A relatively recent concept is that vascular dysfunction plays a key role in cognitive impairment, as well as stroke. Impaired neurovascular coupling, probably in part through activation of the angiotensin-1 (AT1) receptor, is central to cerebrovascular dysfunction.1 Reactive oxygen species (ROS) clearly are important mediators of the deleterious vascular effects of angiotensin II. The evidence seemed to favor the concept that angiotensin II, perhaps through activation of NAD(P)H oxidase, releases superoxide, which scavenges nitric oxide to produce cerebral vascular dysfunction.1
But just when we thought that we understood mechanisms by which angiotensin II produces cerebrovascular dysfunction, Capone et al. in this issue present compelling evidence that products of cyclooxygenase (COX) metabolism are important facilitating factors for angiotensin II signaling in cerebral blood vessels. The authors report that prostaglandin E2 (PGE2) and the type 1 PGE2 (EP1) receptor, are required for endothelial dysfunction and impaired neurovascular coupling induced by acute administration of angiotensin II.2 Because the hypothesis is novel and important for our understanding of angiotensin II effects, it is desirable to have multiple lines of evidence to support the conclusion. This indeed the authors have accomplished, as they use several genetically altered mice and pharmacological inhibitors to build their case.
EP1 Receptors and COX-1 in angiotensin II-induced vascular dysfunction
COX-1 is involved in synthesis and release of an endothelium-derived contracting factor.3 Pressor responses to angiotensin are attenuated in COX-1 knockout mice and in mice treated with a COX-1 inhibitor, whereas opposite effects (augmentation of responses to angiotensin) are found with COX-2 inhibitors.4 Interestingly, angiotensin increases COX-1 and decreases COX-2 expression in murine mesenteric arteries, through an ROS-independent mechanism.5 These findings suggest that a product of arachidonic acid metabolism, through COX-1, interacts with angiotensin to induce hypertension.
Similarly, several studies have implicated a prostanoid receptor in vascular dysfunction produced by angiotensin. SQ29548, a thromboxane A and EP1 receptor antagonist, attenuates vascular dysfunction induced by angiotensin.5 Moreover, in mice deficient in thromboxane or EP1 receptors, mortality and the pressor response to infusion of angiotensin are attenuated.6,7
Capone et al.2 demonstrates that COX-1-dependent formation of PGE2 and the EP1 receptor are necessary for angiotensin II-induced impairment in neurovascular coupling and cerebrovascular dysfunction. The authors demonstrated that COX-1 and the EP1 receptor are expressed in microglia and blood vessels respectively. Cerebrovascular dysfunction and impaired neurovascular coupling after an acute infusion of angiotensin II are attenuated in mice treated with a COX-1 inhibitor, but not a COX-2 inhibitor, and in mice deficient in COX-1. Angiotensin II did not increase PGE2 synthesis in brain, but PGE2 superfusion restored susceptibility of cerebral vessels to angiotensin II-induced dysfunction after COX-1 inhibition.
In addition, effects of angiotensin II are reduced in mice treated with an EP-1 receptor antagonist and in EP-1 knockout mice. EP1 receptors are also required for angiotensin II-induced increase of ROS. Therefore, PGE2 (a COX-1 product) and EP1 receptors are required for deleterious effects of angiotensin II in the cerebral circulation.
EP1 receptors are important for the vascular effects of angiotensin II, but what is the mechanism?
Several concurrent mechanisms may explain the crosstalk between EP1 and angiotensin II receptors (Fig. 1). First, Capone et al.2 propose that EP1 receptor activation may increase intracellular calcium concentrations and facilitate activation of the NAD(P)H oxidase. Different results have been published before, where PGE2 attenuated the increase in intracellular calcium induced by angiotensin II in smooth muscle cells from rat preglomerular arterioles8. Second, activation of EP1 receptors may modulate the activity of regulatory kinases or regulators of G protein signaling (RGS proteins) to facilitate intracellular signaling in response to angiotensin II. Thus, angiotensin II may work synergistically with prostaglandins to induce vascular dysfunction. Third, we speculate that EP1 receptors may physically regulate the activity of AT1 receptors through heterodimerization. Both angiotensin and PGE2 receptors are G protein-coupled receptors (GPCR), and it is now known that heterodimerization of GPCRs alters the trafficking and activity of receptors at the plasma membrane.9 For example, AT1 and EP1 heterodimerize and modulate the activity of β adrenergic receptors.9 It is not known, however, if angiotensin II receptors dimerize with EP1 receptors. Finally, it is possible that facilitation of angiotensin II signaling by PGE2 exists only in the context of increased blood pressure.10 It would be of interest to know if EP1 receptor facilitation of angiotensin II-induced vascular dysfunction is present in the absence of hypertension, for example, during non-pressor doses of angiotensin II in vivo.
