Abstract
Neuropoietin (NP) is a member of the gp130 cytokine family that is closely related to cardiotrophin-1(CT-1) and shares functional and structural features with other family members, including ciliary neurotrophic factor (CNTF) and cardiotrophin-like cytokine (CLC). Studies have shown that NP can play a role in the development of the nervous system, as well as affect adipogenesis and fat cell function. However, the signaling mechanisms utilized by NP in adipocytes have not been examined. In our present studies, we demonstrate that NP-induced activation of STAT3 tyrosine phosphorylation is independent of leukemia inhibitory factor receptor (LIFR) phosphorylation and degradation. Although it is widely accepted that NP signals via the LIFR, our studies reveal that NP results in phosphorylation of gp130, but not LIFR. These observations suggest that the profound effects that NP has on adipocytes are not mediated via LIFR signaling.
Keywords: Neuropoietin (NP), adipocytes, leukemia inhibitory factor receptor (LIFR)
Introduction
Neuropoietin (NP) is a gp130 cytokine that was characterized as a regulator of central nervous system development in mice that could modulate motor neuron survival in vitro [1]. NP was shown to be highly expressed in mouse embryonic neuroepithelia [1] and has been shown to be capable of inducing neuroepithelial cells to differentiate into astrocytes [2]. Though the studies of NP action had been limited to the nervous system, studies from our laboratory have also examined the effects of NP in adipocytes [3]. Our data indicate that NP is a potent activator of Signal Transducer and Activator of Transcription (STAT) 3 and MAPK (ERKs 1 and 2) in both 3T3-L1 adipocytes in vitro and adipose tissue in vivo. Moreover, NP was shown to inhibit adipogenesis and have profound effects on insulin signaling both in vitro and in vivo.
Though all members of the gp130 cytokine family utilize glycoprotein 130 (gp130) as a common signal transducer in the receptor complex for signaling, these cytokines exert differential effects on multiple tissues. This specificity can be partially attributed to the presence of other receptor components that are required for activation, including the leukemia inhibitory factor (LIF) receptor and the cytokine's specific α receptor. A common feature of many gp130 cytokines is their shared use of not only gp130, but also of LIFR proteins as components of their receptor complex. Numerous studies have shown gp130 cytokine signaling results in LIFR activation (reviewed in [4]), which is followed by LIFR internalization and degradation in various cell types, including adipocytes [5-7]. It is also well established that gp130 cytokines induce the tyrosine phosphorylation of their recruited signal transducer receptors, including the LIFR, upon ligand binding (reviewed in [4].
NP has been reported to utilize a membrane receptor complex consisting of ciliary neurotrophic factor (CNTF) receptor α, gp130, and the LIF receptor for signal transduction [1]. Our novel observations in this study demonstrate that NP does not induce LIFR activation, as judged by both LIFR degradation and LIFR tyrosine phosphorylation. In addition, NP does not induce the activation of OSMRβ, a receptor used by oncostatin (OSM) that has substantial structural homology to the LIFR [8]. Overall, these studies demonstrate that STAT3 activation induced by NP in adipocytes is not mediated via LIFR signaling.
Materials and Methods
Materials
Dulbecco's Modified Eagle's Media (DMEM) was purchased from Sigma. Bovine and fetal bovine (FBS) sera were purchased from Hyclone. Mouse recombinant NP, mouse recombinant CT-1, mouse recombinant OSM, and mouse recombinant OSMRβ antibodies were all purchased from R&D Systems. Recombinant human OSM was purchased from Invitrogen, and mouse recombinant LIF was purchased from BD Transduction. Cycloheximide was purchased from Sigma, and 4G10 was purchased from Millipore (previously Upstate). Phospho-specific STAT3 (Y705) monoclonal antibody was purchased from BD Transduction. Both the gp130 and LIFR polyclonal antibodies were purchased from Santa Cruz. Protein-A agarose was purchased from Repligen Corporation. Nitrocellulose was purchased from Bio-Rad. The BCA kit and the enhanced chemiluminescence kit were purchased from Pierce. Horseradish peroxidase (HRP)-conjugated secondary antibodies were purchased from Jackson ImmunoResearch Laboratories.
