Abstract
Adherence to HIV medication regimens is a function of multiple dimensions including psychological functioning, social support, adherence self-efficacy and optimism regarding treatment. Active substance use can also negatively affect adherence. An understanding of the nature of the associations among the correlates of adherence can better inform the design of interventions to improve adherence. This study developed an exploratory path model of schedule adherence using data from a sample 130 African-American HIV-positive crack cocaine users on highly active antiretroviral therapy (ART). This model was based on the Transactional Model of Stress and Coping developed by Lazarus and Folkman. Following the theory, the effects of psychological distress on schedule adherence were mediated by patients’ relationship with their doctor and optimism towards antiretroviral treatment. Adherence was also associated with patients’ self-efficacy regarding their medical regimen which, in turn, was associated with their social support.
Introduction
Antiretroviral therapy (ART) is now the standard treatment for HIV-infected persons in the Western world (Gulick et al., 1998; Hogg et al., 1998; Palella et al., 1998). The advancement has had an impact on longevity of people living with HIV infection. The rates of AIDS-related morbidity and mortality have decreased, however to a lesser extent among injection drug users compared to other individuals with HIV infection (Porter et al., 2003; Wood et al., 2003). The aim of ART is to suppress replication of the virus to undetectable levels (<50 copies/ml) (Poppa et al., 2004). One prerequisite for optimal ART efficacy is how well those medications are taken, i.e. level of adherence to medication regimen. Rates of dose adherence (the percentage of prescribed medications actually taken) of at least 95% are needed for prolonged viral suppression, to prevent viral mutation, and to prevent the development of drug resistant viral strains (Paterson et al., 2000; Raboud et al., 2002; Poppa et al., 2004). In general, rates of dose adherence to ART vary between 26% and 85% (Kalichman et al., 1999; Gifford et al., 2000; Paterson et al., 2000; Kleeberger et al., 2001; Nieuwkerk et al., 2001; Golin et al., 2002; Martini et al., 2002; Arnsten et al., 2002; Simoni et al., 2002).
Studies show that illegal drug users are under-represented among eligible persons receiving ART (Anderson et al., 2000; Porter et al., 2003; Wood et al., 2003; Ware et al., 2005). These findings may reflect providers’ hesitance to prescribe antiretrovirals to drug users due to concerns about the potential for adverse drug interactions to occur. Another explanation is the assumption that drug users are less capable than others of adhering to complicated medication regimens (Clarke & Mulcahy, 2000; Ware et al., 2005). This assumption is supported by many studies indicating that adherence to ART is undermined by the use of illicit drugs, especially injection drug use and crack cocaine (Gordillo et al., 1999; Arnsten et al., 2002; Lucas et al., 2001; Stein et al., 2000; Power et al., 2003; Ingersoll, 2004; Sharpe et al., 2004). But there are also studies that show no significant associations between adherence and current illicit drug use (Catz et al., 2000; Stone et al., 2001). However, how, why, and under what circumstances drug use interferes with adherence is not yet well understood.
Adherence to ART is a complex psychosocial behaviour and is a function of several factors. Research has identified a number of psychosocial factors associated with suboptimal adherence to dose instructions. Psychiatric morbidities, such as depression and anxiety, can adversely affect adherence (Gordillo et al., 1999; Holzemer et al., 1999; Arnsten et al., 2002; Sternhell & Corr, 2002; van Servellen et al., 2002; Murphy et al., 2004). Furthermore, studies indicate that psychiatric morbidities may be more common and pronounced in drug users (te Vaarwerk & Gaal, 2001; Nnadi et al., 2002; Valente, 2003). The individual’s attitude, expressed as optimism or pessimism, with regard to their HIV infection and the efficacy of treatment is shown to be associated with adherence (Aversa & Kimberlain, 1996; Bartos & McDonald, 2000; Sternhell & Corr, 2002; Harzke et al., 2004; Nilsson Schönnesson et al., 2007). Several studies suggest that effective patient–physician relationships and communication may improve adherence to ART among patients with HIV (Murri et al., 2002; Wu et al., 2002). More specifically, it has been found that patient perception of being known ‘as a person’ i.e. the essence of patient-centredness (Balint, 1969), is a strong predictor of ART adherence (Bakken et al., 2000; Schneider et al., 2004; Beach et al., 2006).
On the other hand, lack of support from partners and family, friends and healthcare providers have been shown to correlate to suboptimal adherence to dose instructions (Mostashari et al., 1998; Bakken et al., 2000; Catz et al., 2000; Murphy et al., 2000; Safren et al., 2001; Roberts, 2002; Turner, 2002; Power et al., 2003; Murphy et al., 2004). It has been suggested (Welch, 2000) that support may be lower in those with a substance use problem, who, because of their illicit drug use, may not be able to maintain supportive relations. HIV-positive individuals may also experience negative relationships in the form of unsupportive behaviours such as insensitivity and disconnecting (Ingram et al., 1999). Among HIV infected individuals, perceived stigma from treatment providers has been found to be higher in drug users (Surlis & Hyde, 2001) and may be another negative factor influencing adherence to ART.
Patients’ perceived ability to follow a medication regimen, i.e. adherence self-efficacy, is an important factor in adherence. It has been associated with adherence in studies by Catz et al. (2000); Chesney et al. (2000); Gifford et al. (2000); Molassiotis et al. (2002); Godin et al. (2005); and Nilsson Schönnesson et al. (2006).
While some researchers have suggested that demographic variables may be proxies for other factors (Ferguson et al., 2002; Jensen-Fangel et al., 2002), adherence to antiretroviral medications has been shown to be lower in African-Americans (Muma et al., 1995; Kleeberger et al., 2001; Golin et al., 2002; Chander et al., 2006). Negative experiences with, and perceptions of, the health care system may be especially present in African-American drug users (Pach et al., 2003).
In addition to the associations between these factors and adherence, recent studies have examined the associations among the factors that affect adherence to ART. Mizuno et al. (2003) examined depression in a sample of HIV-positive injection drug users and found perceived social support and having a regular place for HIV medical care were significantly correlated with lower levels of depressive symptoms. Ingram et al. (1999) reported associations between positive social support and unsupportive social interactions with depression in a sample of HIV-positive persons. van Servellen et al. (1998) observed an inverse relationship between optimism regarding the future and anxiety and depression and an inverse association between social support and anxiety in a study of HIV-positive women. In a longitudinal study of low-income injection drug users, Knowlton et al. (2001) found lower levels of social support to be marginally predictive of depression. Godin et al. (2005) found a positive attitude towards medication was associated with patients’ satisfaction with their physician, social support, and being optimistic towards life. Self-efficacy with regard to their medication regimen was associated with social support and satisfaction with physician.
