Abstract
Mice of the C57BL/6 strain develop acute ileal inflammation following infection with the protozoan parasite Toxoplasma gondii. This pathology resembles many key features of human Crohn's disease, including a Th1 cytokine profile with high levels of IFN-γ, IL-12 and TNF-α, presence of pathogenic CD4+ T cells, and infiltration of gut flora into inflammed tissue. Using CCR2−/− mice, we identify a role for this chemokine receptor in pathogenesis of inflammatory pathology during T. gondii infection. Lack of CCR2 was associated with low levels of CD103+ T lymphocytes in the intraepithelial compartment, Peyer's patch and lamina propria relative to wild-type animals. Adoptive transfer of wild-type, but not IFN-γ−/−, intraepithelial T lymphocytes converted CCR2 knockout mice from a resistant to susceptible phenotype with respect to parasite-triggered inflammatory gut pathology. These results for the first time demonstrate a role for intraepithelial T lymphocytes in pathogenesis of ileitis triggered by a microbial pathogen.
INTRODUCTION
Certain inbred mice, most notably the C57BL/6 strain, develop severe small intestinal pathology in the ileum following oral infection with the opportunistic protozoan pathogen Toxoplasma gondii 1. Antibody-mediated depletion and gene knockout studies indicate that disease involves excess production of IFN-γ and TNF-α and the activity of CD4+ T cell effectors 1, 2. The ileal inflammation induced following T. gondii infection shares a number of features in common with inflammatory bowel disease (IBD), and in particular with Crohn s disease (CD) which is associated with a hyperinflammatory Th1 cytokine profile and tissue necrosis in the ileum 2, 3.
Dysregulated immune responses to microbial gut flora are implicated in disease pathogenesis during both Toxoplasma infection and IBD. Invasive Enterobacteriaceae can be found at inflamed sites of the small intestine following inoculation with T. gondii, and broad-spectrum antibiotic treatment to eliminate gut flora also prevents pathological consequences of oral infection 4, 5. TLR4-mediated sensing of commensal bacteria has been linked to murine ileitis triggered by Toxoplasma infection 5. Thus, an emerging view is that Toxoplasma infection serves as a trigger for inflammatory pathology caused by intestinal bacteria rather than the parasite itself. In this regard, it is notable that invasive Enterobacteriaceae are also found at inflamed sites during human CD, and a dysregulated balance between commensal flora and pathogenic bacteria species is believed to play a role in emergence of CD 6-8.
A key event in initiation of inflammation in the gut is thought to be activation of pathogenic T cells. In CD, it is believed that lamina propria Th1 and possibly Th17 T lymphocytes contribute to proinflammatory cytokine production 3. In CD patients and in experimental models of CD, upregulation of the Th1 transcription factor T-bet is associated with disease 9. During Toxoplasma infection, pathology fails to develop in the absence of MHC class II-restricted CD4+ T cells 1. The parasite surface protein SAG-1 (p30) has been identified as an antigen recognized by pathogenic CD4+ T cells 10. Other lines of evidence indicate that lamina propria T cells are a source of IFN-γ that mediates pathology, and that CD8+ intraepithelial lymphocytes (IEL) can down-regulate this activity through TGF-β production 11, 12.
Chemokines and their receptors play a dominant role in orchestrating the activity of T cells in IBD, and in particular there is evidence that CCR2 and its ligands are involved in human CD 13. Immunohistochemical staining of gut biopsy samples from Crohn's patients reveals infiltrating CD4+ T cells that are uniformly positive for CCR2 14. Furthermore, disease phenotypes of Crohn's patients have been linked to polymorphisms in CCL2 and CCR2 genes 15, 16. While these studies indicate that CCR2 and its ligands are associated with pro-inflammatory damage in the intestine, their role in the etiopathogenesis of mucosal inflammation is unknown.
Here we show a requirement for CCR2 in Toxoplasma-triggered intestinal pathology. We identify a population of CD103+ IEL that is expanded in dependence upon this chemokine receptor. Most importantly, wild-type (WT), but not IFN-γ−/−, IEL induce severe intestinal damage upon transfer into infected CCR2−/− mice. Our data for the first time show that microbial infection can trigger pathogenic IEL that cause inflammation and necrosis of the small intestine dependent upon their ability to produce IFN-γ.
RESULTS
CCR2−/− mice display increased susceptibility during oral infection with T. gondii
We infected WT and CCR2−/− mice with the type II ME49 Toxoplasma strain to examine the role of the CCR2 chemokine receptor in resistance to the parasite. As reported by others 17, 18 during low dose infection (20 cysts), absence of CCR2 led to increased mortality (Fig. S1A). Cyst numbers were elevated in the brains of surviving animals compared to WT controls (Fig. S1B), consistent with a defect in microbicidal effector activity. When we raised the infectious dose to 100 cysts, both WT and CCR2−/− animals succumbed to infection with the same kinetics (Fig. S1C).
Mice deficient in CCR2 are resistant to Toxoplasma-induced damage to the intestinal mucosa
To understand the role of CCR2 in development of intestinal inflammation during microbial infection, we utilized the ME49 parasite strain to examine pathological consequences of oral infection in WT and CCR2−/− mice. In agreement with previously published data, WT mice developed severe intestinal pathology by day 8 post-infection 1 (Fig. 1A). Inflammation was localized to the ileum and consisted of extensive cellular infiltration into the lamina propria and submucosa, hemorrhage, villus blunting and areas of epithelial layer destruction. This pathology has previously been established to be partly dependent upon CD4+ T cells and IFN-γ 19, 20. In sharp contrast to the pathology induced in WT animals, inflammation in CCR2−/− mice was mild (Fig. 1B; Fig. S2), characterized by low level cellular infiltration into the lamina propria and modest thickening of the villi and submucosa.
