Abstract
In somatosensory cortex (S1) tactile stimulation activates specific regions. The borders between representations of different body parts constrain the spread of excitation and inhibition: connections that cross from one representation to another (cross-border, CB) are weaker than those remaining within the representation (noncross border, NCB). Thus, physiological properties of CB and NCB synapses onto layer 2/3 pyramidal neurons were compared using whole-cell recordings in layer 2/3 neurons close to the border between the forepaw and lower jaw representations. Electrical stimulation of CB and NCB connections was used to activate synaptic potentials. Properties of excitatory (EPSPs) and inhibitory (IPSPs) postsynaptic potentials (PSP) were determined using 3 methods: 1) minimal stimulation to elicit single-fiber responses; 2) stimulation in the presence of extracellular Sr2+ to elicit asynchronous quantal responses; 3) short trains of stimulation at various frequencies to examine postsynaptic potential (PSP) dynamics. Both minimal and asynchronous quantal EPSPs were smaller when evoked by CB than NCB stimulation. However, the dynamics of EPSP and IPSP trains were not different between CB and NCB stimulation. These data suggest that individual excitatory synapses from connections that cross a border (CB) have smaller amplitudes than those that come from within a representation (NCB), and suggest a postsynaptic locus for the difference.
Keywords: EPSPs, horizontal connections, IPSPs, minimal stimulation, layer 2/3
Introduction
Sensory input to the neocortex activates highly ordered representations, referred to as sensory maps. Within primary somatosensory cortex (S1), tactile information from the periphery activates discrete regions that reflect the location on the skin of the stimulus. These sensory maps exist throughout the central nervous system and play vital roles in information processing and integration. Furthermore, these maps are highly dynamic and change their organization (i.e., reorganize) in response to a variety of manipulations that affect the activity patterns into the map (Kaas 1991; Ebner et al. 1997; Buonomano and Merzenich 1998; Merzenich et al. 1988, 1990). Cortical reorganization is correlated with a variety of important clinical phenomena, including “phantom” pain and sensation (Flor et al. 1995; Borsook et al. 1998; Ramachandran and Hirstein 1998) and recovery of function after stroke (Nudo 1997; Friel and Nudo 1998); reorganization is also associated with various forms of sensorimotor learning (Jenkins et al. 1990; Recanzone et al. 1992a, 1992b; Wang et al. 1995; Diamond et al. 1999).
The cellular and synaptic bases of cortical organization and reorganization are incompletely understood. It is clear that a variety of properties of the local circuit within the cortex contribute to both (Buonomano and Merzenich 1998). For example, synaptic potentials are generally larger when evoked by stimulation from within the representation (noncross border, NCB) than when evoked by stimuli from an adjacent representation (cross-border [CB]; Hickmott and Merzenich 1998; Petersen and Sakmann 2000; Burns and Hickmott 2003). Anatomical structures of the local circuit, both dendritic and axonal also are constrained by representational borders (Hickmott and Merzenich 1999; Petersen and Sakmann 2000; Fang et al. 2002; Steen et al. 2007). During reorganization, these physiological and anatomical properties of the cortical circuit can change to reflect the new organization (Buonomano and Merzenich 1998; Hickmott and Merzenich 2002; Hickmott and Steen 2005; Tailby et al. 2005).
Our recent experiments at the forepaw/lower jaw border indicate that the amplitudes of both excitatory (EPSPs) and inhibitory (IPSPs) postsynaptic potentials of layer 2/3 horizontal connections are smaller when evoked by stimuli in an adjacent representation (i.e., from across the border; Hickmott and Merzenich 1998). There are 2 possible mechanisms that might underlie this difference: A lower density of axons that cross the border than do not; or synapses from CB connections have smaller efficacies than those from NCB connections. Labeling of axons with cholera toxin beta and phaseolus leucagglutinin has revealed that there are indeed fewer axons that cross the border (Steen et al. 2007), indicating that the first mechanism contributes to the functional imbalance between CB and NCB stimulation.
In order to examine the second possibility, 3 independent methods for measuring synaptic efficacy were used: 1) minimal stimulation, 2) replacement of extracellular Ca2+ with Sr2+, 3) stimulation using short trains (15 pulses) at various frequencies. EPSPs and IPSPs onto layer 2/3 neurons were analyzed and were compared between CB and NCB stimulation. The amplitudes of EPSPs resulting from minimal stimulation and those evoked in the presence of Sr2+ were significantly smaller for CB than for NCB stimulation, but no such difference was observed for IPSPs. Neither EPSP nor IPSP dynamics differed between CB and NCB synapses.
Materials and Methods
The In Vivo Preparation
All animal procedures are consistent with National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee at the University of California Riverside. Briefly, using standard in vivo extracellular recording methods, the border between the forepaw and lower jaw representations in somatosensory cortex was mapped. Adult Sprague–Dawley rats (>P 90) were anesthetized with pentobarbital (50 mg/kg, intraperitoneal) and mounted in a stereotaxic frame. Supplemental doses of anesthetic were administered as needed. Lidocaine was injected subcutaneously around wound margins and at pressure points. Rectal temperature was maintained at 38 °C with a heating pad.
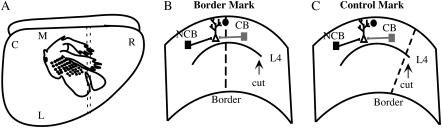
S1 was exposed via a wide craniotomy centered on Bregma, the dura was removed, and the cortex covered with silicone oil. A computer image of the brain surface was recorded using a CCD camera and appropriate software (Pixera, San Jose, CA). Carbon-fiber electrodes (10 μm fiber diameter) designed to generate minimum damage were used for response mapping. The forepaw and jaw were stimulated with a fine glass probe to elicit cutaneous responses in S1. Penetrations were introduced into the forepaw zone, 1–2 mm rostral to Bregma, and subsequent penetrations made more laterally until regions that responded to tactile stimulation of lower jaw were discovered. The location of penetrations was recorded on the computer image of the cortex by using landmarks of the surface vasculature. Penetrations spaced <50 μm apart were then made to locate the border more exactly. Typically, 3 series of penetrations perpendicular to the forepaw/lower jaw border, which runs approximately parallel to the midline, were made at 400- to 500-μm intervals. The recording electrode was then repeatedly dipped in a 1–2% DiI solution (in ethanol) and DiI crystals allowed to deposit on the electrode (DiCarlo et al. 1996). This electrode was used to mark sites along the previously determined border, leaving DiI crystals along the electrode track as a lasting mark. In order to control for any effects of the mapping and marking procedure, we performed the same analyses in a group of control animals. For these animals, the DiI mark was placed 500 μm medial or lateral to the actual border at a nonborder site (see Fig. 1C).
Figure 1.
The in vivo/in vitro preparation. (A) Schematic of a lateral view of the rat cortex), showing the somatotopic map. The border between the forepaw and lower jaw areas is indicated by the filled circle; the dotted lines delineate the orientation and location of a 400-μm-thick slice used for the in vitro analysis. C: caudal, R: rostral, N: medial, L: lateral. (B) Schematic coronal section from rat S1 showing the recording and stimulating configuration in a normal slice. The black circle represents the dye mark defining the location of the forepaw/lower jaw border (dotted line) determined in vivo, the schematic neuron is the neuron recorded from, and the 2 squares the sites of electrical stimulation across the border (“CB stim,” gray) and not across the border (“NCB stim,” black) from the neuron. L4 indicates the position of layer 4 and the arrow points to the cut used to isolate the supragranular layers. (C) Schematic coronal section showing the recording and stimulating configuration in a control slice. Abbreviations are as in (B).
In Vitro Recording
After marking, the animal was decapitated, the brain rapidly removed, and 400-μm-thick coronal slices cut on a vibratome from the marked region of cortex. Marked sites were visualized using an epifluorescence microscope and their location was determined in relation to visible landmarks in the slice. Supragranular layers of the cortex were isolated with a cut parallel to the cortical surface around layer 4 (500–700 μm deep), as was performed previously (Hickmott and Merzenich 1998, 2002; Burns and Hickmott 2003). The cut served to ensure that horizontal connections in layer 2/3 were stimulated. These slices were maintained in standard mammalian bicarbonate buffer (in mM: NaCl, 119; KCl, 2.5; NaH2PO4, 1.25; MgSO4, 1.3; CaCl2, 2.5; NaHCO3, 26.2; glucose, 11; saturated with 95%O2/5%CO2) for intracellular recording at 30.5 °C. Slices were incubated in gas-saturated room-temperature buffer for at least 1 h and then in the recording chamber for at least 30 min before recording. All recordings were from submerged slices that were held in place with a nylon mesh net.
