Abstract
Synthetic materials can be electrospun into submicron or nanofibrous scaffolds to mimic extracellular matrix (ECM) scale and architecture with reproducible composition and adaptable mechanical properties. However, these materials lack the bioactivity present in natural ECM. ECM-derived scaffolds contain bioactive molecules that exert in vivo mimicking effects as applied for soft tissue engineering, yet do not possess the same flexibility in mechanical property control as some synthetics. The objective of the present study was to combine the controllable properties of a synthetic, biodegradable elastomer with the inherent bioactivity of an ECM derived scaffold. A hybrid electrospun scaffold composed of a biodegradable poly(ester-urethane)urea (PEUU) and a porcine ECM scaffold (urinary bladder matrix, UBM) was fabricated and characterized for its bioactive and physical properties both in vitro and in vivo. Increasing amounts of PEUU led to linear increases in both tensile strength and breaking strain while UBM incorporation led to increased in vitro smooth muscle cell adhesion and proliferation and in vitro mass loss. Subcutaneous implantation of the hybrid scaffolds resulted in increased scaffold degradation and a large cellular infiltrate when compared with electrospun PEUU alone. Electrospun UBM/PEUU combined the attractive bioactivity and mechanical features of its individual components to result in scaffolds with considerable potential for soft tissue engineering applications.
Keywords: Biodegradable, elastomer, electrospinning, polyurethane, scaffold, urinary bladder matrix
Introduction
There exists a need for biodegradable materials that exhibit elastomeric mechanical properties and possess bioactive components similar to native tissues. Such materials could be utilized in a large number of applications involving wound healing and tissue engineering. More specifically, elastomeric scaffolds are appropriate for cyclic mechanical conditioning that many believe desirable for soft tissue development [1]. One class of these materials, biodegradable poly(ester-urethane)urea (PEUU), have been noted for their reproducible elastomeric mechanical properties and non-toxic degradation products [2, 3].
While synthetic materials typically offer the advantage of reproducible control of material composition and physical properties, they are also more amenable to many processing methods to produce porous scaffolding appropriate for cell adhesion and growth for tissue reconstruction. One such method, electrospinning, permits fabrication of scaffolds that possess morphologies and mechanical behavior that can mimic those of the native extracellular matrix (ECM) [4, 5]. In addition, electrospinning of aligned PEUU scaffolds can be controlled to produce mechanical anisotropy that approximates certain native tissues [6]. However, these materials still lack some of the native bioactivity and biocompatibility that ECM-based scaffolds possess.
In order to improve upon the bioactivity of electrospun synthetics, electrospinning of natural ECM materials such as type-I collagen and elastin-based materials has been evaluated [5, 7]. In addition, reports have demonstrated fabrication of hybrid scaffolding by blending collagen with synthetic materials such as high-molecular-weight poly(ethylene oxide) to facilitate electrospinning [8]. Collagen was also blended with biodegradable elastomers such as PEUU or equimolar poly(l-lactide-co-ε-caprolactone) to achieve continuous electrospun fibers with improved mechanical properties and cellular adhesion [9, 10]. This method of combining synthetic and biological materials into submicron or nanoscale fibers can be applied to a broad variety of material combinations provided that appropriate solvents are available for the electrospinning process.
Naturally derived ECM scaffolds represent an alternative to synthetic materials and individual highly-purified proteins such as type-I collagen. Scaffolds derived from de-cellularized tissues and organs have been successfully used in a wide array of pre-clinical and clinical applications, including repair of the urinary tract, skin, vasculature, myocardium, esophageal and musculotendinous structures [11–14]. After removal of cells from a tissue, the resulting scaffold is a complex mixture of structural and functional proteins that constitute the ECM. ECM scaffolds are characterized by their ability to support the adhesion, proliferation and differentiation of a variety of cell types both in vitro and in vivo and by their ability to promote constructive tissue remodeling [12, 13, 15–17]. However, ECM-derived scaffolds can be limited to the inherent geometrical and mechanical properties of the tissue from which they are derived and do not possess the same degree of mechanical property control found in synthetic materials. In addition, the mechanical and material properties of ECM-derived scaffolds are subject to biologic variations, dependent upon the age of the host, the tissue of origin and the specific methods of processing.
Urinary bladder matrix (UBM) is an ECM scaffold derived from the porcine urinary bladder which contains an intact basement membrane [18]. UBM contains adhesion molecules such as fibronectin and laminin, structural proteins such as collagen and proteoglycans, and growth factors, chemokines and cytokines that could enhance cellular adhesion, migration and proliferation. UBM scaffolds have been shown to promote constructive tissue remodeling in a variety of tissue locations such as the esophagus [19], myocardium [20, 21] and larynx [22] making it an attractive material for an ECM-based scaffold.
The objective of the present study was to fabricate a superior hybrid natural and synthetic nanofibrous scaffold that combines advantages of each material component. More specifically, the attractive elastomeric properties of a biodegradable polyurethane were combined with the bioactivity and favorable host tissue response found in ECM derived scaffolding. An electrospun biohybrid scaffold was fabricated from a soluble form of UBM and a biodegradable and cytocompatible PEUU. Scaffolds of various UBM/PEUU weight ratios were processed by electrospinning. Resulting fibrous scaffolds were characterized for their fiber morphologies, thermal properties, tensile mechanical properties, mass loss and in vitro and in vivo cyto-compatibility to demonstrate their substantial potential for soft tissue engineering.
Materials and Methods
Poly(ester urethane)urea synthesis
All monomers and reagents were acquired from Sigma unless otherwise noted. 1,4-diisocyantobutane (BDI) and 1,4-butanediamine were purified by vacuum distillation. Poly(ε-caprolactone) diol (PCL, Mn = 2000) was dried under vacuum at 50°C for 48 h. Solvents dimethyl sulfoxide (DMSO) and N,N-dimethylformamide (DMF) were dried on 4-Å molecular sieves. Stannous octoate and 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP, Oakwood) were used as received.