Cell Culture
Murine 3T3-L1 preadipocytes were plated and grown to 2 days post-confluence in DMEM containing 10% bovine serum. Medium was changed every 48 h. Cells were induced to differentiate by changing the medium to DMEM containing 10% fetal bovine serum (FBS), 0.5 mM 3-isobutylmethylxanthine, 1 μM dexamethasone, and 1.7 μM insulin (MDI). After 48 h, this medium was replaced with DMEM supplemented with 10% FBS, and cells were maintained in this medium until utilized for experimentation.
Preparation of Whole Cell Extracts
Cell monolayers of 3T3-L1 adipocytes were harvested in a non-denaturing buffer containing 150 mM NaCl, 10 mM Tris pH 7.4, 1mM EGTA, 1mM EDTA, 1% Triton-X 100, 0.5% Igepal CA-630, 1 μM phenylmethylsulfonyl fluoride (PMSF), 1 μM pepstatin, 50 trypsin inhibitory milliunits of aprotinin, 10 μM leupeptin, and 2 mM sodium vanadate and frozen. Next, the samples were thawed and centrifuged at 14,000 × g at 4°C for 10 minutes. Supernatants containing whole cell extracts were analyzed for protein content using a BCA kit according to the manufacturer's instructions.
Immunoprecipitations of LIFR, OSMRβ, or gp130 from adipocyte extracts
Cell monolayers of 3T3-L1 adipocytes were harvested under non-denaturing conditions following cytokine treatment, and the protein content was determined as described above. The protein extracts were preincubated with protein-A agarose, and the resulting supernatants were then incubated with 5 μg of either the LIFR, OSMRβ, or gp130 antibody for 2 hours at 4°C. Protein-A agarose was added to the mixture, and the samples were incubated for an additional hour. Bound LIFR, OSMRβ, or gp130 and any associated proteins were isolated by pelleting this mixture. The pellets were rinsed twice with phosphate-buffered saline (PBS), and bound proteins were eluted from the agarose by incubation at 100°C for 10 minutes after the addition of Laemmli sample buffer. These samples were separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and analyzed by Western blotting with 4G10 antibody.
Gel Electrophoresis and Western Blot Analysis
Proteins were separated in 7.5% polyacrylamide (acrylamide from Nationl Diagnostics) gels containing sodium dodecyl sulfate (SDS) according to Laemmli [9] and transferred to nitrocellulose membrane in 25 mM Tris, 192 mM glycine, and 20% methanol. Following transfer, the membrane was blocked overnight in 4% milk at 4°C. Results were visualized with horseradish peroxidase (HRP)-conjugated secondary antibodies and enhanced chemiluminescence.
Results
Previous studies demonstrated that several gp130 cytokines, notably LIF, CT-1, and hOSM, induce STAT3 tyrosine phosphorylation that is accompanied by LIFR degradation in adipocytes [5]. Since NP activates STAT3 in adipocytes in vitro and in vivo [3], we examined the ability of this gp130 cytokine to modulate LIFR degradation in adipocytes. Fully differentiated 3T3-L1 adipocytes were treated with cycloheximide (CH) in the presence or absence of LIF or NP. As shown in Figure 1, the expression levels of LIFR in adipocytes treated with LIF dramatically decreased within 1-hr as previously reported [5]. However, NP treatment did not cause a substantial decrease in LIFR degradation. The efficacy of both LIF and NP was shown by the activation of STAT3 Tyr705. We also observed that NP failed to induce LIFR degradation in differentiated OP9 mouse stromal cells (data not shown), a newly characterized adipocyte cell culture model [10].
Fig. 1. NP does not induce LIFR degradation.