Neurons for recording were obtained using blind whole-cell recording (Blanton et al. 1989) from a region near the mark (100–200 μm; measured from the center of the mark with a mark width <20 μm) in cortical layer 2/3. Patch electrodes were pulled on a Flaming/Brown puller (Sutter Instruments, Novato, CA) to an internal tip diameter of 1.5–2.5 μm and filled with 1 of 2 filling solutions. For recording EPSPs it contained in mM: Cs-Gluconate, 128; CsCl, 7; ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid, 1; 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid, 10; adenosine 5′-triphosphate magnesium salt, 2; guanosine 5′-triphosphate sodium salt, 0.2; pH 7.0–7.4. For recording IPSPs, 100 mM CsCl was substituted for 100 mM of the Cs-gluconate. This allowed large-amplitude, depolarizing IPSPs to be recorded at hyperpolarized membrane potentials. For both, Cs-based solution was used to enhance the signal-to-noise ratio of PSPs. Electrodes had tip resistances of 3–6 MΩ. Only neurons with initial resting potentials more negative than −60 mV and stable input resistances of >50 MΩ were used. Any recordings in which the access resistance changed by >15% during the course of the experiment were not used for analysis. Recordings were amplified using an Axoclamp 2B amplifier (Axon Instruments, Sunnyvale, CA) in current clamp mode, digitized at 15 kHz (National Instruments, Austin, TX), and saved to the hard disk of a personal computer (MacIntosh G4, Apple Computer, Inc., Cupertino, CA) using the IgorPro (Wavemetrics, Inc., Portland, OR) data acquisition system. EPSPs were isolated by recording in the presence of 20–25 μM picrotoxin in the bath, whereas IPSPs were isolated by a combination of 10 μM CNQX and 100 μM APV.
Stimulation: “Border” and “Control” Groups
Stimulation was applied via bipolar parylene-coated tungsten electrodes (resistance ∼1 MΩ) with a tip separation of ∼50 μm (FHC). Brief (100 μs, 0.1–0.2 Hz) current pulses were used. Stimulating electrodes were placed in the slice at an equivalent distance from the cortical surface as the recording electrode and were ∼300 μm medial or lateral to the recording site, as measured using an eyepiece graticle. In experimental animals, where the DiI mark is at the border, one electrode was placed such that the border was between the stimulating and recording sites (cross-border, CB) and the other was placed such that the border was not between the sites (NCB; Fig. 1B). Thus, the CB electrode was in a different representation than the recording electrode, whereas the NCB electrode was in the same representation (Fig. 1B). In order to control for effects of the mapping and marking procedure, identical stimulation and recording conditions were used in control animals in which the DiI mark was placed 500 μm away from the border at a nonborder site, so that no border intervened for either stimulus (Fig. 1C). Thus, for these control slices, the designation of “CB” was used for the stimulus that was on the other side of the DiI mark from the recorded cell and “NCB” was used for the stimulus on the same side (Fig. 1C). Based on our previous results, we would expect to see no bias in these control cells (Hickmott and Merzenich 1998). For all groups, CB and NCB stimulation were presented alternately at a rate of 0.1 Hz. Responses to both pathways were obtained for each cell. There were thus 4 groups for each analysis: control EPSPs, border EPSPs, control IPSPs, and border IPSPs.
Analysis of Spontaneous PSPS
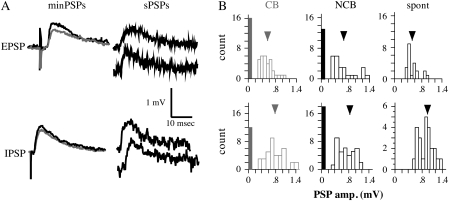
Unstimulated data were obtained to allow analysis of ∼50 spontaneous PSPs. All data were analyzed offline. Events were considered to be spontaneous PSPs based on their kinetics (rise time <2 ms, smooth fall time). First, the base-to-peak amplitude, rise time (from 10% of the peak to 90%) and the fall time tau were determined for 50 spontaneous PSPs. Amplitude was defined as the difference between the peak voltage and the baseline voltage. Baseline voltage was determined by averaging the 10 points before the spontaneous PSP. Rise time was defined as the time required for the spontaneous PSP to go from 10% of the peak amplitude to 90% of the peak amplitude. Fall time tau was calculated by fitting a single exponential to the falling phase of the PSP (starting at the peak) and determining tau (the time required for the voltage to fall from peak to 1/e of the peak) for that curve. The frequency distribution of spontaneous PSP amplitude (Fig. 2B, right) was examined to determine the smallest amplitude spontaneous PSPs that could be reliably distinguished from the noise. This amplitude value ranged from 0.15 to 0.2 mV, and was used to classify minimally stimulated events as failures or nonfailures.
Figure 2.
Examples of minimal PSPs. (A) Left: mean minimal PSPs evoked by CB (gray) and NCB (black) stimulation. Stimulation intensities for minimal EPSPs were 0.016 mA for CB and 0.023 mA for NCB, and for minimal IPSPs were 0.02 mA for CB and 0.025 mA for NCB; stimuli were 300 μm horizontal from the cell. Right: Examples of spontaneous PSPs from the same neurons. (B) Amplitude distributions for minimal PSPs from CB (left) and NCB (center) stimulation, and for spontaneous PSPs (right). Open bars are the numbers of minimal PSPs in the indicated amplitude bins. The filled bars represent the number of events classed as failures, and the arrowheads indicate the mean amplitude.
Minimal Stimulation-Evoked PSPs
Minimal stimulation was used to activate putative single-axon PSPs. To achieve single-axon stimulation, stimulus intensity for both CB and NCB was decreased so that the amplitude of the PSP reached an asymptote. Fifty responses (minimal PSPs) were obtained from both CB and NCB stimulation (cells were current clamped at −75 mV). All data were analyzed offline. Based on the frequency distribution of spontaneous PSP amplitude (Fig. 2B, right), the smallest amplitude PSPs that could be reliably distinguished from the noise were determined. This amplitude value ranged from 0.15 to 0.2 mV. Minimal PSPs that had smaller amplitude than this value were considered to be failures. Typically, >30% of stimuli resulted in failures based on this criterion. To assess the completeness of failure, the failure traces were averaged together and the voltage was determined at the point at which the peak amplitude had been measured for the nonfailure traces. This “failure peak” voltage was compared with a noise voltage value determined by averaging random baseline voltages from an equal number of traces from that cell. There was no significant difference between the “failure peak” and the baseline noise for minimal EPSPS or minimal IPSPs (P > 0.2, paired Student's t-test, data not shown). Thus, we are confident that the events defined as failures were truly failures and not just small, difficult to resolve events.
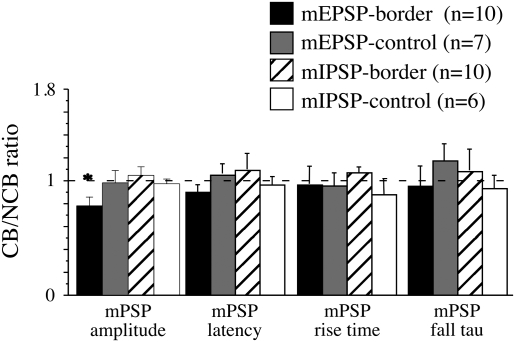
For each nonfailure trace, the amplitude, rise time (10–90%), fall time tau, and latency from stimulus artifact to initial rise were determined for both CB and NCB stimulation. Amplitude, rise time and fall time tau were determined as for spontaneous PSPs (above). Latency was defined as the time between the peak of the stimulus artifact and the onset of the minimal PSP. This onset was conservatively estimated as the point at which the voltage had increased to 10% of the base-peak amplitude (i.e., the same point as was used for the rise time measure). If the latencies of the responses to CB and NCB stimulation differed by more than 15%, it would suggest that the stimulating electrodes were not equidistant from the recording site; such cells were not used for analysis. Note that no cells were discarded from this data set for failing this criterion. For each neuron the mean value from CB and NCB stimulation for each of the above parameters (amplitude, latency, rise time, fall tau) was determined. The CB/NCB ratio was calculated using these mean values (Fig. 3) and was used as a measure of the amount of bias induced at the border: values < 1 reflect CB < NCB, values > 1 reflect CB > NCB and a value = 1 indicates no bias (CB = NCB). The ratio was compared with 1 using a 1-sample t-test. Values from different groups were compared with a 1-way ANOVA, followed by post hoc test (Fisher's protected least-significant difference). P values of < 0.05 taken as significant.
Figure 3.
CB/NCB ratio for the amplitudes, latencies, rise times, and fall taus of minimal PSPs. n = the number of cells for each group. The bars represent the mean CB/NCB ratio. Error bars are the SEM. The dotted line indicates a value of 1, which indicates no difference between the values obtained with CB and NCB stimulation. *Significantly different from other amplitude ratios, P < 0.01, 1-way ANOVA followed by post hoc Fisher's protected least-significant difference (PLSD) tests.
