Abstract
Sepsis refers to a systemic inflammatory response syndrome resulting from a microbial infection. The inflammatory response is partly mediated by innate immune cells (such as macrophages, monocytes and neutrophils), which not only ingest and eliminate invading pathogens, but also initiate an inflammatory response by producing early (e.g., TNF and IFN-gamma) and late (e.g., HMGB1) proinflammatory cytokines. Here we briefly review emerging evidence that support extracellular HMGB1 as a late mediator of experimental sepsis, and discuss therapeutic potential of several HMGB1-inhibiting agents (including neutralizing antibodies and steroid-like tanshinones) in experimental sepsis.
Keywords: innate immune cells, phagocytes, inflammation, cytokines, sepsis, antibodies, HMGB1, tanshinones
INTRODUCTION
In response to microbial infection or injury, the host’s innate immune system mounts an immediate biological response - termed “inflammation” (“set on fire”, in Greek) – to remove invading pathogens and to heal the wound (1). Upon effective pathogen elimination and tissue repair, inflammation normally resolves to restore immunologic homeostasis (2). Otherwise, exogenous pathogens or endogenous proinflammatory mediators can leak into the blood stream, triggering a systemic inflammatory response that may lead to sepsis (1) (Fig. 1). Sepsis refers to a systemic inflammatory response syndrome resulting from a microbial infection. As a continuum of increasing clinical severity, sepsis can progress into “severe sepsis” or “septic shock” (3). Here we briefly review the prevailing theories of sepsis as an uncontrolled systemic inflammatory response, and discuss potential therapeutic agents that target a late mediator of experimental sepsis.
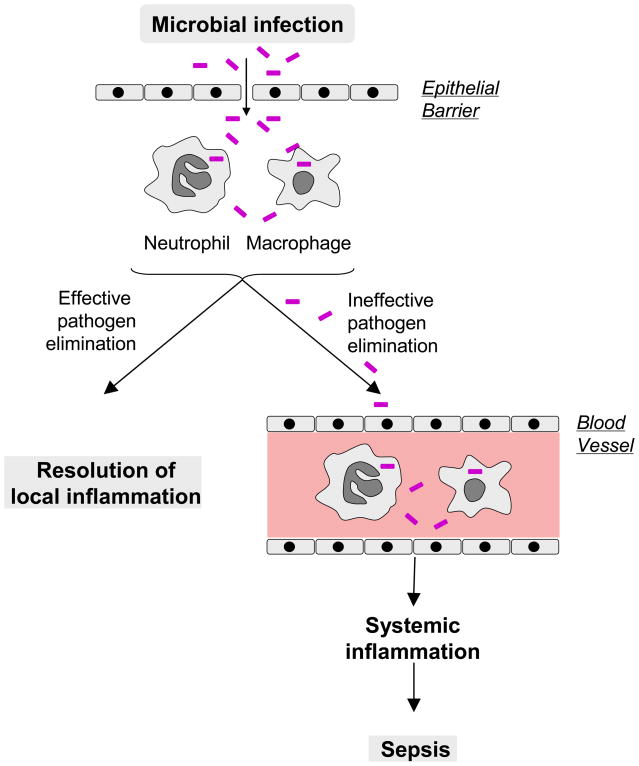
Fig. 1. A microbial infection can trigger a local or systemic inflammatory response.
Upon disruption of epithelial barrier, microbial pathogens invade and elicit an innate immune response at infection site. If invading pathogens are effectively ingested and eliminated by neutrophils and macrophages, local inflammation resolves normally to regain immunological homeostasis. Otherwise, invading pathogens can leak into the blood stream, and trigger a potentially injurious systemic inflammatory response that could lead to sepsis.
INNATE IMMUNE RESPONSE AGAISNT MICROBIAL INFECTION
The innate immune cells (such as macrophages, monocytes and neutrophils) are responsible not only for eliminating invading pathogens, but also for initiating an inflammatory response (1).
Elimination of invading pathogens
Monocytes and neutrophils continuously patrol the body to search for invading pathogens or damaged tissues, and infiltrate into infected/injured tissues upon detecting exogenous microbial products or endogenous chemotactic factors (4). Neutrophils are usually the first to arrive at the infection site, and kill more invading bacteria than other phagocytes (Fig. 1) (5). After engulfing and killing bacteria, however, neutrophils exhaust intracellular enzymes, and subsequently undergo apoptotic cell death. In contrast, monocytes can differentiate into tissue-specific resident macrophages (such as Kupffer cells) once reaching extravascular tissues. Macrophages recognize pathogens or apoptotic cells through opsonins (such as complement or antibodies) (6) or cell surface receptors for phosphatidylserine (PS) (7). After engulfing pathogens or damaged cells, phagocytes eliminate them through reactive oxygen species and hydrolytic enzymes (Fig. 1) (8).
Initiation of inflammatory responses
Innate immune cells are equipped with pattern recognition receptors (such as Toll-like receptor, TLR 2, 4, and 9) (9–11), which specifically recognize molecules shared by a group of related microbes called pathogen-associated molecular patterns (PAMPs). Upon recognition of various PAMPs (such as bacterial peptidoglycan, endotoxin, and CpG-DNA) (11, 12), innate immune cells release a wide array of cytokines and chemokines (13–15). Although an appropriate local inflammation is required to defend against infection or injury, an uncontrolled systemic inflammation may contribute to the pathogenesis of lethal inflammation diseases (such as sepsis).
SEPSIS AS A DYSREGULATED SYSTEMIC INFLAMMATORY RESPONSE
The prevailing theories of sepsis as an dysregulated systemic inflammatory response are supported by extensive studies employing various animal models of sepsis, including endotoxemia and peritonitis induced by cecal ligation and puncture (CLP) (1, 16).
Early pro-inflammatory mediators of experimental sepsis
In animal models of sepsis, a wide array of pro-inflammatory mediators including TNF (17), interleukin (IL)-1 (18), interferon (IFN)-γ (19), and macrophage migration inhibitory factor (MIF) (20, 21) individually or in combination, contribute to the pathogenesis of lethal systemic inflammation. For instance, neutralizing antibodies against endotoxin (22) or TNF (17), reduces lethality in an animal model of endotoxemic/bacteremic shock. However, the early kinetics of systemic TNF accumulation makes it difficult to target in clinical setting (17), prompting a search for other late proinflammatory mediators that may offer a wider therapeutic window (Fig. 2).
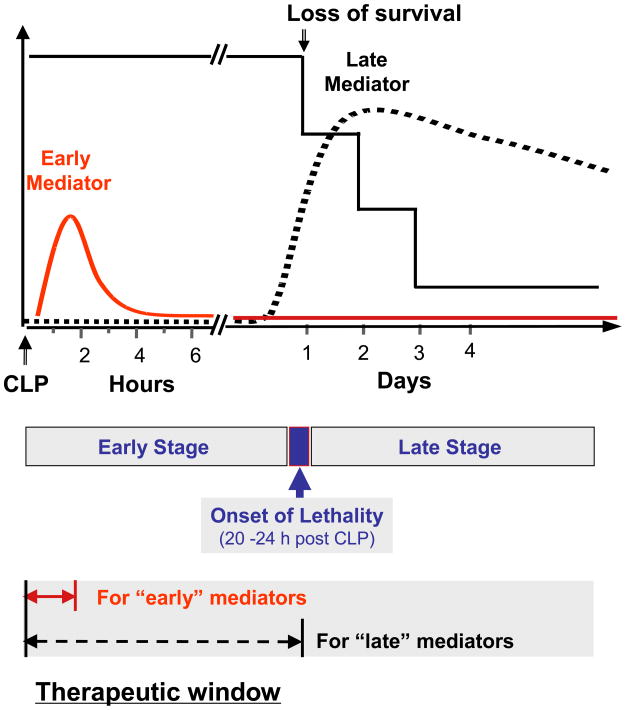
Fig. 2. Early versus late mediators of septic lethality.
Mice subjected to septic insult (such as cecal ligation and puncture, CLP) succumb at latencies of up to 1–3 days. Early cytokines (such as TNF) reach plateau levels in the circulation long before the onset of septic lethality (20–24 h post CLP) during an “early” stage of sepsis. In contrast, late mediators (such as HMGB1) reach peak levels in the circulation immediately after onset of septic lethality during a “late” stage of sepsis. This delayed systemic HMGB1 accumulation parallels with septic lethality, and provides HMGB1 with a wider therapeutic window.
Discovery of HMGB1 as a late mediator of experimental sepsis
In an attempt to broaden the therapeutic window for sepsis, we initiated a search for other macrophage-derived putative mediator released relatively “late” after onset of endotoxemia (23). We stimulated macrophage cultures with bacterial endotoxin, and screened the conditioned media for proteins appearing after 16 hours. Endotoxin induced the appearance of a 30-kDa protein in the conditioned media that was not detectable at earlier time points. The N-terminal amino acid sequence of this 30-kDa protein (i.e., G-K-G-D-P-K-K-P-R-G-K-M-S-S) was identical to a non-histone necleosomal protein termed “high mobility group 1” (HMG-1) (23–25).
Nuclear HMGB1 as a transcription factor
HMG-1 was first purified from nuclei approximately 40 years ago, and termed “high mobility group” (HMG) protein because of its rapid mobility on electrophoresis gels (26). Recently, HMG-1 was renamed as high mobility group box 1 (HMGB1) by a nomenclature committee (27). It is constitutively expressed in many types of cells, and a large “pool” of preformed HMGB1 is stored in the nucleus, owing to the presence of two lysine-rich nuclear localization sequences (28). As an evolutionarily conserved protein, HMGB1 shares 100% homology (in amino acid sequence) between mouse and rat, and a 99% homology between rodent and human (24, 25, 29). It contains two internal repeats of positively charged domains (“HMG boxes” known as “A box” and “B box”) in the N-terminus, and a continuous stretch of negatively charged (aspartic and glutamic acid) residues in the C-terminus. These HMG boxes enable HMGB1 to bind chromosomal DNA, and fulfill its nuclear functions including determination of nucleosomal structure and stability, and regulation of gene expression (30).
