Introduction
Progressive remodeling of the left ventricular (LV) architecture occurs after myocardial infarction (MI). While initially required for maintenance of cardiac output, this response ultimately leads to LV dysfunction and heart failure in the absence of a recurrent ischemic event [1,2]. Even with current optimal therapy, mortality in end-stage-heart-failure amounts to 20–50% per year [3]. Heart transplantation is applied as the last therapeutic option for patients with terminal heart-failure, but requests for organ transplantation far outstrip the number of donor organs. Therefore, new therapeutic strategies are urgently needed in order to ameliorate both patient prognosis and quality of life.
Following MI, dilatation of the LV cavity has the effect of increasing LV wall tension, which triggers further dilatation of the LV cavity, and progression down a spiral of adverse cardiac remodeling towards the advanced stages of cardiac failure [4]. To restore wall tension, the endoventricular circular patch plasty technique (the Dor procedure) [5,6] and partial left ventriculectomy (the Batista procedure) [4] have been clinically implemented for severe cardiac dilation and dysfunction many years after an infarction. Employing a similar strategy to limit the remodeling pathway at an earlier stage, epicardial restraint therapies, such as the Acorn Cardiac Support Device [7], and the Paracor device [8] have been investigated. However, these both apply materials that are non-biodegradable and result in a permanent foreign body encapsulating the epicardium. Using biodegradable and elastic polyester urethane urea, we recently reported that cardiac patch implantation onto a chronic myocardial infarct prevented further cardiac dilatation and improved contraction, while altering LV wall thickness and compliance [9].
Supported by a finite element model simulation [10], another concept in locally treating the failing cardiac wall was proposed where a bulking material is injected into the infarcted left ventricular wall to positively alter cardiac mechanics and result in a potentially beneficial reduction of elevated stresses in the infarcted wall. In this numerical model the local systolic fiber stress distribution was determined in an infarcted LV wall injected with a mechanically passive material. The simulation showed that injection of a volume 4.5% that of the total LV wall volume and with a stiffness (elastic modulus) 20% of the natural LV tissue into the infarct border zone could decrease the fiber stress in the border zone of the infarct by 20% compared to a control simulation in which there was no injection. The mechanical simulation also showed that this attenuation effect on LV wall stress increased with the injection volume and the modulus of the injected material.
Thermally responsive hydrogels are particularly attractive materials for injection therapy following MI since it is possible to inject the necessary fluid volumes from a syringe maintained below body temperature. Upon injection and warming hydrogel mechanical properties are increased, the “holding” of the material at the injection site is facilitated and the mechanical benefit of the injected volume on the cardiac wall is increased [10].
Poly(N-isopropylacrylamide (PNIPAAm) is a popular thermally responsive polymer with a lower critical solution temperature (LCST) of approximately 32°C. However, PNIPAAm is non-biodegradable and since its LCST remains constant, it is not readily cleared from the body at physiologic temperature. Bioabsorbable thermoresponsive polymer systems have been achieved by incorporation of biodegradable segments such as hyaluronic acid [11], gelatin [12], peptides [13] and collagen [14], into PNIPAAm-based polymers. Another approach has been to copolymerize NIPAAm with monomers possessing a hydrolytic polyester side chain, such as 2-hydroxyethyl methacrylate-polylactide (HEMAPLA) [15-17] and poly(ε-caprolactone)-2-hydroxylethyl methacrylate (HEMAPCL) [18, 19]. Cleavage of the hydrophobic polyester side chains will increase the hydrophilicity of the copolymer and raise the LCST above body temperature [15-17], therefore making the polymer soluble.
In this work we have developed a thermally responsive injectable and bioabsorbable hydrogel by copolymerization of N-isopropylacrylamide (NIPAAm), acrylic acid (AAc), and biodegradable monomer hydroxyethyl methacrylate-poly(trimethylene carbonate) (HEMAPTMC). We sought to investigate and tune the molecular design by altering the relative amount of AAc so that a thermoresponsive hydrogel would be achieved with an LCST below body temperature prior to hydrolysis of the poly(trimethylene carbonate) (PTMC) branches, but with an LCST that rose above body temperature with PTMC cleavage. The HEMAPTMC component was selected and synthesized for use since the carbonate bond in PTMC should have a hydrolysis rate that would allow retention of the gel over the several week period that we hypothesize would be necessary for the cardiac application in vivo. After characterizing and optimizing the copolymer structure, the optimized hydrogel was evaluated by injection into chronic rat myocardial infarctions two weeks following coronary ligation, and the resulting cardiac performance and ventricular remodeling were assessed over an 8 week period. Our hypothesis was that injection of the designed thermoreponsive hydrogel would alter the progression of ventricular remodeling, preserving ventricular wall thickness and maintaining contractile function.
Materials & Methods
Materials
Chemicals were purchased from Sigma-Aldrich unless otherwise stated. NIPAAm was purified by recrystallization from hexane and vacuum dried. NIPPAm (50g) was dissolved into 150 mL hexane at 80°C and then recrystallized at room temperature. AAc and 2-hydroxyethyl methacrylate (HEMA) were purified by vacuum distillation at 70°C and 100°C, respectively. Benzoyl peroxide (BPO), stannous 2-ethylhexanoate [Sn(OCt)2], trimethylene carbonate (TMC, Boehringer Ingelheim Chemicals Inc.) were used as received.
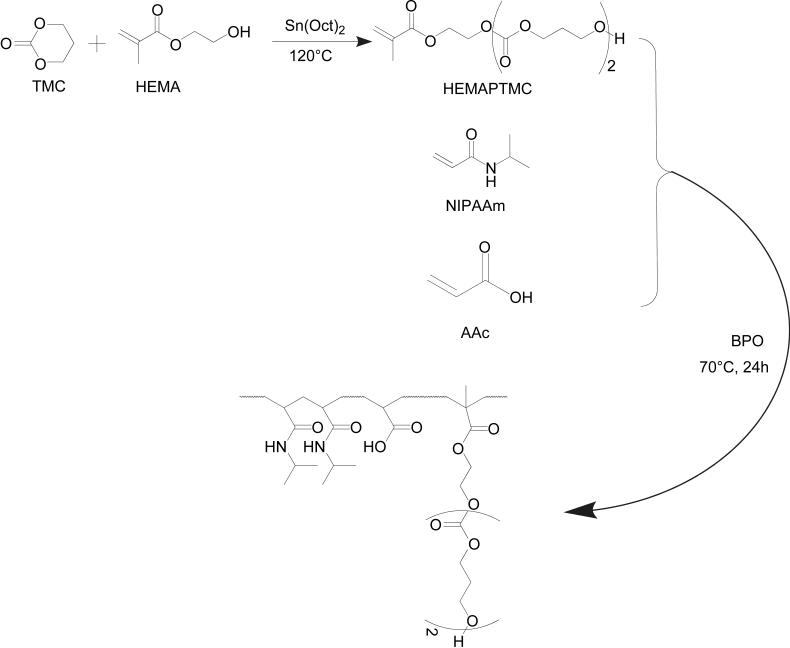
Synthesis of HEMA-polyTMC (HEMAPTMC)
HEMAPTMC was synthesized by ring-opening polymerization of TMC initiated by HEMA with Sn(OCt)2 as a catalyst (Figure 1). Stoichiometric amounts of HEMA and TMC (molar ratio 1:2) were mixed in a flask to which was added anhydrous toluene of equal mass to the TMC/HEMA mixture. Sn(OCt)2 (1 mol% with respect to HEMA) in 1 mL toluene was subsequently added. The reaction was conducted at 120°C for 1.5 h. The mixture was then dissolved in THF and precipitated in water. This precipitation process was repeated twice and the liquid precipitate was then isolated by centrifugation, dissolved in THF, and dried over anhydrous MgSO4. THF was removed by rotary evaporation.