Optimal ART efficacy, i.e. undetectable viral load (HIV-1 RNA<50 copies/ml) (Poppa et al., 2004), requires not only dose adherence, but also schedule adherence (the percentage of medications taken on time) (Nieuwkerk et al., 2001; Nilsson Schönnesson et al., 2004). In contrast to dose adherence, there is no benchmark level of schedule adherence to achieve optimal ART efficacy. Some studies have found a strong correlation between dose and schedule adherence (Kalichman et al., 1999; Arnsten et al., 2002; Simoni et al., 2002), whereas others have not (Nieuwkerk et al., 2001; Stone et al., 2001; Murphy et al., 2004; Nilsson Schönnesson et al., 2004; Nilsson Schönnesson et al., 2006). Yet, studies of contributing factors to suboptimal schedule adherence are rare in the research literature. Whereas correlation between high scores on HIV-related posttraumatic stress symptoms (PTSD) and suboptimal schedule adherence was found in one study (Delahanty et al., 2004), PTSD symptoms predicted positive schedule adherence over time in another (Nilsson Schönnesson et al., 2006). In a longitudinal study, perceived pressures from medical staff to take HIV medications, life stress, ART health concerns, and a belief that ART prolongs one’s life predicted reduced schedule adherence over time (Nilsson Schönnesson et al., 2006).
Thus, for clinicians the issue of adherence in general, and in substance users in particular, is multifactorial in its nature. Several potential points of intervention for improving ART adherence among drug users may exist. Current knowledge could be strengthened by further consideration of the interrelationships among psychological distress, perceived social support, ART optimism, adherence self-efficacy and adherence within a sample of current drug users. The purpose of this study was to develop a multifactorial model of schedule adherence to ART using data from a sample of African–American crack cocaine users. The multifactorial model guided by the Transactional Model of Stress and Coping (Lazarus & Folkman, 1984) was constructed using path modelling.
This paper expands on the work presented by Harzke et al. (2004), who examined the relationships, within a sample of African-American crack cocaine users, between adherence and psychosocial and other measures suggested by the Transactional Model (Lazarus & Folkman, 1984). According to the theory, the stress induced by an event or situation is mediated by the individual’s appraisal of the stressor, by an appraisal of available resources and by coping efforts aimed at managing the stressor. This process of appraisal and coping efforts eventuates in adaptations or outcomes which may include specific health behaviours such as adherence to medication. In a multivariate regression analysis, adherence was found to be a function of the perceived efficacy of antiretroviral medications and simply forgetting to take medications.
Methods
Sample recruitment procedures
A sample of sexually active HIV positive African–American crack cocaine users was recruited by referral from community based HIV service agencies between March 1999 and July 2000 in Houston, Texas. Fliers announcing the study were posted at the agencies and agency personnel were asked to bring the fliers to the attention of their clientele. Once the study was underway and initial recruits were interviewed, participants referred others who they believed might be eligible for the study. Individuals interested in the study were asked to call the study’s data collection centre and respond to a brief screening questionnaire.
Initial screening was conducted by phone or in face-to-face interviews. Potential participants who met initial eligibility requirements were asked to complete a more in-depth screening questionnaire. These persons were also asked to bring medication bottles for all or some of the HIV-related medications they were taking at the time of the study. After giving verbal consent, these individuals were screened by a trained research assistant using a screening questionnaire and asked to show their medication bottles.
Individuals were deemed eligible for the study if they met the following criteria: self-identified as African–American, were at least 18 years of age, were HIV positive and had received treatment with HIV antiretroviral medications for a minimum of three months prior to screening, had used crack cocaine at least once in the 48 hours before screening, were willing to provide a urine sample to confirm recent drug use, had engaged in vaginal or anal sex at least once in the past 7 days, and were willing to sign an informed consent form.
One-hundred-and-thirty-seven (137) individuals were enrolled in the study. An additional 88 persons were screened but did not qualify. There were no significant differences between the qualified and disqualified groups with regard to age, gender, or self-perceived sexual orientation (heterosexual, bisexual, or homosexual).
Once eligible individuals gave their consent for the study, they were administered a multipart interview. Interviews were conducted in private at an office used for data collection. The data collection centre was not affiliated with any provider where participants were receiving medical care or social services. Participants were paid a gratuity of $20 for their time and any expenses they may have incurred travelling to the data collection centre. All research protocols and instruments were approved by committees for the protection of human subjects.
Measures
Participants completed the Elicitation of Compliance and Adherence Behaviors Questionnaire (ECAB). The ECAB is a compilation of other reliable surveys previously administered to drug users at risk for HIV infection (Simpson et al., 1993; Simpson et al., 1994; Camacho et al., 1995; Williams et al., 2000). The instrument was designed to collect sociodemographic data and data related to HIV risk behaviours, antiretroviral therapy, and social support, attitudes towards HIV and antiretroviral medications, and adherence to medication regimens. The ECAB was administered by a trained interviewer and responses were recorded using computer-assisted personal interview (CAPI) software, the Questionnaire Development System (Nova Research Company).
After completion of the ECAB, participants were administered the TCU Self-Rating Form (SRF). The SRF was developed for use in the Improving Drug Abuse Treatment for AIDS-Risk Reduction (DATAR) study (Knight et al., 1994). The SRF measures psychological functioning in several domains. The SRF was self-administered, again using CAPI software.
The following scales were developed from items in the ECAB and SRF with scores computed as the mean of non-missing items. Two measures of psychological distress were defined. Anxiety/Depressive symptoms consisted of eight items measuring symptoms of anxiety or depression: having trouble sleeping, feeling anxious or nervous, having trouble concentrating, feeling tense or keyed up, feeling sad or depressed, feeling tired or run down, worrying or brooding. Each item was scored on a five-point scale from 1=‘strongly disagree’ to 5=‘strongly agree’ (mean=3.1; sd=0.8; a=.86). While anxiety and depression are separate constructs of psychological functioning, they are closely related (Ingersoll, 2004) and so were combined here. HIV-related stress symptoms consisted of three items indicating whether a participant felt stressed since learning of their infection, were fearful of others finding out about their infection, or became concerned whenever someone learned of their infection. Each item was scored on a six-point scale from 1=‘disagree a lot’ to 6=‘agree a lot’ (mean=3.6; sd=1.6; a=.68).