Delayed systemic HMGB1 accumulation
In murine models of endotoxemia and sepsis, HMGB1 is first detectable in the circulation eight hours after the onset of diseases, subsequently increasing to plateau levels from 16 to 32 hours (23, 31). Meanwhile, tissue HMGB1 mRNA levels are increased in various tissues such as muscle, liver, and lung following endotoxemia (32) or burn-induced sepsis (33). This late appearance of circulating HMGB1 precedes and parallels with the onset of animal lethality from endotoxemia or sepsis, and distinguishes itself from TNF and other early proinflammatory cytokines (Fig. 2) (34). In septic patients, circulating HMGB1 levels were also elevated (23, 35, 36), although its levels in un-fractionated crude serum samples did not correlate with disease severity (35, 36). However, HMGB1 levels in the low molecular weight (M.W. < 100 kDa) sub-fraction (following ultrafiltration of serum samples through filters with defined M.W. cut-off) correlated well with the lethal outcome of human sepsis (1, 23). This observation suggested a possibility that HMGB1 may interact with other serum components such as thrombomodulin (37), immunoglobulin (e.g., IgG1) (38) to form large (> 100 kDa) complexes (1).
Protective effects of anti-HMGB1 antibodies
The pathogenic role of HMGB1 as a late mediator of lethal endotoxemia was originally examined using HMGB1-specific neutralizing antibodies, which conferred a dose-dependent protection against lethal endotoxemia (23). In a more clinically relevant animal model of sepsis (induced by CLP), delayed administration of HMGB1-specific neutralizing antibodies beginning 24 h after the onset of sepsis, dose-dependently rescued mice from lethal sepsis (31, 39).
HMGB1-mediated injurious inflammatory responses
Administration of exogenous HMGB1 to mice recapitulates many clinical signs of sepsis including fever (40), derangement of intestinal barrier function (41), and tissue injury (42–45). In the brain, exogenous HMGB1 induces the release of proinflammatory cytokines (46) and excitatory amino acids (such as glutamate) (47) and fever (40). In the lung, HMGB1 induces neutrophil infiltration and acute injury (42–45). Focal administration of HMGB1 near the sciatic nerve induces unilateral and bilateral low threshold mechanical allodynia (48). Similarly, intraperitoneal injection of HMGB1 induces peritoneal infiltration of neutrophils (49), and accumulation of cytokines (e.g., TNF and IL-6) and chemokines (e.g., MCP-1). Taken together, these experimental data establish extracellular HMGB1 as a critical late mediator of experimental sepsis, with a wider therapeutic window than early proinflammatory cytokines (Fig. 3).
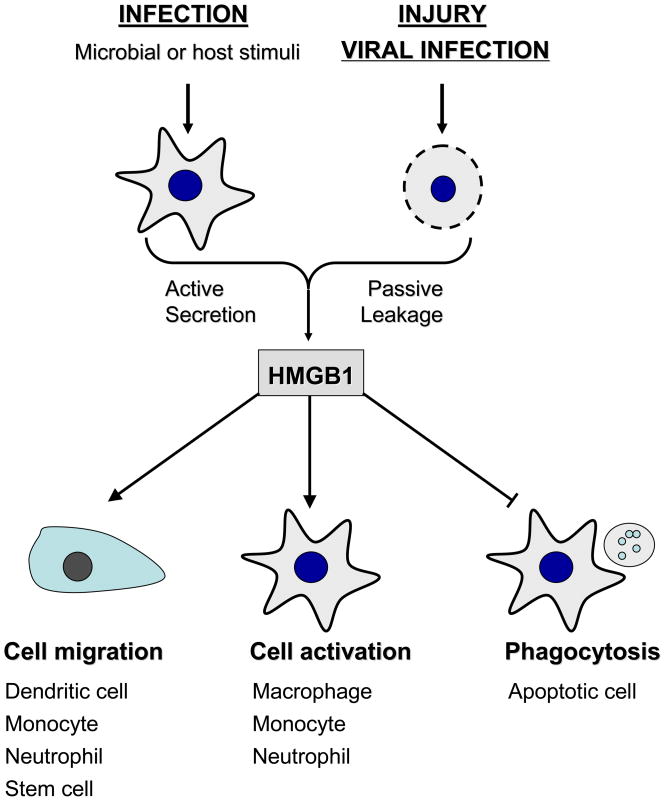
Fig. 3. Extracellular HMGB1 functions as an alarmin signal.
HMGB1 is actively secreted by innate immune cells in response to exogenous microbial products (e.g., LPS or CpG-DNA) or endogenous host stimuli (TNF, IFN-γ, or hydrogen peroxide), and passively released by damaged or virus-infected cells. Extracellular HMGB1 sustains an inflammatory response by stimulating migration of innate immune cells, facilitating innate recognition of bacterial products, activating various innate immune cells, and suppressing phagocytosis of apoptotic cells. Thus, HMGB1 can function as an alarmin signal to recruit, alert and activate various innate immune cells, thereby sustaining potentially injurious inflammatory response.
HMGB1 as an early mediator of ischemic or traumatic injury
In contrast to the delayed systemic HMGB1 accumulation in experimental sepsis, HMGB1 functions as an early mediator in animal models of ischemia/reperfusion (I/R) injury (50–52). Similarly, HMGB1 release may be an early event in patients with hemorrhagic shock (53) or traumatic injury (54), because its circulating levels are elevated within 2–6 hours after onset of these diseases. Prophylactic administration of HMGB1-neutralizing antibody conferred protection against hepatic I/R injury in wild-type mice, but not in TLR4-defective (C3H/HeJ) mutant, implicating a role for TLR4 in HMGB1-mediated hepatic I/R injury (50). In contrast, treatment with HMGB1 antagonist (such as HMGB1 box A) significantly reduced myocardial ischemic injury in wild-type mice, but not in RAGE-deficient mutants, indicating a potential role for RAGE in HMGB1-mediated ischemic injury (55). The potential involvement of RAGE in HMGB1-mediated ischemic injury was further supported by the observation that genetic RAGE deficiency and the decoy soluble RAGE receptor similarly reduced cerebral ischemic injury (56).
In addition, HMGB1-specific neutralizing antibodies have been proven protective against ventilator-induced acute lung injury (57), severe acute pancreatitis (58), and hemorrhagic shock (53), supporting a pathogenic role for extracellular HMGB1 in various inflammatory diseases. Notably, HMGB1 is capable of attracting stem cells (59), and may be important for tissue repair and regeneration (1, 60). For instance, although elevated serum HMGB1 levels were associated with adverse clinical outcomes in patients with myocardial infarction (61), prolonged blockade of HMGB1 with neutralizing antibodies (for 7 days) impaired healing process in animal models of myocardial ischemia/reperfusion. Therefore, like other cytokines, there may be protective advantages of extracellular HMGB1 when released at low amounts (60, 62). It is thus important to pharmacologically modulate, rather than abrogate, systemic HMGB1 accumulation to facilitate resolution of potentially injurious inflammatory response.
EXTRACELLULAR HMGB1 AS AN ALARMIN SIGNAL
Recently, a number of ubiquitous, structurally and functionally diverse host proteins [such as HMGB1 and heat shock protein 72 (Hsp72)] have been categorized as “alarmins” based on the following shared properties (63) (Fig. 2).
1) Active release and passive leakage
As mentioned earlier, innate immune cells actively release HMGB1 in response to exogenous bacterial products (such as endotoxin or CpG-DNA) (23, 64), or endogenous host stimuli (e.g., TNF, IFN-γ, or hydrogen peroxide) (23, 65, 66). Lacking a leader signal sequence, HMGB1 can not be actively secreted via the classical ER-Golgi secretory pathway (23). Instead, activated macrophages/monocytes acetylated HMGB1 at its nuclear localization sequences, leading to sequestration of HMGB1 within cytoplasmic vesicles and subsequent extracellular release (28, 65, 67). In addition, serine phosphorylation might be another requisite step for HMGB1 nucleocytoplasmic translocation (68). The phosphorylation of HMGB1 is potentially mediated by the Calcium/Calmodulin-Dependent Protein Kinase (CaMK) IV (69), because CaMK IV can be translocated to the nucleus following endotoxin stimulation, where it can potentially binds and phosphorylates HMGB1 (69). In addition, HMGB1 can be passively released from necrotic cells (70), or cells infected by viruses (e.g., West Nile, Salmon anemia, Dengue, and influenza viruses) (71–74) or mycobacteria (75, 76), and similarly triggers inflammatory response (Fig. 3).
2). Stimulating cell migration
Accumulating evidence indicate that HMGB1 is capable of stimulating migration of neurite (77), smooth muscle cells (78), tumor cells (79), mesoangioblast stem cells (59, 80), monocytes (81), dendritic cells (82, 83), and neutrophils (49, 84) (Fig. 3). It raises a possibility that extracellular HMGB1 may facilitate recruitment of innate immune cells to sites of infection or injury (85), thereby functioning as a potential host cell-derived chemotactic factor (86).
3). Facilitating innate recognition of microbial products
Recent studies suggested that HMGB1 can bind and facilitate innate recognition of bacterial products (e.g., CpG-DNA or LPS) by innate immune cells (such as macrophages and dendritic cells) (64, 87, 88). In addition, HMGB1 may also bind many endogenous molecules such as thrombomodulin (37), immunoglobulin (e.g., IgG1) (38), IL-1 (89), or nucleosomes derived from apoptotic cells (90). These different host factors have been shown to negatively (37) or positively (89, 90) affect HMGB1-mediated inflammatory responses.
4). Activating innate immune cells
Accumulating evidence have suggested that extracellular HMGB1 binds to the receptor for advanced glycation end products (RAGE), and pattern-recognition receptors such as TLR2 and TLR4 (91, 92). Consequently, HMGB1 activates innate immune cells (91–96) or endothelial cells (97, 98) to produce proinflammatory cytokines, chemokines (95), and adhesion molecules (Fig. 3). In vitro, one of the DNA-binding domains of HMGB1, the “A box”, functions as an antagonist of HMGB1 (31, 99, 100). In contrast, another DNA-binding domain, the “B box”, recapitulates the cytokine activity of full length HMGB1 (101, 102). Interestingly, oxidation of HMGB1 by reactive oxygen species (ROS) enables formation of disulfide bond between thiol group of Cys106 and Cys23 or Cys 45, and consequently abolish HMGB1-mediated immunostimulatory activities (103). Because Cys106 is located within the 18-amino acid cytokine domain of HMGB1 B box, it will be important to investigate whether oxidization similarly affects HMGB1 cytokine activities in future studies.
