Abstract
Cerebral ischemia-elicited inflammatory responses are driven by inflammatory mediators produced both by central (e.g., neurons and microglia) and infiltrating peripheral immune cells (e.g., macrophage/monocyte), and contribute to the evolution of tissue injury. A ubiquitous molecule, spermine, is released from injured cells, and counter-regulates release of various proinflammatory cytokines. However, the spermine-mediated anti-inflammatory activities are dependent on the availability of fetuin-A, a liver-derived negative acute-phase protein. Using an animal model of focal cerebral ischemia (i.e., permanent middle cerebral artery occlusion, MCAo), we found that levels of fetuin-A in the ischemic brain tissue were elevated in a time-dependent manner, starting between 2 and 6 h, peaking around 24 to 48 h, and returning to baseline 72 h after MCAo. When administered peripherally, exogenous fetuin-A gained entry across the BBB into the ischemic brain tissue, and dose dependently reduced brain infarct volume at 24 h after MCAo. Meanwhile, fetuin-A effectively attenuated (i) ischemia-induced HMGB1 depletion from the ischemic core; (ii) activation of centrally (e.g., microglia) and peripherally derived immune cells (e.g., macrophage/monocytes); and (iii) TNF production in ischemic brain tissue. Taken together, these experimental data suggest that fetuin-A protects against early cerebral ischemic injury partly by attenuating the brain inflammatory response.
Keywords: fetuin-A, inflammation, ischemia
Introduction
Cerebral ischemia is frequently caused by occlusion of a cerebral artery, which, if not resolved within a short period of time, will lead to irreversible ischemic injury. Despite advances in acute and prophylactic therapies, stroke still represents the leading cause of long-term disability (500,000 to 700,000 cases per year), and the third most common cause of death (with a mortality rate of 20% to 25%) in the US. A major conceptual advance in understanding the pathophysiology of stroke was the recognition that cerebral ischemic injury comprises two stages: primary tissue damage in the ischemic core and secondary cell injury in the surrounding periphery (Feuerstein et al, 1998).
Outside of the ischemic zone where cells are destined to die (the ‘ischemic core'), lies a ‘periphery zone' where brain cells continue to die for hours, and even days after the onset of ischemia. This progressive cell death is partly mediated by ischemia-elicited inflammatory response that is manifested by accumulation of various proinflammatory cytokines (Feuerstein et al, 1998). For instance, tumor necrosis factor (TNF) is produced in vanishingly small amounts (if any at all) in quiescent neural cells, but its transcription and translation are rapidly upregulated after cerebral ischemia (Botchkina et al, 1997; Meistrell et al, 1997). Its pathogenic role has been supported by the observations that pharmacological inhibition of TNF production (by CNI-1493 or dexamethasone) (Meistrell et al, 1997), or cytokine activity (by neutralizing antibody or soluble receptors) (Meistrell et al, 1997; Barone and Parsons 2000), confers protection against cerebral ischemic injury.
In contrast, HMGB1 is constitutively expressed in quiescent neural cells, and a large pool of pre-formed HMGB1 is stored in the nucleus and cytosol compartments (Merenmies et al, 1991). As a ubiquitous protein, HMGB1 can be secreted by activated macrophages/monocytes (Wang et al, 1999; Rendon-Mitchell et al, 2003), or passively released by necrotic cells (Scaffidi et al, 2002), and amplifies an inflammatory response. For instance, cerebral ischemia induces rapid HMGB1 depletion from the ischemic core in vivo (Qiu et al, 2008). Furthermore, HMGB1-specific neutralizing antibodies and antagonists (e.g., the A-box) are protective against cerebral ischemic injury (Liu et al, 2007; Muhammad et al, 2008), supporting a pathogenic role for HMGB1 in inflammatory diseases.
As a feedback, counter-regulatory mechanism, a ubiquitous biogenic molecule, spermine, accumulates at the sites of injury and inhibits the release of multiple proinflammatory cytokines (such as TNF, inerleulkin-1 (IL-1), macrophage inflammatory protein-1α, and macrophage inflammatory protein-1β) from activated macrophages/monocytes (Zhang et al, 1997; Zhu et al, 2009). However, its anti-inflammatory activities are dependent on the availability of a ubiquitous protein, fetuin-A (Wang et al, 1997; Wang et al, 1998), as spermine fails to inactivate macrophages/monocytes if these cells were deprived of fetuin-A by serum starvation or addition of fetuin-A-neutralizing antibodies (Wang et al, 1997). In contrast, the anti-inflammatory activity of spermine is enhanced by supplementing macrophage cultures with highly purified fetuin-A (Wang et al, 1997), suggesting a potential role for fetuin-A in the regulation of innate immune response.
Fetuin-A is produced primarily by the liver, and its synthesis is downregulated by proinflammatory cytokines (e.g., TNF), thereby being characterized as a negative acute-phase protein (Dziegielewska and Brown 1995). Intriguingly, plasma fetuin-A levels were significantly elevated in patients with ischemic stroke (Weikert et al, 2008; Tuttolomondo et al, 2009), although it is not known whether fetuin-A levels are similarly elevated in ischemic brain tissue of these patients. A wide range of biological functions have been proposed for fetuin-A based on its structural similarities to other proteins, or interaction with cationic molecules. For instance, fetuin-A shares amino-acid sequence similarity to insulin receptor tyrosine kinase (Mathews et al, 1997) and type-II transforming growth factor-β receptor (Demetriou et al, 1996), and has thus been proposed as a natural inhibitor of the insulin-signaling pathway and an antagonist of transforming growth factor-β. Conversely, fetuin-A carries two N-linked three O-linked oligosaccharide chains that terminate with sialic acid residues, thereby bearing a net negative charge at physiological pH. It has been proposed that fetuin-A functions as a carrier of cationic ions (e.g., Ca2+) and other biologically active molecules (e.g., spermine) (Suzuki et al, 1994; Wang et al, 1997). Through its acidic residues, fetuin-A binds Ca2+ and consequently inhibits calcium salt precipitation (apatite formation) during bone formation (Szweras et al, 2002). Similarly, fetuin-A potentially binds spermine to enhance its cellular uptake, thereby enhancing its anti-inflammatory activities (Wang et al, 1997).
At higher concentrations, fetuin-A itself is anti-inflammatory, and effectively inhibits bacterial endotoxin-induced production of proinflammatory mediators (such as TNF, IL-1, and nitric oxide) in macrophage cultures (Dziegielewska et al, 1998). In animal model of carrageenan-induced paw edema, intraperitoneal administration of fetuin-A attenuated local TNF production and inflammation in vivo (Ombrellino et al, 2001). However, the potential role of fetuin-A in cerebral ischemia injury was previously unknown. Here we reported that fetuin-A levels were increased in ischemic brain tissue, and that further elevation of its cerebral levels by peripheral administration conferred a dose-dependent reduction of infarct volume. This protection was associated with parallel attenuation of (i) ischemia-induced HMGB1 depletion from the ischemic core; (ii) activation of centrally (e.g., microglia) and peripherally derived immune cells (e.g., macrophage/monocyte); and (iii) expression of TNF in the ischemic brain. Taken together, these experimental data suggest that fetuin-A protects against cerebral ischemic injury partly by attenuating ischemia-elicited inflammatory response.
