Abstract
Markers of early pancreatic cancer and its precursors are needed to improve the uniformly poor prognosis of this disease. Fatty acid synthase (FAS) catalyzes the synthesis of long-chain fatty acids and is overexpressed in most human solid tumors. We therefore evaluated serum FAS as a marker of pancreatic adenocarcinoma. FAS expression patterns in primary pancreatic adenocarcinomas, intraductal papillary mucinous neoplasms (IPMN), and chronic pancreatitis tissues were analyzed by immunohistochemistry. Serum FAS levels were determined by ELISA in 102 patients with pancreatic adenocarcinomas, in 42 patients with IPMNs, in 27 patients with chronic pancreatitis, and in 39 healthy control subjects. FAS protein was overexpressed in the ductal epithelium of 343 of 399 primary pancreatic adenocarcinomas (86.0%) and 28 of 30 IPMNs (93.3%), and in the islet and ductal cells in 3 of 54 chronic pancreatitis tissues (5.6%), whereas normal ductal epithelium lacked FAS expression. Serum FAS levels were significantly higher in patients with pancreatic ductal adenocarcinoma (first quartile median, 22.0; 4.5 ng/mL), in patients with IPMNs (20.7; 9.4 ng/mL), and in patients with chronic pancreatitis (31.1; 11.9 ng/mL) than in healthy controls (0; 0 ng/mL). FAS levels declined postoperatively in 8 of 9 patients with pancreatic adenocarcinoma and elevations of their preoperative serum FAS. In conclusion, serum FAS levels are elevated in patients with pancreatic cancer and IPMNs and are associated with neoplastic overexpression of FAS.
Introduction
Pancreatic ductal adenocarcinoma is the 4th leading cause of cancer death and one of the most aggressive of the solid cancers. The late presentation and poor response of pancreatic cancer patients to radiation therapy and conventional chemotherapy contribute to the low overall 5-year survival rate of <4% (1–3). Prognosis is greatly improved when patients are diagnosed at an early, operable disease stage. Identification of sensitive and specific biomarkers could likely facilitate early detection and improve outcome in these patients. The early detection of pancreatic cancer is difficult because patients generally present at an advanced disease stage. The identification of patients with an increased risk such as individuals with a family history of the disease (4–8), patients in whom pancreatic cysts are discovered incidentally (9), and possibly patients with other risk factors such as new-onset diabetes (10), may lead to a higher rate of detection of early pancreatic cancer and its precursors if effective early detection strategies are applied to these at-risk populations. Indeed, recent studies have shown that screening using pancreatic imaging tests such as endoscopic ultrasound can identify asymptomatic pancreatic neoplasms in patients with a strong family history of pancreatic cancer and other inherited predisposition syndromes (11, 12). The identification of effective molecular markers of pancreatic neoplasia could improve the early detection of this disease (13). Several new candidate markers have been described in recent years and have been evaluated in pancreatic secretions to detect local pancreatic neoplasia (14), and in serum (15), but more accurate markers are needed if they are going to be used to improve the early detection of pancreatic neoplasia.
Fatty acid synthase (FAS), a metabolic enzyme that catalyzes the synthesis of long-chain fatty acids, is expressed at high levels in a variety of human cancers, including cancer of the breast (16, 17), prostate (18, 19), endometrium (20), ovary (21), colon (22), lung (23, 24), and pancreas (25). Although the mechanism of FAS overexpression is unknown, it seems to be up-regulated during the early stages of tumorigenesis (22, 26–29). This differential expression between normal and neoplastic tissues makes FAS a potential diagnostic tumor marker.
In addition to being overexpressed in malignant tissues, increased FAS levels can also be detected in the circulation in cancer patients (30, 31). A study measuring FAS expression by ELISA in the circulation of 22 breast cancer patients found significantly elevated FAS levels in sera from patients with different clinical stages of breast cancer than in healthy control subjects (31). Tumor expression of FAS is also a useful prognostic indicator in some cancer types, including prostate (18, 32) and breast carcinomas (17), and is linked to proliferation and tumor grade in endometrial carcinomas (20).
Although FAS is found to be overexpressed in many solid tumors, its role in pancreatic cancer has not been extensively evaluated. We evaluated FAS as a marker of pancreatic cancer by using an ELISA to measure FAS levels in the serum of patients with pancreatic cancer, patients with other pancreatic neoplasms, and in normal pancreas disease control subjects. We also analyzed FAS protein expression in primary pancreatic adenocarcinomas by immunohistochemistry.
