Abstract
Background
The growth kinetics of untreated solid organ malignancies are not defined. Radiographic active surveillance (AS) of renal tumors in patient unfit or unwilling to undergo intervention provides an opportunity to quantitate the natural history of untreated localized tumors. Here we report the radiographic growth kinetics of renal neoplasms during a period of surveillance.
Methods
We identified patients with enhancing renal masses who were radiographically observed for at least 12 months. Clinical and pathological records were reviewed to determine tumor growth kinetics and clinical outcomes. Tumor growth kinetics were expressed in terms of absolute and relative linear and volumetric growth.
Results
We identified 172 renal tumors in 154 patients under AS. Median tumor diameter and volume on presentation was 2.0 cm (mean 2.5, range 0.4 - 12.0) and 4.18 cm3 (mean 20.0, range 0.0033 – 904). Median duration of follow-up was 24 months (mean 31, range 12 – 156). A significant association between presenting tumor size and proportional growth was noted, with smaller tumors growing faster than larger tumors. 39% (68/173) of tumors underwent delayed intervention and 84% (57/68) were pathologically malignant. Progression to metastatic disease was noted in 1.3% (2/154) of patients.
Conclusions
We demonstrate the association between a tumor’s volume and subsequent growth with smaller tumors exhibiting significantly faster volumetric growth than larger tumors, consistent with Gompertzian kinetics. Surveillance of localized renal tumors is associated with a low rate of disease progression in the intermediate term and suggests potential over-treatment biases in select patients.
Keywords: Active Surveillance, Growth Kinetics, Kidney Neoplasms
Introduction
The natural history of untreated solid human tumors has not been defined. Most malignancies diagnosed by endoscopic, clinical or radiographic means are promptly treated due to the perceived risk of disease progression, the uncertainty of active surveillance, legal, social and/or financial forces all of which bias toward treatment. However, accumulating data in low risk prostate and kidney cancer suggest that the latency period between local disease and the development of metastases may be longer than believed1, 2.
A significant increase in the age adjusted incidence of localized renal cancer (RC) has been observed due to routine use of cross sectional abdominal imaging to evaluate abdominal symptomatology3. The greatest increase has been noted in patients in the seventh to ninth decades of life4. The increased detection of renal tumors in elderly patients and those with multiple competing disease states has led to the active surveillance (AS) of renal tumors in select patients. Although surgical excision remains the standard of care for localized renal tumors5, several series have examined the intermediate oncologic outcomes of AS and provide valuable insight into the malignant potential of localized renal tumors2, 6. A recent comprehensive analysis of the AS series in kidney tumors noted that the majority of tumors demonstrate slow interval growth, with a mean observed linear growth rate of 0.28 cm/yr over a median follow-up of 32 months2. Further data have noted that a substantial proportion (25-35%) of renal tumors exhibit zero radiographic growth when followed a median of 29 months7.
Presently, AS of renal tumors is reserved for patients who are unfit or unwilling to proceed with surgical intervention, with the primary deterrent being the uncertainty of growth and the risk of metastatic disease. However, recent series have suggested that a period of AS followed by delayed primary intervention is not associated with alterations in treatment plan or stage migration8, 9. An understanding of the growth kinetics of solid tumors will aide in developing thresholds to trigger interventions in patients who choose an initial period of AS. Here we report the largest series investigating the growth kinetics of any solid organ human neoplasm undergoing a period of radiographic active surveillance. Additionally, we summarize pathologic features of tumors undergoing definitive treatment and the clinical outcomes of renal tumors continuing AS.
Methods
Patient Selection
Our IRB approved institutional renal tumor database was reviewed for solid or cystic tumors with enhancing components that were radiographically observed for at least 12 months from January 2000 through July of 2007. All lesions were locally confined to the kidney based upon standard radiographic staging protocols. Patients with hereditary RC were excluded from analysis.
Variables examined included patient age, gender, indication for AS, tumor size (maximal cross-sectional diameter and volume) on presentation, duration of AS, presence of multifocal renal tumors, tumor growth (linear and volumetric), surgical pathology, and local and/or systemic disease progression.
Indications for AS and Delayed Intervention
Indications for AS were categorized as absolute, relative and elective. Absolute indications include patients who were not surgical candidates based upon medical co-morbidities which were deemed prohibitive. Relative indications for AS include the desire to avoid the potential need for renal replacement therapy following treatment of the renal tumor(s). Elective indications for AS include patients who refuse surgical intervention despite being at lower operative and nephrologic risk.
