Abstract
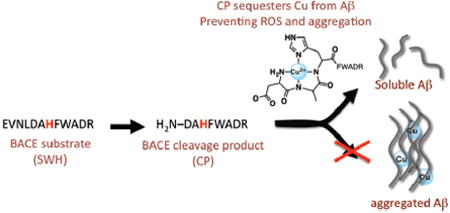
The intersection of the amyloid cascade hypothesis and the implication of metal ions in Alzheimer disease progression has sparked an interest in using metal-binding compounds as potential therapeutic agents. In the present work, we describe a prochelator SWH that is enzymatically activated by β-secretase to produce a high affinity copper chelator CP. Because β-secretase is responsible for the amyloidogenic processing of the amyloid precursor protein, this prochelator strategy imparts disease specificity towards copper chelation not possible with general metal chelators. Furthermore, once activated, CP efficiently sequesters copper from amyloid-β, prevents and disassembles copper-induced amyloid-β aggregation, and diminishes copper-promoted reactive oxygen species formation.
Amyloid plaque formation in the brain plays a central role in the cognitive dysfunction characteristic of Alzheimer’s Disease (AD).1,2 The plaques are composed primarily of 39–43 amino acid amyloid-β peptides (Aβ) that are derived by enzymatic cleavage of the amyloid precursor protein (APP) by β- and γ-secretases.3 The extracellularly released Aβ is then able to bind metal ions such as copper and zinc, which not only exacerbate plaque formation but, in the case of copper, can also contribute to the formation of neurotoxic reactive oxygen species (ROS).4
Currently approved treatments for AD are limited and only temper symptoms without preventing or reversing disease progression. To change this paradigm, a focus on targeting the molecular basis of pathogenesis has lead to searches for secretase inhibitors, particularly of β-secretase (BACE).5 Additionally, with the implication of metals in AD pathogenesis,6 new drugs such as clioquinol and PBT2 that attenuate metal localization have also made their way into clinical trials.7 While general metal chelation strategies may address some of the underlying processes associated with AD, they have a shortcoming in their limited ability to differentiate toxic metals associated with Aβ plaques from those associated with normal metal homeostasis.
We previously introduced the concept of a prochelator as an agent that does not interact with metal ions until activated to its chelator form under specific conditions, for example elevated H2O2 levels.8-10 Here we present a peptide prochelator that is enzymatically activated by BACE to yield a high affinity copper chelator (Fig. 1). The utilization of a prodrug design allows the metal-binding functionality to be activated at the site of Aβ production and specifically target copper in the Aβ-Cu complex. Furthermore, once copper is bound its reactivity to promote ROS formation via Fenton chemistry is significantly reduced.
Figure 1.
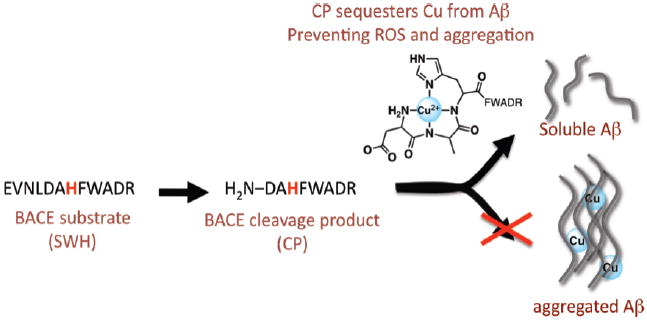
The β-secretase substrate has weak metal affinity, but after selective proteolytic cleavage, the product sequesters copper from Aβ, preventing aggregation. When copper is coordinated to Aβ it catalyzes production of ROS, but sequestration by CP prevents such deleterious reactions.