Materials and Methods
Serum and Tissue Samples
A total of 171 serum samples were analyzed. Fasting preoperative serum samples numbering 127 in total were obtained from patients undergoing pancreaticoduodenectomy at the Johns Hopkins Medical Institutions, including 102 patients with pancreatic adenocarcinoma, 27 with chronic pancreatitis, and 42 with intraductal papillary mucinous neoplasms (IPMN). Postoperative serum samples were obtained from 11 of the patients with pancreatic adenocarcinoma. Postoperative samples were obtained during the patient’s hospitalization. An additional 44 serum samples were obtained from disease controls enrolled in the Cancer of the Pancreas Screening Study 2 (CAPS 2). These disease controls were patients undergoing evaluation of their pancreas for known suspected pancreatic disease. Of the 44 CAPS serum samples analyzed, 3 patients had IPMN, 18 had chronic pancreatitis, and 23 had pancreatic cancer. Serum samples from 39 healthy control individuals were also obtained from the Johns Hopkins Bayview Medical Center General Clinical Research Center. The mean age of the patient group was 66.5 y (range, 47–87 y) and 43.5 y for the healthy control group (range, 21–62 y). All of the samples were collected with approval from the Johns Hopkins Committee for Clinical Investigation.
FAS ELISA
A total of 100 μL of serum were analyzed with a commercially available ELISA kit, FAS-detect ELISA (FASgen), according to the manufacturer’s recommendations.
Briefly, sera were incubated in a 96-well capture plate on a plate shaker for 90 min at room temperature. The plate was then washed five times with wash buffer. FAS enzyme conjugate was added and the plate was incubated for 60 min, and the wash was repeated. Serum FAS levels were visualized by color change upon addition of tetramethyl-benzidine substrate followed by addition of substrate stop solution. Absorbance values were read at 450 nm using a SpectraMax spectrophotometer. FAS concentrations were determined by interpolation from the standard curve.
Pancreatic Adenocarcinoma Tissue Microarrays and FAS Immunohistochemistry
The expression of FAS protein was examined utilizing immunohistochemical labeling of formalin-fixed, paraffin-embedded tissue microarrays using a DAKO Autostainer (DAKO). Thirteen tissue microarrays containing a total of 483 different surgically resected pancreatic ductal adenocarcinomas, IPMNs, and chronic pancreatitis tissues were constructed as previously described (33). Sections were deparaffinized in xylene, hydrated in graded ethanol concentrations, and boiled for 20 min in epitope retrieval buffer. Immunostaining was then done on the DAKO Autostainer using a mouse monoclonal antihuman FAS antibody, FAS-detect IHC (kindly provided by FASgen, Inc.) at a 1:1,000 dilution for an incubation time of 60 min. Labeling was done according to the manufacturer’s protocol using the Envision Plus Detection Kit (DAKO). Nuclei were counterstained with hematoxylin. The relative intensity of labeling (from 0 to 2+) was scored by two observers at a multiheaded microscope. The relative intensity of labeling of the nonneoplastic stroma was evaluated as was the relative intensity of labeling of the neoplastic cells. A score of 0 was assigned if there was no appreciable labeling. In the statistical analyses, labeling was considered positive if the cells labeled at an intensity of 1 or 2+. Labeling of 0 was considered negative.
Western Blot Analysis
Western blot analysis was done using lysates from an immortalized culture established from normal pancreas, HPDE (human pancreatic ductal epithelium from Tsao et al.; ref. 34), and six pancreatic cancer cell lines (Capan1, Capan2, BxPC3, Hs766T, MiaPaca2, and Panc1). A Bradford assay was used to estimate protein concentration, using bovine serum albumin (Invitrogen) as a standard. Equal amounts of protein (40 μg/lane) were separated by 3% to 8% gradient SDS gel electrophoresis (Invitrogen), transferred to nitrocellulose membranes, and incubated in blocking solution (5% milk in TBS with 0.1% Tween 20) for 30 min. Membranes were incubated for 60 min with polyclonal or monoclonal antibodies directed against FAS (FASGen, Inc.) at 1:1,000 dilution or against GAPDH (Cell Signaling) at 1:5,000 dilution. Secondary horseradish peroxidase–linked antibodies (Santa Cruz Biotechnology and Amersham Biosciences) were applied at 1:2,000 dilution and proteins were detected using an ECL kit (Amersham Biosciences).