Biodegradable PEUU was synthesized from PCL and BDI with chain extension by putrescine as previously reported [2]. The reaction occurred as a two-step solution polymerization in DMSO with a monomer feed molar ratio of 2:1:1 BDI:PCL:1,4-butanediamine. After PEUU precipitation and drying, PEUU transparent films were cast from a 3 wt% solution in DMF and dried under vacuum for 48 h.
Urinary bladder matrix preparation
The preparation of the porcine urinary bladder matrix has been previously described [16]. Porcine urinary bladders were harvested from pigs immediately after they were killed. Connective tissue and adipose tissue were removed from the serosal surface and any residual urine was removed by multiple water rinses. The tunica serosa, tunica muscularis externa, the tunica submucosa and most of the muscularis mucosa were mechanically removed and the luminal urothelial cells were dissociated by soaking in 1.0 M saline solution. The remaining basement membrane plus the subjacent tunica propria was referred to as urinary bladder matrix. UBM sheets were disinfected for 2 h on a shaker in a solution containing 0.1% (v/v) peracetic acid, 4% (v/v) ethanol and 95.9% (v/v) sterile water. Peracetic acid residue was removed by washing twice with sterile PBS (pH 7.4) and twice with sterile water for 15 min each time. UBM sheets were subsequently lyophilized and comminuted to form a powder [23]. 1 g lyophilized UBM powder and 100 mg pepsin were added to 100 ml of 0.01 M HCl under mechanical stirring for 48 h at room temperature. After pepsin digestion, the digest was aliquotted and stored at −20° C until use or at 4°C after initial thawing. UBM digest was then neutralized with PBS and lyophilized before electrospinning.
Electrospinning
UBM was dissolved in HFIP at various concentrations (6, 9, 10, 12 and 15 wt%). For hybrid scaffold electrospinning, UBM and PEUU were dissolved and blended in HFIP at a total concentration of 6 wt% at 3 different UBM/PEUU ratios (25, 50, 75 solute wt% UBM relative to PEUU) and then electrospun. A similar technique to that described previously was utilized for electrospinning [10]. The electrospinning process consisted of feeding the polymer or the polymer/ECM solution at 1.0 ml/h through Teflon tubing to a stainless steel capillary (type 316, 1.2-mm I.D.) located 15 cm from an aluminum disc target. Samples were electrospun by charging the polymer solution at 10 kV and the aluminum target at −10 kV. The target was also attached to x–y linear stages repeatedly translating in a square pattern with 5-cm sides to produce scaffolds of uniform thickness of approximately 250 μm. Scaffolds were subsequently dried under vacuum at room temperature for 48 h.
Electrospun UBM/PEUU scaffold characterization
Scaffolds were imaged by scanning electron microscopy (SEM) to characterize scaffold fiber morphology. The samples were attached to aluminum SEM specimen mounting stubs, sputter coated with a Pd/Au alloy, and then examined using a scanning electron microscope (JEOL 6330F). Differential scanning calorimetry (DSC) was performed on a differential scanning calorimeter (Shimadzu, DSC 60) under helium and nitrogen purge. Temperature scanning rates of 20°C/min were employed over a range of −100°C to 200°C. Uniaxial tensile mechanical properties such as tensile strength and breaking strain were measured on an ATS universal testing device using a 10 mm/min crosshead speed according to ASTM D638-98 (n = 4 of each sample type).
In vitro scaffold mass loss was measured in PBS at 37°C over 2 weeks. Dry scaffolds were weighed and immersed in 15 ml PBS (pH 7.4) and incubated at 37°C. Samples were removed at the time points studied and lyophilized for 48 h prior to weighing (n = 4 of each sample type at each time point). Mass loss was calculated as:
| (1) |
where m1 and m2 are the masses of films before and after degradation, respectively.
Cytocompatibility analysis
Scaffold cytocompatibility was determined through in vitro smooth muscle cell culture. Vascular smooth muscle cells (SMCs) were isolated from rat aortas and cultured on tissue culture polystyrene (TCPS) flasks and expanded [24]. Media consisted of Dulbecco's Modified Eagle Medium with 10% fetal bovine serum and 1% penicillin–streptomycin (1 unit penicillin and 1 ug streptomycin/ml medium). SMCs were subcultured and statically seeded at 200 μl of 15 × 104 cells/ml on 7-mm scaffold discs firmly placed into the bottoms of 96-well TCPS plates. Empty TCPS wells were used as controls. Scaffolds were exposed to UV light for 2 h prior to seeding. Media was replaced in the wells every 2 days. Cell viability was evaluated 1, 4 and 8 days after seeding using the MTT mitochondrial activity assay (n = 4 per sample type).
Cell morphology was observed by SEM and confocal microscopy. For SEM, samples were rinsed in PBS, fixed in 2.5% glutaraldehyde and 1% osmium tetraoxide, dehydrated in graded ethanol treatments and then critical point dried and sputter coated with Pd/Au before imaging. For fluorescent imaging, samples were rinsed with PBS, fixed in 2% paraformaldehyde, permeabilized with 0.1% Triton X-100 and then nuclei stained with draq-5 (Biostatus) and f-actin stained with rhodamine phalloidin (Molecular Probes). Representative images on a Leica TCS-SL laser scanning confocal microscope were taken as a series of stacked images.
Subcutaneous implantation
The host tissue response to implanted hybrid scaffolds was determined using a rat subcutaneous implant model. All animal procedures were performed in accordance with the National Institutes of Health guidelines for care and use of laboratory animals and with the approval of the Institutional Animal Care and Use Committee.