Indications for delayed interventions included: interval tumor growth, improved medical risk and/or performance status, interval change in clinical circumstances (ie: development of tumor related symptoms) or patient choice (i.e.: anxiety, familial pressure). Surgical management was categorized as radical or nephron sparing. Nephron sparing techniques included open and laparoscopic partial nephrectomies and ablative techniques. Surgical management was also categorized as minimally invasive or open procedures.
Tumor Growth Kinetics
Radiographic follow-up was performed at 3-6 month intervals using cross-sectional abdominal imaging (CT or MRI). Tumor size was measured as the maximal cross-sectional diameter. Lesion measurements were performed at consistent levels within the kidney by direct comparison to existing studies. Tumor volume was calculated using the maximal cross sectional tumor diameter, with the equation: V = 0.523x3. Absolute tumor growth rate for change in tumor diameter and volume were expressed as the change in tumor size per year, ATD and ATV respectively. Relative change in tumor diameter and volume were expressed as percentage change in tumor size per year, PTD and PTV respectively. Doubling time could not be used to evaluate changes in tumor volume because of the significant number of tumors that did not demonstrate interval growth.
PTD was calculated using the equation:
D0 = tumor diameter at time zero
D1 = tumor diameter at last follow-up
T = duration of follow-up in years
PTV was calculated using the equation:
V0 = tumor volume at time zero
V1 = tumor volume at last follow-up
T = duration of follow-up in years
Pathologic Assessment
Surgical pathology was reviewed for all tumors undergoing definitive treatment. Clinical and pathologic stages were assigned using the 2002 American Joint Committee on Cancer/UICC TNM guidelines. In addition to abdominal cross sectional contrast based imaging, surveillance for metastatic disease in patients continuing AS was performed at six to twelve monthly intervals and included chest x-ray, hepatic function tests, and a bone scan in symptomatic patients. Local progression was defined as the development of previously undetected enhancing renal tumors in the ipsilateral kidney.
Statistical Analysis
Nominal predictors of linear and volumetric growth were assessed using the Wilcoxon rank-sum test or the Kruskal-Wallis test, as appropriate. Continuous predictors of growth were assessed using linear regression. To satisfy the assumptions of linear regression, growth rates were logarithmically transformed. Categorical predictors and outcomes were analyzed using the chisquared test of independence. Descriptive statistics and hypothesis tests were performed using JMP software (SAS, Cary, North Carolina). All tests were two sided with 5% type 1 error. No adjustments were made for multiple testing. Waterfall plots were constructed using Microsoft Excel.
Results
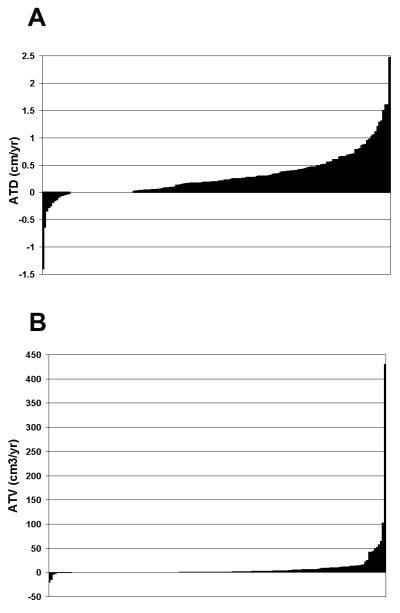
We identified 173 enhancing renal tumors in 154 patients followed radiographically for at least 12 months. Multifocal disease was noted in 7.8% (12/154) of patients, accounting for 31 tumors. Table 1 provides patient demographics and tumor characteristics on presentation. Figure 1 demonstrates the observed ATD (1a) and ATV (1b) rates for individual lesions expressed as a waterfall graph. The majority, 74% (128/173), of tumors demonstrated interval growth, while the remaining 26% (45/173) demonstrated negative or no growth. Median size, on presentation were similar between tumors that did and did not demonstrate interval growth, p = 0.779. Table 2 summarizes ATD, ATV, PTD and PTV for all patients and selected clinicopathologic variables.
Table 1.
