Abstract
Although TLR9 was originally thought to specifically recognize microbial DNA, it is now evident that mammalian DNA can be an effective TLR9 ligand. However, the DNA sequence required for TLR9 activation is controversial as studies have shown conflicting results depending on the nature of the DNA backbone, the route of DNA uptake, and the cell type being studied. In systemic lupus erythematosus (SLE), a major route whereby DNA gains access to intracellular TLR9, and thereby activates dendritic cells (DC), is through uptake as a DNA-containing immune complex (IC). In this report, we used defined dsDNA fragments with a natural (phosphodiester) backbone and show that unmethylated cytosine-guanosine (CpG) dinucleotides within dsDNA are required for murine DC TLR9 activation induced by DNA-containing IC. The strongest activation is seen with dsDNA fragments containing optimal CpG motifs (purine-purine-CpG-pyrimidine-pyrimidine) that are common in microbial DNA but rare in mammalian DNA. Importantly however, activation can also be induced by CpG-rich DNA fragments that lack these optimal CpG motifs and which we show are plentiful in CpG islands within mammalian DNA. No activation is induced by DNA fragments lacking CpG dinucleotides, although this CpG-free DNA can induce DC activation if internalized by liposomal transfection instead of as an IC. Overall, the data suggest that the release of CpG-rich DNA from mammalian DNA may contribute to the pathogenesis of autoimmune diseases such as SLE and psoriasis in which activation of TLR9 in DC by self-DNA has been implicated in disease pathogenesis.
Keywords: autoimmunity, cytokines, dendritic cells, rodent, systemic lupus erythematosus
Introduction
Toll-like receptor 9 (TLR9) was identified as the receptor for oligodeoxynucleotides (ODN) containing unmethylated cytosine-guanosine (CpG) dinucleotides and was originally thought to distinguish pathogen DNA from that of the mammalian host on this basis, as unmethylated CpG dinucleotides are relatively uncommon in mammalian DNA compared with pathogen DNA (1, 2). This discrimination could be further refined by the identification of so-called CpG motifs, where the particular pattern of bases flanking the CpG dinucleotide appeared to further distinguish microbial and mammalian DNA. However, subsequent studies revealed specific instances in which mammalian DNA proved to be a highly effective TLR9 ligand (3–7) and pointed to a critical role for mammalian DNA in the pathogenesis of certain autoimmune diseases including systemic lupus erythematosus (SLE) and psoriasis (8, 9).
As TLR9 is located intracellularly, it is necessary for the DNA to be internalized before it can engage TLR9 (10, 11). This intracellular localization serves to facilitate TLR9 access to pathogen DNA and at the same time restrict TLR9 access to self DNA (6). However in SLE, DNA-containing immune complexes (IC) can activate plasmacytoid DC (pDC) to produce interferon-alpha (IFN-α), a cytokine thought to be important in disease pathogenesis (12). This occurs by uptake and internalization of the IC by a cell surface Fcγ receptor on the DC with subsequent delivery of the DNA to intracellular TLR9 (4, 5, 13). Similarly, autoreactive B cells can be activated through TLR9 by B cell receptor-mediated internalization of mammalian DNA (3, 14). In the autoimmune skin disease psoriasis, the antimicrobial peptide LL37 (also known as CAMP) is released from neutrophils and epithelial cells in response to bacterial infection (7). Self-DNA from apoptotic or necrotic cells in damaged skin binds to the peptide and the peptide-DNA complex can then be internalized and trigger IFN-α production by pDC through TLR9.
The ability of mammalian DNA to activate TLR9 raises questions as to the nature of the DNA sequence involved in this activation. This is controversial, with conflicting results being reported depending on the nature of the DNA backbone, the cell type being studied, and the route of DNA uptake by the cell. The large majority of studies examining the structure-activity relationship of DNA sequence and TLR9 activation have been done using synthetic ODN protected from DNase degradation by the use of phosphorothioate linkages (2, 15). These studies found that unmethylated CpG dinucleotides were important for activation, and also demonstrated that different CpG motifs elicited varying degrees of TLR9 activation.
A CpG motif is defined as the hexamer comprising the two bases to the 5’ and 3’ sides of the unmethylated CpG dinucleotide as well as the CpG dinucleotide itself (16). The optimal CpG motif is species-specific but for mouse TLR9 is purine-purine-CG-pyrimidine-pyrimidine (2). However, the phosphorothioate backbone itself results in a large number of sequence-independent effects (2, 15) and it is thus possible that the DNA sequence requirements for TLR9 activation determined using phosphorothioate ODN may not necessarily reflect the DNA sequences required for TLR9 activation by pathogen or mammalian DNA which utilize natural phosphodiester linkages (17). Indeed, phosphodiester-linked ODNs lacking CpG dinucleotides can have immunostimulatory effects when used at very high concentration (18), or when the cationic lipid transfection reagent N-[1-(2,3-Dioleoyloxy)propyl]-N,N,N-trimethylammonium methylsulfate (DOTAP) is used to increase the amount of ODN internalized and to enforce endosomal translocation of the ODN (19, 20).
A recent study has shown that when phosphodiester ODNs are internalized into DC by DOTAP or by the addition of 3’-poly G tails to enforce endosomal translocation, TLR9 is activated in a CpG-independent manner on the basis of the inherent stimulatory potential of the phosphodiester 2’ deoxyribose backbone itself (21). This specificity analysis was further extended to elegant binding studies dependent on recombinant proteins that incorporated the entire TLR9 ectodomain. On the basis of these results, it was proposed that the CpG-motif dependency of TLR9 activation is restricted to phosphorothioate-modified synthetic DNA (21, 22).
This conclusion differs from that reached in murine B cell studies where TLR9 activation by phosphodiester-linked DNA internalized through the B cell receptor showed a strong preference for unmethylated CpG dinucleotides (14, 23). It is not clear whether this difference reflects an intrinsic difference between TLR9 activation in DC and B cells or whether it is due to the particular routes of DNA internalization. Moreover, the relevance of the binding studies is confounded by recent reports demonstrating that CpG ODNs bind better to a TLR9 cleavage product than to full-length TLR9 (24, 25). Therefore, the importance of CpG content in TLR9 activation of DC when phosphodiester-linked dsDNA is internalized by physiological routes, such as IC uptake via the FcγR, remains controversial. This is an important issue to resolve as it addresses the fundamental question of whether TLR9 detection of mammalian DNA depends on TLR9 location or DNA sequence. If the latter is the case, it then becomes necessary to understand how the particular conditions associated with autoimmune diseases such as SLE and psoriasis enrich for the availability of these stimulatory DNA sequences.
To address these questions in this study, we determined the ability of dsDNA fragments of defined sequence and methylation status to activate TLR9 in murine DC after internalization in the form of an IC. This activation was also compared to that elicited following DNA fragment uptake by liposomal transfection. In addition, the stimulatory capacity of DNA fragments cloned from CpG islands within the mammalian genome was evaluated.
Materials and Methods
Mice
BALB/c wild-type mice were purchased from the Jackson Laboratory. TLR9-deficient mice were backcrossed 11 generations onto the BALB/c genetic background. All mice were maintained at the Boston University School of Medicine Laboratory Animal Sciences Center or at Charles River Laboratories in accordance with the regulations of the American Association for the Accreditation of Laboratory Animal Care. All experimental procedures were approved by the Institutional Animal Care and Use Committee at Boston University School of Medicine.
Reagents
CpG-A ODN 2336 (GGg gac gac gtc gtc gtg GGGGGG) was purchased from Coley Pharmaceutical Group. Capital letters indicate a phosphorothioate backbone, and lowercase letters indicate a phosphodiester backbone. LPS was purchased from Invivogen.
DNA fragments
The CG50 fragment, CGSubOp fragment, and CGneg fragment (Table I) were purified by digestion with BamHI and EcoRI of pLIT-CG50.1 plasmid DNA, pLIT-HIV (CG+) plasmid DNA and pUC-HIV(CG−) plasmid DNA, respectively (14). For the methylation studies, DNA fragments were methylated at their CG residues using M.SssI methylase (CpG methylase), or at their AGCT residues using Alu I methylase (New England BioLabs). To confirm the completeness of methylation induced by M.SssI methylase, the methylated DNA fragments were digested with the methylation sensitive restriction enzyme, HpyCH4IV (New England BioLabs) (14). For studies requiring biotinylated fragments, biotinylation of CG50, CGSubOp, and CGneg was performed by digestion of the fragments with EcoRI and BamHI, followed by filling in 5’ and 3’ overhangs with Klenow polymerase in the presence of biotin-16-2’-deoxy-uridine-5’-triphosphate (Roche). CpG-free pCpG-mcs DNA (Invivogen) was purified using the Qiagen Endfree plasmid kit (Qiagen). The pCpG-mcs DNA was further purified by equilibrium centrifugation in cesium chloride- ethidium bromide gradients. Preparation of the CpG island dsDNA fragments and the small ubiquitin-related modifier (SUMO) dsDNA fragment by PCR has been described in detail previously (23). Biotinylation of these fragments was performed by digestion of the fragments with EcoRI, followed by filling in 5’overhangs with Klenow polymerase in the presence of biotin-16-2’-deoxy-uridine-5’-triphosphate (Roche). Primers and enzymes were removed from all DNAs using the DNA Clean & Concentrator-25™ kit (Zymoresearch). Endotoxin levels in the DNA fragment preparations were measured using the Limulus amebocyte lysate assay (Cambrex) and were less than 0.1 EU/ml.