Materials and methods
Permanent MCAo
This study was approved and performed in accordance with the guidelines for the care and use of laboratory animals at the Feinstein Institute for Medical Research, Manhasset, New York. Permanent focal cerebral ischemia was induced in Lewis rats (male, 270 to 300 g), wild-type (Jackson Laboratory, Bar Harbor, ME, USA), or fetuin-A-deficient, C57BL/6J mice (male, 28 to 30 g obtained from Dr. W Jahnen-Dechent's Laboratory) by permanent middle cerebral artery occlusion (MCAo) as previously described (Meistrell et al, 1997; Ivanova et al, 1998). Briefly, animals were anesthetized with ketamine (75 mg/kg, intramuscular) and the contralateral common carotid arteries and ipsilateral MCA were exposed. The contralateral common carotid artery was occluded for 1 hour while the ipsilateral MCA was permanently occluded by electrocoagulation. After 1 hour temporary occlusion of the common carotid artery was removed and the neck and head incisions were closed immediately afterwards.
Purified bovine fetuin-A, or asialofetuin-A (sialic acid residues of fetuin-A removed by neuraminidase; Calbiochem, San Diego, CA, USA), was dissolved in 1 × phosphate-buffered saline (PBS) solution and administrated intravenously at 15, 30, or 60 mins after MCAo. Twenty-four hours after MCAo, the animals were anesthetized with ketamine and then killed to remove the brain. The brains were cut coronally into 2-mm-thick sections and stained with 2,3,5-triphenyl-2H-tetrazolium chloride (TTC, 2% in 154 mmol/L NaCl) at 37°C for 30 mins. The total cerebral infarct volume was calculated by computerized quantitative planimetry using the following formula: infarct volume=(Thickness of the slice) × (Sum of the infarction area in all brain slices (mm2)) as previously described (Meistrell et al, 1997; Ivanova et al, 1998). As brain edema may affect the accuracy of infarct estimation, we calculated the corrected infarct size using the following formula: Acorrected=Ainfarct × (1−AI−Ac)/Ac, where AI is the ipsilateral hemisphere area and Ac is the contralateral hemisphere area.
SDS–PAGE Analysis of Protein Profiles
For analysis of protein profiles by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE), rats were killed and immediately perfused intracardially with 100 ml PBS (0.1 mol/L, pH 7.4) before harvesting brain tissue. After TTC staining, the ischemic and contralateral non-ischemic brain tissues were excised and separately homogenized in lysis buffer (50 mmol/L Tris–HCl, 0.5% NP-40, 150 mmol/L NaCl, 5 mmol/L EDTA, 1 mmol/L pheynylmethylsulfonyl fluoride, 10 mg/ml leupeptin, 10 mg/ml pepstatin, 0.02% sodium azide, pH 7.4). After brief centrifugation, the soluble lysate was mixed with one volume 2 × SDS loading buffer and resolved on 4% to 20% gradient SDS–PAGE gels.
Western Blot Analysis
Rat fetuin-A-specific antibodies were obtained from Dr Willi Jahnen-Dechent (Germany). Bovine fetuin-A-specific polyclonal antibodies were generated in rabbits as previously described (Ombrellino et al, 2001). Monoclonal antibodies against β-actin were obtained from Abcam (Abcam, Cambridge, MA, USA; cat. no. mAbcam 8226). HMGB1-specific antibodies were generated in rabbit as previously described (Wang et al, 1999). Monoclonal anti-ED1 antibodies were obtained from AbD Serotec (Raleigh, NC, USA; MCA341R). Polyclonal anti-rat TNF antibodies were generated in rabbits as previously described (Meistrell et al, 1997). After SDS–PAGE, the proteins were transferred onto a polyvinylidine fluoride membrane and hybridized with specific primary antibodies. After incubation with the alkaline phosphatase-conjugated secondary antibodies, the signal was detected with a colormetric alkaline phosphatease assay kit (Bio-Rad Laboratories, Hercules, CA, USA). The relative band intensity was quantified by using the NIH image 1.59 software as previously described (Zhu et al, 2009).
Immunohistochemistry
Brain tissues were fixed by sequential intracardiac perfusion with 0.05 mol/L PBS (pH 7.4) containing 0.1% sodium nitrate and heparin, followed by infusion with 2% paraformaldehyde in 0.1 mol/L PBS (pH 7.4) and 4% paraformaldehyde in 0.1 mol/L PBS (pH 7.4). After perfusion, brains were removed and stored in the same fixative solution for 15 mins at 4°C, overnight, and then transferred to a solution of 20% sucrose in PBS, overnight, at 4°C. The frozen sections were cut coronally (20-μm thick) with the cryostat. After quenching endogenous peroxidase activity with 0.3% H2O2 solution, sections were incubated with normal serum (1:20 dilution) for 1 hour and subsequently incubated (4°C, overnight) with primary rabbit polyclonal antibodies against NeuN (Chemicon, Rosemont, IL, USA; MAB 377), HMGB1 (1:500), ED1 (CD68, 1:500; MCA341R; AbD Serotec USA), and TNF (1:500, hamster monoclonal anti-TNF; Genzyme, Cambridge, MA, USA). After several washes in 1 × PBS, the sections were incubated with biotin-conjugated goat anti-rabbit, mouse, or hamster IgG antibodies (for 4 h at 25°C), and subsequently incubated for 15 mins with substrate buffer (0.1 mol/L 3,3′-diaminobenzidine in 0.05 mol/L Tris–HCl buffer, pH 7.4) containing 3% 3-amino-9-ethylcarbazole in N,N-dimethylformamide. For HMGB1 or NeuN immunohistochemistry, Alexa 488-conjugated goat anti-rabbit IgG (Invitrogen, Carlsbad, CA, USA; cat. no. A11029) or Alexa 594-conjugated goat anti-murine IgG (cat. no. A11032; Invitrogen) were used, and images were captured using a fluorescence microscope (Carl Zeiss Microimaging).
Determination of BBB Permeability to FITC-Labeled Fetuin-A
To determine whether fetuin-A gains entry into the ischemic brain, fetuin-A was labeled with fluorescein isothiocyanate (FITC) using the EZ-Label FITC Protein Labeling kit (cat. no. 53004; Pierce Biotechnology, Rockford, IL, USA) according to the manufacturer's instructions. Excess fluorescent dye was removed using the D-Salt Dextran Desalting Columns supplied in the FITC labeling kit. FITC-labeled fetuin-A (10 mg/kg) was administered intravenously at 15 mins after MCAo. At 24 h after MCAo, animals was anesthetized and sequentially perfused with 50 ml saline and 50 ml 4% paraformaldehyde with 0.1 mol/L PBS (pH 7.4). After harvesting, the brain was fixed in 4% paraformaldehyde for additional 2 h and subsequently sectioned (20-μm thick). After air drying for 15 mins, the brain sections were examined for presence of FITC-labeled fetuin-A with a fluorescence microscope (excitation filter, 450∼490 nm; suppression filter, 515∼560 nm).
