Structured Abstract
Objective
To investigate the effects of the antifibrotic drug halofuginone on extracellular matrix production, cell proliferation and apoptosis of cultured myometrial and leiomyoma smooth muscle cells.
Design
Comparative and controlled experimental research study.
Setting
University research laboratory.
Patients
Leiomyoma and myometrial tissues were obtained from 8 different patients at the time of elective hysterectomy.
Main Outcome Measures
The effects of halofuginone on cell proliferation were assessed by tritiated thymidine uptake assays and cell count assays. Effects on TGFβ1, collagen type I, and collagen type III mRNA levels were assessed by quantitative real-time PCR. Effects on apoptosis were assayed using a chemiluminescent assay to measure changes in caspase 3 and 7.
Results
Halofuginone inhibited cell proliferation of both leiomyoma and autologous myometrial cells in a dose-dependent manner by inhibiting DNA synthesis within 24 hrs and later inducing apoptosis (as measured by increased caspase 3/7) by 48-72 hrs. Halofuginone also significantly reduced collagen type I (α1) and collagen type III (α1) mRNA levels, as well as the profibrotic factor TGFβ1 mRNA levels in both cell types.
Conclusions
These results provide evidence to support the use of the antifibrotic drug halofuginone as a novel drug treatment for uterine leiomyomas.
Keywords: halofuginone, leiomyoma, myometrium, collagen, antifibrotic
INTRODUCTION
Uterine leiomyomas are benign tumors originating from a single neoplastic uterine smooth muscle cell (SMC) and are characterized by an excess of extracellular matrix (ECM) production and increased cell proliferation. Although benign, these tumors present a significant health problem to women of reproductive age. Leiomyomas have a reported symptomatic prevalence of up to 25%, but the overall incidence of these tumors in premenopausal women has been reported to be as high as 77% (1-3). Nearly $1.5 billion healthcare dollars are spent yearly towards treatment of these benign tumors that cause abnormal uterine bleeding, severe abdominal pain and pressure, and infertility (4). Available treatments for leiomyomas are limited. Hysterectomy is considered the standard surgical treatment, but less-invasive treatments such as vaginal hysterectomy, laparoscopic excision, uterine artery embolization, and thermoblative therapy have become more common. Unfortunately, these are often followed by a high rate of recurrence and other complications (1,5,6). The only FDA-approved drug therapy for treatment of leiomyomas is gonadotropin-releasing hormone agonist treatment (GnRHα). Although GnRHα treatment is effective in reducing leiomyoma size, this treatment cannot be used as a long term therapy since women experience irreversible bone loss and other painful menopausal side effects (2). In spite of the high morbidity and economic impact of this disease, little is known about the etiology of these tumors. Genetic, endocrine, autocrine, and environmental factors all seem to contribute to leiomyoma tumor initiation and growth, but the precise cause is still not well understood (1).
Two essential features of all leiomyoma tumors are an increase in SMC proliferation and excessive ECM deposition. It is not known exactly why leiomyoma SMCs have a higher rate of proliferation, but one growth factor that appears to play an important role in regulating proliferation of these cells is transforming growth factor-β (TGFβ). TGFβ is a member of the TGFβ superfamily which is comprised of TGFβ I, II and III, bone morphogenic proteins, and activins (7-9). TGFβs have diverse biological activities within the cell including regulation of cell proliferation and extracellular matrix production. Human myometrial and leiomyoma SMCs have been shown to express the mRNA and proteins for TGFβI, -βII, and −βIII as well as their receptors (10-12). Studies have shown that leiomyoma SMCs express higher levels of TGFβ3 mRNA and protein than corresponding myometrium (12,13). These same studies also showed that treatment with exogenous TGFβI and TGFβIII inhibited myometrial SMC proliferation, while TGFβIII increased leiomyoma SMC proliferation (12). In addition to regulating cell proliferation TGFβs have also been shown to increase collagen and fibronectin synthesis by leiomyoma SMCs (12,13).
Antifibrotic drugs are attractive candidates for treatment of uterine leiomyomas. One antifibrotic agent is halofuginone, a small alkaloid molecule isolated from the plant Dichora febrifuga, that has been used as a coccidiostat in chickens since the 1960s (14). Coccidiostats are commonly added to the feed or water of poultry to prevent infection of the gastrointestinal tract by coccidium protozoa. Halofuginone is considered an antifibrotic drug since it has been shown to inhibit collagen type I production, TGFβI signaling, and cell proliferation in a number of mesenchymal cell types (15,16). This drug has recently been shown to be effective in treating fibrotic conditions including scleroderma, radiation-induced fibrosis, pulmonary fibrosis, fibrotic adhesions surrounding surgical implants, SMC proliferation in arteries following angioplasty in rabbits, as well as the growth of prostate tumors in mice (17-21).
We hypothesized that halofuginone would inhibit both proliferation and collagen production in cultured leiomyoma and myometrial SMCs. The effects of increasing concentrations of halofuginone on cell proliferation and apoptosis were measured using DNA synthesis assays and measurement of caspase activity. Real-Time PCR was used to assess effects on TGFβ and collagen mRNA levels. Our results demonstrated that halofuginone can reversibly inhibit proliferation of both myometrial and leiomyoma SMCs, and this inhibition initially involves an inhibition of DNA synthesis followed later by induction of apoptosis. Our results also demonstrate that halofuginone reduced TGFβI, collagen type I(αI) and type III(αI) mRNA production, in both myometrial and leiomyoma SMCs. These results provide evidence to support the use of halofuginone as a novel drug treatment for uterine leiomyomas.
MATERIALS AND METHODS
Tissue Collection
Leiomyoma and myometrial samples were obtained from premenopausal women with symptomatic leiomyomas at the time of elective hysterectomy at either Carle Foundation Hospital (Urbana, Illinois) or the Brigham & Women’s Hospital (Boston, MA). All tissues were collected under a consent for use of discarded human tissue that was approved by the Institutional Review Board at the University of Illinois Urbana-Champaign and Carle Foundation Hospital or by the Brigham & Woman’s Hospital and Harvard Medical School. Each tissue sample was assigned an arbitrary identification number on the day it was received and patient information was known only to the physician. However, the physicians did provide information about the day of the menstrual cycle for all patients. The patients that were consented for use of discarded tissue were all premenopausal and had not been on any hormonal active medications for three months prior to hysterectomy. Cells used in this study were obtained from 8 different patients. Five of the patients were in the proliferative phase of the cycle and three were in the mid to late secretory phase.