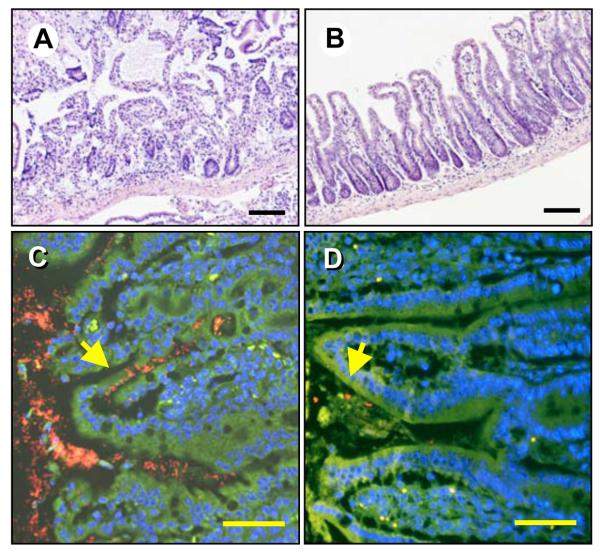
Figure 1.
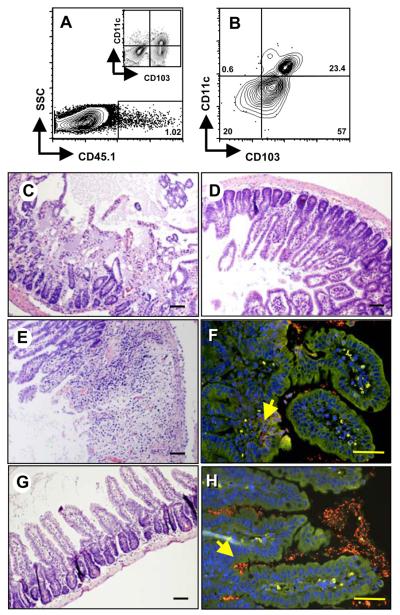
Absence of CCR2 protects against T. gondii-mediated intestinal pathology. WT (A) and CCR2−/− (B) mice were orally infected with 100 ME49 cysts and tissues collected for H & E staining 8 days later. Sections of ileum from Day 8 infected WT (C) and CCR2−/− (D) mice were hybridized with Eub338 (Cy3; red) and non-eub338 (FITC; green) to detect localization of intestinal flora. Sections were counterstained with DAPI (blue) and examined by fluorescence microscopy. Bacteria appear red/orange (yellow arrows). Autofluorescence and non-specific hybridization of the probe appear as yellow/green. Scale bars: 50 μm.
To further substantiate the contribution of CCR2 to pathology following infection, we used fluorescence in situ hybridization (FISH) employing oligonuclotide probes recognizing the 16S ribosomal RNA subunit to identify bacteria present in mucosal tissue. Previous studies have shown that in areas of Toxoplasma-induced intestinal damage, gut flora penetrate mucosal tissue, further enhancing inflammation 4, 21. In agreement with our pathology data, WT mucosa was colonized with high numbers of translocated bacteria (Fig. 1C). In Fig. S3A an enlarged view of the region indicated by the arrow in Fig. 1C is shown. Fig. S4 shows an area of translocated bacteria that appear to have been internalized by resident cells, possibly macrophages. Fig. S5A showns a low power image of infected WT small intestine. Contrasting with these results, bacteria were strictly confined to the lumen of the gut in CCR2−/− mice (Fig. 1D). The yellow arrow in this figure shows luminal bacteria. This area is enlarged in Fig. S3B, and a low power image of the infected CCR2−/− intestine is shown in Fig. S5B. Thus, in the absence of CCR2, mice are protected from inflamatory pathology and accompanying bacterial translocation in the gut.
CCR2 mediates recruitment of CD103+ T cells into mucosal sites
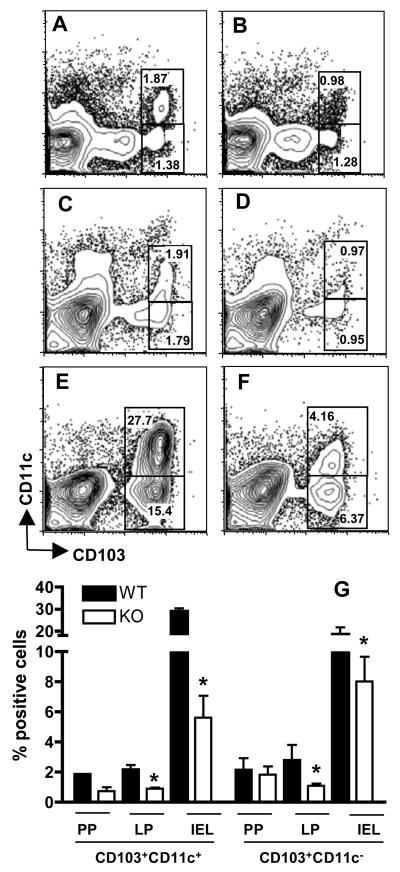
We next investigated the immune cell composition of Peyer's patches (PP), lamina propria (LP) and IEL compartments following infection of WT and CCR2−/− mice, in particular assessing expression of CD103, an integrin associated with homing to the intestine 22. In all mucosal compartments WT mice accumulated more CD103+ cells than their CCR2−/− counterparts (Fig. 2A-F; averaged results of three animals per strain are shown in Fig. 3G). Approximately half of these cells also expressed CD11c, a marker expressed by DC and, importantly, activated T cells resident in the mucosal epithelium 23. The difference between CCR2+/+ and CCR2−/− strains was most striking in the IEL compartment where there were up to six-fold more CD103+CD11c+, and more than double the number of CD103+CD11c− cells in the WT IEL population (Fig. 2E) compared with CCR2−/− IEL (Fig. 2F). The difference in cell number in WT vs. knockout strains likely reflects differences in recruitment or retention of cells in response to infection. This is because the proportion of CD103+ cells was equivalent in noninfected animals in the presence or absence of CCR2 (data not shown).
Figure 2.
Absence of CCR2 results in diminished recruitment of CD103+CD11c+ and CD103+CD11c− cells to sites of infection. CD11c and CD103 expression was examined in Peyer s patches (A and B) lamina propria (C and D) and the intraepithelial lymphocyte compartment (E and F) of 4-day infected WT (A, C, E) and CCR2−/− (B, D, F) mice. The numbers in each rectangle indicate the relative percentage of cells out of the total population. Panel G shows mean values obtained from the Peyer s patches (PP), lamina propria (LP) and intraepithelial lymphocyte (IEL) compartment in a representative experiment using 3 mice per strain (*, p < 0.05).