Asynchronous Quantal PSPs in the Presence of Sr2+ (Asynchronous PSPs)
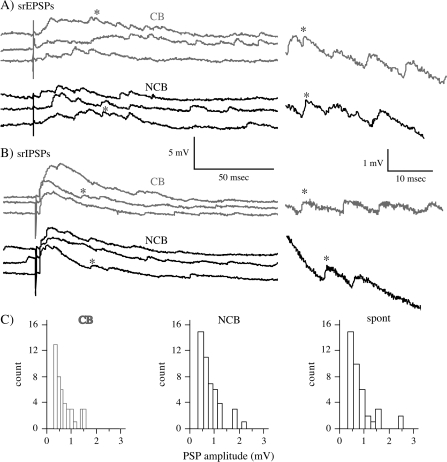
If all or most of the external Ca2+ is replaced by Sr2+, synaptic transmission is affected resulting in asynchronous release of neurotransmitter. These asynchronous release events have been shown to reflect release of neurotransmitter by single quanta. As such, they have been used as a measure of synaptic efficacy (Miledi and Slater 1966; Goda and Stevens 1994; Bender et al. 2006). In these experiments, the external Ca2+ concentration was reduced to 0.5 and 5 mM Sr2+ was included in the bath. PSPs were evoked by alternate CB and NCB stimulation at 0.1 Hz and the stimulus intensity was adjusted to yield small synchronous PSPs (<5 mV) with distinct asynchronous PSPs superimposed on them (see Fig. 4A). Also, sufficient unstimulated data were obtained to allow analysis of ∼50 spontaneous PSPs. Events (spontaneous PSPs and asynchronous PSPs) were selected for analysis based on their kinetics (rise time < 2 ms, smooth fall time). Events falling in a window from the end of the stimulus artifact to 300 ms after the artifact were defined as asynchronous PSPs. Amplitudes of spontaneous PSPs and asynchronous PSPs were measured from baseline just before the event to the peak voltage. For asynchronous PSPs this amplitude measure will be affected by the small change in driving force due to the synchronous depolarization that was evoked simultaneously (Fig. 4A). In order to equalize this small effect between CB and NCB stimulation, the stimulus intensities were independently adjusted so that the magnitude of the synchronous PSP was approximately equal. Thus, the effect on the CB/NCB ratio for these amplitudes would be minimal. Furthermore, the synchronous PSP is small (<5 mV), therefore we calculate that there would be a <5% difference in amplitude for an asynchronous PSP at the peak of this synchronous depolarization versus one at baseline (based on the difference in driving force between peak and baseline). Values from ∼50 asynchronous PSPs were obtained and the mean amplitude determined for both CB and NCB stimulation. The CB/NCB ratio was determined for each group from these mean values. The ratio was compared with one using a 1-sample t-test. Values from different groups were compared planned Student's t-tests. P values of < 0.05 taken as significant.
Figure 4.
Examples of asynchronous PSPs evoked in 0.5 mM Ca2+/5 mM Sr2+ buffer. (A) Asynchronous EPSPs recorded in the presence of 20 μM picrotoxin for CB (gray) and NCB (black) stimulation. Stimuli were 0.066 mA for CB and 0.048 mA for NCB; stimuli were 300 μm from the cell. On the right are expanded traces with the asterisk marking the corresponding events. (B) Asynchronous IPSPs evoked in the presence of 10 μM CNQX plus 100 μM APV. Stimuli were 0.051 mA for CB and 0.04 for NCB. Conventions are as in (A). (C) Amplitude distributions for asynchronous EPSPs and spontaneous EPSPs from the same cell as pictured in “A”. Left (gray): asynchronous EPSPs from CB stimulation, middle (black); asynchronous EPSPs from NCB stimulation, right: spontaneous EPSPs.
Analysis of PSP Dynamics
The third method used to measure synaptic efficacies relies on using short (15 pulses) trains of isolated EPSPs and IPSPs to analyze their short-term dynamics. In cortical synapses, such trains typically evoke depression of the PSPs. For low train frequencies (<20 Hz), synapses with low release probability show greater depression than those with high release probability. Furthermore, decreasing release probability at a given synapse, such as by lowering the external Ca2+ concentration, causes an increase in depression (Markram and Tsodyks 1996b; Tsodyks and Markram 1997). Thus, we can infer differences in release probability based on differences in the rate of depression of PSPs during such a train.
PSPs were evoked in the same cells and with the same configuration (CB and NCB) as for minimal stimulation, except that the stimulus intensity of each was raised independently to yield a PSP of 1–2 mV. Five trains of 15 PSPs were evoked at frequencies of 5, 10, and 20 Hz and the traces averaged for each frequency (Fig. 6A). From these averages, 2 paired-pulse ratios (PPRs) were determined: the ratio of the second PSP to the first (PPR-2; Fig. 6C), and the ratio of the steady-state response amplitude (defined as the average of the last 3 responses in the train) to the first response (PPR-SS; Fig. 6D). Thus, information on both rapid and sustained dynamics was measured. Mean PPR values from CB and NCB were compared using Student's t-test, P < 0.05 as significant.
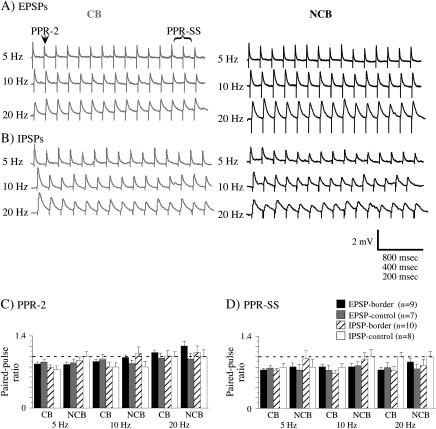
Figure 6.
Examples of trains of EPSPs (A) and IPSPs (B) evoked by CB (gray) and NCB (black) stimulation at various frequencies. The time scale is equal to 800, 400, 200, ms for 5, 10, 20 Hz, respectively. Two ratios were used to quantify these trains: PPR-2, in which the amplitude of the second PSP was divided by the first; and PPR-SS, in which the mean amplitude of the last 3 PSPs was divided by the amplitude of the first. The mean values of PPR-2 (C) and PPR-SS are plotted in (C) and (D). The error bars are the SEM. n = the number of cells in each group. The dotted line indicates a value of 1, which indicates no difference in amplitude between first and second PSPs (PPR-2) or first and last PSPs (PPR-SS). Note that no values differ significantly between corresponding CB and NCB stimulation (1-way ANOVA).
All data presented is expressed as mean ± SEM. n represents the number of neurons sampled to generate a particular mean.
Results
All neurons were recorded using blind whole-cell recording, and the morphologies of neurons were not determined. The presence of Cs+ in the recording solution made it impossible to use the characteristics of spike trains to differentiate regular spiking (pyramidal) cells from fast spiking (nonpyramidal). Based on our previous data in which cell identity was determined (Hickmott and Merzenich 1999; Hickmott 2005; Hickmott and Steen 2005), the majority of neurons analyzed were pyramidal cells (∼80%). Thus, the data presented should be taken to reflect characteristics of horizontal connections onto pyramidal neurons.
Four experimental groups were analyzed: control EPSPs, border EPSPs, control IPSPs, and border IPSPs. For numbers of cells and experiments for each group, see Table 2. The difference between the “control” and “border” groups was the location of the cells with respect to the border. In “border” groups, cells were close (<200 μm horizontal distance) to the DiI-marked border (Fig. 1B), whereas in “control” groups cells were close (<200 μm horizontal distance) to a DiI mark placed in the middle of the representation (either forepaw or lower jaw; Fig. 1C). Thus, for ease of comparison, in these control slices the designation of “CB” was used for the stimulus that was on the other side of the DiI mark from the recorded cell and “NCB” was used for the stimulus on the same side (Fig. 1C), even though there was no actual border at the site. These control animals controlled for possible effects of the mapping and marking procedure. All neurons analyzed were in the supragranular layers (<500 μm from the cortical surface). The depths of neurons from the cortical surface, their distance from the DiI mark (which marks the border in the experimental groups), and the distance for CB and NCB stimulation are presented in Table 1 and did not differ significantly among the 4 groups (1-way ANOVA, P > 0.05). Note that DiI marks were typically <20 μm in width and that distances measured with respect to the mark were from the center of the mark. Neurons had resting potentials <−60 mV and input resistances >50 MΩ. These values are summarized in Table 1 and did not differ significantly among the 4 groups (1-way ANOVA, P > 0.05). The properties of the various PSPs are summarized in Table 2.
Table 2.