Patient Demographics and Tumor Characteristics on Presentation
| Gender | |
| Female (%) | 43 (28) |
| Male (%) | 111 (72) |
|
| |
| Age (yr) | Median 71 |
| Mean 69 | |
| Range 35 - 88 | |
|
| |
| Tumor size | |
| Maximal diameter (cm) | Median 2.00 |
| Mean 2·45 | |
| Range 0·40 - 12·00 | |
| Volume (cm3) | Median 4·18 |
| Mean 20·0 | |
| Range 0·033 - 904 | |
|
| |
| Radiographic characteristics | |
| Solid (%) | 147 (85) |
| Partially Cystic(%) | 26 (15) |
| Solitary lesion (%) | 142 (82) |
| Multifocal lesions (%) | 31 (18) |
|
| |
| Indication for AS | |
| Elective (%) | 115 (75) |
| Relative (%) | 21 (13) |
| Absolute (%) | 18 (12) |
|
| |
|
Duration of Follow-Up (months) |
Median 24 |
| Mean 31 | |
| Range 12 - 156 | |
Figure 1.
Observed ATD (panel A) and ATV (panel B) of all tumors undergoing active surveillance. Shaded areas at or below the zero axis represents no or negative interval tumor growth. Shaded area above the zero axis represents positive interval tumor growth.
Table 2.
Tumor Growth Rates: Overall and Associations with Clinicopathologic Variables
| Overall Growth per Year (n=173) | ||||
|---|---|---|---|---|
| ATD | ATV | PTD | PTV | |
| Median | 0.145 | 3 | 9.6 | 36 |
| Mean (STD) | 0.285(0.41) | 17.0(71.6) | 15(23) | 104(250) |
| Range | −1.40 - 2.47 | −20.0 - 431 | −42.2 - 160 | −81 - 2043 |
| Growth Rates and Clinicopathologic Variables | ||||
|---|---|---|---|---|
| ATD | ATV | PTD | PTV | |
| Gender | ||||
| Male (n=111) | 0.19 (0.29) | 1.19 (9.49) | 9.02 (15.0) | 33.2 (109.8) |
| Female (n=43) | 0.19 (0.34) | 2.00 (5.93 | 10.6 (17.4) | 36.9 (105.5) |
| p value | 0.312 | 0.151 | 0.501 | 0.558 |
| Age (n=173) | ||||
| R2 | 0.0164 | 0.000161 | 0.00186 | 0.0130 |
| p value | 0.114 | 0.876 | 0.596 | 0.160 |
| Size | ||||
| <2cm (n=71) | 0.25 | 2.67 | 0.22 | 3.55 |
| 2-4cm (n=80) | 0.30 | 17.23 | 0.11 | 2.07 |
| >4cm (n=22) | 0.37 | 62.77 | 0.07 | 0.52 |
| p value | 0.783 | 0.0001 | 0.025 | 0.017 |
| Multifocality | ||||
| Solitary (n=160) | 0.28 | 12.6 | 0.15 | 2.45 |
| Multifocal (n=12) | 0.28 | 5.4 | 0.18 | 3.01 |
| p value | 0.577 | 0.966 | 0.219 | 0.183 |
| Pathology | ||||
| Benign (n=9) | 0.13 | 3.72 | 0.23 | 8.25 |
| Low Grade (n=40) | 0.34 | 32.2 | 0.15 | 1.67 |
| High Grade (n=12) | 0.53 | 16.5 | 0.36 | 3.98 |
| p value | 0.629 | 0.230 | 0.396 | 0.405 |
ATD = Absolute tumor diameter (change in tumor diameter per year)
ATV = Absolute tumor volume (change in tumor volume per year)
PTD = Percent tumor diameter (% change in tumor diameter per year – relative change)
PTV = Percent tumor volume (% change in tumor volume per year – relative change)
Of the 173 tumors undergoing a period of AS, 39% (68/173) of tumors eventually underwent treatment. Table 3 compares clinical parameters and observed growth kinetics between patients continuing AS and those undergoing delayed intervention. Median duration of AS prior to treatment was 21 months (mean 25, range 12 - 96). Indications for terminating AS were patient choice 62% (42/68), medical clearance 7% (5/68), interval tumor growth 29% (20/68), and spontaneous retroperitoneal bleed 1% (1/68). Compared to those that continued AS, patients who underwent delayed intervention were statistically younger and had tumors with higher interval growth rates. Despite a period of active surveillance the majority, 57% (39/68), of patients undergoing delayed intervention were treated with a minimally invasive approach and most, 74% (50/68), underwent nephron sparing approaches. Table 4 demonstrates tumor pathology in those with delayed intervention. Malignant renal cell carcinoma was confirmed in 84% (57/68). Histologic subtype, pathologic stage, and nuclear grade of tumors undergoing extirpative surgery are presented in Table 4.
