Abstract
Effector CD8 T cell recruitment into the skin in response to antigen challenge requires prior CXCL1/KC-directed neutrophil infiltration. Mechanisms inducing CXCL1 production and the dynamics of neutrophil-CD8 T cell interactions during elicitation of antigen-specific responses in the skin were investigated. CXCL1 and CXCL2/MIP-2 were produced within 3–6 hours of antigen challenge at 10-fold higher levels in skin challenge sites of antigen-sensitized vs. non-sensitized mice. In the challenge sites of sensitized mice this production decreased at 6–9 hours post-challenge to near the levels observed in skin challenge sites of non-sensitized mice but rose to a second peak 12 hours after challenge. The elevated early neutrophil chemoattractant production at 3–6 hours after skin challenge of sensitized animals required both IFN-γ and IL-17, produced by distinct populations of antigen-primed CD8 T cells in response to antigen challenge. Although induced by the antigen-primed CD8 T cells, the early CXCL1 and CXCL2 production was accompanied by neutrophil but not CD8 T cell infiltration into the skin antigen challenge site. Infiltration of the CD8 T cells into the challenge site was not observed until 18–24 hours after challenge. These results demonstrate an intricate series of early interactions between antigen-specific and innate immune components that regulate the sequential infiltration of neutrophils and then effector T cells into the skin to mediate an immune response.
Keywords: Skin, CD8 T cells, neutrophils, IL-17, IFN-γ
A characteristic feature of inflammation is leukocyte infiltration into the tissue site. Neutrophils are typically the first leukocytes recruited to infiltrate tissue inflammation in response to the production of chemoattractants binding CXCR1 and CXCR2 that are constitutively expressed on circulating neutrophils (1–6). In addition to directing recruitment, neutrophil chemoattractants such as IL-8 and CXCL1/KC activate neutrophils to release granules containing many chemokines, cytokines, and extracellular matrix degrading enzymes (3). The release of these mediators amplifies inflammation by recruiting other leukocyte populations into the tissue site and inflammation is often attenuated by antagonism of the initial neutrophil infiltration. In contrast to the constitutive expression of chemokine receptors by neutrophils, T cells are directed to peripheral tissues through the induced expression of specific integrins and chemokine receptors that complement the ligands expressed in that tissue. Human and mouse CD4 T memory cells trafficking to the skin express CCR4 and are directed to the skin through endothelial cell production of CCL17/TARC (7, 8). The requirements for antigen-primed CD8 T cell trafficking into the skin during elicitation of cutaneous immune responses are not as clearly defined as for CD4 T cells.
Contact hypersensitivity (CHS) is a T cell mediated inflammatory response in the skin to cutaneous sensitization and subsequent challenge with a hapten. Hapten application to the skin triggers antigen acquisition by epidermal and dermal dendritic cells, including Langerhans cells, that migrate to the skin draining lymph nodes and prime hapten-specific T cell populations (9–12). Following subsequent skin contact with the hapten, the primed T cells infiltrate the challenge site and are activated to express effector functions, including IFN-γ and TNFα production, that mediate the characteristic tissue edema/spongiosis of the CHS response that peaks 18–48 hours after challenge. Hapten-specific CD8 T cells are the primary effector cells of CHS responses to many haptens, including dinitrofluorobenzene (DNFB), oxazolone (Ox) and urushiol, the reactive hapten in poison ivy (13). CHS responses to these haptens are absent or substantially decreased in mice treated with CD8 T cell depleting antibodies and in mice with targeted gene deletions that result in the absence of mature CD8 T cell development (14–16). Consistent with this effector function, sensitization with DNFB and other haptens results in the activation and expansion of hapten-specific CD8 T cells producing IFN-γ whereas hapten-primed CD4 T cells produce IL-4, IL-5 and IL-10 (16–18).
The factors directing antigen-primed CD8 T cells into challenged skin to mediate an immune response remain poorly understood. Previous studies indicated the inhibition of CHS responses when hapten sensitized mice were treated with CXCL1-specific antibodies at the time of hapten challenge (19). CXCL1 does not directly recruit hapten-primed CD8 T cells into the challenge site but recruits neutrophils that, in turn, induce effector CD8 T cell infiltration into the site to mediate the response. Furthermore, the amount of CXCL1 produced and the intensity of neutrophil infiltration into the skin following hapten challenge directly influences the number of effector T cells infiltrating the challenge site and the magnitude of the immune response elicited (20). It has been presumed that hapten application mediates an early inflammatory response that includes CXCL1 production although the specific factors inducing this CXCL1 as well as the site of production during elicitation of CHS remain unknown. In the current study, we investigated these aspects of the initiation of the response as well as the relationship between this chemokine production and subsequent neutrophil and CD8 T cell infiltration into the hapten challenge site during CHS responses. The results indicate that hapten challenge is rapidly followed by stimulation of distinct populations of primed effector CD8 T cell populations to produce IFN-γ and IL-17. Both of these cytokines are required to induce the chemoattractants directing neutrophil infiltration into the challenge site. These results indicate that the early antigen-specific CD8 T cell production of IFN-γ and IL-17 triggers the innate immune response that subsequently directs the effector CD8 T cells into the skin to elicit the response.
Materials and Methods
Mice
BALB/c (H-2d) and C57/BL6 (H-2b) mice were obtained through the National Cancer Institute (Frederick, MD), CD4−/−, CD8−/−, and RAG-1−/− mice on the C57/BL6 background and IFN-γ−/− mice on the BALB/c background from the Jackson Laboratory (Bar Harbor, ME), and IL-17−/− mice on the BALB/c background from Dr. Yoichiro Iwakura, (University of Tokyo, Tokyo, Japan). Female mice, 8–10 weeks of age, were used throughout these studies. All studies were approved by the Institutional Animal Care and Use Committee of the Cleveland Clinic.
Hapten sensitization and elicitation of CHS
Mice were sensitized to 2,4-dinitrofluorobenzene (DNFB) or oxazolone (Ox) by painting the shaved abdomen with 25 μl 0.25% DNFB (Sigma Aldrich, St. Louis, MO) or 25 μl 1% Ox (Sigma Aldrich) and 10 μl to each paw on days 0 and +1 (16). On day +5 hapten sensitized and control, non-sensitized mice were challenged on each side of each ear with 10 μl DNFB or Ox. Ear thickness was measured using an engineer’s micrometer (Mitutoyo, Elk Grove Village, IL) and expressed in units of 10−4 in. The ear swelling response is given as the mean increase of each group of 4 individual animals ± SEM.
Antibodies
For immunohistochemistry and flow cytometry the following antibodies were used: anti-CD3, anti-CD8, and anti-CD45 mAb (BD Pharmingen, San Diego, CA), rat anti-mouse Gr-1 mAb (eBioScience, San Diego, CA). Anti-mouse IL-17 mAb was purchased from Southern Biotech (Birmingham, AL). Purified mAb YTS 191.1.2 and GK1.5 (anti-mouse CD4) and YTS 169 and TIB-150 (anti-mouse CD8) for in vivo treatment were purchased from BioXCell (West Lebanon, NH). RB6.8C5 (anti-mouse Gr-1 mAb), XMG1.2 (anti-mouse IFN-γ mAb), and 2C11 (anti-mouse CD3 mAb) were purified from culture supernatants by protein G chromatography.
Mice were depleted of CD4+ T cells by injecting 100 μg each of YTS 191 and GK1.5, i.p. on three consecutive days before hapten sensitization (16–18). Mice were injected with 100 μg each of YTS 169 and TIB-150 to deplete CD8+ T cells. Both CD4 and CD8 T cells were depleted by injecting mice with 200 μg anti-CD3 mAb 2C11 on three consecutive days before hapten sensitization. Mice were injected with 150 μg of RB6.8C5 i.p. on days +4 and +5 following hapten sensitization to deplete neutrophils (19, 20). In each experiment, treated sentinel mice were used to evaluate the efficiency of CD4+ or CD8+ T cell depletion by antibody staining and flow cytometry analysis of spleen and lymph node cells (LNC) and was always >95% when compared to cells from control, rat IgG treated mice.
Quantitation of CXCL1 and CXCL2 production by immunoassay
Production of CXCL1 and CXCL2 in skin following hapten application was determined using immunoassay kits (R & D Systems, Minneapolis, MN). Hapten challenged skin was removed, homogenized in 500 μl PBS with 0.01M EDTA and a proteinase inhibitor cocktail (10 μg/ml PMSF, 2 μg/ml aprotinin, 2 μg/ml leupeptin, 100 μg/ml Pefabloc SC, 100 μg/ml chymostatin), and 1 ml of 1.5% Triton-X in PBS was added with shaking at 4°C for 30 min. After pelleting cell debris, the supernatants were collected and the total protein concentration quantified using a Coomassie Plus Protein Assay Reagent Kit (Pierce, Rockford, IL). For each experiment, all samples were diluted to an equivalent total protein concentration.
T cell transfer
BALB/c mice were depleted of CD4 or CD8 T cells prior to sensitization to DNFB or Ox. On day +4 following sensitization, 4 × 106 LNC aliquots were transferred i.v. to naïve recipients that had been challenged on the shaved abdomen with 25 μl of 0.2% DNFB one hour before cell transfer. The challenged skin was excised 6 hours after challenge and CXCL1 production determined.