Figure 1.
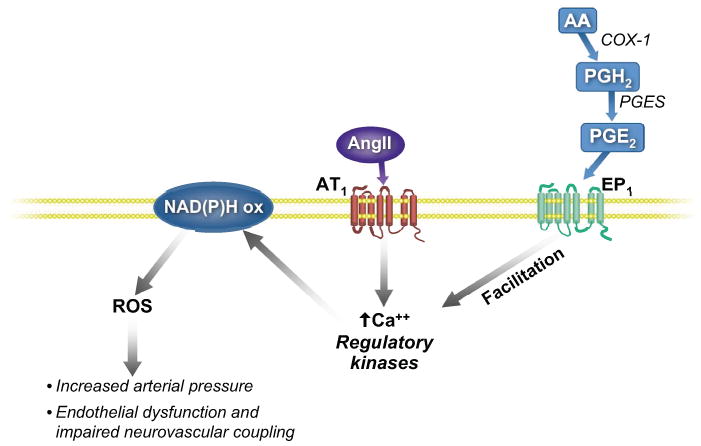
Prostaglandin E2 (PGE2) is a product of metabolism of arachidonic acid (AA) by cyclooxygenase I (COX-1) and the prostaglandin E synthase (PGES), perhaps in microglia. Activation of prostaglandin EP1 receptors by PGE2 on cerebral blood vessels, may increase the intracellular concentrations of calcium (Ca++) or facilitate the activation of regulatory kinases by angiotensin II (Ang II) type I (AT1) receptors, which are required for activation of the NAD(P)H oxidase. Reactive oxygen species (ROS) derived from the active NAD(P)H oxidase, are associated with increased arterial pressure, endothelial dysfunction, and impaired neurovascular coupling.
Is prostaglandin signaling detrimental for cerebral vascular function?
Although the observation that PGE2 contributes to angiotensin-induced cerebral vascular dysfunction is convincing,2 several other lines of evidence point towards a beneficial role of PGE2 and EP1 receptors in vascular function. Mice deficient in microsomal PGE2 synthase have a profound pressor response and increased oxidative stress in aorta after treatment with non-pressor doses of angiotensin.10 Moreover, PGE2 evoked dilation of mouse basilar artery, ex vivo.11 EP1 receptors also facilitate NO release during neurogenic vasodilation in porcine basilar arteries.12 Finally, in a rat model of hypertension associated with increased formation of angiotensin II, COX 1 inhibition did not attenuate vascular dysfunction or hypertension.13 The conflicting results may be explained by differences in model (in vivo vs. ex vivo preparations), vascular bed (cerebral cortical vessels vs. basilar arteries or systemic vessels), and duration of treatment (acute vs. chronic). Therefore, studies are needed to clarify the protective or deleterious effects of different isoforms of PGE2 synthase and EP1 receptors in function of cerebral and other vessels during chronic infusions of angiotensin II.
Perspective
Capone et al.2 provide evidence for a crucial role of prostanoid metabolism and signaling in regulation of cerebrovascular function. Inhibition of COX-1 or EP1 receptors attenuated the deleterious vascular effects of angiotensin II. Development of specific PGES inhibitors might be a therapeutic target for cerebrovascular disease. It will be of interest to clarify mechanisms responsible for crosstalk between prostaglandin and angiotensin II pathways especially in chronic hypertension.
Acknowledgments
We thank Drs. Mark Chappell, Frank Faraci and Rory Fisher for their valuable suggestions.
Sources of Funding: This work was supported by a Fulbright Scholarship and AHA Predoctoral Fellowship 0815525G (R. Pena-Silva), a Carver Program of Excellence, and NIH grants NS-24621 and HL-62984 (D. Heistad).
