Abstract
Eleven authenticated botanicals used in the traditional Chinese medicine Huo-Luo-Xiao-Ling Dan were screened for ligands to cyclooxygenase (COX) using pulsed ultrafiltration liquid chromatography-mass spectrometry, and a mass spectrometry-based enzyme assay was used to determine the concentration of each of 17 ligands that inhibited COX-1 or COX-2 by 50% (IC50). Acetyl-11-keto-β-boswellic -boswellic acid, acid, acetyl-α-boswellic acid, acetyl-β-boswellic acid, and betulinic acid were COX-1 selective inhibitors with IC50 values of approximately 10 μM. Senkyunolide O and cryptotanshinone were COX-2 selective inhibitors with IC50 values of 5 and 22 μM, respectively. Roburic acid and phenethyl-trans-ferulate inhibited COX-1 and COX-2 equally. COX inhibition and the IC50 values of most of these natural product ligands have not been reported previously.
Keywords: Cyclooxygenase, COX-2, drug discovery, botanical dietary supplements, senkyunolide O, cryptotanshinone
1. Introduction
Rheumatoid arthritis and osteoarthritis are the most common form of arthritis and are the major causes of morbidity, limitation of physical activity and health care utilization, especially in the elderly [1]. Although there is no cure, medications including steroids, non-steroidal anti-inflammatory drugs and opioids are commonly used for the treatment of arthritis. Since most of these drugs are associated with undesirable side effects such as gastrointestinal disturbances [2], new anti-inflammatory drugs are needed and complementary and alternative medicines are being sought [3].
An example of a botanical dietary supplement used to treat arthritis and related disorders is Huo-Lou-Xiao-Lin Dan (HLXL) [4]. HLXL contains 11 Chinese herbs including Ruxiang (Boswellia carterii Birdw., defatted gum resin); Qianghuo (Notopterygium incisum Ting ex H.T. Chang., root and rhizome); Danggui (Angelica sinensis (Oliv.) Diels., root); Baishao (Paeonia lactiflora Pall., root); Gancao (Glycyrrhiza uralensis Fisch., root); Yanhusuo (Corydalis yanhusuo W.T. Wang., root); Danshen (Salvia miltiorrhiza Bge., root); Chuanxiong (Ligusticum chuanxiong S.H. Qiu., root); Qinjiao (Gentiana macrophylla Pall., root); Guizhi (Cinnamomum cassia Presl., twigs); and Duhuo (Angelica pubescens Maxim., root) [2, 5]. To facilitate in vitro, in vivo and clinical investigations of safety and efficacy of this botanical dietary supplement [2, 5], we prepared a chemically standardized HLXL product employing a HPLC fingerprint method [2]. However, to be able to replicate the biological/pharmacological profiles in subsequent studies, it is essential to standardize HLXL both biologically and chemically [6-8]. Furthermore, mechanism of action studies would benefit from the identification of pharmacologically active compounds.
In support of biological and chemical standardization studies of HLXL employing bioassays relevant to arthritis of the knee, as well as to assist in determining potential mechanisms of action of the clinical preparation, extracts of the 11 plant components of HLXL, their isolated chemical compounds and the crude extract of HLXL were tested for anti-inflammatory activities. The isolated compounds included steroids, terpenes, alkaloids, flavonoids, glycolated compounds, and acids. This paper describes cyclooxygenase (COX)-1 and COX-2 screening studies of HLXL and its constituent plants and compounds using pulsed ultrafiltration liquid chromatography-mass spectrometry (LC-MS), the identification of COX ligands, and the determination of the concentration of each ligand that inhibits COX-1 or COX-2 by 50% (IC50).
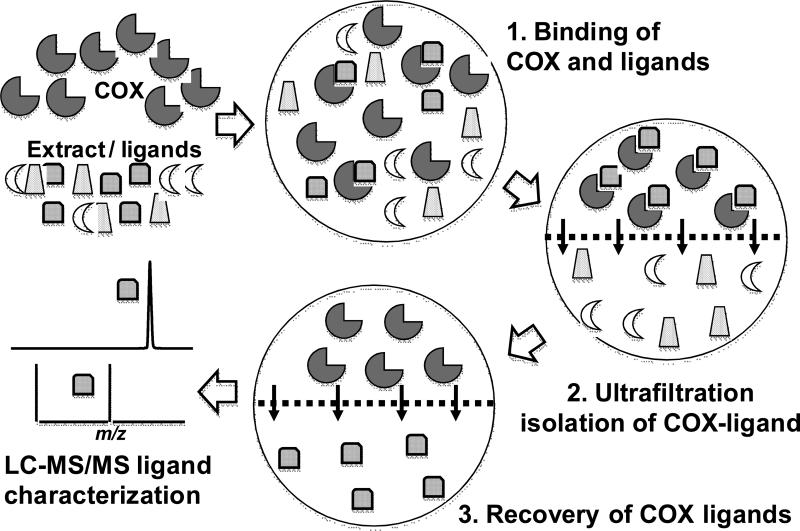
Pulsed ultrafiltration LC-MS is an established approach for the screening of complex mixtures such as combinatorial libraries or natural product extracts for the discovery of ligands to macromolecular targets such as enzymes (see outline of the pulsed ultrafiltration LC-MS approach in Figure 1) [9-15]. By applying this technique, we identified 17 ligands in extracts of HLXL and its constituent plants that bind to COX-2 and characterized additional ligands which will require further isolation and structure determination. Through the use of a COX functional assay based on LC-tandem mass spectrometry (LC-MS-MS), the inhibitory activities of these ligands towards COX-1 and COX-2 were determined. Selective and non-selective COX-1 and COX-2 inhibitors were identified with IC50 values as low as 5 μM. Together, the COX inhibitors in HLXL might be responsible, at least in part, for the anti-inflammatory activity of this traditional Chinese medicine.
Figure 1.