Stereotactically Guided Microinjections of Fetuin-Specific Neutralizing Antibodies
To confirm the neuroprotective role of fetuin-A in cerebral ischemia, we examined the effect of intracerebroventricular (ICV) administration of fetuin-specific neutralizing antibodies on the progression of the cerebral ischemic injury. Fetuin-A-specific neutralizing antibodies were generated in rabbits as previously described (Wang et al, 1997; Ombrellino et al, 2001). As previously described (Meistrell et al, 1997; Ivanova et al, 1998), male Lewis rats (270 to 300 g) were anesthetized and placed in a stereotactic head frame (Stoelting Co., Wood Dale, IL, USA). The incisor bar was adjusted until the plane defined by the lambda and bregma was parallel to the base plate. A microsurgical craniotomy was performed 1.7 mm anterior to bregma and 5 mm right of the midline, and the tip of a 29-gauge needle was advanced to a 2-mm depth to the dural opening. Solution of fetuin-specific IgGs or irrelevant rabbit IgGs (200 μg) prepared in 20 μl of sterile saline (NaCl; 154 mmol/L) were injected over 3 mins and the needle was left undisturbed for 5 mins and then removed. An hour later, the animals were subjected to MCAo and the brains were harvested to determine infarct volume 24 h later.
Measurement of Organ Blood Flow
A radiolabeled microsphere technique was used to measure organ blood flow as previously described (Li et al, 2007). Briefly, a bolus of cerium-141-labeled microspheres (4.0 μCi; DuPont NEN, Boston, MA, USA) was injected into the left ventricle where they were mixed uniformly with the oxygenated blood at the root of the aorta, and subsequently distributed via aortic blood flow to the capillary beds within each organ. The reference blood sample was withdrawn from the femoral arterial catheter (at a rate of 0.7 ml/minute) with a pump (Harvard Apparatus, Holliston, MA, USA), after which isotonic sodium chloride solution was infused manually to replace the volume of blood lost. At 60 mins after infusion, animals were killed to harvest various organs for measurement of radioactivity using an automatic gamma counter (1470 Wizard; Wallac, Gaithersburg, MD, USA).
Statistical Analysis
Data are expressed as mean±s.d. of at least two to three independent experiments (n=2 to 3). One-way analysis of variance was used for comparison among all different groups. When the analysis of variance was significant, post hoc testing of differences between groups was performed using Tukey's test. A P-value <0.05 was considered statistically significant.
Results
Cerebral Fetuin-A Levels were Elevated after Focal Cerebral Ischemia
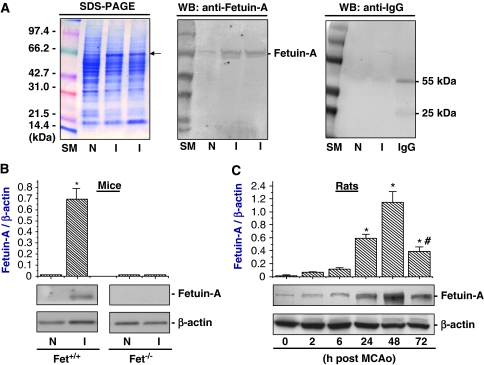
To understand the pathogenesis of cerebral ischemic injury, we evaluated potential protein profile changes in ischemic brain tissue by SDS–PAGE. At 24 h after MCAo, there was a substantial increase in the level of a 63-kDa protein in ischemic brain tissue (‘I') as compared with that of the contralateral non-ischemic (‘N') brain tissue (Figure 1A, left panel). This 63-kDa protein was specifically detected by rat fetuin-A-specific monoclonal antibodies (Figure 1A, middle panel) as well as bovine fetuin-A-specific polyclonal antibodies (data not shown). The cross-reactivity was not entirely unexpected, as rat and bovine fetuin-A share >60% identity in their amino-acid sequences (Dziegielewska and Brown, 1995). It was previously shown that levels of other plasma proteins such as immunoglobulin (IgG) were also elevated in the ischemic core area at 1 to 3 days after temporal MCAo, an animal model of ischemia/reperfusion injury (Czurko and Nishino, 1994). However, levels of IgG were not elevated at 24 h after permanent MCAo, as judged by Western blotting analysis using anti-rat IgG antibodies (Figure 1A, right panel). Our experimental results are consistent with an earlier observation that IgG was detected in ischemic brain tissue of neonatal rats, but not in adult animals (3 weeks old) at 24 h after permanent MCAo (Muramatsu et al, 1997).
Figure 1.
Cerebral fetuin-A levels were elevated after cerebral ischemia. (A) Comparison of protein profiles by SDS–PAGE and Western blotting analysis. Note that the level of the 63-kDa protein was dramatically higher in the ischemic (‘I') than in the contralateral non-ischemic (‘N') brain tissue. This 63-kDa protein was specifically detected by Western blotting analysis using rat fetuin-A-specific monoclonal antibodies. (B) Comparison of cerebral fetuin-A levels between wild-type and fetuin-A-deficient, C57BL/6J mice. Levels of fetuin-A in ischemic (‘I') and contralateral non-ischemic (‘N') brain tissue were determined by Western blot analysis at 24 h after MCAo, and expressed as mean±s.d. of two independent experiments (n=2). *P<0.01 versus ‘Fet+/+, N', ‘Fet−/−, N', or ‘Fet−/−, I'. (C) Fetuin-A levels in ischemic brain increased in a time-dependent manner. At various time points after the onset of MCAo, levels of fetuin-A in ischemic brain tissue were determined by Western blot analysis and expressed as mean±s.d. of two independent experiments (n=2). *P<0.01 versus ‘0 h after MCAo' #P<0.01 versus ‘48 h after MCAo'.
To further confirm the identity of this 63-kDa protein, we compared the levels of fetuin-A in ischemic brain tissues between wild-type and fetuin-A-deficient C57BL/6J mice. As expected, levels of fetuin-A in ischemic brain tissue of wild-type, but not fetuin-A-deficient, mice were similarly elevated at 24 h after MCAo (Figure 1B), confirming that fetuin-A levels were significantly elevated after focal cerebral ischemia. Furthermore, levels of fetuin-A in ischemic brain tissue were increased in a time-dependent manner, significantly elevated at 24 h, and peaked at 48 h after MCAo (Figure 1C). Subsequently, fetuin-A levels were significantly decreased at 72 h after MCAo (Figure 1C), suggesting a possibility that fetuin-A only temporally gains entry into the ischemic brain tissue across the blood–brain barrier (BBB).
