Abstract
Oxygen radicals, and other reactive oxygen species, may play an important role in the pathophysiology of atherosclerosis, stroke, and other cardiovascular diseases. Mechanisms that account for oxidative stress in different cardiovascular diseases are diverse. For example, increases in activity of NAD(P)H oxidase, “uncoupling” of nitric oxide synthase, and maladaptive changes in expression of antioxidants all can contribute to increases in oxidative stress. We have observed very different patterns of pro-and antioxidant mechanisms that contribute to increases in oxygen radicals in atherosclerotic plaques, hemorrhagic strokes, and aortic valve stenosis. A disappointment, in relation to the hypothesis that oxygen radicals contribute to cardiovascular risk, is that many studies indicate that antioxidant vitamins fail to reduce the risk of cardiovascular disease. We speculate, however, that better understanding of mechanisms that lead to increases in oxidative stress in different cardiovascular diseases may lead to more effective antioxidant prevention or treatment of diseases.
Keywords: oxidative stress, antioxidant mechanisms, aortic valve stenosis, stroke
Introduction
Superoxide and other reactive oxygen species (ROS) are increased in arteries in several major cardiovascular diseases (1,2). This finding led to one of the most intriguing hypotheses in contemporary vascular biology: ROS play a critical role in pathophysiology of atherosclerosis, stroke, hypertension, and other cardiovascular diseases.
In the past, the concept that ROS, including oxygen radicals, may affect the heart and blood vessels focused on 1) generation of ROS by leukocytes, and 2) vascular damage during ischemia/reperfusion. Leukocytes release ROS at a high concentration which kill bacteria, and thereby may produce vascular damage. In addition, reperfusion of blood after ischemia generates ROS, and reperfusion may thereby produce damage to reperfused tissues, including heart, brain, and liver.
It is now clear that ROS play a far more important role than previously recognized, in normal signaling (3) and physiological mechanisms, as well as in pathophysiology. Low levels of ROS play an important role in normal cellular signaling pathways (4), and modulate growth and apoptosis of endothelium and of vascular and cardiac muscle. These changes are important during development, and also during adaptive changes that lead to hypertrophy and remodeling.
High levels of ROS are generated in many disease states, including atherosclerosis and hypertension, and contribute to endothelial dysfunction, and to risk of cardiovascular consequences. It is now widely accepted that excessive superoxide inactivates nitric oxide and contributes to endothelial dysfunction (e.g., 5), which is a risk factor for cardiovascular disease. A more recent hypothesis is that superoxide dismutases may augment endothelium-mediated vasodilatation, by generation of hydrogen peroxide (6) as well as by dismutation of superoxide. In some vascular beds, hydrogen peroxide appears to be an endothelium-derived relaxing factor. The interaction of ROS antioxidants and endothelium is complex and important.
This review will focus on the role of ROS (especially superoxide) in cardiovascular diseases.
Mechanisms of oxidative stress
Two mechanisms that may lead to increases in superoxide in cardiovascular diseases are increased generation or decreased dismutation (or inactivation) of superoxide. The usual mechanism for increases in superoxide in cardiovascular disease is by increased generation. Many enzymes (including NAD(P)H oxidase, xanthine oxidase, cyclooxygenase, “uncoupled” nitric oxide synthases) can generate superoxide and may contribute to increases in superoxide in cardiovascular diseases (7). Leukocytes, in inflammatory states, also may contribute to increases in superoxide.
Although decreased dismutation or inactivation of superoxide may produce high levels of superoxide, this mechanism appears to be a less common cause for oxidative stress in disease states than increased generation of superoxide. In fact, oxidative stress typically stimulates antioxidant mechanisms, including superoxide dismutases (SODs), so oxidative stress usually is associated with a compensatory increase in levels of SODs. Redox/inflammatory signal sensitive regions in the promoter regions increase expression of SODs during oxidative stress (8,9). There are some exceptions to this concept. For example, in some studies, oxidative stress associated with heart failure and aging appears to be due in part to decreased levels of SODs, as well as increased generation of ROS.
Mice with heterozygous or homozygous deficiency of MnSOD, CuZnSOD, or ecSOD have been generated and are used to examine cardiovascular consequences of increased levels of superoxide (10). In humans, however, heterozygous or homozygous deficiency of antioxidant genes is rare. Gene variants of SODs, however, may reduce efficacy of antioxidants. For example, ecSODR213G is a common gene variant which impairs the localization of the protein, and reduces the protective effect of ecSOD against vascular dysfunction. Of interest is the finding that the risk of cardiovascular disease is increased in people with ecSODR213G (11).