Perceived social support was measured by three scales. Presence of social support consisted of two items measuring the extent participants could share their personal thoughts with someone else and extent they had someone who tried to understand them. Items were measured on a ten-point scale ranging from 1=‘rarely true’ to 10=‘usually true’ (mean=7.1; sd=3.2; a=.80). Social isolation consisted of three items, using the ten-point scale, that indicated participants’ spending time alone, not telling people about themselves, and not having much in common to talk about with other people (mean=5.1; sd=2.9; a=.83). Patient–doctor relationship consisted of six items asking if participants’ understood their doctor when talking about medications, if the doctor explained how to take medications, if they trusted their doctor, if their doctor understood how they felt, if their doctor spent enough time with them, and whether they saw the same doctor. Responses for each item were recorded using a 10-point scale ranging from 1=‘strongly disagree’ to 10=‘strongly agree’ (mean=8.5; sd=1.8; a=.84).
Adherence self-efficacy was computed from three items related to participants’ uncertainty in their ability to refill prescriptions, in knowing when to take their medicines, and how to take them. Items were measured using the ten-point ‘strongly disagree’, ‘strongly agree’ scale (mean=8.5; sd=2.2; a=.74) and were reversed scored so that higher scores indicated greater adherence self-efficacy.
ART optimism was formed from three items assessing whether participants felt more hopeful than two years before, felt better since taking their medications and felt that HIV and AIDS would become manageable conditions in next five years. It was measured using the ten-point ‘strongly disagree’, ‘strongly agree’ scale (mean=7.5; sd=2.2; a=.65).
For each of the antiretroviral medications they reported taking, participants were asked how often they had missed taking the medication at the proper time. The question was asked without reference to a specific length of time from the prescribed time or a specific time entire period before the interview. Possible responses ranged from 1=‘never’ to 5= ‘always’. Scores were reversed so that higher scores indicated better adherence, and schedule adherence was computed as the mean of the scores reported for each medication taken.
Analyses
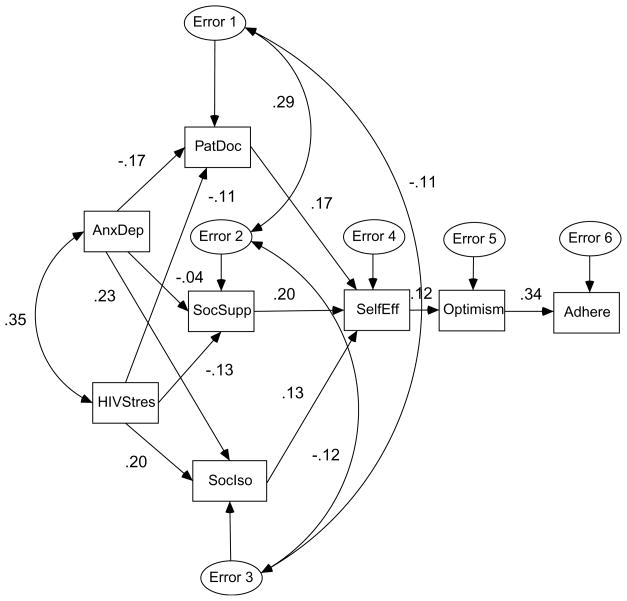
The sample for this study consisted of 130 individuals who completed both the ERCAB and SRF. Associations between the variables were assessed by analysing the path model presented in Figure 1. In this model, based on the transactional theory, adherence (Adhere) was hypothesized to be a function of ART optimism (Optimism). Optimism, in turn, was hypothesized to be a function of adherence self-efficacy (SelfEff). Adherence self-efficacy was hypothesized to be a function of the patient–doctor relationship (PatDoc), social support (SocSupp), and social isolation (SocIso). Each of the perceived social support measures was hypothesized to be a function of anxiety/depressive symptoms (AnxDep) and HIV-related stress symptoms (HIVStress). This model represents a situation in which stressors are mediated by levels of support, adherence self-efficacy and view of ART. The model also included covariances between anxiety/depressive symptoms and HIV-related stress symptoms and the error terms for each of the perceived social support variables.
Figure 1.
Full Path Model (standardized regression coefficients and correlation between AnxDep and HIVStres and error terms for support measures).
Using this full model, standardized regression coefficients and p values were computed for each path. Correlations between anxiety/depressive symptoms and HIV-related stress syndromes and between the error terms for each of the support measures were also computed. The coefficients and correlations are shown in Figure 1. Paths with a p value<.05 were retained and the resulting model analysed. Modification indices were also computed for each model. These gave the extent to which the model chi-square would be reduced by the inclusion of additional paths in the model. This process was repeated until only significant paths remained and no additional paths were suggested. Goodness of fit of the final model was assessed by model chi-square and the goodness of fit index (GFI). The GFI can range from 0 to 1 with 1 indicating a perfect fit. The significance of the change in chi-square given the change in the degrees of freedom was used to assess the improved fit of a reduced model. Descriptive analyses were conducted using SPSS (SPSS, Inc.) and the path models were analysed using AMOS (SmallWaters Corp.).
Results
The majority (74%) of participants were male, had not been married (55%), and relied on Medicaid to pay for their medications (55%) and to pay for their medical treatments (56%). Participants had an average age of 40.0 years (s.d.=6.8 years) and 11.7 years of education (s.d.=2.4 years). Ten participants (8%) were employed either part time or full time. Self-perceived sexual orientation was evenly divided across the sample (36% heterosexual, 33% homosexual, and 31% bisexual). One quarter (25%) considered themselves homeless. Ten (8%) participants had injected drugs in the previous 30 days, and one quarter (25%) had received drug treatment in the previous six months. Seventeen participants (13%) stated they had never missed taking their medication at the proper time. Schedule adherence was not significantly associated with any of these characteristics.
Chi-square for the full model was 60.8 with 13 degrees of freedom (p<.001). The GFI for this model was .91. This model accounted for 5% of the variance in patient-doctor relationship, 2% in social support, 12% in social isolation, 2% in ART optimism, and 12% in schedule adherence. Two iterations of the model were run before a model with no insignificant links and no additional links based on the modification indices was derived. The second iteration of the model (not shown) had a chi-square of 21.9 with 17 degrees of freedom. The GFI for this model was .96.