5). Inhibiting phagocytotic elimination of apoptotic neutrophils
As mentioned earlier, macrophages recognize apoptotic cells through cell surface receptors for phosphatidylserine (PS). Interestingly, HMGB1 could interact with PS on cell surface of apoptotic neutrophils, and consequently inhibit phagocytotic elimination of apoptotic neutrophils by macrophages (Fig. 3) (104). Inefficient elimination of apoptotic cells may lead to excessive accumulation of late apoptotic and/or secondary necrotic cells, which may passively release proinflammatory mediators (such as HMGB1) (105). Considered together, these studies indicate that extracellular HMGB1 can function as an alarmin signal to recruit, alert and activate innate immune cells, thereby sustaining a potentially injurious inflammatory response.
POTENTIAL HMGB1-INHIBITING THERAPEUTIC AGENTS
With a limited number of effective therapies available for patients with sepsis, it is important to search for other agents capable of inhibiting clinically accessible late mediators. Below is a list of agents that have been proven protective against experimental sepsis partly through attenuating systemic HMGB1 accumulation (Table 1).
Table 1.
Potential therapeutic agents for experimental sepsis.
| Agents | Conferred Protection Against | By inhibiting HMGB1 | References |
|---|---|---|---|
| Antibodies | |||
| Anti-IFN-γ | Sepsis | release | 106 |
| Anti-HMGB1 | Endotoxemia and Sepsis | activity | 23, 31, 39 |
| Intravenous immunoglobulin (IVIG) | Sepsis | release | 108 |
| Anti-coagulant agents | |||
| Anti-thrombin III | Endotoxemia | release | 110 |
| Thrombomodulin | Endotoxemia | activity | 37 |
| Endogenous hormones | |||
| Insulin | Endotoxemia | release | 111, 112 |
| Vasoactive intestinal peptide | Endotoxemia and Sepsis | release | 113, 114 |
| Ghrelin | Sepsis | release | 115, 116, 117 |
| Vagus nerve stimulation | |||
| Electrical | Endotoxemia and Sepsis | release | 118 |
| Chemical (nicotine, GTS-21, choline) | Endotoxemia and Sepsis | release | 119, 121, 122, 123 |
| Mechanical (transcutaneous) | Endotoxemia and Sepsis | release | 120 |
| Chinese herbal components | |||
| Danshen (TSN IIA-SS) | Endotoxemia and Sepsis | release | 129 |
| Green tea (EGCG) | Endotoxemia and Sepsis | release | 130, 131 |
| Others | |||
| Ethyl pyruvate | Endotoxemia and Sepsis | release | 135, 136 |
| Stearoyl lysophosphatidylcholine | Endotoxemia and Sepsis | release | 124–126 |
Antibodies
Anti-IFN-gamma antibodies
As mentioned earlier, pro-inflammatory cytokines (such as IFN-γ) effectively stimulate innate immune cells to actively release HMGB1 (65). In animal model of sepsis, intravenous administration of IFN-gamma antibodies (1.2 mg/kg), immediately or 24 h after CLP, reduced peritoneal and serum HMGB1 levels, and attenuated CLP-induced animal mortality (106). It suggests that specific inhibition of HMGB1-stimulating proinflammatory cytokines may attenuate sepsis-induced HMGB1 accumulation, thereby protecting animals against lethal sepsis. In parallel with CLP-induced systemic HMGB1 accumulation, nucleocytoplasmic shuttling of HMGB1 occurs in alveolar macrophages at 24 h post CLP (107). The sepsis-induced HMGB1 translocation was associated with a significant decrease in TNF production (107), suggesting that HMGB1 deprivation is associated with macrophage suppression. The potential role for nuclear HMGB1 in the regulation of innate immune function (107) was further supported by the observation that HMGB1 binds to a cis-acting regulatory element (spanning from −157 to −137 bp of the 5′-flanking region) of the TNF gene to facilitate its transcription (96).
Intravenous immunoglobulin
Intravenous immunoglobulin (IVIG) refers to immunoglobulins (IgG, antibodies) pooled from the plasma of many healthy blood donors. It is usually given intravenously as a plasma protein replacement therapy to patients with various inflammatory diseases due to acute infections, autoimmune, or immune deficiencies. A recent study indicated that IVIG dose-dependently protected rats against sepsis-induced lung injury and lethality by attenuating systemic HMGB1 release (108). The mechanisms by which IVIG suppresses systemic HMGB1 release remains poorly understood. Notably, it has recently been found that human IgGs can bind to HMGB1, and potentially interfere with ELISA detection of HMGB1 (38). It is not known whether IVIG indeed attenuate systemic HMGB1 accumulation, or merely interfere with ELISA detection of HMGB1 (1).
Anti-coagulant agents
As a major regulator of hemostasis, thrombin is pro-coagulant by activating blood-clotting factors (Va, VIIIa and XI), cleaving fibrinogen to form a fibrin clot, and stimulating platelet aggregation. Its activities can be inhibited by various anti-coagulant factors such as anti-thrombin and thrombomodulin.
Anti-thrombin III
Antithrombin inhibits the pro-coagulant activities of thrombin upon interaction with heparin or related glycosaminoglycans. Although anti-thrombin III (AT-III) failed to reduce mortality rate in large sepsis clinical trial (109), a recent study suggested that AT-III could attenuate endotoxin-induced systemic HMGB1 accumulation, and reduced endotoxemic lethality (110). The mechanisms by which AT-III inhibits HMGB1 release remains to be further investigated.
Thrombomodulin
Thrombomodulin can bind thrombin to inhibit its pro-coagulant activities, and enhance its capacities to activate a plasma anticoagulant, activated protein C. Interestingly, human soluble thrombomodulin (ART-123) can physically bind to HMGB1 protein, thereby inhibiting HMGB1-mediated inflammatory response. Furthermore, ART-123 conferred significant protection against lethal endotoxemia (37), but it is not yet known whether ART-123 is protective in clinically relevant animal models of sepsis.
Endogenous hormones
Insulin
A recent study indicated that hyperglycemia, induced by infusion of glucose immediately following endotoxemia, aggravated endotoxin-induced HMGB1 release and lung injury (111). In contrast, intensive blood glucose control by insulin conferred protection against endotoxin-induced acute lung injury, and endotoxemic lethality (111). It is currently unknown whether the observed protective effects are dependent on insulin’s anti-inflammatory activities, or its blood glucose-modulating properties (112).
Neuropeptides
Vasoactive intestinal peptide (VIP) is a short-lived small peptide hormone produced by the gut, pancreas and brain. It can induce smooth muscle relaxation, and is involved in communication between brain neurons. In animal models of sepsis induced by CLP, administration of VIP attenuated systemic HMGB1 accumulation, and consequently reduced animal lethality (113). Another member of the VIP family, the pituitary adenylate cyclase-activating polypeptide (PACAP), has also been shown protective against lethal endotoxemia partly by attenuating systemic HMGB1 accumulation (114). Another neuropeptide, urocortin, belongs to the corticotropin-releasing factor family. It is expressed in the brain, and may be responsible for regulation of appetite. It is also a potent and long-lasting hypotensive agent and increases coronary blood flow. In animal models of sepsis induced by CLP or bacteremia, administration of urocortin also attenuated systemic HMGB1 accumulation, and consequently reduced animal lethality (113), supporting a therapeutic potential for neuropeptides in the treatment of lethal systemic inflammatory diseases.
Ghrelin
Ghrelin is a stomach-derived hormone that is responsible for regulating the appetite – increasing it before eating and decreasing it afterward. Intriguingly, plasma ghrelin levels are significantly decreased in septic animals (115), and administration of ghrelin promoted a dose-dependent protection against sepsis-induced acute lung injury and lethality (115–117). Ghrelin may exert its protective effects through multiple mechanisms, such as by attenuating systemic HMGB1 release, and by facilitating bacterial elimination (117). Intriguingly, ghrelin may attenuate systemic accumulation of proinflammatory cytokines partly via the vagus nerve (116), suggesting that pharmacologic stimulation of the vagus nerve may be an effective therapy for experimental sepsis.
Vagus nerve stimulation
Recent evidence suggests that the central nervous system can attenuate peripheral innate immune response through efferent vagus nerve signals to tissue-resident macrophages (118). This effect is mediated by the principle neurotransmitter of the vagus nerve, acetylcholine, which inactivates macrophages via nicotinic cholinergic receptors (118). Indeed, stimulation of the vagus nerve by physical methods (e.g., electrical or mechanical) (119, 120) or chemical agents (such as cholinergic agonists, nicotine, choline and GTS-21) (121–123) conferred protection against lethal endotoxemia and sepsis partly by attenuating systemic HMGB1 accumulation.
A chemical derivative of choline, stearoyl lysophosphatidylcholine, has also been proven protective against experimental sepsis by stimulating neutrophils to destroy ingested bacteria in an H2O2-dependent mechanism (124). However, stearoyl LPC also confers protection against lethal endotoxemia (124), implying that it may exert protective effects through an additional, bactericidal-independent mechanism (125). Indeed, administration of stearoyl LPC significantly attenuated circulating HMGB1 levels (126), indicating that stearoyl LPC protects against experimental sepsis partly by facilitating elimination of invading pathogens, and partly by attenuating systemic HMGB1 accumulation (125).
Chinese medicinal herbs
Traditional herbal medicine has formed the basis of folk remedies for various inflammatory ailments. Among several dozens commonly used Chinese herbs (127), we found that aqueous extracts of Danggui (Angelica sinensis), Green tea (Camellia sinensis), and Danshen (Saliva miltorrhiza) efficiently inhibited endotoxin-induced HMGB1 release, and protected animals against experimental sepsis (128–130).
Danggui
Danggui has been traditionally used to treat gynecological disorders (such as abnormal menstruation). Its aqueous extract dose-dependently inhibited LPS-induced HMGB1 release in macrophage and monocyte cultures, partly by interfering with HMGB1 cytoplasmic translocation (128). Furthermore, Danggui extract rescued mice from lethal sepsis even when the first dose was given at 24 h post onset of disease (128). The active components responsible for these beneficial effects remain a subject of future investigation.