Analysis of tissue infiltrating cells
After 6 or 18 hours, the challenged skin of sensitized and non-sensitized, mice was excised and incubated in 0.5% dispase (Invitrogen, Carlsbad, CA) for 18 hours at 4°C. The next day, the epidermis was separated from the dermis and incubated in 0.5% trypsin (Sigma Aldrich, St. Louis, MO) for 60 min. at 37° C, 5% CO2. The epidermis and dermis were each pressed through dialysis tubing and the cells were incubated in 0.2% DNase (Roche, Indianapolis, IN) for 10 min. at room temperature and 1 × 106 cell aliquots were washed in staining buffer (Dulbecco’s PBS with 2% FCS/0.2% NaN3) and incubated in 100 μl Fc block (BD Pharmingen) diluted 1:100 in the staining buffer for 20 min on ice. To examine leukocytes infiltrating the skin challenge site, cell aliquots were washed and stained with fluorochrome-labeled anti-mouse mAb to CD45, CD3 and Gr-1. After 30 min., the cells were washed, resuspended in staining buffer and analyzed by two-color flow cytometry using a FACScan and CellQuest software (Becton-Dickinson, San Jose, CA). The cells were gated to exclude non-viable cells and sample data were collected on 20,000 cells.
Histological analyses
To detect leukocyte infiltration into challenged skin, the tissue was excised, fixed in 10% formalin, embedded in paraffin, and 8 μm sections were stained with hematoxylin and eosin. To detect chemokine protein, the excised skin was fixed with 10% formalin and 8 μm paraffin embedded sections were mounted onto slides. The slides were deparaffinized, rehydrated and boiled in an antigen retrieval solution (Biogenex, San Ramon, CA). Slides were stained with CXCL1-specific (5 μg/ml) goat antiserum diluted in PBS/1% BSA solution overnight at 4°C. Control slides were incubated with normal goat serum (Vector, Burlingame, CA). Primary antibody binding was detected using biotinylated rabbit anti-goat IgG followed by streptavidin horse radish peroxidase and developed using the substrate chromagen 3,3′-diaminobenzidine (DAB). For staining to detect neutrophils or CD8 T cells, excised skin was embedded in OCT compound (Sakura Finetek U.S.A., Torrence, CA), frozen in liquid nitrogen, and 8 μm sections mounted onto slides. The slides were dried, fixed in acetone, air-dried, and rehydrated with PBS before immersion in 0.03% H2O2 for 10 min. Slides were stained with CD8 (10μg/ml) or RB6-8C5 antibody (10μg/ml) diluted in PBS/1% BSA and control slides were incubated with rat IgG. Primary antibody was detected using biotinylated rabbit anti-rat IgG and developed as above. After a final wash in dH2O, slides were counter-stained with hematoxylin, rinsed and dehydrated for mounting. Images were captured using ImagePro Plus 5.0 (Media Cybernetics, Silver Spring, MD).
Analysis of gene expression by quantitative RT-PCR
Whole cell RNA was obtained by dissolving homogenates from challenged skin in TRIZOL reagent (Invitrogen Life Tec hnologies, Carlsbad, CA) with subsequent chloroform extraction. cDNA was synthesized from 2 μg RNA using the TaqMan Reverse Transcription Reagent Kit (Applied Biosystems, Foster City, CA). PCR was performed using custom primers and FAM dye-labeled probes (Applied Biosystems) for mouse TNFα, IFN-γ, IL-17, CXCL1/, CXCL2, IL-10, IL-21, and Mrpl 32 (gene assay ID#: Mm00443258_m1, Mm00801778_m1, Mm00439619_m1, Mm00433859_m1, Mm00436450_m1, Mm00439616_m1, Mm00517640_m1, and, Mm00777741_sH, respectively). The comparative CT method for relative quantitation of cytokine gene expression was used where log measurements for each sample are made during amplification and the expression level of the Mrpl 32 housekeeping gene is subtracted from the expression level for each test cytokine gene. For each test cytokine, the expression level of a single RNA sample prepared from the challenged skin of sensitized, untreated wild-type mice was used as the calibrator and was arbitrarily set at 1.0 and the expression levels of all other samples were then normalized to the calibrator. Duplicate runs of each individual RNA sample prepared from a single mouse of 3–4 mice per group were tested and the data from 3–4 RNA samples for each group are expressed as mean test cytokine expression level ± SEM.
Enumeration of hapten-specific T cells producing IFN-γ or IL-17
ELISPOT assays were performed as described (21). Briefly, ELISPOT plates (Unifilter 350, Polyfiltronics, Rockland, MA) were coated with 4 μg/ml IFN-γ or 2 μg/ml IL-17 mAb and blocked with 1% BSA in PBS. LNC from sensitized or nonsensitized wild-type BALB/c, IFN-γ−/−, or IL-17−/− mice were prepared on day +5 as responder cells. Spleen cells from naïve mice were incubated with 50 μg/ml mitomycin C for 30 min. at 37°C, washed and labeled with DNBS for 1 hour for use as stimulator cells. Stimulator cells were plated at 5 × 105 cells/well with 2.5 × 105 responder cells/well in serum-free HL-1 medium (Bio-Whittaker, Walkersville, MD) supplemented with 1mM L-glutamine and 1mM antibiotic. After 24 hours cells were removed by extensive washing with PBS/0.05% Tween-20 (PBS-T). Biotinylated anti-IFN-γ mAb (2 μg/ml) or anti-IL-17 mAb (1 μg/ml) was added and the plate incubated overnight at 4°C. The plate was washed with PBS-T and conjugated streptavidin-alkaline phosphatase added to each well. After 2 hrs. at room temperature, the plates were washed with PBS-T and nitroblue tetrazolium/5-bromo-4-cholor-30-indolyl substrate (Bio-Rad Laboratories, Hercules, CA) added. The resulting spots were counted with an ImmunoSpot Series I Analyzer (Cellular Technology Ltd., Cleveland, OH).
Statistical analysis
Statistical analysis to assess differences between experimental groups was performed using Students’ t test. Differences were considered significant when P < 0.05.
Results
Bimodal production of CXCL1/KC and CXCL2/MIP-2 during elicitation of CHS
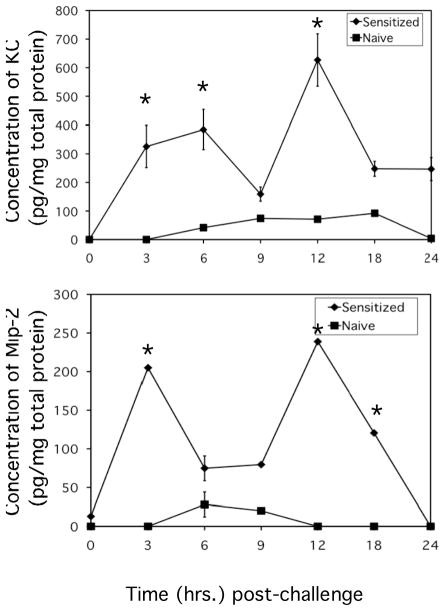
The temporal production of the neutrophil chemoattractants CXCL1 and CXCL2 in skin challenge sites during elicitation of CHS was investigated. Groups of DNFB-sensitized and naïve/non-sensitized mice were challenged with DNFB and at various times post-challenge tissue homogenates of the skin challenge site were prepared and the production of the neutrophil chemoattractant proteins was tested. In naïve mice DNFB application induced low levels of CXCL1 and CXCL2, first evident 6 hours later and maintained at low levels before falling to background levels by 12–24 hours after application (Figure 1A and B). In contrast, hapten challenge of sensitized mice induced bimodal production of CXCL1 and CXCL2 with production evident as early as 3 hours post-challenge and at levels 6–10 fold higher than observed in challenged skin of naïve mice at any time. CXCL1 production reached peak at 6 hours post-challenge and then fell near to levels observed in naïve mice and then increased again to peak levels at 12 hours after challenge followed by a second decline. CXCL2 production reached peak 3 hours after challenge of sensitized mice, decreased and then reached a second peak at 12 hours post-challenge.
Figure 1.
Rapid production of CXCL1/KC and CXCL2/MIP-2 in antigen challenged skin of sensitized mice. BALB/c mice were sensitized with 0.25% DNFB on days 0 and +1. On day +5 after sensitization, mice were challenged on a shaved square area of trunk skin with 0.2% DNFB. Challenged areas of skin were removed from DNFB-sensitized (-◆-) or non-sensitized (-■-) mice at the indicated times post-challenge and prepared tissue homogenates were tested for concentrations of CXCL1 (A) or CXCL2 (B) by ELISA. The mean concentration ± SEM for 4 individual mice per time-point is shown. *p ≤ 0.01.