Footnotes
Disclosures: No relevant conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Chrissobolis S, Faraci FM. The role of oxidative stress and NADPH oxidase in cerebrovascular disease. Trends Mol Med. 2008;14:495–502. doi: 10.1016/j.molmed.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Capone C, Faraco G, Anrather J, Zhou P, Iadecola C. Cox-1-derived prostaglandin E2 and EP1 receptors are required for the cerebrovascular dysfunction induced by angiotensin II. Hypertension. 2010 doi: 10.1161/HYPERTENSIONAHA.109.145813. current issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang D, Feletou M, Levens N, Zhang JN, Vanhoutte PM. A diffusible substance(s) mediates endothelium-dependent contractions in the aorta of SHR. Hypertension. 2003;41:143–148. doi: 10.1161/01.hyp.0000047651.45322.16. [DOI] [PubMed] [Google Scholar]
- 4.Qi Z, Hao CM, Langenbach RI, Breyer RM, Redha R, Morrow JD, Breyer MD. Opposite effects of cyclooxygenase-1 and -2 activity on the pressor response to angiotensin II. J Clin Invest. 2002;110:61–69. doi: 10.1172/JCI14752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Virdis A, Colucci R, Fornai M, Duranti E, Giannarelli C, Bernardini N, Segnani C, Ippolito C, Antonioli L, Blandizzi C, Taddei S, Salvetti A, Del TM. Cyclooxygenase-1 is involved in endothelial dysfunction of mesenteric small arteries from angiotensin II-infused mice. Hypertension. 2007;49:679–686. doi: 10.1161/01.HYP.0000253085.56217.11. [DOI] [PubMed] [Google Scholar]
- 6.Francois H, Athirakul K, Mao L, Rockman H, Coffman TM. Role for thromboxane receptors in angiotensin-II-induced hypertension. Hypertension. 2004;43:364–369. doi: 10.1161/01.HYP.0000112225.27560.24. [DOI] [PubMed] [Google Scholar]
- 7.Guan Y, Zhang Y, Wu J, Qi Z, Yang G, Dou D, Gao Y, Chen L, Zhang X, Davis LS, Wei M, Fan X, Carmosino M, Hao C, Imig JD, Breyer RM, Breyer MD. Antihypertensive effects of selective prostaglandin E2 receptor subtype 1 targeting. J Clin Invest. 2007;117:2496–2505. doi: 10.1172/JCI29838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Purdy KE, Arendshorst WJ. Prostaglandins buffer ANG II-mediated increases in cytosolic calcium in preglomerular VSMC. Am J Physiol. 1999;277:F850–F858. doi: 10.1152/ajprenal.1999.277.6.F850. [DOI] [PubMed] [Google Scholar]
- 9.Barnes PJ. Receptor heterodimerization: a new level of cross-talk. J Clin Invest. 2006;116:1210–1212. doi: 10.1172/JCI28535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jia Z, Guo X, Zhang H, Wang MH, Dong Z, Yang T. Microsomal prostaglandin synthase-1-derived prostaglandin E2 protects against angiotensin II-induced hypertension via inhibition of oxidative stress. Hypertension. 2008;52:952–959. doi: 10.1161/HYPERTENSIONAHA.108.111229. [DOI] [PubMed] [Google Scholar]
- 11.Fang X, Faraci FM, Kaduce TL, Harmon S, Modrick ML, Hu S, Moore SA, Falck JR, Weintraub NL, Spector AA. 20-Hydroxyeicosatetraenoic acid is a potent dilator of mouse basilar artery: role of cyclooxygenase. Am J Physiol Heart Circ Physiol. 2006;291:H2301–H2307. doi: 10.1152/ajpheart.00349.2006. [DOI] [PubMed] [Google Scholar]
- 12.Jadhav V, Jabre A, Chen MF, Lee TJ. Presynaptic prostaglandin E2 EP1-receptor facilitation of cerebral nitrergic neurogenic vasodilation. Stroke. 2009;40:261–269. doi: 10.1161/STROKEAHA.108.516104. [DOI] [PubMed] [Google Scholar]
- 13.Cheng ZJ, Finckenberg P, Louhelainen M, Merasto S, Tikkanen I, Vapaatalo H, Mervaala EM. Cardiovascular and renal effects of cyclooxygenase inhibition in transgenic rats harboring mouse renin-2 gene (TGR[mREN2]27) Eur J Pharmacol. 2003;461:159–169. doi: 10.1016/s0014-2999(03)01307-4. [DOI] [PubMed] [Google Scholar]