Green tea
Green tea brewed from the leaves of the plant, Camellia sinensis, contains a class of biologically active polyphenols called catechins. Accounting for 50–80% of the total catechin, EGCG, is effective in attenuating endotoxin-induced HMGB1 release by macrophage and monocytes (130). In addition, EGCG dose-dependently inhibited HMGB1-induced release of TNF, IL-6, and nitric oxide in macrophage cultures (130). Interestingly, EGCG completely abrogated accumulation/clustering of exogenous HMGB1 on macrophage cell surface (130), suggesting that EGCG inhibits HMGB1 cytokine activities by preventing its cell surface accumulation/clustering. In vivo, EGCG (10 mg/kg, intraperitoneally) improved animal survival in a rat model of CLP-induced experimental sepsis (131). Similarly, repeated administration of EGCG conferred a dose-dependent protection against lethal endotoxemia, and rescued mice from lethal sepsis even when the first dose of EGCG was given at +24 h after onset of sepsis (130). Consistently, delayed administration of EGCG significantly attenuated circulating levels of HMGB1, as well as surrogate markers of experimental sepsis (such as IL-6 and KC) (130, 132). Considered together, these experimental data indicate that EGCG protects mice against lethal sepsis partly by attenuating systemic HMGB1 accumulation, and partly by inhibiting HMGB1-mediated inflammatory response.
Danshen
Another Chinese herb, Danshen has been widely used in China for patients with cardiovascular disorders (133, 134). It contains abundant red pigments (termed tanshinone I, tanshinone IIA, and cryptotanshinone), which effectively attenuated LPS-induced HMGB1 release (129). A water-soluble derivative (sodium sulfonate) of tanshinone IIA, TSN IIA-SS, at concentrations (100 μM) that completely abrogated LPS-induced HMGB1 release, only partially attenuated LPS-induced release of four out of 62 cytokines [e.g., IL-12p70, IL-1α, platelet factor 4 (PF-4), and MCP-5] (129), indicating a specificity for TSN IIA-SS in inhibiting LPS-induced HMGB1 release. Despite a structural resemblance (i.e., the presence of a four-fused-ring structure) between tanshinones and steroidal anti-inflammatory drugs (such as dexamethasone and cortisone), tanshinones inhibit LPS-induced HMGB1-release in a glucocoticoid receptor-independent mechanism (129). More importantly, repeated administration of TSN IIA-SS beginning at +24 h, followed by additional doses at +48, +72 and + 96 h after the onset of sepsis, dose-dependently rescued mice from lethal sepsis (129). Notably, administration of TNS IIA-SS dose-dependently attenuated circulating HMGB1 levels in septic mice (129), suggesting that TSN IIA-SS confer protection against experimental sepsis partly by inhibiting systemic HMGB1 accumulation.
Others
Ethyl pyruvate is an aliphatic ester derived from endogenous substance, pyruvic acid, which is a final product of glycolysis and the starting substrate for the tricarboxylic acid cycle (135). It dose-dependently inhibits LPS-induced release of early (e.g., TNF) and late proinflammatory cytokines (e.g., HMGB1), and protected mice against experimental sepsis, even when treatment is started as late as 12–24 hours after the onset of disease (136).
CONCLUSIONS AND PERSPECTIVES
For complex systemic inflammatory diseases such as sepsis, it appears difficult to translate successful animal studies into clinical applications (1). For instance, although neutralizing antibodies against endotoxin (22) or cytokines (e.g., TNF) (17, 137) are protective in animal models of endotoxemia or bacteremia, these agents failed in sepsis clinical trials (138–140). This failure partly reflects the complexity of the underlying pathogenic mechanisms of sepsis, and the consequent heterogeneity of the patient population (3, 141). It may also be attributable to pitfalls in the selection of: 1) feasible therapeutic targets or drugs; 2) optimal doses and timing of drugs; and 3) non-realistic clinical outcome measures (such as mortality rates) (3, 141).
Intensive pre-clinical animal studies have established HMGB1 as a late mediator of experimental sepsis with a wider therapeutic window (142, 143). First, circulating HMGB1 levels are elevated in a delayed fashion in endotoxemic and septic animals. Second, administration of exogenous HMGB1 to mice induces fever, derangement of intestinal barrier function, and tissue injury. Third, administration of anti-HMGB1 antibodies or inhibitors rescues mice from lethal experimental sepsis even when the first dose is given 24 hours after onset of sepsis. Will HMGB1 ever become a clinically feasible therapeutic target for human sepsis? This question can not be answered until HMGB1-inhibiting agents have been tested for efficacy in large clinical trials.
One of the most selective HMGB1 inhibitor, TSN IIA-SS, has already been used in China as a medicine for patients with cardiovascular disorders (133). Even in septic animals, TSN IIA-SS reduced total peripheral vascular resistance, and yet increased cardiac stroke volume and cardiac output (129). Because HMGB1 may function as a myocardial depressant factor by reducing contractility of cardiac myocytes (144), it is plausible that TSN IIA-SS improves cardiovascular function partly by attenuating HMGB1 release. The dual effects of TSN IIA-SS in attenuating late inflammatory response and improving cardiovascular function make it a promising therapeutic agent for sepsis. It is thus important to further investigate the intricate mechanisms by which various agents attenuate systemic HMGB1 release, and explore their therapeutic potential in future clinical studies.
Acknowledgments
Acknowledgments of funding: Work in authors’ laboratory was supported by grants from the National Institutes of Health, National Institute of General Medical Science (R01GM063075, R01GM070817, to HW).
References
- 1.Wang H, Zhu S, Zhou R, Li W, Sama AE. Therapeutic potential of HMGB1-targeting agents in sepsis. Expert Rev Mol Med. 2008;10:e32. doi: 10.1017/S1462399408000884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 3.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, Calandra T, Dhainaut JF, Gerlach H, Harvey M, Marini JJ, Marshall J, Ranieri M, Ramsay G, Sevransky J, Thompson BT, Townsend S, Vender JS, Zimmerman JL, Vincent JL. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 4.Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nat Immunol. 2005;6:1182–1190. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- 5.Scapini P, Lapinet-Vera JA, Gasperini S, Calzetti F, Bazzoni F, Cassatella MA. The neutrophil as a cellular source of chemokines. Immunol Rev. 2000;177:195–203. doi: 10.1034/j.1600-065x.2000.17706.x. [DOI] [PubMed] [Google Scholar]
- 6.Mosser DM. Receptors on phagocytic cells involved in microbial recognition. Immunol Ser. 1994;60:99–114. [PubMed] [Google Scholar]
- 7.Wu Y, Tibrewal N, Birge RB. Phosphatidylserine recognition by phagocytes: a view to a kill. Trends Cell Biol. 2006;16:189–197. doi: 10.1016/j.tcb.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Liles WC. Immunomodulatory approaches to augment phagocyte-mediated host defense for treatment of infectious diseases. Semin Respir Infect. 2001;16:11–17. doi: 10.1053/srin.2001.22724. [DOI] [PubMed] [Google Scholar]
- 9.Brightbill HD, Libraty DH, Krutzik SR, Yang RB, Belisle JT, Bleharski JR, Maitland M, Norgard MV, Plevy SE, Smale ST, Brennan PJ, Bloom BR, Godowski PJ, Modlin RL. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science. 1999;285:732–736. doi: 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- 10.Poltorak A, He X, Smirnova I, Liu MY, Huffel CV, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 11.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 12.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002;20:709–760. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 13.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 14.Baggiolini M, Loetscher P. Chemokines in inflammation and immunity. Immunol Today. 2000;21:418–420. doi: 10.1016/s0167-5699(00)01672-8. [DOI] [PubMed] [Google Scholar]
- 15.Balkwill F. Cytokines--soluble factors in immune responses. Curr Opin Immunol. 1988;1:241–249. doi: 10.1016/0952-7915(88)90008-8. [DOI] [PubMed] [Google Scholar]
- 16.Wichterman KA, Baue AE, Chaudry IH. Sepsis and septic shock--a review of laboratory models and a proposal. J Surg Res. 1980;29:189–201. doi: 10.1016/0022-4804(80)90037-2. [DOI] [PubMed] [Google Scholar]
- 17.Tracey KJ, Fong Y, Hesse DG, Manogue KR, Lee AT, Kuo GC, Lowry SF, Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987;330:662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- 18.Dinarello CA, Thompson RC. Blocking IL-1: interleukin 1 receptor antagonist in vivo and in vitro. Immunol Today. 1991;12:404–410. doi: 10.1016/0167-5699(91)90142-G. [DOI] [PubMed] [Google Scholar]