Tissue samples were minced manually and then digested gently overnight in DMEM medium supplemented with 5% fetal calf serum (FBS) and 5% bovine calf serum (BCS) (Atlanta Biologicals, Atlanta, GA) and containing 100 U/mL of collagenase (cat#17018-029, Invitrogen, Carlsbad, CA) at 37°C in a 5% CO2 incubator. Once digested, cells were plated into 75cm2 flasks and grown in this same medium as described in our earlier study (12). Cells were used in experiments at passages 2-8. All cell culture maintenance and experiments were conducted at 37°C and 5% CO2.
Effects of Halofuginone on DNA Synthesis
Leiomyoma and autologous myometrial SMCs from eight different patients were plated at 2,000 cells per well in 96-well plates (Nalge Nunc International, Rochester, NY) and allowed to reach 80% confluency. Six wells each of the leiomyoma and myometrial SMCs were then treated with DMEM containing 10% serum plus 0, 10, 25, 50, 75, 100, 150, or 200 ng/mL of halofuginone (Hoechst-Maion-Roussel, Strasbourg, France) dissolved in dimethyl sulfoxide (DMSO) (Sigma, St. Louis, MO) vehicle for 24h. 0.4μCi [3H] thymidine was added per well in the last 6 hours of treatment. At the end of the treatment period, cells harvested and counted in a MicroBeta liquid scintillation counter. A total of eight experiments were carried out using six wells per treatment group per experiment.
Effects of Halofuginone Treatment on Collagen and TGFβ
Leiomyoma and myometrial SMCs established from 3 different patients were plated in 60cm2 dishes and allowed to reach 80% confluency. Once cells reached 80% confluency they were treated with DMEM containing 10% serum plus 0, 25, 100, or 200 ng/mL of halofuginone for 24h. After treatment medium was removed and cells were harvested in TRIzol™ Reagent (Invitrogen, Carlsbad, CA) for RNA isolation and Real-Time PCR (RT-PCR). A total of three experiments were performed.
cDNA Synthesis and Quantitative Reverse-Transcriptase PCR
Total RNA was extracted from cells using TRIzol™ and transcribed into cDNA using the iScript™ cDNA Synthesis Kit following the manufacturer’s instructions (cat#170-8890, Bio-Rad, Hercules, CA). Synthesized cDNA was then used for TaqMan® Gene Expression Assays real-time PCR analysis (Applied Biosystems, Foster City, CA) using the 20X Assays-on-Demand™ Gene Expression Assays (Applied Biosystems) for COLLAGEN I (Hs00164004_m1), COLLAGEN III (Hs00164103_m1), TGFβ1 (Hs99999918_m1), TGFβ3 (Hs00234245_m1) and 18S (Hs99999901_s1). Reference sequences are listed in Table 1. Real-time PCR amplification and detection were performed in ABI Prism 384-well Clear Optical Reaction Plates (Applied Biosystems). Amplification conditions included: hold 10 min at 95°C, 40 thermal cycles of denaturing 15 sec at 95°C and anneal/extend for 1 min at 60°C. Relative fold induction levels were calculated using the Comparative CT Method as outlined in the ABI Sequence Detector User Bulletin #2 (www.appliedbiosystems.com). 18S ribosomal RNA gene expression served as an endogenous control.
Table 1.
Applied Biosystems Assays-on-Demand™ Gene Expression Sequences
| Gene | Reference Sequence |
|---|---|
| COLLAGEN I | AGACGAAGACATCCCACCAATCACC |
| COLLAGEN III | ACAACAGGAAGCTGTTGAAGGAGGA |
| TGFβ I | GACATCAACGGGTTCACTACCGGCC |
| TGFβ III | GCTGGCGGAGCACAACGAACTGGCT |
| 18S | GGAGGGCAAGTCTGGTGCCAGCAGC |
Effect of Halofuginone on Caspase 3/7 Production
Leiomyoma and corresponding myometrial SMC lines from three different patients were used between passages 3-5. Cells were plated at 2,000 per well and allowed to attach for 24h. Both cell types where then treated for 0, 12, 24, 48, and 72 hours with 100μL of medium containing 0, 25, 100, or 200ng/mL of halofuginone in DMSO vehicle. Control cells were treated with the same amount of DMSO vehicle alone. A 1μM Staurosporine (Sigma) treatment for 20h was used as a positive control as recommended by the Caspase Glo® 3/7 Assay Technical Bulletin (Promega, Madison, Wisconsin). After halofuginone treatment, cells were treated with 100μL of the Caspase Glo® 3/7 Reagent according to the manufacturer’s instructions. Caspase 3/7 activity was detected in quadruplicate wells for each treatment in relative luminescence units (RLU) using the Bio-Tek Synergy HT Reader (Bio-Tek, Winooski, Vermont). A total of three experiments were carried out.
Effect of Halofuginone on Cell Proliferation
Leiomyoma and myometrial SMC lines from three different patients were plated at 200,000 cells/dish and allowed to attach for 48h in DMEM supplemented with 10% serum. On day 1, cells were treated with 75ng/mL of halofuginone in 10% serum-containing DMEM, and two dishes were harvested to obtain an initial cell count. On day 3, one pair of dishes was counted and the remaining dishes were given fresh medium containing 75ng/mL halofuginone. On day 5, one pair of dishes was counted and all remaining dishes were given fresh 10% serum DMEM without halofuginone to see if the cells could resume growth after the removal of halofuginone. On day7 and day 9, one pair of dishes was counted while the remaining dishes were again given fresh 10% serum DMEM without halofuginone. Leiomyoma and myometrial SMCs that were not treated with halofuginone are not shown in the results as these cells reached 100% confluency by day 3 (data not shown). A total of five experiments were carried out for leiomyoma cells and three for myometrial cells.