Table 3.
Predictors of Delayed Intervention.
| Continued AS | Delayed Intervention | p value | |
|---|---|---|---|
| Age (yr) | Median 74 Mean 72 Range 35 - 87 |
Median 66 Mean 63 Range 35 - 88 |
0·0001 |
| Gender (male/total) | 73% (65/89) | 71% (46/65) | 0·856 |
| Size at Presentation (cm) | Median 2·0 Mean 2·49 Range 0·8 - 9 |
Median 2 Mean 2·38 Range 0·4 - 12 |
0·442 |
| Volume at Presentation (cm3) | Median 4·2 Mean 17·4 Range 0·27 - 381 |
Median 4·4 Mean 24·0 Range 0·0033 - 903 |
0·442 |
|
Duration of Follow-Up (months) |
Median 29 Mean 35 Range 12 - 156 |
Median 21 Mean 25 Range 12 - 96 |
0·0007 |
| Zero Growth | 30% (32/105) | 19% (13/68) | 0·112 |
| ATD (cm/yr) | Median 0·17 Mean 0·24 Range −0·63 - 2·47 |
Median 0·26 Mean 0·34 Range −1·4 - 1·6 |
0·023 |
| ATV (cm3/yr) | Median 1.06 Mean 5.74 Range −20.0 - 102.8 |
Median 2.48 Mean 11.3 Range −15 - 430 |
0.075 |
| PTD (%) | Median 6.90 Mean 11.9 Range −25 - 75 |
Median 12.8 Mean 19.9 Range −42 - 160 |
0.015 |
| PTV (%) | Median 24 Mean 78 Range −52 - 1374 |
Median 52 Mean 143 Range −80 - 2043 |
0·026 |
ATD = Absolute tumor diameter (change in tumor diameter per year)
ATV = Absolute tumor volume (change in tumor volume per year)
PTD = Percent tumor diameter (% change in tumor diameter per year – relative change)
PTV = Percent tumor volume (% change in tumor volume per year – relative change)
Table 4.
Pathologic Assessment in 68 patients undergoing active surveillance with delayed intervention.
| Pathology | |
| Benign | 9 (13) |
| Oncocytoma | 7 (10) |
| Angiomyolipoma | 2 (3) |
| Malignant | 57 (84) |
| Histologic subtype | |
| Clear cell | 39 (68) |
| Papillary | 15 (26) |
| Chromophobe | 2 (4) |
| Collecting duct | 1 (2) |
| Stage | |
| T1a | 36 (86) |
| T1b | 3 (7) |
| T2 | 1 (2) |
| T3a | 1 (2) |
| T3b | 1 (2) |
| Nuclear grade | |
| 1 | 19 (36) |
| 2 | 21 (40) |
| 3 | 11 (21) |
| 4 | 2 (4) |
| Unavailable | 2 (3) |
Recurrent disease was noted in 1 patient 15 months following delayed intervention. The patient initially presented with multifocal papillary RCC which was treated with NSS. An ipsilateral lesion in the renal remnant developed subsequently. The patient has elected to observe the recurrent tumor and has not demonstrated systemic progression in over 38 months. Progression to metastatic disease was noted in 1.3% (2/154) of patients. Of the two patients developing metastatic disease, one demonstrated disease progression following delayed intervention and one during continued AS.
Discussion
Increased detection of asymptomatic localized incidental solid tumors in several organ systems have been observed including prostate, breast, thyroid, kidney, and lung1, 3, 10-12. The inference that early detection is consistently associated with improved outcomes is confounded by the limitations of most surgical data sets. Even in contemporary surgical series, tremendous treatment biases exist including lead time, length time, selection, and researcher bias. Moreover, surrogate endpoints of success often lead to conclusions or inferences that may not be fully substantiated. The net result is that many surgical interventions may risk over or under-treating the inherent biology of an asymptomatic localized tumor. Moreover, while an “orderly” molecular progression may lead to sequential stage progression of localized tumors13, particularly egregious concurrent genetic insults may result in tumors that are systemic from their inception14.