Experimental design of pulsed ultrafiltration LC-MS screening of solutions for ligands to cyclooxygenases. 1. COX is incubated with a mixture of potential ligands; 2. COX-ligand complexes are separated from unbound compounds by using ultrafiltration; 3. COX ligands are released by denaturing the protein with organic solvent; 4. ligands are characterized using LC-MS/MS and identified by comparison to standards.
2. Materials and methods
2.1. Chemicals and reagents
All 11 medicinal plants were procured and authenticated by Dr. Chen Shilin who is a botanist and the Director of the Institute of Medicinal Plants Development at the Academy of Chinese Medicine in Beijing, China. The 11 individual plant components were extracted and formulated into HLXL under GMP conditions by Phytoway (Changsha, Hunan China). Briefly, each of the 11 botanical powders was macerated separately in 70% aqueous acetone overnight at a ratio of 250 g of herb/L solvent. The supernatant was obtained by filtration, and the macerate was extracted twice more with 70 % aqueous acetone and filtered. The combined filtrates for each botanical were concentrated to dryness using a combination of rotary evaporation at 50 °C and lyophilization. HLXL was formulated by compounding the extracts of these 11 medicinal herbs.
Ovine COX-1, ovine COX-2, human recombinant COX-2, arachidonic acid, prostaglandin-E2 (PGE2), and [d4]-PGE2 were purchased from Cayman Chemicals (Ann Arbor, MI). The cofactors (-)epinephrine and hematin [16], and the positive control reference standards resveratrol and indomethacin were purchased from Sigma-Aldrich (St. Louis, MO). The positive control reference standard celecoxib was purchased from 3B PharmaChem International (Wuhan, China). All organic solvents were HPLC grade or better and were purchased from Thermo Fisher (Hanover Park, IL). Purified water was prepared by using a Millipore Milli-Q purification system (Millipore, Billerica, MA).
The standards for each of the COX ligands were either isolated or purchased from commercial sources. The isolated compounds were identified by comparison of their NMR and mass spectra with published data. Acetyl-11-keto-β-boswellic acid, β-boswellic acid, acetyl-α-boswellic acid, acetyl-β-boswellic acid [17, 18], betulinic acid [19], senkyunolide O [20], roburic acid [21], phenethyl trans-ferulate [22], and cryptotanshinone [23] were isolated and identified based on published procedures. All the COX ligands for testing were >95% pure by LC-MS. All other chemicals and solvents were ACS reagent grade, unless stated otherwise.
2.2. Pulsed ultrafiltration LC-MS screening for COX-1 and COX-2 ligands
Extracts and standards were dissolved in dimethylsulfoxide at concentrations of 10 mg/mL and 1 mg/mL, respectively. Mixtures of 10 standards at 100 μg/mL each were screened for relative affinities to COX-2. Incubation mixtures were prepared by adding 1 μL of the dimethylsulfoxide standard mixture solution into 134 μL of PBS buffer (pH 7.4) and 15 μL of enzyme solution containing 600 pmol COX-2. For each standard, the final concentration was ~0.7 μg/mL. Celecoxib at a final concentration of 0.33 μg/mL was used as a positive control. Control analyses were carried out using denatured COX-2 prepared by boiling in water for 10 min.
After incubation in the dark for 1 h at 37 °C, ultrafiltration was carried out using a Microcon (Millipore, Bedford, MA) YM-30 centrifugal filter containing a regenerated cellulose ultrafiltration membrane with a 30,000 MW cut-off at 13,000 g for 10 min at 4 °C. The membrane was pre-washed with 150 μL binding buffer. The sample was spin-rinsed 3 times with 150 μL PBS buffer to remove unbound compounds. Then, each filter was transferred to a new centrifuge tube to reduce background due to non-specific binding to the apparatus. Ligands were dissociated from COX-2 by incubation for 10 min at room temperature with 400 μL methanol/water (90:10, v/v). The dissociated ligands were collected by ultrafiltration. This dissociation and collection procedure was repeated, and the ultrafiltrates were combined for each sample, dried under a stream of dry nitrogen, and reconstituted in 60 μL 50% aqueous methanol immediately prior to LC-MS analysis. A summary of the pulsed ultrafiltration LC-MS screening assay is shown in Figure 1.
Three LC-MS analyses (see details below) were carried out for each sample consisting of 1) the unprocessed sample to obtain a LC-MS profile; 2) the ultrafiltrate after incubation with active COX; and 3) the control ultrafiltrate after incubation with denatured COX. Specific binding was determined based on an increase in peak area in the LC-MS chromatogram of a ligand in the ultrafiltrate of the COX-2 incubation compared to that obtained after incubation with denatured COX-2. If the peak area of a ligand in the incubation with active COX-2 exceeded the peak area observed in the control incubation by at least 2-fold, then the IC50 values and selectivity of that ligand were determined using COX-1 and COX-2 functional assays.
2.3. COX functional assay
The inhibitory activities of each COX ligand towards COX-2 and COX-1 were determined using a functional assay based on the formation of PGE2 which is a stable oxidation product resulting from COX oxidation of arachidonic acid. Briefly, 2 μL of a 100 μM hematin (co-factor) solution and 10 μL of a 40 mM L-epinephrine (co-factor) solution were mixed with 146 μL of a 100 mM Tris.HCl buffer (pH 8.0). Then, 20 μL of COX-2 (0.2 μg) or COX-1 (0.1 μg) solution was added, and the mixture was incubated at room temperature for 2 min. The COX ligand (2 μL in dimethylsulfoxide) was added to give a 10 μM concentration for initial testing or one of 11 other concentrations for IC50 determination, and the mixture was preincubated at 37 °C for 10 min to allow for time-dependent enzyme inactivation [24]. Celecoxib and indomethacin were used as positive controls for the functional inhibition assays of COX-2 and COX-1, respectively. Negative controls were identical except that 2 μL of dimethylsulfoxide containing no test compound was used.