In order to generate a prochelator that would be a good substrate for BACE and that would release a copper chelating agent as the product, we used the known APP Swedish mutant sequence (EVNLDAEF, representing residues 668–675 and abbreviated SW) as a blueprint, since it is a better substrate for BACE than native APP.11 The cleavage site is located between the leucine and aspartic acid residues. For the prochelator SWH, we replaced the second glutamic acid of SW with a histidine so that BACE cleavage would release the chelator peptide CP, with the N-terminal sequence DAHF. Peptides with an N-terminal free amine and a histidine in the third position are known as ATCUN motifs (amino terminal Cu and Ni binding), and are found natively on human serum albumin (HSA), one of the proteins responsible for binding Cu2+ in serum.12 To facilitate concentration determination and product identification, residues WHDR and WADR were incorporated onto the ends of the SW peptide and the SWH prochelator peptide13, respectively, to give the final sequences in Table 1. The H was changed to A in SWH to avoid metal binding. Peptides were prepared by standard Fmoc solid-phase peptide synthesis.
Table 1.
Description of peptides discussed in the text, along with conditional stability constants for Cu2+ at pH 7.4 (log K’). “Ac” denotes an N-terminal acetyl cap and “-NH2” a C-terminal amide.
Conversion of SWH to CP was achieved by incubating SWH with BACE and analyzing aliquots at various time intervals by liquid-chromatography-mass spectrometry (LC-MS). The tryptophan tag in both SWH and CP allowed for a direct comparison of peak areas at 280 nm on the LC trace, while the in-line electrospray mass spectrometer provided mass identification of the products to confirm that cleavage occured at the intended location. This analysis provided initial rates of 0.2% per min for both SWH and SW at 155 μM substrate concentration, indicating that SWH is as good a substrate as SW for BACE (Supp Info).
Once activated, the cleavage product CP has a high affinity for Cu2+. Competition studies with nitrilotriacetic acid in HEPES buffer at pH 7.4 provide a conditional stability constant (K’) corrected for the ternary NTA(Cu)(HEPES) complex of 1012.6, which is similar to other reports on the ATCUN motif found in HSA.14 In contrast, SWH at pH 7.4 is unable to compete with even the weak Cu2+ ligand glycylglycine (Supp Info). This result indicates that copper binding by prochelator SWH would be inconsequential in a biological system. The summary of relevant conditional stability constants provided in Table 1 predicts that CP should be able to strip Cu2+ from Aβ.
As predicted and shown by fluorescence titration experiments in the Supp Info, Cu2+ readily transfers from Aβ to CP. The binding of paramagnetic metal ions to peptides quenches the fluorescence of aromatic residues tryptophan and tyrosine, and indeed emission from the sole Tyr in Aβ decreases in the presence of Cu2+. When Trp-containing CP is added to this solution, the fluorescence signal remains quenched until more than 1 equiv of CP is added, after which point emission increases linearly with CP concentration. This response is consistent with CP extracting Cu2+ from Aβ, which prevents Trp emission until the concentration of CP exceeds that of Cu2+. When the experiment is repeated using SWH as the competitor, fluorescence increases linearly with added SWH, confirming that the prochelator is unable to strip Cu2+ from Aβ, as predicted from the thermodynamic data.
By sequestering Cu2+ from Aβ, CP also displays an ability to inhibit Cu2+-induced Aβ aggregate formation, as verified by the light-scattering turbidity assay shown in Fig. 2. As expected, SWH is unable to inhibit fibril formation while CP shows a protective effect at 1:1 CP:Cu stoichiometry. This result is consistent with peptides of similar sequence reported by others.16 Predictably, CP is not as effective at binding Zn2+ and preventing Zn2+-induced aggregation. Importantly, the reaction mixture from SWH and BACE incubations (but not SW + BACE) is able to both prevent and disaggregate preformed Aβ-Cu aggregates, as shown by the green bars in Fig. 2.
Figure 2.