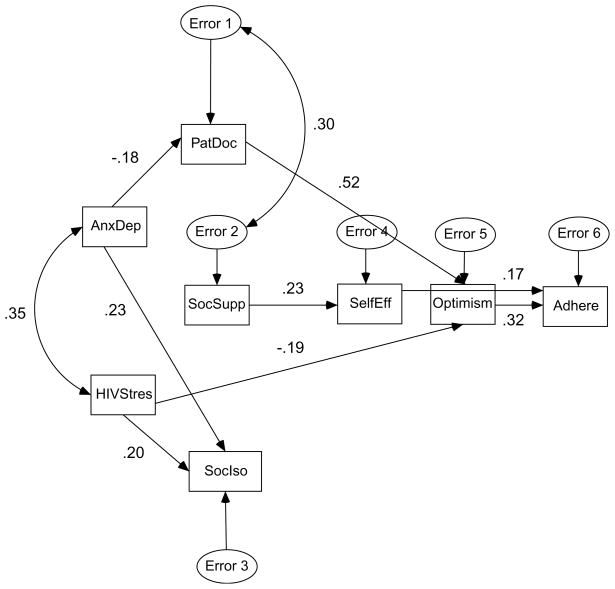
The final path model is shown in Figure 2. Again, the figure presents the standardized regression coefficients for each path and the correlation between anxiety/depressive symptoms and HIV-related stress symptoms and between the error terms for the patient–doctor relationship and presence of social support. For the final model, chi-square was 17.4 with 18 degrees of freedom (p=.50), and the GFI was .97, indicating the model fit well. Based on changes in chi-square and degrees of freedom, the final model provided a significantly improved fit in comparison to the two earlier models. Schedule adherence was positively associated with ART optimism and adherence self-efficacy. ART optimism was positively associated with patient–doctor relationship and inversely associated with HIV-related stress symptoms. Adherence self-efficacy was positively associated with presence of social support. Patient–doctor relationship was inversely associated with anxiety/depressive symptoms. Social isolation was positively associated with anxiety/depressive symptoms and with HIV-related stress symptoms. The correlation between anxiety/depressive symptoms and HIV-related stress symptoms was .35. The correlation between the error terms for the patient–doctor relationship and presence of social support was .30. The final model accounted for 3% of the variance in patient–doctor relationship, 32% of the variance in ART optimism, 5% of the variance in adherence self-efficacy, 12% of the variance in social isolation, and 13% of the variance in schedule adherence.
Figure 2.
Final Path Model (standardized regression coefficients and correlation between AnxDep and HIVStres).
Discussion
This study was undertaken to examine the correlates of schedule adherence to ART. A path analysis approach provided a means for assessing a theoretical model in which variables serve as independent variables in one context and as dependent variables in another. Study variables were taken from the Transactional Model of Stress and Coping and included measures of psychological distress, social support, adherence self-efficacy and ART optimism. The sample for this study consisted of African-American current crack cocaine users, a population potentially especially at risk of suboptimal adherence. Thirteen percent of participants reported always taking each of their medications at the proper time.
Consistent with other studies, the paths in the final model indicate that schedule adherence may be improved by increasing patient’s optimism about their medication regimen (Godin et al., 2005; Nilsson Schönnesson et al., 2007) and their confidence in carrying it out, i.e. adherence self-efficacy (Catz et al., 2000; Gifford et al., 2000; Molassiotis et al., 2002; Godin et al., 2005). Our data also demonstrate that it takes a good patient–doctor relationship and a satisfying social network, respectively, in order to do so. When the patient perceives the doctor as being trustworthy/reliable and acknowledging the patient as a whole person, i.e. being patient-centred, (s)he infuses hope and optimism in the patient about his/her health and ultimately being adherent to medication. This finding suggests that efforts to improve adherence should not only be focused on the patient as such but also ‘to improve upon the health professional’s ability to make a patient feel known “as a person”’ (Beach et al., 2006).
Our finding supports those of Godin et al. (2005) that the presence of social support is important for patient’s adherence self-efficacy. It suggests that support and encouragement from significant others are vital to persons with HIV infection to be able to sustain their confidence in themselves to be adherent. To improve the patient’s sense of adherence self-efficacy, group interventions could be a strategy to promote psychological/emotional social support.
While the quality of the patient–doctor relationship is lowered by increased level of anxiety/depressive symptoms, presence of social support was not associated with either measure of psychological distress. The link between anxiety/depressive symptoms and lesser quality of patient–doctor relationship may be a reflection of patients’ perception of their doctor as being insensitive to their psychological distress. The correlation between anxiety/depressive symptoms and HIV-related stress symptoms suggests that doctors’ (or other health care personnel for that matter) should address whether anxiety/depressive symptoms are of a more general character or associated with HIV-related stress symptoms. The link between HIV-related stress symptoms and ART optimism suggests that addressing such stress symptoms may be an avenue that doctors may consider to improve patient’s schedule adherence.
Although the scientific literature has emphasized the role played by psychological distress in adherence, the results of this study suggest that it is through the patient’s relationship with their doctor and their optimism regarding their treatment that anxiety/depressive symptoms influence schedule adherence. The effect of HIV-related stress symptoms on schedule adherence is indirect through its link with ART optimism.
While not associated with any other measure, social isolation was a function of anxiety/depressive symptoms and HIV-related stress symptoms. Isolation is a way of coping with stressors, and minimizing the sources of stress may have beneficial effects beyond the factors studied here.
There were limitations to the study. The study was conducted using a crossectional sample, so we can only describe associations among the factors studied, not causal relationships. Sample size may also have been a limiting factor in determining the significance of paths in the model. Adherence and each of the other variables studied were measured based on patients’ self reports. The accuracy of these reports may have been affected by recall bias (the tendency to remember certain events or experiences) and the desire to give socially acceptable answers. As the data collection centre was not affiliated with medical care or social service agencies; this may have decreased the occurrence of socially desirable answers. This study focused on schedule adherence and did not include other types of adherence such as dose or dietary adherence. As noted, the measure of schedule adherence employed did not consider the length of time that actually passed before the medication was taken and covered adherence during the whole time before a participant’s interview. Thus the actual time frames involved in participants’ reported adherence were not similar across participants.