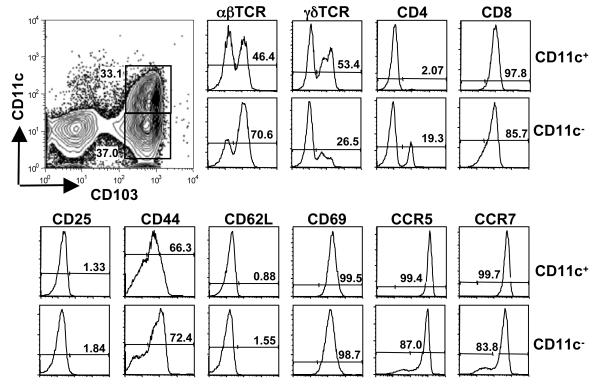
Figure 3.
CD11c+ and CD11c− populations of CD103+ cells express T cell lineage markers. WT animals were orally infected with T. gondii, and then IEL prepared 4 days later. Four-color staining was used to identify cell surface molecules. Cells were stained for CD11c and CD103, and then for either TCRαβ and TCRγδ or CD4 and CD8. The activation status of the cells was determined by staining with CD25, CD44, CD62L and CD69, and chemokine receptor expression was examined by staining for CCR5 and CCR7.
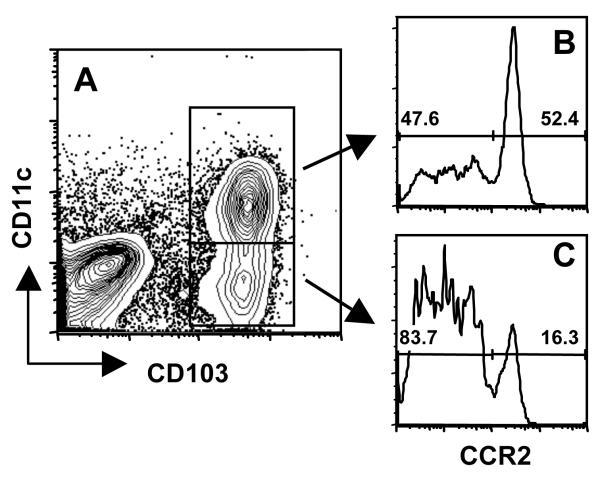
We further examined the phenotype of the CD103+ populations that were recruited in dependence upon CCR2 (Fig. 3). The CD11c+ population was uniformly positive for CD8, and was approximately equally divided between αβ and γδ TCR expression. Both populations also expressed the T cell molecule CD3 (Fig. S6A and B). The elevated percentage of αβ T cells may be the result of Toxoplasma-driven recruitment of these cells to the site of infection. The cells expressed a partially activated phenotype, in that they were CD25 and CD62L negative, but positive for CD69. The CD103+CD11c+ population also expressed high levels of CCR5 and CCR7. The CD103+ cells that lacked expression of CD11c possessed similar phenotypic characteristics, although the distribution of T cells markers differed. Thus, amongst CD103+CD11c− cells, 70% were positive for the αβTCR, and approximately 20% were CD4 positive. We then determined levels of CCR2 on the CD103+ populations. In cells co-expressing CD11c, approximately half expressed CCR2. In the CD103+CD11c− population, approximately 16% stained positive for CCR2 (Fig. 4). Lastly, we examined the phenotype of the CD103−CD11c− population. A subpopulation (17%) of these cells expressed CD3 (Fig. S6C), but the majority stained positive for the epithelial marker EpCAM (Fig. S6D). We conclude that most of these cells are of epithelial origin.
Figure 4.
Expression of CCR2 by IEL in the small intestine. IEL isolated from WT ilea on Day 4 post-infection were triple-stained for CD103, CD11c and CCR2. (A) CD11c vs. CD103 staining profile of isolated IEL. CCR2 surface expression is shown on double positive (CD11c+ CD103+) IEL in panel B, and single positive (CD11c-CD103+) CCR2 levels are shown in panel C.
WT IEL are skewed to a proinflammatory phenotype following T. gondii infection
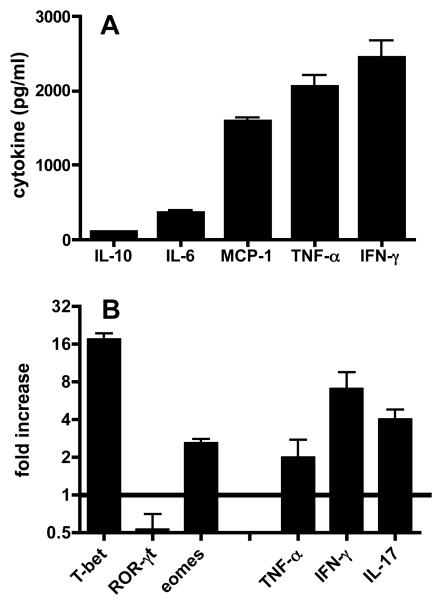
Toxoplasma-induced ileal pathology is dependent upon proinflammatory mediators that include IFN-γ and TNF-α, but production of these mediators during infection in the small intestine has not been examined. Here, we cultured gut biopsy samples from infected mice and examined cytokine release. While this approach does not identify the specific cell source of the cytokines measured, it allows an assessment of the overall cytokine environment in the intestine of animals undergoing infection. As shown in Fig. 4A, high levels of IFN-γ and TNF-α were produced. In addition, cultures contained high amounts of MCP-1, the major chemokine ligand of CCR2. In contrast, only low amounts of IL-10 and IL-6 were produced.
To further characterize the functional phenotype of the IEL population we performed real-time PCR on IEL isolated from noninfected and infected WT mice. Transcripts for signature T cell transcription factors were examined. The data in Fig. 4B show a major increase in expression of the Th1 master regulator T-bet relative to IEL from non-infected mice. In comparison with IEL from noninfected mice, levels of the Th-17 associated transcription factor RORγt were down-regulated after infection, but eomesodermin, a regulator of CD8+ T cell differentiation, was modestly elevated. In the same samples, upregulation of mRNA for IFN-γ, TNF-α and IL-17 was detected. We conclude that IEL from infected mice express an overwhelmingly proinflammatory transcriptional program, associated in particular with increased expression of the transcription factor T-bet.