Properties of minimal PSPs, asynchronous PSPs and their associated spontaneous PSPs
| EPSP-border (n = 10 (5)) | EPSP-control (n = 7 (4)) | IPSP-border (n = 11 (6)) | IPSP-control (n = 6 (4)) | ||
| Minimal PSPs | Rise-CB (ms) | 1.17 ± 0.14 | 1.29 ± 0.11 | 1.79 ± 0.11 | 1.80 ± 0.2 |
| Rise-NCB (msec) | 1.09 ± 0.09 | 1.34 ± 0.13 | 1.85 ± 0.19 | 1.83 ± 0.19 | |
| Tau-CB (ms) | 16 ± 4 | 17 ± 3 | 10 ± 4 | 11 ± 3 | |
| Tau-NCB (ms) | 20 ± 6 | 18 ± 3 | 10 ± 3 | 12 ± 2 | |
| Amp-CB (mV) | 0.54 ± 0.04* | 0.68 ± 0.04 | 0.66 ± 0.05 | 0.64 ± 0.04 | |
| Amp-NCB (mV) | 0.69 ± 0.05 | 0.73 ± 0.12 | 0.69 ± 0.04 | 0.65 ± 0.03 | |
| Spont. PSPs | Rise (ms) | 1.15 ± 0.12 | 1.26 ± 0.12 | 1.10 ± 0.12 | 1.16 ± 0.14 |
| Tau (ms) | 11 ± 1 | 10 ± 2 | 9 ± 1 | 10 ± 1 | |
| Amp (mV) | 0.58 ± 0.04 | 0.60 ± 0.07 | 0.66 ± 0.06 | 0.72 ± 0.1 | |
| (n = 9 (6)) | (n = 9 (4)) | ||||
| Asynch. PSPs | Rise-CB (ms) | 0.58 ± 0.05 | 0.59 ± 0.03 | ||
| Rise-NCB (ms) | 0.62 ± 0.09 | 0.57 ± 0.06 | |||
| Tau-CB (ms) | 10 ± 2 | 13 ± 3 | |||
| Tau-NCB (ms) | 11 ± 2 | 10 ± 2 | |||
| Amp-CB (mV) | 0.46 ± 0.05** | 0.58 ± 0.07 | |||
| Amp-NCB (mV) | 0.56 ± 0.06 | 0.58 ± 0.07 | |||
| Spont. PSPs (in Sr) | Rise (ms) | 0.62 ± 0.07 | 0.67 ± 0.07 | ||
| Tau (ms) | 9 ± 2 | 11 ± 2 | |||
| Amp (mV) | 0.63 ± 0.06 | 0.66 ± 0.09 |
Note: Means ± SEM are presented; n represents the number of neurons sampled; the number in parenthesis after the n value represents the number of experiments performed to obtain that number of cells. *P < 0.01, **P < 0.05; both significantly different with respect to the corresponding NCB value. Significance determined with 1-way ANOVA followed by Fisher's PLSD (minimal PSP data) or Student's t-test (asynchronous PSP data).
Table 1.
Recording and stimulating distances, membrane properties
| Depth from surface (μm) | Distance from border (μm) | Stim. distance (μm) | Resting potential (mV) | Input resistance (MΩ) | |
| EPSP border (n = 23) | 290.9 ± 7.0 | 160.5 ± 8.6 | 298.5 ± 7.6 | −75.1 ± 4.7 | 78.0 ± 8.9 |
| EPSP control (n = 10) | 296.7 ± 8.0 | 176.7 ± 6.2 | 303.3 ± 12.0 | −75.0 ± 2.8 | 92.6 ± 9.9 |
| IPSP border (n = 22) | 305.9 ± 8.1 | 150.0 ± 8.8 | 293.5 ± 7.7 | −75.2 ± 3.9 | 81.6 ± 4.9 |
| IPSP control (n = 11) | 291.2 ± 9.5 | 166.0 ± 11.1 | 292.7 ± 13.1 | −76.4 ± 6.6 | 78.3 ± 10.1 |
Note: Means ± SEM are presented; n represents the number of neurons sampled. Note that for EPSP border and IPSP border the data are from minimal PSP, asynchronous PSP and train experiments. For controls data are from minimal PSP and train experiments only.
Minimal PSPs
To evoke minimal PSPs, the stimulus intensity for CB and NCB stimulation was reduced until the resulting PSP amplitude reached an asymptote. Note that the stimulating intensity varied considerably, particularly with different stimulating electrodes; however, the mean values needed to evoke minimal PSPs did not differ significantly between CB and NCB stimulation for EPSPs or IPSPs (mean for minimal EPSPs: 0.033 ± 0.016 mA, mean for IPSPs 0.039 ± 0.02 mA). Such a stimulus intensity resulted in a significant number of failures (>30%), and the stimulated responses closely resemble spontaneously occurring responses in the same cells (Fig. 2A,B); the amplitudes, rise times and fall times of minimal PSPs were not significantly different among the 4 groups (1-way ANOVA, P > 0.05; see Table 2). Thus, the minimal stimulation paradigm effectively stimulated single axons onto the recorded cell, and the minimal PSPs are a reasonable measure of synaptic efficacy.
To determine whether there was a bias in the amplitude of the minimal PSPs associated with the border, the CB/NCB ratio was calculated for various parameters of the PSP (Fig. 3). Ratios significantly different from 1 (asterisk) indicate a significant difference between minimal PSPs evoked by CB and NCB stimulation. Only the ratio for the minimal EPSP amplitude for neurons close to the border (left plot, black bar) was significantly different from one, indicative of bias. This CB/NCB ratio for minimal EPSPs was also significantly different from the ratios from the other groups (1-way ANOVA). The CB/NCB ratio was less than 1 because the amplitude of the minimal EPSPs evoked by CB stimulation was significantly smaller than that evoked by NCB stimulation (Table 2). Neither controls, nor minimal IPSPs showed any bias. Thus, this measure of synaptic efficacy suggests that CB synapses have lower efficacies than NCB.
Asynchronous PSPs
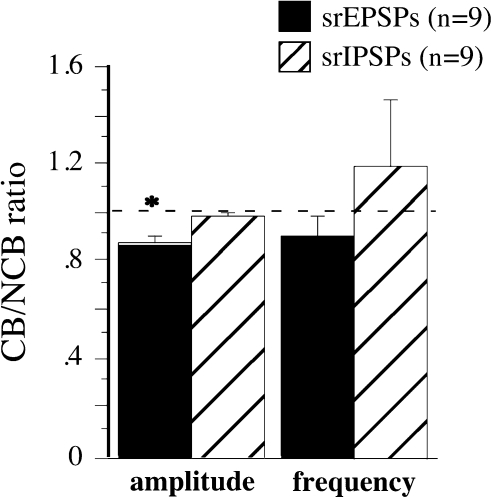
Replacement of external Ca2+ with Sr2+ allows the examination of evoked asynchronous quantal events; these events were analyzed in an attempt to confirm and extend the data obtained from minimal PSPs. Note that only neurons close to the border were examined, as the controls from all other analyses have indicated no biasing at a control site (Figure 3, 6; (Hickmott and Merzenich 1998, 1999). To evoke asynchronous PSPs, the stimulus intensity was adjusted to evoke synchronous PSPs < 5 mV in amplitude with equal amplitudes for CB and NCB stimulation. This paradigm produced significant numbers of resolvable asynchronous PSPs superimposed on the synchronous PSP (Fig. 4A). As for minimal PSPs, the intensities required varied, but did not differ significantly between CB and NCB stimulation. The amplitude distributions of the asynchronous PSPs were similar to those for spontaneous PSPs in the same cell (Fig. 4C), and their mean amplitudes and kinetics were not significantly different from spontaneous PSPs recorded in the presence of strontium (unpaired t-test, P > 0.05; in Table 2 compare Asynch. PSPs to Spont. PSPs [in Sr]). The frequencies of the asynchronous PSPs (Fig. 5, right) did not differ significantly between CB and NCB stimulation for either EPSPs (Fig. 5, black bars) or IPSPs (Fig. 5, hatched bars). However, the mean asynchronous PSP amplitude (Fig. 5, left) was smaller for CB than for NCB stimulation for EPSPs (Fig. 5, black bars), but not for IPSPs (Fig. 5, hatched bars). Such a difference in amplitude has been interpreted previously to reflect a difference in synaptic efficacy with a postsynaptic locus (Miledi and Slater 1966; Goda and Stevens 1994; Bender et al. 2006). Thus, this analysis confirms the conclusions obtained using minimal PSP analysis and suggests a postsynaptic difference between CB and NCB EPSPs.
Figure 5.
Mean CB/NCB ratio of asynchronous EPSP (black) and asynchronous IPSP (hatched) amplitude and frequency. n = the number of cells for each group and the error bars are the SEM. The dotted line indicates a value of 1, which indicates no difference between the values obtained with CB and NCB stimulation. *Significantly different from 1 (P < 0.01, 1-sample t-test).