Statistical Analysis
Descriptive statistical values and plots were generated using the Microsoft Excel software package or the Partek Genomics Suite ver. 6.03. Statistical analysis was done using unpaired Student’s t test (two-tailed). Mann-Whitney (nonparametric test) and Pearson χ2 tests were done using SPSS 10.0 software statistical program. Differences were considered significant at P < 0.05.
Results
FAS Serum Levels
Serum FAS levels were analyzed in patients from the following subgroups: pancreatic ductal adenocarcinoma, IPMN, chronic pancreatitis, and normal pancreas healthy controls. The median and first quartile FAS level in patients with pancreatic ductal adenocarcinoma were 22.0 and 4.5 ng/mL; for patients with IPMN, 20.7 and 9.4 ng/mL; for patients with chronic pancreatitis, 31.1 and 11.9 ng/mL; and for healthy controls, 0 and 0 ng/mL (Fig. 1). FAS levels were significantly higher in patients with pancreatic cancer than in healthy controls (P < 0.05, Student’s t test). FAS levels were also significantly higher in patients with an IPMN or with chronic pancreatitis than in healthy controls. There was no relationship between FAS levels and age or gender. The distribution of FAS levels in healthy controls was skewed with the majority of individuals having an undetectable level.
Figure 1.
Dot plot of serum FAS levels (in ng/mL) in pancreatic cancer, IPMN, chronic pancreatitis, and healthy control patients.
Diagnostic Accuracy of Serum FAS in Pancreatic Cancer Patients
To determine the sensitivity of elevated serum FAS as a diagnostic test for pancreatic cancer, we designated 14.7 ng/mL, a level 2 SDs above the mean FAS level in the healthy control group, as a cutoff. Using this level, we found that 60 of 102 patients with pancreatic adenocarcinoma had an elevated FAS level (sensitivity, 58.8%) and 47 of 66 healthy control patients did not have an elevated FAS level (specificity, 71.2%).
To determine if elevated FAS serum levels in pancreatic cancer patients could be directly attributed to elevated FAS within the patient’s tumor, we compared FAS levels in 11 preoperative serum samples to corresponding postoperative serum samples from patients undergoing a Whipple resection for pancreatic cancer. We found that of 11 patients with paired preoperative and postoperative samples, 8 of 9 patients (88.9%) had a reduced FAS level in their postoperative serum compared with their preoperative serum, 2 patients had undetectable FAS before and after surgery, and 1 patient had an elevation after surgery. In this patient the post op sample was drawn ~3 months after surgery, raising the possibility that tumor recurrence had begun (Fig. 2).
Figure 2.
Comparison of preoperative (left) and postoperative (right) serum FAS levels in pancreatic cancer patients.
FAS Immunohistochemistry
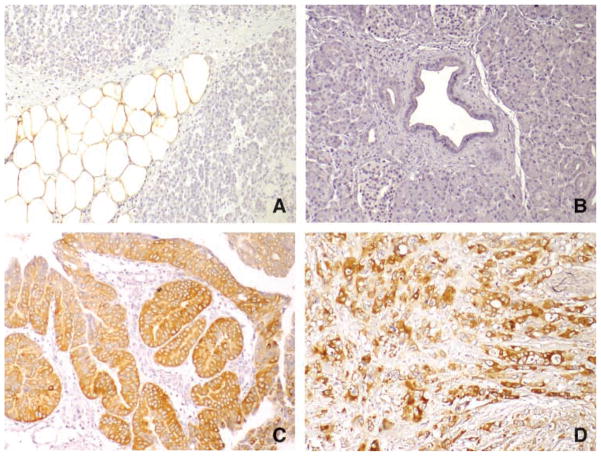
FAS tissue expression was investigated by immunohistochemistry on paraffin-embedded, formalin-fixed, surgically resected pancreatic adenocarcinoma, IPMN, and chronic pancreatitis tissues. A strong FAS signal detected in normal adipocytes within the pancreas served as an internal positive control (Fig. 3A). No FAS-specific labeling was seen in stromal cells or in normal pancreatic ductal cells (Fig. 3B). Labeling when present was seen diffusely in the pancreatic adenocarcinomas and IPMNs. A positive cytoplasmic FAS signal was detected in the ductal cells of 28 of 30 (93.3%) IPMNs (Fig. 3C). Of these, 11 (36.7%) exhibited weak immunohistochemical labeling and 17 (56.7%) exhibited strong immunohistochemical labeling. FAS tissue expression in IPMN tumors correlated with histologic grade (P = 0.001; Table 1) and with the presence of an associated invasive cancer (P = 0.001; Table 1). FAS labeling was also detected in the ductal cells of 343 of 399 (86.0%) primary pancreatic adenocarcinomas (Fig. 3D). Of these cases, 238 (59.6%) exhibited weak positive immunohistochemical labeling and 105 (26.3%) exhibited strong positive immunohistochemical labeling. We found that FAS tissue expression in pancreatic cancers correlated with vascular invasion (P = 0.002) but did not correlate with histologic grade (Table 2). Weak positive FAS labeling was observed in the islet and ductal cells in 3 of 54 (5.6%) chronic pancreatitis tissues (data not shown). Interestingly, there was no correlation between FAS levels in the circulation and intensity of labeling of the patients’ associated neoplasm (Table 3).