T. gondii-primed WT IEL induce IFN-γ-dependent pathological damage in CCR2−/− mice
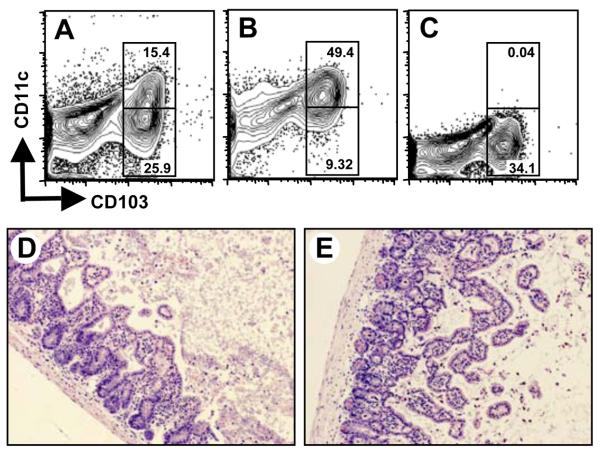
IEL have been linked to protective immune responses in the gut during T. gondii infection 12, 24, 25, but here we show an association between parasite-induced pathology and CCR2-dependent IEL recruitment. To establish a causal link between pathology and T. gondii-primed IEL, we determined if adoptive transfer of WT IEL was sufficient to mediate intestinal damage in CCR2−/− mice. First, we confirmed that primed IEL were capable of homing to the intestinal epithelium following transfer. Accordingly, IEL were isolated from Day 4-infected CD45.1 congenic mice and adoptively transferred into Day 4-infected C57BL/6 (CD45.2) mice. As shown in Fig. 6A, the transferred CD45.1+ cells trafficked to the intestinal epithelium within 24 hr of injection. Gating on this population revealed that both CD11c+ and CD11c− populations of IEL were capable of migrating back to the IEL compartment (Fig. 6B). Notably, there was minimal homing of CD103− cells back to the intestine. This is consistent with the known role of CD103 as a mucosal adressin molecule.
Figure 6.
WT CD103+ cells traffic to the IEL compartment and mediate IFN-γ-dependent ileal pathology in CCR2−/− mice. IEL were isolated from Day 4 infected C57BL/6 background CD45.1 congenic mice. Cells were stained for CD11c and CD103 to confirm IEL phenotype (A inset) then i.v. injected into Day 4-infected C57BL/6 (CD45.2) recipient mice. IEL were isolated from recipients 24 hr later, and CD45.1 cells tracked by flow cytometry, as shown in (A). Gating on CD45.1 IEL, then examining expression of CD11c and CD103 (B) determined the phenotype of the transferred cells. Pathological damage in the intestine was evaluated 5 days post-transfer. WT (C) but not CCR2−/− (D) mice display characteristic inflammatory pathology. CCR2−/− mice reconstituted with IEL from Day 4-infected WT mice develop WT-like pathology (E) with concomitant translocation of bacteria into the intestinal mucosa (F). In contrast, transfer of IEL from Day 4-infected IFN-γ−/− animals into CCR2−/− mice does not trigger pathological damage (G) and bacteria remain in the lumen of the intestine (H). Arrows in F and H point to bacteria, visible as yellow staining in the submucosa (F) and lumen (H). The scale bars in each panel indicate 50 μm.
To directly determine if IEL mediate intestinal pathology, IEL from Day 4-infected WT mice were transferred into Day 4-infected CCR2−/− recipients. Mice were euthanized 5 days post transfer, and pathology assessed. WT mice developed typical pathological changes in the small intestine (Fig. 6C) whereas damage in the ileum of CCR2−/− mice was mild (Fig. 6D). Dramatically, CCR2−/− mice receiving Day 4 post-infection WT IEL developed severe intestinal inflammation similar to that of WT mice (Fig. 6E; Fig. S2). To further substantiate this result, tissues were subjected to FISH analysis to determine the status of gut flora in intestinal tissues. Fig. 6F shows that translocated bacteria are present in the lamina propria of CCR2−/− mice reconstituted with T. gondii-elicited WT IEL. An expanded view of the area indicated by the yellow arrow that points out translocated bacteria is shown in Fig. S7A.
Because IEL from infected animals produce large amounts of IFN-γ, a cytokine implicated in Toxoplasma-induced immunopathology 1, 2, we sought to determine whether IFN-γ production by CD103+ IEL accounted for their pathogenicity by repeating the transfer experiments using cells from IFN-γ−/− mice as donors. Strikingly, CCR2−/− mice receiving IEL from IFN-γ−/− mice did not develop inflammatory pathology (Fig. 6G; Fig. S2). We subjected tissue sections to FISH analysis, and, as predicted, bacteria remained luminal in animals receiving IFN-γ−/− IEL, consistent with a lack of damage to the intestinal epithelium (Fig. 6H and Fig. S7B). We conclude that IFN-γ is necessary for the pathogenicity of CCR2-dependent IEL.
Finally, we used our adoptive transfer model to ask whether damage to the intestine was mediated by CD11c+ or CD11c− IEL populations. IEL from Day 4 infected WT mice (Fig. 7A) were immunomagentically separated into CD11c+ (Fig. 7B) and CD11c− (Fig. 7C) fractions. These were adoptively transferred into Day 4 infected CCR2−/− mice and small intestines were collected for histopathological evaluation 5 days later. Mice receiving single positive cells (CD103+ CD11c−) developed intestinal inflammation as characterized by extensive immune cell infiltrates, tissue hemorrhaging and villus fusion (Fig. 7D; Fig. S2). A similar outcome was observed in the mice receiving double positive (CD103+ CD11c+) IEL (Fig. 7E; Fig. S2). Control Day 9 infected CCR2−/− mice not receiving WT IEL showed minimal pathology indistinguishable from that shown in Fig. 1B (data not shown). We conclude that both CD11c+ and CD11c− IEL populations possess pathogenic activity in this model.
Figure 7.