Each COX inhibition reaction was initiated by adding 20 μL of arachidonic acid (5 μM, final concentration) and terminated after 2 min by adding 20 μL 2.0 M HCl. A 10 μL aliquot of [d4]-PGE2 (100 ng/mL) was added as a surrogate standard. Each sample was extracted using 800 μL hexane/ethyl acetate (50:50, v/v), and the organic phase was removed, evaporated to dryness, and reconstituted in 100 μL methanol/water (50:50, v/v) immediately prior to quantitative analysis of PGE2 and [d4]-PGE2 using LC-MS-MS as described previously [25].
The inhibitory potency of each test sample was determined by comparing the amount of PGE2 produced with that of a negative control. For IC50 value determinations, 11 different concentrations of the test compound were assayed three times. The inhibition curves were plotted using Graph Pad Prism 5 software (Mountain View, CA), and the IC50 values of each compound for the inhibition of human COX-2 and ovine COX-1 were determined.
2.4 Mass spectrometry
A Micromass (Manchester, UK) Q-TOF-2 high resolution hybrid mass spectrometer equipped with electrospray and a Waters (Bedford, MA) Alliance 2690 HPLC system was used for pulsed ultrafiltration mass spectrometric screening. HPLC separations were carried out using a Waters Xterra C18 column (2.1 × 100 mm, 3 μm) at a flow rate of 0.2 mL/min. The mobile phase consisted of a 50 min linear gradient from 20% to 100% acetonitrile in 0.5% aqueous acetic acid. Ligands were ionized using negative ion electrospray. As an alternative to electrospray, positive ion atmospheric pressure photoionization (APPI) was used during LC-MS-MS with an Agilent (Santa Clara, CA) 6410 triple quadrupole mass spectrometer equipped with an Agilent 1200 HPLC system. During APPI, HPLC separations were carried out using a linear gradient from 60% to 90% methanol in water.
For the COX functional assay, HPLC separations were carried out using a Shimadzu (Columbia, MD) Prominence HPLC system with a Waters XTerra MS C18 (2.1×50 mm, 3.5 μm) analytical column, and an isocratic mobile phase consisting of acetonitrile/aqueous 0.1% formic acid (35:65; v/v) at a flow rate of 200 μL/min. Negative ion electrospray tandem mass spectrometry and collision-induced dissociation with selected reaction monitoring (SRM) were used with an Applied Biosystems (Foster City, CA) API 4000 triple quadrupole mass spectrometer with a nitrogen gas collision energy of -23 eV. The SRM transitions of m/z 351 to m/z 271 for PGE2 and m/z 355 to m/z 275 for the surrogate standard [d4]-PGE2 were used based on the method of Cao et al. [25].
Extracts of the 11 plant components of HLXL were assayed for the presence of the COX ligands using LC-MS-MS with electrospray or APPI. High resolution tandem mass spectrometry was used with the QTOF-2 mass spectrometer for elemental composition determination and structural analysis. After the identification of the botanicals containing each ligand, the compounds were identified, when possible, by comparison to known compounds. The structures of previously unknown compounds or those for which no authentic standards were available are the subject of on-going investigation and are not reported here.
The amount of each COX ligand in HLXL was determined using LC-MS-MS with an Agilent 6410 triple quadrupole mass spectrometer and a model 1200 HPLC system or a Shimadzu IT-TOF mass spectrometer and a Prominence HPLC system. Negative ion electrospray was used for all compounds except for senkyunolide O and cryptoshinone, which were assayed using positive ion APPI. A Waters XTerra MS C18 (2.1 × 100 mm, 3.5 μm) analytical column was used for HPLC separations. The mobile phase consisted of a 60 min linear gradient from 20 to 100% acetonitrile containing 0.1% aqueous formic acid for the organic acids or a 30 min linear gradient from 20 to 60% acetonitrile containing 0.1% aqueous formic acid for all other ligands except senkyunolide O and cryptoshinone which were measured using a 15 min linear gradient from 70 to 90% acetonitrile in water. HLXL was dissolved in methanol at 4 mg/mL, and standard curves were prepared using either oleanolic acid or resveratrol at 0.1 ng/mL as an internal standard.
3. Results
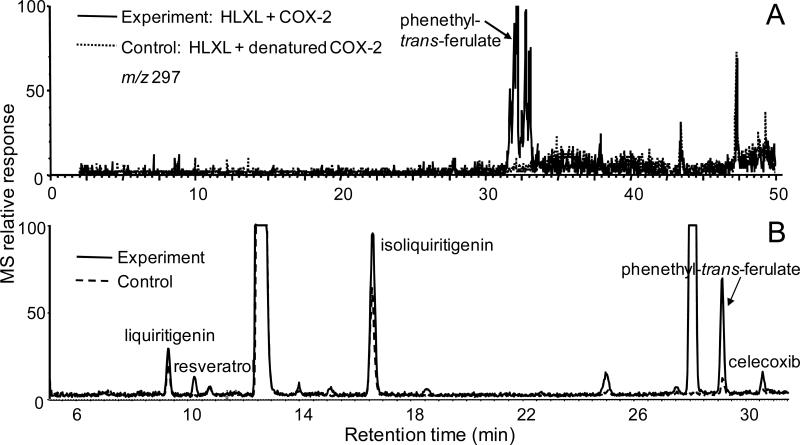
Extracts HLXL, the 11 plant components of HLXL and mixtures of standard compounds were screened for ligands to COX-2 using pulsed ultrafiltration LC-MS. Enhancement of LC-MS peak heights for ultrafiltrates from incubations with active COX-2 compared with those from incubations using denatured COX-2 indicated the presence of ligands to COX-2. For example, phenethyl-trans-ferulate was detected in HLXL as a COX-2 ligand using pulsed ultrafiltration LC-MS (Figure 2A). Based on the elemental compositions of this and other ligands, which were derived from accurate mass measurements, and based on compounds reported to occur in the botanicals comprising HLXL, standards with these properties were obtained for additional testing. To confirm that the standard compounds were also ligands of COX-2, they were screened for COX-2 binding as mixtures using pulsed ultrafiltration LC-MS. As examples, phenethyl trans-ferulate, liquiritigenin and isoliquiritigenin showed peak enhancement due to specific binding to COX-2 (Figure 2B). However, only ligands showing ≥40% peak enhancement compared to the control chromatograms, such as phenethyl-trans-ferulate and isoliquiritigenin, were selected for COX inhibition studies using a functional enzyme assay. Liquiritigenin which is an isomer of isoliquiritigenin did not show sufficient binding to COX-2 to qualify for additional measurements (Figure 2B). Some compounds that are known to be constituents of HLXL based on the literature were screened as a library of HLXL compounds, even though they were not detected during pulsed ultrafiltration LC-MS screening with electrospray. The compounds senkyunolide O and cryptotanshinone could not be detected using electrospray but were determined to be COX-2 ligands using pulsed ultrafiltration LC-MS screening with APPI instead of electrospray.