The generalizablility of these results will be limited by the fact that the sample consisted of African-Americans in one city only, recruited non-randomly. The present study did not include physical correlates of adherence such as HIV viral load, CD4 cell counts, and medication side effects. It is possible that measures of anxiety and depressive symptoms based on physical problems (feeling tired or run down, having trouble sleeping) might overlap with symptoms of HIV disease and/or medication side effects. Sociodemographic variables were not included in the model. As noted, there was no direct association between sample characteristics studied and adherence. However, paths between these characteristics and the other variables studied should be considered in future research. For example, depression has been shown to vary by gender in HIV-positive patients (Turner et al., 2003).
Finally, the results presented here were not intended to be a definitive model of schedule adherence to antiretroviral therapy medication but rather one possible representation of adherence. Nor was this a test of the full TMSC theory. For example, the model considered here did not include measures of coping efforts as explicit coping items were not included in the original study instrument. However, these results suggest that schedule adherence, like dose adherence, to ART in drug users is a multidimensional process. Several researchers have called for consideration of drug and mental health treatment along with HIV treatment (Knowlton et al., 2001; Lucas et al., 2001; Nnadi et al., 2002; Turner et al., 2003). Others have suggested screening by clinicians and physicians for depression and psychosocial variables (Valente, 2003; Ingersoll, 2004). Clinicians will need technical knowledge and skill with behaviour change to promote adherence in HIV-positive drug users (Cohn, 2002). Other potential interventions may include peer counselling and social support (Welch, 2000). These efforts will be helped by knowledge of the mechanisms by which the correlates of adherence may operate. Results presented here suggest that schedule adherence may be increased by improving adherence self-efficacy and treatment optimism and by alleviating psychological distress. But they also suggest that factors directly linked to adherence may in turn be influenced by other factors. Our study suggests that the quality of the patient–doctor relationship plays an indirect, but important role in schedule adherence. Thus, educational interventions of doctors to promote patient-centred medicine should be encouraged and evaluated.
In conclusion, there is an increasing realization that achieving effective adherence to ART in drug users may require the coordination of services among various actors addressing different correlates of adherence. The effects of these correlates on adherence may not always be direct. For clinicians, improving adherence may involve a process of addressing these intermediate variables. Understanding the nature of these associations can better inform the development of potential adherence interventions.
Acknowledgments
The authors wish to acknowledge the helpful comments of Dr. Michael Ross of the University of Texas School of Public Health in the development of this manuscript. This research was supported by a grant from the National Institute on Drug Abuse, R01 DAO14485. The opinions expressed herein are solely those of the authors.
References
- Andersen R, Bozzette S, Shapiro M, St Clair P, Morton S, Crystal S, Goldman D, Wenger N, Gifford A, Leibowitz A, Asch S, Berry S, Nakazono T, Heslin K, Cunningham W. Access of vulnerable groups to antiretroviral therapy among persons in care for HIV disease in the United States. Health Services Research. 2000;35(2):389–416. [PMC free article] [PubMed] [Google Scholar]
- Aversa SL, Kimberlain C. Psychological aspects of antiretroviral medication use among HIV patients. Patient Education and Counseling. 1996;29:207–19. doi: 10.1016/0738-3991(96)00910-x. [DOI] [PubMed] [Google Scholar]
- Arnsten JH, Demas PA, Grant RW, Gourevitch MN, Farzadegan H, Howard AA, Schoenbaum EE. Impact of active drug use on antiretroviral therapy adherence and viral suppression in HIV-infected drug users. Journal of General Internal Medicine. 2002;17(5):377–81. doi: 10.1046/j.1525-1497.2002.10644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakken S, Holzemer WL, Brown MA, Powell-Cope GM, Turner JG, Inouye J, Nokes KM, Corless IB. Relationships between perception of engagement with health care provider and demographic characteristics, health status, and adherence to therapeutic regimen in persons with HIV/AIDS. AIDS Patient Care and STDs. 2000;14(4):189–97. doi: 10.1089/108729100317795. [DOI] [PubMed] [Google Scholar]
- Balint E. The possibilities of patient-centred medicine. Journal of the Royal College of General Practitioners. 1969;17(82):269–76. [PMC free article] [PubMed] [Google Scholar]
- Bartos M, McDonald K. HIV as identity, experience or career. AIDS Care. 2000;12(3):299–306. doi: 10.1080/09540120050042954. [DOI] [PubMed] [Google Scholar]
- Beach MC, Keruly J, Moore RD. Is the quality of the patient-provider relationship associated with better adherence and health outcomes for patients with HIV? Journal of General Internal Medicine. 2006;21(6):661–65. doi: 10.1111/j.1525-1497.2006.00399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho M, Williams M, Vogtsberger K, Simpson D. Cognitive readiness of drug injectors to reduce AIDS risks. American Journal of Addictions. 1995;4(1):49–55. [Google Scholar]
- Catz SL, Kelly JA, Bogart LM, Benotsch EG, McAuliffe TL. Patterns, correlates, and barriers to medication adherence among persons prescribed new treatments for HIV disease. Health Psychology. 2000;19(2):124–33. [PubMed] [Google Scholar]