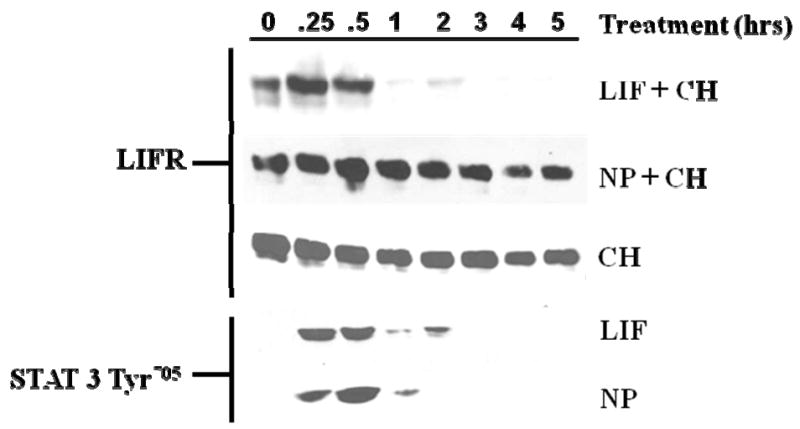
Fully differentiated 3T3-L1 adipocytes were treated with either 1 nM LIF or 1 nM NP in the presence of 5 μM cycloheximide (CH). Whole cell extracts were prepared from adipocytes that were untreated (0) or treated at the indicated times. 125 μg of extract was separated by SDS-PAGE, transferred to nitrocellulose, and subjected to Western blot analysis. This is a representative experiment independently performed three times.
It is well established that gp130 cytokines induce the tyrosine phosphorylation of their recruited signal transducer receptors, including the LIFR, upon ligand binding (reviewed in [4]). Since our results indicated that NP did not induce LIFR degradation, we examined the ability of NP to induce the tyrosine phosphorylation of the LIFR, since NP has been reported to signal via the LIFR [1]. The ability of gp130 cytokines to phosphorylate the LIFR was examined by immunoprecipitating LIFR from whole cell extracts and immunoblotting with 4G10, an anti-phosphotyrosine antibody. As shown in Figure 2A, both LIF and hOSM, but not NP, induced the phosphorylation of the LIFR, and this effect was not attributed to differences in LIFR expression. The lack of LIFR phosphorylation following mOSM stimulation supports previous studies that revealed an alternative receptor complex in murine cells for this cytokine [11]. The efficacy of each cytokine was shown by the activation of STAT3 Tyr705. These surprising observations indicated that although previously reported to signal via the LIFR [1], NP does not induce phosphorylation of the LIFR. Hence, we examined the ability of NP to activate oncostatin M receptor β (OSMRβ), which is similar in structure to the LIFR and is utilized by mOSM for signal transduction [8]. The results in Figure 2B demonstrate that mOSM, but not NP, induced the tyrosine phosphorylation of the OSMRβ. The efficacy of each cytokine was shown by the activation of STAT3 Tyr705. Although NP did not induce LIFR or OSMRβ activation, the results in Figure 2C demonstrate that NP was capable of inducing gp130 tyrosine phosphorylation in a manner equivalent to CT-1, LIF, and mOSM.
Fig. 2. NP treatment induces the tyrosine phosphorylation of gp130, but not LIFR or OSMRβ in 3T3-L1 adipocytes.