- 19.Heinzel FP. The role of IFN-gamma in the pathology of experimental endotoxemia. J Immunol. 1990;145:2920–2924. [PubMed] [Google Scholar]
- 20.Calandra T, Echtenacher B, Roy DL, Pugin J, Metz CN, Hultner L, Heumann D, Mannel D, Bucala R, Glauser MP. Protection from septic shock by neutralization of macrophage migration inhibitory factor. Nat Med. 2000;6:164–170. doi: 10.1038/72262. [DOI] [PubMed] [Google Scholar]
- 21.Bozza M, Satoskar AR, Lin G, Lu B, Humbles AA, Gerard C, David JR. Targeted disruption of migration inhibitory factor gene reveals its critical role in sepsis. J Exp Med. 1999;189:341–346. doi: 10.1084/jem.189.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis CE, Brown KR, Douglas H, Tate WJ, III, Braude AI. Prevention of death from endotoxin with antisera. I. The risk of fatal anaphylaxis to endotoxin. J Immunol. 1969;102:563–572. [PubMed] [Google Scholar]
- 23.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, Tracey KJ. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 24.Ferrari S, Ronfani L, Calogero S, Bianchi ME. The mouse gene coding for high mobility group 1 protein (HMG1) J Biol Chem. 1994;269:28803–28808. [PubMed] [Google Scholar]
- 25.Paonessa G, Frank R, Cortese R. Nucleotide sequence of rat liver HMG1 cDNA. Nucleic Acids Res. 1987;15:9077. doi: 10.1093/nar/15.21.9077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johns EW. History, Definitions and Problems. In: Johns EW, editor. The HMG Chromosomal Proteins. London: Academic Press Inc. (London) Ltd; 1982. pp. 1–8. [Google Scholar]
- 27.Bustin M. Revised nomenclature for high mobility group (HMG) chromosomal proteins. Trends Biochem Sci. 2001;26:152–153. doi: 10.1016/s0968-0004(00)01777-1. [DOI] [PubMed] [Google Scholar]
- 28.Bonaldi T, Talamo F, Scaffidi P, Ferrera D, Porto A, Bachi A, Rubartelli A, Agresti A, Bianchi ME. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J. 2003;22:5551–5560. doi: 10.1093/emboj/cdg516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wen L, Huang JK, Johnson BH, Reeck GR. A human placental cDNA clone that encodes nonhistone chromosomal protein HMG-1. Nucleic Acids Res. 1989;17:1197–1214. doi: 10.1093/nar/17.3.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bustin M. At the crossroads of necrosis and apoptosis: signaling to multiple cellular targets by HMGB1. Sci STKE. 2002;2002:E39. doi: 10.1126/stke.2002.151.pe39. [DOI] [PubMed] [Google Scholar]
- 31.Yang H, Ochani M, Li J, Qiang X, Tanovic M, Harris HE, Susarla SM, Ulloa L, Wang H, DiRaimo R, Czura CJ, Wang H, Roth J, Warren HS, Fink MP, Fenton MJ, Andersson U, Tracey KJ. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci U S A. 2004;101:296–301. doi: 10.1073/pnas.2434651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lang CH, Silvis C, Deshpande N, Nystrom G, Frost RA. Endotoxin stimulates in vivo expression of inflammatory cytokines tumor necrosis factor alpha, interleukin-1beta, -6, and high-mobility-group protein-1 in skeletal muscle. Shock. 2003;19:538–546. doi: 10.1097/01.shk.0000055237.25446.80. [DOI] [PubMed] [Google Scholar]
- 33.Fang WH, Yao YM, Shi ZG, Yu Y, Wu Y, Lu LR, Sheng ZY. The significance of changes in high mobility group-1 protein mRNA expression in rats after thermal injury. Shock. 2002;17:329–333. doi: 10.1097/00024382-200204000-00016. [DOI] [PubMed] [Google Scholar]
- 34.Wang H, Yang H, Czura CJ, Sama AE, Tracey KJ. HMGB1 as a Late Mediator of Lethal Systemic Inflammation. Am J Respir Crit Care Med. 2001;164:1768–1773. doi: 10.1164/ajrccm.164.10.2106117. [DOI] [PubMed] [Google Scholar]
- 35.Sunden-Cullberg J, Norrby-Teglund A, Rouhiainen A, Rauvala H, Herman G, Tracey KJ, Lee ML, Andersson J, Tokics L, Treutiger CJ. Persistent elevation of high mobility group box-1 protein (HMGB1) in patients with severe sepsis and septic shock. Crit Care Med. 2005;33:564–573. doi: 10.1097/01.ccm.0000155991.88802.4d. [DOI] [PubMed] [Google Scholar]
- 36.Angus DC, Yang L, Kong L, Kellum JA, Delude RL, Tracey KJ, Weissfeld L. Circulating high-mobility group box 1 (HMGB1) concentrations are elevated in both uncomplicated pneumonia and pneumonia with severe sepsis. Crit Care Med. 2007;35:1061–1067. doi: 10.1097/01.CCM.0000259534.68873.2A. [DOI] [PubMed] [Google Scholar]
- 37.Abeyama K, Stern DM, Ito Y, Kawahara K, Yoshimoto Y, Tanaka M, Uchimura T, Ida N, Yamazaki Y, Yamada S, Yamamoto Y, Yamamoto H, Iino S, Taniguchi N, Maruyama I. The N-terminal domain of thrombomodulin sequesters high-mobility group-B1 protein, a novel antiinflammatory mechanism. J Clin Invest. 2005;115:1267–1274. doi: 10.1172/JCI22782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Urbonaviciute V, Furnrohr BG, Weber C, Haslbeck M, Wilhelm S, Herrmann M, Voll RE. Factors masking HMGB1 in human serum and plasma. J Leukoc Biol. 2007;81:67–74. doi: 10.1189/jlb.0306196. [DOI] [PubMed] [Google Scholar]
- 39.Qin S, Wang H, Yuan R, Li H, Ochani M, Ochani K, Rosas-Ballina M, Czura CJ, Huston JM, Miller E, Lin X, Sherry B, Kumar A, Larosa G, Newman W, Tracey KJ, Yang H. Role of HMGB1 in apoptosis-mediated sepsis lethality. J Exp Med. 2006;203:1637–1642. doi: 10.1084/jem.20052203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Connor KA, Hansen MK, Rachal PC, Deak MM, Biedenkapp JC, Milligan ED, Johnson JD, Wang H, Maier SF, Tracey KJ, Watkins LR. Further characterization of high mobility group box 1 (HMGB1) as a proinflammatory cytokine: central nervous system effects. Cytokine. 2003;24:254–265. doi: 10.1016/j.cyto.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 41.Sappington PL, Yang R, Yang H, Tracey KJ, Delude RL, Fink MP. HMGB1 B box increases the permeability of Caco-2 enterocytic monolayers and impairs intestinal barrier function in mice. Gastroenterology. 2002;123:790–802. doi: 10.1053/gast.2002.35391. [DOI] [PubMed] [Google Scholar]
- 42.Abraham E, Arcaroli J, Carmody A, Wang H, Tracey KJ. HMG-1 as a mediator of acute lung inflammation. J Immunol. 2000;165:2950–2954. doi: 10.4049/jimmunol.165.6.2950. [DOI] [PubMed] [Google Scholar]
- 43.Ueno H, Matsuda T, Hashimoto S, Amaya F, Kitamura Y, Tanaka M, Kobayashi A, Maruyama I, Yamada S, Hasegawa N, Soejima J, Koh H, Ishizaka A. Contributions of high mobility group box protein in experimental and clinical acute lung injury. Am J Respir Crit Care Med. 2004;170:1310–1316. doi: 10.1164/rccm.200402-188OC. [DOI] [PubMed] [Google Scholar]
- 44.Lin X, Yang H, Sakuragi T, Hu M, Mantell LL, Hayashi S, Al Abed Y, Tracey KJ, Ulloa L, Miller EJ. {alpha}-Chemokine receptor blockade reduces high mobility group box 1 protein-induced lung inflammation and injury and improves survival in sepsis. Am J Physiol Lung Cell Mol Physiol. 2005;289:L583–L590. doi: 10.1152/ajplung.00091.2005. [DOI] [PubMed] [Google Scholar]
- 45.Rowe SM, Jackson PL, Liu G, Hardison M, Livraghi A, Solomon GM, McQuaid DB, Noerager BD, Gaggar A, Clancy J, O’Neal W, Sorscher EJ, Abraham E, Blalock JE. Potential Role of High Mobility Group Box 1 in Cystic Fibrosis Airway Disease. Am J Respir Crit Care Med. 2008;178(8):822–31. doi: 10.1164/rccm.200712-1894OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Agnello D, Wang H, Yang H, Tracey KJ, Ghezzi P. HMGB1, a DNA-binding protein with cytokine activity, induces brain TNF and IL-6 production, and mediates anorexia and taste aversion. Cytokine. 2002;18:231–236. doi: 10.1006/cyto.2002.0890. [DOI] [PubMed] [Google Scholar]
- 47.Pedrazzi M, Raiteri L, Bonanno G, Patrone M, Ledda S, Passalacqua M, Milanese M, Melloni E, Raiteri M, Pontremoli S, Sparatore B. Stimulation of excitatory amino acid release from adult mouse brain glia subcellular particles by high mobility group box 1 protein. J Neurochem. 2006;99:827–838. doi: 10.1111/j.1471-4159.2006.04120.x. [DOI] [PubMed] [Google Scholar]
- 48.Chacur M, Milligan ED, Gazda LS, Armstrong C, Wang H, Tracey KJ, Maier SF, Watkins LR. A new model of sciatic inflammatory neuritis (SIN): induction of unilateral and bilateral mechanical allodynia following acute unilateral peri-sciatic immune activation in rats. Pain. 2001;94:231–244. doi: 10.1016/S0304-3959(01)00354-2. [DOI] [PubMed] [Google Scholar]