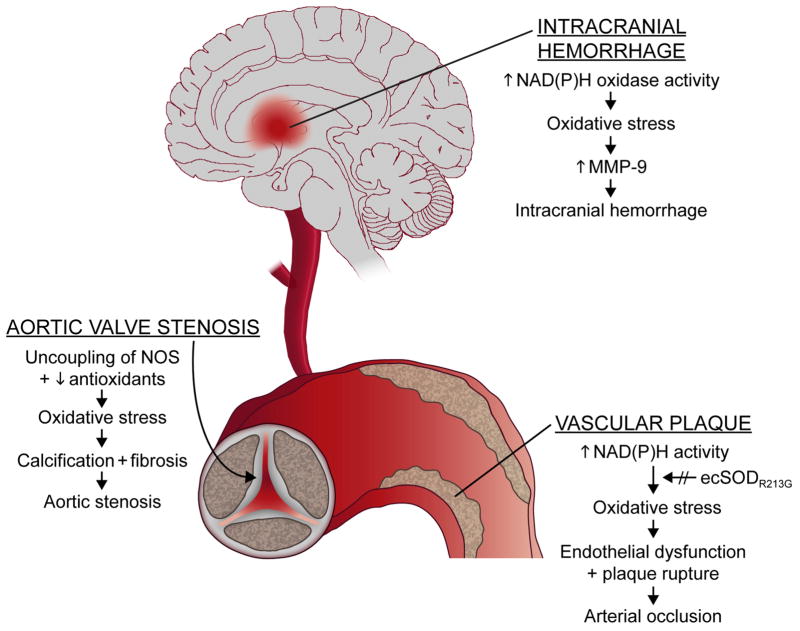
In this review, we will focus on some of our recent studies of oxidative stress in cardiovascular diseases. We will use three diverse examples to illustrate mechanisms by which oxidative stress may be increased by a variety of mechanisms, and have differing consequences in different tissues (Fig. 1). An underlying question will be whether oxidative stress, which clearly is increased in several major cardiovascular diseases, is a cause or an association. Because many studies indicate that antioxidant vitamins fail to reduce risk of cardiovascular diseases (e.g., 12), it is important to consider whether oxidative stress does indeed play a causative role in cardiovascular diseases.
Figure 1.
Examples of diverse mechanisms by which oxidative stress may be increased in different tissues, with different consequences. In the aortic valve, oxidative tress appears to be produced by uncoupling of nitric oxide synthase (NOS) and reduction in antioxidant mechanisms (superoxide dismutases--SODs--and catalase). Oxidative stress in intracranial hemorrhage appears to be produced in part by increases in NAD(P)H oxidase activity. In arterial plaques, in coronary and other arteries, oxidative stress appears to be produced primarily by increases in NAD(P)H activity. Increases in superoxide (O2.−) are augmented by a gene variant (ecSODR213G) that is common in humans.
Calcific Aortic Stenosis
Aortic valvular stenosis, which is sometimes described as senile degenerative calcific stenosis, is an extremely important, and greatly under-studied clinical problem. The most frequent cardiovascular operation in the U.S. is coronary artery bypass grafting (which is decreasing in number) and the second most frequent operation is replacement of cardiac valves, primarily the stenotic aortic valve (13). The prevalence of calcific aortic valve stenosis, and the number of operations to replace the valve, is increasing as our population ages.
The histological appearance of stenotic aortic valves resembles atherosclerotic lesions, with lipids, inflammatory cells, neoangiogenesis, and calcification (14). Risk factors for aortic stenosis and atherosclerosis also are similar, and include older age, hypercholesterolemia, hypertension, smoking, and diabetes (13,15).
Based on similarity of aortic stenosis and atherosclerosis, in relation to histological appearance and risk factors, one might suggest that aortic stenosis is simply atherosclerosis of the valve. There are major differences, however, between the pathophysiology of aortic stenosis and atherosclerosis (Fig. 1). We will summarize some of those differences below.
Oxidative stress
Superoxide is increased in stenotic aortic valves (16), as well as atherosclerotic vascular plaques. We stumbled on the finding in genetically altered mice, which are hypercholesterolemic (because they are deficient in lipoprotein receptors -- LDLr−/−), and especially prone to atherosclerosis (because they have apolipoprotein B100 instead of apoB48). We found that about one-third of old LDLr−/−, apoB100/100 mice develop severe aortic stenosis, and have high levels of superoxide in the aortic valve (16).
Superoxide levels also are increased in stenotic aortic valves from humans (17). We found, in stenotic valves removed during surgical replacement of the aortic valve, that superoxide is increased greatly near calcified regions of the valve. Others (18) also found, in valves obtained at surgery or autopsy, that oxidative stress is increased in stenotic aortic valves. Thus, in calcified stenotic aortic valves as well as in atherosclerotic lesions, oxidative stress is increased.
But, there are important differences in mechanisms that account for oxidative stress in aortic valves and in atherosclerotic arteries (Fig. 2) (17). In calcific aortic stenosis, increased production of superoxide may be mediated by “uncoupling” of nitric oxide synthase (NOS), as NOS primarily produces superoxide instead of nitric oxide (19,20); NAD(P)H expression and activity do not appear to be increased in aortic valves (17). In striking contrast, increased expression and activity of NAD(P)H oxidase appears to be a major mechanism for oxidative stress in atherosclerotic lesions (1).
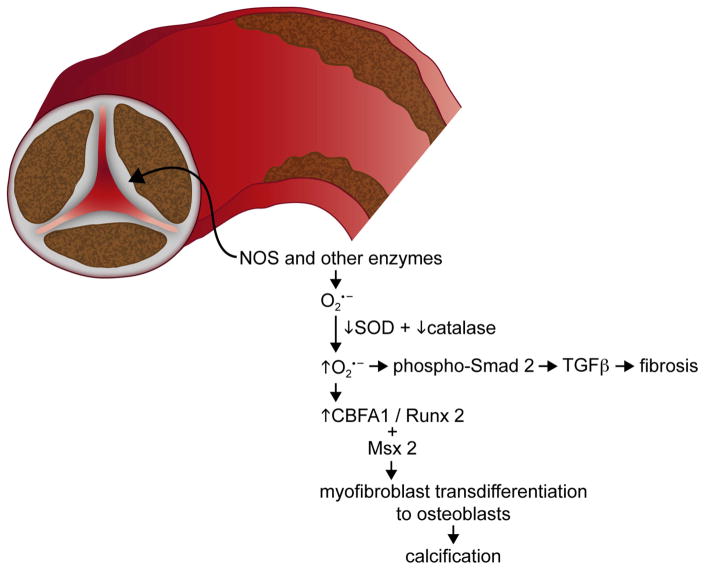
Figure 2.