Figure 2.
Pulsed ultrafiltration LC-MS screening of compounds that bind to COX-2. Enhancement of peak heights in the experiment using active COX-2 compared with the control containing denatured enzyme indicates specific binding to COX-2. (A) Pulsed ultrafiltration LC-MS screening of HLXL for COX-2 ligands. The computer-reconstructed mass chromatogram of m/z 297 shows the detection of the deprotonated molecule of the COX-2 ligand phenethyl trans-ferulate. (B) Total ion chromatogram of a mixture of COX-2 ligands (0.7 μg/mL each) including the HLXL compounds liquiritigenin, isoliquiritigenin and phenethyl trans-ferulate. Resveratrol and celecoxib were included in the assay as positive controls. The peaks eluting at approximately 12 and 28 min are impurities in the mobile phase or ion source, and the peak eluting at 25 min is p-hydroxyphenethyl anisate which not in HLXL but was included in this particular assay as another positive control.
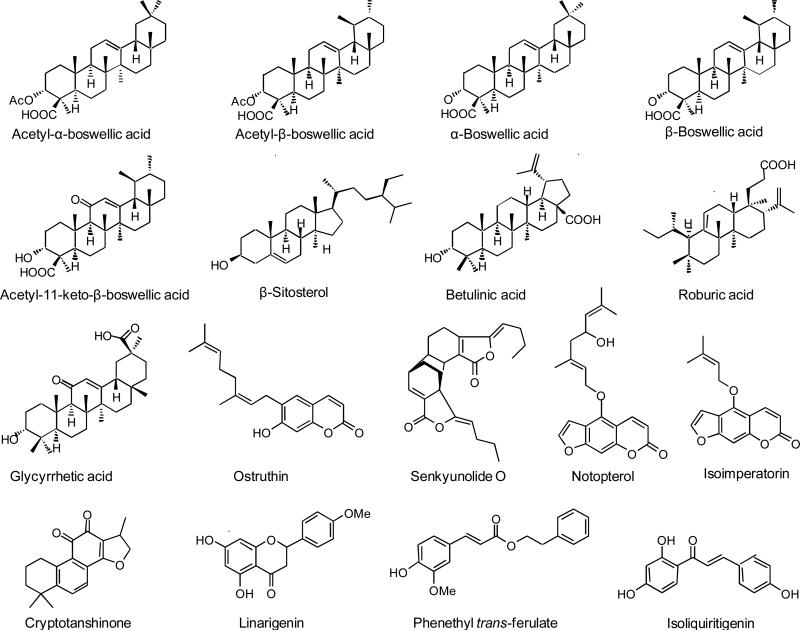
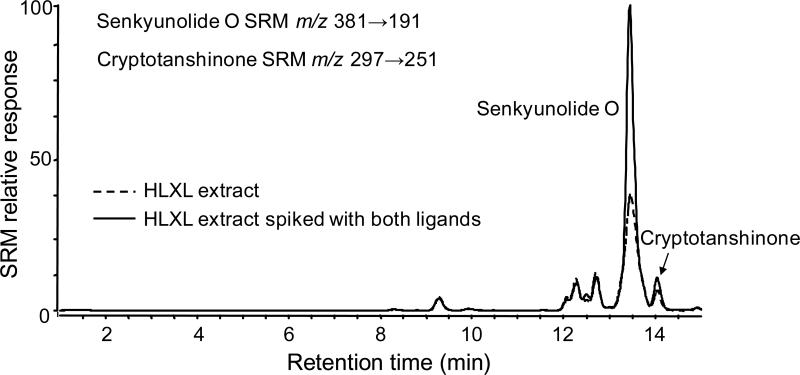
As shown in Figure 3, 17 compounds were identified as COX-2 ligands. To confirm that each of these ligands was derived from a plant component of HLXL, its extract was spiked with each of these standards and analyzed using LC-MS or LC-MS-MS using electrospray or APPI. Enhancement of specific peaks in the mass chromatograms or the selected reaction monitoring chromatograms of the extracts of plant components by these standards confirmed that they were present in the extracts. For example, Figure 4 shows enhancement of peaks in the LC-MS-MS chromatogram corresponding to senkyunolide O and cryptotanshonone when HLXL was spiked with standards of these compounds. Some ligands for COX-2 were detected using pulsed ultrafiltration LC-MS that did not correspond to compounds for which standards were available (data not shown). Chemical fractionation studies are in progress to provide sufficient quantities of these compounds for identification using NMR and mass spectrometry.
Figure 3.
Chemical structures COX-2 ligands from HLXL identified using pulsed ultrafiltration LC-MS with comparison to standards.
Figure 4.
Identification of senkyunolide O and cryptotanshinone in HLXL using LC-MS-MS. In addition to demonstrating identical elemental compositions using high resolution mass spectrometry with accurate mass measurements and identical tandem mass spectra, co-chromatography was demonstrated using LC-MS-MS. Note that the signals for the SRM transitions corresponding to senkyunolide O and cryptotanshinone have been summed into one chromatogram.