While the treatment of symptomatic or potentially symptomatic localized lesions provides both oncologic control and palliation of tumor related symptoms, the primary goal of treating incidental lesions is to alter the natural history or course of the disease. The inability to accurately match surgical treatment to a tumor’s biology is becoming more apparent in oncology. While increased detection of incidental small renal tumors has been associated with a downward stage migration over the last two decades15, treatment of these incidental tumors has not translated into a decrease in cancer specific deaths from RCC, suggesting the possible over-treatment of small, potentially indolent renal tumors16. The independent impact of patient age and medical co-morbidities on overall survival following nephrectomy was recently reported by Berger et al who demonstrate that limited life expectancy and competing medical co-morbidities can reduce the survival benefit otherwise rendered by nephrectomy in patients with renal tumors17. For these reasons the practice of AS of small renal tumors in now being implemented in select patients. However, prior to the routine initiation of AS in acceptable surgical candidates, thresholds for terminating AS and implementing definitive intervention and the risk of progression to metastatic disease should be defined.
While no standards currently exist, the threshold for intervention is largely based upon a number of radiographic features including an absolute size cutoff, tumor growth kinetics, patient/physician perceptions of risk, or a combination therein. A tumor size cutoff of 3 cm has been proposed for initiating treatment of renal masses in patients with von Hippel-Lindau disease18. This threshold was developed based on a low propensity for lesions <3cm to metastasize in VHL patients. While, parallels between the AS of hereditary renal tumors to those with sporadic lesions should be made with caution, molecular data suggest that most sporadic and hereditary clear cell RCC exhibit similar genetic or epigenetic silencing of VHL as a primary defect. Data which describe the low metastatic potential of small bilateral, multifocal RCC in VHL should therefore be cautiously extended to sporadic cases, which are often unilateral and unifocal. Additionally, while mathematical models of tumor growth suggest that stage progression is related to tumor volume19, 20, the recognized heterogenous behaviors of sporadic human malignancies does not support the use of a threshold for intervention based on size alone. For these reasons tumor growth kinetics, determined during a period of AS, may provide a better indication of a tumor’s biologic potential.
When terminating AS based upon tumor growth kinetics several factors should be taken into account including the manner in which growth kinetics are reported (linear versus volumetric), the potential for stage migration during AS, and the growth rates of tumors associated with the development of metastatic disease. Tumor growth kinetics can be evaluated and expressed in several different ways. Linear growth kinetics are based upon changes in maximal axial tumor diameter. When using axial diameter, it is assumed that the tumor is spherical and grows in a uniform fashion in all directions, a hypothesis that has never been confirmed. Nonetheless, measuring the maximal cross sectional diameter of a lesion over time is simple, and provides a relatively reproducible means of assessing growth. A potential drawback of examining tumor growth kinetics in terms of linear growth is that linear growth is not reflective of overall change in tumor volume. This is because calculating the volume of a sphere requires cubing the diameter. For example, a change in maximal axial diameter of a renal mass from 1 cm to 2 cm does not represent doubling the volume but rather a change from 0.52cm3 to 4.19cm3 or a 700% increase in tumor volume. However, a 1 cm change in maximal tumor diameter from 4 cm (33 cm3) to 5 cm (65 cm3) only represents a 97% change in tumor volume. Another potential benefit of using tumor volume instead of axial diameter is that tumor volume provides a better representation of changes in total tumor cell number. Here we analyze tumor growth kinetics in relation to both tumor diameter and volumetric changes. While we could find no correlation between presenting tumor diameter and ATD; ATV, PTD and PTV were significantly associated with tumor size on presentation, with smaller tumors growing proportionally faster than larger tumors. This finding is suggestive of Gompertzian growth kinetics, which theorizes that a tumor’s growth rate is initially exponential and then decreases with increasing size21.
Although the relationship between tumor volume at presentation and observed growth kinetics has been examined previously in non-functioning pituitary adenomas and parathyroid tumors, these series are limited due to sample size and the assumption that the growth kinetics of benign tumors is reflective of malignant disease22, 23. To date, the largest body of data on the growth kinetics of solid organ tumors is based upon renal tumors. In a study by Abou Youssif et al, the relationship between tumor volume at presentation and observed tumor growth kinetics was evaluated in 44 renal tumors24. During a median follow up of 41 months, the median change in tumor volume was 0.83 cc/year was not found to be significantly associated with the tumor volume at presentation. Unfortunately, these authors did not evaluate changes in volume relative to presenting tumor size as was done here. Another study by Bratslavsky et al evaluated the observed growth rates of renal tumors in von Hippel-Lindau patients. These authors noted a significant association between tumor volume on presentation and changes in tumor volume during observation, with smaller tumors growing faster than larger tumors25.
