Abstract
We provide evidence that two members of the intracellular phospholipase A2 family, namely calcium-dependent group IVA (cPLA2 GIVA) and calcium-independent group VIA (iPLA2 GVIA) may play important roles in Wallerian degeneration in the mouse sciatic nerve. We assessed the roles of these PLA2s in cPLA2 GIVA−/− mice, and mice treated with a selective inhibitor of iPLA2 GVIA (FKGK11). Additionally, the effects of both these PLA2s were assessed by treating cPLA2 GIVA−/− mice with the iPLA2 inhibitor. Our data suggest that iPLA2 GVIA may play more of a role in the early stages of myelin breakdown, while cPLA2 GIVA may play a greater role in myelin clearance by macrophages. Our results also show that the delayed myelin clearance and Wallerian degeneration after sciatic nerve crush injury in mice lacking cPLA2 and iPLA2 activities is accompanied by a delay in axon regeneration, target re-innervation and functional recovery. These results indicate that the intracellular PLA2s (cPLA2 GIVA and iPLA2 GVIA) contribute significantly to various aspects of Wallerian degeneration in injured peripheral nerves, which is then essential for successful axon regeneration. This work has implications for injury responses and recovery after peripheral nerve injuries in humans, as well as for understanding the slow clearance of myelin after CNS injury and its potential consequences for axon regeneration.
Keywords: axon regeneration, myelin, macrophage, phagocytosis, phospholipase A2, sciatic nerve injury
Introduction
Peripheral nerve injury leads to rapid axonal degeneration and removal of degenerating myelin sheaths distal to the site of lesion (Waller, 1850; George and Griffin, 1994). This process, known as Wallerian degeneration, includes rapid axonal degeneration and myelin clearance. Axonal degeneration occurs within the first 48 h after injury (Beirowski et al., 2005) and appears to require calcium-mediated proteolytic activity (Schlaepfer and Bunge, 1973; George et al., 1995). Myelin degradation is a relatively slower process (lasting several days) that is not yet fully understood but appears to require the contribution of both Schwann cells and macrophages (Stoll et al., 2002). Peripheral nerve myelin, which contains axon growth inhibitory molecules, has been shown using in vitro neurite growth assays to be as inhibitory as CNS myelin (David et al., 1995). The clearance of myelin, which contains these axon growth inhibitors, appears to be essential for successful axon regeneration after peripheral nerve crush injury (Schafer et al., 1996). Furthermore, the slow clearance of myelin in the CNS after injury may contribute to the failure of axon regeneration (David and Lacroix, 2003). Therefore, understanding the molecular control of myelin clearance after peripheral nerve injury may provide insights into promoting more rapid myelin clearance and axon regeneration in the CNS.
A number of factors, such as chemokines and cytokines, have been reported to influence macrophage responses in Wallerian degeneration (Carroll and Frohnert, 1998; Toews et al., 1998; Shamash et al., 2002; Stoll et al., 2002; Tofaris et al., 2002; Perrin et al., 2005). In this article we examined the role of phospholipase A2 (PLA2) enzymes in different stages of Wallerian degeneration. PLA2 enzymes hydrolyze the ester bond at the sn-2 position of phospholipids to generate a free fatty acid [e.g. arachidonic acid (AA)], and a lysophospholipid [e.g. lysophosphatidylcholine (LPC)]. There are several forms of mammalian PLA2s that are grouped into secreted (sPLA2) and intracellular PLA2s that include calcium-dependant (cPLA2) and calcium-independent (iPLA2) forms (Murakami et al., 1997; Six and Dennis, 2000; Schaloske and Dennis, 2006). PLA2s play a normal physiological role in phospholipid metabolism, membrane turnover, host defence, and signal transduction (Dennis, 1997; Brown et al., 2003). However, they have also been implicated in inflammation in a variety of tissues and organs, including the nervous system (Murakami, 2004; Farooqui and Horrocks, 2006; Yaksh et al., 2006). We showed previously that sPLA2 group IIA (GIIA) and cPLA2 GIVA are expressed by Schwann cells and macrophages after sciatic nerve injury (De et al., 2003). In addition, treatment of C57BL/6 mice, which have a naturally occurring null mutation of sPLA2 GIIA (Kennedy et al., 1995), with a chemical inhibitor (AACOCF3) that blocks all members of the intracellular PLA2 family (cPLA2 and iPLA2) leads to reduced clearance of myelin debris (De et al., 2003). As this inhibitor was infused into the cut segment of the nerve with an osmotic pump, the effects of this non-specific inhibitor could only be assessed on Wallerian degeneration for up to 7 days. The differential roles, if any, of the two main intracellular PLA2s (cPLA2 GIVA and iPLA2 GVIA) on Wallerian degeneration and axon regeneration is still not known.
We used cPLA2 GIVA null mice and systemic treatment with a novel selective iPLA2 GVIA inhibitor to dissect out the roles of cPLA2 GIVA and iPLA2 GVIA and their combined effects in various stages of Wallerian degeneration and subsequent axon regeneration in the mouse sciatic nerve for up to 21 days. We show that cPLA2 GIVA and iPLA2 GVIA appear to play differential roles in different aspects of myelin breakdown and clearance and that both are required for efficient Wallerian degeneration. Lack of activity of one or both of these PLA2s leads to delayed axon regeneration, target re-innervation and functional recovery.
Materials and Methods
Surgery
All surgical procedures were approved by the McGill University Animal Care Committee and followed the guidelines of the Canadian Council on Animal Care.
The C57BL/6 mouse strain has a naturally occurring null mutation of one of the major forms of inflammatory sPLA2 (group IIA) (Kennedy et al., 1995). This mouse strain was therefore used to assess the role of the intracellular PLA2s (cPLA2 and iPLA2) in Wallerian degeneration using cPLA2 null mice on the C57BL/6 background, and treating wild-type C57BL/6 mice with an iPLA2 inhibitor (see below).
Adult female mice (10–12 weeks old) were anesthetized with ketamine: xylazine: acepromazine (50 : 5 : 1 mg/kg), and the right sciatic nerve exposed and crushed with liquid nitrogen-cooled fine forceps (Dumont no 5) for 30 s. The lesion site was about 45 mm from the tip of the third digit. The crush site was labelled with one 10-0 suture through the epineurium of the peroneal branch used for analysis.
cPLA2 GIVA−/− mice on the C57BL/6 background and wild-type littermates generated by breeding heterozygotes were used to study the role of cPLA2 GIVA. The generation of these cPLA2 GIVA−/− mice and their initial characterization has been described previously (Uozumi et al., 1997). The role of iPLA2 GVIA was assessed by treating C57BL/6 mice with daily intraperitoneal injections of a novel fluoroketone that is a selective inhibitor for iPLA2 GVIA (FKGK11; 200 μl of a 2 mM solution). FKGK11 is a fluoroketone that is highly selective for iPLA2 GVIA as it shows >95% inhibition of iPLA2 at 0.091 mole fraction as compared to only 17% for cPLA2. At this high concentration of substrate, values below 25% are not considered significant; the Xi(50) value of FKGK11 for iPLA2 = 0.0073 ± 0.00074. Xi(50) is the mole fraction of the inhibitor in the total substrate interface required to inhibit the enzyme by 50%, indicating that FKGK11 is a potent inhibitor of iPLA2 (Baskakis et al., manuscript submitted). Mice in the control group were treated with the vehicle used to suspend the inhibitor, i.e. PBS containing 5% Tween 80. In addition, one group of cPLA2 GIVA−/− mice were also given FKGK11 at the same dose to assess the combined effects of cPLA2 GIVA and iPLA2 GVIA.
Functional evaluation
Prior to lesion and at 7, 11, 14, 17 and 21 days following crush injury, mice were allowed to walk down a 40 cm long track after inking their hind paws. From the footprints, the sciatic functional index (SFI) was calculated using the formula developed by de Medinaceli and co-workers (de Medinaceli et al., 1982) and modified for mice by Insierra and co-workers (Inserra et al., 1998).