Table I.
DNA fragments used in this study
| Size (bp) | % GC | Optimala CpG |
Non- optimalb CpG |
M.SssI site (cg) |
Alu I site (agct) |
|
|---|---|---|---|---|---|---|
| CG50 | 607 | 52.0 | 50 | 0 | 50 | 0 |
| CGSubOp | 727 | 52.8 | 0 | 27 | 27 | 6 |
| CGneg | 629 | 42.4 | 0 | 0 | 0 | - |
| pCpG-mcs | 3070 | 38.9 | 0 | 0 | 0 | - |
| CpG island Clone-11 |
573 | 58.6 | 2 | 42 | 44 | - |
| CpG island Clone-12 |
453 | 52.5 | 0 | 19 | 19 | - |
| CpG island Clone-14 |
597 | 49.6 | 1 | 38 | 39 | - |
| CpG island Clone-15 |
587 | 56.7 | 3 | 34 | 37 | - |
| CpG island Clone-23 |
574 | 52.6 | 0 | 40 | 40 | - |
| SUMO | 618 | 33.0 | 0 | 1 | 1 | - |
Optimal CpG: purine-purine-unmethylated cytosine–guanine-pyrimidine-pyrimidine
Non-optimal CpG: unmethylated cytosine–guanine, with flanking bases different from optimal CpG motif.
Antibodies
The anti-DNA monoclonal antibody (mAb) PA4 (26) and the anti-biotin mAb 1D4 (23) have been described previously. The mAbs were purified using a protein A (GE Healthcare) affinity column. Endotoxin levels in the mAb preparations were less than 0.1 EU/ml.
Formation of DNA immune complexes and DNA-DOTAP complexes
DNA IC were formed by incubating various concentrations of the DNA fragments with the anti-DNA mAb PA4 in complete RPMI 1640 medium (10% fetal bovine serum, 2 mM L-glutamine, 50 µM 2-mercaptoethanol, 100 U/ml penicillin and 100 µg/ml streptomycin) for 1 h at 37°C prior to addition of the DNA IC to the DC cultures. Biotinylated DNA IC were formed by incubating various concentrations of the biotinylated DNA fragments with the anti-biotin mAb 1D4 in complete RPMI 1640 medium for 1 h at 37°C prior to addition of the biotinylated DNA IC to the DC cultures.
DNA-DOTAP complexes were formed by incubating the DNA fragments with DOTAP (Karl-Roth) at a DNA: DOTAP weight ratio of 1:2 in 50 µl of 150 mM NaCl, pH 7.4 buffered with 20 mM HEPES. After 15 min, 100 µl of complete RPMI 1640 medium was added. 100 µl of this DNA-DOTAP mixture in a total well volume of 200 µl was used in the subsequent DC activation assays.
DNA competition ELISA
Maxi Sorp immuno plates (NUNC) were coated with the anti-DNA mAb PA4 (2 µg/ml) and incubated overnight at 4°C. Plates were washed 3 times with PBS/0.05% Tween, blocked with PBS/1% BSA for 2 h at room temperature, and then unlabeled DNA fragments at various concentrations were added to the plates. After 1h, biotinylated CGSubOp (5 ng/ml) or biotinylated CG50 (5 ng/ml) was added and incubated for 4 h at room temperature. Plates were then washed 3 times, incubated with streptavidin-conjugated horseradish peroxidase (BD Biosciences) for 20 min, washed, and developed with TMB substrate (BD Biosciences). The reaction was stopped by H2SO4, and absorbance was measured at 450 nm. The percent binding was calculated as the absorbance in the presence of competitor DNA relative to the absorbance in the absence of competitor DNA.
DC preparation
Bone marrow cells were seeded at 1.5 × 106 cells/ml in complete RPMI 1640 supplemented with 5–10% conditioned medium from B16 cells transfected with fms-like tyrosine kinase 3 ligand (Flt3L or FL) . The FL B16 cells were originally made by Dr. H. Chapman (27) and kindly provided by Dr. U. Von Andrian (Harvard Medical School). The cells were used for experiments after 8 days at which time more than 90% were CD11c positive, of which 15%–40% displayed a plasmacytoid DC phenotype (CD11cpos CD45RAhigh B220high CD11blow) and the remainder displayed a conventional DC phenotype (CD11cpos CD45RAlow B220low CD11bhigh).
Cell sorting
To obtain purified pDC (CD11cpos B220high CD11blow) and cDC (CD11cpos B220negative CD11bhigh) populations for certain experiments, FL-DC from 8 day cultures were stained with anti-B220-PE-Cy5, anti-CD11c-PE and anti-CD11b-FITC (all from BD Biosciences), and the DC populations separated using a MoFlo cell sorter (DakoCytomation).
DC stimulation
Prior to setting up each assay, the FL-DCs were routinely checked by flow cytometry for the relative percentages of pDCs and cDCs and the respective DC activation status after staining with anti-CD11c-PE, biotinylated anti-CD45RA followed by streptavidin-PE-Cy5, anti-B220-FITC, anti-CD11b-FITC and anti-CD62L-FITC antibodies (all from BD Biosciences). 3 × 105 FL-DCs were seeded in 96 well round bottomed plates and cultured in complete RPMI with the relevant stimuli in a total well volume of 200 µl. In some experiments, IFN-β was added to the cultures 2 h before addition of the TLR ligands, DNA and DNA-ICs. After 18 h to 24 h, the supernatants were collected for cytokine measurement. After collection of the supernatants, the FL-DCs were labeled with a combination of anti-B220-PE-Cy5 and anti-CD40-FITC (both from BD Biosciences), detected with a FACScan flow cytometer (BD Biosciences) and analyzed with FlowJo software (Tree Star).
DNA binding and uptake
Biotinylated CGneg DNA fragments (5 µg/µl) were incubated with Qdot® 655 streptavidin conjugate (20 nM, Invitrogen) for 30 min at 37°C. Qdot-labeled CGneg-DOTAP complexes (CGneg 1000 ng/ml + DOTAP 2 µg/ml), Qdot-labeled CGneg ICs (CGneg 1000 ng/ml + PA4 10 µg/ml), and Qdot-labeled CGneg alone (1000 ng/ml) were then added to FL-DC. After 2 h at 37°C, the cells were washed 5 times with 3% FBS in PBS and labeled with anti-B220-FITC and anti-CD11b-PE. The cells were detected with a FACScan flow cytometer and analyzed with FlowJo software.
Cytokine measurement
Cytokine levels in the tissue culture supernatants were measured using in-house ELISAs developed using commercially available antibodies as previously described (28). The detection limit of the IL-6 ELISA is 16 pg/ml. The detection limit of the IFN-α ELISA is 80 pg/ml.
Statistical analysis
Statistical analysis was performed using the Wilcoxon signed-rank test. p values less than 0.05 were considered significant.