Statistical Analysis for PCR, Cell Proliferation and Caspase Assays
All PCR experiments were carried out in quadruplicate with each of 3 different cell lines from 3 different patients used as replicates. The difference between the threshold cycle of the target gene and 18S was used to determine statistical significance (ΔCT) between treatments. Threshold cycle (CT) is defined as the cycle number where transcripts are in the linear phase of amplification. All data were first transformed (2-ΔCT) and normalized to the control treatment expression so that they could be expressed as relative fold differences. Standard error for each treatment was determined using individual transformed 2-ΔCT values that had been normalized to the control treatment for each of the 3 replicates. Data were transformed using a log10 transformation, and then all points were compared using ANOVA (α=0.05).
Analysis of the proliferation assays was performed using Kruskal-Wallis ANOVA with Dunn’s test using the STATA software program (CRC, College Station, TX). An α=0.05 was considered statistically significant. All caspase experiments were carried out in triplicate for each of the three cell lines, with results representing the average RLU from all 3 experiments. Caspase data were transformed using a log10 transformation, and then multiple analyses were carried out using ANOVA (α=0.05). Cell count results represent the average of 3-5 experiments using three different leiomyoma or myometrial SMC lines. Cell count numbers were transformed using a log10 transformation and all time points were compared using an ANOVA (α=0.05) test. All statistical analyses in this manuscript were analyzed using SAS v.9.1 (SAS Institute, Inc, Cary, NC). The statistical significance between treatments is indicated in the figures by different letters above treatments.
RESULTS
Effect of Halofuginone on Myometrial and Leiomyoma SMC Proliferation
To assess the effects of halofuginone on leiomyoma and myometrial SMC proliferation, cells were treated with increasing concentrations of halofuginone for 24h and analyzed for changes in DNA synthesis. There was a dose-dependent decrease in DNA synthesis at concentrations of halofuginone above 25 ng/mL for both myometrial and leiomyoma SMCs (Figure 1). We next tested whether the inhibitory effect of halofuginone on proliferation of leiomyoma or myometrial SMCs was reversible and whether treated cells could recover and resume proliferating once halofuginone was removed. Cell proliferation experiments were carried out using leiomyoma and myometrial SMCs from several different patients treated with 75ng/mL of halofuginone. This concentration of halofuginone was chosen because it caused an approximately 50-60% inhibition of DNA synthesis in the thymidine uptake assays. Cell numbers did not change between days 1-3 in cultures treated with halofuginone, in contrast to untreated cells that had already reached confluence at this time (data not shown). This confirmed the results of the tritiated thymidine uptake assays that showed inhibition of DNA synthesis within the first 24 hrs of treatment. Cell numbers of both types of treated cells were markedly decreased by day 5 of treatment, suggesting that by this time there was probably a significant effect on apoptosis (Figure 2). Cells were then placed in fresh, halofuginone-free medium on day 5, and cell populations were increased on days 7 and 9 showing that the inhibitory effect of halofuginone on cell proliferation was reversible. Cell morphology appeared normal throughout the 5 days of halofuginone treatment and the 4 day recovery period (Figure 2).
Figure 1. Halofuginone inhibits DNA synthesis in leiomyoma and myometrial cells.
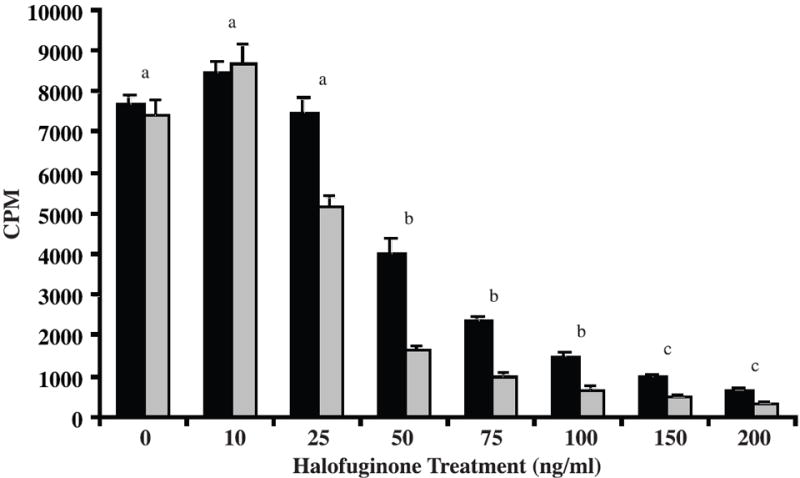
Leiomyoma (black bars) and myometrial (grey bars) SMCs were treated with increasing concentrations of halofuginone for 24 hrs and tritiated thymidine incorporation was measured by liquid scintillation. Tritiated thymidine incorporation is reported as counts per minute (CPM). Eight experiments were carried out with six samples per treatment for each experiment. Statistically significant differences between treatments are indicated by different lower case letters (p≤0.05).
Figure 2. Halofuginone-mediated inhibition of leiomyoma and myometrial cell proliferation is reversible.