Recovery of pain sensitivity was tested on mice by pinching the most distal portion of the last three toes (third, fourth and fifth) of the injured hind limb with forceps. Digits 1 and 2 were not tested since they are partially innervated by the saphenous nerve. The first day after the lesion on which foot withdrawal was observed was recorded (Siconolfi and Seeds, 2001). All analyses were carried out blind.
Double immunofluorescence
Mice were deeply anesthetized as described above at 6 h, 2, 5, 7 and 21 days after the crush injury and transcardially perfused with 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB). A 5 mm length of the sciatic nerve distal to the site of crush was cryoprotected and longitudinal cryostat sections (10 μm) collected on permanent positive-charged surface slides. The crush site was identified using a 10-0 suture. Tissue sections were blocked in 0.1% Triton-X 100 and 1% normal goat serum and incubated overnight with polyclonal anti-cPLA2 (Santa Cruz Biotechnology, 1 : 75) or anti-iPLA2 (Cayman Chemicals, 1 : 500), combined with monoclonal antibodies specific for Schwann cells (mouse anti-S100β, Sigma, 1 : 200) or macrophages (rat anti-Mac-1, Serotec 1 : 200). This was followed by incubation with donkey anti-rabbit fluorescein-conjugated secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA, 1 : 400) combined with a donkey anti-mouse or anti-rat rhodamine-conjugated secondary antibody (Jackson ImmunoResearch Laboratories, 1 : 400). Tissue sections were examined using a Zeiss AxioSkop 2 FS Plus microscope (Carl Zeiss Canada, Toronto, Canada) equipped with flurorescence optics (filter set 9 and 15).
Assessment of axonal regeneration and skin re-innervation
Four days after the nerve crush, animals (n = 3 per group) were deeply anesthetized and transcardially perfused with 4% PFA as described above. A 5 mm length of the sciatic nerve distal to the site of crush injury was prepared for cryostat sectioning and serial cross sections (10 μm) taken from 3 mm distal to the crush were collected on positively charged glass slides. Tissue sections were blocked in 0.1% Triton-X 100 with 2% goat normal serum for 4 h and incubated overnight at 4°C with rabbit polyclonal antibodies against GAP-43 (1 : 500, Chemicon) and a monoclonal mouse anti-S100β (Sigma, 1 : 200) followed by a 1 h incubation at room temperature with goat anti-rabbit fluorescein-conjugated secondary antibody (1 : 500) combined with a donkey anti-mouse rhodamine-conjugated secondary antibody (1 : 400) (Jackson ImmunoResearch Laboratories). Axonal regeneration was assessed by counting the number of GAP-43 positive and S100 negative fibres at 3 mm distal to the crush site.
Twenty one days after the crush injury, animals (n = 6 per group) were perfused with 4% PFA, and plantar pads from the injured hind paw were harvested, post-fixed, cryoprotected and cut with a cryostat (25 μm). Tissue sections were immunostained as described above using rabbit polyclonal antibodies against PGP 9.5 (1 : 500, Chemicon, Temecula, CA), a marker for all types of nerve fibres, followed by a fluorescein-conjugated goat anti-rabbit secondary antibody.
Histological analysis
At 7 days (n = 3 per group) and 21 days (n = 6 per group) after the crush injury, mice were deeply anesthetized and perfused with 0.1 M phosphate buffer followed by 3% glutaraldehyde and 0.5% paraformaldehyde in 0.1 M phosphate buffer. The distal segment of the sciatic nerve was cut into 1 mm segments, post-fixed in 2% osmium tetroxide for 2 h, and embedded in Epon. Cross sections (1 μm) of the nerve were cut and stained with 1% toluidine blue for light microscopy.
Images of the whole sciatic nerve sampled at 3 mm distal to the site of crush were captured at × 10 with a Retiga 1300C digital camera (QImaging Corp., Burnaby, British Columbia) using a Zeiss AxioSkop II (Carl Zeiss Canada Ltd., Toronto) light microscope to assess the area of the nerve. In addition, six sets of images chosen by random sampling of squares representing at least 40% of the nerve cross-sectional area were also acquired at × 100. These images were used to calculate the numbers of myelinated degenerating fibres and macrophages. Macrophages were identified by their ‘foamy’ morphology in 1 μm thick Epon embedded cross sections of the nerve stained with toluidine blue. The ‘foamy’ morphology which is due to the presence of end-products of myelin/lipid degradation, is widely used to identify phagocytic macrophages (Boven et al., 2006). Degenerating fibres that were not phagocytosed were distinguished into two types: ones with disrupted myelin sheaths; ones with more compact myelin sheaths. The latter were identified as fibres in the process of degeneration because of changes in the axoplasm or presence of additional whorls of compact myelin. Degenerating fiber counts, are expressed as a percentage of the total number of fibres in the uninjured sciatic nerve. All analyses were carried out blind.
Electron microscopy
Ultrathin cross sections of Epon embedded sciatic nerves (n = 3 for each group) at a distance of 4 mm distal to the crush site were cut at 7 days after the lesion. Sections were stained with lead citrate and viewed with a Philips CM 10 electron microscope. Large single unmyelinated axons ensheathed by Schwann cells were counted in each nerve in a total area of 2.7 × 104 μm2. Data are presented as the mean ± SEM. Statistical analysis was performed as described below.
Quantitative real-time PCR
A 10 mm length of nerve distal to the lesion was harvested from uninjured mice and at 1 day after crush injury and RNA extracted using the RNeasy Lipid Tissue kit (Qiagen, Mississauga, Ontario, Canada). Nerves from eight mice were pooled for each group. A reverse transcription (RT) reaction was then carried out using Omniscript® RT kit (Qiagen, Mississauga, ON) according to the manufacturer's protocol. One μl of the RT product was added to 24 μl of Brilliant SYBR Green quantitative PCR Master Mix (Stratagene), and QRT-PCR was done to analyse the expression of IL-1β and MCP-1 (MX4000 apparatus, Stratagene). The primers 5′-TCAGGCAGGCAGTATCACT-3′ (sense) and 5′-CACGGGAAAGACACAGGTAGCT-3′ (antisense); and 5′-GAGAGCTACAAG AGGATCACCA-3′ (sense) and 5′-GTATGTCTGGACCCATTCCTTC-3′ (antisense) were used to amplify IL-1β and MCP-1 cDNA, respectively. Peptidylprolyl isomerase A (PPIA) was used as a housekeeping gene using the following primers: 5′-AGCATACAGGTCCTGGCATC-3′ (sense) and 5′-TTCACCTTCCCAAAGACCAC-3′ (antisense). The amount of cDNA was calculated based on the threshold cycle (CT) value, and was standardized by the amount of house-keeping gene using the 2−ΔΔCT method (Livak and Schmittgen, 2001).
Statistical analyses
Data are shown as mean ± SEM. The histological data was analysed by the Student's t-test. Statistical analyses of functional recovery were performed using two-way repeated-measures ANOVA with post-hoc Tukey's test for multiple comparisons. Differences were considered significant at P < 0.05.
Results
Expression of cPLA2 GIVA and iPLA2 GVIA in Wallerian degeneration
We first assessed using immunofluorescence the localization of cPLA2 GIVA and iPLA2 GVIA in Wallerian degeneration after sciatic nerve crush injury. No detectable immunostaining for cPLA2 GIVA was found in the sciatic nerve of uninjured mice (Fig. 1A). Six hours after injury, cPLA2 GIVA was exclusively observed in the site of the crush and was co-localized with S100β staining, indicating that the early expression of cPLA2 GIVA is in Schwann cells (Fig. 1B–D). Expression of cPLA2 GIVA progressively extended caudal to the crush starting at Day 2 after injury and occupied most of nerve length by Day 5 (Fig. 1O and P). Double immunofluorescence revealed that at 2 days after injury cPLA2 GIVA was expressed mainly in Schwann cells, while at 5 days post lesion ∼60% of the cPLA2+ cells were macrophages (Fig. 1E–G, O and P; supplemental table). At 21 days post-injury, immunoreactivity for cPLA2 was back to normal, i.e. almost undetectable (Supplemental Fig. 1A).