PSP Dynamics
Another paradigm that has been used to assess differences in synaptic efficacy relies on analysis of the dynamics of repetitively evoked PSPs. Trains of 15 PSPs were evoked in cells close to (experimental) and far from (control) the border at 3 frequencies: 5, 10, and 20 Hz (Fig. 6). The amplitudes of PSPs after the first showed increases, decreases or no change, although depression was most frequently observed (Fig. 6). The ratio of the second PSP to the first was used as a measure of rapid dynamics and is referred to as PPR-2. As a measure of the steady-state dynamics, the ratio of the mean amplitude of the last 3 PSPs to the amplitude of the first PSP was used, and is referred to as PPR-SS. These ratios were examined for all 3 frequencies for the 4 groups of neurons studied (Fig. 6C,D). In most cases, PPRs less than or equal to 1 were observed, reflecting decreases in amplitude across the train or no change across the train. This pattern was observed for both short-term (PPR-2) and steady-state (PPR-SS) dynamics. When the ratios for CB and NCB stimulation were compared (paired t-test), no significant differences were observed between ratios evoked by CB and NCB stimulation.
Discussion
We have demonstrated that intracortical excitatory, but not inhibitory synapses onto layer 2/3 neurons have different properties depending on whether their axons must cross a functional border (CB) or not (NCB). Three standard methods were used to assess the efficacy and short-term dynamics of isolated EPSPs and IPSPs: minimal stimulation, replacement of Ca2+ with Sr2+ and short trains of stimulation.
Minimal Stimulation and Differences in Excitatory Synaptic Contacts
Minimal stimulation, which activates PSPs associated with a single axon, has previously been used to characterize synaptic changes associated with LTP and also those associated with cortical reorganization (Markram and Tsodyks 1996a; Finnerty et al. 1999). The minimal PSPs observed in this paper closely resembled unitary events recorded in rat S1 in previous studies (Thomson et al. 1988; Volgushev et al. 1995; Thomson et al. 1996; Finnerty et al. 1999). Minimal PSPs had fluctuating amplitudes, including failures, and the amplitude did not decrease with further reductions in stimulus intensity. Furthermore, the stimulated minimal PSPs were indistinguishable from spontaneous PSPs in the same cell (Fig. 2, Table 2). Thus, we are confident that the minimal PSPs analyzed were a good approximation of a single-fiber response in these cells. Minimal EPSPs evoked by CB stimulation were consistently smaller in amplitude than those evoked by NCB stimulation, but with no other difference in properties, whereas minimal IPSPs showed no such difference (Figs 2, 3, Table 2). These data are consistent with an overall smaller synaptic efficacy for excitatory synapses for axons that cross a representational border in cortex.
These minimal PSPs reflect activation of a single axon synapsing onto the target cell; as such, they reflect properties of single synapses made by the axon and also the number and distribution of those synapses. Thus, the smaller minimal PSPs from CB stimulation could reflect a smaller synaptic efficacy, a smaller number of synaptic contacts per axon or a different distribution of synaptic contacts on the target cell. Furthermore, the observed difference between CB and NCB stimulation could result from differences in presynaptic and/or postsynaptic processes.
Differences in Asynchronous EPSPs: Mechanisms Underlying Differences in Excitatory Synaptic Contacts
PSPs evoked in low Ca2+/high Sr2+ bathing medium were used to further characterize the difference in EPSPs. By removing most of the extracellular Ca2+ in the presence of high Sr2+, synaptic release is altered such that the previously synchronous release of neurotransmitter becomes asynchronous. As shown in Figure 4, asynchronous EPSPs and IPSPs could be resolved from both CB and NCB stimulation. The asynchronous PSPs had similar amplitudes and kinetics to spontaneous PSPs from the same cells (Fig. 4C, Table 2). Because the mean rate of spontaneous PSPs was low (mean = 1.3 Hz for EPSPs, 1.6 Hz for IPSPs) and the duration of the window for counting the asynchronous PSPs was short (300 ms after the artifact), it is unlikely that the sample of asynchronous PSPs was significantly contaminated with spontaneous PSPs.
The results obtained using this approach confirmed and extended the conclusions based on minimal stimulation. Unitary asynchronous EPSPs evoked by CB stimulation were smaller than those evoked by NCB, but there was no such difference for IPSPs (Fig. 5). This finding allows us to infer that the smaller size of CB EPSPs is most likely due to a difference at single synaptic contacts, rather than in the overall number of synaptic contacts. Similar asynchronous events have been shown to reflect quantal release from single terminals and their amplitudes have been used as a measure of quantal amplitude at single synaptic contacts (Miledi and Slater 1966; Xu-Friedman and Regehr 2000). Thus, differences in their amplitude have been interpreted as reflecting differences in postsynaptic properties of single synaptic contacts (Miledi and Slater 1966; Goda and Stevens 1994; Bender et al. 2006). Based on these previous data, the difference in the amplitude of asynchronous events between CB and NCB synapses is most likely associated with a difference in the properties of single excitatory synaptic contacts, and most likely is based on some difference in postsynaptic properties of single synapses, either their relative efficacies or their locations on the postsynaptic cell.
The previous analyses do not rule out the possibility that the CB and NCB synapses have different distributions on the target cell's dendrites. However, a more detailed examination of the kinetics of minimal and asynchronous EPSPs can be used to do so. If CB synaptic contacts tended to be further away from the site of recording in the soma than NCB synaptic contacts, the CB responses would be smaller than the NCB because of greater electrotonic attenuation of their synaptic potentials (Williams and Stuart 2002). The data do not support this hypothesis, however, because the rise times of minimal PSPs and asynchronous PSPs were not different between CB and NCB stimulation (Table 2). If the CB PSPs were generated further from the soma, then they should also exhibit slower rise times, as EPSPs from more distal synapses have slower rise times in neocortical pyramidal cells (Williams and Stuart 2002). Because this was not observed, it is likely that the differences between CB and NCB populations do represent actual properties of synapses and not differential distribution.
No Differences in Short-Term Dynamics
A wide variety of terms and techniques have been used to describe the effects of repetitive stimulation of synapses, including short-term plasticity, paired-pulse behavior augmentation, train behavior, repetitive stimulation behavior, etc (Zucker and Regehr 2002). In this paper the term short-term dynamics is used to describe the changes in synaptic responses during the course of a short train of stimuli at varying interstimulus intervals. Both paired-pulse and steady-state processes were analyzed using this technique.
These various techniques have been used on both EPSPs and IPSPs at a variety of synapses (Tsodyks and Markram 1997; Reyes et al. 1998; Gupta et al. 2000). The PSPs either depress or facilitate during the course of the train, reaching a steady-state amplitude. In general the major determinant of these dynamics is based on the presynaptic release properties of the synapses involved (Markram and Tsodyks 1996a; Dobrunz and Stevens 1997; Tsodyks and Markram 1997; Rozov et al. 2001; Zucker and Regehr 2002). Although postsynaptic mechanisms, particularly receptor desensitization and saturation, have also been demonstrated (Zucker and Regehr 2002). Synapses that have a high probability of release typically are depressing, and thus exhibit paired-pulse and steady-state ratios <1, whereas those with low release probability are facilitating and exhibit ratios >1. Note that release probability is not the only factor contributing to presynaptic modulation of short-term dynamics; activation of presynaptic metabotropic receptors for both glutamate and GABA can lead to a reduction in neurotransmitter release for both excitatory and inhibitory terminals (Zucker and Regehr 2002).
There is a great deal of variability in synaptic dynamics in a given neuron population; a single neuron can have facilitating dynamics on some of its targets and depressing on others, conversely a single target can have facilitating or depressing dynamics expressed by its various inputs (Markram et al. 1998; Reyes et al. 1998; Gupta et al. 2000). Thus, the identity of both the presynaptic and postsynaptic partner determines the synaptic dynamics. As shown in Figure 6, PSPs evoked by both CB and NCB stimulation exhibited depressing or flat dynamics (PPR-2 and PPR-SS ≤ 1), particularly at lower frequencies, as has been observed for pyramidal to pyramidal and some pyramidal to interneuron (particularly multipolar interneurons) synapses (Reyes et al. 1998; Markram et al. 2004). Furthermore, no differences in dynamics were observed between CB and NCB stimulation for PSPs at any frequency tested (Fig. 6C,D). These data suggest that the presynaptic properties of horizontal axons onto layer 2/3 cells are similar for the CB and NCB projections.