Figure 3.
Immunohistochemical analysis of FAS protein expression in tissue microarrays. A. Normal pancreatic adipose tissue with strong labeling of FAS. B. Normal pancreatic ductal cells and surrounding stromal cells do not label for FAS. C. IPMN is strongly positive for FAS in the neoplastic pancreatic ductal cells. D. Pancreatic ductal adenocarcinoma cells are strongly positive for FAS.
Table 1.
FAS immunohistochemical expression in intraductal papillary mucinous neoplasm
| Variables | FAS expression |
Total | P | |
|---|---|---|---|---|
| No/weak expression | Strong expression | |||
| Dysplasia of IPMN | 0.001* | |||
| Mild dysplasia | 4 | 0 | 4 | |
| Moderate dysplasia | 9 | 3 | 12 | |
| Severe dysplasia | 2 | 12 | 14 | |
| Association with invasive cancer | 0.001* | |||
| With cancer | 2 | 11 | 13 | |
| Without cancer | 13 | 4 | 17 | |
Significant at the level of <0.05.
Table 2.
FAS immunohistochemical expression in pancreatic ductal adenocarcinoma
| Variables | FAS expression |
Total | P | |
|---|---|---|---|---|
| No/weak expression | Strong expression | |||
| Differentiation | 0.68 | |||
| Well | 9 | 3 | 12 | |
| Moderate | 151 | 49 | 200 | |
| Poorly | 122 | 51 | 173 | |
| Tumor extension | 0.49 | |||
| Localized (pT1 & pT2) | 12 | 5 | 17 | |
| Extended (pT3) | 270 | 98 | 368 | |
| Lymph node metastasis | 0.32 | |||
| Absent | 46 | 14 | 60 | |
| Present | 236 | 89 | 325 | |
| Vascular invasion* | 0.002† | |||
| Absent | 145 | 31 | 176 | |
| Present | 128 | 60 | 188 | |
| Perineural invasion | 0.53 | |||
| Absent | 24 | 6 | 30 | |
| Present | 258 | 97 | 355 | |
Evaluation for vascular invasion was available 364 of 385 patients.
Significant at the level of <0.05.
Table 3.
Correlation of FAS immunohistochemical expression with serum FAS ELISA
| FAS ELISA+* | FAS ELISA − | Total | P | |
|---|---|---|---|---|
| Pancreatic adenocarcinoma | 0.44 | |||
| FAS IHC strong+ | 5 | 1 | 6 | |
| FAS IHC weak+/− | 8 | 4 | 12 | |
| IPMN | ||||
| FAS IHC strong+ | 3 | 2 | 5 | 0.36 |
| FAS IHC weak+/− | 3 | 0 | 3 | |
Abbreviation: IHC, immunohistochemistry
FAS ELISA is considered positive at a level of >14.7 ng/mL.
We also examined FAS expression in microdissected pancreatic tissues and in pancreatic cancer cell lines. Previously generated serial analysis of gene expression analysis (35) revealed that the mean FAS RNA expression level in microdissected pancreatic cancer cell lines (n = 24) was 2.6-fold higher than in microdissected normal pancreatic ductal cells (n = 2). The normal pancreatic ductal cell line HPDE (34) expressed a slightly higher FAS level than microdissected normal ductal cells. Western blot analysis of FAS protein expression in six pancreatic cancer cell lines and HPDE cells was consistent with the serial analysis of gene expression data, in which FAS expression in cancer cell lines was variable, and expression in HPDE cells was comparable with that in the moderately expressing cell lines. The pancreatic cancer cell line Panc1 strongly overexpressed FAS protein (Fig. 4).