Both CD11c+ and CD11c− IEL subsets contribute to intestinal inflammation. IEL were prepared from Day 4-infected mice, then CD11c+ and CD11c− populations were isolated employing anti-CD11c Ab and immunomagnetic beads. (A) Starting population of IEL; (B) CD103+ population; (C) CD103− population. The cells shown in panels B and C were adoptively transferred into Day 4-infected CCR2−/− animals and small intestine tissue was evaluated 5 days later by H & E staining. Micrographs showing the outcome of transfers in mice receiving CD11c+ CD103+ (D) or CD11c− CD103+ (E) IEL demonstrate that both cell subsets induce inflammatory pathology to a similar degree. Images are representative of 5 independent experiments.
DISCUSSION
Here we demonstrate an essential role for IEL in proinflammatory intestinal pathology induced by infection with T. gondii. Damage to the intestinal mucosa was furthermore dependent upon ability of the cells to produce IFN-γ. In addition, emergence of pathogenic IEL during infection required expression of CCR2. Consequently, mice lacking CCR2 were protected from development of ileal inflammation. CD4+ T lymphocytes have previously been implicated in Toxoplasma-induced intestinal pathology, but this study now shows that the IEL compartment, mostly composed of CD8+ T cells, also possess pathogenic activity during infection.
Lack of CCR2 in Toxoplasma-infected mice was recently associated with failure to recruit antimicrobe inflammatory monocytes and increased pathology in several tissues, including the intestine 17. In the present study we also found that CCR2−/− mice are more susceptible to infection using a low parasite innoculum, as measured by increased mortality and significantly higher parasite burdens in the surviving mice. However, following high dose infection we found that deletion of CCR2 mediates protection against pathology in the small intestine. In agreement with us, another recent study found that CCR2−/− mice are resistant to Toxoplasma-induced damage to the gut 18. Issues of parasite dose may underlie differences reported in these studies regarding parasite-induced pathology in CCR2−/− mice. Low infection inoculums employed by Dunay et al. 17 may trigger CCR2-dependent inflammatory monocytes whose absence leads to uncontrolled parasite replication associated with tissue destruction at the site of infection. Under higher infection conditions, CCR2 appears to be important in recruitment and activation of IEL that cause proinflammatory pathology in the intestine.
The CCR2-dependent IEL identified in this study were a mixed population. While the cells uniformly expressed CD103, approximately half were positive for CD11c, a molecule known to be expressed on activated T cells in the intestinal mucosa 23. Our data show that both CD11c+ and CD11c− IEL induce damage, but further studies are needed to determine if this is an activity mediated by αβ or γδ T lymphocytes that are present in both populations.
The CD103 molecule, also known as αE, forms a heterodimer with the integrin β7 subunit. Binding of CD103/β7 to epithelial cell E-cadherin is important in homing and retention of lymphocytes and dendritic cells to the intestinal mucosa 22. There is evidence that CD103 is involved in intestinal graft-versus-host disease pathology and TNP-OVA-induced colitis in IL-2-deficient mice 26, 27. Other studies suggest that CD103+ T cells regulate pathology in TNF-mediated experimental colitis, and there is evidence that CD103+ DC control disease in T cell transfer models of colitis 28, 29. Thus, whether CD103 is associated with inflammation or prevention of pathology most likely depends upon the effector function of the cells expressing this intestinal homing molecule.
Although we found that IEL contribute to pathology in the gut during infection, this compartment has been linked to protection and maintenance of homeostasis during T. gondii infection 12, 24, 25. While LP CD4+ T cells were found to synergize with intestinal epithelial cells for proinflammatory cytokine production, IEL secretion of TGF-β down-regulated the response, and in vivo transfer studies suggested a role for this anti-inflammatory cytokine in preventing parasite-induced ileitis 12, 20, 25. Furthermore, γδ T cells have been associated with epithelial barrier function during Toxoplasma infection 30. Other studies have shown that adoptive transfer of IEL protects against infection and induces long-term immunity against Toxoplasma 24, 31, 32. Consistent with our studies, IEL effector activity in these cases was associated with production of IFN-γ 24.
The divergence between the protective function of the IEL compartment reported previously 24 and the clear pathologic activity reported here may be a consequence of differences in parasite doses employed in each case. Whereas we isolated IEL from animals undergoing high dose infection, earlier studies employed IEL from animals undergoing low dose infection. We hypothesize that exposure to a low parasite dose elicits IEL activity that mediates protection against inflammatory pathology, and that high parasite doses triggers a switch to IEL that mediate inflammatory pathology in the intestine.
Based upon previous studies in animal models, IBD pathogenesis has been attributed to the activity of lamina propria CD4+ cells responding to gut flora 33. Here, we show that IEL induce similar pathology. Because the overwhelming majority of these cells express the CD8 molecule, it is likely that this IEL subset mediates disease. In particular, the CD11c+ IEL that are approximately 98% positive for CD8 transfer pathology upon inoculation into CCR2−/− mice. However, we cannot completely exclude a role for CD4+ T lymphocytes because these cells comprise approximately 20% of the CD103+CD11c− population that also mediates damage.
There is increasing evidence that CD8+ T cells may trigger pathogenic CD4+ responses during IBD, possibly by damaging the epithelium and allowing access of luminal bacteria or by releasing activating cytokines 33. In a hapten-induced colitis model, hapten-sensitized CD8+ T cells were identified as the earliest initiators of infection 30,. In another study, transgenic CD8 T cells specific for influenza hemagglutinin A (HA) that had developed in the absence of cognate antigen were able to mediate severe intestinal destruction after transfer into transgenic mice expressing an HA transgene in the intestinal epithelial compartment 31. The results of the present study reinforce and extend the hypothesis that pathogenic CD8+ T cells are initiators of intestinal pathology. Importantly, our study provides evidence that IEL themselves mediate ileal damage triggered by microbial infection. Furthermore, our results reveal for the first time chemokine receptor CCR2 as a key player in this pathology.
Unraveling the roles of IEL in the pathogenesis of intestinal inflammation is a complex task. The IEL compartment is diverse, consisting of T cells expressing αβ and γδ T cell receptors 34. Most IEL express the CD8 molecule, yet of these, some are generated independently of classical major histocompatibility complex (MHC) class I molecules 35, 36. Some IEL are generated in the thymus, but other subpopulations are believed to derive from cryptopatches in the intestinal mucosa 22. By examining expression of chemokine receptors and T cell receptors, as well as the need for MHC in generating the cells, it should be possible to elucidate requirements for local recruitment and expansion of protective or pathogenic IEL. In turn, this can be expected to shed light on pathogenesis of IBD.
