Abstract
Transplantation is the treatment of choice for patients with end-stage organ failure. Its success is limited by side effects of immunosuppressive drugs, such as inhibitors of the calcineurin pathway that prevent rejection by reducing synthesis of interleukin-2 by T cells. Moreover, none of the existing drugs efficiently prevent the late development of chronic rejection. Blocking the CD28-mediated T cell costimulation pathway is a non toxic alternative immunosuppression strategy that is currently achieved by blockade of CD80/86, the counter receptors for CD28 on antigen-presenting cells.. However interaction of CD80/86 with CTLA-4 is required for immune regulation. Therefore CD28 blockade, instead of CD80/86 blockade, might preserve regulatory signals mediated by CTLA-4 and favor immune regulation. By using monovalent antibodies, we identified true CD28 antagonists inducing a CTLA-4-dependent decreased T cell function compatible with regulatory T cell (Treg) suppression. In transplantation experiments in primates, blocking CD28 augmented intragraft and peripheral blood regulatory T cells, induced molecular signatures of immune regulation and prevented graft rejection and vasculopathy in synergy with calcineurin inhibition. These findings suggest that targeting costimulation blockade at CD28 favors CTLA-4-dependent immune regulation and promotes allograft survival.
Introduction
T cells were identified as major players in immune responses after allotransplantation and in autoimmunity. T cell activation is induced by specific antigen recognition and reinforced by engagement of costimulatory molecules that regulate their differentiation into either pathogenic effector cells or anti-inflammatory regulatory cells. Costimulation by CD28 and CTLA-4 contributes to determining this balance after initial antigen exposure. The current paradigm holds that constitutively expressed CD28 binds CD80/86 to provide a co-stimulatory signal important for sustaining T cell proliferation and proinflammatory responses (4). Furthermore, although CD28 signals are critical for regulatory T cell (Treg) homeostasis (5), CD28 engagement by CD80/86 molecules can inhibit Treg activity (6). CTLA-4, the other CD80/86 ligand, delivers antiproliferative signals to T cells (7), triggers indoleamine 2,3-dioxygenase (IDO) (8) production in antigen-presenting cells (APCs) and is essential for the suppressive function of Tregs (9) and the induction of tolerance to allografts (10, 11). Targeting the CD28-CD80/86 pathway in patients with CTLA-4-Ig reagents (Belatacept, Abatacept, CD80/86 antagonists) is a promising alternative to current immunosuppressive treatments in autoimmunity (1, 2) and renal transplantation (3). However, CD80/86-specific blocking strategies inhibit CTLA-4 signals crucial to the function of Tregs and do not reproducibly induce transplant tolerance (12, 13). We thus hypothesized that blocking CD28 without affecting CTLA-4 could be an effective strategy for modulating immune responses by preventing the maturation of pathogenic effectors while preserving the function of Tregs. In this study, we used non-cross-linking selective CD28 antagonists and showed that this treatment decreased the allogeneic immune response against kidney or heart transplant and prolonged allograft survival in two primate models.
Results
CTLA-4 dependent and independent components of CD28 blockade
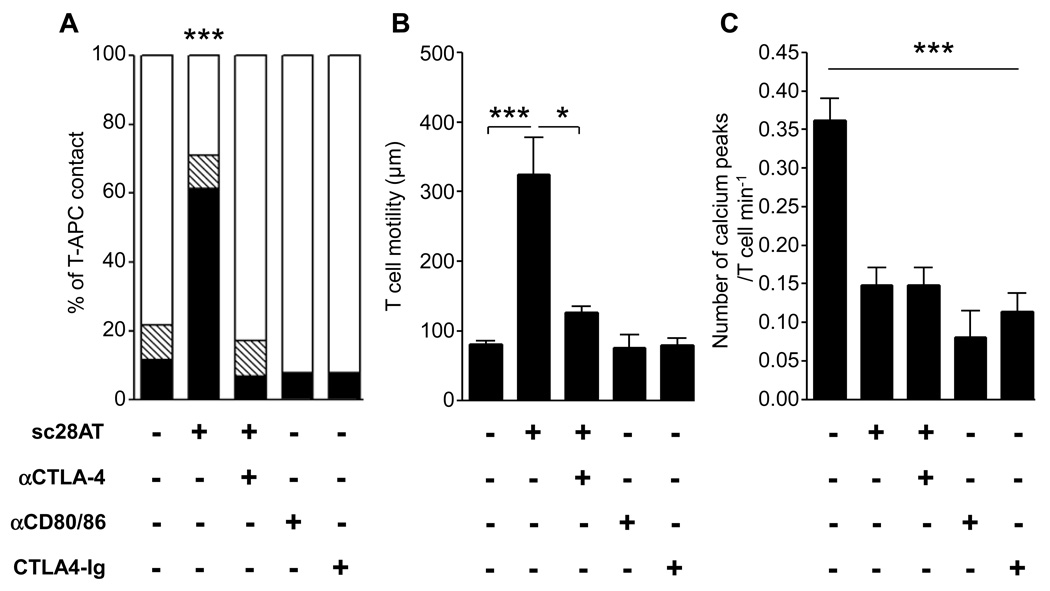
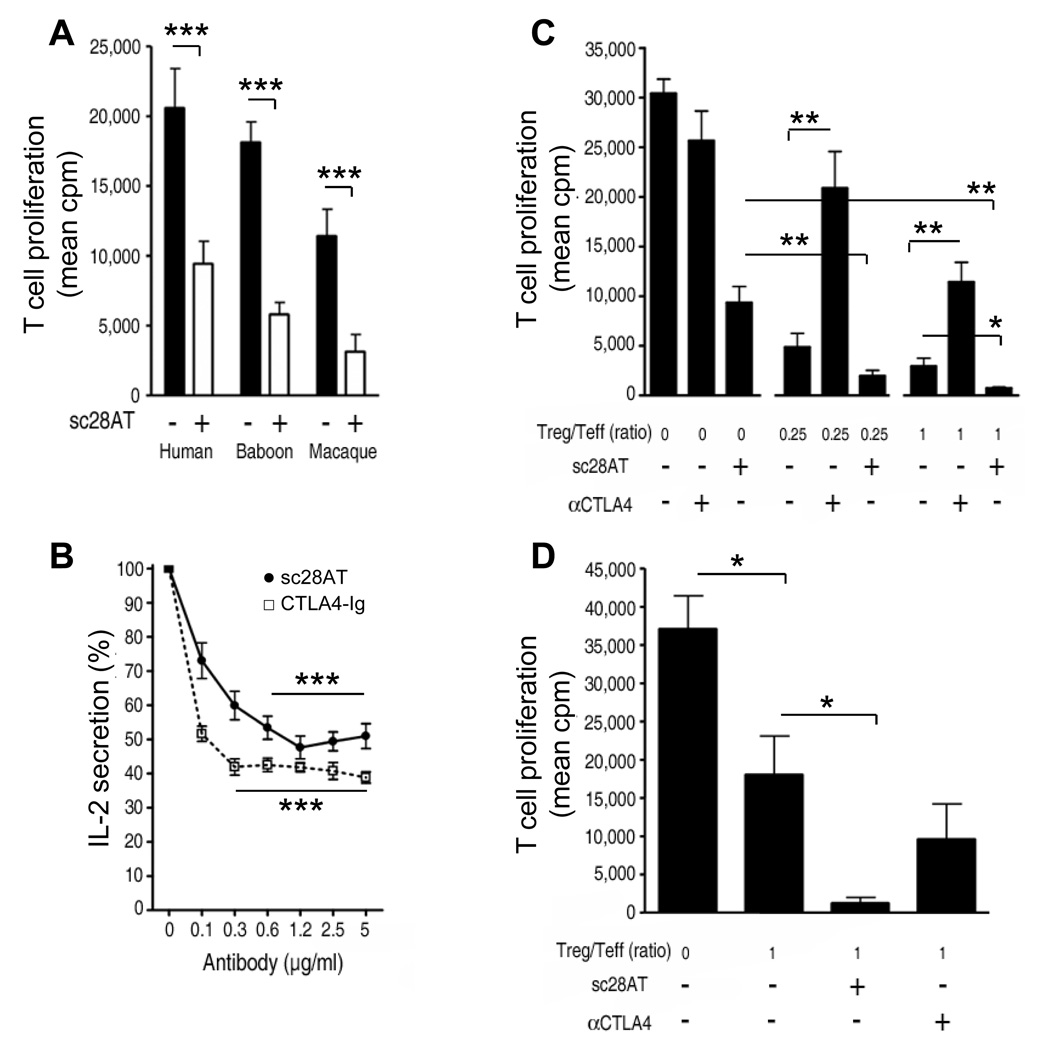
Sc28AT, a monovalent fusion antibody, competes with CD80/86 for binding to CD28 (14). Since the binding epitope is different from the epitope of superagonistic CD28 antibodies (15) (Fig. S1A), sc28AT did not induce TCR-independent activation and proliferation of human T cells (Fig. S1B) or human Treg (Fig. S1C) in vitro. We investigated in vitro the consequences of selective CD28 blockade with sc28AT on cognate human T cell interactions with B cells used as APCs by live-cell dynamic microscopy. Sc28AT prevented the formation of stable T cell-APC conjugates, increased T cell motility and reduced T cell activation as measured by calcium flux (Fig. 1A–C and Movie S 1–Movie S 3). The effect of sc28AT on conjugate formation and T cell motility was abolished by the simultaneous blockade of CTLA-4 (Fig. 1A, B), suggesting that interaction of CTLA-4 with CD80/86 was essential for sc28AT-mediated activity. Even though a minority of T cells still established prolonged contacts with APCs in the presence of sc28AT, these contacts resulted in greatly reduced calcium influx as compared with T cells interacting with APCs in the absence of CD28 blockade, and this reduction was not reversed by CTLA-4 blockade (Fig. 1C and Fig. S2). The importance of CTLA-4-CD80/86 interaction for T cell motility was confirmed after CD80/86 targeting by either CD80/86 antibodies or CTLA4-Ig (Fig. 1B), which did not modify T cells/APCs contacts but prevented T cell activation (Fig. 1, A, C). These data reveal CTLA-4-dependent (promotes T cell motility and inhibits T-APC contacts) and -independent (inhibition of T cell activation) components in the action of monovalent anti-CD28 antibodies. Furthermore, sc28AT reduced the proliferation of human and primate T cells in mixed lymphocyte reactions (MLR; Fig. 2A) and decreased cytokine release after stimulation of Jurkat T cells with superantigens (Fig. 2B), showing that inhibiting long-term T-APCs contacts by specifically antagonizing CD28-CD80/86 interaction does suppress effector T cell activation.