Results
Defined ICs containing optimal CpG motifs activate DCs through TLR9 and induce different cytokine profiles from plasmacytoid and conventional DCs
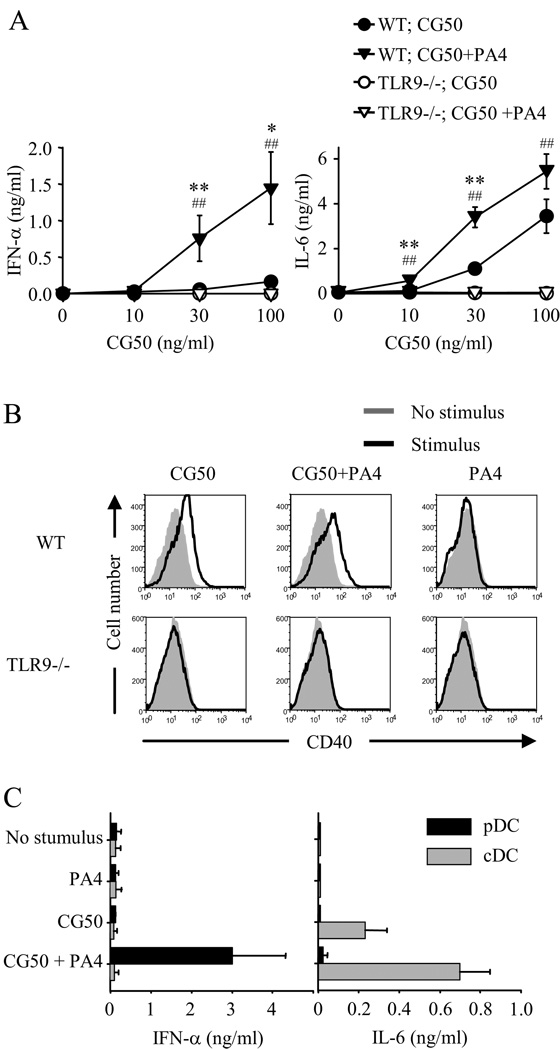
Before directly comparing dsDNA fragments of different sequence, it was necessary to demonstrate in our system that DNA fragment-containing ICs could in fact activate DCs and that TLR9 was involved in this process. To do this, we isolated a 660 bp EcoRI/BamHI DNA restriction fragment containing an array of 50 CpG motifs from the plasmid pMCG50 (29), as previously described (14) (Table I). The CpG motifs in this fragment, referred to as CG50, are unmethylated and have the optimal flanking bases (GACGTT) to activate murine TLR9. Immune complexes were formed by incubating the CG50 fragment with the IgG2a anti-DNA monoclonal antibody (mAb) PA4 (26). Dendritic cells for use in these studies were generated by culturing mouse bone marrow cells in vitro with fms-like tyrosine kinase 3 ligand (Flt3L or FL) which leads to the development of a mixed population of plasmacytoid DCs (pDC) and conventional DCs (cDC), collectively referred to as FL-DC. These pDC and cDC show equivalent properties to pDC and cDC isolated directly from mouse spleen (30).
The addition of the CG50-PA4 ICs to the FL-DC cultures induced the production of IFN-α and IL-6 (Fig. 1A), and upregulation of the co-stimulatory molecule CD40 (Fig. 1B). CG50 alone (in the absence of PA4) induced CD40 upregulation and a lower level of IL-6 production but did not induce IFN-α production. These effects were completely TLR9 dependent as no activation was seen in FL-DC derived from TLR9-deficient mice (Fig. 1, A and B). To determine the DC subset responsible for the cytokine production, pDC and cDC were purified from FL-DC cultures by cell sorting and stimulated with CG50-PA4 ICs (Fig. 1C). This revealed that pDC were the source of IFN-α and cDC the major source of IL-6.
FIGURE 1.
Immune complexes containing optimal CpG motifs activate FL-DCs through TLR9 and induce different cytokine profiles from plasmacytoid and conventional DCs. A and B, Bone-marrow-derived FL-DCs from wild-type (WT) BALB/c or TLR9-deficient (TLR9−/−) mice were incubated with indicated concentrations of the DNA fragment CG50 in the presence or absence of the anti-DNA mAb PA4 (10 µg/ml). A, IFN-α and IL-6 concentrations in supernatants collected after 24 h were measured by ELISA. Data shown are the mean ± SEM of six experiments (WT) and four experiments (TLR9−/−). *, p < 0.05 versus WT;CG50; **, p< 0.01 versus WT;CG50; ##, p < 0.01 versus TLR9−/−;CG50+PA4. B, After removal of supernatants for cytokine analysis, the cells were analyzed by flow cytometry for CD40 expression, with stimulus-induced staining intensity compared with staining intensity of the nonstimulated cultures. C, FL-DCs from wild-type BALB/c mice were prepared and pDC (B220high, CD11bnegative, and CD11cpositive) and cDC (B220negative, CD11bhigh and CD11cpositive) were separated by cell sorting. The cells were stimulated with CG50 (100 ng/ml) in the presence or absence of PA4 (10 µg/ml). IFN-α and IL-6 concentrations in supernatants collected after 24 h were measured by ELISA. Data represent mean ± SEM of two experiments.
CpG content is required for TLR9-dependent DC activation induced by DNA-containing IC
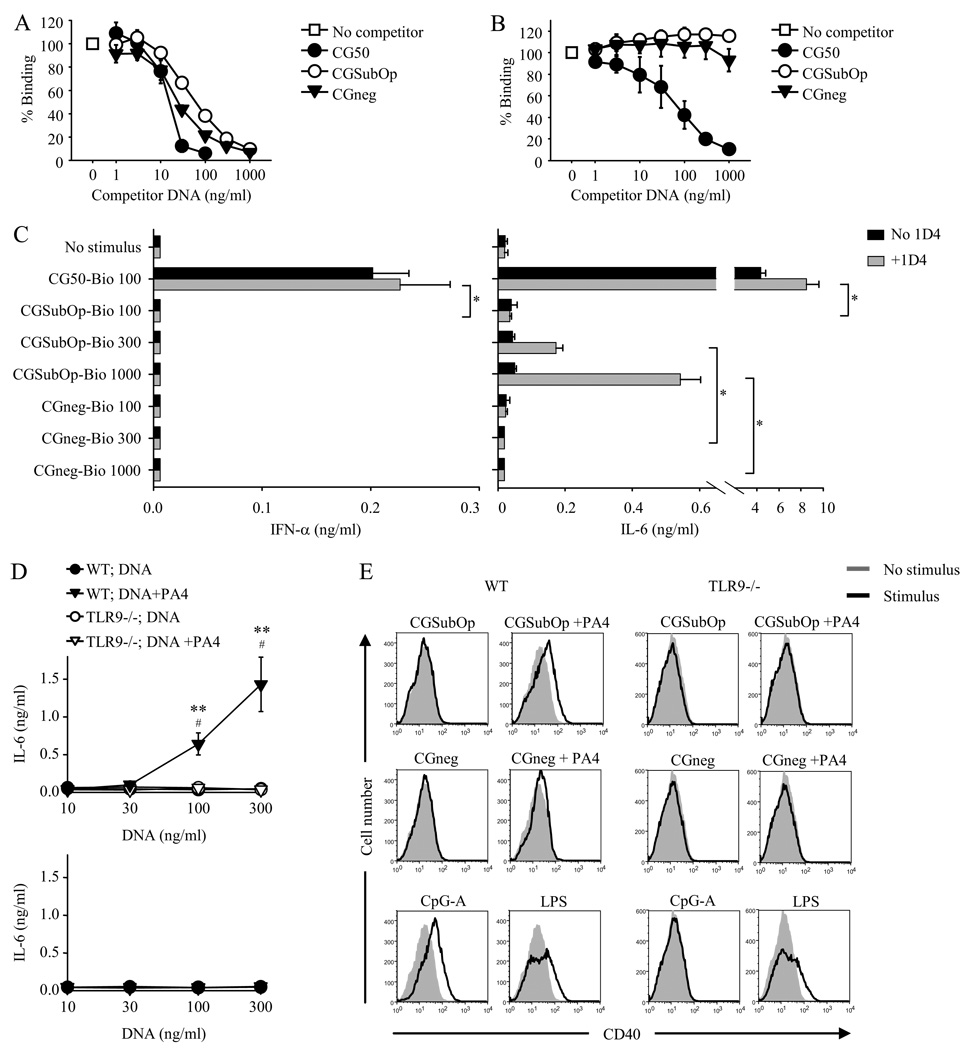
To evaluate the role of CpG content, we compared the stimulatory capacity of ICs formed with three dsDNA fragments of similar size but different sequence (Table I) (14). These were (i) the 660 base pair (bp) CG50 fragment that contains 50 optimal CpG motifs; (ii) a 726 bp fragment derived from the promoter region of HIV-1 that contains 27 unmethylated CpG dinucleotides but no optimal CpG motifs (referred to as CG SubOp); and (iii) a 629 bp fragment derived from the HIV-1 gag gene that contains no CpG dinucleotides (referred to as CGneg). Our initial plan was to compare the stimulatory capacity of the different fragments in IC formed with the PA4 anti-DNA mAb. However, we first used a competition ELISA to evaluate the relative binding affinity of the fragments to PA4. The competition ELISA was performed by measuring the ability of the individual fragments to compete with biotin-labeled CGSubOp or with biotin-labeled CG50 for binding to plate-bound PA4. Unexpectedly, although the binding of CGSubOp and CGneg to PA4 was equivalent, CG50 bound to PA4 with greater affinity (Fig. 2, A and B). Given the increased affinity of CG50 for PA4, the DNA fragment-PA4 system could not be used for comparing the stimulatory capacity of CG50 to CGSubOp or CGneg because it would not be possible to determine with certainty whether any differences observed in activation induced by CG50-PA4 ICs were due to the differences in CpG content per se or simply to a greater extent of DNA internalization.
FIGURE 2.