The specific botanicals of HLXL which contain the 17 COX-2 ligands were identified and summarized in Table 1. The COX-2 ligands were determined to be constituents of 6 of the 11 botanicals used to produce HLXL. Specifically, there were 5 boswellic acids derived from Boswellia carterii, 4 compounds from Glycyrrhiza incisum, 4 compounds from Notopterygium incisum, 2 compounds from Salvia miltiorrhiza, and 1 compound each from Gentiana macrophylla and Ligusticum chuangxiong.
Table 1.
HLXL compounds determined to be ligands of COX-2 using pulsed ultrafiltration LC-MS (>40% bound to ovine COX-2 during pulsed ultrafiltration LC-MS)
| Compound Name | Plant Source |
|---|---|
| Senkyunolide O | Ligusticum chuanxiong |
| Betulinic acid | Glycyrrhiza uralensis |
| Glycyrrhetic acid | Glycyrrhiza uralensis |
| Isoliquiritigenin | Glycyrrhiza uralensis |
| Linarigenin | Glycyrrhiza uralensis |
| Ostruthin | Notopterygium incisum |
| Notopterol | Notopterygium incisum |
| Isoimperatorin | Notopterygium incisum |
| Phenethyl trans-ferulate | Notopterygium incisum |
| Cryptotanshinone | Salvia miltiorrhiza |
| β-Sitosterol | Salvia miltiorrhiza |
| Acetyl-11-keto-β-boswellic acid | Boswellia carterii |
| β-Boswellic acid | Boswellia carterii |
| Acetyl-β-boswellic acid | Boswellia carterii |
| α-Boswellic acid | Boswellia carterii |
| Acetyl-α-boswellic acid | Boswellia carterii |
| Roburic acid | Gentiana macrophylla |
Although pulsed ultrafiltration LC-MS is an efficient screening assay for the detection and characterization of ligands to COX-2 in mixtures and botanical extracts [12], this screening assay does not indicate whether or not the ligands are inhibitors of COX-2. Therefore, a functional COX-2 assay was used to determine if each ligand was also a COX-2 inhibitor. Since many COX-2 inhibitors also inhibit COX-1, functional COX-1 assays were carried out. Out of 17 COX-2 ligands found in HLXL, 10 of these compounds were confirmed to be COX-2 inhibitors, and 7 compounds also inhibited COX-1 (Table 2). The ligands α-boswellic acid, glycyrrhetic acid, β-sitosterol, isoimperatorin, linarigenin, notopterol, and ostruthin did not inhibit COX-1 or COX-2 in the functional assays. These compounds probably bound to COX-2 non-specifically so that enzymatic activity was not affected.
Table 2.
Inhibitors of COX-1 and COX-2 in HLXL identified by using a combination of pulsed ultrafiltration LC-MS (Table 1) and COX functional assays.
| Compound (10 μM) | Inhibition of COX-1 (%) | Inhibition of COX-2 (%) |
|---|---|---|
| Mean ± std. dev. (N=3) | Mean ± std. dev. (N=3) | |
| β-Boswellic acid | 42 ± 15 | 17 ± 15 |
| Acetyl-α-boswellic acid | 74 ± 3 | 19 ± 5 |
| Isoliquiritigenin | 0 ± 10 | 23 ± 3 |
| Acetyl-11-keto-β-boswellic acid | 39 ± 5 | 23 ± 15 |
| Acetyl-β-boswellic acid | 31 ± 10 | 25 ± 6 |
| Phenethyl-trans-ferulate | 44 ± 3 | 25 ± 10 |
| Cryptotanshinone | 0 ± 22 | 31 ± 20 |
| Betulinic acid | 32 ± 5 | 33 ± 5 |
| Roburic acid | 33 ± 8 | 45 ± 5 |
| Senkyunolide O | 0 ± 5 | 52 ± 20 |
| Resveratrol (reference compound) | 86 ± 7 | 90 ± 5 |
| Celecoxib (33 μM) (positive control) | 68 ± 5 | 95 ± 3 |
| Celecoxib (46 nM) (positive control) | 0 ± 10 | 50 ± 10 |
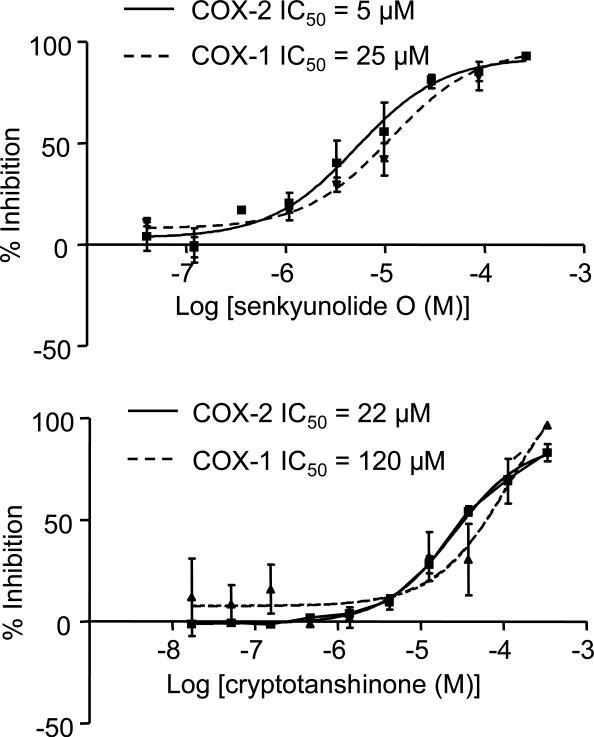
For each compound in Table 2 that showed COX-1 or COX-2 inhibition exceeding 30% at a concentration of 10 μM, the IC50 values were determined. Out of these 11 COX inhibitors, all compounds except for isoliquiritigenin showed significant COX inhibition and were tested further. The IC50 values for these 9 COX inhibitors are summarized in Table 3. As examples of the IC50 value determinations, the data for inhibition of the COX-1 and COX-2 by senkyunolide O and cryptotanshinone are shown in Figure 5. By comparing the ratios of the IC50 values for each inhibitor toward COX-1 and COX-2, the selectivity of each COX inhibitor was determined (Table 3). Note that the known natural product COX inhibitor resveratrol and the COX-2 inhibitor celecoxib were used as positive controls in these assays and are included in Table 2 and Table 3.