Figure 1. CTLA-4-dependent impairment of T-APC contact after selective CD28 blockade.
(A) Time-lapse microscopy of cognate contacts between EBV-specific T cells and B-EBV APCs in the presence of the indicated Ab. Results are shown as % of cells establishing short (≤ 5 min.; black), medium (between 5 and 15 min., hatched) and long (≥ 15 min., white) contacts over 20 min. (B) T cells were cultured as in (A) and the cumulative distance moved of individual cells was recorded to assess T cell motility. (C) T cells were cultured as in (A) and calcium peaks were recorded. * and *** indicate a significant difference at P<0.05 and 0.001, respectively.
Figure 2. Monovalent CD28 antagonists block allorecognition but do not impede the function of Treg cells in vitro.
(A) Mixed-lymphocyte reaction using human (n=10), baboon (n=15) or macaque (n=9) PBMC. Black bars: mean ±SD in control conditions (mouse irrelevant IgG1). White bars: mean ± SD with 10 µg/ml sc28AT. (B) IL-2 secretion by Jurkat T cells stimulated with bacterial superantigen (staphylococcal enterotoxin E, SEE) and Raji B cells in the presence of sc28AT (n=8) or CTLA4-Ig (n=3). Results are expressed as percentage of IL-2 secretion observed in the absence of Ab (100%). (C) Suppressive activity of human Treg is not impeded by CD28 blockade. Tregs were added to CD4+CD25− T cells stimulated with allogeneic irradiated PBMC at the indicated ratio in the presence of 10µg/ml of CD28 or CTLA-4 blocking antibodies. Results are mean cpm ± SD of one representative assay out of 3. (D) Suppressive activity of human Treg pre-treated with CD28 or CTLA-4 blocking Ab. Treg were first cultured with allogeneic mature DC in the presence of sc28AT or anti-CTLA-4 Fab fragments (10µg/ml) for 18h, washed and assessed in a suppression assay. Results are mean cpm ± SD of a representative assay out of 3. *, ** and *** indicate a significant difference at p<0.05, 0.01 and 0.001, respectively.
Compatibility of CD28 blockade with Treg function
Immunosuppressive drugs might either block or synergize with the suppressive activity of Tregs. It was therefore important to analyze the consequences of CD28 blockade on the function of Tregs. The suppression by Tregs of the proliferation of naïve CD4+ T cells in response to allogeneic irradiated PBMC synergized with sc28AT whereas addition of CTLA-4 antagonists blocked the suppression (Fig. 2C). To examine the effect of sc28AT specifically on Tregs, Tregs were first activated with allogeneic mature dendritic cells (mDC) in the presence of sc28AT and secondarily tested for their ability to suppress the proliferation of naïve T cells stimulated with allogeneic mDC. Treg suppression was significantly increased when Tregs were primed in the presence of sc28AT whereas it was not modified by CTLA-4 blockade (Fig. 2D). Taken together, these data suggest that signals through CTLA-4 and CD28 regulate positively and negatively, respectively, the regulatory activity of Treg. In particular, the enhanced Treg immunosuppressive capacity observed following CD28 blockade with sc28AT in vitro prompted us to examine the potential effect of this CD28 antagonist in vivo.
Selective CD28 blockade in transplantation
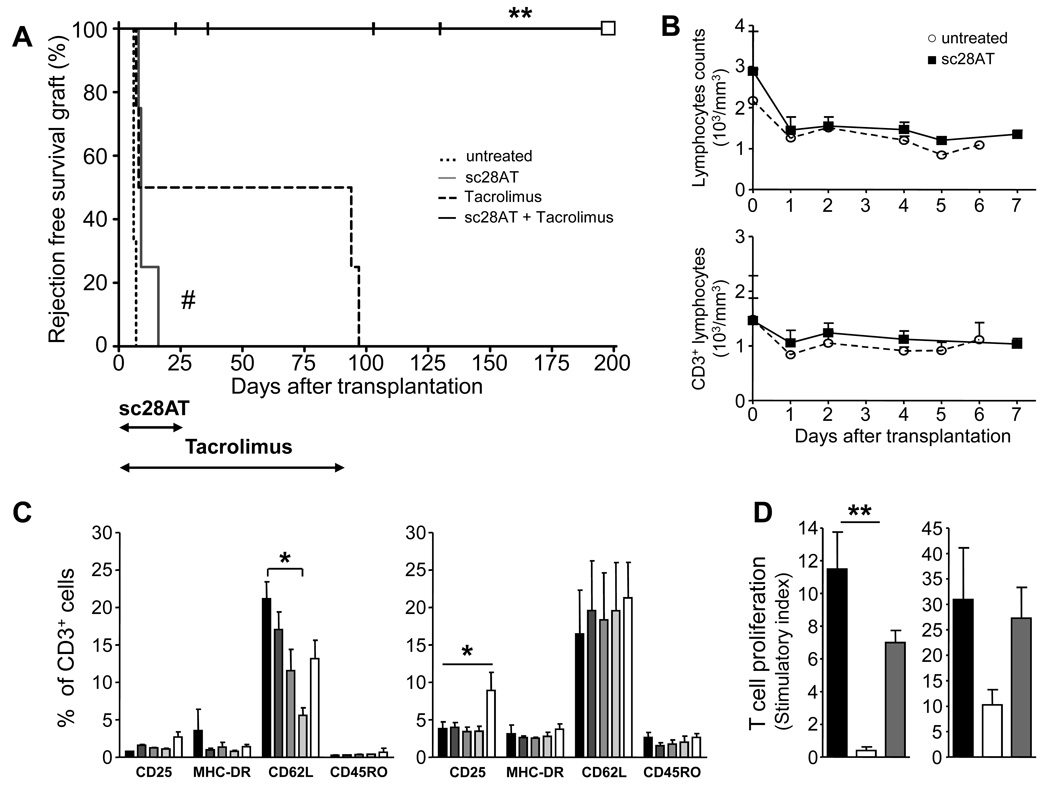
To investigate the action of CD28 blockade in vivo, we used a life-sustaining kidney transplant model in baboons. The animals were divided into four groups (Fig. 3A): 1) Without immunosuppressive treatment, kidney transplant were rejected within a week (median graft survival of 6 days) with acute biopsy-proven cellular rejection (n=3). 2) With monotherapy with the calcineurin inhibitor Tacrolimus for 90 days, 50% of recipients developed a renal graft failure with a biopsy-proven cellular acute rejection during the first week after transplantation. The other 50% had stable kidney function over the 3-month experiment but developed cellular acute rejection within a week following withdrawal of the drug. The median survival time in this group was 47 days (n=4; rejection at 6, 7, 94, 97 days). 3) Sc28AT induction monotherapy for 25 days (see pharmacokinetic profiles in Fig. S3A and description of target cells in Fig. S3C) modestly, but significantly, prolonged graft survival (n=4; median survival time of 11 days). 4) Combined administration of Tacrolimus (0 to 90 days) and sc28AT (0 to 25 days) resulted in a significant increase in graft survival (n=5; median survival time of 103 days: 23, 36, 103, 130, 269). Importantly, no rejection episode (clinical or biopsy-proven) developed in any of these bitherapy recipients, even after complete withdrawal of immunosuppression at day 90. However, three animals in this group were euthanized at day 23, 36 and 130 from pyelonephritis or acute tubular necrosis and one animal was lost at day 103 from an anesthetic accident. Anatomopathological analyses excluded rejection in these animals.
Figure 3. Administration of sc28AT and Tacrolimus prevents kidney allograft rejection in baboons.
(A) Rejection-free survival after renal allotransplantation for baboons without therapy (n=3) or treated with a 25 day induction therapy with sc28AT alone (n=4), Tacrolimus for 90 days (n=4) and sc28AT + Tacrolimus (25 and 90 days, respectively; n=5). Vertical hash marks, death of recipients without graft rejection as assessed by histology. White square, remaining living baboon. **, P=0.008 versus Tacrolimus monotherapy. ##, P=0.01 versus untreated controls. (B) Total lymphocyte counts (means ± SD, upper panel) and CD3+ T cell counts (lower panel) in untreated recipients (n=3) and recipients treated with sc28AT monotherapy (n=4). (C) Phenotype of blood T cells during the first week post transplantation in controls (n=3; left panel) and sc28AT monotherapy recipients (n=4; right panel). Percentages of T cells expressing the specified marker on day 0, 1, 2, 4 and 6 from left (black) to right (white). *, P<0.05. (D) Donor-specific hyporesponsiveness. PBMC were harvested from baboons in the sc28AT + Tacrolimus group and tested in MLR against donor cells before transplantation (black bars) and at day 90 (white bars) and against 3rd party cells at day 90 (grey bars). Results are mean stimulatory index ± SD of triplicate wells. Two experiments are shown. **, P=0.0014.
No differences were observed between groups with regards to CD3+, CD4+ or CD8+ T cell infiltration into the graft. In contrast, CD20+ infiltrating B cells were barely detectable after sc28AT treatment alone or in combination with Tacrolimus whereas they were abundant in kidney graft biopsies from untreated animals or animals treated with Tacrolimus alone (Fig. S4). Furthermore, mRNA levels of inflammatory cytokines IL-6 and IFNγ were reduced in kidney graft biopsies one week after transplantation in animals treated with sc28AT in comparison with control untreated animals (Fig. S5). In contrast, mRNA levels of TGFβ as well as CD25, CTLA-4, Foxp3 and HO-1 were increased in sc28AT-treated animals (Fig. S5).
Absolute numbers of total lymphocytes and CD3+ T cells varied slightly within the normal range after sc28AT treatment (Fig. 3B) (16), indicating that sc28AT did not induce T cell depletion. In addition, the expression of activation markers on T cells was not markedly modified by CD28 blockade (Fig. 3C, and Fig. S6A), although we observed an increase in the percentage of CD25+ T cells one week after transplantation in the sc28AT group (Fig. 3C, right panel). Serum levels of IFNγ, TNFα, IL-2, IL-4, IL-5 or IL-6 cytokines were low and similar in animals receiving sc28AT and controls (Fig. S6B). Thus, interaction of sc28AT with CD28 on T cells did not result in polyclonal T cell activation.