CpG content is required for TLR9-dependent DC activation induced by DNA-containing IC. A and B, Increasing concentrations of unlabeled dsDNA fragments CG50, CGSubOp and CGneg were added to plate-bound PA4. After 1 h, biotinylated CGSubOp (5 ng/ml) (A), or biotinylated CG50 (5 ng/ml) (B) was added. The binding of biotinylated CGSubOp (A), or biotinylated CG50 (B), to PA4 was detected with streptavidin-HRP and TMB substrate. Data represent mean ± SEM of three experiments for both A and B. C, Biotinylated DNA fragments (100, 300, or 1000 ng/ml) with or without the anti-biotin mAb 1D4 (3 µg/ml) were added to FL-DC from wild type BALB/c mice. IFN-α and IL-6 concentrations in supernatants collected after 24 h were measured by ELISA. Data represent mean ± SEM of four experiments. *, p < 0.05. D, Bone-marrow-derived FL-DCs from wild-type (WT) BALB/c or TLR9-deficient (TLR9−/−) mice were incubated with indicated concentrations of the DNA fragments CGSubOp and CGneg in the presence or absence of the anti-DNA mAb PA4 (10 µg/ml). IL-6 concentrations in supernatants collected after 24 h were measured by ELISA. Data shown are the mean ± SEM of five experiments (WT) and three experiments (TLR9−/−). **, p< 0.01 versus WT;DNA; #, p < 0.05 versus TLR9−/−;DNA+PA4. E, After removal of supernatants for cytokine analysis, the cells were analyzed by flow cytometry for CD40 expression, with stimulus-induced staining intensity compared with staining intensity of the nonstimulated cultures.
We therefore used an alternative method to form DNA fragment ICs. The individual DNA fragments were labeled at the 5’ and 3’ ends with a single biotin molecule at each end, and are referred to as CG50-bio, CGSubOp-bio, and CGneg-bio. IC were formed by incubating the biotin-labeled DNA fragments with an IgG2a anti-biotin mAb, 1D4 (23). Formation of the immune complex therefore depends on the affinity of the anti-biotin mAb for the attached biotin which is independent of the DNA fragment itself. The individual biotin-labeled DNA-fragment ICs elicited markedly different responses when added to FL-DC cultures (Fig. 2C). Even at the lowest DNA fragment concentration of 100 ng/ml, the CG50-bio-1D4 ICs induced IFN-α and high levels of IL-6 production. The CGSubOp-bio-1D4 ICs induced lower levels of IL-6 and only at the higher DNA fragment concentrations. The CGneg-bio-1D4 ICs failed to induce IL-6 production at any of the DNA fragment concentrations.
To confirm the observed differences between CGSubOp and CGneg, we also compared the stimulatory capacity of these DNA fragments when the IC were formed with the anti-DNA mAb PA4 instead of with 1D4. This approach could be used because, as previously noted, the affinity of CGSubOp for PA4 was equivalent to that of CGneg (Fig. 2, A and B). When added to the FL-DC cultures, the CGSubOp-PA4 ICs induced IL-6 production whereas the CGneg-PA4 ICs did not (Fig. 2D). Similarly, upregulation of the co-stimulatory molecule CD40 was only induced by the CGSubOp-PA4 ICs (Fig. 2E). The induction of both cytokine production and co-stimulatory molecule expression was completely TLR9-dependent (Fig. 2, D and E).
Overall, these data demonstrate that CpG content is required for TLR9-dependent DC activation by DNA-containing IC. In addition, although optimal CpG motifs induce the strongest response, effective activation is also induced by DNA that contains unmethylated CpG dinucleotides but lacks the optimal motifs.
Methylation of CpG dinucleotides abrogates the stimulatory capacity of DNA that contains unmethylated CpG dinucleotides but lacks optimal CpG motifs
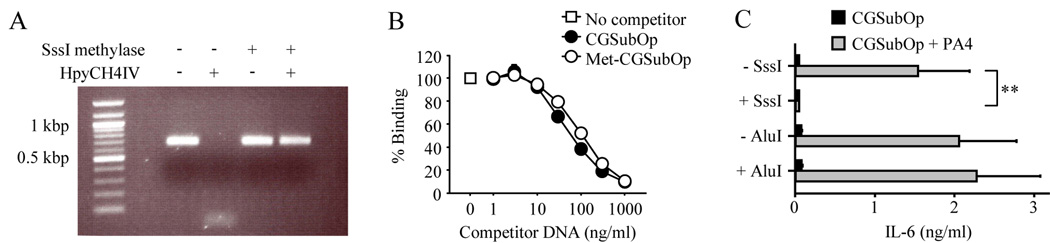
If the difference between the stimulatory capacity of the CGSubOp-PA4 ICs and the lack of stimulatory capacity of the CGneg-PA4 ICs resides in the unmethylated CpG dinucleotides within the CGSubOp DNA fragment, then methylation of the CpG dinucleotides in CGSubOp should eliminate its ability to activate FL-DC. To initially confirm that the methylation procedure was working adequately, CG50 was treated with the CpG methylase, M.SssI, which methylates all cytosine residues within the double-stranded dinucleotide recognition sequence 5'…CG…3' (31). This procedure eliminated unmethylated CpGs, as shown by the complete resistance of CpG methylase-treated CG50 to the restriction endonuclease HpyCH4IV which cuts DNA at unmethylated CpG sites flanked 5’ by an adenosine base, but does not cut at methylated CpG sites (Fig. 3A). CGSubOp was then methylated with the CpG methylase, M.SssI, using the same procedure as was used for CG50. In a competition ELISA, CpG methylase-treated CGSubOp (referred to as Met-CGSubOp) bound to the anti-DNA mAb PA4 at least as well as untreated CGSubOp (Fig. 3B). However, whereas CGSubOp-PA4 ICs induced IL-6 production when added to FL-DC cultures, no IL-6 production was induced by Met-CGSubOp-PA4 ICs (Fig. 3C). As an additional control to demonstrate the specificity of the methylation effect, we also tested the stimulatory capacity of CGSubOp after treatment with Alu I methyltransferase, which methylates the cytosine residue of AGCT sequences, but does not methylate cytosine residues in CpG dinuceotides. Alu I methyltransferase treatment of CGSubOp, which contains 6 Alu I methyltransferase sites (Table I), did not affect the ability of CGSubOp-PA4 ICs to induce IL-6 production (Fig. 3C). Overall these data demonstrate that unmethylated CpG dinucleotides are critical determinants of the ability of CGSubOp-PA4 ICs to activate DCs through TLR9.
FIGURE 3.
Methylation of CpG dinucleotides abrogates the stimulatory capacity of DNA that contains unmethylated CpG dinucleotides but lacks optimal CpG motifs. A, The 660 bp DNA fragment CG50 was treated, or not treated, with M.SssI methylase (CpG methylase) and then digested, or not digested, with the methylation sensitive restriction endonuclease, HpyCH4 IV. B, Increasing concentrations of unlabeled CGSubOp and CGSubOp treated with M.SssI methylase (Met-CGSubOp) were added to plate-bound PA4. After 1 h, biotinylated CGSubOp (5 ng/ml) was added. The binding of biotinylated CGSubOp to PA4 was detected with streptavidin-HRP and TMB substrate. Data represent mean ± SEM of three experiments. C, CGSubOp fragments were treated, or not treated, with M.SssI methylase or Alu I methylase. FL-DC from wild type BALB/c mice were then incubated with the variously treated CGSubOp fragments alone (300 ng/ml) or with CGSubOp-ICs (300 ng/ml of CGSubOp plus 10 µg/ml of PA4). IL-6 concentrations in supernatants collected after 24 h were measured by ELISA. Data represent mean ± SEM of five experiments. **, p< 0.01.
CpG-free DNA internalized into DCs by liposomal transfection is stimulatory, in contrast to CpG-free DNA internalized in an IC which is not stimulatory
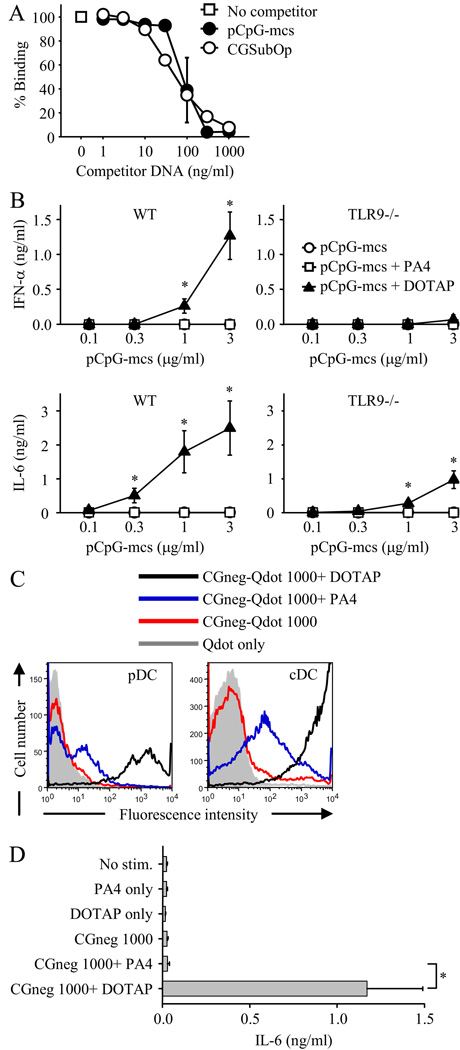
Previous studies have shown that CpG-free phosphodiester ODN can activate TLR9 when the ODN are internalized into DCs by liposomal transfection with DOTAP or by the addition of 3’ poly G tails to the ODN (20, 21). However, we found that the double-stranded CpG-free fragment CGneg could not activate when internalized into DCs as an IC (Fig. 2, C–E). To directly compare the stimulatory capacity of CpG-free DNA internalized by DCs through these different routes, we used two different CpG-free fragments. The one fragment was CGneg. The other was a 3070 bp CpG-free plasmid from Invivogen, termed pCpG-mcs.