Fig. 1.
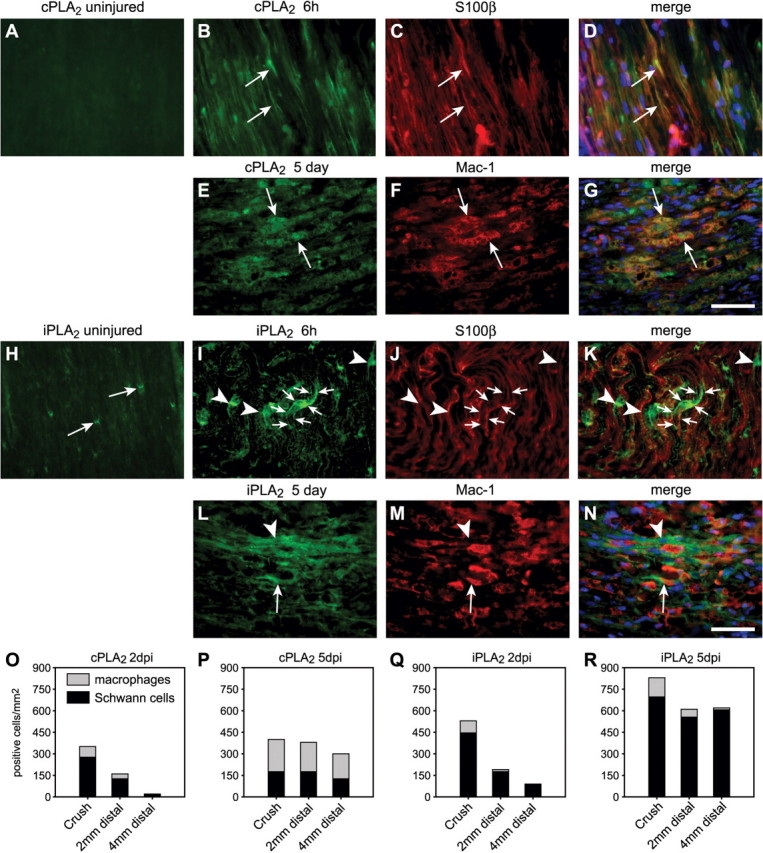
Expression of cPLA2 GIVA and iPLA2 GVIA in the uninjured and injured mouse sciatic nerve. (A) cPLA2 GIVA is not expressed in detectable levels in the uninjured mouse sciatic nerve. (B–D) As early as 6 h after crush injury cPLA2 GIVA is expressed near the site of lesion in S100β+ Schwann cells (arrows). (E–G) After 5 days, cPLA2 GIVA expression is robust mainly in Mac-1+ phagocytic macrophages (arrows). (H) In uninjured sciatic nerve, iPLA2 GVIA is expressed at the paranodal loop regions of the myelin sheath (arrows). (I–K) At 6 h after crush injury, iPLA2 immunoreactivity spreads from the paranodal regions along the length of the myelin sheath (arrows). Tissue section double-labelled with S100β. (L–N) At 5 days after injury, iPLA2 immunoreactivity was found both in Mac-1+ macrophages (arrow) and Mac-1 negative elongated cells. The latter are Schwann cells based on labelling with S100β (data not shown). Scale Bars = 50 μm. (O–R) Quantification of the number of cPLA2+ (O) and iPLA2+ (Q) Schwann cells (black bars) and macrophages (grey bars) at 2 days post injury (dpi) show a greater number of these cells at the site of crush and decrease distally along the nerve. At 5 dpi, 60% of the cPLA2 + cells are macrophages (P), while iPLA2 is expressed mainly by Schwann cells (R). Data is the mean from three experiments. See supplemental table.
Immunoreactivity for iPLA2 GVIA in the uninjured sciatic nerve was detectable in the paranodal loop regions of the myelin sheath (Fig. 1H). Six hours after the injury, iPLA2 GVIA immunoreactivity at the lesion site was more intense and spread beyond the paranodal region along the myelin sheath (Fig. 1I–K). At 2 days after injury, iPLA2 immunoreactivity extended distal to the site of crush, occupying most of entire length by Day 5 post-injury (Fig. 1Q and R). iPLA2 was expressed mainly in Schwann cells in the injured nerve, although some macrophages were also iPLA2+ (Fig. 1I–N, Q and R; supplemental table). By Day 21 after crush, iPLA2 GVIA immunoreactivity was again restricted to the paranodal regions of the myelin sheath (Supplemental Fig. 1B).
cPLA2 GIVA and iPLA2 GVIA are involved in Wallerian degeneration
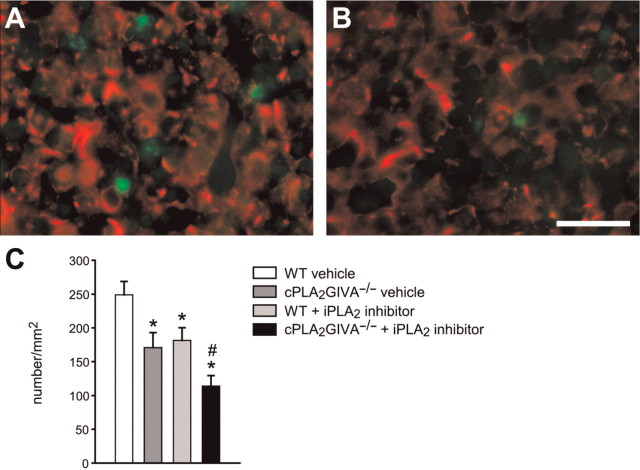
To obtain direct evidence whether cPLA2 GIVA and iPLA2 GVIA play a role in Wallerian degeneration, we crushed the right sciatic nerve in cPLA2 GIVA−/− mice and C57BL/6 mice receiving a daily injection of FKGK11, a novel selective iPLA2 inhibitor (n = 3 per group). Seven days post-injury, sciatic nerves from wild-type control animals showed advanced signs of breakdown of compact myelin and myelin phagocytosis (Fig. 2A and 5A). In wild-type-injured nerves, toluidine-blue stained sections 3 mm caudal to the crush displayed numerous phagocytic macrophages, which appear to have phagocytosed most of the degenerating myelin (∼90% of the total fibres) (Fig. 2A, 2E–G). The presence of degenerating fibres with compact myelin sheaths was scarce (Fig. 2G). Sciatic nerves from mice lacking cPLA2 GIVA or treated with iPLA2 inhibitor (FKGK11) showed delayed signs of myelin breakdown (Fig. 2B and C) and contained fewer phagocytic macrophages (40% reduction in cPLA2 GIVA−/− mice, and 30% reduction in mice treated with the FKGK11; Fig. 2E) and greater numbers of unphagocytosed nerve fibres with disrupted myelin sheaths that are still in the process of degeneration (100% increase in cPLA2 GIVA−/−, and 50% in mice treated with FKGK11; Fig. 2F). In addition, the number of unphagocytosed fibres that still had compact myelin sheaths was about 7.5 and 13-fold-higher in mice lacking cPLA2 GIVA and in wild-type mice treated with the FKGK11, respectively (Fig. 2G). The fibres with the compact myelin sheaths in these mice are not likely to be newly regenerated and remyelinated axons because of their large diameter and the thickness of their myelin sheaths. Furthermore, the axoplasm shows signs of degeneration and there are often additional myelin whorls—evidence that these fibres are in the process of degeneration (Fig. 5B and D). Regenerating axons at this early stage (7 days) after injury are not yet myelinated (see section below). These data suggest that although both intracellular forms of PLA2 are involved in Wallerian degeneration, iPLA2 GVIA is likely to play a greater role in the early stages of myelin breakdown, while cPLA2 GIVA may be more involved in the later stages of myelin clearance by macrophages.
Fig. 2.