To investigate the alloreactivity of peripheral T cells in kidney recipients that received sc28AT and Tacrolimus bitherapy and failed to reject the allograft, we performed ex-vivo mixed lymphocyte reaction. The proliferative response against donor cells was reduced following combination therapy whereas the alloreactive response against cells from a third party animal was preserved, suggesting the acquisition of donor-specific hyporesponsiveness in animals treated with sc28AT and Tacrolimus (Fig. 3D).
Increase in Tregs following transplantation and specific CD28 blockade
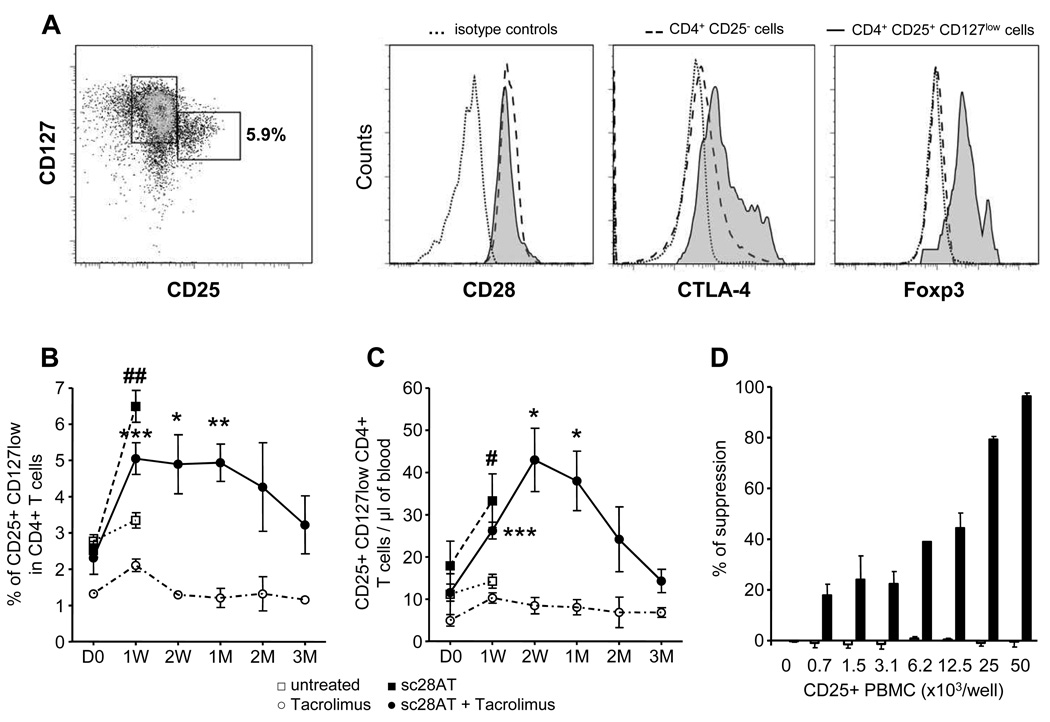
We determined levels of Tregs using multi-parameter flow cytometry. CD4+CD25+CD127lo Tregs also expressed Foxp3, CTLA-4 and CD28 (Fig. 4A). After administration of sc28AT, alone or in combination with Tacrolimus, the percentage of Tregs increased more than twofold within a week to reach 5 to 6% of CD4+ T cells as compared with 3% in untreated recipients and 2% in recipients treated with Tacrolimus alone (Fig. 4B). Absolute Treg counts similarly increased two- to three-fold in recipients treated with sc28AT alone or together with Tacrolimus as compared with their pre-treatment levels, and with untreated or Tacrolimus-treated animals (Fig. 4C). Animal receiving Tacrolimus alone did not display any change in levels of Treg during the 3-month treatment (Fig. 4B–C). We investigated whether these phenotypically defined Tregs were functional. CD25+ PBMC harvested from control Tacrolimus-treated ungrafted baboons were unable to suppress Teff cell proliferation (Fig. 4D). This is in agreement with a previous report showing that CD4+CD25+ T cells are barely detectable in peripheral blood of naïve baboons and this cell population does not exhibit suppressive activity (17). In contrast, CD25+ PBMC obtained 90 days after transplant from kidney allograft recipients treated with sc28AT in combination with Tacrolimus were very effective at suppressing Teff proliferation, even at low Treg:Teff ratios (Fig. 4D).
Figure 4. Treg cell enrichment in the peripheral blood of kidney allograft baboon recipients treated with sc28AT.
(A) CD25+ CD127 lo Treg cells analyzed in blood by flow cytometry after gating on CD3+ CD4+ cells. These cells also expressed CD28, and intra-cellular CTLA-4 and Foxp3. (B-C) Kinetics of CD4+CD25+Foxp3+CD127lo Treg levels in blood, percentage of CD4+ T cells (B) or absolute number (C), in control untreated animals (n=3), sc28AT monotherapy (n=4), Tacrolimus monotherapy (n=4 until week 1 and then n=2), sc28AT + Tacrolimus (n=5 up to 2 weeks, 4 at 1 month and then n=3). D: days, W: weeks, M: month. # and ##, significant difference with the untreated group (P=0.02 and P=0.0011, respectively). ***, ** and * significant difference with the Tacrolimus monotherapy group (P<0.05, 0.01 and 0.001, respectively). (D) Suppressive activity of CD25+ PBMC from sc28AT + Tacrolimus recipients at day 90 after transplantation (black bars, n=3) or from control ungrafted baboons treated with Tacrolimus (white bars, n=4).
The increase in Tregs detected post-transplantation was not due to non-specific expansion of existing CD4+CD25+Tregs by sc28AT since infusion of sc28AT at the same dose in non-transplanted baboons during a week did not elicite changes to the frequency of Tregs (Fig. S7).
T cells expressing Foxp3 and CTLA-4 accumulate in the kidney allograft after CD28 inhibition
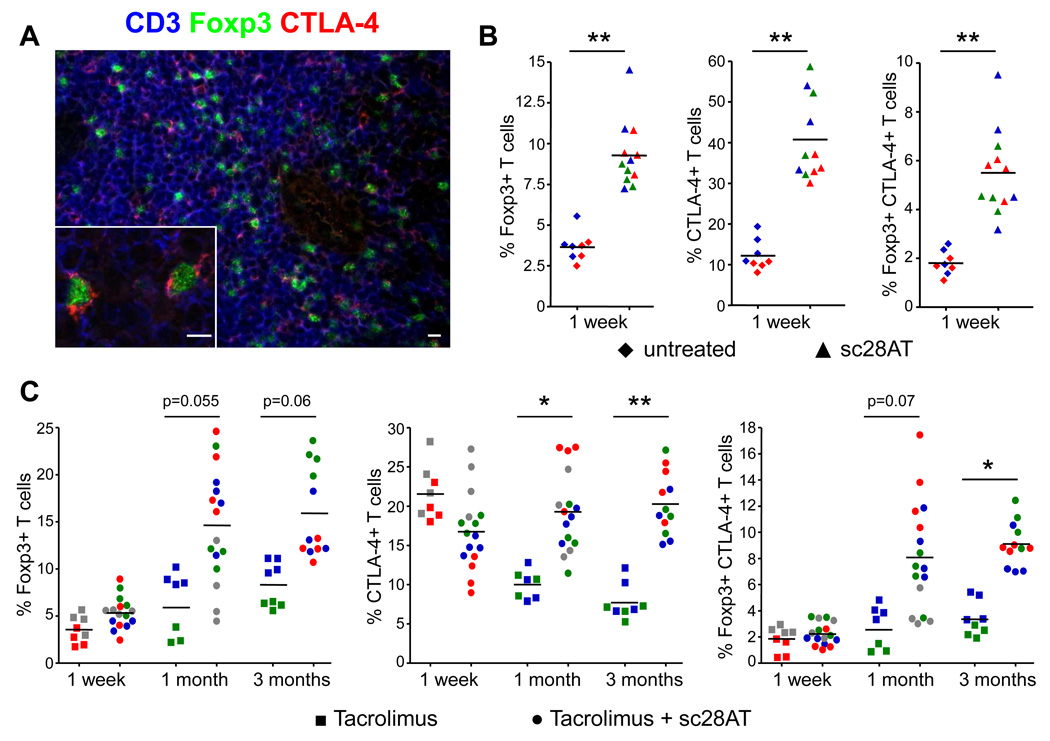
In order to better characterize the localization of Tregs in kidney graft recipients that received a bitherapy, we examined kidney biopsies for the presence of cells expressing the Treg markers Foxp3 and CTLA-4. We observed increased infiltration with T cells expressing Foxp3, CTLA-4 or both molecules one week after transplantation in animals treated with sc28AT monotherapy as compared with untreated controls (Fig. 5A, B). In recipients that received sc28AT plus Tacrolimus, the number of infiltrating Tregs expressing Foxp3 and CTLA-4 was initially low and similar to levels observed in recipients treated with Tacrolimus alone (Fig. 5C). However, numbers of infiltrating Tregs increased two- to three-fold within 1 to 3 months after transplantation in recipients treated with sc28AT and Tacrolimus, and Treg infiltration in kidney grafts was dramatically higher in bitherapy recipients in comparison with recipients treated with Tacrolimus alone (Fig. 5C).
Figure 5. Increased Treg cell infiltration in kidney allografts following selective CD28 blockade with sc28AT.
(A) Confocal-like microscopy analysis of a kidney graft biopsy from a sc28AT + Tacrolimus treated recipient one month post transplantation. Blue, CD3 staining; green, Foxp3 staining; red, CTLA-4 staining. Scale bars, 10µm. (B) Quantitative evaluation of graft infiltration by T cells expressing Foxp3, CTLA-4 or both in control and sc28AT monotherapy recipients 1 week post transplant (expressed as % of CD3+ T cells). Data from individual animals are represented with a specific color and the 4 individual data points represent quadruplicate evaluations performed on two different tissue sections of the same animal. (C) Same as (B) in recipients treated with Tacrolimus alone or sc28AT + Tacrolimus at 1 week, 1 month and 3 months post-transplant. * and ** indicate a significant difference at P<0.05 and 0.01, respectively.