pCpG-mcs bound comparably to the CpG-containing DNA fragment CGSubOp in a competition ELISA measuring binding affinity to the anti-DNA mAb PA4 , demonstrating that pCpG-mcs could form ICs with PA4 (Fig. 4A). The pCpG-mcs-PA4 ICs did not induce either IFN-α or IL-6 when added to FL-DC cultures, even at pCpG-mcs concentrations as high as 3 µg/ml (Fig. 4B). In contrast, pCpG-mcs internalized with DOTAP induced both IFN-α and IL-6 with the activation being mostly, but not completely, TLR9-dependent (Fig. 4B). The TLR9-independent component of activation might represent DOTAP-mediated transport of pCpG-mcs to other DNA recognition receptors, such as the cytosolic DNA-dependent activator of IFN-regulatory factors (DAI, also called Z-DNA binding protein 1 and DLM-1) (32) or the cytosolic dsDNA receptor absent in melanoma 2 (AIM2) (33–36).
FIGURE 4.
CpG-free DNA internalized into DCs by liposomal transfection is stimulatory, in contrast to CpG-free DNA internalized in an IC which is not stimulatory. A, Increasing concentrations of the unlabeled DNA fragments pCpG-mcs and CGSubOp were added to plate-bound PA4. After 1 h, biotinylated CGSubOp (5 ng/ml) was added. The binding of biotinylated CGSubOp to PA4 was detected with streptavidin-HRP and TMB substrate. Data represent mean ± SEM of two experiments. B, pCpG-mcs alone, pCpG-mcs plus PA4 (10 µg/ml) or pCpG-mcs plus DOTAP (at weight ratio of 1:2) were added to FL-DC from wild-type (WT) BALB/c or TLR9-deficient (TLR9−/−) mice. IFN-α and IL-6 concentrations in supernatants collected after 24 h were measured by ELISA. Data represent mean ± SEM of three experiments. *, p < 0.05 versus pCpG-mcs and pCpG-mcs+PA4. C, Biotinylated CGneg was labeled with streptavidin-coated fluorescent Qdots. Qdot-labeled CGneg-DOTAP complexes (CGneg 1000 ng/ml + DOTAP 2 µg/ml), Qdot-labeled CGneg ICs (CGneg 1000 ng/ml + PA4 10 µg/ml), and Qdot-labeled CGneg alone (1000 ng/ml) were added to FL-DC for 2 h at 37°C. The extent of Qdot-labeled CGneg internalization or binding to pDC and cDC was determined using flow cytometry to measure fluorescence intensity. Data represent one of three representative experiments. D, CGneg alone (1000 ng/ml), CGneg-ICs (CGneg 1000 ng/ml + PA4 10 µg/ml) and CGneg-DOTAP complexes (CGneg 1000 ng/ml + DOTAP 2 µg/ml) were added to FL-DC from wild-type BALB/c mice. IL-6 concentrations in supernatants collected after 24 h were measured by ELISA. Data represent mean ± SEM of three experiments. *, p < 0.05
One possible explanation for why DOTAP-mediated internalization of CpG-free DNA is stimulatory whereas IC-mediated internalization is not, is that the amount of DNA able to be internalized by IC might be relatively limited as compared with DOTAP-mediated internalization. To evaluate this possibility, CGneg-bio was fluorescently labeled using Qdot® 655 streptavidin conjugate, and CGneg alone, CGneg-PA4 ICs, and CGneg-DOTAP were added to FL-DC cultures. The extent of binding and/or uptake of CGneg was at least 100-fold greater with CGneg-DOTAP than with CGneg-PA4 ICs in both pDC and cDC (Fig. 4C). At the same DNA concentration used in the binding studies (1000 ng/ml), CGneg-PA4 ICs did not induce cytokine production by FL-DCs whereas CGneg internalized with DOTAP did induce activation (Fig. 4D).
Endogenous mammalian CpG-rich sequences activate DCs
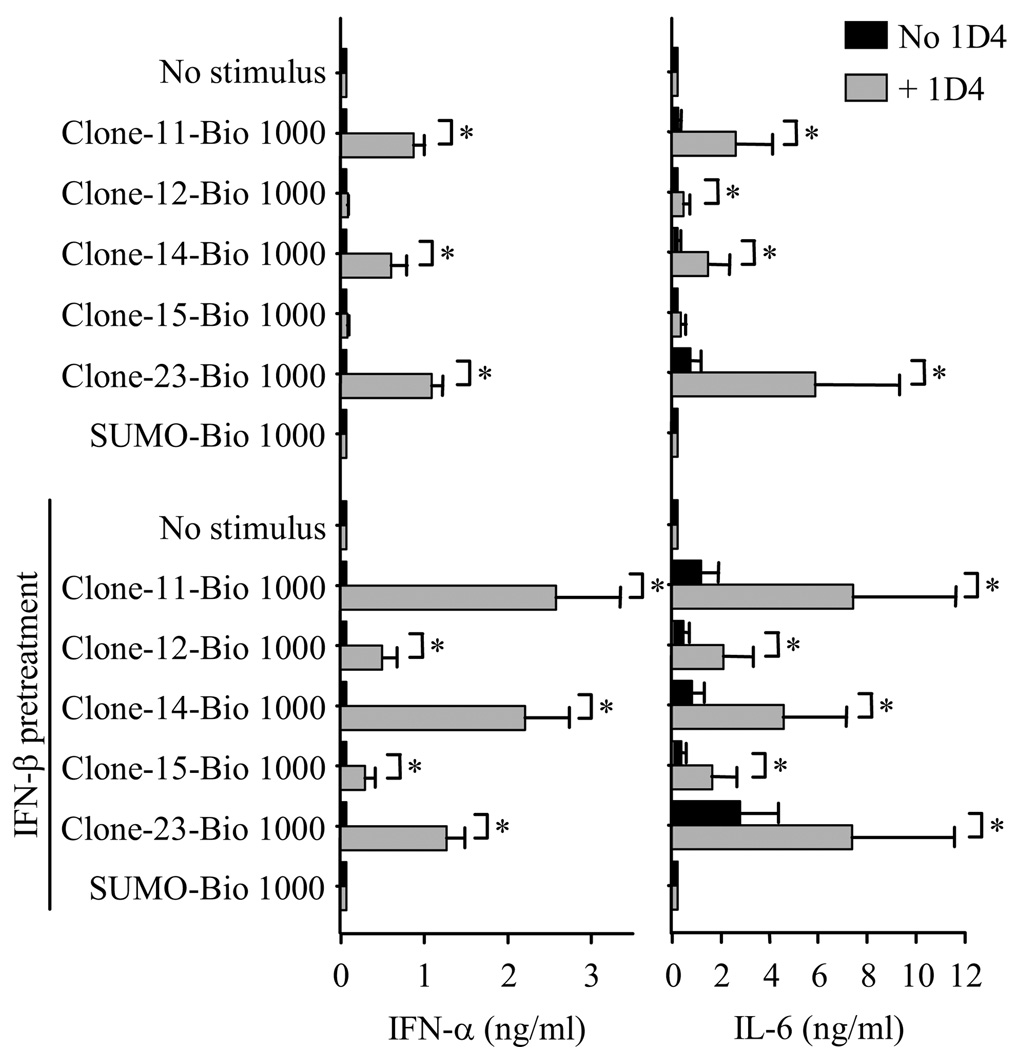
Our studies to this point had shown that unmethylated CpG dsDNA sequences were required for DC activation by DNA-containing IC. However, in order to demonstrate the relevance of this finding to human autoimmune disease, it was important to show that similar stimulatory DNA sequences were present in mammalian DNA. Although mammalian DNA in general has far fewer unmethylated CpG dinucleotides than bacterial DNA, the promoter regions of mammalian genes contain areas called CpG islands that are CpG rich and unmethylated (37). In the context of CpG islands, CpG-rich fragments are defined as DNA fragments having a GC content greater than 50% and a ratio of observed-to-expected CpGs greater than 0.5 (38). To determine whether CpG-rich dsDNA fragments derived from mammalian cells can activate DC, we used dsDNA fragments isolated from a mouse CpG island library (Table I). These fragments, termed clones 11, 12, 14, 15 and 23, have recently been shown by our group to activate autoreactive B cells following internalization through the B cell receptor (23). They contain plentiful CpG dinucleotides but few or no optimal CpG motifs. A dsDNA fragment similar in size but containing only a single CpG dinucleotide, termed SUMO, was used as a control (Table I). The fragments were biotinylated at the 5’ end with a single biotin molecule and ICs were formed by incubating the biotin-labeled DNA fragments with the anti-biotin mAb 1D4. Addition of the biotinylated CpG island fragments alone (in the absence of 1D4) to FL-DC cultures induced no IFN-α production and very low level or no IL-6 production (Fig. 5). However, 3 of the 5 biotinylated CpG island fragments complexed with 1D4 induced substantial amounts of both IFN-α and IL-6. In contrast, the control SUMO fragment induced no cytokine production, either on its own or complexed with 1D4. These data demonstrate that immune complexes containing CpG-rich dsDNA fragments from mammalian DNA can induce DC activation.