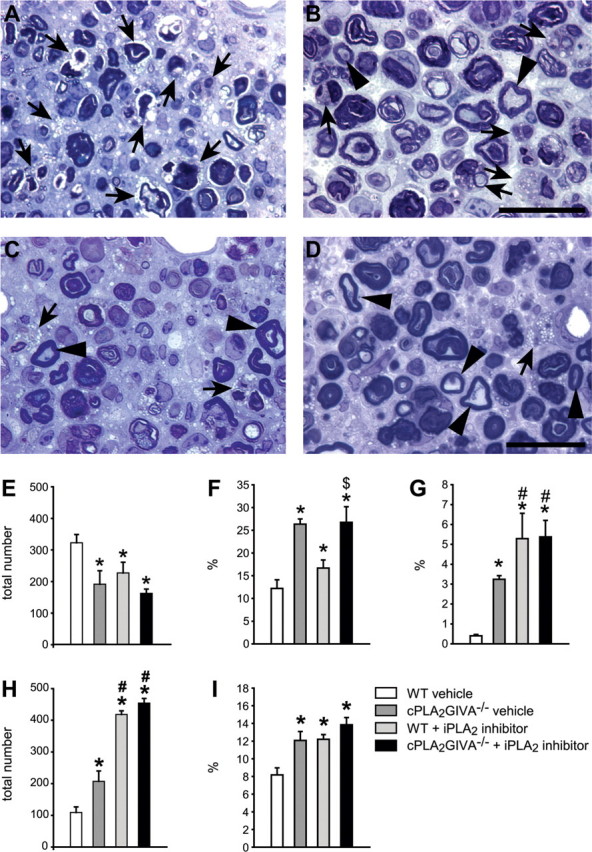
Effect of cPLA2 GIVA and iPLA2 GVIA on Wallerian degeneration in the sciatic nerve. (A) Epon embedded toluidine blue-stained cross section of the lesioned sciatic nerve of a wild-type mouse 7 days after nerve crush injury shows marked disruption of myelin and phagocytosis by macrophages (arrows). In contrast, the sciatic nerve of a cPLA2 GIVA−/− mouse (B), wild-type mouse treated with the iPLA2 inhibitor (FKGK11) (C), or cPLA2 GIVA−/− mouse treated with FKGK11 (D), show large numbers of well preserved intact myelin profiles (arrowheads), and fewer phagocytic macrophages (arrows). Graphs show the quantification of the total number of phagocytic macrophages (E), degenerating fibres with disrupted myelin sheaths (F) and fibres with intact myelin sheaths (G) in cross-sections of lesioned sciatic nerves from wild-type vehicle-treated mice, cPLA2 GIVA−/−, wild-type mice treated with the iPLA2 inhibitor, and cPLA2 GIVA−/− mice treated with the iPLA2 inhibitor at 7 days after crush injury. Values in F and G represent the number of degenerating fibres with disrupted myelin sheath (F) and fibres with compact myelin sheaths (G), shown as a percentage of the total number of fibres in the uninjured nerve. Note the marked reduction in the number of macrophages in the cPLA2 GIVA null mice and iPLA2 inhibitor treated mice as compared to the wild-type mice (E), and the marked slowing of myelin breakdown and phagocytosis (F and G). The quantification at 21 days after crush injury of the total number of phagocytic macrophages (H) and degenerating fibres with disrupted myelin sheath (I) shown as a percentage of the total number of myelinated fibres in the uninjured nerve. Note the markedly increased persistence of phagocytic macrophages (H) in mice treated with the iPLA2 inhibitor as compared to the cPLA2 null mice and injured wild-type controls. Bars = 25 μm; *P < 0.05 for experimental groups versus wild-type (vehicle) controls; $ = P < 0.05 for indicated groups versus FKGK11-treated mice; # = P < 0.05 for indicated groups versus cPLA2−/− mice.
Fig. 5.
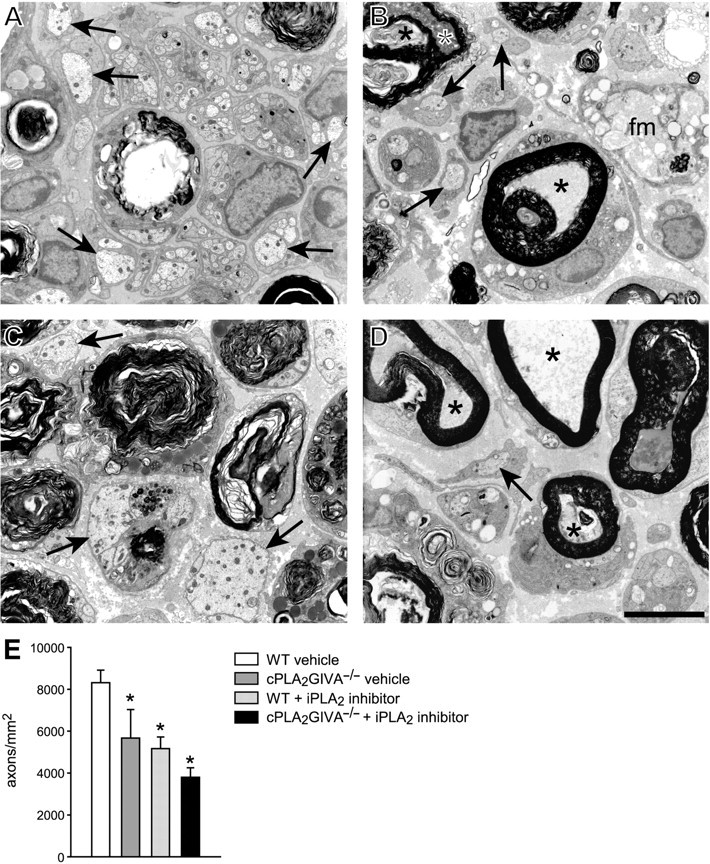
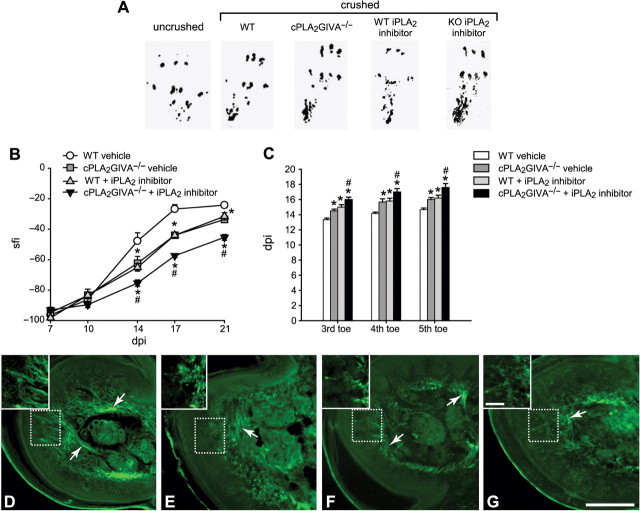
Electron micrographs of nerves 4 mm distal to the crush site at 7 days after injury from wild-type (WT) vehicle-treated mouse (A), WT iPLA2 inhibitor treated (B), cPLA2−/−mouse (C), and cPLA2−/−mouse treated with the cPLA2 inhibitor (D). Note the single large diameter, regenerated axons that are not yet myelinated (arrows), Note also the presence of degenerating fibres with compact myelin sheaths (*) in nerves of WT mice treated with the iPLA2 inhibitor (B) and cPLA2−/− mice treated with the iPLA2 inhibitor (D). A ‘foamy’ macrophage (fm) is seen in B. (E) Graph showing quantification of these regenerating axons (n = 3); *P < 0.05 as compared to WT injured controls. Scale Bar = 5 μm.
We then assessed whether the lack of activity of both cPLA2 GIVA and iPLA2 GVIA leads to a greater delay in Wallerian degeneration. For these experiments, cPLA2 GIVA−/− mice were given daily injections of FKGK11. At 7 days post injury, sciatic nerve sections from animals lacking activity of both PLA2s showed a reduction in the number of macrophages that was similar to that of cPLA2 GIVA null mice (Fig. 2). They also had similar numbers of degenerating fibres with disrupted myelin sheaths as cPLA2 GIVA−/− mice (Fig. 2F and G) while the number of fibres with compact myelin sheaths were similar to that of mice treated with FKGK11 (Fig. 2F and G). The presence of degenerating nerve fibres with compact myelin sheaths was also evident by electron microscopy (Fig. 5D). These results suggest that iPLA2 may contribute more to the early phase of myelin breakdown, while cPLA2 may contribute more to myelin phagocytosis by macrophages.