Figure 4.
Western blot analysis of FAS protein levels in pancreatic cancer cell lines and immortalized normal pancreas controls.
Discussion
Markers for early detection of pancreatic cancer are urgently needed to improve the prognosis in patients with this highly aggressive disease. Development of a noninvasive diagnostic test could facilitate screening of high-risk individuals, such as those with a family history of pancreatic cancer. Identification of serum protein markers of pancreatic cancer could help provide such a noninvasive diagnostic screening tool.
This study found that elevated FAS protein levels could be detected in the serum of patients with pancreatic cancer and patients with precancerous lesions of the pancreas.
FAS protein overexpression was localized to neoplastic cells in the pancreas and its overexpression was found in early lesions as well as invasive cancers. These findings agree with previous reports in other cancer types suggesting that FAS up-regulation occurs early in tumorigenesis (16, 19, 27–29) and suggest that serum FAS could aid in the early detection of pancreatic cancer. FAS was similarly elevated in pancreatic cancer patients, patients with IPMNs, and patients with chronic pancreatitis compared with healthy controls, suggesting that FAS detection cannot be used for distinguishing pancreatic cancer from patients with other pancreatic diseases. However, it is possible that serum FAS levels could be helpful in identifying and following patients with IPMNs. Indeed, FAS tissue expression in IPMN tumors correlated with histologic grade (P = 0.001) and with the presence of an associated invasive cancer (P = 0.001; Table 1).
The elevated serum FAS levels in pancreatic cancer and IPMN patients were supported by immunohistochemistry data, which revealed FAS overexpression in the majority of tumors (86.0% and 93.3%, respectively) we examined. Although serum FAS levels were elevated in the majority of chronic pancreatitis patients we examined, only 5.6% of cases were found to overexpress FAS in regions of pancreatitis by immunohistochemistry. Pancreatic adipocytes were often not present in the chronic pancreatitis cores, but when adipocytes were included in the tissue cores they uniformly expressed FAS, suggesting that such an analysis may not have been helpful. One explanation for the increase in serum FAS in pancreatitis patients in the absence of overexpression in pancreatitis tissues is that the inflammatory process associated with chronic pancreatitis caused necrosis of surrounding pancreatic adipocytes, releasing FAS into the circulation. If so, the amount of pancreatic fat could also influence circulating FAS levels.
We found that serum FAS levels decreased in 8 of 9 of pancreatic cancer patients following surgical resection of their tumor. In five of these patients the FAS level was undetectable in postoperative serum. These results suggest that the tumor was the primary source of circulating FAS in these patients.
One obstacle to the use of FAS as a serum marker of pancreatic cancer is that FAS is expressed in normal adipocytes and therefore patients may have elevated serum FAS levels in the absence of pancreatic disease. For example, FAS gene expression and FAS protein activity are increased by insulin, and FAS gene expression is elevated in adipocytes in patients with type 2 diabetes (36, 37). FAS is also elevated in an experimental model of renal failure (38, 39) and is overexpressed in hepatocytes in animal models of nonalcoholic steatohepatitis (40, 41). However, adipocyte FAS expression alone does not seem to be sufficient to contribute to circulating FAS levels. Although FAS alone cannot be used to accurately diagnose pancreatic cancer (sensitivity, 58.8%; specificity, 71.2%), it may improve diagnostic utility when used in combination with other markers.
Our immunohistochemistry data support a role for FAS up-regulation in highly proliferative cells. FAS overexpression was localized to neoplastic ductal cells in pancreatic cancers and IPMNs, whereas FAS was overexpressed in ductal and islet cells in a small percentage of chronic pancreatitis cases examined. Although the mechanisms of FAS up-regulation in cancer are not completely understood, it is thought that FAS is overexpressed in rapidly proliferating and hyperplastic cells due to their increased energy requirement and increased need for lipid synthesis. Others have reported FAS overexpression in nonmalignant, highly proliferative lesions as well as malignant tissues (17, 18, 27, 42, 43). FAS overexpression is also correlated with the proliferation marker Ki-67 in many cancers including pancreatic cancer (25) and endometrial carcinoma (20). FAS overexpression in pancreatic cancer is also associated with higher histologic grade and overall survival (25), and further studies will help determine the utility of FAS as a prognostic predictor in patients. We found that FAS tissue expression in pancreatic cancers correlated with vascular invasion (P = 0.002) but did not correlate with histologic grade (Table 2).