In the aortic valve, superoxide appears to be generated by NOS (and perhaps other enzymes). Levels of superoxide are augmented by deficiency of SODs and catalase, and signaling cascades are activated that lead to fibrosis (primarily through TGFβ) and calcification of the valve (likely through TGFβ, CBFA1/Runx2, and Msx2).
Antioxidant mechanisms
The role of SODs differs in calcific aortic stenosis and atherosclerotic lesions. In the aortic valve, there is a profound decrease in expression of all three isoforms of superoxide dismutase (SODs) in calcified regions of the aortic valve, and SOD activity is decreased (17). Thus, decreases in SODs contribute to oxidative stress in the aortic valve. We were surprised by the finding, because in most tissues with increased oxidative stress, there is a protective, compensatory increase in levels of SODs (21). In contrast to decreases in SODs in calcific aortic stenosis, SODs have been reported to increase (or not change) in atherosclerotic vascular lesions. Thus, there are fundamental differences in mechanisms that lead to increased oxidative stress in the aortic valve and atherosclerotic vascular lesions (22).
Furthermore, consequences of oxidative stress may differ in aortic stenosis and in atherosclerotic vascular lesions. Many proteins and transcription factors that play a key role in fibrosis and calcification are redox-sensitive (23,24). In the aortic valve, it is likely that both fibrosis and calcification contribute to restrictions in motion of the cusps, reduction in valve orifice, and progression to severe stenosis. In contrast, in arteries, increases in fibrosis of atherosclerotic plaques probably are protective, by transformation of a vulnerable plaque to a stable lesion. Thus, the consequences of increases in pro-fibrotic and pro-calcific gene expression are site specific and depend on the function of the tissue. Appreciation of such complexities is critical when designing therapies for patients with comorbid conditions, such as atherosclerosis and aortic valve stenosis, where treatment that improves valve function may increase the propensity for plaque rupture or instability.
Oxidative stress, in addition to contributing to fibrosis, may activate matrix metalloproteinases (MMPs) in the aortic valve and arteries (25). In the valve, MMPs may play a permissive role in expansion of calcification of the valve, and degraded fragments of collagen and elastin also may increase pro-calcific signaling in valvular interstitial cells. Activation of MMPs in arteries probably is harmful in a different way, by contributing to plaque rupture (25).
Treatment
HMGCoA-reductase inhibitors (statins) are undergoing clinical studies to test the hypothesis that statins may slow the progression of aortic stenosis (e.g., 26,27). The rationale is strongly supported by the enormous benefit of statins in reduction of cardiovascular events, and by findings in retrospective studies. But on closer examination, the logic may be flawed, in applying effects of statins on arteries to effects on valves. Regression of atherosclerotic lesions in arteries is accompanied by progression of fibrosis, and perhaps of calcification. Fibrosis in blood vessels during treatment with statins probably is beneficial, by stabilizing the vulnerable plaque, but it is likely that fibrosis is harmful in the aortic valve, by contributing to progression of stenosis. Thus, based on findings in arteries, it is difficult to predict whether statins will prove to be useful or harmful in treating aortic stenosis.
We have studied “Reversa” mice (16) with aortic stenosis which have a genetic “switch,” in which hypercholesterolemia can be effectively treated, without statins. Our preliminary data (28) suggest that normalization of cholesterol in the presence of early lipid deposition and calcification of the aortic valve completely prevents development and progression of aortic stenosis. The findings imply that aggressive reduction of lipids in patients with hypercholesterolemia, if started early, may be useful in slowing the progression of aortic stenosis.
Cause or association?
We and others have demonstrated that oxidative stress is associated with calcific aortic stenosis, in mice and humans. It will be of great interest and importance to determine whether superoxide contributes to initiation and progression of aortic stenosis, perhaps by an effect on redox-sensitive genes that modulate fibrosis and calcification. Or, alternatively, superoxide may not produce aortic stenosis and, perhaps, aortic stenosis per se increases oxidative stress.
Our preliminary evidence suggests that superoxide in the aortic valve increases before the valve becomes stenotic, which is compatible with a causal role for superoxide (and/or other ROS) in aortic stenosis. Interventions, not an association, will be the key in determining whether oxidative stress is cause or effect for aortic stenosis. The finding, that treatment of hypercholesterolemia with a genetic switch reduces superoxide in the aortic valve and stops development of stenosis, also suggests a causal role for superoxide (28). Tempol (which reduces superoxide, but increases hydrogen peroxide) failed to slow the development of aortic valve disease in rabbits (18). Because rabbits do not develop hemodynamically significant aortic stenosis, however, and because tempol increases hydrogen peroxide, the finding is not definitive, in relation to the role of ROS in progression of aortic stenosis. It will be of great interest to see whether appropriate antioxidants slow the progression of aortic stenosis in an experimental model that develops hemodynamically significant aortic stenosis.