Indoleamine 2,3-dioxigenase expression in kidney transplant after CD28 inhibition
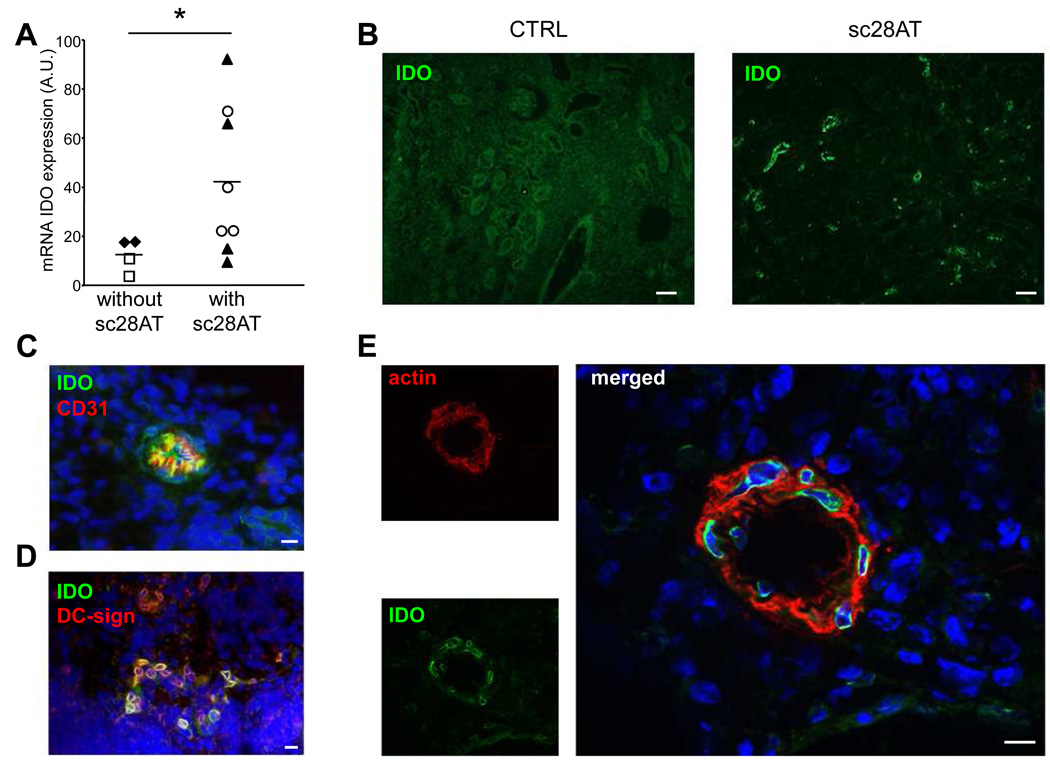
Indoleamine 2,3-dioxigenase (IDO) is an enzyme that is upregulated after engagement of CD80/86 on APCs by CTLA-4 on Teff or Treg cells (18). IDO limits T cell responses by tryptophan deprivation and by the pro-apoptotic action of tryptophan catabolites (19). Comparison of IDO mRNA levels in groups treated with or without sc28AT (alone or in combination with Tacrolimus) showed that selective CD28 blockade was accompanied by a notable increase in intragraft IDO mRNA expression (Fig. 6A). IDO expression was detectable by fluorescence microscopy in graft biopsies from sc28AT-treated recipients (Fig. 6B) in association with CD31+ endothelial cells (Fig. 6C), DC-sign+ infiltrating cells (representing a subset of immature myeloid cells; Fig. 6D) and actin+ smooth muscle cells (Fig. 6E). IDO expression could not be detected in graft biopsies from untreated recipients or from recipients treated with Tacrolimus alone (Fig. 6B and data not shown).
Figure 6. Expression of indoleamine 2,3-dioxigenase (IDO) in allograft of sc28AT-treated recipients.
(A) qPCR measurement of IDO mRNA transcripts one week post-transplantation in kidney graft biopsies from control untreated (♦, n=2) or Tacrolimus alone-treated recipients (□, n=2), and from sc28AT alone (▲, n=4) or sc28AT + Tacrolimus recipients (○, n=4). Each point represents the mean of duplicate measurements. *, P=0.04 (B) Immunohistology of biopsies from a control and a sc28AT-treated recipient labeled with an anti-IDO antibody (green) one week post transplantation. Scale bar, 100 µm. (C) Co-staining of IDO (green) and CD31, an endothelial cell marker (red), showing a small blood vessel. (D) Co-staining of IDO (green) and DC-sign, a C-type lectin expressed by immature myeloid cells (red), in the renal parenchyma. (E) Co-staining of IDO (green) and smooth muscle actin (red), showing co-localization in an arteriole. Nuclei are in blue. Scale bars in C, D and E, 10µm.
CD28 blockade inhibits acute and chronic rejection of heart allografts in macaques in synergy with calcineurin inhibition
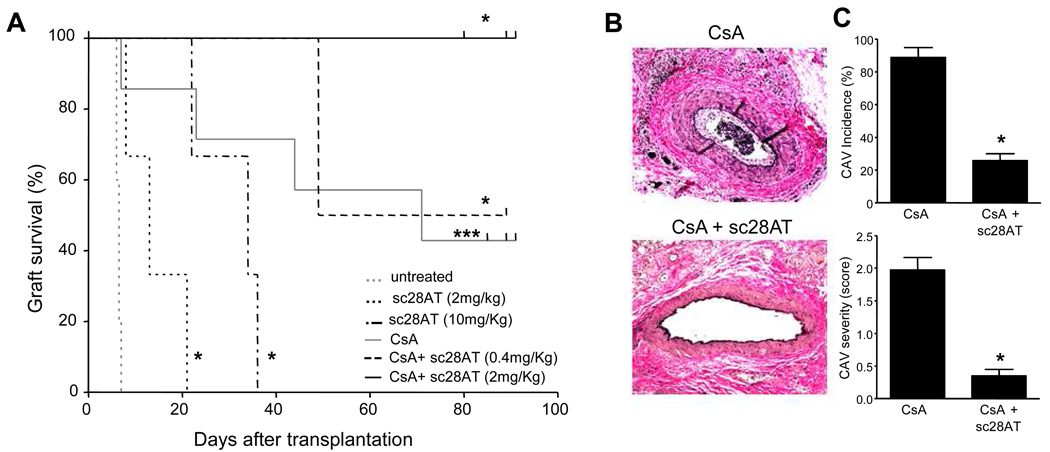
We tested sc28AT in a more stringent model of heterotopic heart transplantation in the cynomolgus macaque (20), using sc28AT alone or in combination with a calcineurin inhibitor (Cyclosporine A). The pharmacokinetic profiles are presented in Fig. S3B. Sc28AT bound to target T cells in the blood and showed tissue penetration in the spleen, lymph nodes and to some extent in the thymus (Fig. S3D). In macaques treated for 20 days with sc28AT monotherapy at 2mg/kg (n=3) or 10 mg/kg (n=3), cardiac allografts had a median survival of 13 and 34 days, respectively (Fig. 7A). Thus, treatment with sc28AT alone resulted in a significant prolongation of graft survival as compared with untreated monkeys (median graft survival time of 6 days, n=5). Another group received Cyclosporine A monotherapy that was dosed at 10–25 mg/kg i.m. daily to achieve therapeutic trough levels >400 ng/ml (21). Four out of eight treated animals exhibited symptomatic acute allograft rejection on days 7, 23, 44 and 71. One recipient died of an infection on day 26. The other three recipients exhibited graft survival over 80 days without any evidence of clinical rejection and transplanted hearts were electively explanted around day 90.
Figure 7. Selective CD28 inhibition prolongs cardiac allograft survival and prevents cardiac allograft vasculopathy in macaques.
(A) Cardiac allograft survival for monkeys without therapy (n=5) or treated with sc28AT monotherapy at 2 mg/kg/day (n=3) or 10 mg/kg/day (n=3), Cyclosporine A monotherapy (n=6), Cyclosporine A + sc28AT at 0.4 mg/kg bitherapy (n=2) or Cyclosporine A + sc28AT at 2mg/kg bitherapy (n=3). *, P<0.05 and ***, P<0.001 (indicated group versus control untreated recipients). (B) A representative vessel from a cardiac allograft treated with Cyclosporine A (day 72, left panel) shows grade 2 cardiac allograft vasculopathy (CAV) with distinct neointimal thickening and 10–50% (estimated at 25% in this instance) luminal narrowing. In contrast, a representative graft artery from a recipient treated with sc28AT + Cyclosporine A shows an absence of neointimal proliferation (day 80, lower panel). (C) CAV incidence and severity at day 70–90, graded as described in Methods, were significantly lower (P<0.05) in CD28 blockade plus Cyclosporine A combination therapy (n=4) as compared with Cyclosporine A alone (n=5).
When Cyclosporine A was combined with sc28AT at 0.4 mg/kg daily, one out of two animals developed symptomatic rejection at day 49. Importantly, when sc28AT was dosed at 2 mg/kg daily and combined with Cyclosporine A, all three recipients displayed prolonged graft survival over 80 days. One animal was euthanized with a beating graft at day 80 due to a lymphoma and the two remaining functional heart grafts were electively explanted around day 90 in the absence of any detectable clinical rejection. ISHLT rejection scores were consistently lower in protocol biopsies and explanted grafts from monkeys treated with sc28AT + Cyclosporine A as compared with animals treated with Cyclosporine A alone (Fig. S8). Whereas all grafts treated with Cyclosporine A monotherapy exhibited severe cardiac allograft vasculopathy (CAV) at the time of explant, CAV incidence and severity were significantly reduced when sc28AT was combined with Cyclosporine A (Fig. 7B, C).
In addition, long-term graft survival induced by Cyclosporine A and CD28 blockade was associated with a control of alloantibody production that was otherwise induced in heart graft recipients under monotherapy during the first month following transplantation (Fig. S9). We also analyzed levels of CD4+CD25+CD127lo Tregs in the blood of two sc28AT monotherapy-treated recipients by flow cytometry. As expected, more than 80% of CD4+CD25+CD127lo Tregs expressed Foxp3 and more than 50% expressed CTLA-4. Tregs represented 3.5±0.1% of CD4+ T cells prior to transplantation and their level increased to reach 6.3±0.4% of CD4+ T cells at 3 weeks post transplantation in sc28AT-treated recipients.
The synergy observed between sc28AT and CsA suggested that sc28AT might be used as calcineurin (CNI) sparing regimen to reduce allograft rejection while decreasing CNI toxicity. We therefore explored whether sc28AT combined with a subtherapeutic CsA regimen (CsA tapered to trough levels of 100–300 ng/ml within two weeks after transplant) modulates alloimmunity and CAV. Relative to monotherapy with sc28AT (MST 22 days, range 8–36, n=6) or subtherapeutic CsA (MST 38 days, range 12–49, n=7), addition of CD28 blockade prolonged graft survival (MST 59 days, range 21->88, n=6, p<0.05). (Fig. S10A). Importantly, subtherapeutic CsA-treated grafts showed severe CAV as early as two weeks after transplantation (d14 CAV score: 1.5±0.8) whereas added CD28 blockade was associated with significantly attenuated CAV scores (d14, 0±0.01, p=0.036) (Fig. S10B).