We then assessed changes in the sciatic nerves at 21 days after crush (n = 6 per group), when almost all of the myelin debris has been removed and axon regeneration has occurred in the wild-type control mice. At this time point, and in contrast to 7 days post injury, sciatic nerves from cPLA2 GIVA−/− mice and wild-type littermates treated with FKGK11 showed a significant increase in the number of macrophages and degenerating fibres with disrupted myelin sheaths (Fig. 2H and I), further indicating that Wallerian degeneration was delayed with the lack of cPLA2 GIVA or iPLA2 GVIA activity. Most of the phagocytic macrophages have been cleared from the nerve at 21 days in wild-type mice (Fig. 2H). Interestingly, mice treated with FKGK11 had 2-fold higher number of phagocytic macrophages than cPLA2 GIVA−/− mice, suggesting that iPLA2 GVIA could also contribute to the efflux of macrophages from the nerve at later stages of Wallerian degeneration. Similar changes were also observed in mice lacking both PLA2 activities (Fig. 2H).
These data provide direct evidence that cPLA2 GIVA and iPLA2 GVIA play an important role in myelin breakdown and phagocytosis after peripheral nerve injury, and the lack of their activity results in impaired Wallerian degeneration.
Altered expression of pro-inflammatory mediators in the absence of cPLA2 GIVA and/or iPLA2 GVIA activity
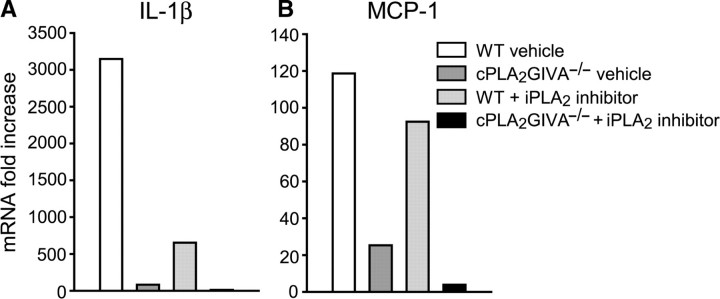
Chemokines and cytokines are important regulators of the immune response. Among them, IL-1β and MCP-1 play a crucial role in the recruitment and activation of macrophages in injured peripheral nerve (Shamash et al., 2002; Perrin et al., 2005). We therefore assessed, whether the decreased macrophage influx observed in cPLA2 GIVA−/− mice and in mice treated with the iPLA2 GVIA inhibitor was related to a reduction in the expression of these inflammatory mediators. Q-PCR performed on samples harvested at 1 day after crush (a pool of eight nerves per group) revealed a marked upregulation in IL-1β (3147-fold) and MCP-1 (118-fold) mRNA expression in the crushed sciatic nerve of WT-vehicle treated mice. In mice lacking cPLA2 GIVA, the mRNA expression of IL-1β and MCP-1 was markedly less that in the injured vehicle treated wild-type (Fig. 3A and B), while wild-type mice treated with the iPLA2 inhibitor also showed lower levels of IL-1β mRNA but little change in MCP-1 (Fig. 3A and B). The mRNA levels of IL-1β and MCP-1 were even lower in mice lacking both cPLA2 GIVA and iPLA2 activities (Fig. 3). The expression of these molecules was not completely blocked, as their levels were still 9-fold higher than in uninjured nerves, suggesting that other factors also contribute to this effect.
Fig. 3.
Q-PCR analyses of IL-1β and MCP-1 mRNA at 1 day after sciatic nerve crush injury. Values are expressed as fold increase over uninjured wild-type nerves. Note the marked increase in expression of IL-1β and MCP-1 after injury in wild-type vehicle treated mice. In contrast, the expression of IL-1β and in MCP-1 mRNA in mice lacking cPLA2 GIVA is markedly less than in injured wild-type nerve. In wild-type mice treated with the iPLA2 inhibitor, the expression of IL-1β mRNA is also lower but there is only a slight change in MCP-1. The expression of IL-1β and MCP-1 mRNA is more drastically decreased in cPLA2 null mice treated with the iPLA2 inhibitor as compared to injured wild-type vehicle-treated mice. PPIA is used as a housekeeping gene.
Axonal regeneration and functional recovery after sciatic nerve injury is impaired in the lack of cPLA2 GIVA and/or iPLA2 GVIA activity
We next carried out experiments to assess whether the reduction in myelin clearance by macrophages in the absence of cPLA2 GIVA or iPLA2 GVIA activity influences axon regeneration since peripheral nerve myelin contains molecules that inhibit axonal growth. We first quantified the number of GAP-43 positive axons that were S100β negative (Fig. 4A–C) in sciatic nerve cross-sections at a distance of 3 mm distal to the site of crush, 4 days after injury. As S100β-positive non-myelinating Schwann cells may also express GAP-43 (Curtis et al., 1992), we only quantified GAP-43 labelled rounded profiles that were S100β-negative to ensure we were counting regenerating axons and not Schwann cells (Fig. 4A). Lack of the cPLA2 GIVA or iPLA2 GVIA activity resulted in ∼30% reduction in the number of regenerating axons (Fig. 4C). In cPLA2−/− mice treated with the iPLA2 inhibitor (i.e. lacking both PLA2s), the number of regenerating fibres was reduced even further to 55% of control values (Fig. 4C). To further confirm these results, we quantified the number of regenerated axons by electron microscopy in cross-sections of the sciatic nerve 4 mm distal to the crush site, at 7 days after injury. At this time point, many regenerated axons were present in the wild-type-vehicle treated injured nerve but these newly regenerated single large diameter axons were not yet myelinated. Mice lacking cPLA2 GIVA or treated with the iPLA2 inhibitor had significantly fewer regenerated axons (∼40%) as compared to the wild-type-vehicle treated mice (Fig. 5B, C, E). In addition, nerves from cPLA2 GIVA null mice treated with the iPLA2 inhibitor showed an even greater reduction in the number of single, large diameter regenerated axons (∼60%) (Fig. 5D and E). These results provide direct evidence that the lack of intracellular forms of PLA2 lead to delayed axonal regeneration after sciatic nerve injury.
Fig. 4.
Axon regeneration assessed 3 mm distal to the crush site at 4 days after sciatic nerve crush injury. Micrographs of cross-sections of sciatic nerve from a WT vehicle-treated mouse (A) and a cPLA2 GVIA−/− mouse treated with iPLA2 inhibitor (B) double-labelled with GAP-43 (green) and S100β (red). Regenerating axons are identified as profiles that are labelled with GAP-43 but not S100β. (C) Graph showing the quantification of GAP-43+ regenerating axons. Note that, there is a significantly lower number of regenerating axons in cPLA2 GIVA−/− mice and wild-type mice treated with the iPLA2 inhibitor as compared to wild-type injured controls (*P < 0.05). There is also a further reduction in the number of regenerating axons in cPLA2 GIVA−/− mice treated with the iPLA2 inhibitor; #P < 0.05 versus cPLA2 null; and WT+iPLA2 inhibitor). Bar = 20 μm.
We further evaluated the rate of functional recovery after sciatic nerve crush injury in mice lacking cPLA2 GIVA or iPLA2 GVIA activity. The walking track method in which foot-prints are analysed was used to assess motor recovery. After sciatic nerve crush, the SFI calculated from foot-prints by measuring the distance between the first and the fifth toes and the length of the footprint (Fig. 6A), drops immediately to a negative value of about −100, indicating a marked disability in the injured paw (Fig. 6B). This deficit recovers towards the normal value of 0 when axons regenerate and appropriately reinnervate their targets (Fig. 6B). Wild-type control littermates underwent a fast recovery in the SFI from 10 to 17 days post injury, showing toe spreading and plantar length close to that of the uninjured paw (Fig. 6A and B). Mice lacking cPLA2 GIVA or iPLA2 GVIA activity showed significantly slower recovery in SFI compared to control injured mice (Fig. 6A and B). This deficit in functional recovery was even greater in mice lacking both cPLA2 GIVA and iPLA2 GVIA activity (Fig. 6A and B).