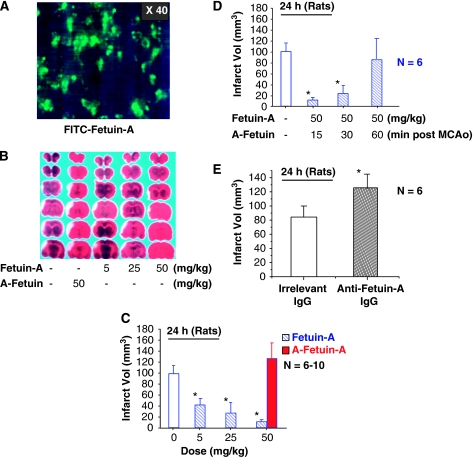
To determine whether peripheral fetuin-A can gain entry across the BBB into ischemic brain tissue, FITC-labeled fetuin-A was administered intravenously at 15 mins after MCAo and brain tissue was examined for presence of fluorescence signals at 24 h after MCAo. As shown in Figure 2A, fluorescence signals were observed in the ischemic brain region, indicating that peripherally administered fetuin-A gained entry across the BBB into the ischemic brain tissue. In contrast, fluorescence signal was not detected in ischemic brain tissue at 24 h after intravenous administration of FITC-labeled rat IgG (data not shown), indicating that not all circulating proteins can easily gain entry into the ischemic brain tissue.
Figure 2.
Peripheral administration of fetuin-A reduced infarct volume at 24 h after MCAo. (A). Detection of exogenous fetuin-A by dye trace technique. FITC-labeled fetuin-A was administered intravenously at 15 mins after MCAo and brain sections were examined for fluoresecence signal 24 h later. Note that fetuin-A-associated fluorescence signals are observed in ischemic brain regions. (B). Representative TTC staining of brain sections. At 24 h after MCAo, brain tissues were harvested, sectioned, and stained with TTC. Note that the area of the infarct (shown as white) region is significantly smaller in animals treated with fetuin-A. (C, D) Effects of intravenous administration of fetuin-A on ischemic brain infarct volume. Note that total infarct volume (mean±s.d. of 6 to 10 animals per group) is reduced by peripheral administration of fetuin-A (but not asialofetuin-A, ‘A-fetuin-A') in a dose (C) and time (D)-dependent manner. *P<0.01 versus the control vehicle group (‘0 mg/kg fetuin-A'). (E) Effect of ICV administration of fetuin-A-specific antibodies on ischemic brain infarct volume. Note that total infarct volume (mean±s.d.) is significantly increased by ICV administration of fetuin-A-specific neutralizing antibodies (200 μg, 20 μl). *P<0.05 versus the ‘irrelevant IgG' group.
Peripheral Administration of Fetuin-A Reduced Infarct Volume
To understand the role of fetuin-A in cerebral ischemic injury, we determined whether fetuin-A-deficient mice were more susceptible to cerebral ischemic insult. Compared with age- and sex-matched, wild-type C57BL/6J mice, body weight was significantly higher in fetuin-A-knockout mice (25.67±0.64 g for wild-type, male, 10-weekold mice, n=10, versus 27.85±0.97 for fetuin-A- knockout, male, 10-week-old mice, n=10; P<0.01), suggesting that fetuin-A deficiency might be associated with metabolic abnormalities. As a potential consequence, as compared with sex- and body-matched (male, 27 to 29 g), wild-type C57BL/6J mice, infarct volume was not significantly larger in fetuin-A-knockout mice (11.8±2.6 mm3 for wild-type mice, n=5, versus 6.9±3.6 mm3 for fetuin-A-knockout mice, n=5). Notably, in the absence of fetuin-A, another member of the fetuin family, fetuin-B (Olivier et al, 2000), which shares >42% to 46% homology in amino-acid sequence with fetuin-A, might function as a redundant mechanism to compensate for the deficiency of fetuin-A. Like its fetuin-A counterpart, fetuin-B is also predominantly expressed in liver and is present abundantly in the circulation of all animal species examined, including rat (200 to 300 μg/ml) and mouse (150 μg/ml) (Olivier et al, 2000). As fetuin-A/fetuin-B double knockout mice are not yet available, we continued to investigate the potential role of fetuin-A in cerebral ischemia using a rat model of permanent MCAo.
In the rat model of MCAo, permanent cerebral ischemia induced a distinct infarct in the right cortex (Figure 2B), with an average infarct volume of 100±25 mm3 (Figure 2C and 2D). Treatment of animals with fetuin-A at 15 mins after MCAo promoted dose-dependent protection against cerebral ischemic injury, with >90% reduction of infarct volume by 50 mg/kg fetuin-A at 24 h after MCAo (Figure 2C). It was previously shown that the permeability of BBB was transiently elevated (3- to 4-fold between 5 and 25 h after MCAo) (Belayev et al, 1996) and returned to baseline at 72 h after MCAo. The parallel, time-dependent elevation of the BBB permeability and fetuin extravasation/ accumulation in ischemic brain tissue supports a possibility that breakdown of the BBB is required for fetuin-A to gain entry into the brain. As a possible consequence, the protective effects were no longer significant (infarct volume=28.4±9.6 mm3 for the control group, n=5, versus infarct volume=32.1±13.1 mm3 for the fetuin-A group, 50 mg/kg) at 7 days after MCAo. In a parallel study, we found that repetitive administration of fetuin-A (100 mg/kg) at 24, 48, and 72 h after the onset of peritoneal infection (induced by cecal ligation and puncture) promoted a long-lasting protection against lethal systemic inflammation at 14 day after cecal ligation and puncture (Wang et al, unpublished observations). As it is unknown whether circulating fetuin-A can still gain entry into the ischemic brain after BBB function restores between 48 and 72 h after MCAo (Belayev et al, 1996), it is important to determine through further studies whether repetitive administration of fetuin-A confers a long-lasting protection against cerebral ischemic injury.
Even when administered 30 mins after MCAo, fetuin-A (50 mg/kg) still significantly reduced infarct volume at 24 h after MCAo (Figure 2D), suggesting that intravenous administration of fetuin-A is protective against early cerebral ischemic injury. To confirm the neuroprotective role of fetuin-A in cerebral ischemia, fetuin-A-specific neutralizing antibodies were intracerebroventricularly (ICV) administered at 1 hour before MCAo and infarct volume was assessed 24 h later. Compared with animals treated with equal amount of control IgG, the infarct volume was significantly increased by ICV administration of fetuin-A-specific neutralizing antibodies (Figure 2E), supporting a neuroprotective role for fetuin-A during an early stage of cerebral ischemia.