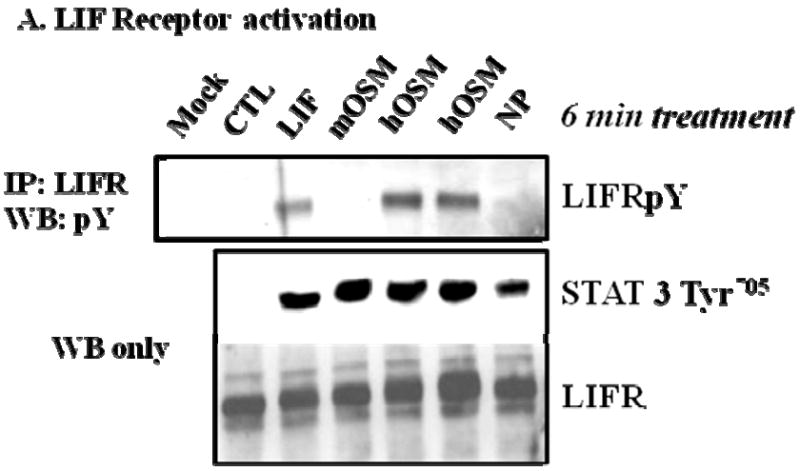
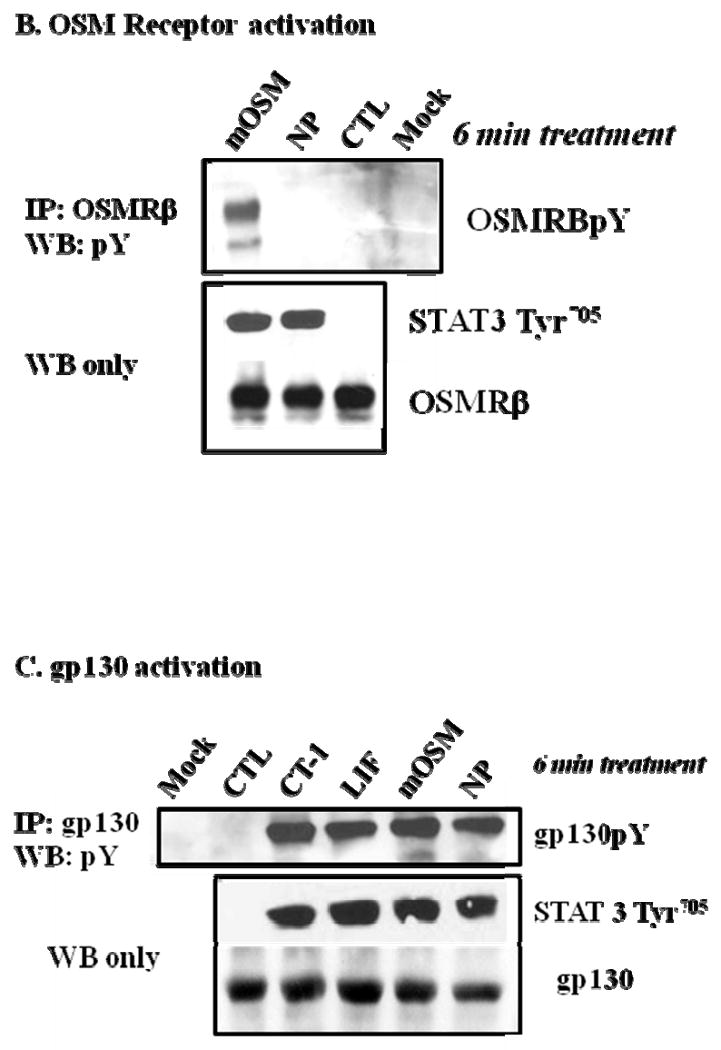
(A) Whole cell extracts were isolated from fully differentated 3T3-L1 adipocytes that were harvested after vehicle treatment (CTL) or an acute (6 min.) treatment with a 1 nM of either CT-1, LIF, mOSM, hOSM, or NP. Each extract was subjected to immunoprecipitation (IP) with an antibody directed against the LIFR (A), OSMRβ (B), or gp130 (C) and Western blotting analysis with 4G10, an anti-phosphotyrosine antibody. Either 700 μg (A), 600 μg (B), or 650 μg (C) of protein was used for IP, and 90 μg of protein was used for Western blotting. For each IP experiment, a mock control that contained everything but cell extract was used. Each figure is a representative experiment independently performed four times.
Discussion
Although NP was originally characterized as a cytokine that utilized the LIFR for signal transduction [1], our novel data indicate that NP does not induce the phosphorylation or degradation of the LIFR (Figures 1 and 2A) in the same manner as other gp130 cytokines in adipocytes. Although NP induces gp130 and STAT3 tyrosine phosphorylation, this occurs in a manner that is unaccompanied by the activation of either the LIFR or OSMRβ. While several lines of evidence support significant roles for NP in neuronal development [1; 2], the actions of NP in other tissues have not been studied. However, NP has important functions in adipose tissue, including the ability to modulate fat cell development and insulin sensitivity [3]. Despite these significant effects, the mechanisms underlying NP signal transduction action in adipocytes have not been previously examined.