METHODS
Mice
Six to 8 week old female C57BL/6, Swiss Webster, IFN-γ−/− and CCR2−/− mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Breeding colonies of B6.SJL-PtprcaPep3b/BoyJ (CD45.1 congenic) and CCR2−/− mice were established in the Transgenic Mouse Facility at the Cornell University College of Veterinary Medicine. Animals were housed under specific pathogen-free conditions at the Cornell University College of Veterinary Medicine animal facility, which is accredited by the American Association of Laboratory Animal Care.
Parasites and infections
Cysts of the type II low virulence T. gondii strain, ME49 were obtained from chronically infected (>1 month) Swiss Webster mice by homogenizing brains in sterile PBS. Homogenate was passaged through an 18 gauge needle and cysts were enumerated by phase contrast microscopy. Age matched mice were infected with 100 cysts by oral gavage.
Cell isolation
Small intestines were removed, cleaned of mesentery and fat, and flushed with 37°C PBS. Mesenteric lymph nodes were excised and the fat removed before cutting into small fragments and homogenizing though a 70 μm filter (BD, Franklin Lakes, NJ). Peyer's patches were removed and passed through a 70 μm filter to yield a single cell suspension. Isolation of IEL was performed as previously described 37. Briefly, intestines were cut into 5 cm lengths and opened longitudinally on a sterile plastic sheet. The mucosal layer, containing epithelial cells and IEL, was scraped off with a blunt scalpel. The cells were washed by centrifugation and resuspended in RPMI containing 10% FBS and 10 mM dithioerythritol (Sigma, Saint Louis, MO) pre-warmed to 37°C. The cells were incubated for 20 min with magnetic stirring. Cells were filtered through a prewashed glass wool column to remove contaminating enterocytes and cell debris. The IEL eluted from the column were further purified by discontinuous Percoll gradient separation. Lamina propria leukocytes were isolated according to standard techniques 38. Briefly, intestinal tissue with the mucosal layer removed was cut into 5 mm fragments and cells were liberated from the tisses by digestion (37°C, 2 hr) in RPMI, 10% FBS, 100 U/ml collagenase (Sigma), and 15 μg/ml DNAse (Sigma). Cells were separated from contaminating debris by discontinuous Percoll gradient separation.
Gut biopsy culture
Intestines were removed from infected WT mice at day 4 post-infection and flushed extensively with PBS containing antibiotics. Intestines were opened longditudinally and a biopsy punch was used to collect uniform pieces of tissue. Intestinal sections were incubated overnight in cDMEM (37°C, 4% CO2). The supernatants were collected and levels of cytokine determined using the cytometric Bead Array (CBA), following the manufacturer's instructions (BD).
Semi-quantitative real time PCR
Semi-quantitative PCR was performed as described elsewhere 39. RNA was extracted from IEL using Trizol (Sigma) and reverse transcribed into cDNA according to standard protocols. PCR was performed on cDNA using primers specific for TNF-α, IFN-γ, T-bet, Foxp3, and eomesodermin. SYBR green 1 (Invitrogen, Carlsbad, CA) was employed to quantitate amplification. Fluorescence was measured using an Applied Biosystems 7700 sequence detector. All samples were amplified in triplicate and normalized to GAPDH.
Flow cytometry
Single cell suspensions obtained from lamina propria, Peyer's patch and IEL compartments were incubated in ice-cold FACS buffer (PBS, 1% BSA, 0.01% NaN3) containing 10% normal mouse serum to block Fc receptor binding (30 min, 4°C). Cells were pelleted by centrifugation and resuspended in optimal concentrations of fluorochrome-conjugated antibodies in ice-cold FACS buffer to stain surface molecules. The antibodies used in this study were anti-TCRγδ, anti-CD3, anti-CD4, anti-CD8, anti-CD11c and anti-EpCAM conjugated to fluorescein isothyocyanate; anti-CD62L, anti-CCR5, anti-CCR7 and anti-CD45.1 conjugated to phycoerythrin; anti-TCRαβ, anti-CD69, anti-CD25 conjugated to PerCP; anti-CD11c and anti-CD44 conjugated to allophycocyanin. Antibodies were purchased from either Biolegend (San Diego, CA) or eBioscience (San Diego, CA). CCR2 was detected using rat anti-mouse CCR2 followed by anti-rat AF488 secondary antibody (Invitrogen, Carlsbad, CA), prior to staining with other antibodies. Antibodies were incubated with cells for 30 min at 4°C. After washing, at least 50,000 cells per sample were collected for analysis on a BD FACSCalibur flow cytometer. Data analysis was performed using FlowJo software (Ashland, OR).
Adoptive transfer
5 × 106 total IEL recovered from Day 4-infected mice were transferred into infected CCR2−/− recipents by intravenous retro-orbital injection under anaesthesia. On day 9 post-infection, intestines were removed and fixed in 10% neutral buffered formaldehyde. Some paraffin-embedded tissue sections were stained with Hematoxylin and Eosin and examined for pathological changes, and others were subjected to fluorescence in situ hybridization. In some experiments whole IEL isolated from Day 4 infected WT mice were incubated with anti-CD11c beads (Miltenyi Biotec, Auburn, CA) according to manufacturer s instructions. Cells were separated into CD11c+ and CD11c− subpopulations using an AutoMacs separator (Miltenyi Biotec). Aliquots of the unseparated population as well as the IEL subsets were stained with CD11c and CD103 and analyzed by flow cytometry to evaluate the efficiency of the magnetic separation. 2 × 106 IEL of each subset were transferred into Day 4 infected CCR2−/− mice. Ilea were collected 5 days after adoptive transfer, formalin fixed and processed for histological evaluation.
Fluorescence in situ hybridization (FISH)
FISH was performed as previously described 8. Paraffin-embedded sections were de-paraffinized and rehydrated by serial immersion in xylene and graded alcohols, then finally water. Sections were incubated with FISH probes (EUB338, or non-EUB338) labelled on the 5′ end with either FITC or Cy3 (Integrated DNA Technologies, Coralville, IA) at 5 μg/ml in hybridization buffer (42°C, 14 hr). Sections were washed in hybridization buffer to remove un-bound probe, rinsed in sterile water, air-dried and mounted with Prolong® Antifade Gold (Molecular Probes, Carlsbad, CA). Images were collected with a BX51 microscope (Olympus, Center Valley, PA) equipped with a DP70 camera using DP Controller Software (version 1.1.1.65; Olympus) and DP Manager software (version 1.1.1.71; Olympus).