Serum FAS has potential as a noninvasive diagnostic marker of pancreatic disease. Further studies are needed to validate the utility of FAS as an early detection marker of pancreatic cancer, but its overexpression in precursors of pancreatic cancer such as IPMN lesions and chronic pancreatitis suggest that it could potentially be used in conjunction with other markers to follow patients at high risk of pancreatic disease.
Acknowledgments
Grant support: National Cancer Institute grant (CA120432, CA62924), the V foundation and the Michael Rolfe Foundation.
Footnotes
Disclosure of Potential Conflicts of Interest
S. Medghalchi: Employment and ownership interest, FASgen, Inc. F. Kuhajda: Ownership interest, FASgen, Inc.
References
- 1.Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics. CA Cancer J Clin. 2000;50:7–33. doi: 10.3322/canjclin.50.1.7. [DOI] [PubMed] [Google Scholar]
- 2.Nitecki SS, Sarr MG, Colby TV, van Heerden JA. Long-term survival after resection for ductal adenocarcinoma of the pancreas. Is it really improving? Ann Surg. 1995;221:59–66. doi: 10.1097/00000658-199501000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567–79. doi: 10.1016/s1091-255x(00)80105-5. [DOI] [PubMed] [Google Scholar]
- 4.Klein AP, Brune KA, Petersen GM, et al. Prospective risk of pancreatic cancer in familial pancreatic cancer kindreds. Cancer Res. 2004;64:2634–8. doi: 10.1158/0008-5472.can-03-3823. [DOI] [PubMed] [Google Scholar]
- 5.Rosty C, Goggins M. Early detection of pancreatic carcinoma. Hematol Oncol Clin North Am. 2002;16:37–52. doi: 10.1016/s0889-8588(01)00007-7. [DOI] [PubMed] [Google Scholar]
- 6.Tersmette AC, Petersen GM, Offerhaus GJ, et al. Increased risk of incident pancreatic cancer among first-degree relatives of patients with familial pancreatic cancer. Clin Cancer Res. 2001;7:738–44. [PubMed] [Google Scholar]
- 7.Brentnall TA, Bronner MP, Byrd DR, Haggitt RC, Kimmey MB. Early diagnosis and treatment of pancreatic dysplasia in patients with a family history of pancreatic cancer. Ann Intern Med. 1999;131:247–55. doi: 10.7326/0003-4819-131-4-199908170-00003. [DOI] [PubMed] [Google Scholar]
- 8.Murphy KM, Brune KA, Griffin C, et al. Evaluation of candidate genes MAP2K4, MADH4, ACVR1B, and BRCA2 in familial pancreatic cancer: deleterious BRCA2 mutations in 17% Cancer Res. 2002;62:3789–93. [PubMed] [Google Scholar]
- 9.Fernandez-del Castillo C, Targarona J, Thayer SP, Rattner DW, Brugge WR, Warshaw AL. Incidental pancreatic cysts: clinicopathologic characteristics and comparison with symptomatic patients. Arch Surg. 2003;138:427–3. doi: 10.1001/archsurg.138.4.427. discussion 433–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chari ST, Leibson CL, Rabe KG, Ransom J, de Andrade M, Petersen GM. Probability of pancreatic cancer following diabetes: a population-based study. Gastroenterology. 2005;129:504–11. doi: 10.1053/j.gastro.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Canto MI, Goggins M, Hruban RH, et al. Screening for early pancreatic neoplasia in high-risk individuals: a prospective controlled study. Clin Gastroenterol Hepatol. 2006;4:766–81. doi: 10.1016/j.cgh.2006.02.005. quiz 665. [DOI] [PubMed] [Google Scholar]
- 12.Canto MI, Goggins M, Yeo CJ, et al. Screening for pancreatic neoplasia in high-risk individuals: an EUS-based approach. Clin Gastroenterol Hepatol. 2004;2:606–21. doi: 10.1016/s1542-3565(04)00244-7. [DOI] [PubMed] [Google Scholar]