- 49.van Zoelen MA, Yang H, Florquin S, Meijers JC, Akira S, Arnold B, Nawroth PP, Bierhaus A, Tracey KJ, van der PT. Role of Toll-like receptors 2 and 4, and the receptor for advanced glycation end products (RAGE) in HMGB1 induced inflammation in vivo. Shock. 2009 doi: 10.1097/SHK.0b013e318186262d. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, Yang H, Li J, Tracey KJ, Geller DA, Billiar TR. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201:1135–1143. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu H, Chen G, Wyburn KR, Yin J, Bertolino P, Eris JM, Alexander SI, Sharland AF, Chadban SJ. TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest. 2007;117:2847–2859. doi: 10.1172/JCI31008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu K, Mori S, Takahashi HK, Tomono Y, Wake H, Kanke T, Sato Y, Hiraga N, Adachi N, Yoshino T, Nishibori M. Anti-high mobility group box 1 monoclonal antibody ameliorates brain infarction induced by transient ischemia in rats. FASEB J. 2007;21:3904–3916. doi: 10.1096/fj.07-8770com. [DOI] [PubMed] [Google Scholar]
- 53.Yang R, Harada T, Mollen KP, Prince JM, Levy RM, Englert JA, Gallowitsch-Puerta M, Yang L, Yang H, Tracey KJ, Harbrecht BG, Billiar TR, Fink MP. Anti-HMGB1 neutralizing antibody ameliorates gut barrier dysfunction and improves survival after hemorrhagic shock. Mol Med. 2006;12:105–114. doi: 10.2119/2006-00010.Yang. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peltz ED, Moore EE, Eckels PC, Damle SS, Tsuruta Y, Johnson JL, Sauaia A, Silliman CC, Banerjee A, Abraham E. HMGB1 is markedly elevated within six hours of mechanical trauma in humans. Shock. 2009 doi: 10.1097/shk.0b013e3181997173. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Andrassy M, Volz HC, Igwe JC, Funke B, Eichberger SN, Kaya Z, Buss S, Autschbach F, Pleger ST, Lukic IK, Bea F, Hardt SE, Humpert PM, Bianchi ME, Mairbaurl H, Nawroth PP, Remppis A, Katus HA, Bierhaus A. High-mobility group box-1 in ischemia-reperfusion injury of the heart. Circulation. 2008;117:3216–3226. doi: 10.1161/CIRCULATIONAHA.108.769331. [DOI] [PubMed] [Google Scholar]
- 56.Muhammad S, Barakat W, Stoyanov S, Murikinati S, Yang H, Tracey KJ, Bendszus M, Rossetti G, Nawroth PP, Bierhaus A, Schwaninger M. The HMGB1 receptor RAGE mediates ischemic brain damage. J Neurosci. 2008;28:12023–12031. doi: 10.1523/JNEUROSCI.2435-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ogawa EN, Ishizaka A, Tasaka S, Koh H, Ueno H, Amaya F, Ebina M, Yamada S, Funakoshi Y, Soejima J, Moriyama K, Kotani T, Hashimoto S, Morisaki H, Abraham E, Takeda J. Contribution of high-mobility group box-1 to the development of ventilator-induced lung injury. Am J Respir Crit Care Med. 2006;174:400–407. doi: 10.1164/rccm.200605-699OC. [DOI] [PubMed] [Google Scholar]
- 58.Sawa H, Ueda T, Takeyama Y, Yasuda T, Shinzeki M, Nakajima T, Kuroda Y. Blockade of high mobility group box-1 protein attenuates experimental severe acute pancreatitis. World J Gastroenterol. 2006;12:7666–7670. doi: 10.3748/wjg.v12.i47.7666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Palumbo R, Sampaolesi M, De Marchis F, Tonlorenzi R, Colombetti S, Mondino A, Cossu G, Bianchi ME. Extracellular HMGB1, a signal of tissue damage, induces mesoangioblast migration and proliferation. J Cell Biol. 2004;164:441–449. doi: 10.1083/jcb.200304135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li W, Sama AE, Wang H. Role of HMGB1 in cardiovascular diseases. Curr Opin Pharmacol. 2006;6:130–135. doi: 10.1016/j.coph.2005.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kohno T, Anzai T, Naito K, Miyasho T, Okamoto M, Yokota H, Yamada S, Maekawa Y, Takahashi T, Yoshikawa T, Ishizaka A, Ogawa S. Role of high-mobility group box 1 protein in post-infarction healing process and left ventricular remodelling. Cardiovasc Res 15. 2008;81(3):565–73. doi: 10.1093/cvr/cvn291. [DOI] [PubMed] [Google Scholar]
- 62.Takahashi K, Fukushima S, Yamahara K, Yashiro K, Shintani Y, Coppen SR, Salem HK, Brouilette SW, Yacoub MH, Suzuki K. Modulated inflammation by injection of high-mobility group box 1 recovers post-infarction chronically failing heart. Circulation. 2008;118:S106–S114. doi: 10.1161/CIRCULATIONAHA.107.757443. [DOI] [PubMed] [Google Scholar]
- 63.Oppenheim JJ, Yang D. Alarmins: chemotactic activators of immune responses. Curr Opin Immunol. 2005;17:359–365. doi: 10.1016/j.coi.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 64.Ivanov S, Dragoi AM, Wang X, Dallacosta C, Louten J, Musco G, Sitia G, Yap GS, Wan Y, Biron CA, Bianchi ME, Wang H, Chu WM. A novel role for HMGB1 in TLR9-mediated inflammatory responses to CpG-DNA. Blood. 2007;110(6):1970–1981. doi: 10.1182/blood-2006-09-044776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rendon-Mitchell B, Ochani M, Li J, Han J, Wang H, Yang H, Susarla S, Czura C, Mitchell RA, Chen G, Sama AE, Tracey KJ, Wang H. IFN-gamma Induces High Mobility Group Box 1 Protein Release Partly Through a TNF-Dependent Mechanism. J Immunol. 2003;170:3890–3897. doi: 10.4049/jimmunol.170.7.3890. [DOI] [PubMed] [Google Scholar]
- 66.Tang D, Shi Y, Kang R, Li T, Xiao W, Wang H, Xiao X. Hydrogen peroxide stimulates macrophages and monocytes to actively release HMGB1. J Leukoc Biol. 2007;81:741–747. doi: 10.1189/jlb.0806540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gardella S, Andrei C, Ferrera D, Lotti LV, Torrisi MR, Bianchi ME, Rubartelli A. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep. 2002;3:955–1001. doi: 10.1093/embo-reports/kvf198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Youn JH, Shin JS. Nucleocytoplasmic shuttling of HMGB1 is regulated by phosphorylation that redirects it toward secretion. J Immunol. 2006;177:7889–7897. doi: 10.4049/jimmunol.177.11.7889. [DOI] [PubMed] [Google Scholar]
- 69.Zhang X, Wheeler D, Tang Y, Guo L, Shapiro RA, Ribar TJ, Means AR, Billiar TR, Angus DC, Rosengart MR. Calcium/calmodulin-dependent protein kinase (CaMK) IV mediates nucleocytoplasmic shuttling and release of HMGB1 during lipopolysaccharide stimulation of macrophages. J Immunol. 2008;181:5015–5023. doi: 10.4049/jimmunol.181.7.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 71.Chu JJ, Ng ML. The mechanism of cell death during West Nile virus infection is dependent on initial infectious dose. J Gen Virol. 2003;84:3305–3314. doi: 10.1099/vir.0.19447-0. [DOI] [PubMed] [Google Scholar]
- 72.Joseph T, Cepica A, Brown L, Ikede BO, Kibenge FS. Mechanism of cell death during infectious salmon anemia virus infection is cell type-specific. J Gen Virol. 2004;85:3027–3036. doi: 10.1099/vir.0.80091-0. [DOI] [PubMed] [Google Scholar]
- 73.Chen LC, Yeh TM, Wu HN, Lin YY, Shyu HW. Dengue virus infection induces passive release of high mobility group box 1 protein by epithelial cells. J Infect. 2008;56:143–150. doi: 10.1016/j.jinf.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 74.Alleva LM, Budd AC, Clark IA. Systemic release of high mobility group box 1 protein during severe murine influenza. J Immunol. 2008;181:1454–1459. doi: 10.4049/jimmunol.181.2.1454. [DOI] [PubMed] [Google Scholar]
- 75.Hofner P, Seprenyi G, Miczak A, Buzas K, Gyulai Z, Medzihradszky KF, Rouhiainen A, Rauvala H, Mandi Y. High mobility group box 1 protein induction by Mycobacterium bovis BCG. Mediators Inflamm. 2007;2007:53805. doi: 10.1155/2007/53805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Grover A, Taylor J, Troudt J, Keyser A, Sommersted K, Schenkel A, Izzo AA. Mycobacterial infection induces the secretion of high-mobility group box 1 protein. Cell Microbiol. 2008;10:1390–1404. doi: 10.1111/j.1462-5822.2008.01135.x. [DOI] [PubMed] [Google Scholar]
- 77.Fages C, Nolo R, Huttunen HJ, Eskelinen E, Rauvala H. Regulation of cell migration by amphoterin. J Cell Sci. 2000;113 ( Pt 4):611–620. doi: 10.1242/jcs.113.4.611. [DOI] [PubMed] [Google Scholar]
- 78.Degryse B, Bonaldi T, Scaffidi P, Muller S, Resnati M, Sanvito F, Arrigoni G, Bianchi ME. The high mobility group (HMG) boxes of the nuclear protein HMG1 induce chemotaxis and cytoskeleton reorganization in rat smooth muscle cells. J Cell Biol. 2001;152:1197–1206. doi: 10.1083/jcb.152.6.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huttunen HJ, Fages C, Kuja-Panula J, Ridley AJ, Rauvala H. Receptor for advanced glycation end products-binding COOH-terminal motif of amphoterin inhibits invasive migration and metastasis. Cancer Res. 2002;62:4805–4811. [PubMed] [Google Scholar]
- 80.Palumbo R, Galvez BG, Pusterla T, De Marchis F, Cossu G, Marcu KB, Bianchi ME. Cells migrating to sites of tissue damage in response to the danger signal HMGB1 require NF-kappaB activation. J Cell Biol. 2007;179:33–40. doi: 10.1083/jcb.200704015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rouhiainen A, Kuja-Panula J, Wilkman E, Pakkanen J, Stenfors J, Tuominen RK, Lepantalo M, Carpen O, Parkkinen J, Rauvala H. Regulation of monocyte migration by amphoterin (HMGB1) Blood. 2004;104:1174–1182. doi: 10.1182/blood-2003-10-3536. [DOI] [PubMed] [Google Scholar]
- 82.Yang D, Chen Q, Yang H, Tracey KJ, Bustin M, Oppenheim JJ. High mobility group box-1 protein induces the migration and activation of human dendritic cells and acts as an alarmin. J Leukoc Biol. 2007;81:59–66. doi: 10.1189/jlb.0306180. [DOI] [PubMed] [Google Scholar]