Delayed intervention was performed in 39% (68/173) of patients in the current series. Interestingly, tumor size at initial presentation was not significantly associated with future intervention. Observed tumor growth kinetics were significantly greater in patients who eventually underwent delayed intervention compared to patients who continued surveillance. This finding suggests that physicians’ and patients’ treatment decisions are being influenced by a tumor’s observed growth kinetics. Unfortunately while it is assumed that active tumor growth kinetics suggest unfavorable biological activity, a direct correlation is yet to be demonstrated2. In fact, Kunkle et al reported, no difference in the incidence of benign and malignant pathology between tumors on AS demonstrating interval growth and those which did not exhibit interval growth. Additionally, in tumors which did demonstrate interval growth, the rate of tumor growth does not predict malignant histology2, 26.
The primary argument against AS of renal tumors is the potential for stage progression, especially in otherwise acceptable surgical candidates. Local stage progression represents clinical or pathologic upstaging of disease incurred during a period of active surveillance. While pathologic upstaging in renal tumors treated promptly has been reported in up to 31-38% of patients27, 28, a recent report on the delayed treatment of 54 pathologically confirmed renal cell carcinomas noted the incidence of pathologic upstaging to be 6%8. Additionally, treatment options offered were not altered in a single patient based upon interval changes in radiographic appearance during the period of delay prior to intervention. Progression to systemic disease is of much greater consequence due to the poor survival associated with metastatic renal cancer. In agreement with previous reports, the rate of progression to metastatic disease in current study was low at 1.3% (2/154). It is important to note that all reported cases of progression to metastatic disease have been associated with interval tumor growth2, 24, 26, 29, 30, and that the observed linear growth rate in these tumors ranged from 0.20 – 1.3 cm/yr. However, the incidence of metastatic disease in populations of patients undergoing AS of renal tumors is likely higher than reported due to several factors including the relative short duration of follow-up, inclusion of benign disease in some series, small tumor size, selective intervention of tumor demonstrating accelerated growth kinetics, and the selection bias inherent to retrospective data sets.
Although the data presented in the current manuscript suggests that tumor size at presentation is significantly associated with observed growth kinetics, several limitations warrant consideration. First, the presented patient cohort represents a select and heterogeneous population of renal tumors undergoing at least 12 months of radiographic observation for a number of indications. While the growth kinetics were compared between tumors based upon size at presentation, the growth kinetics of individual tumors were not evaluated over time. Thus, future evaluation with extended follow up of the growth kinetics of individual tumors will be needed to validate or refute the current findings. Despite these limitations, this series represents the largest to date reporting radiographic surveillance of a solid human malignancy.
Conclusions
A significant association between tumor size at presentation and subsequent growth kinetics was noted, with small tumors growing proportionately faster than larger tumors. This finding is suggestive of Gompertzian growth kinetics, which theorizes that a tumor’s growth rate is initially exponential and then decreases with increasing size. Although the available data suggests that the majority of small asymptomatic renal tumors may be observed with a low risk of disease progression, the excellent oncologic outcomes obtained with prompt surgical intervention continues to imply that extirpative therapy in acceptable candidates remains standard. Identification of clinical, radiographic, pathologic and molecular correlates of a tumor’s biologic potential is essential to avoid potential over-treatment of otherwise indolent asymptomatic tumors.
Condensed Abstract.
Observed growth kinetics of renal tumors has a significant association with tumor volume at presentation, with greater proportional growth in small tumors compared to larger tumors. Surveillance of clinically localized renal tumors is associated with a low rate of disease progression in the intermediate term and suggests potential over-treatment biases in select patients.