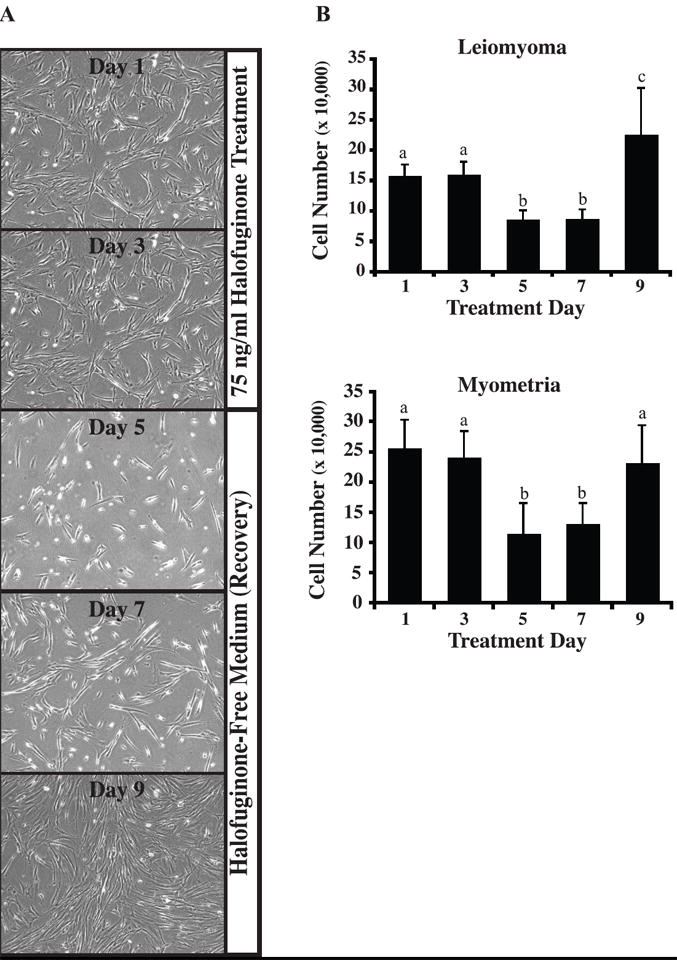
Following treatment of leiomyoma or myometrial cells with 75 ng/mL halofuginone for 5 days, cells were placed in fresh growth medium lacking halofuginone and allowed to recover for 4 days. (A) The photographs shown are representative of the cell cultures used to determine total cell numbers. (B) Total cell number was determined every two days using a hemocytometer. Three to five experiments were carried out with two dishes per time point and treatment group for each experiment. Cells not treated with halofuginone reached confluence by day 3 (300,000-400,000 cells) and are not shown. Statistically significant differences between treatments are indicated by different lower case letters (p≤0.05).
Effect of Halofuginone on Apoptosis of Myometrial and Leiomyoma SMCs
To determine whether extended halofuginone treatment reduced cell number through apoptosis, we performed caspase 3/7 activity assays on leiomyoma and autologous myometrial SMCs treated with halofuginone for up to 72h. Results showed that there were no differences in caspase 3/7 activity levels between leiomyoma and autologous myometrial SMCs for any of the concentrations of halofuginone tested at any of the time points. Treatment with a low dose (25ng/mL) of halofuginone did not increase caspase 3/7 levels at any point during the 72-hour treatment period (Figure 3a). However, treatment with 100ng/mL of halofuginone increased caspase 3/7 activity levels after 48h (Figure 3b). Treatment with a high dose of 200 ng/mL of halofuginone increased caspase 3/7 levels at both the 48h and 72h time points, and to a greater extent than the 100ng/mL dose. The levels of caspase 3/7 measured in response to 25, 100, or 200ng/mL halofuginone were significantly different (p=0.05) from each other. Overall, caspase 3/7 activity levels were increased in response to halofuginone treatment in a dose-dependent manner but the increases were only apparent after 48h of treatment.
Figure 3. Caspase 3/7 activity levels in leiomyoma and myometrial SMCs are elevated only after treatment with high concentrations of halofuginone for greater than 24 hours.
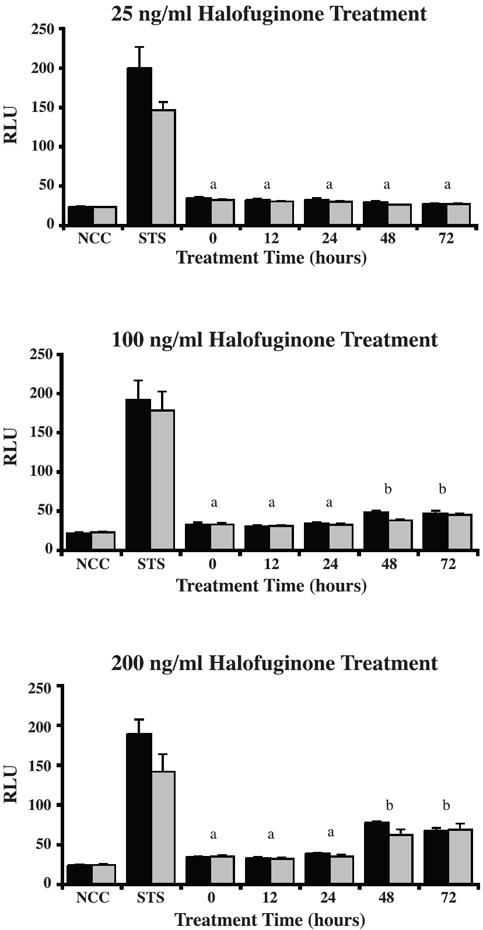
(A) Treatment with 25 ng/mL of halofuginone for 12, 24, 48, and 72 hours showed no difference in caspase 3/7 activity between leiomyoma (black bars) and myometrial SMCs (grey bars), and no difference between any treatment and control. (B) Treatment with 100 ng/mL of halofuginone, or (C) 200 ng/mL of halofuginone for 12, 24, 48 and 72 hours showed a significant increase in caspase 3/7 activity at 48 and 72 hours compared to untreated, 12, and 24 hour treatments for both leiomyoma and myometrial SMCs. STS= 1 μM Staurosporine treatment (positive control). NCC= Caspase 3/7 activity in 10% serum-containing medium without any cells (negative control). Results are from three experiments with four samples for each experiment. Statistically significant differences between treatments are indicated by different lower case letters (p≤0.05).
Halofuginone Alters TGFβ and Collagen mRNA Levels in Leiomyoma and Myometrial SMCs
Autologous leiomyoma and myometrial SMC cell lines from four different patients treated with 0, 25, 100, or 200ng/mL of halofuginone for 24h were analyzed for differences in collagen type I(αI), collagen type III(αI), TGFβI, and TGFβIII mRNA by Real Time-PCR. Halofuginone decreased not only collagen type I(αI), but also collagen type III(αI) mRNA levels in both leiomyoma and myometrial SMCs (Figure 4). However, the decrease in collagen mRNA levels in response to halofuginone was dose-dependent only in leiomyoma SMCs. TGFβI mRNA levels were also decreased in both leiomyoma and myometrial SMCs in response to halofuginone (Figure 5) but halofuginone treatment did not significantly decrease TGFβIII mRNA levels in either leiomyoma or myometrial SMCs.