Figure 1.
Synthetic scheme for HEMAPTMC and the copolymer poly(NIPAAm-co-AAc-co-HEMAPTMC)
Synthesis of poly(NIPAAm-co-AAc-co-HEMAPTMC)
Poly(NIPAAm-co-AAc-co-HEMAPTMC) copolymers were synthesized by free radical polymerization (Scheme 2). Monomers (NIPAAm, AAc, HEMAPTMC) were dissolved in 1,4-dioxane to form a 5 wt% solution containing BPO (7.2×10-3 mol/mol monomer). The polymerization was carried out at 70°C for 24 h under argon atmosphere. The copolymer was precipitated in hexane and further purified by precipitation from THF into diethyl ether. The purified copolymer was vacuum dried.
Material characterization
1H-NMR and 13C-NMR spectra of HEMAPTMC and the poly(NIPAAm-co-AAc-co-HEMAPTMC) copolymers were recorded with a 300 MHz BRUKER spectrometer using CD3Cl or DMSO-d6 as a solvent. AAc content in the copolymers was determined by titration, in which copolymers were dissolved into deionized water with a concentration of 16.7 wt% at 4°C and titrated with NaOH (0.1M) with phenolphthalein as a pH indicator. Molecular weight of the various polymers was determined by gel permeation chromatography (GPC, Waters Breeze System, Waters 1515 HPLC Pump, Waters 2414 differential refractometer). The copolymers were dissolved in THF with a concentration of 1 mg/mL and the GPC tests were made at 35°C with THF as a solvent. A poly(methyl methacrylate) standard kit (Fluka, ReadyCal Set Mp 500-2,700,000) was used for molecular weight-elution volume calibration. LCSTs of the copolymer solutions in PBS (16.7 wt %, pH 7) were studied by measuring UV-optical absorption at 500 nm over a temperature range of 0 to 45°C. The LCST of each copolymer was determined (n=4) by determining the temperature at which the absorbance of the copolymer solution reached half of its maximal value, during the phase transition.
Gelation properties of the copolymer solutions (PBS, 16.7 wt%) were studied by incubating glass vials (3 mL) containing 1 mL solution in a 37°C water bath. The water content of the hydrogel at body temperature and mechanical properties were measured after 24 h incubation. Water content was defined as (w2-w1)/w2 × 100%, where w2 and w1 are wet mass and dry mass of the hydrogel, respectively. To measure hydrogel mechanical (tensile) properties, the hydrogel was cut into rectangular strips 1 mm thick, 4 mm wide and 25 mm long. Samples (n=4) were loaded in a water bath test cell equilibrated to 37±2°C and attaching preheated grips to each end. An ATS 1101 Universal Testing Machine equipped with a 10 lb load cell was utilized with a cross-head speed of 6 cm/min.
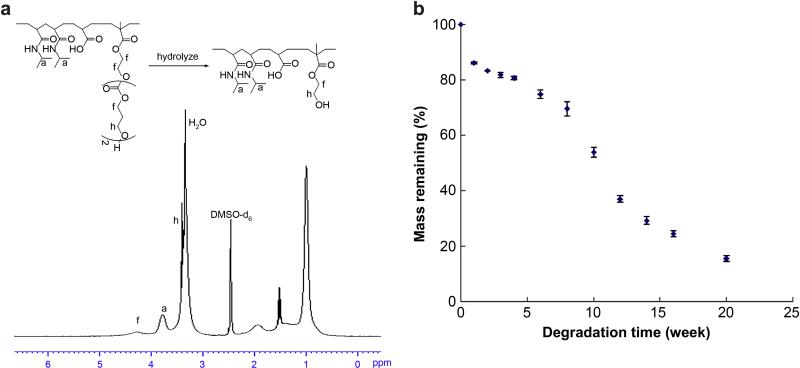
Hydrogel degradation rate was quantified by mass loss measurement. Hydrogels with known initial dry masses (~60 mg) were immersed into 7 mL PBS (pH 7, replaced weekly) at 37°C. At pre-defined time points over a 20 week period the hydrogels (n=4) were lyophilized and the relative mass loss recorded.
Cytotoxicity assay
The cytotoxicity of the polymer degradation products was assessed by measuring the metabolic viability of cells cultured with medium supplemented with degradation products [16]. To verify this result cells were also observed under fluorescence microscopy after live/dead staining with a Promokine® Live/Dead Cell Staining Kit. Live/dead cells were observed with green/red fluorescence respectively using excitation at 480 nm or 540 nm.
The polymer degradation solution was prepared by hydrolysis of the copolymer in a 1.0 M NaOH solution at 4°C for 5 d to cleave the PTMC side chains, followed by removal of NaOH from the solution using an Amberlite® IR-120H ion-exchange resin (Aldrich). The solution was then mixed with 10x EMEM culture medium (BioWhittaker®, Lonza) at a volume ratio of 9:1. Rat vascular smooth muscle cells (RSMCs) were isolated according to the method of Ray et al. from recently deceased animals that had been utilized in other protocols [20]. Cells were cultured in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) to the fifth passage and seeded into a 24-well tissue culture plate at a seeding density of 15,000/well. The copolymer degradation solution with EMEM was added into each well to obtain a final concentration of 5 mg/mL. For control purposes, culture medium without added degradation solution was used. Cell metabolic viability was measured (n=4) using an MTS assay kit (Promega CellTiter 96® Cell Proliferation Assay) to quantify mitochondrial activity.