- 13.Goggins M. Identifying molecular markers for the early detection of pancreatic neoplasia. Semin Oncol. 2007;34:303–10. doi: 10.1053/j.seminoncol.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsubayashi H, Canto M, Sato N, et al. DNA methylation alterations in the pancreatic juice of patients with suspected pancreatic disease. Cancer Res. 2006;66:1208–17. doi: 10.1158/0008-5472.CAN-05-2664. [DOI] [PubMed] [Google Scholar]
- 15.Goggins M. Molecular markers of early pancreatic cancer. J Clin Oncol. 2005;23:4524–31. doi: 10.1200/JCO.2005.19.711. [DOI] [PubMed] [Google Scholar]
- 16.Milgraum LZ, Witters LA, Pasternack GR, Kuhajda FP. Enzymes of the fatty acid synthesis pathway are highly expressed in in situ breast carcinoma. Clin Cancer Res. 1997;3:2115–20. [PubMed] [Google Scholar]
- 17.Alo PL, Visca P, Marci A, Mangoni A, Botti C, Di Tondo U. Expression of fatty acid synthase (FAS) as a predictor of recurrence in stage I breast carcinoma patients. Cancer. 1996;77:474–82. doi: 10.1002/(SICI)1097-0142(19960201)77:3<474::AID-CNCR8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 18.Epstein JI, Carmichael M, Partin AW. OA-519 (fatty acid synthase) as an independent predictor of pathologic state in adenocarcinoma of the prostate. Urology. 1995;45:81–6. doi: 10.1016/s0090-4295(95)96904-7. [DOI] [PubMed] [Google Scholar]
- 19.Swinnen JV, Roskams T, Joniau S, et al. Overexpression of fatty acid synthase is an early and common event in the development of prostate cancer. Int J Cancer. 2002;98:19–22. doi: 10.1002/ijc.10127. [DOI] [PubMed] [Google Scholar]
- 20.Pizer ES, Lax SF, Kuhajda FP, Pasternack GR, Kurman RJ. Fatty acid synthase expression in endometrial carcinoma: correlation with cell proliferation and hormone receptors. Cancer. 1998;83:528–37. [PubMed] [Google Scholar]
- 21.Gansler TS, Hardman W, III, Hunt DA, Schaffel S, Hennigar RA. Increased expression of fatty acid synthase (OA-519) in ovarian neoplasms predicts shorter survival. Hum Pathol. 1997;28:686–92. doi: 10.1016/s0046-8177(97)90177-5. [DOI] [PubMed] [Google Scholar]
- 22.Rashid A, Pizer ES, Moga M, et al. Elevated expression of fatty acid synthase and fatty acid synthetic activity in colorectal neoplasia. Am J Pathol. 1997;150:201–8. [PMC free article] [PubMed] [Google Scholar]
- 23.Visca P, Sebastiani V, Botti C, et al. Fatty acid synthase (FAS) is a marker of increased risk of recurrence in lung carcinoma. Anticancer Res. 2004;24:4169–73. [PubMed] [Google Scholar]
- 24.Orita H, Coulter J, Tully E, Kuhajda FP, Gabrielson E. Inhibiting fatty acid synthase for chemoprevention of chemically induced lung tumors. Clin Cancer Res. 2008;14:2458–64. doi: 10.1158/1078-0432.CCR-07-4177. [DOI] [PubMed] [Google Scholar]
- 25.Alo PL, Amini M, Piro F, et al. Immunohistochemical expression and prognostic significance of fatty acid synthase in pancreatic carcinoma. Anticancer Res. 2007;27:2523–7. [PubMed] [Google Scholar]
- 26.Kusakabe T, Nashimoto A, Honma K, Suzuki T. Fatty acid synthase is highly expressed in carcinoma, adenoma and in regenerative epithelium and intestinal metaplasia of the stomach. Histopathology. 2002;40:71–9. doi: 10.1046/j.1365-2559.2002.01289.x. [DOI] [PubMed] [Google Scholar]
- 27.Piyathilake CJ, Frost AR, Manne U, et al. The expression of fatty acid synthase (FASE) is an early event in the development and progression of squamous cell carcinoma of the lung. Hum Pathol. 2000;31:1068–73. doi: 10.1053/hupa.2000.9842. [DOI] [PubMed] [Google Scholar]
- 28.Kuhajda FP. Fatty-acid synthase and human cancer: new perspectives on its role in tumor biology. Nutrition. 2000;16:202–8. doi: 10.1016/s0899-9007(99)00266-x. [DOI] [PubMed] [Google Scholar]