FIGURE 5.
Endogenous mammalian CpG-rich sequences activate DCs. Biotinylated CpG island DNA fragments (clones 11, 12, 14, 15 and 23) and the CpG-poor SUMO fragment, all at 1000 ng/ml, with or without the anti-biotin mAb 1D4 (3 µg/ml) were added to FL-DC from wild type BALB/c mice. Experiments were done in the absence or the presence of a 2 h pretreatment of the FL-DC with IFN-β (300 U/ml). IFN-α and IL-6 concentrations in supernatants collected after 24 h were measured by ELISA. Data represent the mean ± SEM of three experiments. . *, p < 0.05
DC priming with type I IFN enhances responses to CpG-rich dsDNA but not to CpG -poor dsDNA
Type I IFN (IFN-α or IFN-β) overproduction has been linked to the pathogenesis of SLE (39–41), and priming of human DC with type I IFN enhances responses to DNA-containing IC (13, 42). To determine whether type I IFN priming would modify DC responses to DNA-containing IC in our system, FL-DC were pretreated with IFN-β for 2 hours and then stimulated with IC containing the various biotinylated DNA fragments. IC containing the CpG island fragments induced a higher level of IFN-α and IL-6 production from IFN-β pretreated FL-DC than from FL-DC not pretreated with IFN-β (Fig. 5). Notably, IFN-β pretreatment enabled the two CpG island fragment-IC (clone 12 and clone 15) that were not stimulatory in the absence of type I IFN pretreatment to induce effective DC activation. IFN-β pretreatment also enabled the CpG island fragments alone (in the absence of 1D4) to induce modest levels of IL-6 production. However, even with IFN-β pretreatment, IC containing the CpG-poor fragment SUMO failed to induce cytokine production.
Discussion
In the autoimmune disease SLE, DNA-containing IC are thought to contribute to disease pathogenesis through the activation of intracellular TLR9 in pDCs. To determine the requirement for DNA sequence in the activation of TLR9 under these conditions, we used dsDNA fragments of defined sequence and methylation status to activate TLR9 in murine DC after internalization in the form of an IC. The data demonstrate that unmethylated CpG dinucleotides are required for this activation. Importantly, although DNA containing optimal CpG motifs induces the strongest TLR9 activation, effective activation is also induced by DNA that contains unmethylated CpG dinucleotides but lacks these optimal motifs.
This finding implies that before self DNA can induce DC activation, DNA containing unmethylated CpG dinucleotides first needs to be released into the extracellular environment where it can bind with autoantibody to form an IC. Indeed, there is some evidence that this may occur in patients with SLE (43–46). Although unmethylated CpG dinucleotides are much less common in mammalian DNA as compared with pathogen DNA, they are found at high concentration in regions of the mammalian genome referred to as CpG islands (37, 47). Mammalian CpG islands could thus represent one potential source of this G-C enriched DNA and our study shows that CpG-rich DNA obtained from CpG islands is capable of effective DC activation after internalization as an IC. Optimal CpG motifs are not required for this DC activation as at least two CpG island clone (Clones 12 and 23, Table I) that contain CpG dinucleotides but lack optimal motifs can induce IFN-α and IL-6 production. Our group has recently reported that this CpG island DNA is also able to activate autoreactive B cells (23), which could be the initiating event leading to the production of the autoantibodies required for immune complex formation.
It is not known how CpG island DNA might become accessible to the immune system in patients with SLE. However, CpG islands show the properties that coincide with enhanced sensitivity to nucleases relative to bulk DNA (47), and so could be preferentially released during apoptosis and accumulate as a result of the impaired apoptotic cell clearance that characterizes SLE (48). It is also conceivable that there might be an element of selectivity at the level of immune complex formation. Anti-DNA autoantibodies from lupus patients show preferential binding to GC-rich DNA fragments (49), and certain anti-dsDNA autoantibodies from mouse lupus models bind to G-C much more strongly than to other base combinations (50, 51). In the current study we found that the anti-DNA Ab PA4 has a greater affinity for the DNA fragment CG50 containing optimal CpG motifs than for the DNA fragments CGSubOp or CGneg. One possible explanation for why anti-DNA antibodies might preferentially bind CpG-rich DNA is that B cells with this binding specificity might be preferentially activated due to synergy between the B cell receptor and TLR9.
Our data demonstrates that there are differences in DNA sequence requirements for TLR9 activation in DC depending on the route of DNA internalization. Unmethylated CpG dinucleotides are absolutely required for TLR9 activation if the DNA fragments are internalized in the form of an IC, whereas CpG-free DNA can activate TLR9 if the DNA fragments are internalized by liposomal transfection with DOTAP. Presumably TLR9 activation in this setting is being mediated through non-CG bases and/or direct recognition by TLR9 of the DNA sugar backbone 2’ deoxyribose (20, 21). One possible explanation for the difference between immune complex mediated- and DOTAP mediated- internalization might be the absolute concentration of DNA fragment delivered to intracellular TLR9. We found that DNA fragment binding and/or uptake was at least 100-fold greater with DOTAP mediated- internalization. This interpretation would be consistent with previous studies using phosphorothioate ODN showing that much higher concentrations of CpG-free ODN are required to induce TLR9 activation as compared to ODN containing CpG motifs (52).
However, it is necessary to consider additional possibilities. The precise intracellular location at which TLR9 engages its ligand plays an important role in determining the functional outcome (53, 54), and there may well be differences in intracellular trafficking of the DNA fragments depending on whether they are internalized as an immune complex or with DOTAP. Also, cationic lipids such as DOTAP form a highly ordered multilamellar structure with DNA which can fuse with endosomal vesicles to form large stable aggregates (53, 55–57). In this way, DOTAP might affect both the DNA structure and the kinetics of DNA longevity in endosomes (53). In addition, DOTAP has been reported to possess potent adjuvant activity in its own right through induction of the extracellular-signal-regulated kinase (ERK) pathway (58), and it is conceivable that this could modulate the DC response to DNA fragments particularly as ERK activation has been reported to upregulate TLR9 expression and increase the response to CpG ODN (59).
Although immune complex formation markedly enhanced the stimulatory capacity of the DNA fragments, certain of the CpG island fragments induced low-level, but consistent, IL-6 production even in the absence of antibody. This was enhanced by type I IFN pretreatment of the FL-DC. Additional studies will be needed to determine whether there are other immunological consequences of this antibody-independent uptake of DNA in SLE. For example, a recent study has shown that DNA from gut flora can directly activate TLR9 in DCs in the lamina propria and thereby limit the production of T regulatory cells (60). This effect was present whether or not DOTAP was used to internalize the DNA. We found that pretreatment of FL-DC with type I IFN also enhanced their response to DNA-containing IC, and this may reflect the in vivo situation in many SLE patients where the DC may be exposed to elevated levels of circulating type I IFN. This result was anticipated as other studies examining DC activation by DNA-containing IC had shown similar enhancement (13, 42). However, it is notable in our study that type I IFN only enhanced the response if the DNA within the DNA- IC contained CpG dinucleotides. This effect was particularly remarkable for two of the CpG island clones that did not induce cytokine production in the absence of type I IFN pretreatment but induced both IFN-α and IL-6 with type I IFN pretreatment. Importantly however, type I IFN did not enhance the response to DNA-IC that contained CpG-free DNA, in contrast to what was observed for TLR9 activation in type I IFN-pretreated B cells (23).
In summary, the data demonstrate that unmethylated CpG dinucleotides within phosphodiester dsDNA are required for TLR9 activation in DC induced by DNA-containing IC. The data also show that CpG islands within mammalian DNA represent one possible source of this stimulatory CpG-rich DNA. Our study provides new information on the DNA sequence requirements within natural phosphodiester dsDNA for the activation of TLR9 in DCs and is consistent with the concept that, in normal circumstances, the immune system is protected from inadvertent activation by self DNA both by the intracellular location of TLR9 and the DNA sequence required for TLR9 activation. It will be important to try and understand how different forms of cell death might contribute to the release of CpG-rich DNA in autoimmune diseases such as SLE and psoriasis.