Sprague–Dawley rats (Charles River Laboratories) weighing less than 500 g were anesthetized via induction of isofluorane (2% in oxygen), shaved and prepped for surgery. Bi-lateral subcutaneous pockets were created in the subcutaneous tissue by making incisions of approximately 5 mm in length caudal to the scapula. Scaffold discs (10-mm diameter) were placed in the subcutaneous pocket and secured at two locations with 2-0 prolene sutures. Test samples consisted of PEUU, UBM/PEUU (25:75) and UBM/PEUU (75:25) scaffolds sterilized by exposure to UV light for 2 h and randomly assigned to either the left or right subcutaneous pocket. Incisions were closed using a non-absorbable suture and the animals were recovered from anesthesia and allowed normal ambulation and diet for the remainder of the study period. Bupronex (0.02 mg) was administered subcutaneously the day of surgery and one day post-surgery. Gentamycin (2 mg) was administered subcutaneously the day of the surgical procedure and for two days post-surgery. The animals survived for 28 days. Following killing, the operative site was identified and the excised tissues were placed in 10% neutral buffered formalin fixative. The harvested specimens were then trimmed, embedded in paraffin, sectioned and stained with Masson's Trichrome stain.
Statistics
Results for the mechanical and in vitro testing are displayed as the mean ± standard deviation. Linearity of mechanical properties and mass loss were measured with Pearson's correlation. One-factor analysis of variance (ANOVA) was utilized to evaluate cell viability and cell growth using the Neuman–Keuls test for post hoc assessments of the differences between samples.
Results and Discussion
Electrospun UBM scaffolds
In the present study, the electrospinning of UBM digest alone was evaluated using an appropriate processing solvent such as HFIP. Solution concentration has been shown to be a significant variable which affects scaffold morphology when electrospinning [10]. Therefore, the effect of UBM concentration on resulting scaffold morphology while holding all other process variables constant was investigated. UBM was dissolved in HFIP at various concentrations (6, 9, 10, 12 and 15 wt%) and electrospun. At a UBM concentration of 6 wt%, electrospinning of continuous fibers was not achieved and instead electrospraying of UBM particulates occurred. Electrospinning of fibers was possible at UBM concentrations of 9 wt% and greater but UBM agglomerates or “beads on a string” morphologies were present at all concentrations. Scanning electron micrographs demonstrating electrospun UBM scaffold microstructures are shown in Fig. 1. The “beads on a string” morphology could be attributed to instabilities in the Taylor cone due to electrospraying of UBM droplets, insoluble UBM, or a combination of both phenomena. At UBM concentrations too low for sufficient protein chain entanglements, electrospraying of droplets can occur. The “beads on a string” morphology was more evident in the 9 and 10 wt% samples. At 12 and 15 wt%, the presence of UBM agglomerates was more pronounced. After pepsin solubilization, UBM may still possess undigested proteins which may not be soluble in the electrosprocessing solvent. In addition, at concentrations greater than 15 wt% the solution was too viscous to feed through the nozzle. The presence of beads or agglomerates can disrupt the fibrous architecture resulting in decreased tensile strength and elongation. Therefore, the absence of continuous artifact free electrospun UBM fibers was a factor in transitioning to a hybrid scaffold approach that consisted of combining soluble UBM with a synthetic biodegradable elastomer.
Figure 1.
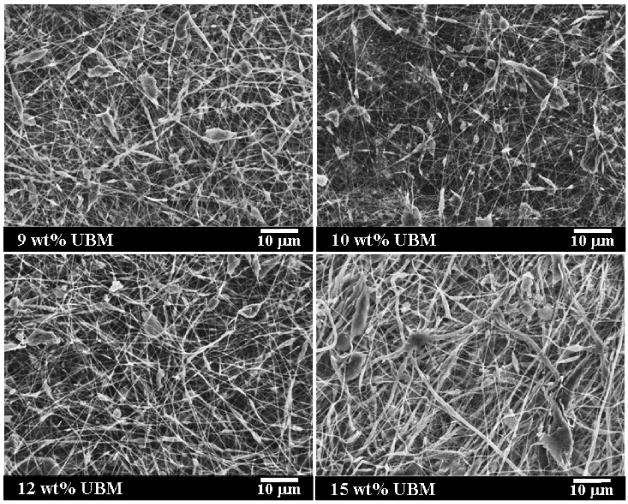
Morphologies of electrospun UBM scaffolds from 9 (a), 10 (b), 12 (c) and 15 wt% (d) UBM in HFIP by SEM. Scale bar = 10 μm, 1400 × magnification.
Electrospun UBM/PEUU scaffold fabrication and analysis
In contrast to electrospinning UBM alone, electrospinning of UBM/PEUU blends produced continuous fibers at total solute concentrations as low as 6 wt%. Continuous fibers were observed at UBM/PEUU ratios of 25:75, 50:50 and 75:25, as shown in Fig. 2. It is likely that PEUU helped stabilize the spinning stream and assisted in UBM solubilization. For UBM/PEUU 75:25, the scaffold morphology demonstrated a collection of larger and smaller sized fibers which may have indicated some degree of phase separation between UBM and PEUU at this ratio.
Figure 2.
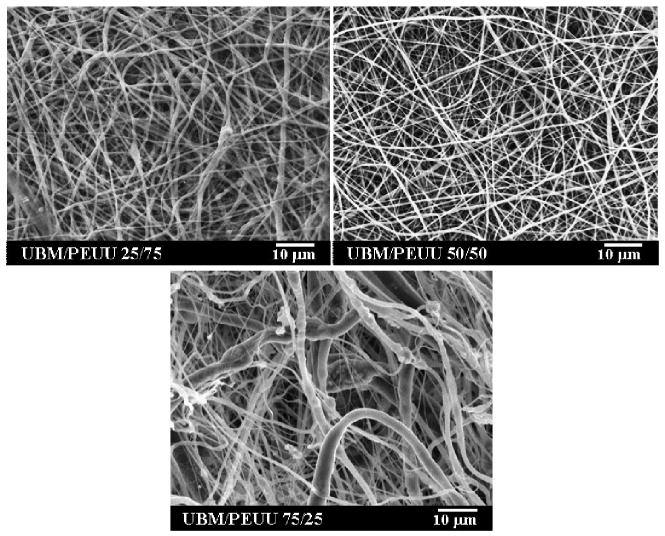
SEMs exhibiting continuous fiber morphologies of electrospun UBM/PEUU at 25, 50 and 75% UBM relative to PEUU. Scale bar = 10 μm, 1400 × magnification.
