata A, et al. Tumor anti-angiogenic effect and mechanism of action of [delta]-tocotrienol. Biochem. Pharmacol. 2008;76:330–339. doi: 10.1016/j.bcp.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 188.Liu HK, et al. Inhibitory effects of gamma-tocotrienol on invasion and metastasis of human gastric adenocarcinoma SGC-7901 cells. J. Nutr. Biochem. 2009 doi: 10.1016/j.jnutbio.2008.11.004. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 189.Yap WN, et al. Gamma-tocotrienol suppresses prostate cancer cell proliferation and invasion through multiple-signalling pathways. Br. J. Cancer. 2008;99:1832–1841. doi: 10.1038/sj.bjc.6604763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ngah W, et al. Effect of tocotrienols on hepatocarcinogenesis induced by 2-acetylaminofluorene in rats. Am. J. Clin. Nutr. 1991;53:1076S–1081S. doi: 10.1093/ajcn/53.4.1076S. [DOI] [PubMed] [Google Scholar]
- 191.Iqbal J, et al. Suppression of diethylnitrosamine and 2-acetylaminofluorene-induced hepatocarcinogenesis in rats by tocotrienol-rich fraction isolated from rice bran oil. Eur. J. Cancer Prev. 2004;13:515–520. doi: 10.1097/00008469-200412000-00009. [DOI] [PubMed] [Google Scholar]
- 192.Hiura Y, et al. Specific accumulation of gamma- and delta-tocotrienols in tumor and their antitumor effect in vivo. J. Nutr. Biochem. 2008;8:607–613. doi: 10.1016/j.jnutbio.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 193.Eichholzer M, et al. Prediction of male cancer mortality by plasma levels of interacting vitamins: 17-year follow-up of the prospective Basel study. Int. J. Cancer. 1996;66:145–150. doi: 10.1002/(SICI)1097-0215(19960410)66:2<145::AID-IJC1>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 194.Taylor PR, et al. Prospective study of serum vitamin E levels and esophageal and gastric cancers. J. Natl Cancer Inst. 2003;95:1414–1416. doi: 10.1093/jnci/djg044. [DOI] [PubMed] [Google Scholar]
- 195.Mahabir S, et al. Dietary alpha-, beta-, gamma- and delta-tocopherols in lung cancer risk. Int J Cancer. 2008;123:1173–1180. doi: 10.1002/ijc.23649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Knekt P, et al. Vitamin E and cancer prevention. Am. J. Clin. Nutr. 1991;53:283S–286S. doi: 10.1093/ajcn/53.1.283S. [DOI] [PubMed] [Google Scholar]
- 197.Greenwald P, et al. Diet and cancer prevention. Eur. J. Cancer. 2001;37:948–965. doi: 10.1016/s0959-8049(01)00070-3. [DOI] [PubMed] [Google Scholar]
- 198.Moyad MA, et al. Vitamin E, alpha- and gamma-tocopherol, and prostate cancer. Semin. Urol. Oncol. 1999;17:85–90. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.