Serotonin-induced valvulopathy
High levels of serotonin, in patients with carcinoid tumors or during treatment with fen-phen or pergolide, may contribute to a proliferative valvulopathy. Serotonin is released from carcinoid tumors into venous blood. The tricuspid and pulmonic valves are especially likely to develop disease, presumably because levels of serotonin in the blood are highest in the right side of the heart, and serotonin is metabolized in the pulmonary circulation before reaching valves on the left side of the heart.
In preliminary studies, we have found that high concentrations of serotonin increase levels of superoxide in normal tricuspid and pulmonic valves obtained from humans (29). When we incubated the valves in a monoamine oxidase (MAO) inhibitor, to increase levels of serotonin and thus oxidative stress, we were surprised to find that the MAO inhibitor reduced the oxidative stress. The findings suggest that production of oxidative stress in human cardiac valves by serotonin may be mediated in part by metabolism of serotonin.
As discussed above, in relation to aortic stenosis, redox-sensitive genes may play a key role in modification of extracellular matrix in cardiac valves. Thus, it is possible that oxidative stress, produced by MAO-mediated metabolism of serotonin, contributes to tricuspid and pulmonic valvulopathy in patients with carcinoid tumor, or during treatment with fen-phen or pergolide. Interventions in vivo with an MAO inhibitor and/or an antioxidant will be required to establish a causal relationship.
Hemorrhagic stroke
Spontaneous intracranial hemorrhage (ICH) accounts for only 10–15% of strokes, but has a very high mortality. The major risk factor for ICH is hypertension. There has been little progress in understanding the pathophysiology and treatment of ICH compared with ischemic stroke, in part because there is no experimental model of spontaneous ICH in hypertensive mice.
Stroke-prone spontaneously hypertensive rats (SHRSP) have been exceedingly useful in studying stroke, but they generally develop ischemic infarcts and, when hemorrhage occurs, ICH usually is secondary to hemorrhagic transformation from an ischemic infarct. In addition, hemorrhagic strokes in SHRSP occur primarily in the cerebrum, not in the brain stem, cerebellum, and basal ganglia (which are the primary sites of hemorrhage in hypertensive humans).
We have developed two models of ICH in hypertensive mice. First, we found that, in a genetic model of hypertension, double transgenic mice (R+A+) that overexpress human renin (R+) and angiotensinogen (A+), administration of a high-salt diet and an inhibitor of nitric oxide synthase (L-NAME) produces severe hypertension and spontaneous ICH (30,31). The distribution of ICH within the brain is similar to that observed in hypertensive patients. Second, we have developed recently another model of ICH in hypertensive mice. In wild-type mice, chronic hypertension was produced with infusion of angiotensin, and with L-NAME, and acute increases in blood pressure were produced by injections of angiotensin twice daily, to simulate acute increases in pressure in patients with chronic hypertension. Our preliminary findings (unpublished data) indicate that, when acute increases in pressure and chronic hypertension are combined, there is a very high incidence of ICH.
Oxidative stress
A major goal of our studies of stroke-prone hypertensive mice is to test the hypothesis that oxidative stress contributes to spontaneous ICH. The hypothesis is that, in addition to producing endothelial dysfunction and many other effects, oxidative stress activates matrix metalloproteinases (MMPs) (32), and may thereby produce vascular rupture and ICH. This mechanism is attractive because activation of MMPs is redox-sensitive (25,32). Furthermore, hypertension (and especially hypertension produced by angiotensin II) increases ROS (33), so there is a milieu that is favorable for generation of ROS in hypertension, with activation of MMPs.
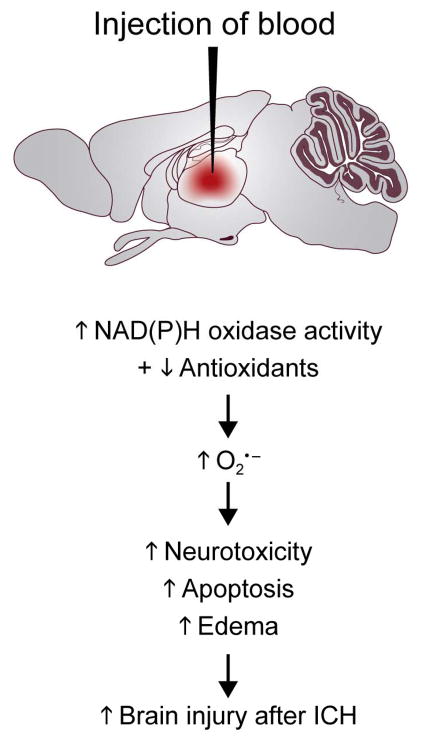
The most frequently used method in experimental animals to examine consequences of ICH is to inject blood or bacterial collagenase (which dissolves extracellular matrix and lamina of blood vessels, and produces ICH) directly into the brain (e.g., 34). The studies suggest that intraparenchymal blood produces an increase in superoxide, and the increase in superoxide is due (in part) to activation of NAD(P)H oxidase (Fig. 3). In addition, it appears that SODs decrease after injection of blood or lysed erythrocytes in the brain. Thus, oxidative stress in response to ICH appears to be produced both by activation of NAD(P)H oxidase and by decreased levels of SODs.