Peripheral Administration of Fetuin-A did not Affect Cerebral Blood Flow, but Attenuated Ischemia-Elicited HMGB1 Depletion from the Ischemic Core
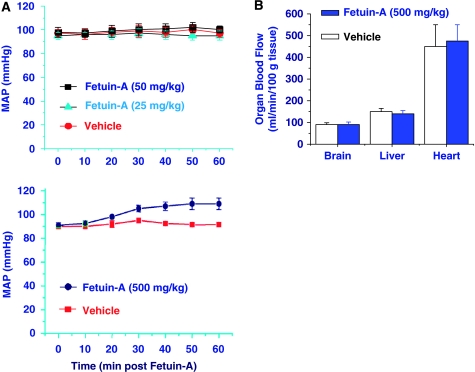
To elucidate the mechanism by which fetuin-A conferred protection against cerebral ischemic injury, we first determined its effects on mean arterial blood pressure (MABP) and cerebral blood flow. At the lower doses (5, 25, and 50 mg/kg) that conferred significant protection against early cerebral ischemia (Figure 2C), fetuin-A did not significantly affect the MABP or cerebral blood flow (Figure 3A, top panel). Even when given at a higher concentration (500 mg/kg), fetuin-A only slightly increased the MABP (Figure 3A, bottom panel), but still did not change cerebral blood flow (Figure 3B). Taken together, these experimental data suggest that fetuin-A-mediated protection was not likely attributable to alteration of physiological parameters (such as MABP and blood flow).
Figure 3.
Peripheral administration of fetuin-A did not affect blood flow. (A) Effect of fetuin-A administration on MABP. Rats were intravenously administered fetuin-A at the indicated doses and MABP was monitored over 60 mins. At lower doses (25 or 50 mg/kg) fetuin-A did not significantly alter the MABP (mean±s.d., n=3). (B) Effect of fetuin-A on cerebral blood flow. Blood flow in major organs was measured at 60 mins after intravenous fetuin-A administration. Note that intravenous administration of fetuin-A did not affect cerebral blood flow.
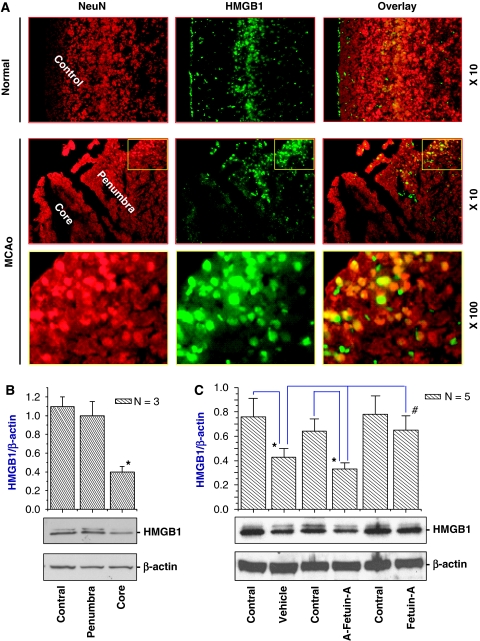
We then examined the effect of fetuin-A on HMGB1 expression levels in the ischemic brain tissue. Consistent with earlier reports (Merenmies et al, 1991), HMGB1 was detected in both nucleus and cytoplasm of neural cells (Figure 4A). After cerebral ischemic insult, it was barely detected in the ischemic core region (Figure 4A) and its relative HMGB1 content in the ischemic core, but not the periphery, was significantly decreased (Figure 4B). Interestingly, peripheral administration of fetuin-A, but not asialofetuin-A, significantly attenuated ischemia-elicited decrease of HMGB1 content in the ischemic core (Figure 4C), suggesting that fetuin-A exerts neuroprotective effect partly by inhibiting ischemia-elicited HMGB1 depletion.
Figure 4.
Peripheral administration of fetuin-A attenuated ischemia-induced HMGB1 release from the ischemic core. (A) Immunohistochemical analysis of HMGB1 in ischemic (‘MCAo') and non-ischemic (‘Normal') brain tissue. Note that HMGB1 was found in both nucleus and cytosol of NeuN-positive cells in normal brain, or in the periphery of ischemic brain tissue. In contrast, HMGB1 was barely detected in the ischemic core at 24 h after MCAo. (B) Western blot analysis of HMGB1 content in the ischemic core. (C) Peripheral administration of fetuin-A attenuated ischemia-induced HMGB1 depletion. *P<0.05 versus the control (contralateral) hemisphere; #P<0.05 versus vehicle or A-fetuin-A.
Administration of Fetuin-A Suppresses TNF Production in Ischemic Brain Tissue
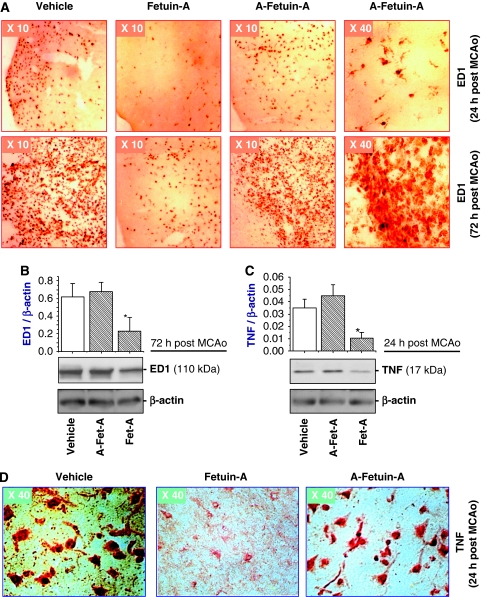
To further elucidate the mechanisms underlying fetuin-A-mediated protection, we determined the effect of fetuin-A on immune cell activation in the ischemic brain tissue. As a specific marker for cell activation, ED1 was detected in the ischemic brain region on microglia-like cells at 24 h after MCAo (Figure 5A, upper panels), and on macrophage-like cells at 72 h after MCAo (Figure 5A, bottom panels). However, treatment of animals with fetuin-A (50 mg/kg), but not asialofetuin-A, led to dramatic reduction of ED1 expression in both microglia-like and macrophage/monocyte-like cells in the ischemic region (Figure 5A). Consistently, relative ED1 expression levels in ischemic brain tissue were significantly reduced by fetuin-A (50 mg/kg), but not asialofetuin-A (Figure 5B).
Figure 5.
Peripheral administration of fetuin-A attenuated ED1 and TNF expression in ischemic brain tissue. (A) Immunohistochemical analysis of ED1 expression. Note that ED1 was detected on microglia-like cells at 24 h after MCAo and on macrophage/monocyte-like cells at 72 h after MCAo. (B, C) Western blot analysis of ED1 and TNF expression levels. The levels of ED1 or TNF in ischemic brain tissue were determined by Western blot analysis and expressed as mean±s.d. of two independent experiments (n=2). *P<0.05 versus the ‘vehicle' group. (D) Immunohistochemical analysis of TNF expression. Note that fetuin-A administration dramatically attenuated TNF expression levels in ischemic brain tissue.
In agreement with previous report (Botchkina et al, 1997), TNF was detected in both the ischemic core (Figure 5C and 5D) and the surrounding periphery (data not shown) at 24 h after MCAo. In the center of ischemic core, most TNF-positive cells showed the typical morphology of neural cells. In the outermost layer of the core, TNF staining was also found in cells morphologically similar to microglia (data not shown). Treatment with fetuin-A (50 mg/kg), but not asialofetuin-A, dramatically decreased TNF immunoreactivity both in the ischemic core (Figure 5D) and the peripheral regions (data not shown). Consistently, levels of TNF expression in ischemic brain tissue were significantly decreased by treatment with fetuin-A (50 mg/kg) but not asialofetuin-A (50 mg/kg) (Figure 5C), suggesting that fetuin-A protects against early cerebral ischemic injury partly by inhibiting TNF expression.