Pathology Scoring
Pathology was scored between 0 (not apparent) and 4 (severe) for 5 criteria. These were villus fusion/blunting, lamina propria inflammation, sloughing of epithelial tips, necrosis of villus tips and transmural inflammation 40. Individual mice were assigned a cumulative score out of a maximum of 20. Data were graphed with each point representing an individual mouse and the bar as the mean of each group.
Statistical analyses
Student's t test was used to analyze statistical differences between groups. Values for p < 0.05 were considered significant. All experiments were repeated a minimum of three times. Pathology scores were assessed for significance using a 1-way ANOVA test.
Supplementary Material
Figure 5.
IEL are heavily skewed to a proinflammatory cytokine and transcription factor profile following parasite infection. (A) Cytokine secretion from mucosal tissue was detected by cytometric bead array on supernatants from overnight culture of gut biopsy samples. (B) RNA extracted from IEL both noninfected and infected WT mice was subjected to real time-PCR amplification and normalized to GAPDH. The data are expressed relative to transcript levels in noninfected animals (defined as 1). Error bars represent SD values from triplicate samples.
Acknowledgements
This work was supported by a grant from the National Institutes of Health (AI06492 to E. Denkers). J. Leng was supported by a Genomics Scholars Award from the Cornell University Center for Vertebrate Genomics. M. Craven received support from the Morris Animal Foundation.
Footnotes
The authors have no competing financial interests.
REFERENCES
- 1.Liesenfeld O, Kosek J, Remington JS, Suzuki Y. Association of CD4+ T cell-dependent, interferon-gamma-mediated necrosis of the small intestine with genetic susceptibility of mice to peroral infection with Toxoplasma gondii. J Exp Medx. 1996;184:597–607. doi: 10.1084/jem.184.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liesenfeld O. Oral infection of C57BL/6 mice with Toxoplasma gondii: a new model of inflammatory bowel disease? J Infect Dis. 2002;185(Suppl 1):S96–101. doi: 10.1086/338006. [DOI] [PubMed] [Google Scholar]
- 3.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–34. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 4.Heimesaat MM, et al. Gram-negative bacteria aggravate murine small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii. J Immunol. 2006;177:8785–95. doi: 10.4049/jimmunol.177.12.8785. [DOI] [PubMed] [Google Scholar]
- 5.Heimesaat MM, et al. Exacerbation of murine ileitis by Toll-like receptor 4 mediated sensing of lipopolysaccharide from commensal Escherichia coli. Gut. 2007;56:941–8. doi: 10.1136/gut.2006.104497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnich N, Darfeuille-Michaud A. Role of bacteria in the etiopathogenesis of inflammatory bowel disease. World J Gastroenterol. 2007;13:5571–6. doi: 10.3748/wjg.v13.i42.5571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darfeuille-Michaud A, et al. Presence of adherent Escherichia coli strains in ileal mucosa of patients with Crohn's disease. Gastroenterology. 1998;115:1405–13. doi: 10.1016/s0016-5085(98)70019-8. [DOI] [PubMed] [Google Scholar]
- 8.Baumgart M, et al. Culture independent analysis of ileal mucosa reveals a selective increase in invasive Escherichia coli of novel phylogeny relative to depletion of Clostridiales in Crohn's disease involving the ileum. Isme J. 2007;1:403–18. doi: 10.1038/ismej.2007.52. [DOI] [PubMed] [Google Scholar]
- 9.Neurath MF, et al. The transcription factor T-bet regulates mucosal T cell activation in experimental colitis and Crohn's disease. J Exp Med. 2002;195:1129–43. doi: 10.1084/jem.20011956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rachinel N, et al. The induction of acute ileitis by a single microbial antigen of Toxoplasma gondii. J Immunol. 2004;173:2725–35. doi: 10.4049/jimmunol.173.4.2725. [DOI] [PubMed] [Google Scholar]
- 11.Luangsay S, et al. CCR5 mediates specific migration of Toxoplasma gondii-primed CD8 lymphocytes to inflammatory intestinal epithelial cells. Gastroenterology. 2003;125:491–500. doi: 10.1016/s0016-5085(03)00903-x. [DOI] [PubMed] [Google Scholar]
- 12.Mennechet FJ, et al. Intestinal intraepithelial lymphocytes prevent pathogen-driven inflammation and regulate the Smad/T-bet pathway of lamina propria CD4+ T cells. Eur J Immunol. 2004;34:1059–67. doi: 10.1002/eji.200324416. [DOI] [PubMed] [Google Scholar]
- 13.Zhong W, et al. Chemokines orchestrate leukocyte trafficking in inflammatory bowel disease. Front Biosci. 2008;13:1654–64. doi: 10.2741/2789. [DOI] [PubMed] [Google Scholar]
- 14.Connor SJ, et al. CCR2 expressing CD4+ T lymphocytes are preferentially recruited to the ileum in Crohn's disease. Gut. 2004;53:1287–94. doi: 10.1136/gut.2003.028225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herfarth H, Pollok-Kopp B, Goke M, Press A, Oppermann M. Polymorphism of CC chemokine receptors CCR2 and CCR5 in Crohn's disease. Immunol Lett. 2001;77:113–7. doi: 10.1016/s0165-2478(01)00199-7. [DOI] [PubMed] [Google Scholar]
- 16.Herfarth H, et al. Polymorphism of monocyte chemoattractant protein 1 in Crohn's disease. Int J Colorectal Dis. 2003;18:401–5. doi: 10.1007/s00384-003-0477-0. [DOI] [PubMed] [Google Scholar]