Figure 4. Halofuginone reduces collagen type I(αI) and collagen type III(α1) mRNA levels in leiomyoma and myometrial SMCs.
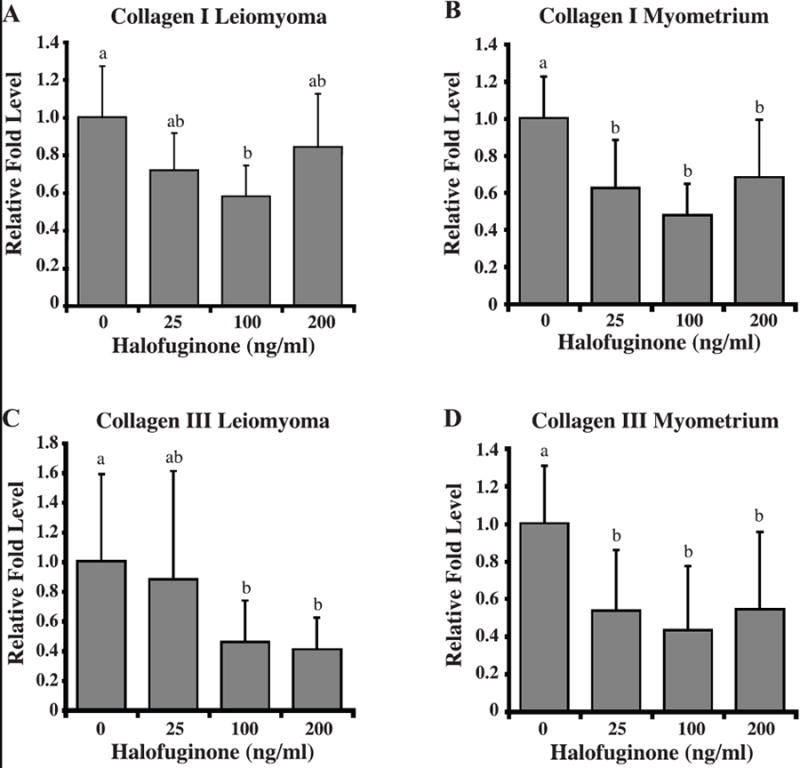
Collagen type I(αI) transcript levels in (A) leiomyoma and (B) myometrial cells and collagen type III(αI) transcript levels in (C) leiomyoma and (D) myometrial cells treated with increasing concentrations of halofuginone were determined by real-time PCR. Relative fold levels for each transcript were normalized to the level of the 18S RNA present in each sample. Results are from three experiments with three samples per experiment. Statistically significant differences between treatments are indicated by different lower case letters (p≤0.05).
Figure 5. Halofuginone reduces TGFβI, but not TGFβIII transcript levels in leiomyoma and myometrial SMCs after a 24 hour treatment with halofuginone.
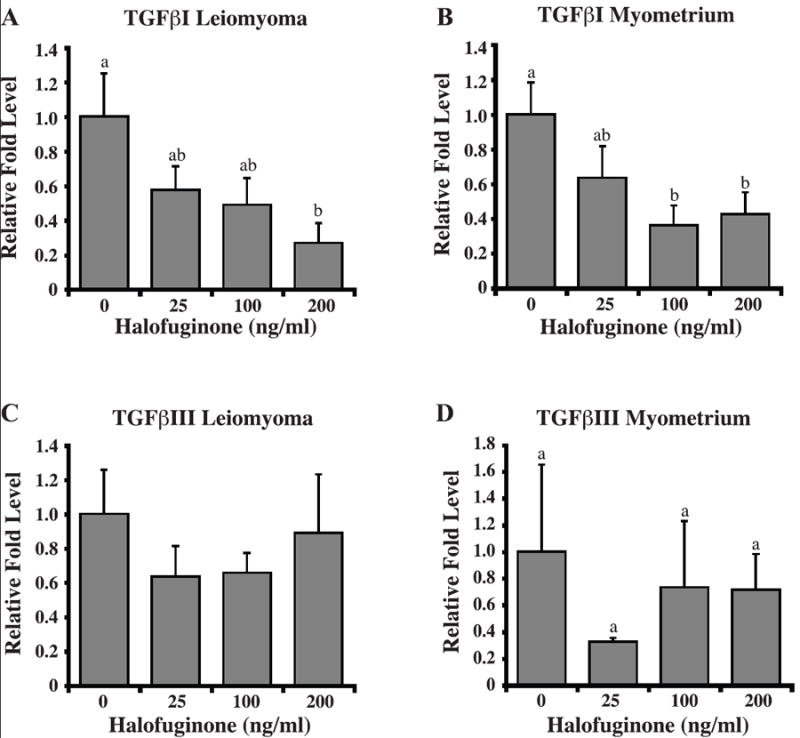
TGFβI transcript levels in (A) leiomyoma and (B) myometrial cells and TGFβIII transcript levels in (C) leiomyoma and (D) myometrial cells treated with increasing concentrations of halofuginone were determined by real-time PCR. Relative fold levels for each transcript were normalized to the level of 18S RNA present in each sample. Results are from three experiments with three samples for each experiment. Statistically significant differences between treatments are indicated by different lower case letters (p≤0.05).
DISCUSSION
In the present study we demonstrated that halofuginone inhibits both myometrial and leiomyoma SMC proliferation. Halofuginone inhibited DNA synthesis within 24 hours of treatment in both leiomyoma and myometrial SMCs in a dose-dependent manner at concentrations above 25ng/mL. Halofuginone did not increase caspase 3/7 levels, a marker of apoptosis, in either cell type until after 48h of treatment and only at higher doses of 100 or 200 ng/ml. The inhibition of DNA synthesis at low concentrations, such as 25ng/mL, coupled with a modest increase in caspase activity (apoptosis) after 72h of treatment, suggests that halofuginone reduces cell proliferation through both mechanisms in uterine SMCs. These results are consistent with the findings of Gavish et al. (21) who observed high levels of apoptosis in prostate cancer cell xenografts after exposure to high doses of halofuginone for 2 months. The increase in apoptosis observed in this study and by Gavish et al. (21) is different from another recent study that reported a decrease in apoptosis in T cells treated with halofuginone. Leiba et al. (22) showed that halofuginone inhibited apoptosis in normal, activated T cells. These results suggest that the induction of apoptosis by halofuginone may be a cell type-specific response. Our results showed that increased levels of caspase 3/7 activity, and therefore apoptosis, occurred only at higher concentrations of halofuginone and after 48-72 h of exposure.