CD8 T cells mediate the early CXCL1 and CXCL2 production in sensitized mice
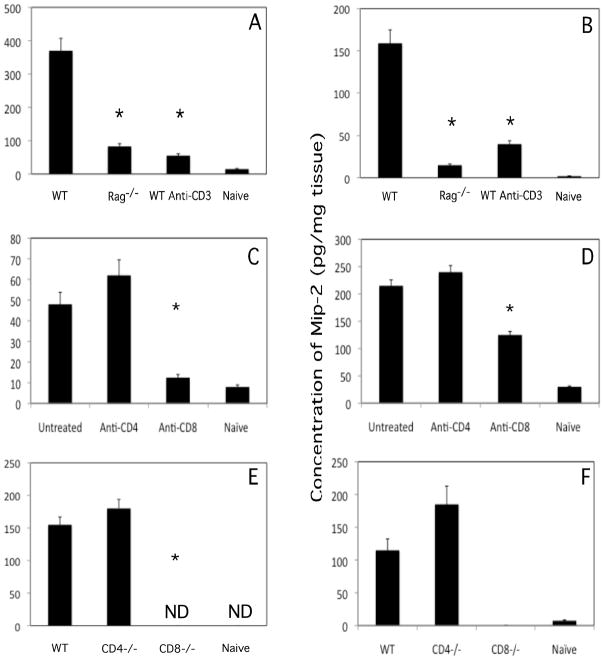
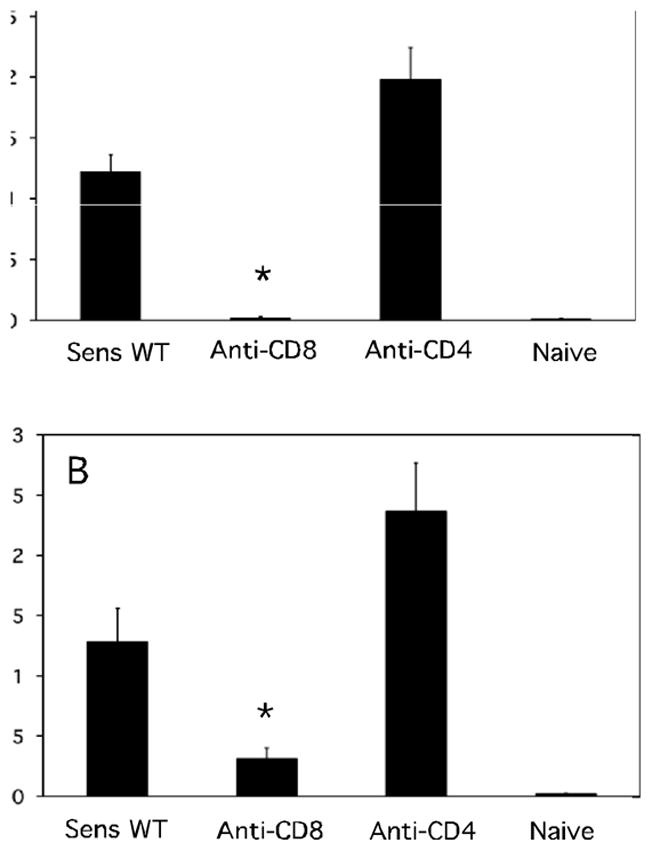
The high levels of CXCL1 and CXCL2 produced shortly after challenge of hapten-sensitized mice suggested the ability to quickly recognize and react to the hapten. In sensitized mice treated with both anti-CD4 plus anti-CD8 mAb to deplete T cells prior to DNFB sensitization, CXCL1 levels induced by antigen challenge were virtually identical to those of non-sensitized naïve mice following challenge (results not shown). Similarly, DNFB challenge of sensitized B6.RAG-1−/− mice or sensitized wild-type C57BL/6 mice depleted of T cells by treatment with anti-CD3 mAb induced markedly decreased levels of CXCL1 and CXCL2 6 hours after challenge when compared to the levels induced by challenge of sensitized wild-type mice (Figure 2A and B). The role of CD4 vs. CD8 T cells in this early neutrophil chemoattractant production was then tested. First, groups of mice were treated with control rat IgG or with specific mAb to deplete either CD4 or CD8 T cells prior to sensitization with DNFB. Skin was excised either 3 or 6 hours after challenge to test CXCL1 and CXCL2 production, respectively. Following skin challenge of sensitized mice depleted of CD8 but not CD4 T cells, CXCL1 production was decreased to naïve levels (Figure 2C). CXCL2 production was equivalent in sensitized animals treated with control rat IgG or CD4 T cell depleting mAb but was significantly reduced in sensitized animals depleted of CD8 T cells (Figure 2D). Similarly, early CXCL1 and CXCL2 production was not detected after challenge of sensitized CD8-deficient mice but was slightly enhanced in sensitized CD4-deficient mice when compared to levels in sensitized wild-type animals (Figure 2E and F).
Figure 2.
CD8 T cells mediate CXCL1/KC and CXCL2/MIP-2 production within 6 hours of antigen challenge of sensitized mice. (A and B) Groups of wild-type C57BL/6 were treated with rat IgG or anti-CD3 mAb to deplete T cells. These mice and a group of B6.RAG-1−/− mice were sensitized and challenged with DNFB. (C and D) Groups of 4 BALB/c mice were depleted of CD4 or CD8 T cells before DNFB sensitization and challenge. (E and F) Groups of wild-type C57BL/6, B6.CD4−/− and B6.CD8−/− mice were sensitized and challenged with DNFB. Tissue homogenates were prepared 6 hours after DNFB challenge of sensitized and naïve mice and tested by ELISA for production of CXCL1 (A, C, and E) or CXCL2 (B D, and F). The mean concentration of chemokine ± SEM for 4 individual mice in each group is shown. *p ≤ 0.01; ND, not detected.
Antigen-specificity of early neutrophil chemoattractant production following challenge to elicit CHS
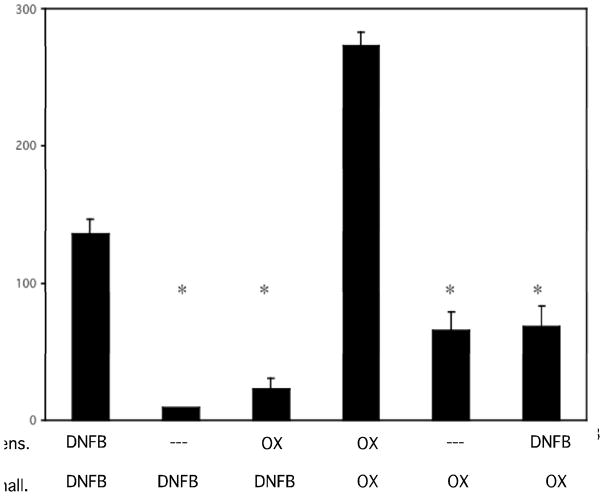
Since the early CXCL1 and CXCL2 production was dependent on CD8 T cells from hapten sensitized mice, the antigen specificity of this production was tested. First, mice were sensitized with DNFB or with Ox and on day +5 the animals were challenged with either the sensitizing hapten or with the irrelevant hapten. Challenged skin was excised from all mice 6 hours after challenge and the levels of CXCL1 production were tested in tissue homogenates. Sensitization and challenge with DNFB induced high levels of CXCL1 production (136.4 ± 10.1 pg/mg lysate protein) and higher levels (272.9 ± 11.5 pg/mg lysate protein) were induced by sensitization and challenge with Ox (Figure 3). Low levels of CXCL1 were induced in naïve mice when either DNFB (9.6 ± 0.3 pg/mg lysate protein) or Ox (65.2 ± 13.6 pg/mg lysate protein) was applied to the skin. Ox challenge of DNFB sensitized mice induced CXCL1 levels similar to Ox application to naïve animals and DNFB challenge of Ox sensitized mice induced levels similar to DNFB application to naïve animals.
Figure 3.
CXCL1/KC production 6 hours after skin challenge of sensitized mice is antigen-specific. Groups of BALB/c mice were sensitized with either 0.25% DNFB or 1% Ox on days 0 and +1 and challenged with either 0.2% DNFB or 1% Ox as indicated. Challenged skin was removed 6 hours post-challenge and prepared tissue homogenates were tested for concentrations of CXCL1 by ELISA. *p ≤ 0.005.
As a second approach, separated populations of CD4 and CD8 T cells from naïve, DNFB-sensitized, or Ox-sensitized donors were transferred to naïve mice and the production of CXCL1 was determined 6 hours after recipient skin challenge with DNFB (Figure 4). Transfer of DNFB-immune CD8 but not CD4 T cells induced high levels of CXCL1 production in response to DNFB challenge of the recipient skin. Low to absent CXCL1 production was observed following DNFB challenge in recipients of CD8 T cells from either Ox-immune or naïve donors. These results indicate that primed CD8 T cell induction of early CXLC1 production in response to hapten challenge to elicit CHS is hapten-specific.
Figure 4.