Relationship to Previous Data
Our previous data demonstrated that the population synaptic responses, assayed by high-intensity (multiaxon) electrical stimulation, showed smaller excitatory and inhibitory responses for CB stimulation (Hickmott and Merzenich 1998; Burns and Hickmott 2003). These earlier data suggested that either the mean individual synaptic strengths of excitation and inhibition were smaller for CB stimulation, or that there were fewer CB connections than NCB targeting a given neuron close to the border. Bulk labeling of axons close to the forepaw/lower jaw border demonstrated the second possibility, but did not differentiate between excitatory and inhibitory axons (Steen et al. 2007). The data from this paper indicate that for excitation (EPSPs) the first possibility, that individual excitatory synapses were weaker for CB stimulation, was also observed in these horizontal pathways (Fig. 3, 5). However, there was no significant difference observed in the properties of inhibitory synapses (IPSPs) between CB and NCB projections.
Why, then, is inhibition not smaller for CB stimulation at the level of single synaptic contacts? At the normal border, it is possible that the smaller IPSPs previously observed with CB stimulation of many synaptic contacts (Hickmott and Merzenich 1998) resulted from a lower density of inhibitory connections crossing the border (Steen et al. 2007). Thus the individual synaptic contacts could be similar for CB and NCB connections. However, the bias of IPSPs observed at the normal border (CB < NCB) has been shown to change very rapidly following denervation of the forelimb, suggesting that the efficacy of inhibitory synaptic contacts can be rapidly regulated (Hickmott and Merzenich 2002). Synaptic plasticity of isolated IPSPs has recently been demonstrated directly for these horizontal connections (Paullus and Hickmott 2007). In general, inhibitory connections in the cortex are known to undergo potentiation and depression based on changes in their activation (Komatsu 1994; Gaiarsa et al. 2002). One possibility is that the inhibitory synaptic contacts recorded based on minimal stimulation and asynchronous quantal release are from a different population of synapses than those evoked by multiaxon stimulation, and this population is either less susceptible to synaptic plasticity or are not activated in ways to induce plasticity. For example, it is likely that minimal IPSPs and asynchronous IPSPs result primarily from activation of proximal synapses, whereas multiaxon evoked IPSPs would include more distal synapses. If the characteristics of synaptic plasticity differ between distal and proximal inhibitory synapses, as has been shown for excitatory synapses (Hardingham et al. 2007), then perhaps the proximal inhibitory synapses are less able to undergo synaptic plasticity.
Relationship to the Functional Organization of S1 at a Representational Border
How do these data relate to the functional organization of S1? It has been demonstrated that excitatory synapses in S1 undergo potentiation and depression when activity patterns to S1 are manipulated. For example, trimming a subset of whiskers induces strengthening of local excitatory connections in layer 2/3 in regions corresponding to the spared whiskers; local excitatory connectivity is reduced in regions corresponding to the deprived. These effects are most prominent at the transition (border) between spared and deprived cortex (Cheetham et al. 2007). Note that in the Cheetham studies, the border is between 2 adjacent columns in the same region of the body (the whisker pad), whereas in our studies the border is between completely different body parts (forepaw and lower jaw) that actually receive their inputs from different thalamic subnuclei. Thus, the 2 borders are not completely analogous. Nevertheless, in each case the transition between areas with differing patterns of activity is an important cue for controlling excitatory response strength. In our system, the border represents a disruption in the ability of excitation to be transferred from one side of the border to the other. Thus, connections that cross the border (CB) would frequently be activated when their targets across the border were inactive. Such a pattern of activity could lead to long-term depression (LTD) of these connections (Hess and Donoghue 1996) and a decrease in synaptic strength for CB synaptic contacts, as was observed (Figs 3 and 5). Alternatively, the difference could reflect potentiation of connections that remain within the representation, because such connections are likely to have correlated firing patterns with their targets. In either case, the locus of the change for our data was apparently postsynaptic. In juvenile S1, LTP exhibited a predominantly presynaptic locus, but postsynaptic components were also observed for distal synapses (Hardingham et al. 2007). However, LTD exhibited additional postsynaptic components as well (Eder et al. 2002; Hardingham et al. 2007). Based on these studies, the hypothesis that our CB connections underwent LTD is more plausible, as the locus of the difference for EPSPs was postsynaptic.
Implications for Cortical Reorganization
One of the important properties of cortical maps is their ability to reorganize in response to changes in incoming activity patterns. Typically, the reorganization entails expansion of more active parts of the representation into areas that have reduced activity. Reorganization can occur very rapidly, on the time scale of minutes, but is also progressive and continues for long periods of time (Merzenich et al. 1983; Calford and Tweedale 1988; Pons et al. 1991; Buonomano and Merzenich 1998; Calford 2002; Hickmott and Merzenich 2002). Given the rapidity of reorganization, there must be pre-existing connections that extend from one region of the representation into an adjacent region. For rapid expansion to occur either excitation in these connections must be enhanced and/or inhibition of these connections must be decreased. This then leads to an increase in the spread of excitation. The smaller efficacy of axons that cross from one representation to another (CB) could provide an important substrate for such expansions. Synapses with lower efficacies generally are more susceptible to potentiation than those with higher efficacies (Bi and Poo 1998; Hardingham et al. 2007). Thus, CB connections, which are weaker than NCB, would be more amenable to potentiation and thus readily available for rapid cortical plasticity. Such a difference in susceptibility to synaptic potentiation is consistent with the progression of CB and NCB responses during reorganization of this border induced by peripheral denervation (Hickmott and Merzenich 2002). Similar changes in synaptic efficacy have been observed during various forms of large-scale reorganization of S1 (Buonomano and Merzenich 1998; Bender et al. 2006; Cheetham et al. 2007). These sorts of mechanisms are likely conserved across various other cortical areas, as horizontal connections of V1 are susceptible to synaptic plasticity (Hirsch and Gilbert 1993) and changes in these synapses have been associated with reorganization of V1 induced by manipulation of peripheral activity (Das and Gilbert 1995a, 1995b; Dreher et al. 2001). Future experiments are necessary to determine whether our CB synapses are more susceptible to synaptic plasticity and how synaptic efficacies are altered during reorganization of the border.
The presence of inhibitory connections that cross the border suggests that these connections could also be a substrate for expansion of representations due to disinhibition. Disinhibition has been implicated as a mechanism for rapid cortical reorganization based on animal (Wall 1988; Tremere et al. 2001a, 2001b) and human (Weiss et al. 2004) studies. The studies in humans are of particular interest, because they also suggest functional consequences of disinhibition and reorganization. For example, temporary loss of arm sensation induced by ischemia (Brasil-Neto et al. 1992; Rossini et al. 1994; Tinazzi et al. 1997) or by local anesthetic injection (Weiss et al. 2004) causes rapid expansion of the adjacent lower lip representation, as assessed by noninvasive functional imaging. The expansion both locally and into adjacent motor areas is consistent with a disinhibitory mechanisms and the expansion is correlated with improvement of 2-point discrimination on the lip and with mislocalization of tactile stimuli on the intact portions of the hand (Weiss et al. 2004). Longer-term changes in tactile perception correlated with cortical reorganization have also been demonstrated with passive tactile coactivation and arm immobilization (Pleger et al. 2003; Lissek et al. 2009). More significant losses of sensory activity, such as amputations, can also induce reorganization of human S1, often with pathological consequences, such as “phantom” pain and sensation (Flor et al. 1995; Flor 2003) or impaired limb use after stroke (Liepert et al. 1998).
Funding
The National Institute of Neurological Disorders and Stroke (NS-42201-01); and UCR bridge and Academic Senate funds.
Acknowledgments
I would like to thank Andrea Nolen, Jeff Paullus, and Azie Jebelli for comments on drafts of this manuscript. Conflict of Interest: None declared.