Experimental animals
Adult female Lewis rats (Harlan Sprague Dawley, Indianapolis, IN) weighing 200-250 g were used. The protocol followed National Institutes of Health (NIH) guidelines for animal care and was approved by the University of Pittsburgh's Institutional Animal Care and Use Committee and Children's Hospital of Pittsburgh Animal Research Care Committee.
Chronic left ventricular infarction model
Anesthesia was induced with 3.0% isoflurane inhalation followed by intubation and respiratory support with a rodent volume-controlled mechanical ventilator. While monitoring with an electrocardiogram and tail cuff blood pressure measurement, a left thoracotomy was performed to expose the heart and the proximal left anterior descending coronary artery was ligated with 7-0 polypropylene. The creation of myocardial ischemia was verified by regional cyanosis and ST segment elevation and the incision was closed in layers with 4-0 continuous silk sutures.
Poly(NIPAAm-co-AAc-co-HEMAPTMC) (86/4/10) hydrogel injection
Two weeks after induction of myocardial infarction, the rats were anesthetized and evaluated with echocardiography to measure infarct size in terms of the percentage of scar area (akinetic or dyskinetic regions) to LV free wall area [21]. A total of 17 rats with infarcts greater than 25% of the LV free wall were randomly divided into two groups: those that would receive hydrogel injections (hydrogel group n=9), and those that would receive the control PBS injections (PBS group; n=8). The infarcted anterior surface of the rat heart was exposed through a left thoracotomy. For a rat in the hydrogel group a total of 500 μL of poly(NIPAAm-co-AAc-co-HEMAPTMC) (86/4/10) solution in PBS (16.7 wt%) was injected into the apical, proximal, lateral, and septal wall regions bordering the infarct as well as into the center of the infarct (5 injections, 100 μL per region). For a rat in the PBS group, 500 μL PBS was injected into the same locations with the same volumes. The incision was closed in layers with 4-0 silk continuous sutures for both groups.
Histology
Eight weeks after injection (10 weeks after myocardial infarction), rats in both surgical groups were anesthetized, and the heart was exposed and arrested by apical injection of 2 mL of a hypothermic arresting solution (68 mM NaCl, 60 mM KCl, 36 mM NaHCO3, 2.0 mM MgCl2, 1.4 mM Na2SO4, 11 mM dextrose, 30 mM butanedione monoxime, and 10,000 U/L of heparin). The heart was explanted and fixed in 2% paraformaldehyde for 2 h and then embedded with optimal cutting temperature compound (Tissue-Tek, Torrance, CA) followed by freezing at - 80°C. Embedded, frozen LV tissues were serially sectioned at 8 μm in the LV transverse direction. Hematoxylin and eosin (H&E) staining and immunohistochemical staining were performed as previously described [22] with antibodies against alpha-smooth muscle actin (α-SMA, Sigma, St Louis, MO), CD 31 (Serotec, Raleigh, NC), caldesmon (Abcam, Cambridge, MA), calponin (Abcam), smooth muscle myosin heavy chain 2 (SMMHC-2, Abcam), SM-22α (Abcam). Nuclei were stained with 4',6-diamidino-2-phenyindole (DAPI, Sigma).
LV wall thickness and capillary density
For each LV sample 5 different microscopic fields at 100x magnification for the wall thickness measurement and 10 different fields at 200x magnification for the capillary density measurement were photographed for 5 rats in each group 8 weeks after the injection procedure. The wall thickness of the infarcted anterior wall where the injections were performed was analyzed using NIH Image software. Capillaries were recognized as tubular structures positively stained for CD31 as previously described [23].
Echocardiography
Echocardiography was performed immediately prior to injection (pre-injection time point, which was 2 weeks post-infarction), as well as 4 and 8 weeks after hydrogel or PBS injection. Rats were anesthetized with isoflurane inhalation. Standard transthoracic echocardiography was performed using the Acuson Sequoia C256 system with 13-MHz linear ultrasonic transducer (15L8; Acuson Corporation, Mountain View, CA) in a phased array format. B-mode measurements on the LV short axis view (papillary muscle level) were performed. The end-diastolic (EDA) and end-systolic (ESA) LV internal cavity areas were measured by tracing the endocardial border. The LV fractional area change (%FAC) was estimated as, %FAC = [(LVEDA – LVESA)/LVEDA] × 100%. All measurements were performed using Scion Image software (Scion Image, Frederick, MD).
Statistics
All data are expressed as means with the standard deviation. Analyses utilized SPSS software (SPSS Inc, Chicago IL). Statistical analyses were performed by ANOVA or 2-way repeated measures ANOVA with Tukey's test applied to investigate specific differences. Statistical significance was defined at p<0.05. The wall thickness and capillary density in each group was compared by Student's t-test.
Results
Synthesis of HEMAPTMC and copolymer
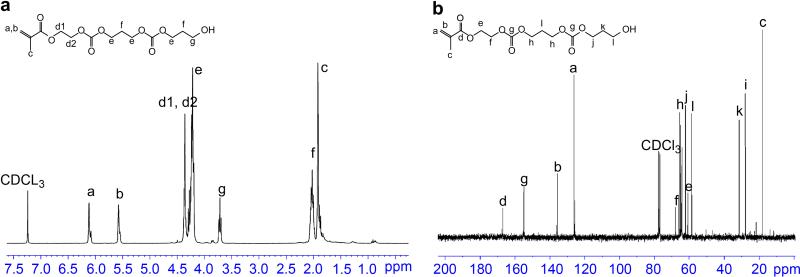
The synthesis of HEMAPTMC was confirmed by the 1H-NMR spectrum of the product (Figure 2a) and the 13C-NMR spectrum (Figure 2b) containing proton peaks and carbon peaks in agreement with the molecular structure of HEMAPTMC. In the 1H-NMR spectrum, HEMA alone would be expected to have two characteristic triple peaks centered at 4.4 ppm and 3.9 ppm for d protons, while for HEMAPTMC the combination of the two d peaks into a single peak at 4.4 ppm provides confirmation of the formation of HEMAPTMC. The chemical structure of HEMAPTMC was further confirmed by the mass spectrum (API-ES positive). Peaks at 254.8 (HEMAPTMC1+Na+), 357.0 (HEMAPTMC2+Na+), 459.0 (HEMAPTMC3+Na+), 561.0 (HEMAPTMC4+Na+) and 663.0 (HEMAPTMC5+Na+) were observed, indicating that the product was a mixture of molecules containing different PTMC lengths. The number average length of PTMC units per monomer was determined from 1H-NMR spectrum (Figure 2a) as 2 by calculation from the ratio of the integrals of hydrogen peaks from PTMC (peak e, f and g) and the double bond hydrogen (CH2=) peak (peak a and b at 5.6 and 6.1 ppm). This PTMC unit number for HEMAPTMC was in agreement with the molar feed ratio of HEMA to TMC (1:2) in the synthesis of HEMAPTMC.