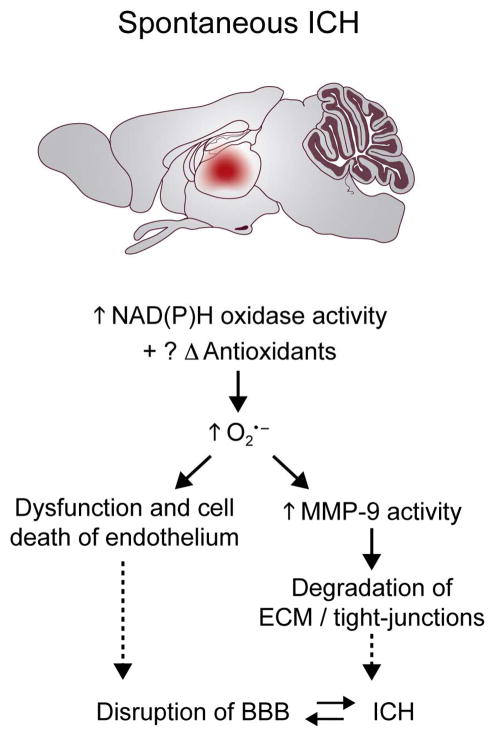
Figure 3.
The most frequently used model of intracranial hemorrhage (ICH) involves injection of blood or collagenase in the striatum. This approach is associated with increased NAD(P)H oxidase activity, reduction of SOD levels, and increased brain injury. We have developed two novel approaches to produce spontaneous ICH in hypertensive mice. NAD(P)H oxidase activity increases before and after spontaneous ICH, but it is not known whether SOD levels change. Oxidative stress activates matrix metalloproteinases (MMPs), and may contribute to both ICH and perhaps disruption of the blood-brain barrier (BBB).
Our studies of spontaneous ICH in hypertensive mice indicate that levels of superoxide in the brain of hypertensive mice increase before ICH, and increase further when mice develop spontaneous ICH (31). The increase in superoxide is due, at least in part, to increased NAD(P)H oxidase activity (Fig. 3). It is not known whether SODs in the brain or its vessels change in response to spontaneous ICH.
Because MMP activity is redox-sensitive, we have measured MMP activity in the brain of hypertensive mice, before and after ICH. Our preliminary data (unpublished) suggest that MMP activity increases in parallel with levels of superoxide: MMP activity increases before spontaneous ICH, and increases further after ICH. Thus, it is likely that oxidative stress, produced by acute increases in pressure during chronic hypertension, increases MMP activity, facilitates proteolysis of extra-cellular matrix in the basement membrane, and contributes to the development of ICH (Fig. 3)
Cause or association?
Our studies indicate that oxidative stress precedes ICH in hypertensive mice, and increases further after ICH. Furthermore, our preliminary data suggest that MMP activity (which is redox-sensitive) increases in parallel with oxidative stress. It is reasonable, therefore, to suggest that oxidative stress may play a key role in ICH during hypertension.
Three approaches will allow a more definitive conclusion about the role of oxidative stress in ICH. First, it will be important to determine which ROS is/are important in ICH. Second, it will be of interest to determine whether an appropriate antioxidant protects against spontaneous ICH. Third, studies of genetically-altered mice in which SODs are overexpressed or knocked out may provide insight into whether oxidative stress is causally related to ICH, or simply an association.
SOD Gene Variant
We have pointed out above that oxidative stress usually is produced by increased generation of superoxide (e.g., by an increase in NAD(P)H oxidase activity, or uncoupling of NOS) and in some disease states (e.g., aortic stenosis and ICH) by decreased levels of SODs. In other research, we have chosen to focus on the role of extracellular SOD (ecSOD), because expression of ecSOD in blood vessels is high, ecSOD protects nitric oxide (NO) against inactivation by superoxide as NO diffuses from endothelium to smooth muscle cells, and ecSOD thereby protects against endothelial dysfunction (35,36).
Gene transfer of ecSOD improves endothelial function in spontaneously hypertensive rats (SHR) and other disease states (e.g., 37,38). Beneficial effects of ecSOD require the heparin-binding domain (37), which mediates binding of ecSOD to heparan sulfate proteoglycans on the cell surface. Bound ecSOD may be endocytosed into cytoplasma, but not translocated to the nucleus (39).
Deletion of the ecSOD gene in mice has little effect under normal conditions. When oxidative stress is increased, however, the gene protects against endothelial dysfunction. Our preliminary data (unpublished) indicate that ecSOD is especially important in protection against endothelial dysfunction in old mice.
We are not aware of a loss-of-function variant of the ecSOD gene in humans. There is however, a gene variant of ecSOD (ecSODR213G) in the heparin-binding domain, which is common, compared with most other gene variants (perhaps 2–5% of humans) (40). The antioxidant activity of ecSOD is not reduced by the ecSODR213G gene variant, but binding to cells is impaired. One might expect, therefore, that the ecSODR213G gene variant would predispose blood vessels to oxidative stress.
Studies of gene variants are of great importance. Science indicated that “human genetic variation” is the “breakthrough of the year” for 2007. We have performed studies to examine mechanisms by which a human gene variant, ecSODR213G, may predispose to disease states.