Scaffold thermal analysis
DSC was utilized to compare the thermal properties of electrospun UBM/PEUU with both UBM and PEUU controls as displayed in Fig. 3. With PEUU control, the DSC spectrum demonstrated a glass transition temperature, Tg, well below room temperature of −56.0°C and a soft segment melting temperature at 41.0°C in Fig. 3b. The UBM control demonstrated a very broad transition ranging between 30°C and 150°C with a peak at 108°C representing the loss of water or dehydration within the UBM (Fig. 3c) [25]. Upon cooling again to -100°C and performing a second heating run on the same UBM control sample this broad peak was not evident (data not shown), which indicated thermally dehydrated collagen. Upon dehydration, the UBM secondary structure and hydrogen bonding would be modified. Therefore, the presence of the broad dehydration peak was utilized to indicate some degree of structure retention in the hybrid materials. Samples containing less than 75% UBM relative to PEUU did not display a significantly large dehydration peak and have been omitted from Fig. 3 for clarity. Electrospun UBM/PEUU 75:25 did demonstrate some degree of retained UBM structure as shown in Fig. 3a. In all hybrid samples investigated there was no significant change in the Tg of the PEUU component which indicated that UBM and PEUU were immiscible.
Figure 3.
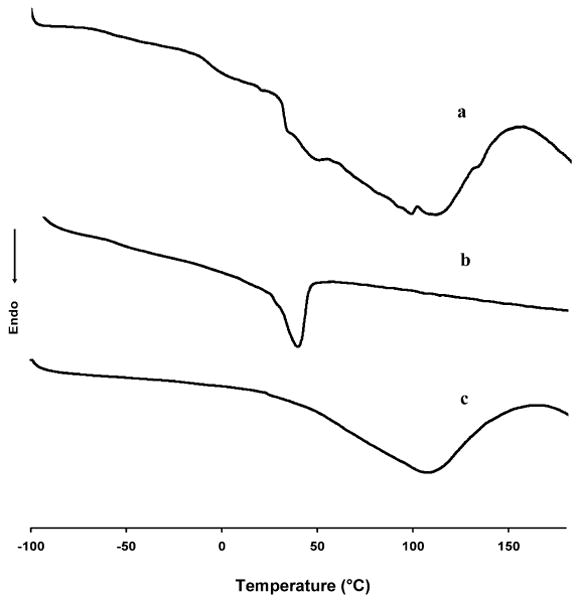
Differential scanning calorimetry spectra for electrospun UBM/PEUU (75:25) (a), electrospun PEUU alone (b) and UBM (c).
Scaffold mechanical properties
After electroprocessing, UBM alone was quickly degraded. Mechanically, it was found to be not ideally suited for use as a load-bearing scaffold which may have been due to the presence of UBM agglomerates within the fibers. Others who have investigated electrospinning of type-I collagen have looked at chemical cross-linking methods such as glutaraldehyde vapor treatment to improve the mechanical properties and extend degradation time of electrospun collagen [5]. However, the introduction of chemical cross-linkers can result in unwanted toxic residuals remaining in the matrix. Also, chemical cross-linking may remove some degree of inherent bioactivity present in the ECM component of the scaffold [26].
PEUU has been demonstrated to possess elastomeric properties suitable for soft tissue engineering and degrade into cytocompatible products [10, 27]. Electrospun UBM/PEUU blends were both strong and distensible with typical stress/strain curves shown in Fig. 4. One can observe a trend of increasing tensile strengths and breaking strains with increasing amounts of PEUU blended with UBM. In fact, approximately linear increases in both tensile strength and breaking strains were observed with increasing concentration of PEUU blended into the matrix (Fig. 5, r2 = 0.93 and r2 = 0.98, respectively). Tensile strengths ranged from 2 to 13 MPa and breaking strains from 38 to 220% with values increasing with higher PEUU content (Fig. 5). The maximum stresses and maximum strains of the biohybrid scaffolds were similar or greater than previously reported values for lyophilized sheets of UBM (0.3–0.4 MPa and 47–67% strain, respectively) [19, 28]. It is believed that addition of larger amounts of UBM may cause some disruptions in the PEUU physical cross-linking or hydrogen bonding (in addition to the mass reduction effect) that could have lead to the reductions in tensile strengths and breaking strains. These trends were also observed previously with increasing amounts of collagen when blended with PEUU and electrospun [10].
Figure 4.
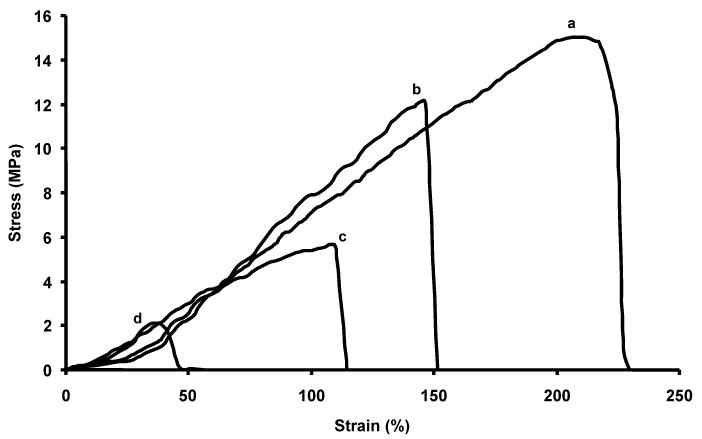
Typical uniaxial stress–strain curves are shown for electrospun PEUU (a), electrospun UBM/PEUU (25:75) (b), electrospun UBM/PEUU (50:50) (c) and electrospun UBM/PEUU (75:25) (d). Each curve represents data plotted from a single sample that best represents each sample type.
Figure 5.