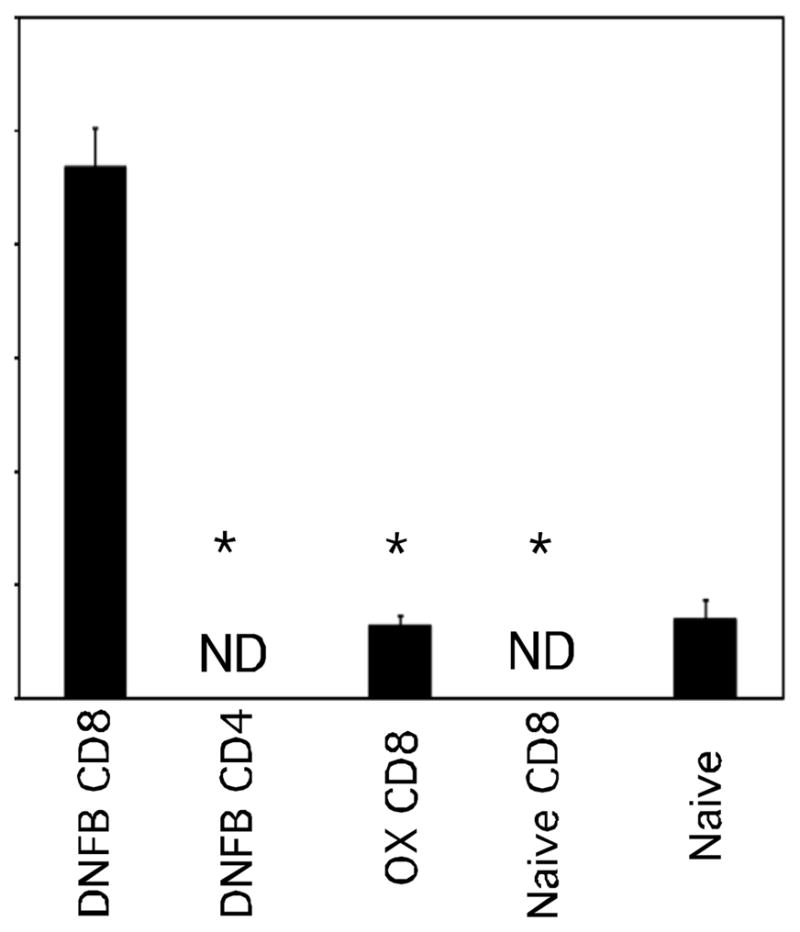
Hapten-primed CD8 T cells transfer antigen-challenge induction of early CXCL1/KC production to naïve mice. T cell donor BALB/c mice were depleted of CD4 or CD8 T cells and sensitized with either 0.25% DNFB or 1% Ox on days 0 and +1. On day +4, groups of 4 naïve BALB/c mice received 4 × 106 LNC aliquots. Recipient mice were challenged with 0.2% DNFB and the concentration of CXCL1 in the challenged skin 6 hours later was tested. The mean concentration ± SEM for 4 individual mice is shown. Results are representative of two individual experiments. *p < 0.05; ND, not detected.
Neutrophils but not CD8 T cells infiltrate challenge sites 6 hours after challenge to elicit CHS
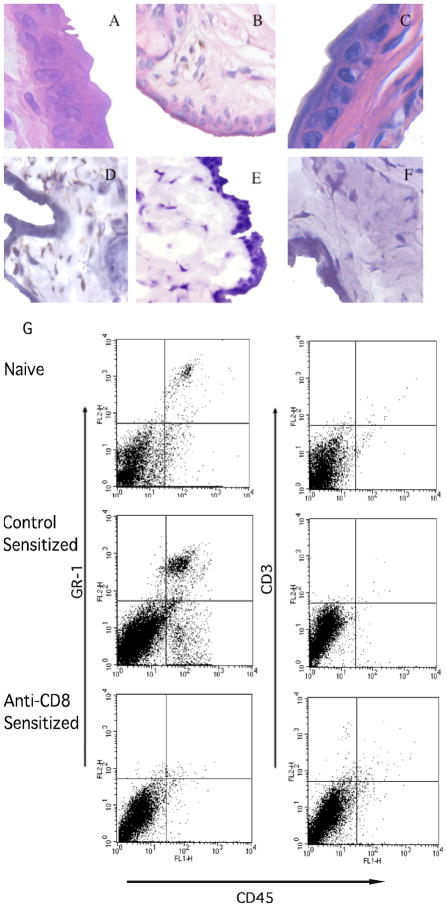
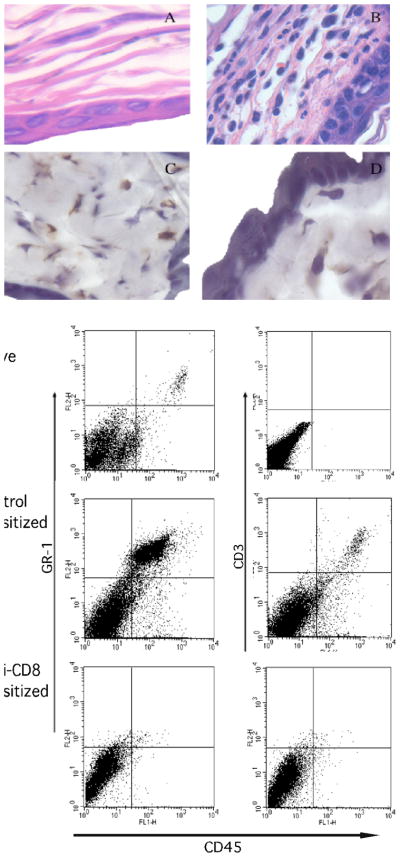
The hapten-primed CD8 T cell mediated neutrophil chemoattractant production within 3–6 hours after challenge of sensitized mice suggested infiltration of both CD8 T cells and neutrophils into the challenged skin at that time. Cellular infiltration into the DNFB challenge site of DNFB-sensitized vs. non-sensitized mice was investigated 6 and 18 hours after DNFB challenge by staining tissue sections with hematoxylin and eosin (Figures 5A–C and 6A and B) or with antibodies to specifically detect infiltrating neutrophils and CD8 T cells (Figures 5D–F and 6C and D). DNFB challenge of naïve mice induced little cellular infiltration (Figure 5A) whereas challenge of sensitized mice induced infiltration with polymorphonuclear leukocytes within 6 hours that stained positively for antibodies that detect neutrophils (Figure 5B and D). Neutrophil infiltration into the skin 6 hours after hapten challenge was dependent on CD8 T cells as this infiltration was absent following challenge of mice depleted of CD8 T cells during DNFB sensitization (Figure 5C and F). In contrast to neutrophils, infiltrating CD8 T cells were not observed 6 hours after challenge of sensitized animals (Figure 5E) but both neutrophils and CD8 T cells were observed 18 hours after challenge of sensitized mice (Figure 6C and D).
Figure 5.
Presence of neutrophils but not CD8 T cells in the skin 6 hrs. after hapten challenge of sensitized mice. BALB/c mice were treated with rat IgG or anti-CD8 mAb and then sensitized with 0.25% DNFB on days 0 and +1. On day +5, sensitized and non-sensitized naïve mice were challenged with 0.2% DNFB. (A-C) Challenged skin was excised 6 hrs. post-challenge and formalin-fixed sections were stained with hematoxylin and eosin. Representative images of sections from naïve (A) and sensitized mice treated with (B) control IgG or (C) CD8-depleting mAb during sensitization are shown. Frozen sections were stained with antibodies to detect (D) neutrophils and (E) CD8+ cells from mice treated with control IgG during sensitization and (F) neutrophils from mice depleted of CD8 T cells before sensitization. Magnification, 400X. (G) The challenged skin was excised and digested 6 hrs. later and prepared cell suspensions were stained with FITC-labeled anti-CD45 mAb and PE-labeled anti-GR-1 mAb or anti-CD3 mAb and analyzed by flow cytometry. Results are representative of 3 individual experiments.
Figure 6.
Both neutrophils and CD8 T cells infiltrate the skin 18 hrs. after hapten challenge. DNFB sensitized and non-sensitized BALB/c mice were challenged with 0.2% DNFB and challenged skin was excised 18 hrs. later and fixed in formalin. Prepared sections were stained with hematoxylin and eosin. Representative images of sections from (A) naïve and (B) sensitized mice are shown. Skin from DNFB sensitized and challenged mice were also fixed in OCT, frozen, and prepared sections were stained with antibodies to detect (C) neutrophils and (D) CD8+ T cells. Magnification, 400X. (E) The skin was excised 18 hrs. after challenge, digested, and prepared cell suspensions were stained with FITC-labeled anti-CD45 mAb and PE-labeled anti-GR-1 mAb or anti-CD3 mAb and analyzed by flow cytometry. Results are representative of 3 individual experiments.
These data were extended using a flow cytometry approach. Skin was excised 6 and 18 hours after challenge and digested to prepare single cell suspensions and cell aliquots were stained with anti-CD45 mAb plus antibodies to detect the presence of neutrophils and T cells in the challenge site. Consistent with the histological analyses, few Gr-1+ cells infiltrated the skin of naïve mice after application of hapten but this infiltration was clearly evident 6 and 18 hours following challenge of sensitized mice and was absent when the animals were depleted of CD8 T cells (Figures 5G and 6E). The Gr-1+ cells were not positive for F4/80 indicating that they were not infiltrating Gr-1+ monocytes or macrophages (data not shown). The skin digestion protocol does not allow for CD8 staining of the cells but CD3 T cell infiltration into the site was evident 18 but not 6 hours after challenge.
CD8 T cells producing IFN-γ and IL-17 stimulate early production of CXCL1 leading to elicitation of the CHS response
Recent studies have indicated a need for hapten-primed T cells producing IL-17 to elicit CHS responses in hapten sensitized mice (22, 23). Previous studies from this laboratory have implicated a role for IFN-γ in the elicitation of CHS (16–18). In order to investigate these previous findings in greater detail, we first tested the elicitation of CHS responses following DNFB sensitization and challenge of wild-type vs. IFN-γ−/− and IL-17−/− mice. The CHS responses to hapten challenge of sensitized IFN-γ−/− and IL-17−/− were markedly decreased when compared to responses elicited in sensitized wild-type mice (Figure 7A). Similarly, treatment of DNFB sensitized wild-type mice with either anti-IFN-γ or anti-IL-17 mAb at the time of hapten challenge significantly attenuated the CHS responses elicited (Figure 7B). These results indicated a requirement for both IFN-γ and IL-17 in the elicitation of CHS responses.