Discussion
Our study tested the immunoregulatory effect of selective CD28 blockade on kidney and hearts allografts in primates. It had been shown previously in rodents that antibody-mediated CD28 downmodulation delayed acute rejection (22) or inhibited chronic rejection (23, 24) after organ transplantation. The lack of a CD28-specific modulating or antagonist antibody for humans or non-human primates has prevented verification of this effect in primates or in man where the role of Tregs in transplant outcome is still a matter of debate (25, 26). We found that sc28AT, a chimeric human/primate CD28 monovalent antagonist antibody (27), selectively blocked CD28 interactions with its ligands without sharing superagonistic activity since it could not stimulate human Teff or Treg cells in vitro, a feature characteristic of superagonistic antibodies. Also, sc28AT did not activate nor deplete T cells in primates in vivo, and did not increase Treg levels in non-transplanted primates. Thus, two major features differentiate sc28AT from a superagonistic antibody. First, sc28AT is monovalent and as such cannot induce CD28 receptor cross-linking (27). Second, it does not bind the target epitope on CD28 that is essential for inducing the TCR-independent superagonistic signal (15). Using sc28AT in vivo, we found that sc28AT synergized with calcineurin inhibitors to promote acceptance of both kidney or heart allografts in non-human primates. Furthermore, because an important goal of clinical transplantation is to eliminate calcineurin inhibitors or limit their use (because of their toxicity), induction treatment with sc28AT was also associated with subtherapeutic regimen of CsA in the heart transplant model. This resulted in a significant delay in the occurrence of first rejection episodes and reduced the severity of CAV. These observations confirmed the synergy between calcineurin inhibition and CD28 blockade. However, since several grafts in this series ultimately rejected it also indicated the importance of a strong immunosuppression in the early post-operation period in this experimental mismatched transplantation setting. Finally, our data from the baboon model suggest that selective blockade of CD28 directly impaired effector T cells while promoting regulation provided by Tregs, CTLA-4 and other immunomodulatory mediators such as IDO, HO-1 and TGF-β.
We showed that sc28AT antagonized T cell activation in vitro by preventing the formation of stable T-APC conjugates and by increasing T cell motility, which was otherwise reduced after cognate interactions with APCs through a T cell receptor (TCR)-induced stop signal (28). Antagonistic anti-CTLA-4 antibodies reversed the effect of sc28AT treatment, in accordance with findings that CTLA-4 overrides the TCR-induced stop signal (28). Similar results were obtained when CTLA-4 engagement was prevented by CTLA4-Ig or anti-CD80/86 antibodies. These data suggest an intrinsic role for CTLA-4 in the T cell inhibitory effect of sc28AT. However, another interpretation might be that in the context of deficient CTLA-4 signals, T cells can overcome CD28 blockade, possibly due to a decrease in the activation threshold, and manage enough signaling to lead to efficient interaction. In contrast, the reduction of calcium peaks in T cells that established a stable contact with APCs despite CD28 blockade could not be reversed by the simultaneous blockade of CTLA-4. Thus, the mechanism of action of selective CD28 blockade on T cells involves both CTLA-4-dependent and CTLA-4-independent components. Notably, sc28AT caused impaired alloreactive T cell proliferation in mixed lymphocyte reaction in vitro, which could contribute to allograft survival in vivo by skewing the Teff/Treg balance towards regulation.
An emerging hypothesis presents CD28 and CTLA-4 molecules as a "rheostat" for targeting T cell responses towards immunity or regulation, respectively (29). If CD28 is blocked, then CTLA-4-CD80/86 interactions would be favored and T cell responses would be shifted towards regulation. In vitro, we could block the suppressive activity of human Tregs with anti-CTLA-4 Fab antibodies, as has been shown in mice (30). In contrast, sc28AT did not block but instead increased the suppressive activity of Tregs in accordance with data showing that CD28 stimulation abolishes the suppressive function of Tregs (4).
In kidney or heart allograft transplantation in primates, acute rejection was prevented, chronic rejection was attenuated and functional Tregs were increased following treatment with sc28AT combined with calcineurin inhibitors. Donor specific hyporesponsiveness was demonstrated in the kidney transplant model. Early graft biopsies showed less infiltration by CD20+ B cells, which have been associated with severe acute rejection (31). The reduction in B cells infiltration might be attributed classically to reduced help by T cells or to a direct effect of Tregs on B cells (32). Indeed, sc28AT treatment favored graft infiltration by T cells expressing Foxp3 and, importantly, CTLA-4, which is associated with the suppressive function of Tregs (9). Finally, examination of graft tissue revealed that selective CD28 blockade resulted in reduced levels of inflammatory cytokines but increased expression of immunoregulatory TGF-β, HO-1 and IDO. The effects on B cells and on Tregs might not be clinically relevant shortly after transplantation at a time when alloreactive T cells are strongly stimulated and Tregs have not yet infiltrated the allograft. Indeed the full control of acute rejection in the early post-transplant period could be achieved only in bitherapy with therapeutic levels of calcineurin inhibitors. However, Tregs (and other induced immune regulation mechanisms) might become relevant to prevent late rejection events since allograft survival persisted after withdrawal of the immunosuppressive treatment in several recipients. In addition, Treg cells could contribute to limit chronic rejection since CAV was consistently reduced in heart grafts in macaques. Furthermore, the exclusive availability of CD80/86 ligands for CTLA-4 binding and the role of CTLA-4 in immune regulation suggest that CTLA-4 could be important in this process.
What mechanisms underlie the induction of Treg cells in sc28AT-treated animals? The observation that the administration of sc28AT to non-grafted primates did not alter the frequency of Tregs excluded a direct effect of the monovalent antibody. Rather, allogeneic T cells might have been driven to differentiate into Tregs in vivo in response to the allograft since CD28 signaling was absent while CTLA-4-CD80/86 interactions could take place. Indeed, although the survival of natural Tregs is strictly CD28-dependent (5), the generation of adaptive Tregs can occur in situations of suboptimal costimulation (33) or in the absence of CD28 signals (34). Additionally, selective CTLA-4 engagement can induce adaptive Tregs with alloantigen specificity (35). The observation that PBMC from kidney graft recipients treated with sc28AT and Tacrolimus were hyporesponsive to donor APCs but not third-party cells is consistent with this possibility. In contrast to clinical immunosuppression achieved by a CD80/86 antagonist, which was not associated with induction of Tregs and not relying on immune regulation (36, 37), our findings are consistent with previously reported downstream mechanisms of suppression attributed to Tregs in transplantation including release of TGF-β, expression of CTLA-4, and induction of IDO and HO-1 enzymes (38).
In summary, our study showed that selective blockade of CD28 costimulation after transplantation reduced alloreactivity and increased the pool of peripheral Tregs. In addition, Tregs accumulated in the graft where they likely modulated pathogenic T cells and promoted prolonged allograft survival. Whether selective CD28 blockade has significant practical advantages relative to CD80/86 blockade (3), as our model predicts, remains to be formally tested. However, the efficacy of CD28 antagonists in combination with conventional immunosuppression to inhibit acute and chronic allograft rejection in primates is promising. Although superagonistic activity has been excluded, any translation of a CD28 antagonist into clinical application will have first to re-evaluate that point.
Materials and Methods
Reagents
A non-activating human CD28-specific scFv antibody fragment was developed from the CD28-specific CD28.3 clone (14), and linked to α-1 anti-trypsin (sc28AT) to prolong its half-life in the serum in vivo (27). Sc28AT was produced by TcL Pharma (Nantes, France) and by a NIH production platform (K. Reimann, Beth Israel Deaconess Medical Center, Boston, MA) from transformed CHO cells and purified from supernatant by ion exchange chromatography. Sc28AT was quantified by two complementary specific sandwich ELISAs (TcL Pharma). Sc28AT cross-reacts with CD28 from cynomolgus monkey, baboon and marmoset but not from dog, rabbit, rat and mouse, and has the same binding affinity as the parental murine Fab fragment (27). Anti-human CTLA-4 antibody (clone 147.1) was provided by Medarex (Princeton, New-Jersey). Fab monovalent fragments were prepared using Immunopure IgG1 Fab preparation kit (Pierce, Rockford, Illinois). Anti-human CD80 (clone M24) and CD86 (clone 1G10) was provided by Innogenetics (Gent, Belgium). CTLA4-Ig (LEA29Y) was prepared from transfected Cos cells in our laboratory. Fluorescent mAbs against human CD3 (SP34-2), CD4 (L200), CD8 (RPA-T8), CD28 (28.6), CD25 (M-A251), CD45RO (UCHL1), CD62L (FMC46), CD127 (hIL-7R-M21), CTLA-4 (BNI3) and HLA-DR (G46-6) were from BD Biosciences (San-Diego, California). Biotinylated anti-human α-1-anti-trypsin (Abcam, Cambridge, MA) was used with Streptavidin-PE (Beckman Coulter, Fullerton, CA) for sc28AT staining on T cells. The APC-conjugated anti-human Foxp3 staining kit (PCH101 and 236A/E7) was purchased from eBioscience (San-Diego, California) and used according to the manufacturer’s instructions. Primary antibodies used for immunohistochemical staining were: rabbit anti-human CD3 (DAKO, Glostrup, Denmark), human IDO-1 (provided by Dr. I. Anegon, Nantes, France (39)), mouse anti-human CD11b (BEAR1; Beckman Coulter, Fullerton, California), human CD20 (L26; DAKO), human CD31 (LCI4; Serotec, Oxford, UK), human CTLA-4 (BNI3; BD Bioscience), human Foxp3 (236A/E7; eBioscience), human DC-SIGN (DCN46; BD Bioscience) and human α-smooth muscle actin (O.N.5; Abcam).
Epitope analysis
The determination of the epitope recognized by the CD28.3 antibody was performed by Agrobio (Orleans, France). Briefly, CD28.3 Fab fragments were added to human CD28 (R&D Systems, Lille, France) immobilized on sepharose. Immune complexes were reduced and alkylated with iodoacetamide (55mM, 1h) before chymotrypsin (1 mg/50mg bound antibody) was added for 4h at 18–25°C. The sepharose was then washed with 25mM ammonium carbonate followed by 50mM glycin, pH 2.5. Eluted peptides where then concentrated on a C18 matrix and analyzed by MALDI-TOF/TOF. The epitope was determined by peptide mass fingerprinting.