Figure 2.
(a) 1H-NMR and (b) 13C-NMR spectra for HEMAPTMC.
Copolymers with different monomer ratios were prepared by free radical polymerization (Figure 1). Table 1 summarizes poly(NIPAAm-co-AAc-co-HEMAPTMC) copolymers synthesized with different AAc feed ratios. All of the copolymers have molecular weights between 20k and 30k, and a polydispersity index of 1.5~2.0. Figure 3 shows typical 1H-NMR and 13C-NMR spectra for a synthesized copolymer. Proton and carbon peaks characteristic of NIPAAm and HEMAPTMC are seen in the spectra. The existence of AAc (-COOH) units in the copolymer was verified and quantified by titration of the polymer solution with NaOH solution (0.1 M). The AAc content obtained by the titration method and the integration ratios of characteristic proton peaks in the 1H-NMR spectra were used to determine copolymer compositions (Table 1). The monomer compositions in the copolymers were found to be close to the feed ratios, with a consistent slight reduction in the measured AAc content from that expected based on the feed ratio.
Table 1.
Properties of poly(NIPAAm-co-AAc-co-HEMAPTMC) copolymers with different feed ratios of AAc.
| Feed ratio NIPAAm/AAc/HEMAPTMC | Yield | Mn g/mol | Mw/Mn | –COOH content, 10–4 mol/g | Polymer composition, NIPAAm/AAc/HEMAPTMC | 37 °C, 16.7 wt% in PBS, pH 7 | LCST 16.7 wt% in PBS, pH 7, °C |
|---|---|---|---|---|---|---|---|
| 87/3/10 | 86% | 27,000 | 1.8 | 1.6 | 88.5/2.1/9.4 | Solid gel | 29.1 ± 0.37* |
| 86/4/10 | 87% | 23,000 | 1.9 | 2.6 | 88.3/3.3/8.4 | Solid gel | 33.1 ± 0.43* |
| 85/5/10 | 84% | 34,000 | 1.5 | 2.8 | 87.0/3.6/9.4 | Cloudy, weak gel | 36.2 ± 0.38* |
| 84/6/10 | 93% | 21,000 | 2.0 | 3.8 | 86.2/4.8/8.9 | Clear solution | 44.5 ± 0.10* |
p < 0.001 versus each of other copolymers.
frozen LV tissues were serially sectioned at 8 μm in the LV transverse direction. Hematoxylin and eosin (H&E) staining and immunohistochemical staining were performed as previously described [22] with antibodies against alpha-smooth muscle actin (α-SMA, Sigma, St Louis, MO), CD 31 (Serotec, Raleigh, NC), caldesmon (Abcam, Cambridge, MA), calponin (Abcam), smooth muscle myosin heavy chain 2 (SMMHC-2, Abcam), SM-22α (Abcam). Nuclei were stained with 4′,6-diamidino-2-phenyindole (DAPI, Sigma).
Figure 3.
(a) 1H-NMR and (b) 13C-NMR spectra for poly(NIPAAm-co-AAc-co-HEMAPTMC) (86/4/10).
Gelation properties, the LCST and optimization of monomer feed ratio
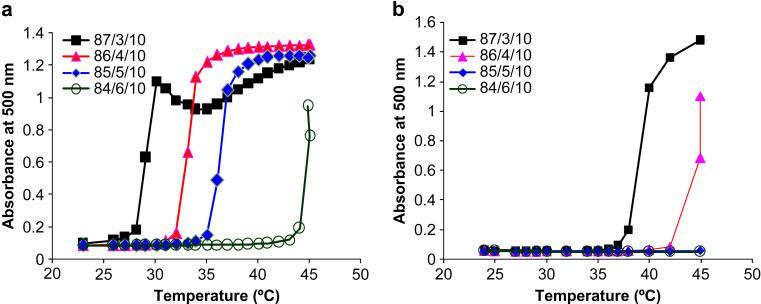
The qualitative gelation properties of the poly(NIPAAm-co-AAc-co-HEMAPTMC) copolymers are summarized in Table 1. When the AAc feed ratio was 3% and 4%, a solid gel could be formed at 37 °C. When the AAc feed ratio was increased to 5%, a fluid-like hydrogel with negligible strength was formed. When the AAc feed ratio was as high as 6%, the copolymer solution remained a clear solution at 37°C, indicating an LCST above 37°C. The calculated LCSTs were determined from the optical data represented in Figure 4a. The temperature at which optical absorption rapidly transitions (the LCST) is seen to increase as the AAc feed ratio of the copolymer is increased. While copolymers with AAc feed ratios of 3, 4 and 5% had LCSTs below 37°C, the copolymer with an AAc feed ratio of 6% had an LCST of 45°C. In addition to evaluating copolymers of poly(NIPAAm-co-AAc-co-HEMAPTMC), copolymers of poly(NIPAAm-co-AAc-co-HEMA) were also synthesized with fixed HEMA molar feed ratios of 10% and with different AAc molar feed ratios (3, 4, 5, and 6%). These HEMA containing copolymers were evaluated since cleavage of the PTMC in the poly(NIPAAm-co-AAc-co-HEMAPTMC) would be expected to result in poly(NIPAAm-co-AAc-co-HEMA). The results in Table 2 present the physical state of various poly(NIPAAm-co-AAc-co-HEMA) solutions at 37°C as well as LCSTs determined from optical absorption measurements (Figure 4b). These data provide guidance as to whether one would expect the corresponding poly(NIPAAm-co-AAc-co-HEMAPTMC) to be soluble after complete removal of its PTMC residues. For these HEMA containing copolymers, when the AAc feed ratio was 3%, the copolymer solution was a cloudy liquid gel at 37°C, while AAc feed ratios of 4, 5 and 6% resulted in solutions that remained clear. This was further confirmed by LCST values, which showed that the copolymer with an AAc feed ratio of 3% had an LCST close to body temperature, while all other copolymers with higher AAc feed ratios had LCSTs well above 37°C. These results demonstrated that in synthesizing the poly(NIPAAm-co-AAc-co-HEMAPTMC), the AAc feed ratio should be higher than 3% to ensure that the polymer will become completely soluble upon cleavage of the PTMC residues.
Figure 4.