Figure 7.
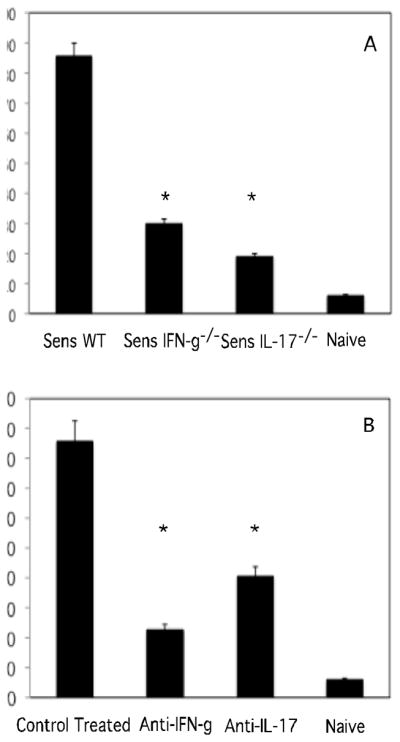
Both IFN-γ and IL-17 are required for elicitation of CHS. (A) Groups of wild-type BALB/c, IFN-γ−/−, and IL-17−/− mice were sensitized with 0.25% DNFB on days 0 and +1. On day +5, sensitized mice and a group of unsensitized/naive mice were challenged on the ears with 0.2% DNFB and the change in ear thickness was measured 24 hours later. (B) Groups of sensitized C57BL/6 mice were treated with 350 μg rat IgG, anti-IFN-γ, or anti-IL-17 i.v. just prior to hapten challenge. The mean increase in ear thickness following hapten challenge is shown in 10−4 in. ± SEM for groups of 4 mice. *p< 0.001.
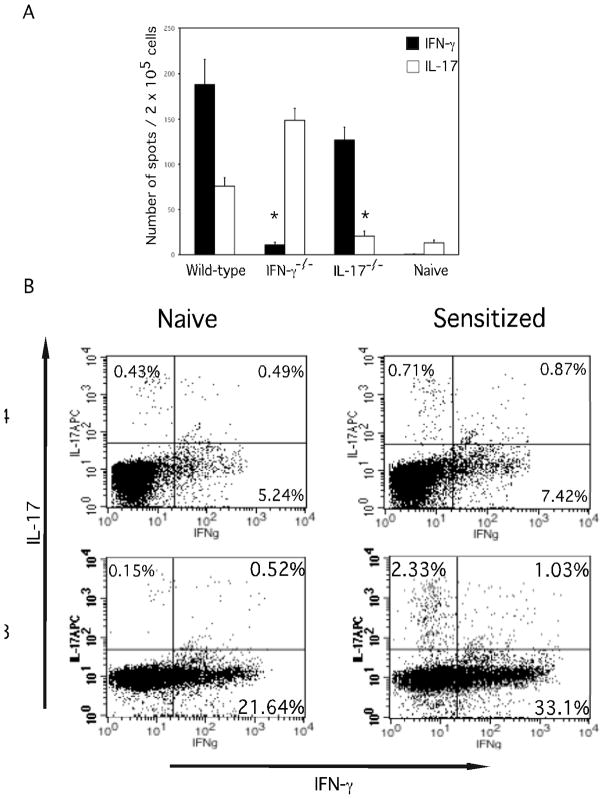
The presence of hapten-specific CD8 T cells producing IFN-γ and IL-17 in the skin draining lymph nodes of sensitized mice was tested by ELISPOT assay. In sensitized wild-type mice, high numbers of hapten-specific CD8 T cells producing IFN-γ were present as well as lower numbers of hapten-specific CD8 T cells producing IL-17 (Figure 8A). In sensitized IL-17−/− mice high numbers of IFN-γ and not IL-17 producing CD8 T cells were present. Alternatively, in sensitized IFN-γ−/− mice high numbers of IL-17 but not IFN-γ producing CD8 T cells were detected. T cells from the skin draining lymph nodes of DNFB sensitized mice were stimulated in vitro, and CD4 and CD8 T cell populations were stained for IFN-γ and IL-17 to detect the cytokines by flow cytometry (Figure 8B). These analyses indicated that separate populations of hapten-primed CD8 T cells produce the IFN-γ and IL-17.
Figure 8.
Hapten sensitization induces separate populations of CD8 T cells producing IFN-γ and IL-17. BALB/c wild-type, IFN-γ−/−, and IL-17−/− mice were sensitized with 0.25% DNFB on days 0 and +1. On day +5, CD8+ T cells were prepared from skin draining lymph nodes and (A) tested in ELISPOT assays with DNBS- and unlabeled spleen cells to enumerate hapten-specific CD8+ T cells producing IFN-γ and IL-17. (B) Lymph node cells from naïve or DNFB sensitized mice were prepared on day +5 and stimulated with PMA and ionomycin and stained to detect intracellular IFN-γ and IL-17 by CD4+ and CD8+ T cell populations. Results are representative of 4 individual experiments for A and 2 individual experiments for B. *p <.0.01
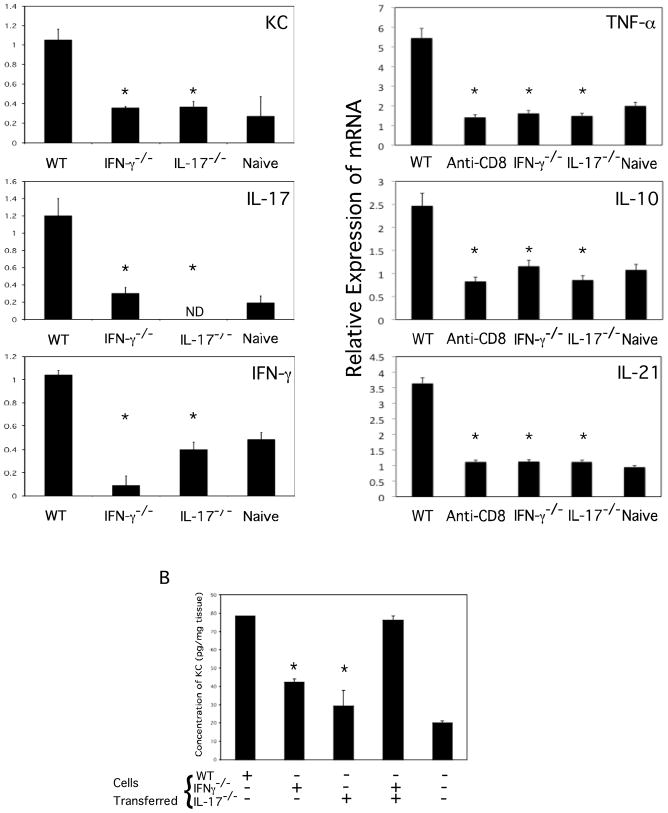
Since primed CD8 T cells are activated within 3–6 hours in the hapten challenge site to induce CXCL1 and CXCL2 production, the expression of IFN-γ and IL-17 in the skin challenge site at early time points during elicitation of CHS was tested. Groups of mice were treated with depleting anti-CD8 or anti-CD4 mAb or with control rat IgG, and were sensitized with DNFB. On day +5 the sensitized and a group of naïve mice were challenged on the skin with DNFB and 6 hours later the challenged skin was retrieved to prepare whole cell RNA. Both IFN-γ and IL-17 mRNA were expressed in the challenge site from sensitized but not naïve mice (Figure 9A and B). Depletion of CD8 T cells before hapten sensitization ablated the appearance of IFN-γ and IL-17 mRNA expression in response to challenge whereas depletion of CD4 T cells increased the mRNA expression of both cytokines.
Figure 9.
CD8 T cell mediated IFN-γ and IL-17 mRNA is expressed by 6 hours in the antigen challenge site during elicitation of CHS. Groups of 4 BALB/c mice were treated with rat IgG, anti-CD4, or anti-CD8 mAb and then sensitized with 0.25% DNFB on days 0 and +1. On day +5, sensitized and non-sensitized naïve mice were challenged with 0.2% DNFB and challenged skin was excised 6 hrs. post-challenge. Whole cell RNA was prepared and used to assess mRNA expression of (A) IFN-γ and (B) IL-17 in the skin challenge site by qRT/PCR. *p < 0.05.
Next, the role of IL-17 and IFN-γ in inducing early CXCL1 and CXCL2 during elicitation of CHS was tested. Treatment of sensitized mice 30 min after hapten challenge with anti-IFN-γ or anti-IL-17 mAb reduced CXCL1 produced in the skin 6 hours later to the levels observed in challenged naïve mice (Figure 10A). Similarly, hapten challenge of either DNFB sensitized IFN-γ−/− or IL-17−/− mice resulted in naïve levels of CXCL1 and CXCL2 at 6 hours post-challenge (Figure 10B and 10C).
Figure 10.