Fig. 6.
Effect of cPLA2 GIVA and iPLA2 GVIA on functional recovery. (A) Representative footprints obtained from uninjured wild-type mice, and 21 days after sciatic nerve crush injury in wild-type mice, cPLA2 GIVA−/− mice, wild-type mice treated with the iPLA2 inhibitor (FKGK11), and cPLA2 GIVA−/− mice treated with FKGK11. At 21 days post-injury, footprints from the injured paw of wild-type mice (WT) display a certain degree of recovery in toe spreading and length of the foot-print due to axonal regeneration and re-innervation. Note that this recovery is impaired in cPLA2 GIVA−/− mice, and wild-type mice treated with the iPLA2 inhibitor (WT FKGK11), and cPLA2 GIVA−/− mice treated with the iPLA2 inhibitor (KO FKGK11) as compared to wild-type mice (WT). Foot-print analysis shows narrower toe spreading and longer footprint length in cPLA2−/− mice and mice treated with the iPLA2 inhibitor than the wild-type controls. (B) Graph showing the recovery of motor function (footprints) after sciatic nerve crush determined by the SFI (described in the Methods section). Delayed recovery of motor function (SFI index) is evident in mice lacking the activity of either cPLA2 GIVA or iPLA2 GVIA or both PLA2s as compared to wild-type controls. (C) Histogram showing the days post-injury after sciatic nerve crush at which the toe pinch reflex was first elicited for the third, fourth and fifth, toes. Recovery of the toe pinch withdrawal reflex is delayed by about 2 days in mice lacking cPLA2 GIVA or wild-type mice treated with the iPLA2 inhibitor. The combined lack of both PLA2 activities results in an even further delay of 3 days. (D–G) Re-innervation of the plantar foot-pads at 21 days after sciatic nerve crush in wild-type (D) cPLA2 GIVA−/− (E), wild-type treated with the iPLA2 inhibitor (F), and cPLA2 GIVA−/− treated with the iPLA2 inhibitor (G). Note that the PGP 9.5 immunoreactive fibres (arrows) are seen around the sweat glands and also close to the sub-epidermal nerve plexus in wild-type animals treated with vehicle (D), whereas fewer labeled fibres are seen in the other groups. Inserts show higher magnification images of the areas outlined by the hatched squares. Scale Bar = 200 μm; scale bar in insert = 50 μm. *P < 0.05 as compared to WT injured controls; #P < 0.05 as compared to cPLA2 null; and WT+iPLA2 inhibitor.
We also examined the recovery of sensory function by means of the toe pinch reflex. After sciatic nerve injury, the pinch reflex response of the third, fourth and fifth, toe was abolished in all animals. Recovery of this response in the fifth toe was observed by 14 days after injury in wild-type mice, and delayed by 2 days in cPLA2 GIVA−/− mice and animals treated with the iPLA2 inhibitor (Fig. 6C). Lack of activity of both PLA2s led to a further delay (3 days) in recovery in pinch reflex response (Fig. 6C).
Finally, we studied the regeneration and innervation of PGP 9.5 positive fibres within the sweat glands (autonomic fibres) and skin of the hind paw (sensory fibres). At 21 days after crush injury, all wild-type control mice showed many PGP 9.5 immunoreactive axonal profiles re-innervating the sweat glands and skin of the injured hind paw (Fig. 6D). In contrast, very few PGP 9.5 immunoreactive fibres were found innervating the sweat glands and skin of cPLA2 GIVA−/− mice (Fig. 6E), mice treated with the iPLA2 inhibitor (Fig. 6F), or cPLA2 GIVA−/− mice treated with the iPLA2 inhibitor (Fig. 6G). These results show that, axon regeneration and re-innervation is delayed in the absence of lack of cPLA2 GIVA and iPLA2 GVIA.
Discussion
The results of this study provide direct evidence that cPLA2 GIVA and iPLA2 GVIA play a significant role in Wallerian degeneration in peripheral nerve injury. This is based on studies done on the cPLA2 GIVA deficient mice and wild-type mice treated with a selective inhibitor for iPLA2 GVIA in which myelin breakdown, macrophage recruitment and myelin phagocytosis are severely impaired. We also show that these changes are accompanied with a deficit in axon regeneration, target re-innervation and functional recovery.
Schwann cells are thought to play a key role in the initiation of myelin degradation after peripheral nerve injury because this process is initiated within the first few hours after injury before the influx of macrophages. We show that Schwann cells express iPLA2 GVIA and cPLA2 GIVA within 6 h after sciatic nerve injury. PLA2s hydrolyze membrane phospholipids to release fatty acids, such as AA, and a lysophospholipid, such as LPC. The latter is a potent demyelinating agent that can initiate myelin breakdown. Myelin is especially susceptible to LPC because, unlike other membranes, it does not possess or is not readily accessible to reacylating enzymes, which limit the damage (Gregson and Hall, 1973). The first signs of myelin degeneration after nerve injury are found at the paranodes and Schmidt–Lantermann incisures, which contain Schwann cell cytoplasm (Williams and Hall, 1971) and are also the first affected after intraneural injection of LPC or Crotalus venom PLA2 (Hall and Gregson, 1971; Gregson and Hall, 1973; Hall, 1989). Therefore, the synthesis of LPC by PLA2s at the paranodes and Schmidt–Lantermann incisures may trigger the onset of myelin breakdown. This is in keeping with our current findings that iPLA2 immunoreactivity spreads from the paranodal regions along the myelin sheath after injury. Interestingly, when iPLA2 GVIA activity was blocked, there was a 13-fold increase in the number of degenerating nerve fibres with intact or compact myelin sheaths as compared to vehicle treated mice, suggesting that iPLA2 GVIA plays a role in the early stages of myelin breakdown. Interestingly, cPLA2 GIVA is also expressed in Schwann cells, especially during the first few hours after injury, although its expression is more robust in macrophages at 5 and 7 days after injury. Mice lacking cPLA2 GIVA, however, showed a 7.5-fold-increase in the numbers of degenerating fibres with compact myelin as compared to wild-type littermates. This suggests that cPLA2 GIVA also plays a role in myelin breakdown, although to a lesser extent than iPLA2 GVIA.
PLA2s expressed in Schwann cells may also trigger the influx of macrophages into the injured tissue. Macrophages participate in Wallerian degeneration and play the main role in the phagocytosis of myelin and axonal debris. We previously reported that AACOCF3, a compound that blocks all members of the intracellular PLA2s, including cPLA2 GIVA and iPLA2 GVIA, reduced ∼60% of the macrophages recruited into the transected sciatic nerve (De et al., 2003). In the present study, a similar reduction in the number of macrophages was observed in cPLA2 GIVA−/− mice treated with the iPLA2 inhibitor. However, when we dissected out the contribution of each of these intracellular PLA2s, we found that cPLA2 GIVA appears to have a greater role in macrophage recruitment, since the number of macrophages was reduced by 40% in cPLA2 GIVA−/− mice, as compared to 30% reduction in animals treated with the iPLA2 inhibitor.