Live cell dynamic microscopy
A human EBV (Epstein-Barr Virus) -specific CD4+CD28+ T cell clone (T-EBV (40); 2.105 cells) was stained with the FURA-2 AM probe (0.5 µM for 30 min; Interchim, Montluçon, France), washed and added to 4.105 human EBV-transformed B lymphoblastoïd cell lines (pool of cells from 3 donors, obtained as described (41)) on a coverslip coated with Poly-L-Lysine (0.001%; Sigma, Saint-Louis, Minnesota). Using the Metafluor image-analysis software (Molecular Devices, Sunnyvale, CA), bright-field and fluorescent images were acquired at 15 second intervals on a Leica microscope (Leica Microsystems, Wetzler, Germany). Individual T cell/APC interactions and individual T cell calcium peaks were recorded manually over a 20 min. incubation period with Metafluor (version 7.1.7) and Metamorph (version 7.5.6; Roper Scientific, Göttingen, Germany) software. A calcium peak was recorded when fluorescence levels reached 2-fold the baseline level. T cells were tracked using the ImageJ free software (version 1.41). The total number of cells analyzed was 60 cells for control condition (from 11 experiments), 31 cells for sc28AT condition (from 7 experiments) and 29 cells for sc28AT + anti-CTLA-4 (from 4 experiments), 13 cells for anti-CD80/86 (from 3 experiments) and 26 cells for CTLA-4Ig condition (from 5 experiments). Antibodies were all used at 10 µg/ml. Data are presented as mean ± SD for each condition.
Mixed Lymphocyte Reactions (MLR)
Peripheral blood mononuclear cells (PBMC) were isolated from whole blood by density centrifugation over Ficoll-Paque (Eurobio, Les Ulis, France). Freshly isolated PBMC were incubated with allogeneic irradiated PBMC (105 cells/well of each cell type) for 5 days at 37°C, 5% CO2, in complete medium (RPMI 1640, 10% heat-inactivated allogeneic pooled sera, 2mM L-glutamine, 100 U/ml penicillin, 0.1 mg/ml streptomycin, 1% nonessential amino acids, 1mM sodium pyruvate and 5mM Hepes, all from Sigma). Cells were pulsed with 1µCi of 3H-thymidine during the final 8 hours of culture and then harvested and counted in a scintillation counter. In other experiments, PBMC were maintained in culture without allogeneic cells and assayed similarly as above.
Cytokine secretion assays
To assess IL-2 production by T cells, 105 Jurkat T cells were stimulated with 2.104 Raji B cells in microtiter plates in the presence of 5 ng/ml staphylococcus enterotoxin E for 48h at 37°C, 5% CO2 in complete medium. IL-2 secretion was evaluated in the supernatant by using the MaxTM Set Deluxe Human IL-2 ELISA Kit (Biolegend, Uithoorn, The Netherlands). To analyze the synthesis of multiple cytokines by human PBMC, 105 PBMC from healthy humans were cultured in triplicate for 5 days in complete medium with control or anti-CD28 antibodies. After 48h, 30µl supernatant was collected from each triplicate, pooled and analyzed for cytokines concentration using a human Th1/Th2 cytokine kit (BD Biosciences).
Suppression assays
All experiments were performed with PBMC obtained from healthy donors. CD4+ T cells were enriched from PBMC by negative selection using CD4+ T cells isolation Kit II (Miltenyi, Bergisch Gladbach, Germany) and an autoMACS separator (Miltenyi). Enriched CD4+ cells were then stained with anti-human CD4, CD25 and CD127 mAbs at 4°C for 30min. CD4+CD25hiCD127lo regulatory T cells and CD4+CD25− naïve T cells were then sorted (purity routinely above 95%) with a high-speed cell sorter (FACSAria; BD Biosciences) and FACSDiva software (BD Biosciences). 2.104 CD4+CD25− cells were co-cultured with 105 allogeneic irradiated PBMC and autologous CD4+CD25hiCD127lo Treg at a 1:1 or 1:0.25 ratio for 5 days at 37°C, 5% CO2 in human complete medium. Blocking Abs were added at the start of the culture at 10µg/ml. Proliferation was assessed by 3H-thymidine incorporation during the last 8 hours of culture.
Allogeneic mature DC (mDC) were generated from monocytes as described (42). Briefly, monocytes were enriched by elutriation (>85% CD14+) and cultured for 6 days in medium supplemented with IL-4 (40ng/ml; R&D Systems, Minneapolis, Minnesota) and GM-CSF (1000 IU/ml; Gentaur, Kampenhout, Belgium). Cells were harvested on day 5 and cultured for 24h with LPS for maturation (1µg/ml; E. coli 0111:B4, Sigma). 5.103 allogeneic mature DC (mDC) were then co-cultured with 5.104 Treg in complete medium with the indicated blocking antibodies (10µg/ml) for 18h. mDC/Treg cells were washed and added to 5.104 CD4+CD25− cells (same donor as Treg) stimulated with 5.103 allogeneic mDC (same donor as mDC used for Treg activation). Cells were then cultured for 5 additional days and proliferative responses were assessed by 3H-thymidine incorporation.
Baboon Treg suppression assays
CD25+ and CD25− cells were prepared from PBMC during the 3rd month post transplantation using FITC anti-human CD25 mAb and specific anti-FITC microbeads (Miltenyi) and positive or negative selection, respectively. To determine suppression, 105 CFSE (CarboxyFluorescein Succinimidyl Ester) -labelled CD25− PBMC were mixed with different numbers of unlabelled CD25+ PBMC (at ratios of 1:0.5 to 1:0.0078) in duplicate wells of a plate previously coated with anti-human CD3 antibodies (10µg/ml; 2h at 37°C). The proliferative response was evaluated after 3 days by measuring the percentage of CFSE-diluted cells by flow cytometry. The percentage of suppression was calculated by comparing the % of proliferated cells (CFSE dilution) in the presence of indicated numbers of CD25+ PBMC to the % of proliferated cells in the absence of CD25+ cells (= maximal proliferation).
Animals
Cynomolgus monkeys (Macaca fascicularis) (2 to 3 kg) were obtained from Covance Research Products (Alice, TX) and Three Springs Scientific Inc (Perkasie, PA). Baboons (Papio anubis) (6 to 15 kg) were obtained from the CNRS primatology center (Rousset, France). Experiments performed on macaques were in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) of the University of Maryland Medical School and carried out in compliance with the HHS/NIH Guide for the Care and Use of Laboratory Animals. Experiments performed in baboons complied with recommendations of the Institutional Ethical Guidelines of the “Institut National de la Santé Et de la Recherche Médicale” (France). The donor-recipient combinations were chosen according to blood group compatibility, major histocompatibility complex (MHC) mismatching by DRB-MHC class II locus typing and verification of MHC incompatibility by MLR (stimulatory index > 5).
Renal transplantation in baboons
Renal allotransplantation was performed in binephrectomized recipients, as described (43). Twenty-four hour diuresis was monitored daily as well as blood urea nitrogen. Transplantectomies were performed when plasma creatinin levels rose up to 400µM and surgical biopsies were performed for histological examination prior to euthanasia. One biopsy fragment was collected for hematoxylin and eosin blinded histological examination by a pathologist. A second fragment was snap-frozen in liquid nitrogen for mRNA extraction and a third fragment was placed in Tissue-Tek (Sakura Finetek, Villeneuve d’Ascq, France) for immunohistochemical staining. Protocol biopsies were performed at one week, one month and 3 months after transplantation. Control groups were either untreated (n=3) or received Tacrolimus alone (Prograf, provided by Astellas, Tokyo, Japan) (n=4). Tacrolimus was given once daily from day 0 to day 90 after transplantation (0.05 to 0.15 mg/kg i.m.) to achieve target therapeutic trough levels (10 to 20 ng/ml). Sc28AT was administered once daily (4mg/kg i.v.) during the first 25 days after transplantation. Blood samples were collected for FACS analysis and assessment of serum levels of sc28AT and cytokines (Non-Human Primate Th1/Th2 cytokine kit; BD Biosciences) before the daily administration of sc28AT.
Cardiac transplantation in cynomolgus monkeys
All recipient animals underwent heterotopic intra-abdominal cardiac allograft transplantation, as described (44). Surgical cardiac biopsies were performed on postoperative days 7, 14, 28 and monthly thereafter until graft explant. Graft function was monitored daily by palpation and implanted telemetry (Data Sciences International, St. Paul, MN). Clinical acute graft rejection was detected as consistent high body temperature (>38.5°C) coupled with either a decrease in graft heart rate (to <120 beats per min., or a drop of >40 beats per min. from a stable baseline) or an increase in graft diastolic pressure of >10 mmHg. Graft rejection was defined as loss of contraction by telemetry and confirmed at explant and was always preceded by signs of acute rejection. Control groups were either left untreated (n=5) or received Cyclosporine A (Neoral, Novartis, Hannover, NJ) (n=6). Cyclosporine A was given once daily (5–25 mg/kg i.m.) to achieve target therapeutic (>400 ng/ml) or subtherapeutic (100–300 ng/ml) trough levels. Sc28AT was given as indicated in Fig. 7. In three therapeutic Cyclosporine A-treated animals, a first episode of symptomatic acute rejection was treated with three daily steroid boluses (Solumedrol, Pharmacia, Kalamozoo, MI; 10 mg/kg). Rejection was reversed in two of the three treated animals. In one therapeutic Cyclosporine A-treated animal, suspected rejection based on histological analysis of the biopsy tissue sample was also treated with a three day course of steroids. One recipient died of infection different than the ones that rejected the heart at day 26 and was excluded from statistical analysis. Cellular infiltrates were analyzed on hematoxylin and eosin-stained paraffin sections and graded for acute rejection by ISHLT (International Society for Heart and Lung Transplantation) criteria (45). Cardiac allograft vasculopathy (CAV) incidence in beating hearts explanted after day 70 (therapeutic cyclosporine A group) or in weekly sample biopsies (subtherapeutic cyclosporine A group) was recorded as percent of arteries and arteriolar vessels involved (CAV score ≥1) at each time point. CAV severity was scored in these explanted hearts as follows: Grade 0, normal arterial morphology; Grade 1, activated endothelial cells with enlarged nuclei and/or adherent leukocytes, without luminal narrowing (<10%); Grade 2, distinct neointimal thickening, luminal narrowing <50%; Grade 3, extensive neointimal proliferation with greater than 50% luminal occlusion. Scoring was independently performed for each explanted heart by three evaluators (TZ, RNP, BN) blinded with respect to the treatment group. The mean CAV score for each biopsy or explant was calculated with the equation: (#grade 0 vessels x0 + #grade 1 vessels x1 + #grade 2 vessels x2 + #grade 3 vessels x3)/total number of arterial vessels scored. Individual means were averaged to calculate the group mean ± SD for each treatment group.