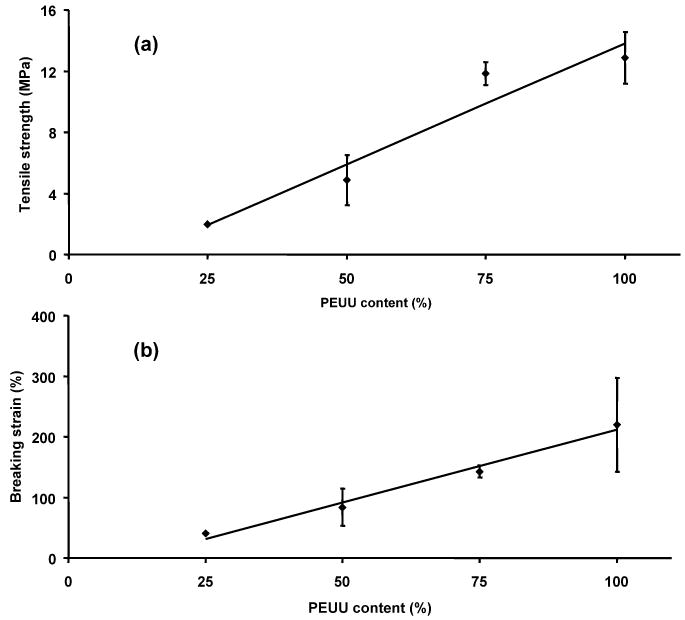
Summary of tensile strengths (a) and breaking strains (b) for electrospun UBM/PEUU scaffolds as a function of PEUU content. r2 = 0.93 (a), r2 = 0.98 (b), P < 0.05.
Scaffold in vitro degradation
In addition to increased tensile properties, hybrid scaffolds were substantially more resistant to hydrolytic degradation in vitro when compared to electrospun UBM alone, which degraded quickly under static aqueous conditions. Hybrid scaffolds experienced increased degradation as the mass fraction of UBM increased. The mass loss of electrospun UBM/PEUU scaffold at 2 weeks in vitro at 37°C in PBS buffer can be observed in Fig. 6. A linear increase in mass loss from 18, 42 and 66% with UBM contents of 25, 50 and 75%, respectively, was demonstrated (Fig. 6, r2 = 0.98). Considering that electrospun PEUU alone experienced a 5% mass loss at 2 weeks and that UBM rich phases would be expected to degrade significantly faster, analysis of data in Fig. 6 suggests that at least 10% of UBM should remain at any concentration studied after 2 weeks. This retained UBM might impart bioactive properties to the matrix and may be shielded from degradation and solubilization by the slower degrading PEUU, The retained UBM could also consist of UBM fragments that have become physically entrapped in the micropores of the fibrous matrix. It is important to note that this degradation measured in vitro is occurring in the absence of major enzymatic activity, fluid convection or load-bearing function which could significantly accelerate material degradation in vivo.
Figure 6.
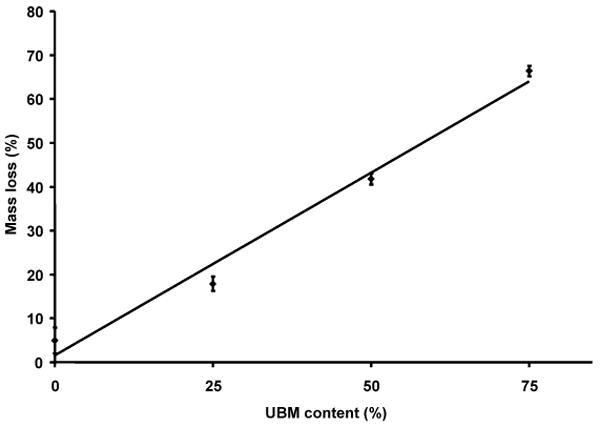
In vitro degradation of electrospun UBM/PEUU as a function of UBM content. Mass loss was measured after incubating samples at 2 weeks in DPBS buffer at 37°C (r2 = 0.98, P < 0.05).
Electrospun UBM/PEUU in vitro cytocompatibility
Hybrid scaffold cytocompatibility was evaluated by statically seeding and culturing primary rat vascular smooth muscle cells on scaffold samples. Electrospun UBM/PEUU at all concentrations supported cell attachment and proliferation. Viable cell numbers on the scaffolds were measured at 1, 4 and 8 days after cell seeding using the MTT mitochondrial activity assay with the results summarized in Fig. 7. Values were compared with TCPS control wells and electrospun PEUU alone at each time point. Cell adhesion 1 day after cell seeding was significantly higher for samples containing UBM compared with TCPS controls (P < 0.05). Cell proliferation at day 4 was significantly higher for UBM samples in comparison to TCPS and electrospun PEUU alone (P < 0.05). At day 8, cell numbers were significantly greater for electrospun UBM/PEUU 25:75 compared with electrospun PEUU and significantly greater for electrospun UBM/PEUU 75:25 compared with either TCPS control or electrospun PEUU alone (P < 0.05).
Figure 7.
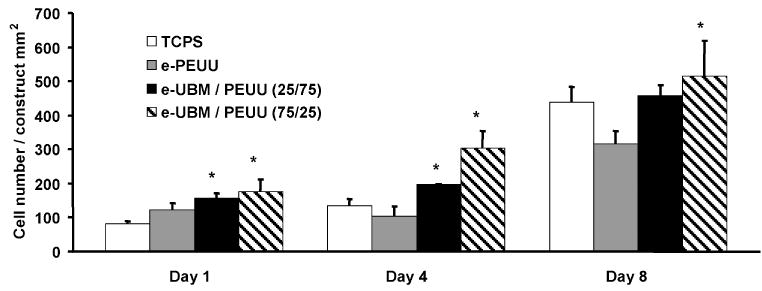
Vascular smooth muscle cell adhesion and proliferation on electrospun UBM/PEUU scaffolds of 0, 25 and 75% UBM and tissue culture polystyrene as a control. Cells were statically seeded on the scaffolds and cultured for 8 days in vitro. Viability was measured using the MTT mitochondrial activity assay. Scaffolds containing UBM exhibited significantly higher cell numbers (*P < 0.05) compared with TCPS and electrospun PEUU controls.