Acknowledgments
We thank Alyssa Brown, Patricia Busto and Tina Ling for technical assistance.
Abbreviations
- CpG
cytosine linked to guanosine by a phosphate bond
- cDC
conventional dendritic cell
- DC
dendritic cell
- DOTAP
N-[1-(2,3-Dioleoyloxy)propyl]-N,N,N-trimethylammonium methylsulfate
- FL
fms-like tyrosine kinase 3 ligand
- FL-DC
DC obtained from bone marrow cells cultured in FL
- IC
immune complex
- ODN
oligodeoxynucleotide
- pDC
plasmacytoid dendritic cell
- SA-HRP
streptavidin-conjugated horseradish peroxidase
- SLE
systemic lupus erythematosus
Footnotes
Publisher's Disclaimer: "This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org."
This work was supported by a grant from the National Institutes of Health AR050256. C. Richez was supported by grants from Société Française de Rhumatologie, CHU de Bordeaux, and Réseau Rhumatologie.
References
- 1.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 2.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu. Rev. Immunol. 2002;20:709–760. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 3.Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–607. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 4.Boule MW, Broughton C, Mackay F, Akira S, Marshak-Rothstein A, Rifkin IR. Toll-like receptor 9-dependent and -independent dendritic cell activation by chromatin-immunoglobulin G complexes. J. Exp. Med. 2004;199:1631–1640. doi: 10.1084/jem.20031942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Means TK, Latz E, Hayashi F, Murali MR, Golenbock DT, Luster AD. Human lupus autoantibody-DNA complexes activate DCs through cooperation of CD32 and TLR9. J. Clin. Invest. 2005;115:407–417. doi: 10.1172/JCI23025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barton GM, Kagan JC, Medzhitov R. Intracellular localization of Toll-like receptor 9 prevents recognition of self DNA but facilitates access to viral DNA. Nat. Immunol. 2006;7:49–56. doi: 10.1038/ni1280. [DOI] [PubMed] [Google Scholar]
- 7.Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang YH, Homey B, Cao W, Wang YH, Su B, Nestle FO, Zal T, Mellman I, Schroder JM, Liu YJ, Gilliet M. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 8.Marshak-Rothstein A, Rifkin IR. Immunologically active autoantigens: the role of toll-like receptors in the development of chronic inflammatory disease. Annu. Rev. Immunol. 2007;25:419–441. doi: 10.1146/annurev.immunol.22.012703.104514. [DOI] [PubMed] [Google Scholar]
- 9.Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat. Rev. Immunol. 2008;8:594–606. doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- 10.Ahmad-Nejad P, Hacker H, Rutz M, Bauer S, Vabulas RM, Wagner H. Bacterial CpG-DNA and lipopolysaccharides activate Toll-like receptors at distinct cellular compartments. Eur. J. Immunol. 2002;32:1958–1968. doi: 10.1002/1521-4141(200207)32:7<1958::AID-IMMU1958>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 11.Latz E, Schoenemeyer A, Visintin A, Fitzgerald KA, Monks BG, Knetter CF, Lien E, Nilsen NJ, Espevik T, Golenbock DT. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat. Immunol. 2004;5:190–198. doi: 10.1038/ni1028. [DOI] [PubMed] [Google Scholar]
- 12.Bave U, Magnusson M, Eloranta ML, Perers A, Alm GV, Ronnblom L. Fc gamma RIIa is expressed on natural IFN-alpha-producing cells (plasmacytoid dendritic cells) and is required for the IFN-alpha production induced by apoptotic cells combined with lupus IgG. J. Immunol. 2003;171:3296–3302. doi: 10.4049/jimmunol.171.6.3296. [DOI] [PubMed] [Google Scholar]
- 13.Vallin H, Blomberg S, Alm GV, Cederblad B, Ronnblom L. Patients with systemic lupus erythematosus (SLE) have a circulating inducer of interferon-alpha (IFN-alpha) production acting on leucocytes resembling immature dendritic cells. Clin. Exp. Immunol. 1999;115:196–202. doi: 10.1046/j.1365-2249.1999.00772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viglianti GA, Lau CM, Hanley TM, Miko BA, Shlomchik MJ, Marshak-Rothstein A. Activation of autoreactive B cells by CpG dsDNA. Immunity. 2003;19:837–847. doi: 10.1016/s1074-7613(03)00323-6. [DOI] [PubMed] [Google Scholar]
- 15.Kindrachuk J, Potter J, Wilson HL, Griebel P, Babiuk LA, Napper S. Activation and regulation of toll-like receptor 9: CpGs and beyond. Mini Rev. Med. Chem. 2008;8:590–600. doi: 10.2174/138955708784534481. [DOI] [PubMed] [Google Scholar]
- 16.Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nat. Rev. Drug Discov. 2006;5:471–484. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 17.Muller T, Hamm S, Bauer S. TLR9-mediated recognition of DNA. Handb. Exp. Pharmacol. 2008;183:51–70. doi: 10.1007/978-3-540-72167-3_3. [DOI] [PubMed] [Google Scholar]
- 18.Elias F, Flo J, Lopez RA, Zorzopulos J, Montaner A, Rodriguez JM. Strong cytosine-guanosine-independent immunostimulation in humans and other primates by synthetic oligodeoxynucleotides with PyNTTTTGT motifs. J. Immunol. 2003;171:3697–3704. doi: 10.4049/jimmunol.171.7.3697. [DOI] [PubMed] [Google Scholar]
- 19.Yasuda K, Yu P, Kirschning CJ, Schlatter B, Schmitz F, Heit A, Bauer S, Hochrein H, Wagner H. Endosomal Translocation of Vertebrate DNA Activates Dendritic Cells via TLR9-Dependent and -Independent Pathways. J. Immunol. 2005;174:6129–6136. doi: 10.4049/jimmunol.174.10.6129. [DOI] [PubMed] [Google Scholar]
- 20.Yasuda K, Rutz M, Schlatter B, Metzger J, Luppa PB, Schmitz F, Haas T, Heit A, Bauer S, Wagner H. CpG motif-independent activation of TLR9 upon endosomal translocation of "natural" phosphodiester DNA. Eur. J. Immunol. 2006;36:431–436. doi: 10.1002/eji.200535210. [DOI] [PubMed] [Google Scholar]
- 21.Haas T, Metzger J, Schmitz F, Heit A, Muller T, Latz E, Wagner H. The DNA sugar backbone 2' deoxyribose determines toll-like receptor 9 activation. Immunity. 2008;28:315–323. doi: 10.1016/j.immuni.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 22.Wagner H. The sweetness of the DNA backbone drives Toll-like receptor 9. Curr. Opin. Immunol. 2008;20:396–400. doi: 10.1016/j.coi.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 23.Uccellini MB, Busconi L, Green NM, Busto P, Christensen SR, Shlomchik MJ, Marshak-Rothstein A, Viglianti GA. Autoreactive B cells discriminate CpG-rich and CpG-poor DNA and this response is modulated by IFN-alpha. J. Immunol. 2008;181:5875–5884. doi: 10.4049/jimmunol.181.9.5875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ewald SE, Lee BL, Lau L, Wickliffe KE, Shi GP, Chapman HA, Barton GM. The ectodomain of Toll-like receptor 9 is cleaved to generate a functional receptor. Nature. 2008;456:658–662. doi: 10.1038/nature07405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park B, Brinkmann MM, Spooner E, Lee CC, Kim YM, Ploegh HL. Proteolytic cleavage in an endolysosomal compartment is required for activation of Toll-like receptor 9. Nat. Immunol. 2008;9:1407–1414. doi: 10.1038/ni.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monestier M, Novick KE, Losman MJ. D-penicillamine- and quinidine-induced antinuclear antibodies in A.SW (H-2s) mice: similarities with autoantibodies in spontaneous and heavy metal-induced autoimmunity. Eur. J. Immunol. 1994;24:723–730. doi: 10.1002/eji.1830240335. [DOI] [PubMed] [Google Scholar]
- 27.Shi GP, Villadangos JA, Dranoff G, Small C, Gu L, Haley KJ, Riese R, Ploegh HL, Chapman HA. Cathepsin S required for normal MHC class II peptide loading and germinal center development. Immunity. 1999;10:197–206. doi: 10.1016/s1074-7613(00)80020-5. [DOI] [PubMed] [Google Scholar]
- 28.Yasuda K, Richez C, Maciaszek JW, Agrawal N, Akira S, Marshak-Rothstein A, Rifkin IR. Murine Dendritic Cell Type I IFN Production Induced by Human IgG-RNA Immune Complexes Is IFN Regulatory Factor (IRF)5 and IRF7 Dependent and Is Required for IL-6 Production. J. Immunol. 2007;178:6876–6885. doi: 10.4049/jimmunol.178.11.6876. [DOI] [PubMed] [Google Scholar]