LCST determination by measurement of copolymer solution optical absorption as a function of temperature. For each copolymer a representative curve is shown. (a) Copolymers poly(NIPAAm-co-AAc-co-HEMAPTMC) with varying AAc feed ratio; (b) copolymers poly(NIPAAm-co-AAc-co-HEMA) with varying AAc feed ratio.
Table 2.
Properties of poly(NIPAAm-co-AAc-co-HEMA) copolymers with different feed ratios of AAc.
| Feed ratio NIPPAm/AAC/HEMA | Yield | Mn | Mw/Mn | –COOH content, 10–4 mol/g | Polymer composition, NIPAAm/AAc/HEMA | 37 °C, 16.7 wt% in PBS, pH 7 | LCST 16.7 wt% in PBS, pH 7, °C |
|---|---|---|---|---|---|---|---|
| 87/3/10 | 96% | 21,000 | 2.1 | 1.7 | 88.2/1.9/9.9 | Cloudy, weak gel | 39.3 ± 0.42* |
| 86/4/10 | 89% | 29,000 | 1.7 | 2.3 | 86.9/2.5/10.6 | Clear solution | 43.5 ± 0.84* |
| 85/5/10 | 92% | 21,000 | 1.9 | 2.9 | 87.0/3.2/9.8 | Clear solution | >45 |
| 84/6/10 | 96% | 22,000 | 1.9 | 3.6 | 85.6/4.0/10.4 | clear solution | >45 |
p < 0.001 between 87/3/10 and 86/4/10.
Based on these results, to move forward towards the application of a poly(NIPAAm-co-AAc-co-HEMAPTMC) hydrogel in the treatment of myocardial infarcts, the optimal monomer feed ratio for NIPAAm, AAc and HEMAPTMC was considered to be 86/4/10. The resulting copolymer should provide an initial LCST between room temperature and body temperature, and theoretically be degradable to a soluble polymer with an LCST above body temperature. Further characterization studies utilized this copolymer.
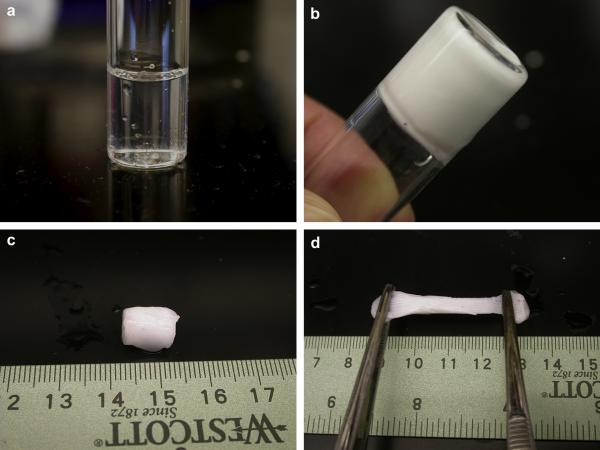
A gross observation of the gelation process for the poly(NIPAAm-co-AAc-co-HEMAPTMC) (86/4/10) hydrogel solution (16.7 wt% in PBS) is shown in Figure 5a-b. The sol-gel transition occurred immediately when the solution was immersed into the water bath of 37°C. After incubation for 10 min, a highly flexible gum-like material was formed (Figure 5c-d). Upon continued warming in the water bath, the hydrogel gradually shrank, excluding water from inside, and became completely stable after 24 h. The final equilibrated water content of the hydrogel was measured as 60±5% and the maximum tensile strength of the equilibrated hydrogel was found to be 6.1±2.0 kPa with plastic deformation occurring beyond the maximum tensile strength at approximately 25% strain.
Figure 5.
Gelation properties of the (NIPAAm-co-AAc-co-HEMAPTMC) (86/4/10) hydrogel. (a) Cooled hydrogel solution; (b) after incubation in 37 °C water bath for 30 sec; (c) hydrogel formed after 10 min; (d) stretching of the hydrogel that was formed after 10 min.
In vitro degradation
The in vitro degradation properties of the poly(NIPAAm-co-AAc-co-HEMAPTMC) (86/4/10) hydrogel was first evaluated by hydrolysis in NaOH (1M) at room temperature for 5 days to theoretically cleave the PTMC residues, representing extensive degradation of the copolymer. In Figure 6a the H1-NMR spectrum of this hydrolyzed copolymer is shown to exhibit a peak characteristic of PTMC at 4.3 ppm that was decreased compared with the NMR spectrum before degradation as shown in Figure 3a. The hydrolyzed copolymer gave a clear solution at 37°C, demonstrating an LCST well above 37°C. A second evaluation of in vitro degradation of the hydrogel was performed in PBS at 37°C with the resulting mass loss curve shown in Figure 6b, the hydrogel was gradually solubilized at a much lower rate than in the NaOH solution, with mass loss of over 85% by 20 weeks.
Figure 6.
(a) 1H-NMR spectrum of degraded poly(NIPAAm-co-AAc-co-HEMAPTMC) (86/4/10); (b) mass loss of poly(NIPAAm-co-AAc-co-HEMAPTMC) (86/4/10) hydrogel in PBS at 37 °C.
Degradation product cytotoxicity
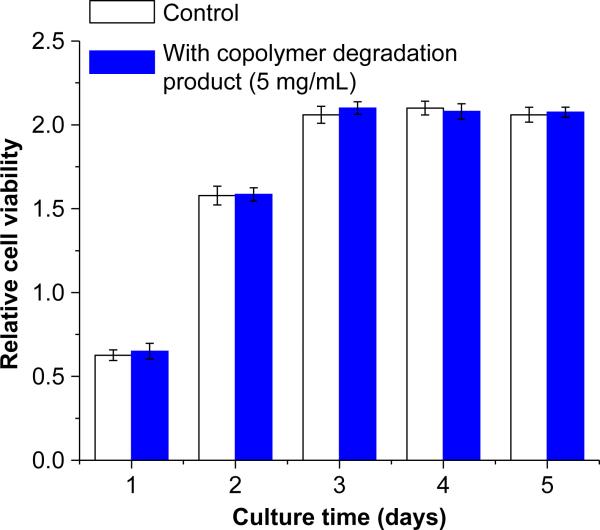
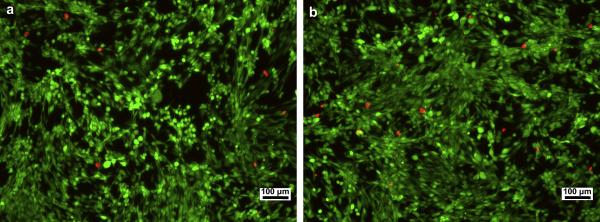
With RSMC mitochondrial activity serving as an index for cell viability, Figure 7 demonstrates a lack of toxic effect of degradation product containing medium on RSMC culture. This result was further verified by fluorescent live/dead staining of RSMC cultures under control or degradation product containing culture medium (Figure 8). For both culture media, dead cells marked by the red color were seen in low numbers and no difference was apparent in the relative number of dead cells viewed over several culture wells.