The improved cytocompatibility results for the hybrid scaffolds could be attributed to retained bioactivity in the electrospun UBM or greater numbers of adhesive peptides present in the UBM component of the hybrid scaffold. The presence of cell adhesion sites on collagen and other proteins naturally present within the UBM (e.g., fibronectin, laminin) could have resulted in the increased cell-adhesion response. Sites present on enzymatically digested proteins such as fibronectin, laminin and collagens, all present in the UBM scaffold, have also been shown to have bioactivity [29, 30]. Bioactive peptides as a result of enzymatic digestion and the presence of growth factors that may have survived the digestion and acidic conditions could have led to the increased cellular proliferation observed for the hybrid scaffold [31].
A scanning electron micrograph and a fluorescent image of smooth muscle cells cultured on electrospun UBM/PEUU 75:25 are shown in Fig. 8. The cells appear spread near the scaffold surface and protrude beneath some fiber layers in the electron micrograph. It is thought that the cells can push and enzymatically degrade their way into the bioactive nanofiber matrix. The decreased tensile strength and increased degradation of the UBM/PEUU 75:25 may have facilitated increased cell infiltration in this scaffold when compared to scaffolds with a higher PEUU content. This behavior could explain the increased cell numbers at day 8 compared with electrospun UBM/PEUU 25:75 and PEUU alone. Results have also shown that cell infiltration into electrospun synthetics was difficult or required extended culture periods [32]. This difficulty can be overcome by a combined electrospinning and cell electrospraying method. Previously, a microintegration approach was demonstrated by which SMCs were seeded simultaneously while electrospinning PEUU to achieve highly cellularized constructs in vitro [33]. This process may also apply to the hybrid scaffolds developed here.
Figure 8.
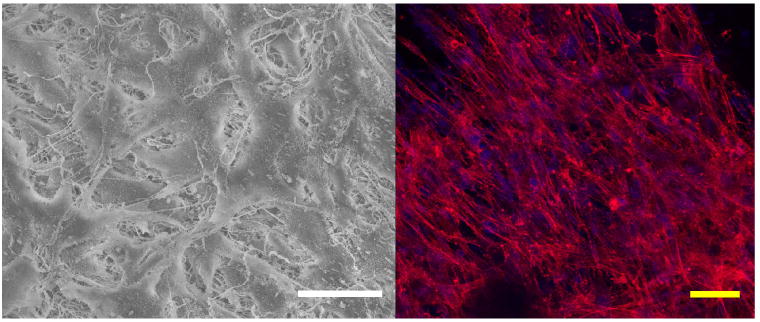
Representative smooth muscle cell morphology cultured on electrospun UBM/PEUU (75:25) as imaged by SEM (left panel, 550× magnification) and fluorescence (right panel, 400× magnification). Scale bars = 50 μm. This figure is published in colour at http://www.ingenta.com
Electrospun UBM/PEUU host tissue response
The host tissue response and in vivo degradation of electrospun PEUU and UBM/PEUU hybrid scaffolds were evaluated using a rat subcutaneous model. There was no gross evidence of infection at the time of harvest at 28 days after implantation for both PEUU and UBM/PEUU. The host tissue response to the electrospun PEUU was characterized by encapsulation of the scaffold by mononuclear cells with little or no scaffold invasion or degradation as shown in Fig. 9a. UBM/PEUU (25:75) scaffolds were characterized by a thick cellular layer surrounding the material with small amounts of cellular infiltration when compared to the PEUU scaffold alone, as well as some evidence of scaffold layer delamination, as shown in Fig. 9b. Degradation into fibrous lamellae was more pronounced in the UBM/PEUU (75:25) scaffolds when compared to PEUU alone or UBM/PEUU (25:75), which were characterized by a dense cellular infiltrate and reduced areas of acellular scaffold (Fig. 9c).
Figure 9.