Table 3.
IC50 values for COX-1 and COX-2 inhibitors in HLXL
| Compound | IC50 COX-1 (μM, N=3) | IC50 COX-2 (μM, N=3) | COX-2 selectivity (IC50 COX-1 / IC50 COX-2) |
|---|---|---|---|
| Senkyunolide O | 25 ± 10a | 5 ± 5 | 5.00 |
| Roburic acid | 5 ± 2 | 9 ± 3 | 0.56 |
| Cryptotanshinone | > 100 | 22 ± 10 | COX-2 specific |
| Phenethyl trans-ferulate | 18 ± 5 | 31 ± 15 | 0.58 |
| Acetyl-β-boswellic acid | 8 ± 5 | 73 ± 20 | 0.11 |
| Acetyl-11-keto-β-boswellic acid | 8 ± 3 | 85 ± 20 | 0.09 |
| Betulinic acid | 20 ± 3 | > 100 | COX-1 specific |
| β-Boswellic acid | 15 ± 5 | > 100 | COX-1 specific |
| Acetyl-α-boswellic acid | 9 ± 4 | > 100 | COX-1 specific |
| Resveratrol (reference) | 0.86 ± 0.1 | 3 ± 2.06 | 0.3 |
| Celecoxib (positive control) | 30 ± 15.30 | 0.05 ± 0.03 | 600.00 |
Mean ± std. dev.
Figure 5.
Determination of IC50 values of cryptotanshinone and senkyunolide O for the inhibition of COX-1 and COX-2. Both compounds showed selectivity for COX-2 inhibition.
Quantitative analyses of the COX inhibitors in HLXL were carried using LC-MS-MS. The most abundant COX inhibitors in HLXL were acetyl-11-keto-β-boswellic acid at 2.98 μg/mg (wt/wt) and senkyunolide O at 1.90 μg/mg. The next most abundant COX inhibitors were cryptoshinone (0.610 μg/mg), betulinic acid (0.604 μg/mg), acetyl-β-boswellic acid (0.552 μg/mg), acetyl-α-boswellic acid (0.385 μg/mg), and roburic acid (0.307 μg/mg). The least abundant COX inhibitors in HLXL were phenethyl trans-ferulate at 0.141 μg/mg, isoliquiritigen at 0.119 μg/mg and β-boswellic acid at 0.078 μg/mg. Overall, COX inhibitors comprised 0.77% of HLXL by weight.
4. Discussion
The IC50 of celecoxib, the COX-2 selective positive control compound, was 50 nM for human COX-2 and 30 μM for ovine COX-1. These data are similar to those of Penning, et al., who reported celecoxib IC50 values of 40 nM for COX-2 and 15 μM for COX-1 [26]. The 600-fold selectivity of celecoxib for COX-2 (COX-1 IC50 / COX-2 IC50) measured in this functional assay is also consistent with other previously reported COX-2 selectivities of >300 for celecoxib [27, 28]. The natural product standard used in this study, resveratrol, inhibited COX-1 and COX-2 with IC50 values of 0.86 ± 0.7 μM and 3.06 ± 2 μM, respectively. For comparison, Kang et al. [29], reported similar values of 0.83 ± 0.44 μM for COX-1 and 0.99 ± 0.40 μM for COX-2.
Five boswellic acids isolated from Boswellia carterii were found to be COX-2 ligands. Except for α-boswellic acid, four of these compounds showed significant COX inhibition in the functional assay (Tables 2 and 3). As indicated by their COX-1 IC50 values of ≤15 μM and COX-2 IC50 values of ≥73 μM, β-boswellic acid, acetyl-α-boswellic acid, acetyl-β-boswellic acid, and acetyl-11-keto-β-boswellic acid selectively inhibited COX-1. With the exception of roburic acid, which was the only COX inhibitor identified from Gentiana macrophylla, the boswellic acids were the most potent inhibitors of COX-1 identified in HLXL. These results are similar to those of Siemoneit et al. [30], who reported that acetyl-11-keto-β-boswellic acid binds to the active site of COX-1 and inhibits its activity with an IC50 of 6 μM in an assay using human platelets.
Several of the other COX inhibitors identified during this investigation have been reported to be anti-inflammatory agents and COX inhibitors. Although Yogeeswari et al. [31], reported that betulinic acid has anti-inflammatory activity, they did not report any COX inhibition. In our study, betulinic acid showed weak inhibition of COX-1 (IC50 = 20 μM) and almost no inhibition of COX-2 (Table 3). Among the of most potent COX inhibitors we found in HLXL, roburic acid non-selectively inhibited COX-1 and COX-2 with IC50 values of 5 μM and 9 μM, respectively. To the best of our knowledge, no COX inhibition by roburic acid has been reported previously. Zschocke et al. [32], reported that phenethyl-trans-ferulate is the most potent COX inhibitor in Notopterygium incisum. This is consistent with our findings that phenethyl-trans-ferulate is a non-selective COX inhibitor with a COX-1 IC50 of 18 μM and a COX-2 IC50 of 31 μM (Table 3).
The COX-2 selective natural products that were identified, senkyunolide O and cryptotanshinone, were found in Ligusticum chuangxiiong and Salvia miltiorrhiza, respectively. These COX inhibitors showed ≥5-fold selectivity toward the inhibition of human COX-2 over COX-1 (Table 3). To the best of our knowledge, senkyunolide O has not been reported previously to be a COX-2 selective inhibitor. Jin et al. [33], reported that cryptotanshinone inhibits COX-2 in rat and in cell models, however, they did not report IC50 values for inhibition of COX-1 or COX-2.