- Chander G, Lau B, Moore RD. Hazardous alcohol use: A risk factor for non-adherence and lack of suppression in HIV infection. Journal of Acquired Immune Deficiency Syndromes. 2006;43(4):411–17. doi: 10.1097/01.qai.0000243121.44659.a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesney MA, Ickovics JR, Chambers DB, Gifford AL, Neidig J, Zwickl B, Wu AW. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: The AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG) AIDS Care. 2000;12(3):255–66. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- Clarke SM, Mulcahy FM. Antiretroviral therapy for drug users. International Journal of STD & AIDS. 2000;11(10):627–31. doi: 10.1258/0956462001914913. [DOI] [PubMed] [Google Scholar]
- Cohn JA. HIV-1 infection in injection drug users. Infectious Disease Clinic of North America. 2002;16(3):745–70. doi: 10.1016/s0891-5520(02)00012-0. [DOI] [PubMed] [Google Scholar]
- Delahanty DL, Bogart LM, Figler JL. Posttraumatic stress disorder symptoms, salivary cortisol, medication adherence, and CD4 levels in HIV-positive individuals. AIDS Care. 2004;16(2):247–60. doi: 10.1080/09540120410001641084. [DOI] [PubMed] [Google Scholar]
- Ferguson TF, Stewart KE, Funkhouser E, Tolson J, Westfall AO, Saag MS. Patient-perceived barriers to antiretroviral adherence: associations with race. AIDS Care. 2002;14(5):607–17. doi: 10.1080/0954012021000005434. [DOI] [PubMed] [Google Scholar]
- Gifford AL, Bormann JE, Shively MJ, Wright BC, Richman DD, Bozzette SA. Predictors of self-reported adherence and plasma HIV concentrations in patients on multidrug antiretroviral regimens. Journal of Acquired Immune Deficiency Syndromes. 2000;23(5):386–95. doi: 10.1097/00126334-200004150-00005. [DOI] [PubMed] [Google Scholar]
- Godin G, Coté J, Naccache H, Lambert D, Trottier S. Prediction of adherence to antiretroviral therapy: A one-year longitudinal study. AIDS Care. 2005;17(4):493–504. doi: 10.1080/09540120412331291715. [DOI] [PubMed] [Google Scholar]
- Golin CE, Liu H, Hays RD, Miller LG, Beck K, Ickovics J, Kaplan AH, Wenger NS. A prospective study of predictors of adherence to combination antiretroviral medication. Journal of General Internal Medicine. 2002;17(10):756–65. doi: 10.1046/j.1525-1497.2002.11214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordillo V, del Amo J, Soriano V, Gonzalez-Lahoz J. Sociodemographic and psychological variables influencing adherence to antiretroviral therapy. AIDS. 1999;13(13):1763–69. doi: 10.1097/00002030-199909100-00021. [DOI] [PubMed] [Google Scholar]
- Gulick RM, Mellors JW, Havlir D, Eron J, Gonzalez C, McMahon D, Jonas L, Meibohm A, Holder D, Schleif WA, Condra JH, Emini EA, Isaacs R, Chodakewitz JA, Richman DD. Simultaneous vs sequential initiation of therapy with indinavir, zidovudine, and lamivudine for HIV-1 infection: 100 week follow-up. Journal of the American Medical Association. 1998;280(1):35–41. doi: 10.1001/jama.280.1.35. [DOI] [PubMed] [Google Scholar]
- Harzke AJ, Williams ML, Nilsson-Schönnesson L, Ross MW, Keel KB. Psychosocial factors associated with adherence to antiretroviral medications in a sample of HIV-positive African American drug users. AIDS Care. 2004;16(4):458–70. doi: 10.1080/09540120410001683394. [DOI] [PubMed] [Google Scholar]
- Hogg RS, Heath K, Yip B, Craib KJP, O’Shaughnessy MV, Schecter MT, Montaner JSG. Improved survival among hiv-infected individuals following initiation of antiretroviral therapy. Journal of the American Medical Association. 1998;279(6):450–54. doi: 10.1001/jama.279.6.450. [DOI] [PubMed] [Google Scholar]
- Holzemer WL, Corless IB, Nokes KM, Turner JG, Brown MA, Powell-Cope GM, Inouye J, Henry SB, Nicholas PK, Portillo CJ. Predictors of self-reported adherence in persons living with HIV disease. AIDS Patient Care and STDs. 1999;13(3):185–97. doi: 10.1089/apc.1999.13.185. [DOI] [PubMed] [Google Scholar]
- Ingersoll K. The impact of psychiatric symptoms, drug use, and medication regimen on non-adherence to HIV treatment. AIDS Care. 2004;16(2):199–211. doi: 10.1080/09540120410001641048. [DOI] [PubMed] [Google Scholar]
- Ingram KM, Jones DA, Fass RJ, Neidig JL, Song YS. Social support and unsupportive social interactions: their association with depression among people living with HIV. AIDS Care. 1999;11(3):313–29. doi: 10.1080/09540129947947. [DOI] [PubMed] [Google Scholar]
- Jensen-Fangel S, Pedersen L, Pedersen C, Larsen CS, Tauris P, Moller A, Sorensen HT, Obel N. The effect of race/ethnicity on the outcome of highly active antiretroviral therapy for human immunodeficiency virus type 1–infected patients. Clinical Infectious Diseases. 2002;35(12):1541–48. doi: 10.1086/344769. [DOI] [PubMed] [Google Scholar]
- Kalichman S, Ramachandran B, Catz S. Adherence to combination antiretroviral therapies in HIV patients of low health literacy. Journal of General Internal Medicine. 1999;14(5):267–73. doi: 10.1046/j.1525-1497.1999.00334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleeberger C, Phair J, Strathdee S, Detels R, Kingsley L, Jacobson L. Determinants of heterogeneous adherence to HIV-antiretroviral therapies in the Multicenter AIDS Cohort Study. Journal of Acquired Immune Deficiency Syndromes. 2001;26(1):82–92. doi: 10.1097/00126334-200101010-00012. [DOI] [PubMed] [Google Scholar]
- Knight K, Holcom M, Simpson D. TCU Psychosocial Functioning and Motivation Scales: Manual of Psychometric Properties. Fort Worth, TX: Institute of Behavioral Research at Texas Christian University; 1994. [Google Scholar]
- Knowlton AR, Latkin CA, Schroeder JR, Hoover DR, Ensminger M, Celentano DD. Longitudinal predictors of depressive symptoms among low income injection drug users. AIDS Care. 2001;13(5):549–59. doi: 10.1080/09540120120063197. [DOI] [PubMed] [Google Scholar]
- Lazarus R, Folkman S. Stress, Appraisal, and Coping. New York, NY: Springer; 1984. [Google Scholar]