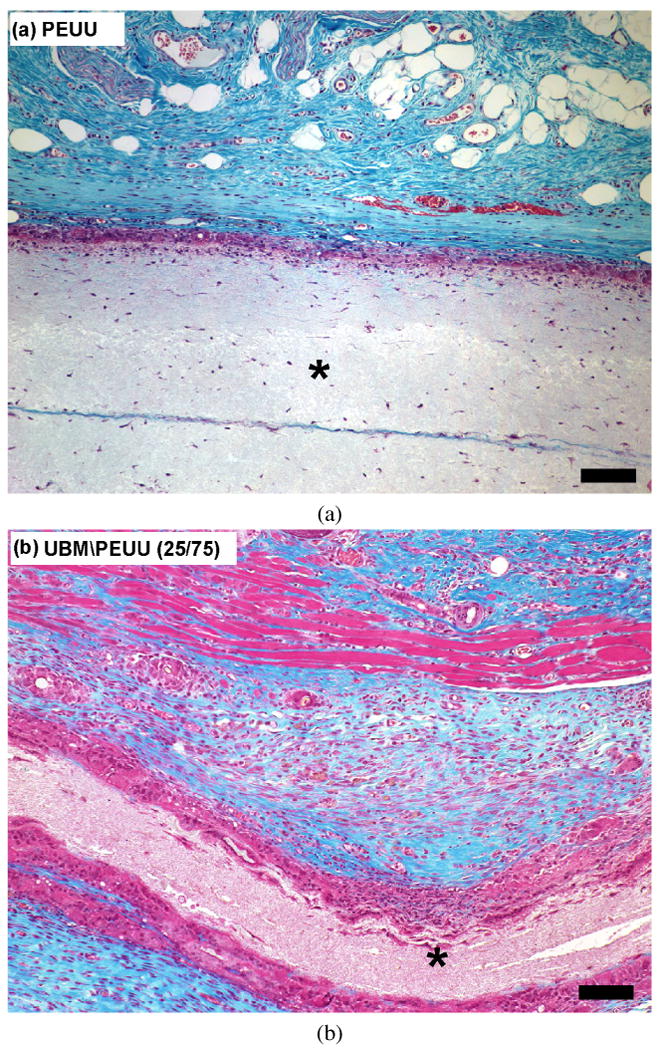
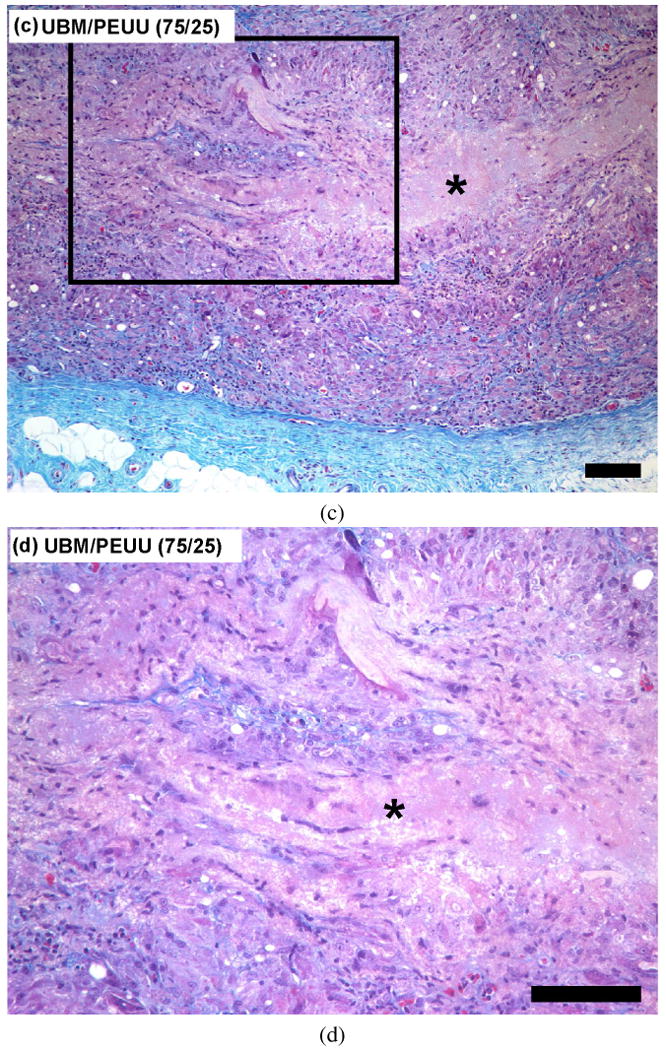
Masson's Trichrome stain of cross-sectional views of scaffolds implanted in a subcutaneous rat pocket for 28 days: (a) PEUU scaffold 10×; (b) UBM/PEUU (25:75) scaffold 10×. An asterisk (*) denotes scaffold material, scale bars = 100 μm. This figure is published in colour at http://www.ingenta.com (c) UBM/PEUU (75:25) 10×; (d) UBM/PEUU (75:25) 20× magnification of box shown in (c).
The increase in scaffold degradation and cellular infiltration with increasing UBM concentration in vivo is consistent with the degradation rates and cellular adhesion and proliferation observed in vitro. UBM/PEUU (75:25) scaffolds were degraded and possessed cells throughout the cross-section of the material with new ECM present that was most likely deposited by the cellular infiltrate (Fig. 9d). The degree of cellular infiltration appears to be directly related to the amount of UBM incorporated and the degradation of the materials and likely there was positive feedback between these processes. The morphological appearance of the scaffolds after 28 days in a subcutaneous location showed that the addition of the UBM to the electrospun scaffolds resulted in changes in the host tissue response. Future studies will be necessary to characterize the types of cells that are recruited as a result of the UBM component of the hybrid materials. It is also important to note that although the initial mechanical properties can be controlled during the manufacturing, long-term outcome of the scaffold properties will ultimately depend upon the host tissue response and tissue remodeling.
Conclusions
A soluble form of the urinary bladder ECM was combined with a biodegradable and cytocompatible polyurethane through electrospinning to create elastomeric biohybrid scaffolds. This hybrid scaffolding material resulted in increased mechanical robustness and flexibility from the synthetic component and increased cell adhesion, proliferation and in vitro degradation from the natural component. The hybrid scaffold also showed increased degradation and changes in the host tissue response in vivo. These hybrid scaffolds have the potential to be utilized as synthetic versions of ECM scaffolds in soft tissue engineering applications where sufficient strength, elasticity, and ECM induced tissue remodeling may be required for a positive structural and functional tissue engineering outcome.
Table 1.
Summary of uniaxial tensile properties and in vitro degradation of electrospun UBM/PEUU
| % UBM/PEUU | Tensile strength (MPa) | Breaking strain (%) | Mass loss at 2 wks (%) |
|---|---|---|---|
| 0:100 | 12.9 ± 1.7 | 220 ± 77 | 5 ± 3 |
| 25:75 | 11.8 ± 0.7 | 143 ± 10 | 18 ± 2 |
| 50:50 | 4.9 ± 1.6 | 83 ± 31 | 42 ± 1 |
| 75:25 | 2.0 ± 0.1 | 40 ± 0.6 | 66 ± 1 |
Acknowledgments
J. J. S. and D. O. F. contributed equally to this manuscript. This work was supported by the National Institutes of Health (#HL069368) and the National Tissue Engineering Center. We acknowledge the staff at the Center for Biologic Imaging at the University of Pittsburgh for their assistance. We thank Drs. Jianjun Guan, Kazuro Fujimoto and Yi Hong for their support and guidance on this project. We also thank Dr. Hongbin Jiang, Aaron Dean and Jolene Valentin for their invaluable assistance with the animal study.