Discussion
The BBB normally prevents cells of the central nervous system from coming into contact with most proteins in the blood, but it becomes leaky and permeable to a number of circulatory proteins after cerebral ischemic insult. For instance, albumin accumulates in the infarct zone and confers neuroprotection by serving as an antioxidant or carrier of free fatty acids or Ca2+ (Belayev et al, 1996). In this study, we showed that peripherally administered fetuin-A similarly gained entry across the BBB into ischemic brain tissue. As a possible consequence, levels of fetuin-A in the ischemic core were increased in a time-dependent manner, starting approximately between 2 and 6 h, peaking between 24 and 48 h, and returning to baseline at 72 h after MCAo. This time-dependent increase in cerebral fetuin-A levels parallels with the transient elevation of the BBB permeability (Belayev et al, 1996), which was shown to increase at 4 h (by 1.8-fold), peak (3 to 4 folds) between 5 to 25 h, and return (1.6-fold) to baseline at 72 h after MCAo (Belayev et al, 1996). Thus, like neurons of the developing brain, which take up fetuin-A from the cerebrospinal fluid during development (Saunders et al, 1994), adult brain tissue transiently recruits fetuin-A from the circulation after cerebral ischemic insult.
Intriguingly, it has been suggested that fetuin-A may determine the fate of neurons during brain development (Saunders et al, 1994), as fetuin-A is more frequently found on ganglion neurons that tend to die at a much lower rate (than those fetuin-A-negative ganglion neurons) (Saunders et al, 1994). Here we showed that fetuin-A is protective against cerebral ischemic insult, as peripheral administration of fetuin-A immediately (15 to 30 mins) after MCAo led to a dose-dependent reduction in infarct volume. The mechanisms underlying fetuin-A-mediated protection may be complex, but may partly be attributable to its anti-inflammatory effects. For instance, peripheral administration of fetuin-A not only effectively attenuated ischemia-induced HMGB1 depletion from the ischemic core, but also dramatically reduced TNF expression levels in the ischemic brain tissue.
We and others discovered that HMGB1 is released by activated innate immune cells (Wang et al, 1999) or necrotic cells (Scaffidi et al, 2002) and functions as an important mediator of lethal systemic inflammation (Wang et al, 2009) and cerebral ischemic injury (Yang et al, 2009). Extracellular HMGB1 activates innate immune cells and endothelial cells to produce proinflammatory cytokines, chemokines, tissue factors, and adhesion molecules (Andersson et al, 2000; Lv et al, 2009). Indeed, ICV administration of HMGB1 induces cerebral expression of proinflammatory cytokines (e.g., TNF, IL-6) (O'Connor et al, 2003). Notably, HMGB1 is rapidly depleted (within 1 hour after MCAo) from the ischemic core (Qiu et al, 2008), as a possible consequence of both active secretion or passive leakage from injured neurons. It is thus plausible that HMGB1 rapidly released from the ischemic core area may be spilled into surrounding periphery, where it mediates a potentially injurious inflammatory response. Afterwards, TNF expression is elevated, starting at 3 h, peaking at 12 h, and persisting for several days after MCAo (Botchkina et al, 1997). It is thus plausible that HMGB1 massively released from the ischemic core may orchestrate an inflammatory response in the surrounding periphery region (Kim et al, 2006).
Immediately after cerebral ischemia, the brain mounts a rigorous inflammatory response driven by the centrally and peripherally derived immune cells. Within the first few hours of cerebral ischemia, several types of neural cells (e.g., microglia and neurons) become activated to produce TNF and other proinflammatory cytokines (Botchkina et al, 1997). Subsequently, polymorphonuclear cells infiltrate into the ischemic brain tissue within 12 to 48 h (Akopov et al, 1996), followed by an influx of monocytes and macrophages over a period of one to several days (Figure 6). In agreement with these observations, we observed a marked increase in the number of ED1-positive microglia-like cells in the ischemic region at 24 h after MCAo, and a continuous increase in the number of ED1-positive macrophage/monocyte-like cells at 3 day after MCAo. However, peripheral administration of fetuin-A effectively attenuated ischemia-elicited activation of these centrally and peripherally derived immune cells. Taken together, these observations suggest that fetuin-A confers neuroprotective effects partly by attenuating ischemia-elicited HMGB1 release, activation of centrally and peripherally derived cells, and production of proinflammatory cytokines in ischemic brain tissue.
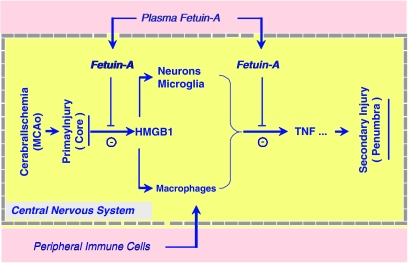
Figure 6.
Hypothetical role of fetuin-A in cerebral ischemic injury. Sudden termination of blood flow to an area of the brain, such as by MCAo, leads to rapid primary ischemic injury through excitotoxicity and oxidative stress. This rapid primary ischemic injury is accompanied by rapid HMGB1 release/leakage from the ischemic neurons. Extracellular HMGB1 may diffuse into the periphery region, where it orchestrates a rigorous inflammatory response driven both by the centrally and peripherally derived cells. Meanwhile, cerebral ischemia induces transient increase in BBB permeability, allowing entry of circulating proteins (e.g., fetuin-A) and peripheral immune cells (such as macrophage/monocytes). Peripheral administration of fetuin-A increased cerebral fetuin-A levels and consequently attenuated (i) ischemia-elicited HMGB1 release; (ii) activation of centrally and peripherally derived immune cells; and (iii) expression of proinflammatory cytokines in the ischemic brain tissue. The mechanisms by which fetuin-A inhibits cerebral HMGB1 depletion and TNF expression remains an important subject of future studies.
Nevertheless, this study cannot eliminate other potential mechanisms underlying fetuin-A-mediated neuroprotective effects. Extensive research has established a pathogenic role for Ca2+ overload and excitotoxicity in primary ischemic injury (Lee et al, 1999). Specifically, engagement of ionotropic N-methyl--aspartate receptor by glutamate or spermine (Zubrow et al, 2000) leads to Ca2+ influx and immediate activation of damaging proteases that compromise the functional and structural integrity of neuronal cells. In addition, spermine can be enzymatically converted by polyamine oxidases into cytotoxic metabolites (e.g., 3-aminopropanal), which mediate cytotoxicity through direct chemical modification of cell membrane proteins (Ivanova et al, 1998). In light of the capacity of fetuin-A to bind Ca2+ and spermine (Suzuki et al, 1994; Wang et al, 1997), it is not yet known whether fetuin-A can compete for binding of Ca2+ and/or spermine with other damaging enzymes (such as polyamine oxidase, Ca2+-dependent proteases), thereby tilting the balance to neuroprotection by inhibiting N-methyl--aspartate receptor receptor and/or excessive inflammatory response.