Acknowledgments
Research Support: This publication was supported in part by grant number P30 CA006927 from the National Cancer Institute. Its contents are solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. Additional funds were provided by Fox Chase Cancer Center via institutional support of the Kidney Cancer Keystone Program
References
- 1.Carter HB, Kettermann A, Warlick C, Metter EJ, Landis P, Walsh PC, et al. Expectant management of prostate cancer with curative intent: an update of the Johns Hopkins experience. J Urol. 2007;178(6):2359–64. doi: 10.1016/j.juro.2007.08.039. discussion 64-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chawla SN, Crispen PL, Hanlon AL, Greenberg RE, Chen DY, Uzzo RG. The natural history of observed enhancing renal masses: meta-analysis and review of the world literature. J Urol. 2006;175(2):425–31. doi: 10.1016/S0022-5347(05)00148-5. [DOI] [PubMed] [Google Scholar]
- 3.Chow WH, Devesa SS, Warren JL, Fraumeni JF., Jr. Rising incidence of renal cell cancer in the United States. Jama. 1999;281(17):1628–31. doi: 10.1001/jama.281.17.1628. [DOI] [PubMed] [Google Scholar]
- 4.Katz DL, Zheng T, Holford TR, Flannery J. Time trends in the incidence of renal carcinoma: analysis of Connecticut Tumor Registry data, 1935-1989. Int J Cancer. 1994;58(1):57–63. doi: 10.1002/ijc.2910580111. [DOI] [PubMed] [Google Scholar]
- 5.Patard JJ, Pantuck AJ, Crepel M, Lam JS, Bellec L, Albouy B, et al. Morbidity and clinical outcome of nephron-sparing surgery in relation to tumour size and indication. Eur Urol. 2007;52(1):148–54. doi: 10.1016/j.eururo.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 6.Kunkle DA, Egleston BL, Uzzo RG. Excise, ablate or observe: the small renal mass dilemma--a meta-analysis and review. J Urol. 2008;179(4):1227–33. doi: 10.1016/j.juro.2007.11.047. discussion 33-4. [DOI] [PubMed] [Google Scholar]
- 7.Kunkle DA, Crispen PL, Chen DY, Greenberg RE, Uzzo RG. Enhancing renal masses with zero net growth during active surveillance. J Urol. 2007;177(3):849–53. doi: 10.1016/j.juro.2006.10.073. discussion 53-4. [DOI] [PubMed] [Google Scholar]
- 8.Crispen PL, Viterbo R, Fox EB, Greenberg RE, Chen DY, Uzzo RG. Delayed intervention of sporadic renal masses undergoing active surveillance. Cancer. 2008;112(5):1051–7. doi: 10.1002/cncr.23268. [DOI] [PubMed] [Google Scholar]
- 9.Kouba E, Smith A, McRackan D, Wallen EM, Pruthi RS. Watchful waiting for solid renal masses: insight into the natural history and results of delayed intervention. J Urol. 2007;177(2):466–70. doi: 10.1016/j.juro.2006.09.064. discussion 70. [DOI] [PubMed] [Google Scholar]
- 10.Raz DJ, Glidden DV, Odisho AY, Jablons DM. Clinical characteristics and survival of patients with surgically resected, incidentally detected lung cancer. J Thorac Oncol. 2007;2(2):125–30. doi: 10.1097/jto.0b013e31802f1cb1. [DOI] [PubMed] [Google Scholar]
- 11.Wilhelm SM, Robinson AV, Krishnamurthi SS, Reynolds HL. Evaluation and management of incidental thyroid nodules in patients with another primary malignancy. Surgery. 2007;142(4):581–6. doi: 10.1016/j.surg.2007.06.033. discussion 86-7. [DOI] [PubMed] [Google Scholar]
- 12.Gennari R, Veronesi U, Andreoli C, Betka J, Castelli A, Gatti G, et al. Early detection of cancer: ideas for a debate. Crit Rev Oncol Hematol. 2007;61(2):97–103. doi: 10.1016/j.critrevonc.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Bignold LP, Coghlan BL, Jersmann HP. Cancer morphology, carcinogenesis and genetic instability: a background. Exs. 2006;(96):1–24. doi: 10.1007/3-7643-7378-4_1. [DOI] [PubMed] [Google Scholar]
- 14.Fisher B, Redmond CK, Fisher ER. Evolution of knowledge related to breast cancer heterogeneity: a 25-year retrospective. J Clin Oncol. 2008;26(13):2068–71. doi: 10.1200/JCO.2007.14.1804. [DOI] [PubMed] [Google Scholar]