Immunohistochemical staining
Serial frozen sections (10µm) were prepared from surgical or protocol renal biopsies. Slides were air dried at room temperature for 1h before acetone fixation for 10 min at room temperature. Sections were saturated with PBS containing 10% baboon serum, 2% normal goat serum and 4% BSA. Sections were incubated overnight with primary Abs at 4°C, followed by fluorescent secondary Abs and nuclear staining (DAPI; Invitrogen). For intracellular immunostaining (CTLA-4, Foxp3 and IDO), sections were permeabilized with 0.5% Saponin (Sigma) in the saturating solution. Treg infiltration was determined by a triple staining with anti-human CD3 antibodies (followed by anti-rabbit IgG-Alexa 350; Invitrogen), anti-human CTLA-4 (followed by anti-mouse IgG2a Alexa 594; Invitrogen) and anti-human Foxp3 (followed by anti-mouse IgG1-Alexa 488; Invitrogen). The percentage of CD3 cells expressing Foxp3, CTLA-4 or both was quantified manually (by an investigator blinded to the experimental conditions) on four different pictures from two different areas of each tissue section separated by at least 100µm. Indoleamine 2,3-dioxigenase (IDO) localization was determined by double staining with anti-human IDO-1 (followed by anti-rabbit IgG FITC; Jackson ImmunoResearch, West Grove, PA) and anti-human CD31, anti-human DC-SIGN or anti-human α-smooth muscle actin (followed by anti-mouse IgG-Alexa 568; Invitrogen)). The specificity of IDO-1 staining was confirmed by competition experiments with the IDO-1 peptide that had been used to raise the antibody. Slides were analyzed with standard fluorescence microscopy or confocal-like microscopy (Apotome, Carl Zeiss, Oberkochen, Germany) and the AxioVision imaging software (Carl Zeiss, Le Pecq, France).
mRNA analysis
mRNA was extracted from snap-frozen renal biopsies using QIAgen RNA microextraction kit (Qiagen, Courtaboeuf, France) according to the manufacturer’s instructions. The quality and quantity of mRNA was controlled by infrared spectrometry (NanoDrop; Thermo scientific, Wilmington, DE). Messenger RNA was amplified and retrotranscribed with the Omniscript RT kit (Qiagen) and real-time quantitative PCR was then performed as previously described(43), using an ABI Prism 7700-Perkin Elmer Sequence Detection System (Perkin Elmer, Foster City, CA). Amplifications were performed for hypoxanthine phosphoribosyltransferase (HPRT; probe Hs99999909_m1; Applied Biosystems, Foster City, California), IDO (F: 5'-ACGGTCTGGTGTATGAAGGGT-3'; R: 5'-CACGGACTGAGGGATTTGACT-3'), IL2-R (probe Hs00907778_m1, Applied), CTLA-4 (F: 5'-TCTTCATCCCTGTCTTCTCCAA-3'; R: 5'-GGTCAACTCATTCCCCATCA-3'), Foxp3 (F: 5'-CCCTGCCCTTCTCATCCA-3'; R: 5'-GTGGCCCGGATGTGAAAA-3'), Heme Oxygenase 1 (HO-1; probe Hs00157965_m1, Applied), IFN-γ (F: 5'-TGGGTTCTCTTGGCTGTTACTG-3'; R: 5'-TTAATGTCTTCCTTGATGGTCTCC-3') and IL-6 (probe Hs00174131_m1, Applied).
Statistical analyses
Graft survival times were plotted with the Kaplan-Meyer representation, and survival time between different groups was evaluated with a log-rank test. Continuous variables were expressed as mean ± SD unless otherwise indicated and were compared using the Mann-Whitney non parametric test. MLR data were analyzed with unpaired t tests. Discrete variables (i.e. incidence of early rejection) were compared by using a contingency table and the Chi-square test. P values less than 0.05 were considered statistically significant. All statistical analyses were performed on a personal computer with the statistical package SPSS for Windows XP (Version 11.0, SPSS, Chicago, IL, USA) or GraphPad InStat (version 5.1, GraphPad Software, San Diego, CA, USA).
Supplementary Material
Footnotes
Author Contributions: N.P. performed baboon experiments, in vitro experiments, interpreted the data and prepared the manuscript. A.A. initiated and designed the m. fascicularis study, performed experiments, interpreted the data and prepared the manuscript. T.Z. conducted heart transplantations and histology analysis in m. fascicularis. N.D. and C.M. performed experiments. B.N. conducted heart transplantations and histology analysis in m. fascicularis. X.T. conducted kidney transplantations in baboon. G.W. assisted with heart transplantations in m. fascicularis. K.R. performed anatomopathological analyses of kidney biopsies. J.H. assisted with kidney transplantations in baboon. B.M. prepared and purified recombinant molecules. G.K conducted kidney transplantations in baboon. F.C. prepared antibodies and antibody fragments, performed ELISA. E.A-L. assisted with immunohistological analyses. J-P.S. analyzed the data and edited the manuscript. R.P. founded the research, designed m. fascicularis experiments, analyzed data and edited the manuscript. G.B. organized and designed baboon experiments, supervised analyses and edited the manuscript. B.V. founded the research, designed experiments, performed experiments, analyzed the data and prepared the manuscript.
References and notes
- 1.Alegre ML, Frauwirth KA, Thompson CB. T-cell regulation by CD28 and CTLA-4. Nat Rev Immunol. 2001;1:220–228. doi: 10.1038/35105024. [DOI] [PubMed] [Google Scholar]
- 2.Kremer JM, Westhovens R, Leon M, Di Giorgio E, Alten R, Steinfeld S, Russell A, Dougados M, Emery P, Nuamah IF, Williams GR, Becker JC, Hagerty DT, Moreland LW. Treatment of rheumatoid arthritis by selective inhibition of T-cell activation with fusion protein CTLA4Ig. N Engl J Med. 2003;349:1907–1915. doi: 10.1056/NEJMoa035075. [DOI] [PubMed] [Google Scholar]
- 3.Vincenti F, Larsen C, Durrbach A, Wekerle T, Nashan B, Blancho G, Lang P, Grinyo J, Halloran PF, Solez K, Hagerty D, Levy E, Zhou W, Natarajan K, Charpentier B. Costimulation blockade with belatacept in renal transplantation. N Engl J Med. 2005;353:770–781. doi: 10.1056/NEJMoa050085. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, Shimizu J, Sakaguchi S. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998;10:1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 5.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 6.Zheng Y, Manzotti CN, Liu M, Burke F, Mead KI, Sansom DM. CD86 and CD80 differentially modulate the suppressive function of human regulatory T cells. J Immunol. 2004;172:2778–2784. doi: 10.4049/jimmunol.172.5.2778. [DOI] [PubMed] [Google Scholar]
- 7.Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H, Mak TW. Lymphoproliferative disorders with early lethality in mice deficient in CTLA-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 8.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 9.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 10.Zheng XX, Markees TG, Hancock WW, Li Y, Greiner DL, Li XC, Mordes JP, Sayegh MH, Rossini AA, Strom TB. CTLA4 signals are required to optimally induce allograft tolerance with combined donor-specific transfusion and anti-CD154 monoclonal antibody treatment. J Immunol. 1999;162:4983–4990. [PubMed] [Google Scholar]
- 11.Tsai MK, Ho HN, Chien HF, Ou-Yang P, Lee CJ, Lee PH. The role of B7 ligands (CD80 and CD86) in CD152-mediated allograft tolerance: a crosscheck hypothesis. Transplantation. 2004;77:48–54. doi: 10.1097/01.TP.0000107286.21985.EF. [DOI] [PubMed] [Google Scholar]
- 12.Kirk AD, Tadaki DK, Celniker A, Batty DS, Berning JD, Colonna JO, Cruzata F, Elster EA, Gray GS, Kampen RL, Patterson NB, Szklut P, Swanson J, Xu H, Harlan DM. Induction therapy with monoclonal antibodies specific for CD80 and CD86 delays the onset of acute renal allograft rejection in non-human primates. Transplantation. 2001;72:377–384. doi: 10.1097/00007890-200108150-00005. [DOI] [PubMed] [Google Scholar]
- 13.Haanstra KG, Ringers J, Sick EA, Ramdien-Murli S, Kuhn EM, Boon L, Jonker M. Prevention of kidney allograft rejection using anti-CD40 and anti-CD86 in primates. Transplantation. 2003;75:637–643. doi: 10.1097/01.TP.0000054835.58014.C2. [DOI] [PubMed] [Google Scholar]
- 14.Nunes J, Klasen S, Ragueneau M, Pavon C, Couez D, Mawas C, Bagnasco M, Olive D. CD28 mAbs with distinct binding properties differ in their ability to induce T cell activation: analysis of early and late activation events. Int Immunol. 1993;5:311–315. doi: 10.1093/intimm/5.3.311. [DOI] [PubMed] [Google Scholar]
- 15.Luhder F, Huang Y, Dennehy KM, Guntermann C, Muller I, Winkler E, Kerkau T, Ikemizu S, Davis SJ, Hanke T, Hunig T. Topological requirements and signaling properties of T cell-activating, anti-CD28 antibody superagonists. J Exp Med. 2003;197:955–966. doi: 10.1084/jem.20021024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schuurman HJ, Smith HT, Cozzi E. Reference values for clinical chemistry and clinical hematology parameters in baboons. Xenotransplantation. 2004;11:511–516. doi: 10.1111/j.1399-3089.2004.00171.x. [DOI] [PubMed] [Google Scholar]
- 17.Porter CM, Horvath-Arcidiacono JA, Singh AK, Horvath KA, Bloom ET, Mohiuddin MM. Characterization and expansion of baboon CD4+CD25+ Treg cells for potential use in a non-human primate xenotransplantation model. Xenotransplantation. 2007;14:298–308. doi: 10.1111/j.1399-3089.2007.00416.x. [DOI] [PubMed] [Google Scholar]