References
- 1.Niklason LE, Gao J, Abbott WM, Hirschi KK, Houser S, Marini R, Langer R. Science. 1999;284:489. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- 2.Guan J, Sacks MM, Beckman EJ, Wagner WR. J Biomed Mater Res. 2002;61:493. doi: 10.1002/jbm.10204. [DOI] [PubMed] [Google Scholar]
- 3.Guan J, Fujimoto KL, Sacks MS, Wagner WR. Biomaterials. 2005;26:3961. doi: 10.1016/j.biomaterials.2004.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li WJ, Laurencin CT, Caterson EJ, Tuan RS, Ko FK. J Biomed Mater Res. 2002;60:613. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- 5.Matthews JA, Wnek GE, Simpson DG, Bowlin GL. Biomacromolecules. 2002;3:232. doi: 10.1021/bm015533u. [DOI] [PubMed] [Google Scholar]
- 6.Courtney T, Sacks MS, Stankus J, Guan J, Wagner WR. Biomaterials. 2006;27:3631. doi: 10.1016/j.biomaterials.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 7.Huang L, McMillan RA, Apkarian RP, Pourdeyhimi B, Conticello RP, Chaikof EL. Macromolecules. 2000;33:2989. [Google Scholar]
- 8.Huang L, Nagapudi K, Apkarian RP, Chaikof EL. J Biomater Sci Polymer Edn. 2001;12:979. doi: 10.1163/156856201753252516. [DOI] [PubMed] [Google Scholar]
- 9.Kwon IK, Matsuda T. Biomacromolecules. 2005;6:2096. doi: 10.1021/bm050086u. [DOI] [PubMed] [Google Scholar]
- 10.Stankus J, Guan J, Wagner WR. J Biomed Mater Res A. 2004;70:603. doi: 10.1002/jbm.a.30122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Badylak S, Obermiller J, Geddes L, Matheny R. Heart Surg Forum. 2003;6:E20. doi: 10.1532/hsf.917. [DOI] [PubMed] [Google Scholar]
- 12.Badylak SF. Cell Dev Biol. 2002;13:377. doi: 10.1016/s1084952102000940. [DOI] [PubMed] [Google Scholar]
- 13.Badylak SF. Transplant Immunol. 2004;12:367. doi: 10.1016/j.trim.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 14.Badylak SF, Lantz G, Coffey A, Geddes LA. J Surg Res. 1989;47:74. doi: 10.1016/0022-4804(89)90050-4. [DOI] [PubMed] [Google Scholar]
- 15.Badylak SF. In: Extracellular Matrix Scaffolds. Wnek GE, Bowlin GL, editors. Marcel Dekker; New York, NY: 2004. p. 910. [Google Scholar]
- 16.Freytes DO, Badylak SF, Webster TJ, Geddes LA, Rundell AE. Biomaterials. 2004;25:2353. doi: 10.1016/j.biomaterials.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 17.Sarikaya A, Record R, Wu CC, Tullius B, Badylak S, Ladisch M. Tissue Eng. 2002;8:63. doi: 10.1089/107632702753503063. [DOI] [PubMed] [Google Scholar]
- 18.Brown B, Lindberg K, Reing J, Stolz DB, Badylak SF. Tissue Eng. 2006;12:519. doi: 10.1089/ten.2006.12.519. [DOI] [PubMed] [Google Scholar]
- 19.Badylak SF, Vorp DA, Spievack AR, Simmons-Byrd A, Hanke J, Freytes DO, Thapa A, Gilbert TW, Nieponice A. J Surg Res. 2005;128:87. doi: 10.1016/j.jss.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Robinson KA, Li J, Mathison M, Redkar A, Cui J, Chronos NA, Matheny RG, Badylak SF. Circulation. 2005;112:I135. doi: 10.1161/CIRCULATIONAHA.104.525436. [DOI] [PubMed] [Google Scholar]
- 21.Kochupura PV, Azeloglu EU, Kelly DJ, Doronin SV, Badylak SF, Krukenkamp IB, Cohen IS, Gaudette GR. Circulation. 2005;112:I144. doi: 10.1161/CIRCULATIONAHA.104.524355. [DOI] [PubMed] [Google Scholar]
- 22.Ringel RL, Kahane JC, Hillsamer PJ, Lee AS, Badylak SF. J Speech Lang Hearing Res. 2006;49:194. doi: 10.1044/1092-4388(2006/016). [DOI] [PubMed] [Google Scholar]
- 23.Gilbert TW, Stolz DB, Biancaniello F, Simmons-Byrd A, Badylak SF. Biomaterials. 2005;26:1431. doi: 10.1016/j.biomaterials.2004.04.042. [DOI] [PubMed] [Google Scholar]
- 24.Ray JL, Leach R, Herbert JM, Benson M. Methods Cell Sci. 2002;23:185. doi: 10.1023/a:1016357510143. [DOI] [PubMed] [Google Scholar]
- 25.McClain PE, Wiley ER. J Biol Chem. 1972;247:692. [PubMed] [Google Scholar]
- 26.Valentin JE, Badylak JS, McCabe GP, Badylak SF. J Bone Joint Surg Am. 2007;88:2673. doi: 10.2106/JBJS.E.01008. [DOI] [PubMed] [Google Scholar]
- 27.Guan J, Sacks MM, Beckman EJ, Wagner WR. Biomaterials. 2004;25:85. doi: 10.1016/s0142-9612(03)00476-9. [DOI] [PubMed] [Google Scholar]
- 28.Freytes DO, Rundell AE, Vande Geest J, Vorp DA, Webster TJ, Badylak SF. Biomaterials. 2005;26:5518. doi: 10.1016/j.biomaterials.2005.01.070. [DOI] [PubMed] [Google Scholar]
- 29.Sephel GC, Tashiro K, Sasaki M, Kandel S, Yamada Y, Kleinman HK. Dev Biol. 1989;135:172. doi: 10.1016/0012-1606(89)90167-x. [DOI] [PubMed] [Google Scholar]
- 30.Davis GE, Bayless KJ, Davis MJ, Meininger GA. Am J Pathol. 2000;156:1489. doi: 10.1016/S0002-9440(10)65020-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDevitt CA, Wildey GM, Cutrone RM. J Biomed Mater Res A. 2003;67:637. doi: 10.1002/jbm.a.10144. [DOI] [PubMed] [Google Scholar]
- 32.Telemeco TA, Ayres C, Bowlin GL, Wnek GE, Boland ED, Cohen N, Baumgarten CM, Mathews J, Simpson DG. Acta Biomater. 2005;1:377. doi: 10.1016/j.actbio.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 33.Stankus JJ, Guan J, Fujimoto K, Wagner WR. Biomaterials. 2006;27:735. doi: 10.1016/j.biomaterials.2005.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]