- 15.Cooperberg MR, Mallin K, Ritchey J, Villalta JD, Carroll PR, Kane CJ. Decreasing size at diagnosis of stage 1 renal cell carcinoma: analysis from the National Cancer Data Base, 1993 to 2004. J Urol. 2008;179(6):2131–5. doi: 10.1016/j.juro.2008.01.097. [DOI] [PubMed] [Google Scholar]
- 16.worth JM, Miller DC, Daignault S, Hollenbeck BK. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst. 2006;98(18):1331–4. doi: 10.1093/jnci/djj362. [DOI] [PubMed] [Google Scholar]
- 17.Berger DA, Megwalu II, Vlahiotis A, Radwan MH, Serrano MF, Humphrey PA, et al. Impact of Comorbidity on Overall Survival in Patients Surgically Treated for Renal Cell Carcinoma. Urology. 2008 doi: 10.1016/j.urology.2008.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duffey BG, Choyke PL, Glenn G, Grubb RL, Venzon D, Linehan WM, et al. The relationship between renal tumor size and metastases in patients with von Hippel-Lindau disease. J Urol. 2004;172(1):63–5. doi: 10.1097/01.ju.0000132127.79974.3f. [DOI] [PubMed] [Google Scholar]
- 19.Guiot C, Degiorgis PG, Delsanto PP, Gabriele P, Deisboeck TS. Does tumor growth follow a “universal law”? J Theor Biol. 2003;225(2):147–51. doi: 10.1016/s0022-5193(03)00221-2. [DOI] [PubMed] [Google Scholar]
- 20.Plevritis SK, Salzman P, Sigal BM, Glynn PW. A natural history model of stage progression applied to breast cancer. Stat Med. 2007;26(3):581–95. doi: 10.1002/sim.2550. [DOI] [PubMed] [Google Scholar]
- 21.Norton L. A Gompertzian model of human breast cancer growth. Cancer Res. 1988;48(24 Pt 1):7067–71. [PubMed] [Google Scholar]
- 22.Honegger J, Zimmermann S, Psaras T, Petrick M, Mittelbronn M, Ernemann U, et al. Growth modelling of non-functioning pituitary adenomas in patients referred for surgery. Eur J Endocrinol. 2008;158(3):287–94. doi: 10.1530/EJE-07-0502. [DOI] [PubMed] [Google Scholar]
- 23.Parfitt AM, Fyhrie DP. Gompertzian growth curves in parathyroid tumours: further evidence for the set-point hypothesis. Cell Prolif. 1997;30(8-9):341–9. doi: 10.1046/j.1365-2184.1997.00097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abou Youssif T, Kassouf W, Steinberg J, Aprikian AG, Laplante MP, Tanguay S. Active surveillance for selected patients with renal masses: updated results with long-term follow-up. Cancer. 2007;110(5):1010–4. doi: 10.1002/cncr.22871. [DOI] [PubMed] [Google Scholar]
- 25.Bratslavsky G, Albert P, Liu J, Gautam R, Rogers C, Peterson J, Choyke L, Choyke P, Pinto P, Linehan WM. Growth Rates of Hereditary Clear Cell Carcinomas: Influence of Germline Mutations and Body Mass Index. SUO Annula Meeting 2006; Bethesda MD. 2006. [Google Scholar]
- 26.Siu W, Hafez KS, Johnston WK, 3rd, Wolf JS., Jr. Growth rates of renal cell carcinoma and oncocytoma under surveillance are similar. Urol Oncol. 2007;25(2):115–9. doi: 10.1016/j.urolonc.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 27.Roberts WW, Bhayani SB, Allaf ME, Chan TY, Kavoussi LR, Jarrett TW. Pathological stage does not alter the prognosis for renal lesions determined to be stage T1 by computerized tomography. J Urol. 2005;173(3):713–5. doi: 10.1097/01.ju.0000153638.15018.58. [DOI] [PubMed] [Google Scholar]
- 28.Hsu RM, Chan DY, Siegelman SS. Small renal cell carcinomas: correlation of size with tumor stage, nuclear grade, and histologic subtype. AJR Am J Roentgenol. 2004;182(3):551–7. doi: 10.2214/ajr.182.3.1820551. [DOI] [PubMed] [Google Scholar]
- 29.Sowery RD, Siemens DR. Growth characteristics of renal cortical tumors in patients managed by watchful waiting. Can J Urol. 2004;11(5):2407–10. [PubMed] [Google Scholar]
- 30.Lamb GW, Bromwich EJ, Vasey P, Aitchison M. Management of renal masses in patients medically unsuitable for nephrectomy--natural history, complications, and outcome. Urology. 2004;64(5):909–13. doi: 10.1016/j.urology.2004.05.039. [DOI] [PubMed] [Google Scholar]