Figure 7.
Cytotoxity assay of poly(NIPAAm-co-AAc-co-HEMAPTMC) (86/4/10) hydrogel degradation products by cell metabolic activity assessment (MTS assay ) for RSMCs cultured on TCPS. Hydrolyzed hydrogel solution was supplemented into cell culture medium at a final concentration of 5.0 mg/mL.
Figure 8.
Live/dead staining of RSMC cultured on TCPS using (a) untreated culture medium and (b) culture medium containing 5.0 mg/mL hydrolyzed poly(NIPAAm-co-AAc-co-HEMAPTMC) (86/4/10). Culture time of 3 days. Scale bar: 100 μm.
Injection in chronic infarction model: external morphology and histology
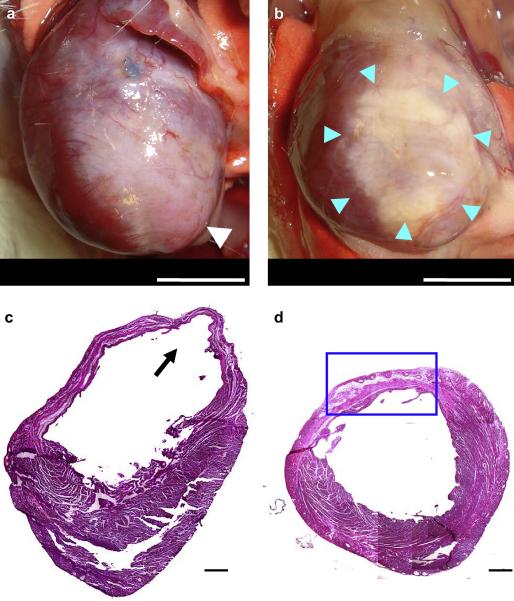
There were no early or late postoperative deaths in either surgical group. After the 8 week evaluation period 3 of 8 animals in the PBS injection group had obvious ventricular aneurysm formation in the apex area (Figure 9a). The other rats in this group did not have obvious aneurysms, but did have well defined scar areas. For the hydrogel injection group the treated infarcts were covered with fat connective tissue with no strong adhesions and no aneurysms (Figure 9b).
Figure 9.
Representative images at 8 weeks following the injection procedure of the anterior view of (a) PBS injected, and (b) poly(NIPAAm-co-AAc-co-HEMAPTMC) (86/4/10) injected hearts. White arrow shows an aneurysm formation in the apex area (a). Blue arrows indicate the injected hydrogel area (b). The composite histological sections of (c) PBS injected and (d) hydrogel injected myocardial walls 8 weeks after injection stained with H&E. Black arrow shows an aneurysm formation (c). Blue box indicates higher magnification area shown in Figure 10 (d). Scale bar: 5 mm in (a, b), 500 μm in (c, d).
In the PBS injected animals, examining H&E stained sections revealed extensive fibrous tissue and decreased tissue thickness in the anterior-lateral wall in all animals and aneurysm formation in the anterior wall in the same animals where this attribute was grossly apparent at the time of explant (Figure 9c). On the other hand, in the hydrogel injected group, the thickness of the ventricular wall was relatively preserved (Figure 9d). The remnant injected hydrogel material appeared to be distributed in the anterior wall and infiltrated with macrophages and fibroblasts. In addition, beneath the distributed hydrogel, a muscle-like layer was observed (Figure 10a). Immunohistochemical staining showed that this muscle-like layer was positive for α-SMA (Figure 10b). In higher magnification images, cellular ingrowth was found inside the hydrogel area, with some regions of this tissue structure staining positively for α-SMA (Figure 11a-d). Further immunohistochemical staining demonstrated that caldesmon, calponin, SM 22α, and SMMHC type II (caldesmon images shown in Figure 12a), all proteins that are associated with contractile function, co-localized with α-SMA positive cells in the hydrogel injected myocardial sections.
Figure 10.
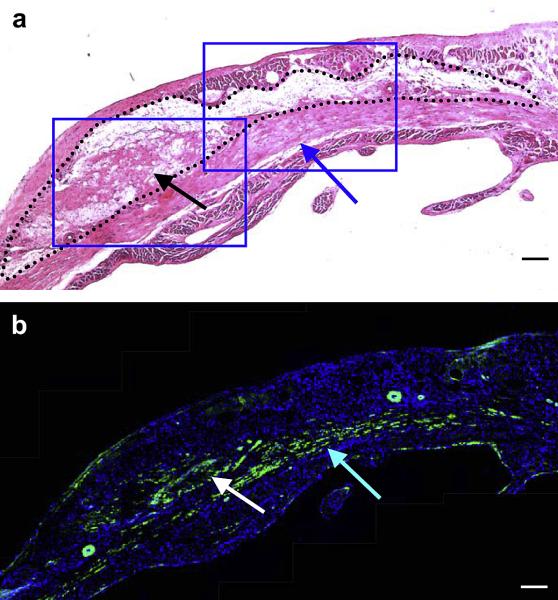
Higher magnification of (a) H&E staining and (b) immunohistochemical staining in the hydrogel injected ventricular wall of sequential sections. Black dots indicate the injected hydrogel area and blue box indicates higher magnification area shown in Figure 11 (a). α-SMA staining appears green and nuclear staining appears blue (b). Black and white arrows in (a) and (b) respectively indicate tissue ingrowth in the hydrogel area, while blue arrows in (a) and (b) indicate smooth muscle populated area beneath the hydrogel area. Scale bars: 100 μm.
Figure 11.
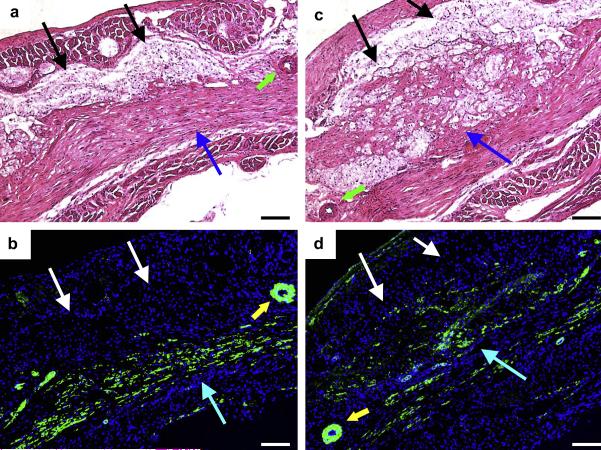
(a) H&E staining and (b) immunohistochemical staining in the left blue boxed area from Figure 10. (c) and (d) represent the same staining order for the right blue boxed area from Figure 10. α-SMA staining appears green and nuclear staining appears blue. Black (a, c) and white (b, d) arrows denote the injected hydrogel area. Blue (a, b, c, d) arrows indicate muscle-like tissue positively staining for α-SMA. Green (a, c) and yellow arrows (b, d) show relatively large arteries with smooth muscle cell walls. Scale bars: 100 μm.