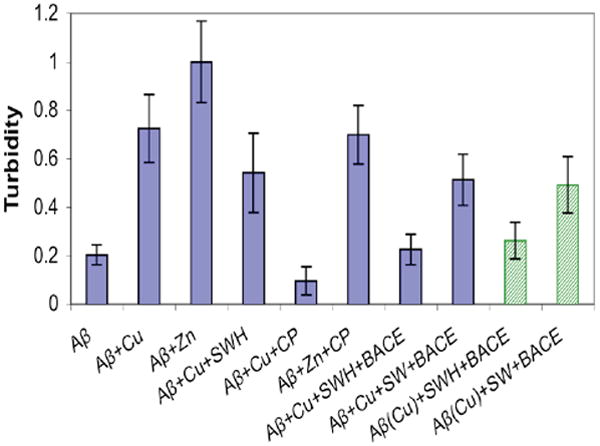
Turbidity of 10 μM Aβ samples in HEPES buffer pH 7.4, as determined by the normalized change in A405nm Where indicated, 1 equiv of Cu(Gly)2, ZnCl2, CP, or SWH were added and incubated at 37 °C for 1 h to monitor aggregation prevention (blue bars). Products from SW or SWH plus BACE reactions were also tested for disaggregation of preformed CuAβ aggregates (green dotted bars).
Along with sequestering copper and preventing fibril formation, CP also shows an ability to prevent ROS formation promoted by copper and Aβ-Cu species. Redox-active Cu2+/+ can catalyze OH• formation from H2O2 in Fenton-like reaction cycles.17 As shown in Fig. 3, CP effectively protects against copper-catalyzed OH• formation, as determined by the deoxyribose assay, a result that is consistent with others in the literature on similar peptide sequences.18 SWH, on the other hand, offers only limited protection, even at concentrations as high as 100 μM. The observed response for SWH may be due to some radical quenching at high concentrations, but the data corroborate the inability of the prochelator to bind copper.
Figure 3.
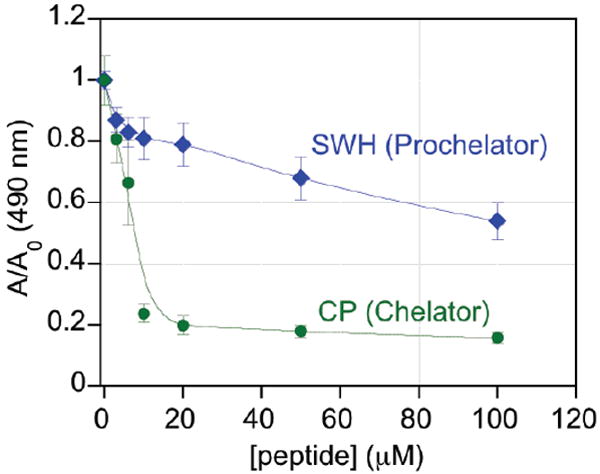
Effect of peptides on deoxyribose degradation by OH• generated by Cu-promoted Fenton chemistry. A decrease in A/A0 indicates a protective effect. Conditions: 100 μM H2O2, 10 μM Cu(SO4), 2 mM ascorbic acid, and 15 mM 2-deoxyribose in 50 mM NaH2PO4 buffered to pH 7.4.
Under reducing conditions, the Aβ-Cu complex reacts with O2 to generate H2O2.19 An Amplex Red assay was used to show that CP also prevents this ROS formation, whereas SWH does not show an inhibitory effect (See Supp Info).
In summary, we present a prochelator SWH that, once activated by BACE, is able to sequester copper from Aβ, prevent and disassemble aggregate formation, and protect against copper-promoted H2O2 and OH• formation. Because these activities require activation by BACE, an enzyme active in AD brains, this strategy imparts site specificity for chelating copper only when BACE activity is elevated. Because a peptide drug is unlikely to cross the blood brain barrier or withstand the multitude of proteases found in the blood, future work includes improving CP’s drug-like properties.
Supplementary Material
Acknowledgments
We thank the National Institutes of Health (grant GM084176), the Sloan Foundation, and the Camille and Henry Dreyfus Foundation for supporting this work.