References
- Bender KJ, Allen CB, Bender VA, Feldman DE. Synaptic basis for whisker deprivation-induced synaptic depression in rat somatosensory cortex. J Neurosci. 2006;26:4155–4165. doi: 10.1523/JNEUROSCI.0175-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi GQ, Poo MM. Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type. J Neurosci. 1998;18:10464–10472. doi: 10.1523/JNEUROSCI.18-24-10464.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanton MG, LoTurco JJ, Kriegstein AR. Whole cell recordings from neurons in slices of reptilian and mammalian cortex. J Neurosci Methods. 1989;30:203–210. doi: 10.1016/0165-0270(89)90131-3. [DOI] [PubMed] [Google Scholar]
- Borsook D, Becerra L, Fishman S, Edwards A, Jennings CL, Stojanovic M, Papinicolas L, Ramachandran VS, Gonzalez RG, Breiter H. Acute plasticity in the human somatosensory cortex following amputation. Neuroreport. 1998;9:1013–1017. doi: 10.1097/00001756-199804200-00011. [DOI] [PubMed] [Google Scholar]
- Brasil-Neto JP, Cohen LG, Pascual-Leone A, Jabir FK, Wall RT, Hallett M. Rapid reversible modulation of human motor outputs after transient deafferentation of the forearm: a study with transcranial magnetic stimulation. Neurology. 1992;42:1302–1306. doi: 10.1212/wnl.42.7.1302. [DOI] [PubMed] [Google Scholar]
- Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Annu Rev Neurosci. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- Burns SA, Hickmott PW. Effect of representational borders on responses of supragranular neurons in rat somatosensory cortex. Brain Res. 2003;985:108–111. doi: 10.1016/s0006-8993(03)03153-6. [DOI] [PubMed] [Google Scholar]
- Calford M, Tweedale R. Immediate and chronic changes in responses of somatosensory cortex in adult flying-fox after digit amputation. Nature. 1988;332:449–449. doi: 10.1038/332446a0. [DOI] [PubMed] [Google Scholar]
- Calford MB. Dynamic representational plasticity in sensory cortex. Neuroscience. 2002;111:709–738. doi: 10.1016/s0306-4522(02)00022-2. [DOI] [PubMed] [Google Scholar]
- Cheetham CE, Hammond MS, Edwards CE, Finnerty GT. Sensory experience alters cortical connectivity and synaptic function site specifically. J Neurosci. 2007;27:3456–3465. doi: 10.1523/JNEUROSCI.5143-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Gilbert CD. Long-range horizontal connections and their role in cortical reorganization revealed by optical recording of cat primary visual cortex. Nature. 1995a;375:780–784. doi: 10.1038/375780a0. [DOI] [PubMed] [Google Scholar]
- Das A, Gilbert CD. Receptive field expansion in adult visual cortex is linked to dynamic changes in strength of cortical connections. J Neurophysiol. 1995b;74:779–792. doi: 10.1152/jn.1995.74.2.779. [DOI] [PubMed] [Google Scholar]
- Diamond ME, Petersen RS, Harris JA. Learning through maps: functional significance of topographic organization in primary sensory cortex. J Neurobiol. 1999;41:64–68. [PubMed] [Google Scholar]
- DiCarlo JJ, Lane JW, Hsiao SS, Johnson KO. Marking microelectrode penetrations with fluorescent dyes. J Neurosci Methods. 1996;64:75–81. doi: 10.1016/0165-0270(95)00113-1. [DOI] [PubMed] [Google Scholar]
- Dobrunz LE, Stevens CF. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron. 1997;18:995–1008. doi: 10.1016/s0896-6273(00)80338-4. [DOI] [PubMed] [Google Scholar]
- Dreher B, Burke W, Calford MB. Cortical plasticity revealed by circumscribed retinal lesions or artificial scotomas. Prog Brain Res. 2001;134:217–246. doi: 10.1016/s0079-6123(01)34016-5. [DOI] [PubMed] [Google Scholar]
- Ebner FF, Rema V, Sachdev R, Symons FJ. Activity-dependent plasticity in adult somatic sensory cortex. Semin Neurosci. 1997;9:47–58. [Google Scholar]
- Eder M, Zieglgänsberger W, Dodt HU. Neocortical long-term potentiation and long-term depression: site of expression investigated by infrared-guided laser stimulation. J Neurosci. 2002;22:7558–7568. doi: 10.1523/JNEUROSCI.22-17-07558.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang PC, Jain N, Kaas JH. Few intrinsic connections cross the hand-face border of area 3b of New World monkeys. J Comp Neurol. 2002;454:310–319. doi: 10.1002/cne.10433. [DOI] [PubMed] [Google Scholar]
- Finnerty GT, Roberts LS, Connors BW. Sensory experience modifies the short-term dynamics of neocortical synapses. Nature. 1999;400:367–371. doi: 10.1038/22553. [DOI] [PubMed] [Google Scholar]
- Flor H. Cortical reorganisation and chronic pain: implications for rehabilitation. J Rehabil Med. 2003;41(Suppl):66–72. doi: 10.1080/16501960310010179. [DOI] [PubMed] [Google Scholar]
- Flor H, Elbert T, Knecht S, Wienbruch C, Pantev C, Birbaumer N, Larbig W, Taub E. Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature. 1995;375:482–484. doi: 10.1038/375482a0. [DOI] [PubMed] [Google Scholar]
- Friel KM, Nudo RJ. Recovery of motor function after focal cortical injury in primates: compensatory movement patterns used during rehabilitative training. Somatosens Mot Res. 1998;15:173–189. doi: 10.1080/08990229870745. [DOI] [PubMed] [Google Scholar]
- Gaiarsa JL, Caillard O, Ben-Ari Y. Long-term plasticity at GABAergic and glycinergic synapses: mechanisms and functional significance. Trends Neurosci. 2002;25:564–570. doi: 10.1016/s0166-2236(02)02269-5. [DOI] [PubMed] [Google Scholar]
- Goda Y, Stevens CF. Two components of transmitter release at a central synapse. Proc Natl Acad Sci USA. 1994;91:12942–12946. doi: 10.1073/pnas.91.26.12942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Wang Y, Markram H. Organizing principles for a diversity of GABAergic interneurons and synapses in the neocortex. Science. 2000;287:273–278. doi: 10.1126/science.287.5451.273. [DOI] [PubMed] [Google Scholar]
- Hardingham NR, Hardingham GE, Fox KD, Jack JJ. Presynaptic efficacy directs normalization of synaptic strength in layer 2/3 rat neocortex after paired activity. J Neurophysiol. 2007;97:2965–2975. doi: 10.1152/jn.01352.2006. [DOI] [PubMed] [Google Scholar]
- Hess G, Donoghue JP. Long-term depression of horizontal connections in rat motor cortex. Eur J Neurosci. 1996;8:658–665. doi: 10.1111/j.1460-9568.1996.tb01251.x. [DOI] [PubMed] [Google Scholar]
- Hickmott PW. Changes in intrinsic properties of pyramidal neurons in adult rat S1 during cortical reorganization. J Neurophysiol. 2005;94:501–511. doi: 10.1152/jn.00924.2004. [DOI] [PubMed] [Google Scholar]
- Hickmott PW, Merzenich MM. Single-cell correlates of a representational boundary in rat somatosensory cortex. J Neurosci. 1998;18:4403–4416. doi: 10.1523/JNEUROSCI.18-11-04403.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickmott PW, Merzenich MM. Dendritic bias of neurons in rat somatosensory cortex associated with a functional boundary. J Comp Neurol. 1999;409:385–399. [PubMed] [Google Scholar]
- Hickmott PW, Merzenich MM. Local circuit properties underlying cortical reorganization. J Neurophysiol. 2002;88:1288–1301. doi: 10.1152/jn.00994.2001. [DOI] [PubMed] [Google Scholar]
- Hickmott PW, Steen PA. Large-scale changes in dendritic structure during reorganization of adult somatosensory cortex. Nat Neurosci. 2005;8:140–142. doi: 10.1038/nn1384. [DOI] [PubMed] [Google Scholar]
- Hirsch JA, Gilbert CD. Long-term changes in synaptic strength along specific intrinsic pathways in the cat visual cortex. J Physiol (Lond). 1993;461:247–262. doi: 10.1113/jphysiol.1993.sp019512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins W, Merzenich M, Ochs M, Allard T, Guic-Robles E. Functional reorganization of primary somatosensory cortex in adult owl monkeys after behaviorally controlled tactile stimulation. J Neurophysiol. 1990;63:82–104. doi: 10.1152/jn.1990.63.1.82. [DOI] [PubMed] [Google Scholar]
- Kaas JH. Plasticity of Sensory and Motor Maps in Adult Mammals. Annu Rev Neurosci. 1991;14:137–166. doi: 10.1146/annurev.ne.14.030191.001033. [DOI] [PubMed] [Google Scholar]
- Komatsu Y. Age-dependent long-term potentiation of inhibitory synaptic transmission in rat visual cortex. J Neurosci. 1994;14:6488–6499. doi: 10.1523/JNEUROSCI.14-11-06488.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepert J, Miltner WH, Bauder H, Sommer M, Dettmers C, Taub E, Weiller C. Motor cortex plasticity during constraint-induced movement therapy in stroke patients. Neurosci Lett. 1998;250:5–8. doi: 10.1016/s0304-3940(98)00386-3. [DOI] [PubMed] [Google Scholar]