- Lucas GM, Cheever LW, Chaisson RE, Moore RD. Detrimental effects of continued illicit drug use on the treatment of HIV-1 infection. Journal of Acquired Immune Deficiency Syndromes. 2001;27(3):251–59. doi: 10.1097/00126334-200107010-00006. [DOI] [PubMed] [Google Scholar]
- Martini M, D’Elia S, Paoletti F, Cargnel A, Adriani B, Carosi G, Mazzotta F, Di Pietro M, Filippini P, Nasta P, Cipriani S, Parazzini F, Agnoletto V. Adherence to HIV treatment: Results from a 1-year follow-up study. HIV Medicine. 2002;3(1):62–64. doi: 10.1046/j.1464-2662.2001.00092.x. [DOI] [PubMed] [Google Scholar]
- Mizuno Y, Purcell DW, Dawson-Rose C, Parsons JT Team, SUDIS. Correlates of depressive symptoms among HIV-positive injection drug users: The role of social support. AIDS Care. 2003;15(5):689–98. doi: 10.1080/09540120310001595177. [DOI] [PubMed] [Google Scholar]
- Molassiotis A, Nahas-Lopez V, Chung WY, Lam SW, Li CK, Lau TF. Factors associated with adherence to antiretroviral medication in HIV-infected patients. International Journal of STD & AIDS. 2002;13(5):301–10. doi: 10.1258/0956462021925117. [DOI] [PubMed] [Google Scholar]
- Mostashari F, Riley E, Selwyn PA, Altice FL. Acceptance and adherence with antiretroviral therapy among HIV-infected women in a correctional facility. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology. 1998;18(4):341–48. doi: 10.1097/00042560-199808010-00005. [DOI] [PubMed] [Google Scholar]
- Muma R, Ross M, Parcel G, Pollard R. Zidovudine adherence among individuals with HIV infection. AIDS Care. 1995;7(4):439–48. doi: 10.1080/09540129550126399. [DOI] [PubMed] [Google Scholar]
- Murphy DA, Roberts KJ, Martin DJ, Marelich W, Hoffman D. Barriers to antiretroviral adherence among HIV-infected adults. AIDS Patient Care and STDs. 2000;14:47–58. doi: 10.1089/108729100318127. [DOI] [PubMed] [Google Scholar]
- Murphy DA, Marelich WD, Hoffman D, Steers WN. Predictors of antiretroviral adherence. AIDS Care. 2004;16(4):471–84. doi: 10.1080/09540120410001683402. [DOI] [PubMed] [Google Scholar]
- Murri R, Antinori A, Ammassari A, Nappa S, Orofino G, Abrescia N, Mussini C, Monforte AD, Wu AW for the AdICoNA Study Group. Physician estimates of adherence and the patient-physician relationship as a setting to improve adherence to antiretroviral therapy. Journal of Acquired Immune Deficiency Syndromes. 2002;31(Suppl 3):S158–S162. doi: 10.1097/00126334-200212153-00015. [DOI] [PubMed] [Google Scholar]
- Nieuwkerk PT, Sprangers MA, Burger DM, Hoetelmans RM, Hugen PW, Danner SA, van Der Ende ME, Schneider MM, Schrey G, Meenhorst PL, Sprenger HG, Kauffmann RH, Jambroes M, Chesney MA, de Wolf F, Lange JM Project, ATHENA. Limited patient adherence to highly active antiretroviral therapy for HIV-1 infection in an observational cohort study. Archives of Internal Medicine. 2001;161(16):1962–68. doi: 10.1001/archinte.161.16.1962. [DOI] [PubMed] [Google Scholar]
- Nilsson Schönnesson L, Ross MW, Williams M. The HIV medication self-reported non-adherence reasons (SNAR) index and its underlying psychological dimensions. AIDS and Behavior. 2004;8(3):293–301. doi: 10.1023/B:AIBE.0000044076.98833.64. [DOI] [PubMed] [Google Scholar]
- Nilsson Schönnesson L, Diamond PM, Ross MW, Williams M, Bratt G. Baseline predictors of three types of antiretroviral therapy (ART) adherence: A 2-year follow-up. AIDS Care. 2006;18(3):246–53. doi: 10.1080/09540120500456631. [DOI] [PubMed] [Google Scholar]
- Nilsson Schönnesson L, Williams M, Ross MW, Diamond PM, Keel B. Three types of adherence to HIV antiretroviral therapy and their associations with AIDS diagnosis, medication side effects, beliefs about antiretroviral therapy, and belief about HIV disease. International Journal of STD & AIDS. 2007;18:369–373. doi: 10.1258/095646207781024757. [DOI] [PubMed] [Google Scholar]
- Nnadi CU, Better W, Tate K, Herning RI, Cadet JL. Contribution of substance abuse and HIV infection to psychiatric distress in an inner-city African-American population. Journal of the National Medical Association. 2002;94(5):336–43. [PMC free article] [PubMed] [Google Scholar]
- Pach A, Cerbone FG, Gerstein DR. A qualitative investigation of antiretroviral therapy among injection drug users. AIDS and Behavior. 2003;7(1):87–100. doi: 10.1023/a:1022517608578. [DOI] [PubMed] [Google Scholar]
- Palella FJ, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. New Eng-land Journal of Medicine. 1998;338(13):853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- Paterson DL, Swindells S, Mohr J, Brester M, Vergis EN, Squier C, Wagener MM, Singh N. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Annals of Internal Medicine. 2000;133(1):21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- Poppa A, Davidson D, Deutsch J, Godfrey D, Fisher M, Head S, Horne R, Sherr L. British HIV Association (BHIVA)/British Association for sexual health and HIV BASHH guidelines on provision of adherence support to individuals receiving antiretroviral therapy (2003) HIV Medicine. 2004;5(s2):46–60. doi: 10.1111/j.1468-1293.2004.00215.x. [DOI] [PubMed] [Google Scholar]
- Porter K, Babiker A, Bhaskaran K, Darbyshire J, Pezzotti P, Walker AS. Determinants of survival following HIV-1 seroconversion after the introduction of HAART. Lancet. 2003;362(9392):1267–74. doi: 10.1016/s0140-6736(03)14570-9. [DOI] [PubMed] [Google Scholar]
- Power R, Koopman C, Volk J, Israelski DM, Stone L, Chesney MA, Spiegel D. Social support, substance Use, and denial in relationship to antiretroviral treatment adherence among HIV-infected persons. AIDS Patient Care and STDs. 2003;17(5):245–52. doi: 10.1089/108729103321655890. [DOI] [PubMed] [Google Scholar]
- Raboud JM, Harris M, Rae S, Montaner JSG. Impact of adherence on duration of virological suppression among patients receiving combination antiretroviral therapy. HIV Medicine. 2002;3(2):118–24. doi: 10.1046/j.1468-1293.2002.00109.x. [DOI] [PubMed] [Google Scholar]