Induction of early CXCL1 and CXCL2 during elicitation of CHS requires IFN-γ and IL-17. (A) On day +5, groups of DNFB-sensitized C57BL/6 mice were treated with 250 μg rat IgG, anti-IFN-γ, or anti-IL-17 i.p. just prior to hapten challenge. (B and C) Groups of 4 wild-type BALB/c, IFN-γ−/− and IL-17−/− mice were sensitized with 0.25% DNFB on days 0 and +1. Challenged skin was excised at 6 hrs. post-challenge and prepared tissue homogenates tested for levels of (A and B) CXCL1 and (C) CXCL2 by ELISA. The mean chemokine concentration ± SEM for 4 individual mice is shown. Results are representative of two individual experiments. *p < 0.03.
The recruitment and activation of hapten-primed CD8 T cells producing only IFN-γ or IL-17 within the challenge site was then investigated. DNFB sensitized wild-type, IFN-γ−/−, and IL-17−/− mice were challenged with DNFB and 6 hours later the skin was removed and prepared RNA was analyzed for expression of the cytokine genes as an indication of the activation of the CD8 T cells in the site (Figure 11A). Again, the absence of CD8 T cells producing either IFN-γ or IL-17 resulted in naïve levels of CXCL1 expression in response to challenge. Although increased numbers of IL-17 producing CD8 T cells were induced by sensitization of IFN-γ−/− vs. wild-type mice (Figure 8A), expression of IL-17 mRNA during challenge of the sensitized IFN-γ−/− mice was reduced to the levels observed following challenge of the naïve mice. Similarly, challenge of sensitized IL-17−/− mice induced reduced levels of IFN-γ mRNA when compared to the levels induced following challenge of sensitized wild-type mice. High levels of mRNA expression of TNFα, IL-10 and IL-21 were also present In the skin challenge site of hapten-sensitized mice 6 hours after challenge and these levels were decreased to those observed following challenge of naïve mice in wild-type mice depleted of CD8 T cells as well as following challenge of sensitized IFN-γ−/− and IL-17−/− mice (Figure 11A).
Figure 11.
Both hapten-specific CD8 T cell derived IFN-γ and IL-17 are required to stimulate proinflammatory cytokines in response to hapten challenge. (A) Wild-type BALB/c, IFN-γ−/−, and IL-17−/− mice were sensitized with 0.25% DNFB on days 0 and +1. On day +5, sensitized and non-sensitized naïve mice were challenged with 0.2% DNFB and challenged skin was excised 6 hrs. post-challenge. Whole cell RNA was prepared and used to assess mRNA expression of CXCL1, IL-17, IFN-γ, TNFα, IL-10 and IL-21 in the skin challenge site by qRT/PCR. (B) CD8 T cell donor BALB/c wild-type, IFN-γ−/−, and IL-17−/− mice were depleted of CD4 T cells and sensitized with 0.25% DNFB on days 0 and +1. On day +4, groups of 4 naïve BALB/c mice received 5 × 106 LNC aliquots and were challenged with 0.2% DNFB. CXCL1 concentrations in the challenged skin 6 hours later were determined by ELISA. The mean concentration ± SEM is shown. Results are representative of two individual experiments. *p < 0.02. ND, not detectable.
Finally, the ability of transferred CD8 T cells from sensitized wild-type, IFN-γ−/− and IL-17−/− mice were compared for the ability to induce CXCL1 production 6 hours after hapten skin challenge of naïve mice. As previously observed (Figure 4), transfer of immune CD8 T cells from sensitized wild-type to naïve mice induced high levels of CXCL1 production in the challenged skin (Figure 11B). Transfer of CD8 T cells from sensitized IFN-γ−/− or IL-17−/− mice induced low levels of the chemokine that were similar to those observed in the skin of naïve mice challenged with the hapten. However, transfer of CD8 T cells from sensitized IFN-γ−/− plus CD8 T cells from sensitized IL-17−/− induced CXCL1 levels that were similar to those observed following challenge of sensitized wild-type mice.
Discussion
The trafficking of antigen-primed T cells through the vascular endothelium and into parenchymal tissue during elicitation of immune responses requires a complex series of cellular and molecular interactions. The expression and role of tissue specific adhesion molecules, chemokines, and their respective receptors have been documented for CD4 T cells infiltrating the skin and intestines (7, 24, 25). For elicitation of many immune responses prior neutrophil infiltration and activation is required for T cell infiltration into peripheral tissue sites. Depletion of neutrophils or inhibition of neutrophil trafficking has been shown to inhibit or attenuate allograft rejection and delayed-type hypersensitivity responses to erythrocyte, tuberculin and viral antigens (2, 26–29). CXCL1-mediated neutrophil infiltration is required for effector CD8 T cell trafficking into hapten challenge sites to mediate CHS and the levels of CXCL1 produced correlate with the infiltration of both neutrophils and hapten-primed CD8 T cells into the challenge site (19, 20). The mechanisms inducing CXCL1 production and neutrophil infiltration into inflammatory sites to initiate T cell recruitment and the elicitation of immune responses in the skin remain unclear and were the focus of the current studies.
The results indicate an intricate series of cellular interactions where production of the chemokines directing neutrophil infiltration into hapten challenge sites during the early stages of CHS is induced by hapten-primed CD8 T cells. The first step in the initiation of this response is the presentation of the hapten in the challenge site to hapten-specific CD8 T cells and their activation to produce IL-17 and IFN-γ These cytokines stimulate the CXCL1 and CXCL2 production that, in turn, direct infiltration of neutrophils into the parenchymal tissue of the challenge site. Both immunohistology and flow cytometry analyses of infiltrating cell populations clearly indicate that hapten-primed CD8 T cell induction of CXCL1 within hours after challenge is accompanied by neutrophil but not CD8 T cell infiltration into the site. This initial neutrophil infiltration is followed several hours later by entry of hapten-primed effector CD8 T cells into the site to mediate the resulting response.
The consequence of neutrophil infiltration and activation in the challenge site at 6 hours post-challenge is a low level edematous response (D. Kish, data not shown). An early hapten-specific tissue-swelling component of CHS responses following hapten sensitization and challenge was originally reported by the Askenase laboratory (30). They have also reported that the induction of this early swelling response is dependent on C5a but, in contrast to the results presented in this report, is independent of antigen-specific T cells (31, 32). This discrepancy is likely due to interpretation of the observed absence of hapten-primed T cell infiltration into the parenchymal tissue. The low magnitude of the swelling response 6 h after challenge reflects this absence and it is not until T cells infiltrate the tissue at later times (12–18 hours) post-challenge that peak levels of edema/swelling are elicited in the challenge site. C5a has chemotactic and activating effects on many different cells including endothelial cells, T cells and neutrophils and the role of C5a as well as the cells expressing C5aR during the generation of this early swelling response in hapten sensitized animals remain unclear.
Two major questions that arises from these studies is what cells present the hapten to the effector CD8 T cell populations and what cells produce the neutrophil chemoattractants in response to the IL-17 and IFN-γ produced by the effector CD8 T cells? It is worth noting that TCR-mediated or mitogenic stimulation of purified effector CD8 T cells does not result in CXCL1 production suggesting that it is not the effector T cells producing CXCL1 in response to hapten skin challenge. Vascular endothelial cells are the first cells that circulating antigen-primed T cells encounter at an inflammatory site prior to infiltration into peripheral tissues. Although direct perfusion of hapten-protein complexes through the blood into the spleen and through the afferent lymph to the nodes following cutaneous hapten application has been previously documented (33), the presentation of hapten by cutaneous vascular endothelial cells has not. Preliminary histological studies suggest that hapten application to the skin results in endothelial cell acquisition of hapten and activation of the CD8 T cells to produce IL-17 and IFN-γ that in turn stimulates the endothelial cells to produce CXCL1 and CXCL2 (D. Kish, unpublished results). During many inflammatory responses various components of tissue injury directly induce neutrophil chemoattractant production. In ischemic tissues, including solid organ grafts, reperfusion rapidly induces endothelial cell production of IL-8, CXCL1, CXCL2 and other neutrophil chemoattractants and is followed by neutrophil infiltration independently of T cells or NK cells (34–37).
Previous studies have documented decreased CHS responses to DNFB and to TNCB in IL-17−/− mice (23). More recently He and coworkers (22) demonstrated the priming of IL-17 and IFN-γ producing CD8 T cells in response to sensitization with DNFB and that treatment of sensitized mice with anti-IL-17 mAb but not with anti-IFN-γ mAb during challenge decreased the CHS response. These results suggested that the IL-17-producing but not the IFN-γ producing CD8 T cells were the effector T cells required to elicit the CHS response to hapten challenge. These results are in contrast to the current studies using both mAb neutralization and CD8 T cells from sensitized IL-17−/− and IFN-γ−/− mice to document the CD8 T cell mediated production of both IL-17 and IFN-γ in the hapten challenge site as well as the requirement for both cytokines to induce the neutrophil chemoattractants required to activate the innate immune component of the CHS response. Most studies investigating the role of IL-17 in inflammatory responses, including autoimmune disease, have focused on CD4+ T (i.e. TH17) cells producing the cytokine (38, 39). Immunofluorescent analysis of T cells in active brain lesions of multiple sclerosis patients revealed both CD4+ and CD8+ T cells co-staining with anti-IL-17 antibodies (40). Whether the hapten-specific CD8 T cell populations producing IL-17 and IFN-γ to induce CXCL1 and CXCL2 early after challenge are a distinct population from the CD8 T cells entering the challenge site at later time points post-challenge to mediate CHS remains unclear. However, transfer of primed CD8 T cells to naïve mice reproduced both the early neutrophil chemoattractant production and the CHS response when the recipients were challenged with hapten.