In this study, pulsed ultrafiltration LC-MS screening combined with a COX functional assay was used to identify COX-1 and COX-2 inhibitors in the 11 botanical constituents of HLXL. Senkyunolide O and cryptotanshinone were identified as COX-2 selective inhibitors, and acetyl-11-keto-β-boswellic acid, β-boswellic acid, acetyl-11-keto-α-boswellic acid, acetyl-β-boswellic acid, and betulinic acid were identified as COX-1 inhibitors. Phenethyl-trans-ferulate and ruburic acid were determined to be non-selective COX inhibitors in HLXL. This combination of selective and non-selective COX inhibitors constitute 0.77% of HLXL by weight and might contribute to its anti-inflammatory activity.
Acknowledgments
This research was supported by grants P01 CA48112 from the National Cancer Institute (NCI) and P01 AT002605 and P50 AT00155 from the National Center for Complementary and Alternative Medicine (NCCAM), the Office of Dietary Supplements (ODS) and the Office for Research on Women's Health (ORWH) of the National Institutes of Health (NIH). The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the NCI, NCCAM, ODS, ORWH, or the NIH.
Abbreviations
- APPI
atmospheric pressure photoionization
- COX
cyclooxygenase
- IC50
ligand concentration that inhibits enzyme by 50%
- PGE2
prostaglandin-E2
- SRM
selected reaction monitoring
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Kvien TK. Epidemiology and burden of illness of rheumatoid arthritis. Pharmacoeconomics. 2004;22:1–12. doi: 10.2165/00019053-200422001-00002. [DOI] [PubMed] [Google Scholar]
- 2.Zhang RX, Fan AY, Zhou AN, Moudgil KD, Ma ZZ, Lee DY, Fong HH, Berman BM, Lao L. Extract of the Chinese herbal formula Huo Luo Xiao Ling Dan inhibited adjuvant arthritis in rats. J Ethnopharmacol. 2009;121:366–71. doi: 10.1016/j.jep.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes PM, Powell-Griner E, McFann K, Nahin RL. Complementary and alternative medicine use among adults: United States, 2002. Adv Data. 2004;343:1–19. [PubMed] [Google Scholar]
- 4.Ho LJ, Lai JH. Chinese herbs as immunomodulators and potential disease-modifying antirheumatic drugs in autoimmune disorders. Curr Drug Metab. 2004;5:181–92. doi: 10.2174/1389200043489081. [DOI] [PubMed] [Google Scholar]
- 5.Lao L, Fan AY, Zhang RX, Zhou A, Ma ZZ, Lee DY, Ren K, Berman B. Anti-hyperalgesic and anti-inflammatory effects of the modified Chinese herbal formula Huo Luo Xiao Ling Dan (HLXL) in rats. Am J Chin Med. 2006;34:833–44. doi: 10.1142/S0192415X06004326. [DOI] [PubMed] [Google Scholar]
- 6.Chadwick LR, Fong HHS. Quality assurance and standardization in herb-drug interaction evaluation and documentation. In: Lam YWF, Huang S-M, Hall SD, editors. Herbal supplement–drug interactions: scientific and regulatory perspectives. Taylor & Francis; New York: 2006. pp. 191–204. [Google Scholar]
- 7.Fong HHS, Pauli GF, Bolton JL, van Breemen RB, Banuvar S, Shulman L, Geller SE, Farnsworth NR. Chapter 2: Evidence-based herbal medicine: challenges in efficacy and safety assessments. In: Leung PC, Fong H, Xue CC, editors. Annals of traditional Chinese medicine – Vol 2, Current review of Chinese medicine: quality control of herbs and herbal medicine. World Scientific; New Jersey: 2006. pp. 11–26. [Google Scholar]
- 8.van Breemen RB, Fong HH, Farnsworth NR. The role of quality assurance and standardization in the safety of botanical dietary supplements. Chem Res Toxicol. 2007;20:577–82. doi: 10.1021/tx7000493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Breemen RB, Huang CR, Nikolic D, Woodbury CP, Zhao YZ, Venton DL. Pulsed ultrafiltration mass spectrometry: a new method for screening combinatorial libraries. Anal Chem. 1997;69:2159–64. doi: 10.1021/ac970132j. [DOI] [PubMed] [Google Scholar]
- 10.Zhao YZ, van Breemen RB, Nikolic D, Huang CR, Woodbury CP, Schilling A, Venton DL. Screening solution-phase combinatorial libraries using pulsed ultrafiltration/electrospray mass spectrometry. J Med Chem. 1997;40:4006–12. doi: 10.1021/jm960729b. [DOI] [PubMed] [Google Scholar]
- 11.Nikolic D, van Breemen RB. Screening for inhibitors of dihydrofolate reductase using pulsed ultrafiltration mass spectrometry. Comb Chem High Throughput Screen. 1998;1:47–55. [PubMed] [Google Scholar]
- 12.Nikolic D, Habibi-Goudarzi S, Corley DG, Gafner S, Pezzuto JM, van Breemen RB. Evaluation of cyclooxygenase-2 inhibitors using pulsed ultrafiltration-mass spectrometry. Anal Chem. 2000;72:3853–59. doi: 10.1021/ac0000980. [DOI] [PubMed] [Google Scholar]
- 13.Gu C, Nikolic D, Lai J, Xu X, van Breemen RB. Assays of ligand-human serum albumin binding using pulsed ultrafiltration and liquid chromatography-mass spectrometry. Comb Chem High Throughput Screen. 1999;2:353–9. [PubMed] [Google Scholar]