- Roberts KJ. Physician-patient relationships, patient satisfaction, and antiretroviral medication Adherence among HIV-infected adults attending a public health clinic. AIDS Patient Care and STDs. 2002;16(1):43–50. doi: 10.1089/108729102753429398. [DOI] [PubMed] [Google Scholar]
- Safren SA, Otto MW, Worth JL, Salomon E, Johnson W, Mayer K, Boswell S. Two strategies to increase adherence to HIV antiretroviral medication: life-steps and medication monitoring. Behavior Research and Therapy. 2001;39(10):1151–62. doi: 10.1016/s0005-7967(00)00091-7. [DOI] [PubMed] [Google Scholar]
- Schneider J, Kaplan SH, Greenfield S, Li W, Wilson IB. Better physician-patient relationships are associated with higher reported adherence to antiretroviral therapy in patients with HIV infection. Journal of General Internal Medicine. 2004;19(11):1096–103. doi: 10.1111/j.1525-1497.2004.30418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe TT, Lee LM, Makashima AK, Elam-Evans LD, Fleming PL. Crack cocaine use and adherence to antiretroviral treatment among HIV-infected black women. Journal of Community Health. 2004;29:117–27. doi: 10.1023/b:johe.0000016716.99847.9b. [DOI] [PubMed] [Google Scholar]
- Simoni JM, Frick PA, Lockhart D, Liebovitz D. Mediators of social support and antiretroviral adherence among an indigent population in New York City. AIDS Patient Care and STDs. 2002;16:431–29. doi: 10.1089/108729102760330272. [DOI] [PubMed] [Google Scholar]
- Simpson D, Knight K, Ray S. Psychosocial correlates of AIDS-risk drug use and sexual behaviors. AIDS Education and Prevention. 1993;5(2):121–30. [PubMed] [Google Scholar]
- Simpson D, Camacho L, Vogtsberger K, Williams M, Stephens R, Jones A, Watsonet D. Reducing AIDS risks through community outreach for drug injectors. Psychology of Addictive Behaviors. 1994;8(2):86–101. [Google Scholar]
- Stein MD, Rich JD, Maksad J, Chen MH, Hu P, Sobota M, Clarke J. Adherence to antiretroviral therapy among HIV-infected methadone patients: Effect of ongoing illicit drug use. American Journal of Drug and Alcohol Abuse. 2000;26(2):195–205. doi: 10.1081/ada-100100600. [DOI] [PubMed] [Google Scholar]
- Sternhell PS, Corr MJ. Psychiatric morbidity and adherence to antiretroviral medication in patients with HIV/AIDS. Australian and New Zealand Journal of Psychiatry. 2002;36(4):528–33. doi: 10.1046/j.1440-1614.2002.00999.x. [DOI] [PubMed] [Google Scholar]
- Stone VE, Hogam JW, Schuman P, Rompalo AM, Howard AA, Korkontzelou C, Smith DK. Antiretroviral regimen complexity, self-reported adherence, and HIV-patients’ understanding of their regimens: survey of women in the HER study. Journal of Acquired Immune Deficiency Syndrome. 2001;28(2):124–31. doi: 10.1097/00042560-200110010-00003. [DOI] [PubMed] [Google Scholar]
- Surlis S, Hyde A. HIV-positive patients’ experiences of stigma during hospitalization. Journal of the Association of Nurses in AIDS Care. 2001;12(6):68–77. doi: 10.1016/S1055-3290(06)60185-4. [DOI] [PubMed] [Google Scholar]
- Turner BJ. Adherence to antiretroviral therapy by human immunodeficiency virus-infected patients. Journal of Infectious Diseases. 2002;185:S143–151. doi: 10.1086/340197. [DOI] [PubMed] [Google Scholar]
- Turner BJ, Laine C, Cosler L, Hauck WW. Relationship of gender, depression, and health care delivery with antiretroviral adherence in HIV-infected drug users. Journal of General Internal Medicine. 2003;18:248–57. doi: 10.1046/j.1525-1497.2003.20122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Vaarwerk MJ, Gaal EA. Psychological distress and quality of life in drug-using and non-drug-using HIV-infected women. European Journal of Public Health. 2001;11(1):109–15. doi: 10.1093/eurpub/11.1.109. [DOI] [PubMed] [Google Scholar]
- Valente SM. Depression and HIV disease. Journal of the Association of Nurses in AIDS Care. 2003;14(2):41–51. doi: 10.1177/1055329002250993. [DOI] [PubMed] [Google Scholar]
- van Servellen G, Sarna L, Nyamathi A, Padilla G, Brecht ML, Jablonski KJ. Emotional distress in women with symptomatic HIV disease. Issues in Mental Health Nursing. 1998;19(2):173–88. doi: 10.1080/016128498249150. [DOI] [PubMed] [Google Scholar]
- van Servellen G, Chang B, Garcia L, Lombardi E. Individual and system level factors associated with treatment nonadherence in human immunodeficiency virus-infected men and women. AIDS Patient Care and STDs. 2002;16(6):269–81. doi: 10.1089/10872910260066705. [DOI] [PubMed] [Google Scholar]
- Ware NC, Wyatt MA, Tugenberg T. Adherence, stereotyping and unequal HIV treatment for active users of illegal drugs. Social Science & Medicine. 2005;61(3):565–76. doi: 10.1016/j.socscimed.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Welch KJ. Correlates of alcohol and/or drug use among HIV-infected individuals. AIDS Patient Care and STDs. 2000;14(6):317–23. doi: 10.1089/10872910050046340. [DOI] [PubMed] [Google Scholar]
- Williams M, Bowen A, Ross M, Freeman R, Elwood W. Perceived compliance with AZT dosing among a sample of African-American drug users. International Journal of STD & AIDS. 2000;11(1):57–63. doi: 10.1258/0956462001914797. [DOI] [PubMed] [Google Scholar]
- Wood E, Montaner JS, Yip B, Tyndall MW, Schechter MT, O’Shaughnessy MV, Hogg RS. Adherence and plasma HIV RNA responses to highly active antiretroviral therapy among HIV-1 infected injection drug users. Canadian Medical Association Journal. 2003;169:656–61. [PMC free article] [PubMed] [Google Scholar]
- Wu AW, Ammassari A, Antinori A. Adherence to antiretroviral therapy: where are we, and where do we go from here? Journal of Acquired Immune Deficiency Syndromes. 2002;31(Suppl 3):95–97. doi: 10.1097/00126334-200212153-00001. [DOI] [PubMed] [Google Scholar]