Cerebral drug delivery is faced with many obstacles due to the unique anatomical and physiological characteristics of the brain and the BBB. It is presently not known whether fetuin-A may be used as a vehicle to deliver therapeutic agents (such as a tetravalent guanylhydrazone compound, CNI-1493) to ischemic brain. We previously found that peripheral administration of a tetravalent guanylhydrazone compound, CNI-1493, effectively inhibited endogenous brain TNF synthesis and conferred significant protection against cerebral ischemic injury if administered at relative high doses (10 mg/kg) (Meistrell et al, 1997). In light of the synergistic inhibition of endotoxin-induced TNF production by fetuin and CNI-1493 in vivo (Wang et al, 1998), it will be important to determine whether fetuin-A facilitates the uptake of CNI-1493 to the CNS, and promotes greater protection against cerebral ischemic injury in future studies.
Acknowledgments
This work was supported by Faculty Award Program of the Feinstein Institute for Medical Research, and National Institute of General Medical Sciences (R01GM063075 and R01GM070817 to HW). We are grateful for Drs Jiantu Che and Michael Beloff for excellent technical assistance with the animal models of cerebral ischemia and stereotactically guided microinjection technique.
Source of Support: This work was supported by the National Institute of General Medical Sciences (R01GM063075 and R01GM070817 to HW).
Footnotes
Disclosure/conflict of interest
KJ Tracey and H Wang are inventors of a US patent application entitled ‘Usage of fetuin in treatment of cerebral ischemic injury (stroke)'.
References
- Akopov SE, Simonian NA, Grigorian GS. Dynamics of polymorphonuclear leukocyte accumulation in acute cerebral infarction and their correlation with brain tissue damage. Stroke. 1996;27:1739–1743. doi: 10.1161/01.str.27.10.1739. [DOI] [PubMed] [Google Scholar]
- Andersson U, Wang H, Palmblad K, Aveberger AC, Bloom O, Erlandsson-Harris H, Janson A, Kokkola R, Zhang M, Yang H, Tracey KJ. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192:565–570. doi: 10.1084/jem.192.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone FC, Parsons AA. Therapeutic potential of anti-inflammatory drugs in focal stroke. Expert Opin Invest Drugs. 2000;9:2281–2306. doi: 10.1517/13543784.9.10.2281. [DOI] [PubMed] [Google Scholar]
- Belayev L, Busto R, Zhao W, Ginsberg MD. Quantitative evaluation of blood–brain barrier permeability following middle cerebral artery occlusion in rats. Brain Res. 1996;739:88–96. doi: 10.1016/s0006-8993(96)00815-3. [DOI] [PubMed] [Google Scholar]
- Botchkina GI, Meistrell ME, Botchkina IL, Tracey KJ. Expression of TNF and TNF receptors (p55 and p75) in the rat brain after focal cerebral ischemia. Mol Med. 1997;3:765–781. [PMC free article] [PubMed] [Google Scholar]
- Czurko A, Nishino H. Appearance of immunoglobulin G and complement factor C3 in the striatum after transient focal ischemia in the rat. Neurosci Lett. 1994;166:51–54. doi: 10.1016/0304-3940(94)90838-9. [DOI] [PubMed] [Google Scholar]
- Demetriou M, Binkert C, Sukhu B, Tenenbaum HC, Dennis JW. Fetuin/alpha2-HS glycoprotein is a transforming growth factor-beta type II receptor mimic and cytokine antagonist. J Biol Chem. 1996;271:12755–12761. doi: 10.1074/jbc.271.22.12755. [DOI] [PubMed] [Google Scholar]
- Dziegielewska KM, Andersen NA, Saunders NR. Modification of macrophage response to lipopolysaccharide by fetuin. Immunol Lett. 1998;60:31–35. doi: 10.1016/s0165-2478(97)00126-0. [DOI] [PubMed] [Google Scholar]
- Dziegielewska KM, Brown WM. Fetuin. Austin, Texas: Landes Co., Springer Verlag; 1995. [Google Scholar]
- Feuerstein GZ, Wang X, Barone FC. The role of cytokines in the neuropathology of stroke and neurotrauma. Neuroimmunomodulation. 1998;5:143–159. doi: 10.1159/000026331. [DOI] [PubMed] [Google Scholar]
- Ivanova S, Botchkina GI, Al-Abed Y, Meistrell M, Batliwalla F, Dubinsky JM, Iadecola C, Wang H, Gregersen PK, Eaton JW, Tracey KJ. Cerebral ischemia enhances polyamine oxidation: identification of enzymatically formed 3-aminopropanal as an endogenous mediator of neuronal and glial cell death. J Exp Med. 1998;188:327–340. doi: 10.1084/jem.188.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JB, Sig CJ, Yu YM, Nam K, Piao CS, Kim SW, Lee MH, Han PL, Park JS, Lee JK. HMGB1, a novel cytokine-like mediator linking acute neuronal death and delayed neuroinflammation in the postischemic brain. J Neurosci. 2006;26:6413–6421. doi: 10.1523/JNEUROSCI.3815-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JM, Zipfel GJ, Choi DW. The changing landscape of ischaemic brain injury mechanisms. Nature. 1999;399 (Suppl:A7–A14. doi: 10.1038/399a007. [DOI] [PubMed] [Google Scholar]
- Li W, Li J, Ashok M, Wu R, Chen D, Yang L, Yang H, Tracey KJ, Wang P, Sama AE, Wang H. A cardiovascular drug rescues mice from lethal sepsis by selectively attenuating a late-acting proinflammatory mediator, high mobility group box 1. J Immunol. 2007;178:3856–3864. doi: 10.4049/jimmunol.178.6.3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Mori S, Takahashi HK, Tomono Y, Wake H, Kanke T, Sato Y, Hiraga N, Adachi N, Yoshino T, Nishibori M. Anti-high mobility group box 1 monoclonal antibody ameliorates brain infarction induced by transient ischemia in rats. FASEB J. 2007;21:3904–3916. doi: 10.1096/fj.07-8770com. [DOI] [PubMed] [Google Scholar]
- Lv B, Wang H, Tang Y, Fan Z, Xiao X, Chen F. High-mobility group box 1 protein induces tissue factor expression in vascular endothelial cells via activation of NF-kappaB and Egr-1. Thromb Haemost. 2009;102:352–359. doi: 10.1160/TH08-11-0759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews ST, Srinivas PR, Leon MA, Grunberger G. Bovine fetuin is an inhibitor of insulin receptor tyrosine kinase. Life Sci. 1997;61:1583–1592. doi: 10.1016/s0024-3205(97)00737-6. [DOI] [PubMed] [Google Scholar]