- 83.Dumitriu IE, Bianchi ME, Bacci M, Manfredi AA, Rovere-Querini P. The secretion of HMGB1 is required for the migration of maturing dendritic cells. J Leukoc Biol. 2007;81:84–91. doi: 10.1189/jlb.0306171. [DOI] [PubMed] [Google Scholar]
- 84.Orlova VV, Choi EY, Xie C, Chavakis E, Bierhaus A, Ihanus E, Ballantyne CM, Gahmberg CG, Bianchi ME, Nawroth PP, Chavakis T. A novel pathway of HMGB1-mediated inflammatory cell recruitment that requires Mac-1-integrin. EMBO J. 2007;26:1129–1139. doi: 10.1038/sj.emboj.7601552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Degryse B, Bonaldi T, Scaffidi P, Muller S, Resnati M, Sanvito F, Arrigoni G, Bianchi ME. The high mobility group (HMG) boxes of the nuclear protein HMG1 induce chemotaxis and cytoskeleton reorganization in rat smooth muscle cells. J Cell Biol. 2001;152:1197–1206. doi: 10.1083/jcb.152.6.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Degryse B, de Virgilio M. The nuclear protein HMGB1, a new kind of chemokine? FEBS Lett. 2003;553:11–17. doi: 10.1016/s0014-5793(03)01027-5. [DOI] [PubMed] [Google Scholar]
- 87.Tian J, Avalos AM, Mao SY, Chen B, Senthil K, Wu H, Parroche P, Drabic S, Golenbock D, Sirois C, Hua J, An LL, Audoly L, La Rosa G, Bierhaus A, Naworth P, Marshak-Rothstein A, Crow MK, Fitzgerald KA, Latz E, Kiener PA, Coyle AJ. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8:487–496. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 88.Silva E, Arcaroli J, He Q, Svetkauskaite D, Coldren C, Nick JA, Poch K, Park JS, Banerjee A, Abraham E. HMGB1 and LPS induce distinct patterns of gene expression and activation in neutrophils from patients with sepsis-induced acute lung injury. Intensive Care Med. 2007 doi: 10.1007/s00134-007-0748-2. [DOI] [PubMed] [Google Scholar]
- 89.Sha Y, Zmijewski J, Xu Z, Abraham E. HMGB1 develops enhanced proinflammatory activity by binding to cytokines. J Immunol. 2008;180:2531–2537. doi: 10.4049/jimmunol.180.4.2531. [DOI] [PubMed] [Google Scholar]
- 90.Urbonaviciute V, Furnrohr BG, Meister S, Munoz L, Heyder P, De Marchis F, Bianchi ME, Kirschning C, Wagner H, Manfredi AA, Kalden JR, Schett G, Rovere-Querini P, Herrmann M, Voll RE. Induction of inflammatory and immune responses by HMGB1-nucleosome complexes: implications for the pathogenesis of SLE. J Exp Med. 2008;205:3007–3018. doi: 10.1084/jem.20081165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E. Involvement of TLR 2 and TLR 4 in cellular activation by high mobility group box 1 protein (HMGB1) J Biol Chem. 2004;279:7370–7377. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- 92.Yu M, Wang H, Ding A, Golenbock DT, Latz E, Czura CJ, Fenton MJ, Tracey KJ, Yang H. HMGB1 signals through Toll-like receptor (TLR) 4 and TLR2. Shock. 2006;26:174–179. doi: 10.1097/01.shk.0000225404.51320.82. [DOI] [PubMed] [Google Scholar]
- 93.Park JS, Gamboni-Robertson F, He Q, Svetkauskaite D, Kim JY, Strassheim D, Sohn JW, Yamada S, Maruyama I, Banerjee A, Ishizaka A, Abraham E. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am J Physiol Cell Physiol. 2006;290:C917–C924. doi: 10.1152/ajpcell.00401.2005. [DOI] [PubMed] [Google Scholar]
- 94.Kokkola R, Andersson A, Mullins G, Ostberg T, Treutiger CJ, Arnold B, Nawroth P, Andersson U, Harris RA, Harris HE. RAGE is the Major Receptor for the Proinflammatory Activity of HMGB1 in Rodent Macrophages. Scand J Immunol. 2005;61:1–9. doi: 10.1111/j.0300-9475.2005.01534.x. [DOI] [PubMed] [Google Scholar]
- 95.Pedrazzi M, Patrone M, Passalacqua M, Ranzato E, Colamassaro D, Sparatore B, Pontremoli S, Melloni E. Selective proinflammatory activation of astrocytes by high-mobility group box 1 protein signaling. J Immunol. 2007;179:8525–8532. doi: 10.4049/jimmunol.179.12.8525. [DOI] [PubMed] [Google Scholar]
- 96.Yamoah K, Brebene A, Baliram R, Inagaki K, Dolios G, Arabi A, Majeed R, Amano H, Wang R, Yanagisawa R, Abe E. High-mobility group box proteins modulate tumor necrosis factor-alpha expression in osteoclastogenesis via a novel deoxyribonucleic acid sequence. Mol Endocrinol. 2008;22:1141–1153. doi: 10.1210/me.2007-0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fiuza C, Bustin M, Talwar S, Tropea M, Gerstenberger E, Shelhamer JH, Suffredini AF. Inflammation-promoting activity of HMGB1 on human microvascular endothelial cells. Blood. 2003;101:2652–2660. doi: 10.1182/blood-2002-05-1300. [DOI] [PubMed] [Google Scholar]
- 98.Treutiger CJ, Mullins GE, Johansson AS, Rouhiainen A, Rauvala HM, Erlandsson-Harris H, Andersson U, Yang H, Tracey KJ, Andersson J, Palmblad JE. High mobility group 1 B-box mediates activation of human endothelium. J Intern Med. 2003;254:375–385. doi: 10.1046/j.1365-2796.2003.01204.x. [DOI] [PubMed] [Google Scholar]
- 99.Kokkola R, Li J, Sundberg E, Aveberger AC, Palmblad K, Yang H, Tracey KJ, Andersson U, Harris HE. Successful treatment of collagen-induced arthritis in mice and rats by targeting extracellular high mobility group box chromosomal protein 1 activity. Arthritis Rheum. 2003;48:2052–2058. doi: 10.1002/art.11161. [DOI] [PubMed] [Google Scholar]
- 100.Yang H, Wang H, Tracey KJ. HMG-1 rediscovered as a cytokine. Shock. 2001;15:247–253. doi: 10.1097/00024382-200115040-00001. [DOI] [PubMed] [Google Scholar]
- 101.Li J, Kokkola R, Tabibzadeh S, Yang R, Ochani M, Qiang X, Harris HE, Czura CJ, Wang H, Ulloa L, Wang H, Warren HS, Moldawer LL, Fink MP, Andersson U, Tracey KJ, Yang H. Structural basis for the proinflammatory cytokine activity of high mobility group box 1. Mol Med. 2003;9:37–45. [PMC free article] [PubMed] [Google Scholar]
- 102.Messmer D, Yang H, Telusma G, Knoll F, Li J, Messmer B, Tracey KJ, Chiorazzi N. High mobility group box protein 1: an endogenous signal for dendritic cell maturation and Th1 polarization. J Immunol. 2004;173:307–313. doi: 10.4049/jimmunol.173.1.307. [DOI] [PubMed] [Google Scholar]
- 103.Kazama H, Ricci JE, Herndon JM, Hoppe G, Green DR, Ferguson TA. Induction of immunological tolerance by apoptotic cells requires caspase-dependent oxidation of high-mobility group box-1 protein. Immunity. 2008;29:21–32. doi: 10.1016/j.immuni.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu G, Wang J, Park YJ, Tsuruta Y, Lorne EF, Zhao X, Abraham E. High Mobility Group Protein-1 Inhibits Phagocytosis of Apoptotic Neutrophils through Binding to Phosphatidylserine. J Immunol. 2008;181:4240–4246. doi: 10.4049/jimmunol.181.6.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bell CW, Jiang W, Reich CF, Pisetsky DS. The Extracellular Release of HMGB1 during Apoptotic Cell Death. Am J Physiol Cell Physiol. 2006;291(6):C1318–325. doi: 10.1152/ajpcell.00616.2005. [DOI] [PubMed] [Google Scholar]
- 106.Yin K, Gribbin E, Wang H. Interferon-gamma inhibition attenuates lethality after cecal ligation and puncture in rats: implication of high mobility group box-1. Shock. 2005;24:396–401. doi: 10.1097/01.shk.0000175556.03300.c6. [DOI] [PubMed] [Google Scholar]
- 107.Pahuja M, Tran C, Wang H, Yin K. Alveolar macrophage suppression in sepsis is associated with high mobility group box a transmigration. Shock. 2008;29(6):754–760. doi: 10.1097/shk.0b013e31815d0c8f. [DOI] [PubMed] [Google Scholar]
- 108.Hagiwara S, Iwasaka H, Hasegawa A, Asai N, Noguchi T. High-dose intravenous immunoglobulin G improves systemic inflammation in a rat model of CLP-induced sepsis. Intensive Care Med. 2008;34(10):1812–1819. doi: 10.1007/s00134-008-1161-1. [DOI] [PubMed] [Google Scholar]
- 109.Abraham E, Reinhart K, Opal S, Demeyer I, Doig C, Rodriguez AL, Beale R, Svoboda P, Laterre PF, Simon S, Light B, Spapen H, Stone J, Seibert A, Peckelsen C, De Deyne C, Postier R, Pettila V, Artigas A, Percell SR, Shu V, Zwingelstein C, Tobias J, Poole L, Stolzenbach JC, Creasey AA. Efficacy and safety of tifacogin (recombinant tissue factor pathway inhibitor) in severe sepsis: a randomized controlled trial. JAMA. 2003;290:238–247. doi: 10.1001/jama.290.2.238. [DOI] [PubMed] [Google Scholar]
- 110.Hagiwara S, Iwasaka H, Matsumoto S, Noguchi T. High dose antithrombin III inhibits HMGB1 and improves endotoxin-induced acute lung injury in rats. Intensive Care Med. 2008;34:361–367. doi: 10.1007/s00134-007-0887-5. [DOI] [PubMed] [Google Scholar]
- 111.Hagiwara S, Iwasaka H, Hasegawa A, Koga H, Noguchi T. Effects of hyperglycemia and insulin therapy on high mobility group box 1 in endotoxin-induced acute lung injury in a rat model. Crit Care Med. 2008;36:2407–2413. doi: 10.1097/CCM.0b013e318180b3ba. [DOI] [PubMed] [Google Scholar]
- 112.Wang H, Zhu S, Ward MF, Gong J, Sama AE. Hyperglycemia aggravates endotoxin-induced high mobility group box 1 protein release: yet another reason not to be too sweet. Crit Care Med. 2008;36:2475–2476. doi: 10.1097/CCM.0b013e318181159c. [DOI] [PubMed] [Google Scholar]