- 17.Dunay IR, et al. Gr1(+) inflammatory monocytes are required for mucosal resistance to the pathogen Toxoplasma gondii. Immunity. 2008;29:306–17. doi: 10.1016/j.immuni.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benevides L, et al. CCR2 receptor is essential to activate microbicidal mechanisms to control Toxoplasma gondii infection in the central nervous system. Am J Pathol. 2008;173:741–51. doi: 10.2353/ajpath.2008.080129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liesenfeld O, et al. TNF-alpha, nitric oxide and IFN-gamma are all critical for development of necrosis in the small intestine and early mortality in genetically susceptible mice infected perorally with Toxoplasma gondii. Parasite Immunol. 1999;21:365–76. doi: 10.1046/j.1365-3024.1999.00237.x. [DOI] [PubMed] [Google Scholar]
- 20.Mennechet FJ, et al. Lamina propria CD4+ T lymphocytes synergize with murine intestinal epithelial cells to enhance proinflammatory response against an intracellular pathogen. J Immunol. 2002;168:2988–96. doi: 10.4049/jimmunol.168.6.2988. [DOI] [PubMed] [Google Scholar]
- 21.Heimesaat MM, et al. Shift towards pro-inflammatory intestinal bacteria aggravates acute murine colitis via Toll-like receptors 2 and 4. PLoS ONE. 2007;2:e662. doi: 10.1371/journal.pone.0000662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johansson-Lindbom B, Agace WW. Generation of gut-homing T cells and their localization to the small intestinal mucosa. Immunol Rev. 2007;215:226–42. doi: 10.1111/j.1600-065X.2006.00482.x. [DOI] [PubMed] [Google Scholar]
- 23.Huleatt JW, Lefrancois L. Antigen-driven induction of CD11c on intestinal intraepithelial lymphocytes and CD8+ T cells in vivo. J Immunol. 1995;154:5684–93. [PubMed] [Google Scholar]
- 24.Buzoni-Gatel D, Lepage AC, Dimier-Poisson IH, Bout DT, Kasper LH. Adoptive transfer of gut intraepithelial lymphocytes protects against murine infection with Toxoplasma gondii. J Immunol. 1997;158:5883–9. [PubMed] [Google Scholar]
- 25.Buzoni-Gatel D, et al. Murine ileitis after intracellular parasite infection is controlled by TGF-beta-producing intraepithelial lymphocytes. Gastroenterology. 2001;120:914–24. doi: 10.1053/gast.2001.22432a. [DOI] [PubMed] [Google Scholar]
- 26.Zhou S, Ueta H, Xu XD, Shi C, Matsuno K. Predominant donor CD103+CD8+ T cell infiltration into the gut epithelium during acute GvHD: a role of gut lymph nodes. Int Immunol. 2008;20:385–94. doi: 10.1093/intimm/dxm153. [DOI] [PubMed] [Google Scholar]
- 27.Ludviksson BR, Strober W, Nishikomori R, Hasan SK, Ehrhardt RO. Administration of mAb against alpha E beta 7 prevents and ameliorates immunization-induced colitis in IL-2−/− mice. J Immunol. 1999;162:4975–82. [PubMed] [Google Scholar]
- 28.Annacker O, et al. Essential role for CD103 in the T cell-mediated regulation of experimental colitis. J Exp Med. 2005;202:1051–61. doi: 10.1084/jem.20040662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leithauser F, et al. Foxp3-expressing CD103+ regulatory T cells accumulate in dendritic cell aggregates of the colonic mucosa in murine transfer colitis. Am J Pathol. 2006;168:1898–909. doi: 10.2353/ajpath.2006.050228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dalton JE, et al. Intraepithelial gammadelta+ lymphocytes maintain the integrity of intestinal epithelial tight junctions in response to infection. Gastroenterology. 2006;131:818–29. doi: 10.1053/j.gastro.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 31.Lepage AC, Buzoni-Gatel D, Bout DT, Kasper LH. Gut-derived intraepithelial lymphocytes induce long term immunity against Toxoplasma gondii. J Immunol. 1998;161:4902–8. [PubMed] [Google Scholar]
- 32.Buzoni-Gatel D, et al. Intraepithelial lymphocytes traffic to the intestine and enhance resistance to Toxoplasma gondii oral infection. J Immunol. 1999;162:5846–52. [PubMed] [Google Scholar]
- 33.Cheroutre H. In IBD eight can come before four. Gastroenterology. 2006;131:667–70. doi: 10.1053/j.gastro.2006.06.041. [DOI] [PubMed] [Google Scholar]
- 34.Ishikawa H, et al. Curriculum vitae of intestinal intraepithelial T cells: their developmental and behavioral characteristics. Immunol Rev. 2007;215:154–65. doi: 10.1111/j.1600-065X.2006.00473.x. [DOI] [PubMed] [Google Scholar]
- 35.Das G, Janeway CA., Jr. Development of CD8alpha/alpha and CD8alpha/beta T cells in major histocompatibility complex class I-deficient mice. J Exp Med. 1999;190:881–4. doi: 10.1084/jem.190.6.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park SH, et al. Selection and expansion of CD8alpha/alpha(1) T cell receptor alpha/beta(1) intestinal intraepithelial lymphocytes in the absence of both classical major histocompatibility complex class I and nonclassical CD1 molecules. J Exp Med. 1999;190:885–90. doi: 10.1084/jem.190.6.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Egan CE, et al. A requirement for the Vgamma1+ subset of peripheral gammadelta T cells in the control of the systemic growth of Toxoplasma gondii and infection-induced pathology. J Immunol. 2005;175:8191–9. doi: 10.4049/jimmunol.175.12.8191. [DOI] [PubMed] [Google Scholar]
- 38.Lefrancois L, Lycke N. Isolation of mouse small intestinal intraepithelial lymphocytes, Peyer's patch, and lamina propria cells. Curr Protoc Immunol. 2001 doi: 10.1002/0471142735.im0319s17. Chapter 3, Unit 3 19. [DOI] [PubMed] [Google Scholar]
- 39.Gavrilescu LC, Butcher BA, Del Rio L, Taylor GA, Denkers EY. STAT1 is essential for antimicrobial effector function but dispensable for gamma interferon production during Toxoplasma gondii infection. Infect Immun. 2004;72:1257–64. doi: 10.1128/IAI.72.3.1257-1264.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson LL, Lanthier P, Hoffman J, Chen W. Vaccination protects B cell-deficient mice against an oral challenge with mildly virulent Toxoplasma gondii. Vaccine. 2004;22:4054–61. doi: 10.1016/j.vaccine.2004.03.056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.