Gp130 is the shared subunit in each respective receptor complex of the gp130 cytokines, but additional receptor components can be responsible for specific actions in particular cell types. Therefore, understanding the signal transducing complex for each cytokine is critical. It is known that NP activates STAT3, as do other gp130 cytokines, in both neuroepithelial cells and adipocytes. As a result of its initial characterization in the nervous system, it is largely accepted that NP-induced STAT3 activation is mediated by a receptor complex composed of LIFR, CNTFRα, and gp130 [1]. However, our investigations show conflicting evidence for LIFR activation by NP in adipocytes. We postulate that NP action on adipocytes is independent of LIFR activation. Although the complete identify of the NP receptor complex in adipocytes has not been characterized, we have shown that gp130, but not LIFR or OSMRβ (Figure 2) plays a role in NP signaling in adipocytes. Further studies are necessary to determine the complex signaling mechanisms of NP in adipocytes, as well other cell types.
Acknowledgments
This work was supported by grant R01 DK052968-11 from the National Institutes of Health to J.M.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Derouet D, Rousseau F, Alfonsi F, Froger J, Hermann J, Barbier F, Perret D, Diveu C, Guillet C, Preisser L, Dumont A, Barbado M, Morel A, deLapeyriere O, Gascan H, Chevalier S. Neuropoietin, a new IL-6-related cytokine signaling through the ciliary neurotrophic factor receptor. Proc Natl Acad Sci U S A. 2004;101:4827–4832. doi: 10.1073/pnas.0306178101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohno M, Kohyama J, Namihira M, Sanosaka T, Takahashi JA, Hashimoto N, Nakashima K. Neuropoietin induces neuroepithelial cells to differentiate into astrocytes via activation of STAT3. Cytokine. 2006;36:17–22. doi: 10.1016/j.cyto.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 3.White UA, Stewart WC, Mynatt RL, Stephens JM. Neuropoietin attenuates adipogenesis and induces insulin resistance in adipocytes. J Biol Chem. 2008;283:22505–22512. doi: 10.1074/jbc.M710462200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zvonic S, Baugh JE, Jr, Arbour-Reily P, Mynatt RL, Stephens JM. Cross-talk among gp130 cytokines in adipocytes. J Biol Chem. 2005;280:33856–33863. doi: 10.1074/jbc.M508020200. [DOI] [PubMed] [Google Scholar]
- 6.Blanchard F, Duplomb L, Wang Y, Robledo O, Kinzie E, Pitard V, Godard A, Jacques Y, Baumann H. Stimulation of leukemia inhibitory factor receptor degradation by extracellular signal-regulated kinase. J Biol Chem. 2000;275:28793–28801. doi: 10.1074/jbc.M003986200. [DOI] [PubMed] [Google Scholar]
- 7.Blanchard F, Wang Y, Kinzie E, Duplomb L, Godard A, Baumann H. Oncostatin M regulates the synthesis and turnover of gp130, leukemia inhibitory factor receptor alpha, and oncostatin M receptor beta by distinct mechanisms. J Biol Chem. 2001;276:47038–47045. doi: 10.1074/jbc.M107971200. [DOI] [PubMed] [Google Scholar]
- 8.Mosley B, De IC, Friend D, Boiani N, Thoma B, Park LS, Cosman D. Dual oncostatin M (OSM) receptors. Cloning and characterization of an alternative signaling subunit conferring OSM-specific receptor activation. J Biol Chem. 1996;271:32635–32643. doi: 10.1074/jbc.271.51.32635. [DOI] [PubMed] [Google Scholar]
- 9.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 10.Wolins NE, Quaynor BK, Skinner JR, Tzekov A, Park C, Choi K, Bickel PE. OP9 mouse stromal cells rapidly differentiate into adipocytes: characterization of a useful new model of adipogenesis. J Lipid Res. 2006;47:450–460. doi: 10.1194/jlr.D500037-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.Ichihara M, Hara T, Kim H, Murate T, Miyajima A. Oncostatin M and leukemia inhibitory factor do not use the same functional receptor in mice. Blood. 1997;90:165–173. [PubMed] [Google Scholar]