- 29.Krieg AM, Wu T, Weeratna R, Efler SM, Love-Homan L, Yang L, Yi AK, Short D, Davis HL. Sequence motifs in adenoviral DNA block immune activation by stimulatory CpG motifs. Proc. Natl. Acad. Sci. U S A. 1998;95:12631–12636. doi: 10.1073/pnas.95.21.12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naik SH, Proietto AI, Wilson NS, Dakic A, Schnorrer P, Fuchsberger M, Lahoud MH, O'Keeffe M, Shao QX, Chen WF, Villadangos JA, Shortman K, Wu L. Cutting edge: generation of splenic CD8+ and CD8− dendritic cell equivalents in Fms-like tyrosine kinase 3 ligand bone marrow cultures. J. Immunol. 2005;174:6592–6597. doi: 10.4049/jimmunol.174.11.6592. [DOI] [PubMed] [Google Scholar]
- 31.Nur I, Szyf M, Razin A, Glaser G, Rottem S, Razin S. Procaryotic and eucaryotic traits of DNA methylation in spiroplasmas (mycoplasmas) J. Bacteriol. 1985;164:19–24. doi: 10.1128/jb.164.1.19-24.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takaoka A, Wang Z, Choi MK, Yanai H, Negishi H, Ban T, Lu Y, Miyagishi M, Kodama T, Honda K, Ohba Y, Taniguchi T. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448:501–505. doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- 33.Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, Fitzgerald KA. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roberts TL, Idris A, Dunn JA, Kelly GM, Burnton CM, Hodgson S, Hardy LL, Garceau V, Sweet MJ, Ross IL, Hume DA, Stacey KJ. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science. 2009;323:1057–1060. doi: 10.1126/science.1169841. [DOI] [PubMed] [Google Scholar]
- 36.Burckstummer T, Baumann C, Bluml S, Dixit E, Durnberger G, Jahn H, Planyavsky M, Bilban M, Colinge J, Bennett KL, Superti-Furga G. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat. Immunol. 2009;10:266–272. doi: 10.1038/ni.1702. [DOI] [PubMed] [Google Scholar]
- 37.Cross SH, Bird AP. CpG islands and genes. Curr. Opin. Genet. Dev. 1995;5:309–314. doi: 10.1016/0959-437x(95)80044-1. [DOI] [PubMed] [Google Scholar]
- 38.Cross SH, Lee M, Clark VH, Craig JM, Bird AP, Bickmore WA. The chromosomal distribution of CpG islands in the mouse: evidence for genome scrambling in the rodent lineage. Genomics. 1997;40:454–461. doi: 10.1006/geno.1996.4598. [DOI] [PubMed] [Google Scholar]
- 39.Baechler EC, Gregersen PK, Behrens TW. The emerging role of interferon in human systemic lupus erythematosus. Curr. Opin. Immunol. 2004;16:801–807. doi: 10.1016/j.coi.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 40.Theofilopoulos AN, Baccala R, Beutler B, Kono DH. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu. Rev. Immunol. 2005;23:307–336. doi: 10.1146/annurev.immunol.23.021704.115843. [DOI] [PubMed] [Google Scholar]
- 41.Banchereau J, Pascual V. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity. 2006;25:383–392. doi: 10.1016/j.immuni.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 42.Vallin H, Perers A, Alm GV, Ronnblom L. Anti-double-stranded DNA antibodies and immunostimulatory plasmid DNA in combination mimic the endogenous IFN-alpha inducer in systemic lupus erythematosus. J. Immunol. 1999;163:6306–6313. [PubMed] [Google Scholar]
- 43.Sano H, Morimoto C. Dna isolated from DNA/anti-DNA antibody immune complexes in systemic lupus erythematosus is rich in guanine-cytosine content. J. Immunol. 1982;128:1341–1345. [PubMed] [Google Scholar]
- 44.Van Helden PD. Potential Z-DNA-forming elements in serum DNA from human systemic lupus erythematosus. J. Immunol. 1985;134:177–179. [PubMed] [Google Scholar]
- 45.Sato Y, Miyata M, Sato Y, Nishimaki T, Kochi H, Kasukawa R. CpG motif-containing DNA fragments from sera of patients with systemic lupus erythematosus proliferate mononuclear cells in vitro. J. Rheumatol. 1999;26:294–301. [PubMed] [Google Scholar]
- 46.Krapf F, Herrmann M, Leitmann W, Kalden JR. Antibody binding of macromolecular DNA and RNA in the plasma of SLE patients. Clin. Exp. Immunol. 1989;75:336–342. [PMC free article] [PubMed] [Google Scholar]
- 47.Antequera F. Structure, function and evolution of CpG island promoters. Cell. Mol. Life Sci. 2003;60:1647–1658. doi: 10.1007/s00018-003-3088-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sheriff A, Gaipl US, Voll RE, Kalden JR, Herrmann M. Apoptosis and systemic lupus erythematosus. Rheum. Dis. Clin. North Am. 2004;30:505–527. doi: 10.1016/j.rdc.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 49.Sano H, Takai O, Harata N, Yoshinaga K, Kodama-Kamada I, Sasaki T. Binding properties of human anti-DNA antibodies to cloned human DNA fragments. Scand. J. Immunol. 1989;30:51–63. doi: 10.1111/j.1365-3083.1989.tb01188.x. [DOI] [PubMed] [Google Scholar]
- 50.Stollar BD, Zon G, Pastor RW. A recognition site on synthetic helical oligonucleotides for monoclonal anti-native DNA autoantibody. Proc. Natl. Acad. Sci. U S A. 1986;83:4469–4473. doi: 10.1073/pnas.83.12.4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eivazova ER, McDonnell JM, Sutton BJ, Staines NA. Specificity and binding kinetics of murine lupus anti-DNA monoclonal antibodies implicate different stimuli for their production. Immunology. 2000;101:371–377. doi: 10.1046/j.1365-2567.2000.00119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vollmer J, Weeratna RD, Jurk M, Samulowitz U, McCluskie MJ, Payette P, Davis HL, Schetter C, Krieg AM. Oligodeoxynucleotides lacking CpG dinucleotides mediate Toll-like receptor 9 dependent T helper type 2 biased immune stimulation. Immunology. 2004;113:212–223. doi: 10.1111/j.1365-2567.2004.01962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Honda K, Ohba Y, Yanai H, Negishi H, Mizutani T, Takaoka A, Taya C, Taniguchi T. Spatiotemporal regulation of MyD88-IRF-7 signalling for robust type-I interferon induction. Nature. 2005;434:1035–1040. doi: 10.1038/nature03547. [DOI] [PubMed] [Google Scholar]
- 54.Guiducci C, Ott G, Chan JH, Damon E, Calacsan C, Matray T, Lee KD, Coffman RL, Barrat FJ. Properties regulating the nature of the plasmacytoid dendritic cell response to Toll-like receptor 9 activation. J. Exp. Med. 2006;203:1999–2008. doi: 10.1084/jem.20060401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zabner J, Fasbender AJ, Moninger T, Poellinger KA, Welsh MJ. Cellular and molecular barriers to gene transfer by a cationic lipid. J. Biol. Chem. 1995;270:18997–19007. doi: 10.1074/jbc.270.32.18997. [DOI] [PubMed] [Google Scholar]
- 56.Radler JO, Koltover I, Salditt T, Safinya CR. Structure of DNA-cationic liposome complexes: DNA intercalation in multilamellar membranes in distinct interhelical packing regimes. Science. 1997;275:810–814. doi: 10.1126/science.275.5301.810. [DOI] [PubMed] [Google Scholar]
- 57.Koltover I, Salditt T, Radler JO, Safinya CR. An inverted hexagonal phase of cationic liposome-DNA complexes related to DNA release and delivery. Science. 1998;281:78–81. doi: 10.1126/science.281.5373.78. [DOI] [PubMed] [Google Scholar]
- 58.Yan W, Chen W, Huang L. Mechanism of adjuvant activity of cationic liposome: phosphorylation of a MAP kinase, ERK and induction of chemokines. Mol. Immunol. 2007;44:3672–3681. doi: 10.1016/j.molimm.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 59.Chen W, Wang J, An H, Zhou J, Zhang L, Cao X. Heat shock up-regulates TLR9 expression in human B cells through activation of ERK and NF-kappaB signal pathways. Immunol. Lett. 2005;98:153–159. doi: 10.1016/j.imlet.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 60.Hall JA, Bouladoux N, Sun CM, Wohlfert EA, Blank RB, Zhu Q, Grigg ME, Berzofsky JA, Belkaid Y. Commensal DNA limits regulatory T cell conversion and is a natural adjuvant of intestinal immune responses. Immunity. 2008;29:637–649. doi: 10.1016/j.immuni.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]