Recruitment of macrophages into the injured distal nerve segment is mediated in part by chemokines and cytokines, which are expressed by Schwann cells as early as 24 h after injury (Toews et al., 1998; Shamash et al., 2002; Perrin et al., 2005). We found, that the expression of IL-1β and MCP-1 mRNA in mice lacking cPLA2 GIVA was markedly less than in injured wild-type nerve. In wild-type mice treated with the iPLA2 inhibitor there was also lower level of IL-1β mRNA but only a slight change in MCP-1. However, the expression of IL-1β and MCP-1 mRNA was more drastically decreased in cPLA2 null mice treated with the iPLA2 inhibitor as compared to injured wild-type vehicle-treated mice. The greater reduction in the expression of MCP-1 in cPLA2 GIVA null mice as compared to mice treated with the iPLA2 inhibitor correlates with the greater reduction in the number of phagocytic macrophages. We previously showed that LPC injected directly into the mouse spinal cord induces the expression of a number of pro-inflammatory chemokines and cytokines including IL-1β and MCP-1, and that neutralizing IL-1β or MCP-1 with blocking antibodies reduced LPC-induced macrophage influx and activation (Ousman and David, 2000, 2001). Furthermore, neutralizing IL-1β and MCP-1 with blocking antibodies also reduced macrophage recruitment, activation and myelin clearance in the injured mouse sciatic nerve (Perrin et al., 2005). As well, AA produced by PLA2s is metabolized by cyclooxygenase enzymes (COX-1 and COX-2) to prostaglandins, which are potent mediators of inflammation that can induce the recruitment of macrophages by promoting vasodilatation and expression of cytokines, such as IL-1β (Rocha et al., 2003). Taken together, these findings suggest that PLA2 enzymes expressed by Schwann cells may induce the recruitment and activation of macrophages into the damaged nerve through a variety of mechanisms.
Activated macrophages play a primary role in removing myelin and axonal debris in the injured nerve. They infiltrate into the lesion site within the first 2 days and spread throughout the distal portion of the nerve beginning by Day 4, peak by 14 days and decrease thereafter (Muller and Minwegen, 1987; Avellino et al., 1995). We show that although macrophages in the injured sciatic nerve express mainly cPLA2 GIVA, some of these cells also express iPLA2 GVIA. Our current data shows that lack of cPLA2 GIVA and/or iPLA2 GVIA activity leads to a marked reduction in the clearance of myelin and axonal debris after sciatic nerve injury. LPC produced by PLA2s binds with high affinity to C-reactive protein and IgM antibodies leading to the activation of the complement pathway, which is crucial for phagocytosis by macrophages (De et al., 2003; Rotshenker, 2003). In addition, LPC produced by iPLA2 may act as an ‘eat me’ signal for macrophages, as has been shown in other experimental models (Lauber et al., 2003). Therefore, cPLA2 GIVA and iPLA2 GVIA expressed in peripheral nerves during Wallerian degeneration may also promote the phagocytic activity of macrophages.
One striking finding we observed is that the lack of iPLA2 resulted in a notable accumulation of macrophages within the nerve at 21 days after injury. This observation might suggest that, iPLA2 may influence the efflux of macrophages from the nerve at later stages of Wallerian degeneration. Previous studies have demonstrated that iPLA2, but not cPLA2, hydrolyzes docosahexaenoic acid (DHA) from phospholipids (Strokin et al., 2003). DHA is the precursor of neuroprotectin D1 and resolvin D, two newly identified lipid mediators that have been shown to play an important role in the resolution of the inflammatory response (Schwab et al., 2007). Therefore, iPLA2 could be involved via these mechanisms in the clearance of macrophages from the injured nerve at late stages of Wallerian degeneration.
We also show that, the impaired Wallerian degeneration associated with the lack of cPLA2 and iPLA2 activity also delays axon regeneration and target re-innervation. The delay in axon regeneration may be due to the poor clearance of myelin-associated glycoprotein (MAG) and oligodendrocyte-myelin glycoprotein (OMgp), two axon growth inhibitors found in peripheral nerve myelin. Our results support earlier work by Schafer and co-workers (Schafer et al., 1996) showing direct evidence for the effects of MAG in retarding axon regeneration in the WldS mouse, in which myelin clearance during Wallerian degeneration is severely impaired. Interestingly, after spinal cord injury where Wallerian degeneration is markedly slow, macrophages do not express cPLA2 GIVA, and only a small population express iPLA2 GVIA (unpublished results). Therefore, strategies that upregulate the expression of these intracellular PLA2s may help speed the breakdown and clearance of myelin in the injured CNS and convert the non-permissive CNS white matter environment into a permissive one.
Funding
Canadian Institutes of Health Research (S.D.); Canadian Institutes of Health Research Postdoctoral Fellowship (R.L.V.).
Supplementary Material
Acknowledgements
The authors thank Ms Hiba Kazak for technical help and Ms Margaret Attiwell for help with the illustrations.
Glossary
Abbreviations:
- cPLA2
calcium-dependent PLA2
- iPLA2
calcium-independent PLA2
- LPC
lysophosphatidylcholine
- PLA2
phospholipase A2
- SFI
sciatic functional index (SFI)
References
- Avellino AM, Hart D, Dailey AT, MacKinnon M, Ellegala D, Kliot M. Differential macrophage responses in the peripheral and central nervous system during wallerian degeneration of axons. Exp Neurol. 1995;136:183–98. doi: 10.1006/exnr.1995.1095. [DOI] [PubMed] [Google Scholar]
- Beirowski B, Adalbert R, Wagner D, Grumme DS, Addicks K, Ribchester RR, et al. The progressive nature of Wallerian degeneration in wild-type and slow Wallerian degeneration (WldS) nerves. BMC Neurosci. 2005;6:6. doi: 10.1186/1471-2202-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boven LA, Van Meurs M, Van Zwam M, Wierenga-Wolf A, Hintzen RQ, Boot RG, et al. Myelin-laden macrophages are anti-inflammatory, consistent with foam cells in multiple sclerosis. Brain. 2006;129:517–26. doi: 10.1093/brain/awh707. [DOI] [PubMed] [Google Scholar]
- Brown WJ, Chambers K, Doody A. Phospholipase A2 (PLA2) enzymes in membrane trafficking: mediators of membrane shape and function. Traffic. 2003;4:214–21. doi: 10.1034/j.1600-0854.2003.00078.x. [DOI] [PubMed] [Google Scholar]
- Carroll SL, Frohnert PW. Expression of JE (monocyte chemoattractant protein-1) is induced by sciatic axotomy in wild type rodents but not in C57BL/Wld(s) mice. J Neuropathol Exp Neurol. 1998;57:915–30. doi: 10.1097/00005072-199810000-00004. [DOI] [PubMed] [Google Scholar]
- Curtis R, Stewart HJ, Hall SM, Wilkin GP, Mirsky R, Jessen KR. GAP-43 is expressed by nonmyelin-forming Schwann cells of the peripheral nervous system. J Cell Biol. 1992;116:1455–64. doi: 10.1083/jcb.116.6.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David S, Lacroix S. Molecular approaches to spinal cord repair. Annu Rev Neurosci. 2003;26:411–40. doi: 10.1146/annurev.neuro.26.043002.094946. [DOI] [PubMed] [Google Scholar]
- David S, Braun PE, Jackson DL, Kottis V, McKerracher L. Laminin overrides the inhibitory effects of peripheral nervous system and central nervous system myelin-derived inhibitors of neurite growth. J Neurosci Res. 1995;42:594–602. doi: 10.1002/jnr.490420417. [DOI] [PubMed] [Google Scholar]
- de Medinaceli L, Freed WJ, Wyatt RJ. An index of the functional condition of rat sciatic nerve based on measurements made from walking tracks. Exp Neurol. 1982;77:634–43. doi: 10.1016/0014-4886(82)90234-5. [DOI] [PubMed] [Google Scholar]