The modulatory effects of IL-17 and IFN-γ during the initiation and course of immune responses are complex. IL-17 stimulates cultured epithelial and venous endothelial cells to produce IL-8 and CXCL1 and IL-17 induced human bronchial epithelial cell production of IL-8 is enhanced by IFN-γ (41–43). However, both in vitro and in vivo studies have indicated that IFN-γ negatively regulates TH17 cell development as well as IL-17 production by differentiated CD4 TH17 cells (43–45). Recent studies have indicated that peptide vaccine-induced CD4 TH17 cells arrive in infected lungs 3 days before CD4 TH1 cells and induce the chemokines mediating TH1 cell recruitment (46). Although hapten-specific CD8 T cells producing IL-17 are induced by sensitization of IFN-γ−/− mice, challenge did not result in detectable activation of these T cells in the challenge site as indicated by the low expression of CXCL1 or IL-17. Similarly, hapten-specific CD8 T cells producing IFN-γ are induced by sensitization of IL-17−/− mice but activation of these T cells at the site of challenge was not detectable by the expression of CXCL1 or IFN-γ. However, transfer of IFN-γ producing CD8 T cells from sensitized IL-17−/− mice with IL-17 producing CD8 T cells from sensitized IFN-γ−/− mice restored early CXCL1 production to hapten challenge. These results not only indicate that both IFN-γ and IL-17 producing CD8 T cells are required to stimulate CXCL1 production at the challenge site but that activation of each cytokine producing CD8 T cell population promotes the recruitment and/or retention of the other at the site.
Although the hapten-primed CD8 T cells induce early CXCL1 and CXCL2 in the hapten challenge site they do not traverse the endothelial barrier into the tissue parenchyma without prior neutrophil infiltration. The neutrophil functions directing antigen-primed T cell infiltration into the site are yet not known. Depletion of neutrophils resulted in slightly increased levels of CXCL1 and CXCL2 production at 6 hours (results not shown), that are likely due to the absence of neutrophil-mediated digestion of the chemokines during transendothelial cell migration (47). Cytokine activation also induces neutrophils to produce T cell chemoattractants such as CXCL9/Mig and CXCL10/IP-10 during transendothelial migration and peripheral tissue infiltration and these chemokines are candidate recruiting factors for directing the subsequent infiltration of antigen-primed T cells into the tissue (48–51). Neutrophil dependent leukocyte infiltration into the murine liver during CMV infection is associated with neutrophil expression of specific matrix metalloproteinases (52), suggesting that digestion and possibly structural alteration of extracellular matrix may be required for T cell infiltration into peripheral tissues during certain immune responses.
The results of these experiments demonstrate a dynamic series of interactions between the innate and adaptive immune responses to achieve antigen-primed T cell infiltration into a tissue site to elicit an immune response. There has been increasing interest in dissecting innate immune mechanisms regulating adaptive responses, particularly in the modulation of antigen-presenting cell function. The current results indicate the requirement for antigen-specific responses to provoke the innate immunity required for T cell infiltration into the tissue site to elicit the immune response. The neutrophil dependency of T cell infiltration into peripheral tissue sites of other immune responses may also involve similar initial T cell control of neutrophil activity. The requirement for such interactions to elicit responses in the skin offers several new points at which regulation of both the adaptive and innate responses can be manipulated in a positive or negative manner to influence the magnitude and course of these immune responses.
Acknowledgments
This work was supported by grants RO1 AI45888 from the National Institutes of Allergy and Infectious Diseases.
The authors thank the staff of the Cleveland Clinic Biological Resources Unit for excellent care of the animals used in this study and Drs. Booki Min and Jeong-Su Do for help with the intracellular cytokine staining and analysis.
Footnotes
Disclosures
The authors have no conflict of interest with the studies reported in this manuscript.
References
- 1.Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines-CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- 2.El-Sawy T, Belperio JA, Strieter RM, Remick DG, Fairchild RL. Inhibition of polymorphonuclear leukocyte-mediated graft damage synergizes with short-term costimulatory blockade to prevent cardiac allograft rejection. Circulation. 2005;112:320–331. doi: 10.1161/CIRCULATIONAHA.104.516708. [DOI] [PubMed] [Google Scholar]
- 3.Jaeschke H, Smith CW. Mechanisms of neutrophil-induced parenchymal cell injury. J Leukoc Biol. 1997;61:647–653. doi: 10.1002/jlb.61.6.647. [DOI] [PubMed] [Google Scholar]
- 4.McColl SR, Clark-Lewis I. Inhibition of murine neutrophil recruitment in vivo by CXC chemokine receptor antagonists. J Immunol. 1999;163:2829–2835. [PubMed] [Google Scholar]
- 5.Miller AL, Strieter RM, Gruber AD, Ho SB, Lukacs NW. CXCR2 regulates respiratory syncytial virus-induced airway hyperreactivity and mucus overproduction. J Immunol. 2003;170:3348–3356. doi: 10.4049/jimmunol.170.6.3348. [DOI] [PubMed] [Google Scholar]
- 6.Moore TA, Newstead MW, Strieter RM, Mehrad B, Beaman BL, Standiford TJ. Bacterial clearance and survival are dependent on CXC chemokine receptor-2 ligands in a murine model of pulmonary Nocardia asteroides infection. J Immunol. 2000;164:908–915. doi: 10.4049/jimmunol.164.2.908. [DOI] [PubMed] [Google Scholar]
- 7.Campbell JJ, Haraldsen G, Pan J, Rottman J, Qin S, Ponath P, Andrew DP, Warnke R, Ruffing N, Kassam N, Wu L, Butcher EC. The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature. 1999;400:776–780. doi: 10.1038/23495. [DOI] [PubMed] [Google Scholar]
- 8.Reiss Y, Proudfoot AE, Power CA, Campbell JJ, Butcher EC. CC chemokine receptor (CCR)4 and the CCR10 ligand cutaneous T cell-attracting chemokine (CTACK) in lymphocyte trafficking to inflamed skin. J Exp Med. 2001;194:1541–1547. doi: 10.1084/jem.194.10.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engeman TM, Gorbachev AV, Gladue RP, Heeger PS, Fairchild RL. Inhibition of functional T cell priming and contact hypersensitivity responses by treatment with anti-secondary lymphoid chemokine antibody during hapten sensitization. J Immunol. 2000;164:5207–5214. doi: 10.4049/jimmunol.164.10.5207. [DOI] [PubMed] [Google Scholar]
- 10.Kripke M, Munn C, Jeevan A, Tang J, Bucana S. Evidence that cutaneous antigen-presenting cells migrate to regional lymph nodes during contact sensitization. J Immunol. 1990;145:2833–2838. [PubMed] [Google Scholar]
- 11.Macatonia SE, Edwards AJ, Knight SC. Dendritic cells and the initiation of contact sensitivity to fluorescein isothiocyanate. Immunology. 1986;59:509–514. [PMC free article] [PubMed] [Google Scholar]
- 12.Macatonia SE, Knight SC, Edwards AJ, Griffiths S, Fryer P. Localization of antigen on lymph node dendritic cells after exposure to the contact sensitizer fluorescein isothiocyanate. J Exp Med. 1987;166:1654–1667. doi: 10.1084/jem.166.6.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Enk AH. Allergic contact dermatitis: understanding the immune response and potential for targeted therapy using cytokines. Mol Med Today. 1997;3:423–428. doi: 10.1016/S1357-4310(97)01087-3. [DOI] [PubMed] [Google Scholar]
- 14.Bour H, Peyron E, Gaucherand M, Garrigue JL, Desvignes C, Kaiserlian D, Revillard JP, Nicolas JF. Major histocompatibility complex class I-restricted CD8+ T cells and class II-restricted CD4+ T cells, respectively, mediate and regulate contact sensitivity to dinitrofluorobenzene. Eur J Immunol. 1995;25:3006–3010. doi: 10.1002/eji.1830251103. [DOI] [PubMed] [Google Scholar]
- 15.Gocinski BL, Tigelaar RE. Roles of CD4+ and CD8+ T cells in murine contact sensitivity revealed by in vivo monoclonal antibody depletion. J Immunol. 1990;144:4121–4128. [PubMed] [Google Scholar]
- 16.Xu H, DiIulio NA, Fairchild RL. T cell populations primed by hapten sensitization in contact sensitivity are distinguished by polarized patterns of cytokine production: Interferon-γ-producing (Tc1) effector CD8+ T cells and Interleukin (Il) 4/IL-10-producing (Th2) negative regulatory CD4+ T cells. J Exp Med. 1996;183:1001–1012. doi: 10.1084/jem.183.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorbachev AV, Heeger PS, Fairchild RL. CD4+ and CD8+ T cell priming for contact hypersensitivity occurs independently of CD40-CD154 interactions. J Immunol. 2001;166:2323–2332. doi: 10.4049/jimmunol.166.4.2323. [DOI] [PubMed] [Google Scholar]