We (YC) made a recombinant adenovirus that expresses ecSODR213G, and compared vascular effects with those of normal ecSOD (41). Binding to the endothelial cells and aorta was much less by ecSODR213G than by normal ecSOD. The important new finding was that, in contrast to beneficial effects of ecSOD on endothelial function and blood pressure in SHR, ecSODR213G had no significant beneficial effects. Similarly, ecSODR213G failed to protect against endothelial dysfunction in heart failure (38) and after endotoxin (42). The demonstration that ecSODR213G fails to protect against endothelial dysfunction provides a possible mechanism by which the ecSODR213G gene variant may predispose humans to vascular disease (11).
What are the clinical implications of these findings? One study demonstrated, in diabetic patients undergoing hemodialysis, that patients with ecSODR213G had increased mortality from ischemic heart disease (43). In addition, in a large association study, the risk of ischemic heart disease was increased in heterozygotes with ecSODR213G (11).
It was of interest, in the large study of subjects with ecSODR213G, that increased risk of ischemic heart disease was not observed until subjects were greater than 70 years old (11). The finding implies that in humans, as in mice, the protective effect of ecSOD is limited until old age, when oxidative stress increases or when cumulative effects of oxidative stress become evident.
As DNA sequencing becomes more efficient, additional human gene variants are being uncovered in large populations (e.g., Ref. 44). Studies in experimental animals of human gene variants will continue to provide mechanistic understanding into physiological function of the genes.
Summary
As illustrated in Figure 1 oxidative stress may contribute to a variety of important cardiovascular diseases. It is quite remarkable that mechanisms that can produce oxidative stress are so diverse, e.g., increased NAD(P)H oxidase activity in arterial plaques and in relation to intracranial hemorrhage, but not in aortic valve stenosis.
It is disappointing that antioxidant vitamins have failed to reduce the risk of cardiovascular diseases. But, considering the complexity and diversity of mechanisms associated with oxidative stress in different cardiovascular diseases, we believe that it is unlikely that non-targeted antioxidant therapy (in terms of specific ROS and subcellular compartmentalization) will prove to be efficacious.
In future studies, it will continue to be of great importance to address three questions. First, what enzymatic mechanisms account for oxidative stress in cardiovascular diseases? Second, based on appropriately targeted antioxidant interventions, is oxidative stress an important cause of cardiovascular disease in experimental animals? Third, with better understanding of mechanisms, will antioxidants be useful in prevention or treatment of cardiovascular diseases in humans?
Figure 4.
As proposed initially by Oury and Crapo (36), ecSOD in blood vessels protects NO from inactivation by superoxide during diffusion from endothelium to smooth muscle cells. Impairment of binding of the ecSODR213G gene variant to vascular cells may lead to failure of this mechanism, increased oxidative stress in arteries, and is associated with increased risk of ischemic heart disease.
Acknowledgments
We thank Shinichiro Iida, Masuo Ohashi, Donald Lund, Frank Faraci, Gary Baumbach, Robert Weiss, and Robert Brooks, who participated importantly in studies described in this review. We thank Arlinda LaRose for typing the manuscript many times, and Teresa Ruggle for preparing the figures. Studies described in this review were supported by National Institutes of Health grants HL-62984 and NS-24621, and funds provided by a Carver Research Program of Excellence.
References
- 1.Griendling KK, Fitzgerald GA. Oxidative stress and cardiovascular injury. Animal and human studies. Circulation. 2003;108:2034–2040. doi: 10.1161/01.CIR.0000093661.90582.c4. [DOI] [PubMed] [Google Scholar]
- 2.Heistad DD. Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol. 2006;26:689–695. doi: 10.1161/01.ATV.0000203525.62147.28. [DOI] [PubMed] [Google Scholar]
- 3.Chen K, Thomas SR, Keaney JF., Jr Beyond LDL oxidation: ROS in vascular signal transduction. Free Radic Biol Med. 2003;35:117–132. doi: 10.1016/s0891-5849(03)00239-9. [DOI] [PubMed] [Google Scholar]
- 4.Dröge W. Free radicals in physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 5.Arimura K, Egashira K, Nakamura R, Ide T, Tsutsui H, Shimokawa H, et al. Increased inactivation of nitric oxide is involved in coronary endothelial dysfunction in heart failure. Am J Physiol Heart. 2001;280:H68–H75. doi: 10.1152/ajpheart.2001.280.1.H68. [DOI] [PubMed] [Google Scholar]