- 113.Chorny A, Delgado M. Neuropeptides rescue mice from lethal sepsis by down-regulating secretion of the late-acting inflammatory mediator high mobility group box 1. Am J Pathol. 2008;172:1297–1307. doi: 10.2353/ajpath.2008.070969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tang Y, Lv B, Wang H, Zuo X, Xiao X. PACAP inhibit the release and cytokine activity of HMGB1 and improve the survival during lethal endotoxemia. Int Immunopharmacol. 2008;8(12):1646–1651. doi: 10.1016/j.intimp.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 115.Wu R, Dong W, Zhou M, Zhang F, Marini CP, Ravikumar TS, Wang P. Ghrelin attenuates sepsis-induced acute lung injury and mortality in rats. Am J Respir Crit Care Med. 2007;176:805–813. doi: 10.1164/rccm.200604-511OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wu R, Dong W, Cui X, Zhou M, Simms HH, Ravikumar TS, Wang P. Ghrelin down-regulates proinflammatory cytokines in sepsis through activation of the vagus nerve. Ann Surg. 2007;245:480–486. doi: 10.1097/01.sla.0000251614.42290.ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chorny A, Anderson P, Gonzalez-Rey E, Delgado M. Ghrelin Protects against Experimental Sepsis by Inhibiting High-Mobility Group Box 1 Release and by Killing Bacteria. J Immunol. 2008;180:8369–8377. doi: 10.4049/jimmunol.180.12.8369. [DOI] [PubMed] [Google Scholar]
- 118.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 119.Huston JM, Ochani M, Rosas-Ballina M, Liao H, Ochani K, Pavlov VA, Gallowitsch-Puerta M, Ashok M, Czura CJ, Foxwell B, Tracey KJ, Ulloa L. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J Exp Med. 2006;203:1623–1628. doi: 10.1084/jem.20052362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Huston JM, Gallowitsch-Puerta M, Ochani M, Ochani K, Yuan R, Rosas-Ballina M, Ashok M, Goldstein RS, Chavan S, Pavlov VA, Metz CN, Yang H, Czura CJ, Wang H, Tracey KJ. Transcutaneous vagus nerve stimulation reduces serum high mobility group box 1 levels and improves survival in murine sepsis. Crit Care Med. 2007;35:2762–2768. doi: 10.1097/01.CCM.0000288102.15975.BA. [DOI] [PubMed] [Google Scholar]
- 121.Wang H, Liao H, Ochani M, Justiniani M, Lin X, Yang L, Al Abed Y, Wang H, Metz C, Miller EJ, Tracey KJ, Ulloa L. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med. 2004;10:1216–1221. doi: 10.1038/nm1124. [DOI] [PubMed] [Google Scholar]
- 122.Pavlov VA, Ochani M, Yang LH, Gallowitsch-Puerta M, Ochani K, Lin X, Levi J, Parrish WR, Rosas-Ballina M, Czura CJ, Larosa GJ, Miller EJ, Tracey KJ, Al Abed Y. Selective alpha7-nicotinic acetylcholine receptor agonist GTS-21 improves survival in murine endotoxemia and severe sepsis. Crit Care Med. 2007;35:1139–1144. doi: 10.1097/01.CCM.0000259381.56526.96. [DOI] [PubMed] [Google Scholar]
- 123.Parrish WR, Rosas-Ballina M, Gallowitsch-Puerta M, Ochani M, Ochani K, Yang LH, Hudson L, Lin X, Patel N, Johnson SM, Chavan S, Goldstein RS, Czura CJ, Miller EJ, Al Abed Y, Tracey KJ, Pavlov VA. Modulation of TNF release by choline requires alpha7 subunit nicotinic acetylcholine receptor-mediated signaling. Mol Med. 2008;14:567–574. doi: 10.2119/2008-00079.Parrish. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yan JJ, Jung JS, Lee JE, Lee J, Huh SO, Kim HS, Jung KC, Cho JY, Nam JS, Suh HW, Kim YH, Song DK. Therapeutic effects of lysophosphatidylcholine in experimental sepsis. Nat Med. 2004;10:161–167. doi: 10.1038/nm989. [DOI] [PubMed] [Google Scholar]
- 125.Wang H, Czura CJ, Tracey KJ. Lipid unites disparate syndromes of sepsis. Nat Med. 2004;10:124–125. doi: 10.1038/nm0204-124. [DOI] [PubMed] [Google Scholar]
- 126.Chen G, Li J, Qiang X, Czura CJ, Ochani M, Ochani K, Ulloa L, Yang H, Tracey KJ, Wang P, Sama AE, Wang H. Suppression of HMGB1 release by stearoyl lysophosphatidylcholine:an additional mechanism for its therapeutic effects in experimental sepsis. J Lipid Res. 2005;46:623–627. doi: 10.1194/jlr.C400018-JLR200. [DOI] [PubMed] [Google Scholar]
- 127.Zhu S, Li W, Li J, Sama AE, Wang H. Caging a beast in the inflammation arena: use of Chinese medicinal herbs to inhibit a late mediator of lethal sepsis, HMGB1. Intern J Clin Exp Med. 2008;1:64–79. [PMC free article] [PubMed] [Google Scholar]
- 128.Wang H, Li W, Li J, Rendon-Mitchell B, Ochani M, Ashok M, Yang L, Yang H, Tracey KJ, Wang P, Sama AE. The Aqueous Extract of a Popular Herbal Nutrient Supplement, Angelica sinensis, Protects Mice against Lethal Endotoxemia and Sepsis. J Nutr. 2006;136:360–365. doi: 10.1093/jn/136.2.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li W, Li J, Ashok M, Wu R, Chen D, Yang L, Yang H, Tracey KJ, Wang P, Sama AE, Wang H. A cardiovascular drug rescues mice from lethal sepsis by selectively attenuating a late-acting proinflammatory mediator, high mobility group box 1. J Immunol. 2007;178:3856–3864. doi: 10.4049/jimmunol.178.6.3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li W, Ashok M, Li J, Yang H, Sama AE, Wang H. A Major Ingredient of Green Tea Rescues Mice from Lethal Sepsis Partly by Inhibiting HMGB1. PLoS ONE. 2007;2:e1153. doi: 10.1371/journal.pone.0001153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wheeler DS, Lahni PM, Hake PW, Denenberg AG, Wong HR, Snead C, Catravas JD, Zingarelli B. The green tea polyphenol epigallocatechin-3-gallate improves systemic hemodynamics and survival in rodent models of polymicrobial sepsis. Shock. 2007;28:353–359. doi: 10.1097/shk.0b013e3180485823. [DOI] [PubMed] [Google Scholar]
- 132.Osuchowski MF, Welch K, Siddiqui J, Remick DG. Circulating cytokine/inhibitor profiles reshape the understanding of the SIRS/CARS continuum in sepsis and predict mortality. J Immunol. 2006;177:1967–1974. doi: 10.4049/jimmunol.177.3.1967. [DOI] [PubMed] [Google Scholar]
- 133.Ji XY, Tan BK, Zhu YZ. Salvia miltiorrhiza and ischemic diseases. Acta Pharmacol Sin. 2000;21:1089–1094. [PubMed] [Google Scholar]
- 134.Cheng TO. Cardiovascular effects of Danshen. Int J Cardiol. 2007;121:9–22. doi: 10.1016/j.ijcard.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 135.Fink MP. Ethyl pyruvate: a novel treatment for sepsis. Curr Drug Targets. 2007;8:515–518. [PubMed] [Google Scholar]
- 136.Ulloa L, Ochani M, Yang H, Tanovic M, Halperin D, Yang R, Czura CJ, Fink MP, Tracey KJ. Ethyl pyruvate prevents lethality in mice with established lethal sepsis and systemic inflammation. Proc Natl Acad Sci U S A. 2002;99:12351–12356. doi: 10.1073/pnas.192222999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Beutler B, Milsark IW, Cerami AC. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985;229:869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- 138.Ziegler EJ, Fisher CJ, Jr, Sprung CL, Straube RC, Sadoff JC, Foulke GE, Wortel CH, Fink MP, Dellinger RP, Teng NN. Treatment of gram-negative bacteremia and septic shock with HA-1A human monoclonal antibody against endotoxin. A randomized, double-blind, placebo-controlled trial. The HA-1A Sepsis Study Group. N Engl J Med. 1991;324:429–436. doi: 10.1056/NEJM199102143240701. [DOI] [PubMed] [Google Scholar]
- 139.Ziegler EJ, McCutchan JA, Fierer J, Glauser MP, Sadoff JC, Douglas H, Braude AI. Treatment of gram-negative bacteremia and shock with human antiserum to a mutant Escherichia coli. N Engl J Med. 1982;307:1225–1230. doi: 10.1056/NEJM198211113072001. [DOI] [PubMed] [Google Scholar]
- 140.Abraham E, Wunderink R, Silverman H, Perl TM, Nasraway S, Levy H, Bone R, Wenzel RP, Balk R, Allred R. Efficacy and safety of monoclonal antibody to human tumor necrosis factor alpha in patients with sepsis syndrome. A randomized, controlled, double-blind, multicenter clinical trial. TNF-alpha MAb Sepsis Study Group. JAMA. 1995;273:934–941. [PubMed] [Google Scholar]
- 141.Cohen J. Adjunctive therapy in sepsis: a critical analysis of the clinical trial programme. Br Med Bull. 1999;55:212–225. doi: 10.1258/0007142991902222. [DOI] [PubMed] [Google Scholar]
- 142.Mantell LL, Parrish WR, Ulloa L. Hmgb-1 as a therapeutic target for infectious and inflammatory disorders. Shock. 2006;25:4–11. doi: 10.1097/01.shk.0000188710.04777.9e. [DOI] [PubMed] [Google Scholar]
- 143.Wang H, Yang H, Tracey KJ. Extracellular role of HMGB1 in inflammation and sepsis. J Intern Med. 2004;255:320–331. doi: 10.1111/j.1365-2796.2003.01302.x. [DOI] [PubMed] [Google Scholar]
- 144.Tzeng HP, Fan J, Vallejo JG, Dong JW, Chen X, Houser SR, Mann DL. Negative inotropic effects of high-mobility group box 1 protein in isolated contracting cardiac myocytes. Am J Physiol Heart Circ Physiol. 2008;294:H1490–H1496. doi: 10.1152/ajpheart.00910.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]