- 18.Xu H, Banerjee A, DiIulio NA, Fairchild RL. Development of effector CD8+ T cells in contact hypersensitivity occurs independently of CD4+ T cells. J Immunol. 1997;158:4721–4728. [PubMed] [Google Scholar]
- 19.DiIulio NA, Engeman TM, Armstrong D, Tannenbaum C, Hamilton TA, Fairchild RL. Groα-mediated recruitment of neutrophils is required for elicitation of contact hypersensitivity. Eur J Immunol. 1999;29:3485–3495. doi: 10.1002/(SICI)1521-4141(199911)29:11<3485::AID-IMMU3485>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 20.Engeman TM, Gorbachev AV, Kish DD, Fairchild RL. The intensity of neutrophil infiltration controls the number of antigen-primed CD8 T cells recruited into cutaneous antigen challenge sites. J Leukoc Biol. 2004;76:941–949. doi: 10.1189/jlb.0304193. [DOI] [PubMed] [Google Scholar]
- 21.Gorbachev AV, Fairchild RL. CD4+ T cells regulate CD8+ T cell-mediated cutaneous immune responses by restricting effector T cell development through a Fas ligand-dependent mechanism. J Immunol. 2004;172:2286–2295. doi: 10.4049/jimmunol.172.4.2286. [DOI] [PubMed] [Google Scholar]
- 22.He D, Wu L, Kim HK, Li H, Elmets CA, Xu H. CD8+ IL-17-producing T cells are important in effector functions for the elicitation of contact hypersensitivity responses. J Immunol. 2006;177:6852–6858. doi: 10.4049/jimmunol.177.10.6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, Sekikawa K, Asano M, Iwakura Y. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–387. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 24.Backkevold ES, Wurbel MA, Kivisakk P, Wain CM, Power CA, Haraldsen G, Campbell JJ. A role for CCR4 in development of mature circulating cutaneous T helper memory cell populations. J Exp Med. 2005;201:1045–1051. doi: 10.1084/jem.20041059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soler D, Humphreys TL, Spinola SM, Campbell JJ. CCR4 versus CCR10 in human cutaneous TH lymphocyte trafficking. Blood. 2003;101:1677–1682. doi: 10.1182/blood-2002-07-2348. [DOI] [PubMed] [Google Scholar]
- 26.Kudo C, Yamashita T, Araki A, Terashita M, Watanabe T, Atsumi M, Tamura M, Sendo F. Modulation of in vivo immune response by selective depletion of neutrophils using a monoclonal antibody, RP-3. I. Inhibition by RP-3 treatment of the priming and effector phases of delayed type hypersensitivity to sheep red blood cells in rats. J Immunol. 1993;150:3728–3738. [PubMed] [Google Scholar]
- 27.Larsen CG, Thomsen MK, Gesser B, Thomsen PD, Deleuran BW, Nowak J, Skodt V, Thomsen HK, Deleuran M, Thestrup-Pedersen K, Harada A, Matsushima K, Menne T. The delayed-type hypersensitivity reaction is dependent on IL-8. Inhibition of a tuberculin skin reaction by an anti-IL-8 monoclonal antibody. J Immunol. 1995;155:2151–2157. [PubMed] [Google Scholar]
- 28.Morita K, Miura M, Paolone DR, Engeman TM, Kapoor A, Remick DG, Fairchild RL. Early chemokine cascades in murine cardiac grafts regulate T cell recruitment and progression of acute allograft rejection. J Immunol. 2001;167:2979–2984. doi: 10.4049/jimmunol.167.5.2979. [DOI] [PubMed] [Google Scholar]
- 29.Tumpey TM, Fenton R, Molesworth-Kenyon S, Oakes JE, Lausch RN. Role for macrophage inflammatory protein 2 (MIP-2), MIP-1α, and interleukin-1α in the delayed hypersensitivity response to viral antigen. J Virol. 2002;76:8050–8057. doi: 10.1128/JVI.76.16.8050-8057.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Loveren H, Askenase PW. Delayed-type hypersensitivity is mediated by a sequence of two different T cell activities. J Immunol. 1984;133:2397–2401. [PubMed] [Google Scholar]
- 31.Tsuji RF, Geba GP, Wang Y, Kawamoto K, Matis LA, Askenase PW. Required early complement activation in contact sensitivity with generation of local C5-dependent chemotactic activity, and late T cell interferon γ: a possible initiating role of B cells. J Exp Med. 1997;186:1015–1026. doi: 10.1084/jem.186.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsuji RF, Kawikova I, Ramabhadran R, Akahira-Azuma M, Taub D, Hugli TE, Gerard C, Askenase PW. Early local generation of C5a initiates the elicitation of contact sensitivity by leading to early T cell recruitment. J Immunol. 2000;165:1588–1598. doi: 10.4049/jimmunol.165.3.1588. [DOI] [PubMed] [Google Scholar]
- 33.Pior J, Vogl T, Sorg C, Macher E. Free hapten molecules are dispersed by way of the blood stream during contact sensitization to fluorescein isothiocyanate. J Invest Dermatol. 1999;113:888–893. doi: 10.1046/j.1523-1747.1999.00770.x. [DOI] [PubMed] [Google Scholar]
- 34.El-Sawy T, Miura M, Fairchild R. Early T cell response to allografts occurring prior to alloantigen priming up-regulates innate mediated inflammation and graft necrosis. Am J Pathol. 2004;165:147–157. doi: 10.1016/s0002-9440(10)63283-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miura M, El-Sawy T, Fairchild RL. Neutrophils mediate parenchymal tissue necrosis and accelerate the rejection of complete major histocompatibility complex-disparate cardiac allografts in the absence of interferon-g. Am J Pathol. 2003;162:509–519. doi: 10.1016/s0002-9440(10)63845-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miura M, Fu X, Zhang QW, Remick DG, Fairchild RL. Neutralization of Groα and macrophage inflammatory protein-2 attenuates renal ischemia/reperfusion injury. Am J Pathol. 2001;159:2137–2145. doi: 10.1016/s0002-9440(10)63065-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rabb H, O’Meara YM, Maderna P, Coleman P, Brady HR. Leukocytes, cell adhesion molecules and ischemic acute renal failure. Kidney Int. 1997;51:1463–1468. doi: 10.1038/ki.1997.200. [DOI] [PubMed] [Google Scholar]
- 38.Bettelli E, Korn T, Kuchroo VK. Th17: the third member of the effector T cell trilogy. Curr Opin Immunol. 2007;19:652–657. doi: 10.1016/j.coi.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 40.Tzartos JS, Friese MA, Raner MJ, Palae J, Newcombe J, Esiri MM, Fugger L. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol. 2008;172:146–155. doi: 10.2353/ajpath.2008.070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kawaguchi M, Kokubu F, Kuga H, Matsaukura S, Hoshino H, Ieki K, Imai T, Adachi M, Huang SK. Modulation of bronchial epithelial cells by IL-17. J Allergy Clin Immunol. 2001;108:804–809. doi: 10.1067/mai.2001.119027. [DOI] [PubMed] [Google Scholar]
- 42.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 43.Park H, Li Z, Yang XO, Chang SE, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cruz A, Khader SA, Torrado E, Fraga A, Pearl JE, Pedrosa J, Cooper AM, Castro AG. IFN-γ regulates the induction and expansion of IL-17-producing CD4 T cells during mycobacterial infection. J Immunol. 2006;177:1416–1420. doi: 10.4049/jimmunol.177.3.1416. [DOI] [PubMed] [Google Scholar]
- 45.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 46.Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, Shen F, Eaton SM, Gaffen SL, Swain SL, Locksley RM, Haynes L, Randall TD, Cooper AM. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8:369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 47.Li Q, Park PW, Wilson CL, Parks WC. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell. 2002;11:635–646. doi: 10.1016/s0092-8674(02)01079-6. [DOI] [PubMed] [Google Scholar]
- 48.Cassatella MA, Gasperini S, Calzetti F, Bertagnin S, Luster AD, McDonald PP. Regulated production of the interferon-gamma-inducible protein-10 (IP-10) chemokine by human neutrophils. Eur J Immunol. 1997;27:111–115. doi: 10.1002/eji.1830270117. [DOI] [PubMed] [Google Scholar]
- 49.Gasperini S, Marchi M, Calzetti F, Laudanna C, Vicentini L, Olsen H, Murphy M, Liao F, Farber J, Cassatella MA. Gene expression and production of the monokine induced by IFN-γ (MIG), IFN-inducible T cell a chemoattractant (I-TAC), and IFN-γ inducible protein-10 (IP-10) chemokines by human neutrophils. J Immunol. 1999;162:4928–4937. [PubMed] [Google Scholar]
- 50.Miura M, Morita K, Kobayashi H, Hamilton TA, Burdick MA, Strieter RM, Fairchild RL. Monokine induced by IFN-γ is a dominant factor directing T cells into murine cardiac allografts during acute rejection. J Immunol. 2001;167:3494–3504. doi: 10.4049/jimmunol.167.6.3494. [DOI] [PubMed] [Google Scholar]
- 51.Molesworth-Kenyon SJ, Oakes JE, Lausch RN. A novel role for neutrophils as a source of T cell-recruiting chemokines IP-10 and Mig during the DTH response to HSV-1 antigen. J Leukoc Biol. 2005;77:552–559. doi: 10.1189/jlb.0904485. [DOI] [PubMed] [Google Scholar]
- 52.Sitia G, Isogawa M, Iannacone M, Campbell IL, Chisari FV, Guidotti LG. MMPs are required for recruitment of antigen-nonspecific mononuclear cells into the liver by CTLs. J Clin Invest. 2004;113:1158–1167. doi: 10.1172/JCI21087. [DOI] [PMC free article] [PubMed] [Google Scholar]