- 6.Morikawa K, Shimokawa H, Matoba T, Kubtoa H, Akaike T, Talukder MAH, et al. Pivotal role of Cu, Zn-superoxide dismutase in endothelium-dependent hyperpolarization. J Clin Invest. 2003;112:1871–1879. doi: 10.1172/JCI19351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leopold JA, Loscalzo J. Oxidative enzymopathies and vascular disease. Arterioscler Thromb Vasc Biol. 2005;25:1332–1340. doi: 10.1161/01.ATV.0000163846.51473.09. [DOI] [PubMed] [Google Scholar]
- 8.Zhao X, Sun G, Zhang J, Strong R, Dash PK, Kan YW, et al. Transcription factor Nrf2 protects the brain from damage produced by intracerebral hemorrhage. Stroke. 2007;38:3280–3286. doi: 10.1161/STROKEAHA.107.486506. [DOI] [PubMed] [Google Scholar]
- 9.St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jäger S, et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcription coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 10.Faraci FM, Didion SP. Vascular Protection: Superoxide dismutase isoforms in the vessel wall. Arterioscler Thromb Vasc Biol. 2004;24:1367–1373. doi: 10.1161/01.ATV.0000133604.20182.cf. [DOI] [PubMed] [Google Scholar]
- 11.Juul K, Tybjaerg-Hansen A, Marklund S, Heegaard NH, Steffensen R, Sillesen H, et al. Genetically reduced anti-oxidative protection and increased ischemic heart disease risk: The Copenhagen City Heart Study. Circulation. 2004;109:59–65. doi: 10.1161/01.CIR.0000105720.28086.6C. [DOI] [PubMed] [Google Scholar]
- 12.Jialal I, Devaraj S. Antioxidants and atherosclerosis: Don’t throw out the baby with the bath water (Editorial) Circulation. 2003;107:926–928. doi: 10.1161/01.cir.0000048966.26216.4c. [DOI] [PubMed] [Google Scholar]
- 13.Rajamannan NM, Bonow RO, Rahimtoola SH. Calcific aortic stenosis: An update. Nat Clin Pract Cardiovasc Med. 2007;4:254–262. doi: 10.1038/ncpcardio0827. [DOI] [PubMed] [Google Scholar]
- 14.Otto CM, Kuusisto J, Reichenbach DD, Gown AM, O’Brien KD. Characterization of the early lesion of ‘degenerative’ valvular aortic stenosis. Histological and immunohistochemical studies. Circulation. 1994;90:844–853. doi: 10.1161/01.cir.90.2.844. [DOI] [PubMed] [Google Scholar]
- 15.Lindroos M, Kupari M, Valvanne J, Strandberg T, Heikkila J, Tilvis R. Factors associated with calcific aortic valve degeneration in the elderly. Eur Heart J. 1994;15:865–870. doi: 10.1093/oxfordjournals.eurheartj.a060602. [DOI] [PubMed] [Google Scholar]
- 16.Weiss RM, Ohashi M, Miller JD, Young SG, Heistad DD. Calcific aortic valve stenosis in old hypercholesterolemic mice. Circulation. 2006;114:2065–2069. doi: 10.1161/CIRCULATIONAHA.106.634139. [DOI] [PubMed] [Google Scholar]
- 17.Miller JD, Chu Y, Brooks RM, Richenbacher WE, Peña-Silva R, Heistad DD. Dysregulation of antioxidant mechanisms contributes to increased oxidative stress in calcific aortic valvular stenosis in humans. J Amer Coll Cardiol. 2008;52:843–850. doi: 10.1016/j.jacc.2008.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liberman M, Bassi E, Martinatti MK, Lario FC, Wosniak J, Jr, Pomerantzeff PM, et al. Oxidant generation predominates around calcifying foci and enhances progression of aortic valve calcification. Arterioscler Thromb Vasc Biol. 2008;28:463–470. doi: 10.1161/ATVBAHA.107.156745. [DOI] [PubMed] [Google Scholar]
- 19.Vasquez-Vivar J, Kalyanaraman B, Martasek P, Hogg N, Masters BS, Karoui H, et al. Superoxide generation by endothelial nitric oxide synthase: The influence of cofactors. Proc Natl Acad Sci USA. 1998;95:9220–9225. doi: 10.1073/pnas.95.16.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milstien S, Katusic Z. Oxidation of tetrahydrobiopterin by peroxynitrite: implications for vascular endothelial function. Biochem Biophys Res Commun. 1999;263:681–684. doi: 10.1006/bbrc.1999.1422. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi S, Inoue N, Azumi H, Seno T, Hirata K, Kawashima S, et al. Expressional changes of the vascular antioxidant system in atherosclerotic coronary arteries. J Atheroscler Thromb. 2002;9:184–190. doi: 10.5551/jat.9.184. [DOI] [PubMed] [Google Scholar]
- 22.Towler DA. Oxidation, inflammation, and aortic valve calcification: Peroxide paves an osteogenic path. J Am Coll Cardiol. 2008;52:851–854. doi: 10.1016/j.jacc.2008.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mody N, Parhami F, Sarafian TA, Demer LL. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic Biol Med. 2001;31:509–519. doi: 10.1016/s0891-5849(01)00610-4. [DOI] [PubMed] [Google Scholar]
- 24.Shao JS, Aly ZA, Lai CF, Cheng SL, Cai J, Huang E, et al. Vascular Bmp Msx2 Wnt signaling and oxidative stress in arterial calcification. Ann N Y Acad Sci. 2007;1117:40–50. doi: 10.1196/annals.1402.075. [DOI] [PubMed] [Google Scholar]