- 18.Munn DH, Sharma MD, Mellor AL. Ligation of B7-1/B7-2 by human CD4+ T cells triggers indoleamine 2,3-dioxygenase activity in dendritic cells. J Immunol. 2004;172:4100–4110. doi: 10.4049/jimmunol.172.7.4100. [DOI] [PubMed] [Google Scholar]
- 19.Grohmann U, Orabona C, Fallarino F, Vacca C, Calcinaro F, Falorni A, Candeloro P, Belladonna ML, Bianchi R, Fioretti MC, Puccetti P. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3:1097–1101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 20.Azimzadeh AM, Pfeiffer S, Wu G, Schroder C, Zorn GL, 3rd, Kelishadi SS, Ozkaynak E, Kehry M, Atkinson JB, Miller GG, Pierson RN., 3rd Alloimmunity in primate heart recipients with CD154 blockade: evidence for alternative costimulation mechanisms. Transplantation. 2006;81:255–264. doi: 10.1097/01.tp.0000190099.62847.e6. [DOI] [PubMed] [Google Scholar]
- 21.Schuurman HJ, Slingerland W, Mennninger K, Ossevoort M, Hengy JC, Dorobek B, Vonderscher J, Ringers J, Odeh M, Jonker M. Pharmacokinetics of cyclosporine in monkeys after oral and intramuscular administration: relation to efficacy in kidney allografting. Transpl Int. 2001;14:320–328. doi: 10.1007/s001470100336. [DOI] [PubMed] [Google Scholar]
- 22.Dengler TJ, Szabo G, Sido B, Nottmeyer W, Zimmerman R, Vahl CF, Hunig T, Meuer SC. Prolonged allograft survival but no tolerance induction by modulating CD28 antibody JJ319 after high-responder rat heart transplantation. Transplantation. 1999;67:392–398. doi: 10.1097/00007890-199902150-00009. [DOI] [PubMed] [Google Scholar]
- 23.Dong VM, Yuan X, Coito AJ, Waaga AM, Sayegh MH, Chandraker A. Mechanisms of targeting CD28 by a signaling monoclonal antibody in acute and chronic allograft rejection. Transplantation. 2002;73:1310–1317. doi: 10.1097/00007890-200204270-00021. [DOI] [PubMed] [Google Scholar]
- 24.Guillonneau C, Seveno C, Dugast AS, Li XL, Renaudin K, Haspot F, Usal C, Veziers J, Anegon I, Vanhove B. Anti-CD28 antibodies modify regulatory mechanisms and reinforce tolerance in CD40Ig-treated heart allograft recipients. J Immunol. 2007;179:8164–8171. doi: 10.4049/jimmunol.179.12.8164. [DOI] [PubMed] [Google Scholar]
- 25.Louis S, Braudeau C, Giral M, Dupont A, Moizant F, Robillard N, Moreau A, Soulillou JP, Brouard S. Contrasting CD25hiCD4+T cells/FOXP3 patterns in chronic rejection and operational drug-free tolerance. Transplantation. 2006;81:398–407. doi: 10.1097/01.tp.0000203166.44968.86. [DOI] [PubMed] [Google Scholar]
- 26.Alvarez CM, Opelz G, Garcia LF, Susal C. Expression of regulatory T-cell-related molecule genes and clinical outcome in kidney transplant recipients. Transplantation. 2009;87:857–863. doi: 10.1097/TP.0b013e318199fa57. [DOI] [PubMed] [Google Scholar]
- 27.Vanhove B, Laflamme G, Coulon F, Mougin M, Vusio P, Haspot F, Tiollier J, Soulillou JP. Selective blockade of CD28 and not CTLA-4 with a single-chain Fv-alpha1-antitrypsin fusion antibody. Blood. 2003;102:564–570. doi: 10.1182/blood-2002-08-2480. [DOI] [PubMed] [Google Scholar]
- 28.Schneider H, Downey J, Smith A, Zinselmeyer BH, Rush C, Brewer JM, Wei B, Hogg N, Garside P, Rudd CE. Reversal of the TCR stop signal by CTLA-4. Science. 2006;313:1972–1975. doi: 10.1126/science.1131078. [DOI] [PubMed] [Google Scholar]
- 29.Sansom DM, Manzotti CN, Zheng Y. What's the difference between CD80 and CD86? Trends Immunol. 2003;24:314–319. doi: 10.1016/s1471-4906(03)00111-x. [DOI] [PubMed] [Google Scholar]
- 30.Tang Q, Boden EK, Henriksen KJ, Bour-Jordan H, Bi M, Bluestone JA. Distinct roles of CTLA-4 and TGF-beta in CD4+CD25+ regulatory T cell function. Eur J Immunol. 2004;34:2996–3005. doi: 10.1002/eji.200425143. [DOI] [PubMed] [Google Scholar]
- 31.Sarwal M, Chua MS, Kambham N, Hsieh SC, Satterwhite T, Masek M, Salvatierra O., Jr Molecular heterogeneity in acute renal allograft rejection identified by DNA microarray profiling. N Engl J Med. 2003;349:125–138. doi: 10.1056/NEJMoa035588. [DOI] [PubMed] [Google Scholar]
- 32.Ludwig-Portugall I, Hamilton-Williams EE, Gottschalk C, Kurts C. Cutting edge: CD25+ regulatory T cells prevent expansion and induce apoptosis of B cells specific for tissue autoantigens. J Immunol. 2008;181:4447–4451. doi: 10.4049/jimmunol.181.7.4447. [DOI] [PubMed] [Google Scholar]
- 33.Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 34.Belghith M, Bluestone JA, Barriot S, Megret J, Bach JF, Chatenoud L. TGF-beta-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat Med. 2003;9:1202–1208. doi: 10.1038/nm924. [DOI] [PubMed] [Google Scholar]
- 35.Li R, Perez N, Karumuthil-Melethil S, Prabhakar BS, Holterman MJ, Vasu C. Enhanced engagement of CTLA-4 induces antigen-specific CD4+CD25+Foxp3+ and CD4+CD25- TGF-beta 1+ adaptive regulatory T cells. J Immunol. 2007;179:5191–5203. doi: 10.4049/jimmunol.179.8.5191. [DOI] [PubMed] [Google Scholar]
- 36.Chavez H, Beaudreuil S, Abbed K, Taoufic Y, Kriaa F, Charpentier B, Durrbach A. Absence of CD4CD25 regulatory T cell expansion in renal transplanted patients treated in vivo with Belatacept mediated CD28-CD80/86 blockade. Transpl Immunol. 2007;17:243–248. doi: 10.1016/j.trim.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 37.Bluestone JA, Liu W, Yabu JM, Laszik ZG, Putnam A, Belingheri M, Gross DM, Townsend RM, Vincenti F. The effect of costimulatory and interleukin 2 receptor blockade on regulatory T cells in renal transplantation. Am J Transplant. 2008;8:2086–2096. doi: 10.1111/j.1600-6143.2008.02377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9:239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hill M, Tanguy-Royer S, Royer P, Chauveau C, Asghar K, Tesson L, Lavainne F, Remy S, Brion R, Hubert FX, Heslan M, Rimbert M, Berthelot L, Moffett JR, Josien R, Gregoire M, Anegon I. IDO expands human CD4+CD25high regulatory T cells by promoting maturation of LPS-treated dendritic cells. Eur J Immunol. 2007;37:3054–3062. doi: 10.1002/eji.200636704. [DOI] [PubMed] [Google Scholar]
- 40.Ibisch C, Saulquin X, Gallot G, Vivien R, Ferrand C, Tiberghien P, Houssaint E, Vie H. The T cell repertoire selected in vitro against EBV: diversity, specificity, and improved purification through early IL-2 receptor alpha-chain (CD25)-positive selection. J Immunol. 2000;164:4924–4932. doi: 10.4049/jimmunol.164.9.4924. [DOI] [PubMed] [Google Scholar]
- 41.Gallot G, Vollant S, Vivien R, Clemenceau B, Ferrand C, Tiberghien P, Gaschet J, Robillard N, Vie H. Selection of Epstein-Barr virus specific cytotoxic T lymphocytes can be performed with B lymphoblastoid cell lines created in serum-free media. Clin Exp Immunol. 2006;144:158–168. doi: 10.1111/j.1365-2249.2006.03035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chauveau C, Remy S, Royer PJ, Hill M, Tanguy-Royer S, Hubert FX, Tesson L, Brion R, Beriou G, Gregoire M, Josien R, Cuturi MC, Anegon I. Heme oxygenase-1 expression inhibits dendritic cell maturation and proinflammatory function but conserves IL-10 expression. Blood. 2005;106:1694–1702. doi: 10.1182/blood-2005-02-0494. [DOI] [PubMed] [Google Scholar]
- 43.Boulday G, Ashton-Chess J, Bernard P, Karam G, Vie H, Moreau A, Minault D, Lorre K, Soulillou JP, Blancho G. Association of rapamycin and co-stimulation blockade using anti-B7 antibodies in renal allotransplantation in baboons. Nephrol Dial Transplant. 2004;19:1752–1760. doi: 10.1093/ndt/gfh126. [DOI] [PubMed] [Google Scholar]
- 44.Pierson RN, 3rd, Chang AC, Blum MG, Blair KS, Scott MA, Atkinson JB, Collins BJ, Zhang JP, Thomas DW, Burkly LC, Miller GG. Prolongation of primate cardiac allograft survival by treatment with ANTI-CD40 ligand (CD154) antibody. Transplantation. 1999;68:1800–1805. doi: 10.1097/00007890-199912150-00026. [DOI] [PubMed] [Google Scholar]
- 45.Billingham ME, Cary NR, Hammond ME, Kemnitz J, Marboe C, McCallister HA, Snovar DC, Winters GL, Zerbe A. A working formulation for the standardization of nomenclature in the diagnosis of heart and lung rejection: Heart Rejection Study Group. The International Society for Heart Transplantation. J Heart Transplant. 1990;9:587–593. [PubMed] [Google Scholar]
- 46.Acknowledgements: We thank A. Laaris, C. Avon, X. Cheng, N. Sangrampurkar, E. Welty, D. Minault, C. Lefeuvre, S. Lebas-Bernardet, P. Hulin (IFR26, Nantes) and T. Haudebourg for technical assistance. Competing interests: BV and JPS are shareholders in TcL Pharma, a company developing CD28 antagonists. Funding: Supported by the ROTRF grant 466230972 to BV, by the Progreffe Foundation (Nantes, France) and by TcL Pharma (Nantes, France). Also supported by the NIH (UO1 AI 066719), an ASTS Mid-Career Award, a contract from the DOD ORD (N00014-04-1-0821), and an AHA Grant-in-Aid, all to RNP; and by the Other Tobacco Related Diseases research grant from the Maryland Restitution Fund Program, to AA and RNP.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.