Footnotes
Supporting Information Available. Full experimental details and additional results are available free of charge via the internet at http://pubs.acs.org.
References
- 1.Hardy J, Selkoe DJ. Science. 2002;297:353. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 2.Crouch PJ, Harding S-ME, White AR, Camakaris J, Bush AI, Masters CL. Int J Biochem Cell Biol. 2008;40:181–198. doi: 10.1016/j.biocel.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 3.Selkoe DJ. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 4.Faller P. ChemBioChem. 2009;10:2837–2845. doi: 10.1002/cbic.200900321. [DOI] [PubMed] [Google Scholar]
- 5.Tomita T. Exp Rev Neurotherap. 2009;9:661–679. doi: 10.1586/ern.09.24. [DOI] [PubMed] [Google Scholar]
- 6.Adlard PA, Bush AI. J Alzheim Dis. 2006;10:145–163. doi: 10.3233/jad-2006-102-303. [DOI] [PubMed] [Google Scholar]
- 7.Bush AI, Tanzi RE. Neurotherapeutics. 2008;5:421–432. doi: 10.1016/j.nurt.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charkoudian LK, Pham DM, Franz KJ. J Am Chem Soc. 2006;128:12424–12425. doi: 10.1021/ja064806w. [DOI] [PubMed] [Google Scholar]
- 9.Dickens MG, Franz KJ. ChemBioChem. 2010;11:59–62. doi: 10.1002/cbic.200900597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.For a review on other multifunctional metal chelators, see: Perez LR, Franz KJ. Dalton Trans. 2010;9:2177–2187. doi: 10.1039/b919237a..
- 11.Lin XL, Koelsch C, Wu SL, Downs D, Dashti A, Tang J. Proc Natl Acad Sci USA. 2000;97:1456–1460. doi: 10.1073/pnas.97.4.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harford C, Sarkar B. Acc Chem Res. 1997;30:123–130. [Google Scholar]
- 13.Turner RT, III, Koelsch G, Hong L, Castenheira P, Ghosh A, Tang J. Biochemistry. 2001;40:10001–10006. doi: 10.1021/bi015546s. [DOI] [PubMed] [Google Scholar]
- 14.Rózga M, Sokolowska M, Protas AM, Bal W. J Biol Inorg Chem. 2007;12:913–918. doi: 10.1007/s00775-007-0244-8. [DOI] [PubMed] [Google Scholar]
- 15.Hatcher LQ, Hong L, Bush WD, Carducci T, Simon JD. J Phys Chem B. 2008;112:8160–8164. doi: 10.1021/jp710806s. [DOI] [PubMed] [Google Scholar]
- 16.Perrone L, Mothes E, Vignes M, Mockel A, Figueroa C, Miquel M-C, Maddelein M-L, Faller P. ChemBioChem. 2009;11:110–118. doi: 10.1002/cbic.200900474. [DOI] [PubMed] [Google Scholar]
- 17.Guilloreau L, Combalbert S, Sournia-Saquet A, Mazarguil H, Faller P. ChemBioChem. 2007;8:1317–1325. doi: 10.1002/cbic.200700111. [DOI] [PubMed] [Google Scholar]
- 18.Bar-Or D, Rael LT, Lau EP, Rao NKR, Thomas GW, Winkler JV, Yukl RL, Kingston RG, Curtis G. Biochem Biophys Res Commun. 2001;856:862. doi: 10.1006/bbrc.2001.5042. [DOI] [PubMed] [Google Scholar]
- 19.Huang XD, Atwood CS, Hartshorn MA, Multhaup G, Goldstein LE, Scarpa RC, Cuajungco MP, Gray DN, Lim J, Moir RD, Tanzi RE, Bush AI. Biochemistry. 1999;38:7609–7616. doi: 10.1021/bi990438f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