This delayed increase in apoptosis was also evident in our longer term cell proliferation experiments. Leiomyoma and myometrial SMCs treated with halofuginone for 5 days did not show a decrease in cell number until after 5 days of treatment. Reversal of the inhibitory effect of halofuginone on cell growth was delayed as there was no increase in cell number until 4 days after removal of halofuginone. Interestingly, cells treated with halofuginone did not appear necrotic or unhealthy during the course of the treatment period (Figure 2), and once halofuginone was removed the cells regained their ability to proliferate as indicated by the significant increase in cell number by day 9 (Figure 2). Halofuginone inhibited DNA synthesis in leiomyoma and myometrial SMCs within 24 h of treatment, but increases in caspase 3/7 levels, and therefore apoptosis, appear to require a longer time of exposure to halofuginone. Other studies have observed a similar inhibition of cell proliferation in response to halofuginone. Haran et al. (23) reported that halofuginone inhibited rat renal fibroblast proliferation in a reversible and dose-dependent manner after 48-192 h of treatment. Another study by Nagler et al. (24) observed a decrease in cultured hepatocellular carcinoma cell proliferation after one week of treatment with halofuginone, and also did not observe any toxic effects to the cells or changes in cell viability.
In addition to inhibiting fibroblast and SMC proliferation, halofuginone has also been shown to be a specific inhibitor of collagen type I synthesis and TGFβ signaling (15,16). We examined the effects of halofuginone on TGFβ I and III mRNA levels in leiomyoma and myometrial SMCs because TGFβI and III have direct positive effects on collagen production in both cell types, and TGFβIII has been shown to increase DNA synthesis in leiomyoma SMCs (12,25). Our results showed that halofuginone specifically decreased TGFβI expression in both leiomyoma and myometrial SMCs. This is in contrast to a recently published study by Popov et al. (26) who found no change in TGFβI mRNA expression in halofuginone-treated rat hepatic satellite cells. However, halofuginone has been shown to inhibit TGFβI signaling in embryonic fibroblasts by interfering with Smad3 activation (16), and as TGFβI production is often dependent upon autocrine stimulation (27), halofuginone could reduce TFGβI production by interfering with the Smad activation pathway. However, halofuginone did not alter TGFβIII mRNA levels in either leiomyoma or myometrial SMCs suggesting that TGFβI and TGFβIII are not regulated in the same manner in these cells.
Real-time PCR analysis of leiomyoma and autologous myometrial SMCs showed an anticipated decrease in collagen type I(αI) mRNA levels in response to treatment with halofuginone. Since collagen type III is another ECM protein that is upregulated in uterine leiomyomas (28, 29), we also examined the effect of halofuginone on collagen type III mRNA levels. The results showed that halofuginone also markedly reduced collagen type III mRNA levels in both leiomyoma and myometrial SMCs, and this inhibitory effect on collagen type III mRNA occurred in a dose-dependent manner in leiomyoma SMCs. The suppression of collagen type III mRNA is in agreement with recent findings by Papov et al. (26) who showed a similar decrease in collagen III mRNA in response to halofuginone in rat hepatic satellite cells. While these rapid decreases in collagen mRNA levels would not immediately be reflected by a decrease in collagen protein expression in the ECM, the levels of newly synthesized, monomeric collagen would be decreased fairly rapidly.
The suppressive effect of halofuginone on collagen production could have multiple effects in leiomyoma and myometrial SMCs. New collagen production is essential for cell proliferation, not only as a scaffold to support cell growth but also as part of a functional signaling pathway. Collagens can signal through the β1 family of integrins and members of the discoidin-domain receptor (DDR) family to convey migration, proliferation, and cell adhesion signals (33-39). Collagens can also bind various signaling molecules such as members of the fibroblast growth factor (FGF) and TGFβ families, and can inhibit or enhance the effects of these growth factors (33-39). The suppression of collagen production by halofuginone could work in two ways. First, it would limit the protein matrix available to leiomyoma SMCs to support growth and migration. Second, it would also reduce the proliferation signals initiated by interactions of collagen with integrins, DDRs, and through binding to members of the FGF and TGFβ growth factor families.
Our studies showed that halofuginone had similar effects on leiomyoma and myometrial SMCs in culture. These results are not very surprising in light of other recent studies that have shown that myometrial and leiomyoma SMCs behave very similarly in culture. A study by Zaitseva et al. (30) examined the gene expression patterns of leiomyoma and myometrial cells in vivo and in vitro using microarray gene analysis. Their results showed that while the gene expression patterns of leiomyoma and myometrial SMCs were quite different in vivo, there were relatively small differences in gene expression between the cell types cultured in vitro. We have observed similar levels of expression of collagen type I and type III mRNAs in cultured leiomyoma and myometrial cells but this is most likely due to the fact that these cells upregulate collagen production significantly in culture in response to being plated on plastic surfaces. In addition, the production of TGFβ-1 was also similar for the two cell types (150-180 pg/ml/24 hrs, (12)). A study by Gross et al (31) showed that while leiomyomas express much higher levels of HMGA2 mRNA in vivo when compared to autologous myometrium, the levels of HMGA2 are quite similar in cultured myometrial and leiomyoma SMCs. Another recent study by Loy et al. (32) examined the effects of the peroxisome proliferator-activated receptor ligand pioglitazone on cultured leiomyoma and myometrial cell proliferation. These investigators also reported similar anti-proliferative effects of pioglitazone on cultured myometrial and leiomyoma SMCs. It will not be possible to determine definitively whether there are differences in the effects of halofuginone on leiomyoma and myometrial cells in vivo until the drug can be tested in an in vivo animal model or in a clinical pilot study.