- 14.Sun Y, Gu C, Liu X, Liang W, Yao P, Bolton JL, van Breemen RB. Ultrafiltration tandem mass spectrometry of estrogens for characterization of structure and affinity for human estrogen receptors. J Am Soc Mass Spectrom. 2005;16:271–9. doi: 10.1016/j.jasms.2004.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu D, Guo J, Luo Y, Broderick DJ, Schimerlik MI, Pezzuto JM, van Breemen RB. Screening for ligands of human retinoid X receptor-αusing ultrafiltration mass spectrometry. Anal Chem. 2007;79:9398–402. doi: 10.1021/ac701701k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noreen Y, Ringbom T, Perera P, Danielson H, Bohlin L. Development of a radiochemical cyclooxygenase-1 and -2 in vitro assay for identification of natural products as inhibitors of prostaglandin biosynthesis. J Nat Prod. 1998;61:2–7. doi: 10.1021/np970343j. [DOI] [PubMed] [Google Scholar]
- 17.Belsner K, Buchele B, Werz U, Syrovets T, Simmet T. Structural analysis of pentacyclic triterpenes from the gum resin of Boswellia serrata by NMR spectroscopy. Magn Reson Chem. 2003;41:115–22. [Google Scholar]
- 18.Culioli G, Mathe C, Archier P, Vieillescazes C. A lupane triterpene from frankincense (Boswellia sp., Burseraceae). Phytochemistry. 2003;62:537–41. doi: 10.1016/s0031-9422(02)00538-1. [DOI] [PubMed] [Google Scholar]
- 19.Peng C, Bodenhausen G, Qiu S, Fong H, Farnsworth N, Yuan S, Zheng C. Computer-assisted structure elucidation: application of CISOC-SES to the resonance assignment and structure generation of betulinic acid. Magn Reson Chem. 1998;36:267–78. [Google Scholar]
- 20.Naito T, Katsuhara T, Niitsu K, Ikeya Y, Okada M, Mitsuhashi H. Phthalide dimers from Ligusticum chuangxiong Hort. Heterocycles. 1991;32:2433–42. [Google Scholar]
- 21.Chen QL, Shi ZY, Tu GZ, Sun WJ. [Studies on the chemical constituents in root of Gentiana macrophylla from Shaanxi]. Zhongguo Zhong Yao Za Zhi. 2005;30:1519–22. [PubMed] [Google Scholar]
- 22.Zdero C, Jakupovic J, Bohlmann F. Diterpenes and other constituents from Pteronia Species. Phytochemistry. 1990;29:1231–45. [Google Scholar]
- 23.Sairafianpour M, Christensen J, Staerk D, Budnik BA, Kharazmi A, Bagherzadeh K, Jaroszewski JW. Leishmanicidal, antiplasmodial, and cytotoxic activity of novel diterpenoid 1,2-quinones from Perovskia abrotanoides: new source of tanshinones. J Nat Prod. 2001;64:1398–1403. doi: 10.1021/np010032f. [DOI] [PubMed] [Google Scholar]
- 24.Cuendet M, Mesecar AD, DeWitt DL, Pezzuto JM. An ELISA method to measure inhibition of the COX enzymes. Nat Protoc. 2006;1:1915–21. doi: 10.1038/nprot.2006.308. [DOI] [PubMed] [Google Scholar]
- 25.Cao H, Xiao L, Park G, Wang X, Azim AC, Christman JW, van Breemen RB. An improved LC-MS/MS method for the quantification of prostaglandins E2 and D2 production in biological fluids. Anal Biochem. 2008;372:41–51. doi: 10.1016/j.ab.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Penning TD, Talley JJ, Bertenshaw SR, Carter JS, Collins PW, Docter S, Graneto MJ, Lee LF, Malecha JW, Miyashiro JM, Rogers RS, Rogier DJ, Yu SS, Anderson GD, Burton EG, Cogburn JN, Gregory SA, Koboldt CM, Perkins WE, Seibert K, Veenhuizen AW, Zhang YY, Isakson PC. Synthesis and biological evaluation of the 1,5-diarylpyrazole class of cyclooxygenase-2 inhibitors: identification of 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]benze nesulfonamide (SC-58635, Celecoxib). J Med Chem. 1997;40:1347–65. doi: 10.1021/jm960803q. [DOI] [PubMed] [Google Scholar]
- 27.Marnett LJ, Kalgutkar AS. Cyclooxygenase 2 inhibitors: discovery, selectivity and the future. Trends Pharmacol Sci. 1999;20:465–9. doi: 10.1016/s0165-6147(99)01385-1. [DOI] [PubMed] [Google Scholar]
- 28.Gierse JK, Koboldt CM, Walker MC, Seibert K, Isakson PC. Kinetic basis for selective inhibition of cyclo-oxygenases. Biochem J. 1999;339:607–14. [PMC free article] [PubMed] [Google Scholar]
- 29.Kang SS, Cuendet M, Endringer DC, Croy VL, Pezzuto JM, Lipton MA. Synthesis and biological evaluation of a library of resveratrol analogues as inhibitors of COX-1, COX-2 and NF-kappaB. Bioorg Med Chem. 2009;17:1044–54. doi: 10.1016/j.bmc.2008.04.031. [DOI] [PubMed] [Google Scholar]
- 30.Siemoneit U, Hofmann B, Kather N, Lamkemeyer T, Madlung J, Franke L, Schneider G, Jauch J, Poeckel D, Werz O. Identification and functional analysis of cyclooxygenase-1 as a molecular target of boswellic acids. Biochem Pharmacol. 2008;75:503–13. doi: 10.1016/j.bcp.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 31.Yogeeswari P, Sriram D. Betulinic acid and its derivatives: a review on their biological properties. Curr Med Chem. 2005;12:657–66. doi: 10.2174/0929867053202214. [DOI] [PubMed] [Google Scholar]
- 32.Zschocke S, Lehner M, Bauer R. 5-Lipoxygenase and cyclooxygenase inhibitory active constituents from Qianghuo (Notopterygium incisum). Planta Med. 1997;63:203–6. doi: 10.1055/s-2006-957653. [DOI] [PubMed] [Google Scholar]
- 33.Jin DZ, Yin LL, Ji XQ, Zhu XZ. Cryptotanshinone inhibits cyclooxygenase-2 enzyme activity but not its expression. Eur J Pharmacol. 2006;549:166–72. doi: 10.1016/j.ejphar.2006.07.055. [DOI] [PubMed] [Google Scholar]