- Meistrell ME, Botchkina GI, Wang H, Di Santo E, Cockroft KM, Bloom O, Vishnubhakat JM, Ghezzi P, Tracey KJ. Tumor necrosis factor is a brain damaging cytokine in cerebral ischemia. Shock. 1997;8:341–348. [PubMed] [Google Scholar]
- Merenmies J, Pihlaskari R, Laitinen J, Wartiovaara J, Rauvala H. 30-kDa heparin-binding protein of brain (amphoterin) involved in neurite outgrowth. Amino acid sequence and localization in the filopodia of the advancing plasma membrane. J Biol Chem. 1991;266:16722–16729. [PubMed] [Google Scholar]
- Muhammad S, Barakat W, Stoyanov S, Murikinati S, Yang H, Tracey KJ, Bendszus M, Rossetti G, Nawroth PP, Bierhaus A, Schwaninger M. The HMGB1 receptor RAGE mediates ischemic brain damage. J Neurosci. 2008;28:12023–12031. doi: 10.1523/JNEUROSCI.2435-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu K, Fukuda A, Togari H, Wada Y, Nishino H. Vulnerability to cerebral hypoxic-ischemic insult in neonatal but not in adult rats is in parallel with disruption of the blood–brain barrier. Stroke. 1997;28:2281–2288. doi: 10.1161/01.str.28.11.2281. [DOI] [PubMed] [Google Scholar]
- O'Connor KA, Hansen MK, Rachal PC, Deak MM, Biedenkapp JC, Milligan ED, Johnson JD, Wang H, Maier SF, Tracey KJ, Watkins LR. Further characterization of high mobility group box 1 (HMGB1) as a proinflammatory cytokine: central nervous system effects. Cytokine. 2003;24:254–265. doi: 10.1016/j.cyto.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Olivier E, Soury E, Ruminy P, Husson A, Parmentier F, Daveau M, Salier JP. Fetuin-B, a second member of the fetuin family in mammals. Biochem J. 2000;350 (Part 2:589–597. [PMC free article] [PubMed] [Google Scholar]
- Ombrellino M, Wang H, Yang H, Zhang M, Vishnubhakat J, Frazier A, Scher LA, Friedman SG, Tracey KJ. Fetuin, a negative acute phase protein, attenuates TNF synthesis and the innate inflammatory response to carrageenan. Shock. 2001;15:181–185. doi: 10.1097/00024382-200115030-00004. [DOI] [PubMed] [Google Scholar]
- Qiu J, Nishimura M, Wang Y, Sims JR, Qiu S, Savitz SI, Salomone S, Moskowitz MA. Early release of HMGB-1 from neurons after the onset of brain ischemia. J Cereb Blood Flow Metab. 2008;28:927–938. doi: 10.1038/sj.jcbfm.9600582. [DOI] [PubMed] [Google Scholar]
- Rendon-Mitchell B, Ochani M, Li J, Han J, Wang H, Yang H, Susarla S, Czura C, Mitchell RA, Chen G, Sama AE, Tracey KJ, Wang H. IFN-gamma induces high mobility group box 1 protein release partly through a TNF-dependent mechanism. J Immunol. 2003;170:3890–3897. doi: 10.4049/jimmunol.170.7.3890. [DOI] [PubMed] [Google Scholar]
- Saunders NR, Sheardown SA, Deal A, Mollgard K, Reader M, Dziegielewska KM. Expression and distribution of fetuin in the developing sheep fetus. Histochemistry. 1994;102:457–475. doi: 10.1007/BF00269578. [DOI] [PubMed] [Google Scholar]
- Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Shimokawa H, Takagi Y, Sasaki S. Calcium-binding properties of fetuin in fetal bovine serum. J Exp Zool. 1994;270:501–507. doi: 10.1002/jez.1402700603. [DOI] [PubMed] [Google Scholar]
- Szweras M, Liu D, Partridge EA, Pawling J, Sukhu B, Clokie C, Jahnen-Dechent W, Tenenbaum HC, Swallow CJ, Grynpas MD, Dennis JW. Alpha 2-HS glycoprotein/fetuin, a transforming growth factor-beta/bone morphogenetic protein antagonist, regulates postnatal bone growth and remodeling. J Biol Chem. 2002;277:19991–19997. doi: 10.1074/jbc.M112234200. [DOI] [PubMed] [Google Scholar]
- Tuttolomondo A, Di Raimondo D, Di Sciacca R, Casuccio A, Bivona G, Bellia C, Barreca L, Serio A, D'Aguanno G, Ciaccio M, Licata G, Pinto A.2009Fetuin-A and CD40L plasma levels in acute ischemic stroke: differences in relation to TOAST subtype and correlation with clinical and laboratory variables Atherosclerosis(in press) [DOI] [PubMed]
- Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, Tracey KJ. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- Wang H, Ward MF, Sama AE. Novel HMGB1-inhibiting therapeutic agents for experimental sepsis. Shock. 2009;32:348–357. doi: 10.1097/SHK.0b013e3181a551bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhang M, Bianchi M, Sherry B, Sama A, Tracey KJ. Fetuin (alpha2-HS-glycoprotein) opsonizes cationic macrophage-deactivating molecules. Proc Natl Acad Sci USA. 1998;95:14429–14434. doi: 10.1073/pnas.95.24.14429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhang M, Soda K, Sama A, Tracey KJ. Fetuin protects the fetus from TNF [letter] Lancet. 1997;350:861–862. doi: 10.1016/S0140-6736(05)62030-2. [DOI] [PubMed] [Google Scholar]
- Weikert C, Stefan N, Schulze MB, Pischon T, Berger K, Joost HG, Haring HU, Boeing H, Fritsche A. Plasma fetuin-a levels and the risk of myocardial infarction and ischemic stroke. Circulation. 2008;118:2555–2562. doi: 10.1161/CIRCULATIONAHA.108.814418. [DOI] [PubMed] [Google Scholar]
- Yang QW, Wang JZ, Li JC, Zhou Y, Zhong Q, Lu FL, Xiang J.2009High-mobility group protein box-1 and its relevance to cerebral ischemia J Cereb Blood Flow Metab(in press) [DOI] [PMC free article] [PubMed]
- Zhang M, Caragine T, Wang H, Cohen PS, Botchkina G, Soda K, Bianchi M, Ulrich P, Cerami A, Sherry B, Tracey KJ. Spermine inhibits proinflammatory cytokine synthesis in human mononuclear cells: a counterregulatory mechanism that restrains the immune response. J Exp Med. 1997;185:1759–1768. doi: 10.1084/jem.185.10.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Ashok M, Li J, Li W, Yang H, Wang P, Tracey KJ, Sama AE, Wang H. Spermine protects mice against lethal sepsis partly by attenuating surrogate inflammatory markers. Mol Med. 2009;15:275–282. doi: 10.2119/molmed.2009.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubrow AB, Numagami Y, Fritz KI, Mishra OP, Delivoria-Papadopoulos M. Spermine dependent activation of the N-methyl--aspartate receptor and the effect of nitric oxide synthase inhibition during hypoxia in the cerebral cortex of newborn piglets. Brain Res. 2000;854:11–18. doi: 10.1016/s0006-8993(99)02252-0. [DOI] [PubMed] [Google Scholar]