In conclusion, we have shown that halofuginone inhibits both leiomyoma and myometrial SMC proliferation by rapidly inhibiting DNA synthesis and later inducing apoptosis. In addition, halofuginone significantly suppressed TGFβI mRNA production. The suppression of TGFβ by halofuginone may be one of the mechanisms by which halofuginone decreases collagen type I(αI) and collagen type III(αI) mRNA levels in uterine SMCs. These results support our hypothesis that halofuginone could be a novel and effective medical therapy for treatment of uterine leiomyomas. Future studies will focus on clarifying the signaling pathways used by halofuginone in exerting its anti-fibrotic and anti-proliferative effects in leiomyoma SMCs.
Acknowledgments
This work was supported by NIH HD046227 (RAN) and a grant from the LAM Foundation (RAN).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Walker CL, Stewart EA. Uterine fibroids: the elephant in the room. Science. 2005;308:1589–1592. doi: 10.1126/science.1112063. [DOI] [PubMed] [Google Scholar]
- 2.Chavez NF, Stewart EA. Medical treatment of uterine fibroids. Clin Obstet Gynecol. 2001;44:372–384. doi: 10.1097/00003081-200106000-00023. [DOI] [PubMed] [Google Scholar]
- 3.Buttram VC, Jr, Reiter RC. Uterine leiomyomata: etiology, symptomatology, and management. Fertil Steril. 1981;36:433–445. doi: 10.1016/s0015-0282(16)45789-4. [DOI] [PubMed] [Google Scholar]
- 4.Zhao SZ, Wong JM, Arguelles LM. Hospitalization costs associated with leiomyoma. Clin Therap. 1999;21:563–575. doi: 10.1016/S0149-2918(00)88309-5. [DOI] [PubMed] [Google Scholar]
- 5.Nowak RA. Identification of new therapies for leiomyomas: what in vitro studies can tell us. Clin Obstet Gynecol. 2001;44:327–334. doi: 10.1097/00003081-200106000-00019. [DOI] [PubMed] [Google Scholar]
- 6.Stewart EA. Uterine fibroids. Lancet. 2001;357:293–298. doi: 10.1016/S0140-6736(00)03622-9. [DOI] [PubMed] [Google Scholar]
- 7.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signaling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 8.Derynck R, Feng XH. TGF-beta receptor signaling. Biochim Biophys Acta. 1997;1333:F105–150. doi: 10.1016/s0304-419x(97)00017-6. [DOI] [PubMed] [Google Scholar]
- 9.Massague J. How cells read TGF-beta signals. Nat Rev Mol Cell Biol. 2000;1:169–178. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- 10.Murphy LJ, Ballejo G. Growth factor and cytokine expression in the endometrium. In: Findlay JK, editor. Molecular biology of the female reproductive system. Academic Press; San Diego: 1994. p. 457. [Google Scholar]
- 11.Tang XM, Dou Q, Zhao Y, McLean F, Davis J, Chegini N. The expression of transforming growth factor-beta s and TGF-beta receptor mRNA and protein and the effect of TGF-betas on human myometrial smooth muscle cells in vitro. Mol Hum Reprod. 1997;3:233–240. doi: 10.1093/molehr/3.3.233. [DOI] [PubMed] [Google Scholar]
- 12.Lee BS, Nowak RA. Human leiomyoma smooth muscle cells show increased expression of transforming growth factor-beta 3 (TGF beta 3) and altered responses to the antiproliferative effects of TGF beta. J Clin Endocrinol Metab. 2001;86:913–920. doi: 10.1210/jcem.86.2.7237. [DOI] [PubMed] [Google Scholar]
- 13.Arici A, Sozen I. Transforming growth factor-β3 is expressed at high levels in leiomyoma where it stimulates fibronectin expression and cell proliferation. Fertil Steril. 2000;73:1006–1011. doi: 10.1016/s0015-0282(00)00418-0. [DOI] [PubMed] [Google Scholar]
- 14.Pines M, Vlodavsky I, Nagler A. Halofuginone: From veterinary use to human therapy. Drug Development Research. 2000;50:371–378. [Google Scholar]
- 15.Granot I, Halevy O, Hurwitz S, Pines M. Halofuginone: an inhibitor of collagen type I synthesis. Biochim Biophys Acta. 1993;1156:107–112. doi: 10.1016/0304-4165(93)90123-p. [DOI] [PubMed] [Google Scholar]
- 16.McGaha TL, Phelps RG, Spiera H, Bona C. Halofuginone, an inhibitor of type-I collagen synthesis and skin sclerosis, blocks transforming-growth-factor-beta-mediated Smad3 activation in fibroblasts. J Invest Dermatol. 2002;118:461–470. doi: 10.1046/j.0022-202x.2001.01690.x. [DOI] [PubMed] [Google Scholar]
- 17.Nagler A, Miao HQ, Aingorn H, Pines M, Genina O, Vlodavsky I. Inhibition of collagen synthesis, smooth muscle cell proliferation, and injury-induced intimal hyperplasia by halofuginone. Arterioscler Thromb Vasc Biol. 1997;17:194–202. doi: 10.1161/01.atv.17.1.194. [DOI] [PubMed] [Google Scholar]
- 18.Pines M, Snyder D, Yarkoni S, Nagler A. Halofuginone to treat fibrosis in chronic graft-versus-host disease and scleroderma. Biol Blood Marrow Transplant. 2003;9:417–425. doi: 10.1016/s1083-8791(03)00151-4. [DOI] [PubMed] [Google Scholar]
- 19.Xavier S, Piek E, Fujii M, Javelaud D, Mauviel A, Flanders KC, et al. Amelioration of radiation-induced fibrosis: inhibition of transforming growth factor-beta signaling by halofuginone. J Biol Chem. 2004;279:15167–15176. doi: 10.1074/jbc.M309798200. [DOI] [PubMed] [Google Scholar]