- Lissek S, Wilimzig C, Stude P, Pleger B, Kalisch T, Maier C, Peters SA, Nicolas V, Tegenthoff M, Dinse HR. Immobilization impairs tactile perception and shrinks somatosensory cortical maps. Curr Biol. 2009;19:837–842. doi: 10.1016/j.cub.2009.03.065. [DOI] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- Markram H, Tsodyks M. Redistribution of synaptic efficacy between neocortical pyramidal neurons. Nature. 1996a;382:807–810. doi: 10.1038/382807a0. [DOI] [PubMed] [Google Scholar]
- Markram H, Tsodyks M. Redistribution of synaptic efficacy: a mechanism to generate infinite synaptic input diversity from a homogeneous population of neurons without changing absolute synaptic efficacies. J Physiol (Paris). 1996b;90:229–232. doi: 10.1016/s0928-4257(97)81429-5. [DOI] [PubMed] [Google Scholar]
- Markram H, Wang Y, Tsodyks M. Differential signaling via the same axon of neocortical pyramidal neurons. Proc Natl Acad Sci USA. 1998;95:5323–5328. doi: 10.1073/pnas.95.9.5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merzenich M, Kaas J, Wall J, Nelson R, Sur M, Felleman D. Topographic reorganization of somatosensory cortical areas 3b and 1 in adult monkeys following restricted deafferentation. Neuroscience. 1983;8:33–55. doi: 10.1016/0306-4522(83)90024-6. [DOI] [PubMed] [Google Scholar]
- Merzenich M, Recanzone G, Jenkins W, Allard T, Nudo R. Cortical representational plasticity. In: Singer PRW, editor. Neurobiology of neocortex. New York: Wiley; 1988. pp. 41–67. [Google Scholar]
- Merzenich M, Recanzone G, Jenkins W, Grajski K. Adaptive mechanisms in cortical networks underlying cortical contributions to learning and nondeclarative memory. Cold Spr Har Symp Quant Biol. 1990;55:873–887. doi: 10.1101/sqb.1990.055.01.082. [DOI] [PubMed] [Google Scholar]
- Miledi R, Slater CR. The action of calcium on neuronal synapses in the squid. J Physiol. 1966;184:473–498. doi: 10.1113/jphysiol.1966.sp007927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudo RJ. Remodeling of cortical motor representations after stroke: implications for recovery from brain damage [news] Mol Psychiatry. 1997;2:188–191. doi: 10.1038/sj.mp.4000188. [DOI] [PubMed] [Google Scholar]
- Paullus JR, Hickmott PW. Inhibitory Long Term Depression in Supragranular Horizontal Connections in the Adult Rat Somatosensory Cortex (S1) Washington, DC: Society for Neuroscience; 2007. Program # 401.18. 2007 Abstract Viewer/Itinerary Planner. Online. [Google Scholar]
- Petersen CC, Sakmann B. The excitatory neuronal network of rat layer 4 barrel cortex. J Neurosci. 2000;20:7579–7586. doi: 10.1523/JNEUROSCI.20-20-07579.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleger B, Foerster AF, Ragert P, Dinse HR, Schwenkreis P, Malin JP, Nicolas V, Tegenthoff M. Functional imaging of perceptual learning in human primary and secondary somatosensory cortex. Neuron. 2003;40:643–653. doi: 10.1016/s0896-6273(03)00677-9. [DOI] [PubMed] [Google Scholar]
- Pons T, Garraghty P, Ommaya A, Kaas J, Taub E, Mishkin M. Massive cortical reorganization after sensory deafferentation in adult macaques. Science. 1991;252:1857–1860. doi: 10.1126/science.1843843. [DOI] [PubMed] [Google Scholar]
- Ramachandran VS, Hirstein W. The perception of phantom limbs. The D.O. Hebb lecture. Brain. 1998;121:1603–1630. doi: 10.1093/brain/121.9.1603. [DOI] [PubMed] [Google Scholar]
- Recanzone G, Merzenich M, Jenkins W. Frequency discrimination training engaging a restricted skin surface results in an emergence of a cutaneous response zone in cortical area 3a. J Neurophysiol. 1992a;67:1057–1070. doi: 10.1152/jn.1992.67.5.1057. [DOI] [PubMed] [Google Scholar]
- Recanzone G, Merzenich M, Jenkins W, Grajski K, Dinse H. Topographic reorganization of the hand representation in cortical area 3b of owl monkeys trained in a frequency-discrimination task. J Neurophysiol. 1992b;67:1031–1056. doi: 10.1152/jn.1992.67.5.1031. [DOI] [PubMed] [Google Scholar]
- Reyes A, Lujan R, Rozov A, Burnashev N, Somogyi P, Sakmann B. Target-cell-specific facilitation and depression in neocortical circuits. Nat Neurosci. 1998;1:279–285. doi: 10.1038/1092. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Martino G, Narici L, Pasquarelli A, Peresson M, Pizzella V, Tecchio F, Torrioli G, Romani GL. Short-term brain ‘plasticity’ in humans: transient finger representation changes in sensory cortex somatotopy following ischemic anesthesia. Brain Res. 1994;642:169–177. doi: 10.1016/0006-8993(94)90919-9. [DOI] [PubMed] [Google Scholar]
- Rozov A, Burnashev N, Sakmann B, Neher E. Transmitter release modulation by intracellular Ca2+ buffers in facilitating and depressing nerve terminals of pyramidal cells in layer 2/3 of the rat neocortex indicates a target cell-specific difference in presynaptic calcium dynamics. J Physiol. 2001;531:807–826. doi: 10.1111/j.1469-7793.2001.0807h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen PA, Mason M, Pham L, Lefebvre Y, Hickmott PW. Axonal bias at a representational border in adult rat somatosensory cortex (S1) J Comp Neurol. 2007;500:634–645. doi: 10.1002/cne.21199. [DOI] [PubMed] [Google Scholar]
- Tailby C, Wright LL, Metha AB, Calford MB. Activity-dependent maintenance and growth of dendrites in adult cortex. Proc Natl Acad Sci USA. 2005;102:4631–4636. doi: 10.1073/pnas.0402747102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AM, Girdlestone D, West DC. Voltage-dependent currents prolong single-axon postsynaptic potentials in layer III pyramidal neurons in rat neocortical slices. J Neurophysiol. 1988;60:1896–1907. doi: 10.1152/jn.1988.60.6.1896. [DOI] [PubMed] [Google Scholar]
- Thomson AM, West DC, Hahn J, Deuchars J. Single axon IPSPs elicited in pyramidal cells by three classes of interneurones in slices of rat neocortex. J Physiol. 1996;496(Pt 1):81–102. doi: 10.1113/jphysiol.1996.sp021667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinazzi M, Zanette G, Polo A, Volpato D, Manganotti P, Bonato C, Testoni R, Fiaschi A. Transient deafferentation in humans induces rapid modulation of primary sensory cortex not associated with subcortical changes: a somatosensory evoked potential study. Neurosci Lett. 1997;223:21–24. doi: 10.1016/s0304-3940(97)13382-1. [DOI] [PubMed] [Google Scholar]
- Tremere L, Hicks TP, Rasmusson DD. Role of inhibition in cortical reorganization of the adult raccoon revealed by microiontophoretic blockade of GABA(A) receptors. J Neurophysiol. 2001a;86:94–103. doi: 10.1152/jn.2001.86.1.94. [DOI] [PubMed] [Google Scholar]
- Tremere L, Hicks TP, Rasmusson DD. Expansion of receptive fields in raccoon somatosensory cortex in vivo by GABA(A) receptor antagonism: implications for cortical reorganization. Exp Brain Res. 2001b;136:447–455. doi: 10.1007/s002210000612. [DOI] [PubMed] [Google Scholar]
- Tsodyks MV, Markram H. The neural code between neocortical pyramidal neurons depends on neurotransmitter release probability. Proc Natl Acad Sci USA. 1997;94:719–723. doi: 10.1073/pnas.94.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volgushev M, Voronin LL, Chistiakova M, Artola A, Singer W. All-or-none excitatory postsynaptic potentials in the rat visual cortex. Eur J Neurosci. 1995;7:1751–1760. doi: 10.1111/j.1460-9568.1995.tb00695.x. [DOI] [PubMed] [Google Scholar]
- Wall J. Variable organization in cortical maps of the skin as an indication of the lifelong adaptive capacities of circuits in the mammalian brain. Trends Neurosci. 1988;11:549–557. doi: 10.1016/0166-2236(88)90184-1. [DOI] [PubMed] [Google Scholar]
- Wang X, Merzenich MM, Sameshima K, Jenkins WM. Remodelling of hand representation in adult cortex determined by timing of tactile stimulation. Nature. 1995;378:71–75. doi: 10.1038/378071a0. [DOI] [PubMed] [Google Scholar]
- Weiss T, Miltner WH, Liepert J, Meissner W, Taub E. Rapid functional plasticity in the primary somatomotor cortex and perceptual changes after nerve block. Eur J Neurosci. 2004;20:3413–3423. doi: 10.1111/j.1460-9568.2004.03790.x. [DOI] [PubMed] [Google Scholar]
- Williams SR, Stuart GJ. Dependence of EPSP efficacy on synapse location in neocortical pyramidal neurons. Science. 2002;295:1845–1846. doi: 10.1126/science.1067903. [DOI] [PubMed] [Google Scholar]
- Xu-Friedman MA, Regehr WG. Probing fundamental aspects of synaptic transmission with strontium. J Neurosci. 2000;20:4414–4422. doi: 10.1523/JNEUROSCI.20-12-04414.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]