Figure 12.
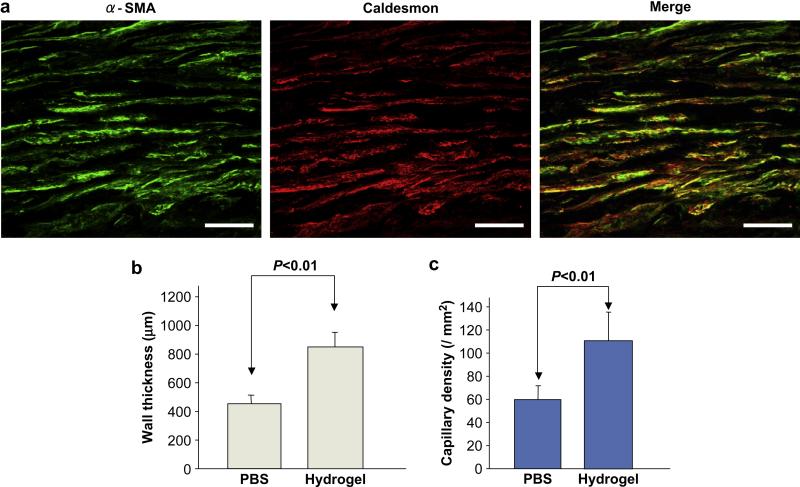
(a) Co-localization of α-SMA and caldesmon with immunohistochemical staining. Scale bars: 50 μm. (b) The left ventricular myocardial wall thickness and (c) capillary density of comparing the hydrogel and PBS injection groups at 8 weeks following injection.
From histological section image analysis, the LV myocardial wall for the hydrogel injection group was found to be thicker than for the PBS injection control group (825 ± 92 vs. 412 ± 104 μm, p<0.01, Figure 12b). The capillary density in the hydrogel group was also significantly higher in comparison to the PBS injection group (110 ± 26 vs. 59 ± 17 / mm2, p<0.01, Figure 12c).
Injection in chronic infarction model: cardiac function
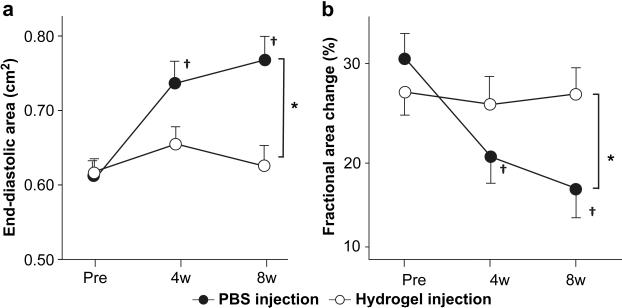
Longitudinal echocardiography showed that the LV EDA increased and the %FAC decreased in the period following the injection procedure (at 4 and 8 weeks, each versus the pre-injection period, p<0.05). The hydrogel group did not experience a change in EDA or %FAC following injection at either of the time points relative to the pre-injection time point. At 8 weeks, the EDA in the PBS group had become significantly larger than that of the hydrogel group, and also the %FAC of the PBS group was also significantly smaller than that of the hydrogel group (Figure 13).
Figure 13.
Echocardiographic assessment of the hydrogel and PBS injection groups during the study period. (a) EDA (end-diastolic area), and (b) % fractional area change (%FAC). *; p<0.05 between groups, †; p<0.05 vs. pre-injection time point within group.
Discussion
We previously reported on the development of a thermoresponsive and biodegradable hydrogel by the copolymerization of NIPAAm, AAc, N-acryloxysuccinimide (NAS) and HEMAPLA[16]. However, application of this hydrogel in vivo for cardiac wall injection therapy was considered non-ideal since in pilot studies with subcutaneous tissue injection it was found to be rapidly resorbed within two days (data not shown). The postulated mechanical benefits of gel injection on the cardiac wall might thus be too transient [10]. In this report, we considered an alternative molecular structure for a thermoresponsive hydrogel where instead of using HEMAPLA, another biodegradable monomer, HEMAPTMC, was synthesized on the theory that the carbonate bond in PTMC residues would experience hydrolysis at a slower rate than the ester bonds in the PLA residue. The synthesis of the HEMAPTMC and the copolymer poly(NIPAAm-co-AAc-co-HEMAPTMC) were confirmed with NMR spectra (Figure 2 and 3). Monomer ratios of copolymers were determined and were found to be similar to the feed ratios (Table 1), with a consistent slight reduction in the AAc content. Previous studies reported that AAc has slightly lower reactivity than NIPAAm in benzene, while HEMA and HEMAPLA, which have similar structures to HEMAPTMC, have close reactivity to NIPAAm[24].
One design objective for the HEMAPTMC-containing hydrogel was that it should be capable of gelation at 37°C, and be slowly solubilized at this temperature as the PTMC residues are hydrolytically cleaved. To achieve this, control of the AAc content in the copolymer poly(NIPAAm-co-AAc-co-HEMAPTMC) is a critical consideration. AAc is a highly hydrophilic monomer since its –COOH residues will be deprotonized into highly hydrated –COO- groups at neutral pH. If the AAc content in the copolymer is too high, the copolymer will be too hydrophilic so that the copolymer solution will have an LCST above 37 °C and will not be able to form a hydrogel at 37 °C. On the other hand, if the AAc content in the copolymer is too low, the copolymer will be too hydrophobic so that even after removal of the PTMC residues the LCST would remain below 37 °C and the copolymer would not become soluble. By performing a set of experiments to explore the gelation properties and LCSTs of various poly(NIPAAm-co-AAc-co-HEMAPTMC) and poly(NIPAAm-co-AAc-co-HEMA) copolymers with different AAc feed ratios (Tables 1 and 2, Figure 4), we were able to determine that an appropriate monomer feed ratio for NIPAAm, AAc and HEMAPTMC was 86/4/10 and the resulting copolymer was adopted for the cardiac application studies.