- 25.Rajagopalan S, Meng XP, Ramasamy S, Harrison DG, Galis ZS. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro: Implications for atherosclerotic plaque stability. J Clin Invest. 1996;98:2572–2579. doi: 10.1172/JCI119076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cowell SJ, Newby DE, Prescott RJ, Bloomfield P, Reid J, Northridge DB, et al. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med. 2005;352:2389–2397. doi: 10.1056/NEJMoa043876. [DOI] [PubMed] [Google Scholar]
- 27.Rossebø AB, Pedersen TR, Boman K, Brudi P, Chambers JB, Egstrup K, et al. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359:1343–1356. doi: 10.1056/NEJMoa0804602. [DOI] [PubMed] [Google Scholar]
- 28.Miller JD, Berry CJ, Serrano KM, Weiss RM, Iyengar NK, Zimmerman KA, et al. Effects of normalizing hyperlipidemia on oxidative stress and disease progression in a mouse model of aortic valve stenosis. Circulation. 2007;116:II–52. (Abstract) [Google Scholar]
- 29.Pena-Silva RA, Miller JD, Heistad DD. Serotonin produces MAO dependent oxidative stress in human heart valves. FASEB J. 2008;22:747.6. doi: 10.1152/ajpheart.00570.2009. (Abstract) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iida S, Baumbach GL, Lavoie JL, Faraci FM, Sigmund CD, Heistad DD. Spontaneous stroke in a genetic model of hypertension in mice. Stroke. 2005;36:1253–1258. doi: 10.1161/01.str.0000167694.58419.a2. [DOI] [PubMed] [Google Scholar]
- 31.Wakisaka Y, Miller JD, Chu Y, Baumbach GL, Wilson S, Faraci FM, et al. Oxidative stress through activation of NAD(P)H oxidase in hypertensive mice with spontaneous intracranial hemorrhage. J Cereb Blood Flow Metab. 2008;28:1175–1185. doi: 10.1038/jcbfm.2008.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu F, Kamada H, Niizuma K, Endo H, Chan PH. Induction of MMP-9 expression and endothelial injury by oxidative stress after spinal cord injury. J Neurotrauma. 2008;25:184–195. doi: 10.1089/neu.2007.0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zimmerman MC, Lazartigues E, Sharma RV, Davisson RL. Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous sytem. Circ Res. 2004;95:210–215. doi: 10.1161/01.RES.0000135483.12297.e4. [DOI] [PubMed] [Google Scholar]
- 34.Tang J, Liu J, Zhou C, Ostanin D, Grisham MB, Granger ND, et al. Role of NADPH oxidase in the brain injury of intracerebral hemorrhage. J Neurochem. 2005;94:1342–1350. doi: 10.1111/j.1471-4159.2005.03292.x. [DOI] [PubMed] [Google Scholar]
- 35.Marklund SL. Extracellular superoxide dismutase and other superoxide dismutase isoenzymes in tissues from nine mammalian species. Biochem J. 1984;222:649–655. doi: 10.1042/bj2220649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oury TD, Day BJ, Crapo JD. Extracellular superoxide dismutase: A regulator of nitric oxide bioavailability. Lab Invest. 1996;75:617–636. [PubMed] [Google Scholar]
- 37.Chu Y, Iida S, Lund DD, Weiss RM, DiBona GF, Watanabe Y, et al. Gene transfer of extracellular superoxide dismutase reduces arterial pressure in spontaneously hypertensive rats: Role of heparin binding domain. Circ Res. 2003;92:461–468. doi: 10.1161/01.RES.0000057755.02845.F9. [DOI] [PubMed] [Google Scholar]
- 38.Iida S, Chu Y, Weiss RM, Kang Y-M, Faraci FM, Heistad DD. Vascular effects of a common gene variant of extracellular superoxide dismutase in heart failure. Am J Physiol Heart. 2006;291:H914–920. doi: 10.1152/ajpheart.00080.2006. [DOI] [PubMed] [Google Scholar]
- 39.Chu Y, Piper R, Richardson S, Watanabe Y, Patel P, Heistad DD. Endocytosis of extracellular superoxide dismutase into endothelial cells: role of the heparin-binding domain. Arterioscler Thromb Vasc Biol. 2006;26:1985–1990. doi: 10.1161/01.ATV.0000234921.88489.5c. [DOI] [PubMed] [Google Scholar]
- 40.Folz RJ, Peno-Green L, Crapo JD. Identification of a homozygous missense mutation (Arg to Gly) in the critical binding region of human EC-SOD gene (SOD3) and its association with dramatically increased serum enzyme levels. Hum Mol Genet. 1994;3:2251–2254. doi: 10.1093/hmg/3.12.2251. [DOI] [PubMed] [Google Scholar]
- 41.Chu Y, Alwahdani A, Iida S, Lund DD, Faraci FM, Heistad DD. Vascular effects of human extracellular SODR213G variant. Circulation. 2005;112:1047–1053. doi: 10.1161/CIRCULATIONAHA.104.531251. [DOI] [PubMed] [Google Scholar]
- 42.Lund DD, Chu Y, Brooks RM, Faraci FM, Heistad DD. Effects of a common human gene variant of ECSOD on endothelial function after endotoxin in mice. J Physiol (London) 2007;584.2:583–590. doi: 10.1113/jphysiol.2007.140830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamada H, Yamada Y, Adachi T, Fukatsu A, Sakuma M, Futenma A, et al. Protective role of extracellular superoxide dismutase in hemodialysis patients. Nephron. 2000;84:218–223. doi: 10.1159/000045580. [DOI] [PubMed] [Google Scholar]
- 44.Dahl M, Bowler RP, Juul K, Crapo JD, Levy S, Nordestgaard BG. Superoxide dismutase 3 polymorphism associated with reduced lung function in two large populations. Am J Respir Crit Care Med. 2008 doi: 10.1164/rccm.200804-549OC. [DOI] [PMC free article] [PubMed] [Google Scholar]