- De S, Trigueros MA, Kalyvas A, David S. Phospholipase A2 plays an important role in myelin breakdown and phagocytosis during Wallerian degeneration. Mol Cell Neurosci. 2003;24:753–65. doi: 10.1016/s1044-7431(03)00241-0. [DOI] [PubMed] [Google Scholar]
- Dennis EA. The growing phospholipase A2 superfamily of signal transduction enzymes. Trends Biochem Sci. 1997;22:1–2. doi: 10.1016/s0968-0004(96)20031-3. [DOI] [PubMed] [Google Scholar]
- Farooqui AA, Horrocks LA. Phospholipase A2-generated lipid mediators in the brain: the good, the bad, and the ugly. Neuroscientist. 2006;12:245–60. doi: 10.1177/1073858405285923. [DOI] [PubMed] [Google Scholar]
- George EB, Glass JD, Griffin JW. Axotomy-induced axonal degeneration is mediated by calcium influx through ion-specific channels. J Neurosci. 1995;15:6445–52. doi: 10.1523/JNEUROSCI.15-10-06445.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George R, Griffin JW. Delayed macrophage responses and myelin clearance during Wallerian degeneration in the central nervous system: the dorsal radiculotomy model. Exp Neurol. 1994;129:225–36. doi: 10.1006/exnr.1994.1164. [DOI] [PubMed] [Google Scholar]
- Gregson NA, Hall SM. A quantitative analysis of the effects of the intraneural injection of lysophosphatidyl choline. J Cell Sci. 1973;13:257–77. doi: 10.1242/jcs.13.1.257. [DOI] [PubMed] [Google Scholar]
- Hall SM. Regeneration in the peripheral nervous system. Neuropathol Appl Neurobiol. 1989;15:513–29. doi: 10.1111/j.1365-2990.1989.tb01251.x. [DOI] [PubMed] [Google Scholar]
- Hall SM, Gregson NA. The in vivo and ultrastructural effects of injection of lysophosphatidyl choline into myelinated peripheral nerve fibres of the adult mouse. J Cell Sci. 1971;9:769–89. doi: 10.1242/jcs.9.3.769. [DOI] [PubMed] [Google Scholar]
- Inserra MM, Bloch DA, Terris DJ. Functional indices for sciatic, peroneal, and posterior tibial nerve lesions in the mouse. Microsurgery. 1998;18:119–24. doi: 10.1002/(sici)1098-2752(1998)18:2<119::aid-micr10>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Kennedy BP, Payette P, Mudgett J, Vadas P, Pruzanski W, Kwan M, et al. A natural disruption of the secretory group II phospholipase A2 gene in inbred mouse strains. J Biol Chem. 1995;270:22378–85. doi: 10.1074/jbc.270.38.22378. [DOI] [PubMed] [Google Scholar]
- Lauber K, Bohn E, Krober SM, Xiao YJ, Blumenthal SG, Lindemann RK, et al. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell. 2003;113:717–30. doi: 10.1016/s0092-8674(03)00422-7. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Muller HW, Minwegen P. Nonresident macrophages in peripheral nerve of rat: effect of silica on migration, myelin phagocytosis, and apolipoprotein E expression during Wallerian degeneration. J Neurosci Res. 1987;18:222–9. doi: 10.1002/jnr.490180132. [DOI] [PubMed] [Google Scholar]
- Murakami M. Hot topics in phospholipase A2 field. Biol Pharm Bull. 2004;27:1179–82. doi: 10.1248/bpb.27.1179. [DOI] [PubMed] [Google Scholar]
- Murakami M, Nakatani Y, Atsumi G, Inoue K, Kudo I. Regulatory functions of phospholipase A2. Crit Rev Immunol. 1997;17:225–83. doi: 10.1615/critrevimmunol.v17.i3-4.10. [DOI] [PubMed] [Google Scholar]
- Ousman SS, David S. Lysophosphatidylcholine induces rapid recruitment and activation of macrophages in the adult mouse spinal cord. Glia. 2000;30:92–104. [PubMed] [Google Scholar]
- Ousman SS, David S. MIP-1alpha, MCP-1, GM-CSF, and TNF-alpha control the immune cell response that mediates rapid phagocytosis of myelin from the adult mouse spinal cord. J Neurosci. 2001;21:4649–56. doi: 10.1523/JNEUROSCI.21-13-04649.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin FE, Lacroix S, Aviles-Trigueros M, David S. Involvement of monocyte chemoattractant protein-1, macrophage inflammatory protein-1alpha and interleukin-1beta in Wallerian degeneration. Brain. 2005;128:854–66. doi: 10.1093/brain/awh407. [DOI] [PubMed] [Google Scholar]
- Rocha PN, Plumb TJ, Coffman TM. Eicosanoids: lipid mediators of inflammation in transplantation. Springer Semin Immunopathol. 2003;25:215–27. doi: 10.1007/s00281-003-0132-4. [DOI] [PubMed] [Google Scholar]
- Rotshenker S. Microglia and macrophage activation and the regulation of complement-receptor-3(CR3/MAC-1)-mediated myelin phagocytosis in injury and disease. J Mol Neurosci. 2003;21:65–72. doi: 10.1385/JMN:21:1:65. [DOI] [PubMed] [Google Scholar]
- Schafer M, Fruttiger M, Montag D, Schachner M, Martini R. Disruption of the gene for the myelin-associated glycoprotein improves axonal regrowth along myelin in C57BL/Wlds mice. Neuron. 1996;16:1107–13. doi: 10.1016/s0896-6273(00)80137-3. [DOI] [PubMed] [Google Scholar]
- Schaloske RH, Dennis EA. The phospholipase A2 superfamily and its group numbering system. Biochim Biophys Acta. 2006;1761:1246–59. doi: 10.1016/j.bbalip.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–74. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamash S, Reichert F, Rotshenker S. The cytokine network of Wallerian degeneration: tumor necrosis factor-alpha, interleukin-1alpha, and interleukin-1beta. J Neurosci. 2002;22:3052–60. doi: 10.1523/JNEUROSCI.22-08-03052.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siconolfi LB, Seeds NW. Mice lacking tPA, uPA, or plasminogen genes showed delayed functional recovery after sciatic nerve crush. J Neurosci. 2001;21:4348–55. doi: 10.1523/JNEUROSCI.21-12-04348.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Six DA, Dennis EA. The expanding superfamily of phospholipase A(2) enzymes: classification and characterization. Biochim Biophys Acta. 2000;1488:1–19. doi: 10.1016/s1388-1981(00)00105-0. [DOI] [PubMed] [Google Scholar]
- Stoll G, Jander S, Myers RR. Degeneration and regeneration of the peripheral nervous system: from Augustus Waller's observations to neuroinflammation. J Peripher Nerv Syst. 2002;7:13–27. doi: 10.1046/j.1529-8027.2002.02002.x. [DOI] [PubMed] [Google Scholar]
- Strokin M, Sergeeva M, Reiser G. Docosahexaenoic acid and arachidonic acid release in rat brain astrocytes is mediated by two separate isoforms of phospholipase A2 and is differently regulated by cyclic AMP and Ca2+ Br J Pharmacol. 2003;139:1014–22. doi: 10.1038/sj.bjp.0705326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toews AD, Barrett C, Morell P. Monocyte chemoattractant protein 1 is responsible for macrophage recruitment following injury to sciatic nerve. J Neurosci Res. 1998;53:260–7. doi: 10.1002/(SICI)1097-4547(19980715)53:2<260::AID-JNR15>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Tofaris GK, Patterson PH, Jessen KR, Mirsky R. Denervated Schwann cells attract macrophages by secretion of leukemia inhibitory factor (LIF) and monocyte chemoattractant protein-1 in a process regulated by interleukin-6 and LIF. J Neurosci. 2002;22:6696–703. doi: 10.1523/JNEUROSCI.22-15-06696.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uozumi N, Kume K, Nagase T, Nakatani N, Ishii S, Tashiro F, et al. Role of cytosolic phospholipase A2 in allergic response and parturition. Nature. 1997;390:618–22. doi: 10.1038/37622. [DOI] [PubMed] [Google Scholar]
- Waller A. Experiments on the section of the glassopharyngeal abd hypoglossal nerves of the frog, and observations of the alterations produced thereby in the structure of their primary fibers. Phil Trans R Soc London (Biol) 1850;140:423–9. [Google Scholar]
- Williams PL, Hall SM. Chronic Wallerian degeneration–an in vivo and ultrastructural study. J Anat. 1971;109:487–503. [PMC free article] [PubMed] [Google Scholar]
- Yaksh TL, Kokotos G, Svensson C, Stephens D, Kokotos CG, Fitzsimmons B, et al. Systemic and intrathecal effects of a novel series of phospholipase A2 inhibitors on hyperalgesia and spinal prostaglandin E2 release. J Pharmacol Exp Ther. 2006;316:466–75. doi: 10.1124/jpet.105.091686. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.