In vitro degradation of the poly(NIPAAm-co-AAc-co-HEMAPTMC) (86/4/10) hydrogel occurred over more than 5 months (Figure 6b), while in vitro results with the HEMAPLA containing hydrogel occurred in 20 days[16]. A specific set of experiments was not performed to determine how fast the PTMC residue was cleaved in PBS solution. Instead, the degradation rate of the hydrogel in PBS, in terms of weight loss, was studied since this is of primary interest with respect to the application. It can be inferred from the degradation test results presented in Figure 6b that after incubation in PBS for five months, more than 80% of the macromolecules of the hydrogel had their PTMC residues cleaved to an extent that allowed the remaining copolymer to become soluble. The speed of the cleavage of the PTMC residue in PBS would be expected to occur on this same time scale of several months. In vivo, the poly(NIPAAm-co-AAc-co-HEMAPTMC) hydrogel was found to be present in the rat ventricular wall injection region at the 8 week post-injection time point utilized in this study (Figures 9 and 10). These experiments showed that the hydrogel had abundant cellular ingrowth in the infarcted myocardial injection site and it is anticipated that the hydrogel in vivo would be degraded faster than in vitro, due to macrophage phagocytic and secretory activity leading to faster hydrolytic cleavage and local removal. The hydrogel showed no in vitro cytotoxicity as evaluated by the cell metabolic viability test, as was the case for the previous hydrogel. As for mechanical properties, the poly(NIPAAm-co-AAc-co-HEMAPTMC) hydrogel was found to be a robust material which could be handled and stretched, as shown in Figure 5.
For therapeutic cardiac wall injection therapy, it has been reported that injection of a fibrin-alginate biocomposite into damaged myocardium showed an increase in LV wall thickness and prevented infarct expansion [25,26]. While alginate alone has been shown to have functional benefit, recent reports of adhesion peptide modified alginate injection do not demonstrate a clear functional benefit of such modification [27,28]. Self-assembling synthetic hydrogels [29] as well as self-assembling peptides carrying specific growth factors have been reported to have beneficial effects on the cardiac wall remodeling process [30]. These latter materials have also been reported as vehicles for the transplantation of skeletal myoblasts into the cardiac wall [31]. Regarding thermoresponsive polymers, a recent report showed that injection of a PNIPPAm-based polymer four days following MI in a rabbit model prevented adverse cardiac remodeling and dysfunction at 30 days following treatment [18]. While similar to the current report, some important differences should be noted. The PNIPPAm-based polymer in the study by Wang et al., was injected earlier in the post-infarct period and at the 30 day follow-up time point no materials appeared to be present at the injected positions [18]. In the current report poly(NIPAAm-co-AAc-co-HEMAPTMC) (86/4/10) injection was performed two weeks post-MI, corresponding to the beginning of the fibrotic phase of remodeling and after the necrotic phase [32]. This time lag may better represent infarcts that would be encountered in patients with sub-acute MI, where the patient may not present clinically until substantial wall remodeling has already occurred [33]. Furthermore, our objective was to have the hydrogel remain over an extended period in the cardiac wall since the remodeling process and the risk for negative remodeling would seem likely to extend beyond the first few weeks. While this has not been demonstrated as beneficial (extended follow up evaluation would be required), we have shown positive effects on function at 8 weeks and the presence of hydrogel remnants at the 8 week time point.
A cellular layer was found at 8 weeks in association with the hydrogel injection area that stained positively for alpha-smooth muscle actin and had co-localized staining for caldesmon, calponin, SM 22α, and SMMHC type II. This staining pattern is consistent with contractile smooth muscle cells. Ultrastructurally mature smooth muscle cells with a contractile phenotype have previously been reported in a rat MI model following treatment with an anti-apoptotic agent [34]. In that study it was hypothesized that apoptosis inhibition would preserve granulation tissue and result in beneficial effects on cardiac remodeling and function. We also previously reported finding smooth muscle cells with a contractile phenotype beneath an elastic, biodegradable poly(ester urethane)urea patch placed onto rat infarcts [9]. These two previous reports may suggest a role for biomaterial-stimulated inflammatory processes in triggering the appearance of these smooth muscle cells, or possibly an effect associated with the alteration of the mechanical environment of the infracted wall. The direct benefit of these smooth muscle cells has not been shown, although their presence contributes to increasing wall thickness.
Several limitations of the current report should be noted. First, the beneficial effects in terms of cardiac function and LV remodeling were only evaluated in the 8 week period following injection. Although the poly(NIPAAm-co-AAc-co-HEMAPTMC) (86/4/10) appears to have substantially remained in this period, the architectural and functional changes should be examined at longer times. There might be an optimal residence time when the hydrogel would have provided its beneficial effect and resorption would be desired. To investigate this effect, the time course of hydrogel degradation might be altered by manipulating the polymer chemistry through the lability of the cleaved side group, while maintaining comparable mechanical properties. A second limitation of this report was associated with the small animal model employed. Since the hydrogel volumes injected and the volume of the infarcted LV wall would be markedly different in the clinical setting, a larger animal model is ultimately needed to more effectively evaluate the promise of this approach. Finally, the specific mechanism by which the hydrogel injection benefited cardiac function is not entirely clear, despite the theoretical model addressing the mechanical benefits [10] that provides a rationale for this approach and several recent experiments showing positive functional effects of LV bulking agents [18, 25, 26]. Quantification of ventricular wall mechanical properties during the implant period, using methods similar to those previously reported [9], would be of value in defining the relationship between residence of the injected gel and cardiac wall stiffness. As mentioned above, the role of the smooth muscle cells found in the injected LV wall on the functional effects observed is not clear, nor is the fate or origin of these cells. Further characterization of these cells would be of interest.
Conclusions
A novel biodegradable monomer, HEMAPTMC, was synthesized and used as a labile element within a thermoresponsive hydrogel, poly(NIPAAm-co-AAc-co-HEMAPTMC). Monomer feed ratios were optimized to make the hydrogel both gellable and ultimately bioabsorbable at body temperature (37°C). The selected poly(NIPAAm-co-AAc-co-HEMAPTMC) (86/4/10) had attractive mechanical properties, exhibited mass loss in vitro over a 20 week period and did not exhibit cytotoxicity. In evaluating this hydrogel in the cardiac injection application for which it was designed, injection of the material prevented ventricular dilation and improved contractile function in a chronic rat infarction model. Further evaluation of this material as a potential treatment for ischemic cardiomyopathy appears to be warranted.
Acknowledgements
This work was supported by the National Institutes of Health, NHLBI, grant #HL069368 and the Commonwealth of Pennsylvania.