- 20.Nagler A, Firman N, Feferman R, Cotev S, Pines M, Shoshan S. Reduction in pulmonary fibrosis in vivo by halofuginone. Am J Respir Crit Care Med. 1996;154:1082–1086. doi: 10.1164/ajrccm.154.4.8887611. [DOI] [PubMed] [Google Scholar]
- 21.Gavish Z, Pinthus JH, Barak V, Ramon J, Nagler A, Eshhar Z, et al. Growth inhibition of prostate cancer xenografts by halofuginone. Prostate. 2002;51:73–83. doi: 10.1002/pros.10059. [DOI] [PubMed] [Google Scholar]
- 22.Leiba M, Cahalon L, Shimoni A, Lider O, Zanin-Zhorov A, Hecht I, et al. Halofuginone inhibits NF-{kappa}B and p38 MAPK in activated T cells. J Leukoc Biol. 2006;80:399–406. doi: 10.1189/jlb.0705409. [DOI] [PubMed] [Google Scholar]
- 23.Haran N, Leschinski L, Pines M, Rapoport J. Inhibition of rat renal fibroblast proliferation by halofuginone. Nephron Exp Nephrol. 2006;104:e35–e40. doi: 10.1159/000093674. [DOI] [PubMed] [Google Scholar]
- 24.Nagler A, Ohana M, Shibolet O, Shapira MY, Alper R, Vlodavsky I, et al. Suppression of hepatocellular carcinoma growth in mice by the alkaloid coccidiostat halofuginone. Eur J Cancer. 2004;40:1397–1403. doi: 10.1016/j.ejca.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 25.Chegini N, Luo X, Ding L, Ripley D. The expression of Smads and transforming growth factor beta receptors in leiomyoma and myometrium and the effect of gonadotropin releasing hormone analogue therapy. Mol Cell Endocrinol. 2003;209:9–16. doi: 10.1016/j.mce.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 26.Popov Y, Patsenker E, Bauer M, Niedobitek E, Schulze-Krebs A, Schuppan D. Halofuginone induces matrix metalloproteinases in rat hepatic stellate cells via activation of p38 and NFkappaB. J Biol Chem. 2006;281:15090–15098. doi: 10.1074/jbc.M600030200. [DOI] [PubMed] [Google Scholar]
- 27.Clark DA, Coker R. Transforming growth factor-beta (TGF-beta) Int J Biochem Cell Biol. 1998;30:293–298. doi: 10.1016/s1357-2725(97)00128-3. [DOI] [PubMed] [Google Scholar]
- 28.Fujita M. Histological and biochemical studies of collagen in human uterine leiomyomas. Hokkaido Igaku Zasshi. 1985;60:602–615. [PubMed] [Google Scholar]
- 29.Stewart EA, Friedman AJ, Peck K, Nowak RA. Relative overexpression of collagen type I and collagen type III messenger ribonucleic acids by uterine leiomyomas during the proliferative phase of the menstrual cycle. J Clin Endocrinol Metab. 1994;79:900–906. doi: 10.1210/jcem.79.3.8077380. [DOI] [PubMed] [Google Scholar]
- 30.Zaitseva M, Vollenhoven BJ, Rogers PA. In vitro culture significantly alters gene expression profiles and reduces differences between myometrial and leiomyoma smooth muscle cells. Mol Hum Reprod. 2006;12:187–207. doi: 10.1093/molehr/gal018. [DOI] [PubMed] [Google Scholar]
- 31.Gross KL, Neskey DM, Manchanda N, Weremowicz S, Kleinman MS, Nowak RA, et al. HMGA2 expression in uterine leiomyomata and myometrium: quantitative analysis and tissue culture studies. Genes Chromosomes Cancer. 2003;38:68–79. doi: 10.1002/gcc.10240. [DOI] [PubMed] [Google Scholar]
- 32.Loy CJ, Evelyn S, Lim FK, Liu MH, Yong EL. Growth dynamics of human leiomyoma cells and inhibitory effects of the peroxisome proliferators-activated receptor ligand, pioglitazone. Mol Hum Reprod. 2005;11:561–566. doi: 10.1093/molehr/gah199. [DOI] [PubMed] [Google Scholar]
- 33.Brooke BS, Karnik SK, Li DY. Extracellular matrix in vascular morphogenesis and disease: structure versus signal. Trends Cell Biol. 2003;13:51–56. doi: 10.1016/s0962-8924(02)00007-7. [DOI] [PubMed] [Google Scholar]
- 34.Henriet P, Zhong ZD, Brooks PC, Weinberg KI, DeClerck YA. Contact with fibrillar collagen inhibits melanoma cell proliferation by up-regulating p27KIP1. Proc Natl Acad Sci U S A. 2000;97:10026–10031. doi: 10.1073/pnas.170290997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koyama H, Raines EW, Bornfeldt KE, Roberts JM, Ross R. Fibrillar collagen inhibits arterial smooth muscle proliferation through regulation of Cdk2 inhibitors. Cell. 1996;87:1069–78. doi: 10.1016/s0092-8674(00)81801-2. [DOI] [PubMed] [Google Scholar]
- 36.Pozzi A, Wary KK, Giancotti FG, Gardner HA. Integrin alpha1beta1 mediates a unique collagen-dependent proliferation pathway in vivo. J Cell Biol. 1998;142:587–594. doi: 10.1083/jcb.142.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vogel WF, Aszodi A, Alves F, Pawson T. Discoidin domain receptor 1 tyrosine kinase has an essential role in mammary gland development. Mol Cell Biol. 2001;21:2906–2917. doi: 10.1128/MCB.21.8.2906-2917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hou G, Vogel WF, Bendeck MP. Tyrosine kinase activity of discoidin domain receptor 1 is necessary for smooth muscle cell migration and matrix metalloproteinase expression. Circ Res. 2002;90:1147–1149. doi: 10.1161/01.res.0000022166.74073.f8. [DOI] [PubMed] [Google Scholar]
- 39.Vogel W, Gish GD, Alves F, Pawson T. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell. 1997;1:13–23. doi: 10.1016/s1097-2765(00)80003-9. [DOI] [PubMed] [Google Scholar]