- 29.Van de Sande T, Roskams T, Lerut E, et al. High-level expression of fatty acid synthase in human prostate cancer tissues is linked to activation and nuclear localization of Akt/PKB. J Pathol. 2005;206:214–9. doi: 10.1002/path.1760. [DOI] [PubMed] [Google Scholar]
- 30.Wang YY, Kuhajda FP, Li J, et al. Fatty acid synthase as a tumor marker: its extracellular expression in human breast cancer. J Exp Ther Oncol. 2004;4:101–10. [PubMed] [Google Scholar]
- 31.Wang Y, Kuhajda FP, Li JN, et al. Fatty acid synthase (FAS) expression in human breast cancer cell culture supernatants and in breast cancer patients. Cancer Lett. 2001;167:99–104. doi: 10.1016/s0304-3835(01)00464-5. [DOI] [PubMed] [Google Scholar]
- 32.Shurbaji MS, Kalbfleisch JH, Thurmond TS. Immunohistochemical detection of a fatty acid synthase (OA-519) as a predictor of progression of prostate cancer. Hum Pathol. 1996;27:917–21. doi: 10.1016/s0046-8177(96)90218-x. [DOI] [PubMed] [Google Scholar]
- 33.Infante JR, Matsubayashi H, Sato N, et al. Peritumoral fibroblast SPARC expression and patient outcome with resectable pancreatic adenocarcinoma. J Clin Oncol. 2007;25:319–25. doi: 10.1200/JCO.2006.07.8824. [DOI] [PubMed] [Google Scholar]
- 34.Liu N, Furukawa T, Kobari M, Tsao MS. Comparative phenotypic studies of duct epithelial cell lines derived from normal human pancreas and pancreatic carcinoma. Am J Pathol. 1998;153:263–9. doi: 10.1016/S0002-9440(10)65567-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones S, Zhang X, Parsons DW, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–6. doi: 10.1126/science.1164368. Epub 2008 Sep 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berndt J, Kovacs P, Ruschke K, et al. Fatty acid synthase gene expression in human adipose tissue: association with obesity and type 2 diabetes. Diabetologia. 2007;50:1472–80. doi: 10.1007/s00125-007-0689-x. [DOI] [PubMed] [Google Scholar]
- 37.Claycombe KJ, Jones BH, Standridge MK, et al. Insulin increases fatty acid synthase gene transcription in human adipocytes. Am J Physiol. 1998;274:R1253–9. doi: 10.1152/ajpregu.1998.274.5.R1253. [DOI] [PubMed] [Google Scholar]
- 38.Szolkiewicz M, Nieweglowski T, Korczynska J, et al. Upregulation of fatty acid synthase gene expression in experimental chronic renal failure. Metabolism. 2002;51:1605–10. doi: 10.1053/meta.2002.36302. [DOI] [PubMed] [Google Scholar]
- 39.Korczynska J, Stelmanska E, Nogalska A, et al. Upregulation of lipogenic enzymes genes expression in white adipose tissue of rats with chronic renal failure is associated with higher level of sterol regulatory element binding protein-1. Metabolism. 2004;53:1060–5. doi: 10.1016/j.metabol.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 40.Ito M, Suzuki J, Tsujioka S, et al. Longitudinal analysis of murine steatohepatitis model induced by chronic exposure to high-fat diet. Hepatol Res. 2007;37:50–7. doi: 10.1111/j.1872-034X.2007.00008.x. [DOI] [PubMed] [Google Scholar]
- 41.Ota T, Takamura T, Kurita S, et al. Insulin resistance accelerates a dietary rat model of nonalcoholic steatohepatitis. Gastroenterology. 2007;132:282–93. doi: 10.1053/j.gastro.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 42.Sebastiani V, Visca P, Botti C, et al. Fatty acid synthase is a marker of increased risk of recurrence in endometrial carcinoma. Gynecol Oncol. 2004;92:101–5. doi: 10.1016/j.ygyno.2003.10.027. [DOI] [PubMed] [Google Scholar]
- 43.Wilentz RE, Witters LA, Pizer ES. Lipogenic enzymes fatty acid synthase and acetyl-coenzyme A carboxylase are coexpressed with sterol regulatory element binding protein and Ki-67 in fetal tissues. Pediatr Dev Pathol. 2000;3:525–31. doi: 10.1007/s100240010116. [DOI] [PubMed] [Google Scholar]